Abstract
Background
Frequent exacerbations of bronchiectasis lead to poor quality of life, impaired lung function, and higher mortality rates. This study aims to evaluate the risk factors associated with readmission within one year due to acute exacerbation of bronchiectasis (AEB).
Methods
A retrospective cohort study was performed on 260 patients with bronchiectasis who were hospitalized in the respiratory and critical care department of a tertiary hospital in China. Univariate and multivariate Cox analyses were used to evaluate the risk factors for readmission within one year.
Results
Readmission within one year was found in 44.6% of 260 patients hospitalized with acute exacerbation of bronchiectasis. The risk factors associated with readmission included age over 65 years (HR = 3.66; 95% CI: 2.30 to 5.85), BMI < 18.5 kg/m2 (HR = 1.71; 95% CI: 1.16 to 2.51), respiratory intensive care unit (RICU) stay during admission (HR = 2.06, 95% CI: 1.16–3.67), involvement of 3 or more lobes on chest high-resolution computed tomography (HRCT) (HR = 1.85; 95% CI, 1.22 to 2.80), chronic Pseudomonas aeruginosa (PA) colonization (HR = 2.29; 95% CI: 1.54 to 3.38), and positive sputum culture results within 24 h after admission (HR = 1.93; 95% CI: 1.27 to 2.94). Long-term oral antibiotics use after discharge was associated with decreased hazard of readmission (HR = 0.34; 95% CI: 0.20 to 0.59).
Conclusions
Patients with bronchiectasis have a high rate of readmission, which is linked to varieties of risk factors, and identifying these risk factors is importance for effectively managing patients with bronchiectasis.
Keywords: Bronchiectasis, Readmission, Hospitalization, Risk factors
Background
Bronchiectasis is defined as the pathological, and often irreversible, dilatation of the tracheobronchial tree that is due to recurrent airway infections and inflammation [1]. Several studies conducted in Western countries have consistently demonstrated a gradual rise in the incidence and economic impact of bronchiectasis, particularly among the elderly population [2–6]. However, the accurate incidence and prevalence of bronchiectasis in China remain inadequately documented and potentially underestimated. In China, bronchiectasis has historically been recognized as one of the prevalent chronic airway diseases [7], and it has been estimated that 1.5% of women and 1.1% of men in the general population have physician-diagnosed bronchiectasis [8]. The number of diagnosed cases of bronchiectasis in China is believed to be steadily increasing, garnering greater attention. This trend can be attributed, at least in part, to the enhanced recognition of the disease, facilitated by the growing utilization of computed tomography (CT) for the assessment of lung disorders [7, 9].
A distinct feature of bronchiectasis is the tendency toward exacerbations. Patients with bronchiectasis have frequent exacerbations that are a cause of significant morbidity and sometimes mortality, and are desirable to prevent [1]. Exacerbations are independently associated with a poor quality of life, decreased lung function, and increased mortality [10–12]. According to previous studies [13, 14], the frequency of readmission after discharge is higher for patients with bronchiectasis with other chronic airway diseases such as chronic obstructive pulmonary disease (COPD) and asthma as well as for those with certain pathogen infections such as Pseudomonas aeruginosa (PA) and fungi. Additionally, decreased lung function, radiological severity, and malnutrition have also been identified as risk factors for readmission. International guidelines for bronchiectasis recommend treatment with inhaled or oral prophylactic antibiotic therapy for patients with 3 or more exacerbations per year with the aim of preventing exacerbations [1]. Exacerbation frequency or time to next exacerbation are the primary end-point in clinical trials evaluating new treatments in adults with bronchiectasis [1, 12]. Hospitalization for intravenous therapy is serious clinical conditions in acute exacerbation of bronchiectasis (AEB) because intravenous therapy, especially intravenous antibiotics is necessary to improve symptoms of AEB (usually more than a few days) (cough, increased sputum or altered viscosity, increased sputum, with or without wheezing, dyspnea, hemoptysis) [15, 16]. In clinical practice, it is important for clinicians to identify patients who are at risk of future exacerbations after last hospitalization of exacerbation in order to target preventative therapies appropriately. This study aims to evaluate the risk factors associated with readmission due to AEB within one year after discharge in patients admitted to a teaching hospital in Shanghai, China.
Methods
Study population
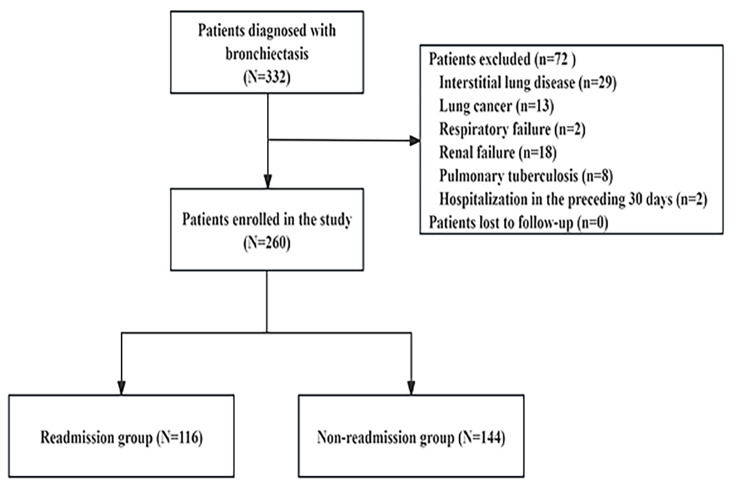
A retrospective cohort study was performed on 260 patients with bronchiectasis who were hospitalized in the Department of Respiratory Medicine, Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine between January 2019 and December 2021 (Fig. 1). Patients over 18 years old, with diagnosis of non-cystic fibrosis bronchiectasis on high-resolution computed tomography (HRCT) scans and follow-up for at least one year, were enrolled in this study to determine risk factors for one-year readmission for acute exacerbations in bronchiectasis. Exclusion criteria in this study are as follows: (1) severe immunosuppression in solid-organ or bone-marrow transplantation or HIV/AIDS; (2) patients with malignancies; (3) severe organ dysfunction; (4) active tuberculosis or nontuberculous mycobacterial infection; (5) patients with mood disturbances or cognitive impairment; (6) cystic fibrosis or traction bronchiectasis due to interstitial lung disease; (7) hospitalization in the preceding 30 days; (8) with incomplete clinical information. This study was approved by the Institutional Review Board of Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine and the Clinical Trial Number was NO. ChiCTR2000033494.
Fig. 1.
Flow diagram depicting patient selection and analysis
Diagnosis of bronchiectasis
Bronchiectasis is defined by bronchial dilatation as suggested by one or more of the following: (1) broncho arterial ratio > 1 (internal airway lumen vs. adjacent pulmonary artery); (2) lack of tapering; (3) airway visibility within 1 cm of costal pleural surface or touching mediastinal pleura.1 The definition of hospitalization for the acute exacerbation of bronchiectasis in this study according to guidelines of British Thoracic Society was as follows: a person with bronchiectasis with the worsening of three or more of the six symptoms of cough, changes in sputum volume, purulent sputum, dyspnea or exercise tolerance, fatigue or discomfort, and hemoptysis > 48 h requiring treatment [17].
Definitions of other variables
Investigators of this study reviewed the electronic records and collected the following variables: demographic information (age, gender, height, weight, body mass index, smoking history, alcohol consumption), previous respiratory infection, chronic bacterial colonization, comorbidities, information of the last hospitalization, and medications after discharge. The diagnosis of COPD in this study was based on medical records. Definitions of chronic PA colonization were highly heterogeneous, with this study defining it as 2 positive cultures at least 3 months apart over 12 months [18]. Long-term oral antibiotics were defined as > 4 weeks. The information of the last hospital admission includes: (1) laboratory tests: blood routine, blood gas analysis, C-reactive protein, procalcitonin, sputum microbial isolation within 24 h of hospitalization; (2) pulmonary function tests: Forced expiratory volume in 1 s (FEV1), Forced vital capacity (FVC), FEV1/FVC ratio, FEV1%predicted; (3) features of HRCT: the number of lesions involving lung lobes, bronchiectasis types; (4) clinical manifestations: sputum characteristics, hemoptysis, cough, dry rales, moist rales; (5) whether to enter Respiratory Intensive Care Unit (RICU). The end point event in this study was one-year readmission for acute exacerbations which was defined as a patient who required intravenous therapy in the hospital for bronchiectasis exacerbation within 1 year since the last hospitalization of bronchiectasis exacerbation. Follow-up to all patient was completed via the Bronchiectasis Specialist Service of Respiratory Clinic and telephone contact, and all patients were followed up once a month. Loss to follow-up was defined in this study as 3 consecutive failures to contact or refusal to follow-up.
Statistical analysis
All analyses were performed using SPSS version 26 (SPSS, Chicago, IL, USA) for Windows. All statistical inferences will be determined using two-sided tests. Continuous variables were assessed using either Student’s t-test or nonparametric tests, depending on the distribution of the data. The data were tabulated as Mean ± SD in the case of quantitative variables and as absolute numbers and percentages in the case of qualitative variables. When using nonparametric methods, median and interquartile range will be provided. A Cox regression model was used to determine the factors that were independently associated with one-year readmission after discharge for acute exacerbations in bronchiectasis. Variables with statistically significant differences in univariate analysis (p < 0.05) or clinical significance were included in the univariate Cox regression analysis. The probability of readmission was analyzed by plotting a Kaplan-Meier curve in a time-to-event analysis. The forward stepwise technique (the LR test) was then used to remove any variables with p > 0.05 from the final model. The hazard ratio (HR) and 95% confidence interval (CI) were calculated for the independent variables, with p < 0.05 considered to be significant.
Results
Demographic
Demographic and partial clinical data of participants in our study were showed in Table 1. More than one-third (44.6%, 116/260) of patients with bronchiectasis were readmitted to hospital within one year, with a median duration of 128.5 days (ranging from 97.5 to 173 days). The mean age was 68.7 ± 9.1 years, and there was a higher readmission rate in women (46.1%, 83/180) compared to men (41.3%, 33/80). Factors such as age over 65 years (73.3%), body mass index (BMI) < 18.5 kg/m2 (38.8%), current smoker (18.1%), and chronic PA colonization (44.0%) were more common in patients who had readmission within one year compared to those without readmission.
Table 1.
Demographic and partial clinical data of participants in our study
| Parameter | All population (n = 260) |
Non-readmission (n = 144) |
Readmission (n = 116) |
statistic | P-value |
|---|---|---|---|---|---|
| Sex, M/F, n | 80/180 | 47/97 | 33/83 | 0.530 | 0.467 |
| Age (years), mean ± SD | 60.5 ± 13.4 | 53.9 ± 12.7 | 68.7 ± 9.1 | -10.553 | < 0.001 |
| Age>65 years, n (%) | 121 (46.5) | 36 (25.0) | 85 (73.3) | 60.180 | < 0.001 |
| BMI (kg/m2), mean ± SD | 20.3 ± 2.3 | 21.2 ± 2.1 | 19.2 ± 2.2 | 7.384 | < 0.001 |
| BMI < 18.5 kg/m2, n (%) | 61 (23.5) | 16 (11.1) | 45 (38.8) | 27.416 | < 0.001 |
| Smoking status, n (%) | 11.776 | 0.003 | |||
| Non-smoker | 156 (60.0) | 83 (57.6) | 73 (62.9) | ||
| Current-smoker | 32 (12.3) | 11 (7.6) | 21 (18.1) | ||
| Ex-smoker | 72 (27.7) | 50 (34.7) | 22 (19.0) | ||
| Current-smoker, n (%) | 32 (12.3) | 11 (7.6) | 21 (18.1) | 6.519 | 0.011 |
| Alcohol abuse, n (%) | 80 (30.8) | 47 (32.6) | 33 (28.5) | 0.530 | 0.467 |
| Chronic PA colonization, n (%) | 61 (23.5) | 10 (6.9) | 51 (44.0) | 49.035 | < 0.001 |
| History of previous respiratory infection, n (%) | 170 (65.4) | 91 (63.2) | 79 (68.1) | 0.684 | 0.434 |
| Previous pertussis, n (%) | 11 (4.2) | 7 (4.9) | 4 (3.4) | 0.064 | 0.801 |
| Previous tuberculosis, n (%) | 74 (28.5) | 38 (26.4) | 36 (31.0) | 0.681 | 0.409 |
| Previous pneumonia, n (%) | 59 (22.7) | 30 (20.8) | 29 (25.0) | 0.636 | 0.425 |
| Previous measles, n (%) | 27 (10.4) | 16 (11.1) | 11 (9.5) | 0.183 | 0.669 |
| Comorbid conditions | |||||
| COPD, n (%) | 46 (17.7) | 8 (5.6) | 38 (32.8) | 32.648 | < 0.001 |
| Asthma, n (%) | 44 (16.9) | 24 (16.7) | 20 (17.2) | 0.015 | 0.902 |
| Chronic sinusitis, n (%) | 62 (23.8) | 34 (23.6) | 28 (24.1) | 0.010 | 0.921 |
| GERD, n (%) | 8 (3.1) | 5 (3.5) | 3 (2.6) | 0.003 | 0.960 |
| ABPA, n (%) | 14 (5.4) | 6 (4.2) | 8 (6.9) | 0.940 | 0.332 |
| Diabetes, n (%) | 23 (8.8) | 13 (9.0) | 10 (8.6) | 0.013 | 0.909 |
| Hypertension, n (%) | 136 (52.3) | 76 (52.8) | 60 (51.7) | 0.029 | 0.866 |
| Coronary heart disease, n (%) | 80 (30.8) | 42 (29.2) | 38 (32.8) | 0.389 | 0.533 |
| Liver disease, n (%) | 22 (8.5) | 13 (9.0) | 9 (7.8) | 0.134 | 0.715 |
| Kidney disease, n (%) | 18 (6.9) | 10 (6.9) | 8 (6.9) | 0 | 0.988 |
| Cerebral vascular disease, n (%) | 89 (34.2) | 46 (31.9) | 43 (37.1) | 0.749 | 0.387 |
| Autoimmune disease, n (%) | 36 (13.8) | 19 (13.2) | 17 (14.7) | 0.115 | 0.735 |
Abbreviations: BMI body mass index, PA Pseudomonas aeruginosa, COPD chronic obstructive pulmonary disease, GERD gastroesophageal reflux disease, ABPA allergic bronchopulmonary aspergillosis
Previous respiratory infections and comorbidities
There was no significant difference in previous respiratory infections between the two groups (63.2% vs. 68.1%, p = 0.434). In readmission group, thirty-six patients (31.0%) had previous tuberculosis, followed by unspecified pneumonia (n = 29, 25.0%), measles (n = 11, 9.5%) and pertussis (n = 4, 3.5%). Comorbidities found in the readmission group included chronic sinusitis (n = 28, 24.1%), asthma (n = 20, 17.2%), gastroesophageal reflux disease (n = 3, 2.6%), allergic bronchopulmonary aspergillosis (n = 8, 6.9%), and autoimmune disease (n = 17, 14.7%). There was a significant difference in comorbid with COPD between the readmission and non-readmission groups (5.6% vs. 32.8%, p < 0.001).
Clinical characteristics during the last admission and medications after discharge
Table 2 showed the clinical manifestations, lung function, laboratory, microbiological, and radiological characteristics during the last hospitalization, and medications after discharge. Variable values in the readmission group were significantly higher than in the non-readmission group including RICU stay during index admission, involvement of three or more lobes on chest HRCT, at least one cystic bronchiectasis on chest HRCT, chronic PA colonization, and inhaling long-acting β agonist (LABA) plus inhaled corticosteroids (ICS) after discharge. FEV1 and FEV1/FVC in readmission group was significantly lower than that in non-readmission group.
Table 2.
Clinical characteristics during the last admission and medications after discharge of participants in our study
| Parameter | All population (n = 260) |
Non-readmission (n = 144) |
Readmission (n = 116) |
statistic | P-value |
|---|---|---|---|---|---|
| RICU during index admission, n (%) | 18 (6.9) | 2 (1.4) | 16 (13.8) | 15.341 | < 0.001 |
| Clinical manifestations | |||||
| Mucopurulent phlegm, n (%) | 224 (86.2) | 122 (84.7) | 102 (87.9) | 0.555 | 0.456 |
| Dry cough, n (%) | 13 (5.0) | 6 (4.2) | 7 (6.0) | 0.472 | 0.492 |
| Hemoptysis, n (%) | 97 (37.3) | 54 (37.5) | 43 (37.1) | 0.005 | 0.943 |
| Dry rales, n (%) | 42 (16.2) | 23 (16.0) | 19 (16.4) | 0.008 | 0.929 |
| Wet rales, n (%) | 130 (50.0) | 71 (49.3) | 59 (50.9) | 0.062 | 0.803 |
| Features of HRCT | |||||
| Emphysema, n (%) | 31 (11.9) | 13 (9.0) | 18 (15.5) | 2.576 | 0.108 |
| Number of lobes involved on chest HRCT, M (Q1, Q3) | 2 (1–2) | 1 (1–2) | 2 (1–4) | -5.190 | < 0.001 |
| Involvement of 3 or more lobes on chest HRCT, n (%) | 58 (22.3) | 9 (6.3) | 49 (42.2) | 48.019 | < 0.001 |
| At least one cystic bronchiectasis, n (%) | 114 (43.8) | 52 (36.1) | 62 (53.4) | 7.843 | 0.005 |
| Types of bronchiectasis on chest HRCT | 14.619 | 0.001 | |||
| Cylindrical, n (%) | 146 (56.2) | 95 (66.0) | 51 (44.0) | ||
| Cystic, n (%) | 83 (31.9) | 39 (27.1) | 44 (37.9) | ||
| Mixed, n (%) | 31 (11.9) | 10 (6.9) | 21 (18.1) | ||
| Lung function indices | |||||
| FEV1 (L), M (Q1, Q3) | 1.59 (1.19-2.00) | 1.82 (1.25–2.04) | 1.38 (1.18–1.89) | -3.447 | 0.001 |
| FEV1%predicted, M (Q1, Q3) | 57.3 (45.1–71.3) | 59.1 (43.9–68.4) | 55.0 (45.2–73.8) | -0.637 | 0.524 |
| FVC (L), M (Q1, Q3) | 1.94 (1.65–2.23) | 1.95 (1.74–2.25) | 1.90 (1.55–2.23) | -0.782 | 0.434 |
| FEV1/FVC, M (Q1, Q3) | 0.85 (0.76–0.96) | 0.89 (0.80-1.00) | 0.81 (0.65–0.91) | -4.123 | < 0.001 |
| Laboratory tests | |||||
| WBC (x 109/L), M (Q1, Q3) | 7.9 (5.9–10.3) | 8.0 (5.9–10.4) | 7.8 (5.9–10.2) | -0.677 | 0.498 |
| NEUT (x 109/L), M (Q1, Q3) | 3.6 (2.8–4.3) | 3.7 (2.9–4.3) | 3.6 (2.7–4.4) | -0.465 | 0.642 |
| CRP (mg/L), M (Q1, Q3) | 17 (10–23) | 17 (10–23) | 16 (10–24) | -0.038 | 0.970 |
| PCT (ng/ml), M (Q1, Q3) | 1.1 (0.6–1.5) | 0.9 (0.4–1.6) | 1.2 (0.7–1.5) | -1.934 | 0.053 |
| Blood gas analysis | |||||
| PaO2 (mmHg), M (Q1, Q3) | 74 (60–82) | 75 (65–83) | 72 (56–82) | -1.311 | 0.190 |
| PaCO2 (mmHg), M (Q1, Q3) | 40 (35–45) | 39 (35–44) | 40 (36–50) | -1.499 | 0.134 |
| SaO2 (%), M (Q1, Q3) | 91 (87–95) | 91.5 (87–95) | 90 (85–95) | -2.134 | 0.033 |
| Hypoxemia, n (%) | 64 (24.6) | 30 (20.8) | 34 (29.3) | 2.488 | 0.115 |
| Hypercapnia, n (%) | 58 (22.3) | 28 (19.4) | 30 (25.9) | 1.527 | 0.217 |
| FiO2, M (Q1, Q3) | 0.29 (0.29–0.33) | 0.29 (0.26–0.33) | 0.33 (0.29–0.35) | -1.872 | 0.061 |
| Positive sputum culture results within 24 h after admission, n (%) | 122 (46.9) | 42 (29.2) | 80 (69.0) | 40.860 | < 0.001 |
| Pseudomonas aeruginosa, n (%) | 38 (14.6) | 6 (4.2) | 32 (27.6) | 28.237 | < 0.001 |
| Haemophilus influenzae, n (%) | 18 (6.9) | 7 (4.9) | 11 (9.5) | 2.130 | 0.144 |
| Acinetobacter baumannii, n (%) | 11 (4.2) | 5 (3.5) | 6 (5.2) | 0.135 | 0.714 |
| Escherichia coli, n (%) | 15 (5.8) | 6 (4.2) | 9 (7.8) | 1.525 | 0.217 |
| Klebsiella pneumoniae, n (%) | 25 (9.6) | 12 (8.3) | 13 (11.2) | 0.610 | 0.435 |
| Staphylococcus aureus, n (%) | 15 (5.8) | 6 (4.2) | 9 (7.8) | 1.525 | 0.217 |
| Medications after discharge | |||||
| Oral corticosteroids, n (%) | 5 (1.9) | 2 (1.4) | 3 (2.6) | 0.060 | 0.807 |
| SABA only, n (%) | 158 (60.8) | 85 (59.0) | 73 (62.9) | 0.411 | 0.522 |
| LAMA only, n (%) | 92 (35.4) | 50 (34.7) | 42 (36.2) | 0.062 | 0.803 |
| LABA plus LAMA, n (%) | 16 (6.2) | 9 (6.3) | 7 (6.0) | 0.005 | 0.943 |
| LABA plus ICS, n (%) | 50 (19.2) | 19 (13.2) | 31 (26.7) | 7.571 | 0.006 |
| LABA plus LAMA plus ICS, n (%) | 7 (2.7) | 3 (2.1) | 4 (3.4) | 0.084 | 0.771 |
| Long-term oral antibiotics, n (%) | 71 (27.3) | 54 (37.5) | 17 (14.7) | 16.891 | < 0.001 |
Abbreviations: RICU respiratory intensive care unit, HRCT high resolution computerized tomography, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, WBC white blood cells, NEUT neutrophil count, CRP C-reactive protein, PCT procalcitonin, PaO2 partial pressure of arterial oxygen, PaCO2 partial pressure of arterial carbon dioxide, SaO2 oxygen saturation, FiO2 fraction of inspiration O2, SABA short-acting β agonist, LAMA long-acting muscarinic antagonist, LABA long-acting β agonist, ICS inhaled corticosteroids
The number of lobes involved on chest HRCT was significantly higher in the readmission group than non-readmission group (2 vs. 1, p < 0.001). Involvement of 3 or more lobes on chest HRCT was more common in patients who had readmission within one year compared to those without readmission (42.2% vs. 6.3%, p < 0.001). Patients in the readmission group had worse lung function and oxygen saturation. There was a significant difference in positive sputum culture results within 24 h after admission between the readmission and non-readmission groups. Among the 144 patients in the non-readmission group, no microorganisms were isolated from the sputum of 102 patients (70.8%). In contrast, among the 116 patients in the readmission group, 80 patients (69.0%) had positive sputum culture results. The most commonly isolated microorganism in the readmission group was PA, found in 32 patients (27.6%), followed by Klebsiella pneumoniae, which was isolated from 13 patients (11.2%).
Risk factors for readmission within one year due to AEB
Given that all patients who used inhaling LABA plus ICS were patients with bronchiectasis combined with COPD or asthma, we were unable to draw the conclusion about the effect of inhaling LABA plus ICS on readmission in patients with bronchiectasis. Therefore, inhaling LABA plus ICS was excluded from Cox analysis.
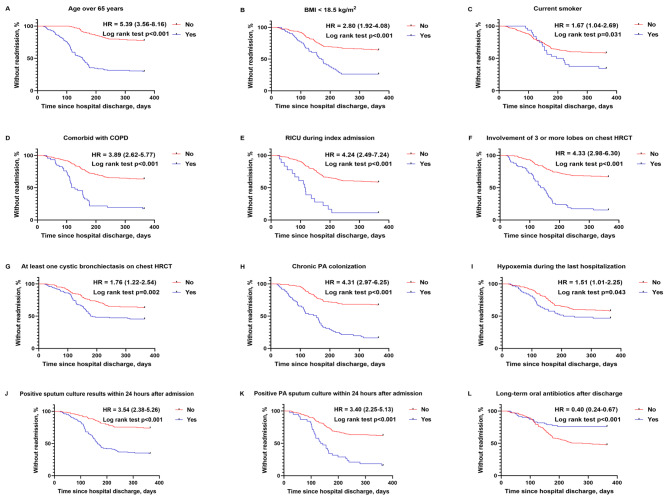
Univariate Cox analysis revealed that age over 65 years (HR, 5.39; 95% CI, 3.56–8.16), BMI < 18.5 kg/m2 (HR = 2.80; 95% CI: 1.92 to 4.08), current smoker (HR = 1.67; 95% CI: 1.04 to 2.69), comorbid with COPD (HR = 3.89; 95% CI: 2.62 to 5.77), RICU stay during index admission (HR = 4.24; 95% CI: 2.49 to 7.24), involvement of three or more lobes on chest HRCT (HR = 4.33; 95% CI: 2.98 to 6.30), at least one cystic bronchiectasis on chest HRCT (HR = 1.76; 95% CI: 1.22 to 2.54), chronic PA colonization (HR, 4.31; 95% CI: 2.97 to 6.25), hypoxemia during the last hospitalization (HR = 1.51; 95% CI: 1.01 to 2.25), positive sputum culture results (HR = 3.54; 95% CI: 2.38 to 5.26), and positive PA sputum culture within 24 h after admission (HR = 3.40; 95% CI: 2.25 to 5.13) were all significantly associated with an increased risk of readmission as shown in Table 3. Long-term oral antibiotics use after discharge was associated with a lower risk of readmission (HR = 0.40; 95% CI: 0.24 to 0.67). Then, Kaplan–Meier survival analysis further confirmed that these variables were significantly associated with readmission (Fig. 2).
Table 3.
Risk factors for readmission within one year due to acute exacerbation of bronchiectasis
| Variables | Univariate Cox analysis | Multivariate Cox analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Age>65 years | 5.39 (3.56 to 8.16) | < 0.001 | 3.66 (2.30 to 5.85) | < 0.001 | |
| BMI < 18.5 kg/m2 | 2.80 (1.92 to 4.08) | < 0.001 | 1.71 (1.16 to 2.51) | 0.007 | |
| Current smoker | 1.67 (1.04 to 2.69) | 0.033 | - | - | |
| Comorbid with COPD | 3.89 (2.62 to 5.77) | < 0.001 | - | - | |
| RICU during index admission | 4.24 (2.49 to 7.24) | < 0.001 | 2.06 (1.16 to 3.67) | 0.014 | |
| Number of lobes involved on chest HRCT | 1.40 (1.26 to 1.55) | < 0.001 | - | - | |
| Involvement of 3 or more lobes on chest HRCT | 4.33 (2.98 to 6.30) | < 0.001 | 1.85 (1.22 to 2.80) | 0.004 | |
| At least one cystic bronchiectasis on chest HRCT | 1.76 (1.22 to 2.54) | 0.002 | - | - | |
| Emphysema | 1.47 (0.89 to 2.43) | 0.135 | - | - | |
| FEV1 | 0.45 (0.29 to 0.69) | < 0.001 | - | - | |
| FEV1/FVC | 0.10 (0.03 to 0.27) | < 0.001 | - | - | |
| Chronic PA colonization | 4.31 (2.97 to 6.25) | < 0.001 | 2.29 (1.54 to 3.38) | < 0.001 | |
| SaO2 during the last hospitalization | 0.95 (0.92 to 0.98) | < 0.001 | - | - | |
| Hypoxemia during the last hospitalization | 1.51 (1.01 to 2.25) | 0.045 | - | - | |
| Positive sputum culture results within 24 h after admission | 3.54 (2.38 to 5.26) | < 0.001 | 1.93 (1.27 to 2.94) | 0.002 | |
| Positive PA sputum culture within 24 h after admission | 3.40 (2.25 to 5.13) | < 0.001 | - | - | |
| Long-term oral antibiotics after discharge | 0.40 (0.24 to 0.67) | 0.001 | 0.34 (0.20 to 0.59) | < 0.001 | |
Abbreviations: BMI body mass index, COPD chronic obstructive pulmonary disease, RICU respiratory intensive care unit, HRCT high-resolution computed tomography, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, PA Pseudomonas aeruginosa, SaO2 oxygen saturation, HR hazard ratio, CI confidence interval
Fig. 2.
The Kaplan-Meier curve shows the probability of readmission for AEB within one year after discharge. AEB acute exacerbation of bronchiectasis, BMI body mass index, COPD chronic obstructive pulmonary disease, RICU respiratory intensive care unit, HRCT high-resolution computed tomography, PA Pseudomonas aeruginosa, HR hazard ratio
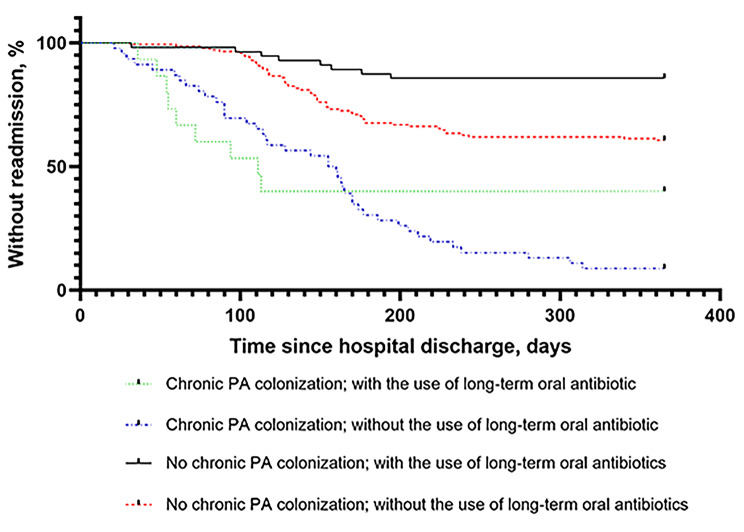
Subgroups were analyzed to determine the effect of chronic PA colonization and long-term oral antibiotics after discharge on readmission. The patients were divided into four groups (chronic PA colonization with long-term oral antibiotic treatment, chronic PA colonization without long-term oral antibiotic treatment, no chronic PA colonization with long-term oral antibiotics, and no chronic PA colonization without long-term oral antibiotics). There was a significant difference in readmission found between the four groups (p < 0.001) (Fig. 3). The patients most likely to be readmitted were those with chronic PA colonization but not receiving long-term oral antibiotic treatment.
Fig. 3.
The Kaplan-Meier curve shows the effect of chronic PA colonization and long-term oral antibiotics after discharge on readmission; p < 0.001. PA Pseudomonas aeruginosa
In the multivariate Cox analysis (Table 3), it was found that age over 65 years (HR = 3.66; 95% CI: 2.30 to 5.85), BMI < 18.5 kg/m2 (HR = 1.71; 95% CI: 1.16 to 2.51), RICU stay during admission (HR = 2.06, 95% CI: 1.16–3.67), involvement of 3 or more lobes on chest HRCT (HR = 1.85; 95% CI, 1.22 to 2.80), chronic PA colonization (HR = 2.29; 95% CI: 1.54 to 3.38), and positive sputum culture results within 24 h after admission (HR = 1.93; 95% CI: 1.27 to 2.94). Additionally, multivariate analyses further demonstrated that long-term oral antibiotics use after discharge (HR = 0.34; 95% CI: 0.20 to 0.59) was a protect factor for readmission.
Discussion
Being a specialized respiratory and critical care department of a tertiary hospital, our department analyzed clinical data of 260 bronchiectasis patients with acute severe exacerbations. Of these patients, 44.6% patients had at least one readmission within 12 months of discharge, with a median duration of 128.5 days (ranging from 97.5 to 173 days). The readmission rate of 44.6% in our study was comparable to rates reported in other series. Readmission rates within 12 months were 46% in both the Roberts et al. study in a single hospital in New Zealand (70/152) and the Wang et al. study in a tertiary hospital in China (46/100) [19, 20]. This high rate of readmission may be attributable to the pathophysiological changes of bronchiectasis, which is defined as irreversibly damaged and dilated bronchi [21].
In our study, readmission within one year of patients with bronchiectasis was associated with age over 65 years. Consistent with the previous studies, bronchiectasis is an age-associated disease and the old age was associated with a poor prognosis [22, 23]. The association between readmission and age can be explained by decreased immunity, comorbidity, and concomitant diseases. Additionally, this study also found that BMI < 18.5 kg/m2 was a risk factor for readmission. The presence of chronic inflammation and recurrent infection in bronchiectasis may compromise skeletal muscle strength and exercise capacity, while a low BMI may indicate sarcopenia and malnutrition, which can worsen the prognosis of bronchiectasis. A Korean study found that underweight was a risk factor for mortality in bronchiectasis [24]. Therefore, nutritional supportive care may help decrease the risk of readmission and improving the prognosis in patients with low weight.
The results of this study shows that the involvement of 3 or more lung lobes on HRCT is a risk factor for readmission within one year. A prospective study also supports this, indicating that the median number of lung lobes affected in readmission patients is higher than in non-readmission patients [25]. The more extensive the bronchiectasis, the poorer the pulmonary structure, the more complications they have, and the greater the risk of readmission due to acute exacerbations. RICU stay during index hospital in this study was also an independent risk factor for readmission. Patients with bronchiectasis, whether it is due to extensive disease involvement or the need for admission to RICU, both indicate severe pulmonary conditions and increased treatment challenges, which increases the risk of future readmissions.
A multicentre European study of 2596 patients with bronchiectasis showed that chronic PA colonization was independently associated with the risk of frequent exacerbations, hospital admissions and worse quality of life [26]. The findings of this study indicate that chronic PA colonization independently increases the risk of readmission, as reported elsewhere [18]. Therefore, eradication treatment for PA infection may improve the prognosis of the bronchiectasis patients [27]. Our study also found that positive sputum culture results within 24 h after admission were associated with readmission. Haemophilus influenzae and PA are reported to be the most frequently isolated pathogens in bronchiectasis patients [22, 28]. A prospective study suggested that microbiomes dominated by Pseudomonas were independently associated with increased hospitalizations [29]. In our study, the most frequent microorganisms isolated in patient cohort were PA, followed by Klebsiella pneumoniae. In contrast, Haemophilus influenzae accounted for 6.9% of isolates in this study. Consistent with Guan W J et al.’s studies [30], Haemophilus influenzae also has a low isolation rate in Guangzhou, China. This might be associated with inappropriate use of antibiotics in China, ethnicity, geographic, and the severity of bronchiectasis.
In this study, Kaplan-Meier curves showed the highest readmission rate in bronchiectasis patients with chronic PA colonization without long-term oral antibiotics, and the lowest risk of readmission in bronchiectasis patients without chronic PA colonization with long-term oral antibiotics. Cox analyses likewise demonstrated that long-term oral antibiotics after discharge had a significant protective effect on readmission in patients with bronchiectasis. This might be that long-term oral antibiotics treatment can decrease airway bacterial loads and reduces markers of the airway and systemic inflammation in patients with bronchiectasis. Some antibiotics, especially macrolide antibiotics, have immunomodulatory properties in addition to their antibacterial and anti-inflammatory properties in patients with or without PA colonization [31]. However, our study did not collect data on the type of oral antibiotics, and the combination of other microbial colonization, making it difficult to draw specific conclusions on their usage.
Bronchiectasis exhibits significant heterogeneity worldwide, influenced by ethnicity, societal factors, and health conditions. This study collected a plethora of variables from a cohort of bronchiectasis patients at a tertiary hospital in China, including demographic data, past infection history, comorbidities, inpatient examination and laboratory results, and post-discharge medication, with the aim of identifying the risk of readmission. The results provide clinicians with comprehensive data, which enables them to more accurately identify risk factors for readmission, optimize disease management, reduce readmissions and alleviate the financial burden on patients as well as the strain on medical resources.
Our study has several limitations. First, as this was a single-center retrospective study, there was an increased risk of selection bias. Patient information was collected by reviewing their medical records, which could have resulted in inaccurate data due to missing records. Therefore, the findings should be interpreted with caution. Second, the analysis included only patients with bronchiectasis admitted to our own institution, so our findings might not be generalizable to populations in other parts of China or populations outside the China. Third, it was not possible to collect patient characteristics after discharge, and therefore we cannot say whether there was instability after discharge that may have caused the readmission. Additionally, our study did not include simultaneous analysis of clinical biological samples. The absence of these clinical data limited our ability to identify other possible risk factors that may be associated with readmission based on previous studies. Finally, this was a relatively small and non-random sample size study. In the future, multi-center clinical studies with larger sample size and longer period of follow-up are needed to further confirmation.
Conclusions
To sum up, this study showed that age over 65 years, BMI < 18.5 kg/m2, RICU stay during index admission, involvement of three or more lobes on chest HRCT, chronic PA colonization, and the positive sputum culture results within 24 h after admission were common risk factors of readmission. Early identification of these risk factors can aid in the development of preventive strategies, reducing acute exacerbation in bronchiectasis patients, improving their quality of life and prognosis.
Acknowledgements
Not applicable.
Abbreviations
- AEB
Acute exacerbation of bronchiectasis
- CT
Computed tomography
- HRCT
High-resolution computed tomography
- PA
Pseudomonas aeruginosa
- BMI
Body mass index
- COPD
Chronic obstructive pulmonary disease
- HR
Hazard ratio
- CI
Confidence interval
- CRP
C-reactive protein
- FEV1
Forced expiratory volume in 1 s
- FiO2
Fraction of inspiration
- FVC
Forced vital capacity
- RICU
Respiratory Intensive Care Unit
- GERD
Gastroesophageal reflux disease
- ABPA
Allergic bronchopulmonary aspergillosis
- PaO2
Partial pressure of arterial oxygen
- PaCO2
Partial pressure of arterial carbon dioxide
- SaO2
Oxygen saturation
- NEUT
Neutrophil count
- PCT
Procalcitonin
- SABA
Short-acting β agonist
- WBC
White blood cells
- LAMA
Long-acting muscarinic antagonist
- LABA
Long-acting β agonist
- ICS
Inhaled corticosteroids
- BCOS
Bronchiectasis-COPD overlap syndrome
Author contributions
YXF, HYZ, LQ and ZHL designed the study. YXF drafted this manuscript. BS, XYY and ZYZ collected clinical resource. LQ, DZW, PYZ and ZHL sought funding. SXZ developed the statistical analysis plan. SYZ revised the manuscript. All authors read and approved the final manuscript.
Funding
This research is funded by the Shanghai Science and Technology Commission Project (21Y11922500; 21S21900200; 21Y21920400; 23S21900600); Shanghai Municipal Health Commission Project (2022XD027; 2022CX010; 20234Y0109); Shanghai Public Health Key Discipline Project (GWVI-11.1-08); Shanghai Shenkang Center Project (SHDC12023106); Shanghai Xuhui District Hospital and Land Cooperation Project (23XHYD-25) and Shanghai Pudong New Area Traditional Chinese Medicine Inheritance Innovation Development Demonstration Pilot Project Construction (YC-2023-0901).
Data availability
The original data supported this study can be looked up in our hospital’s electronic medical record system, further inquiries can be directed to the corresponding author. Datasets are not suitable to be deposited to publicly available repositories due to patient privacy.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, which waived the requirement for patient informed consent because of the anonymous nature of the data. The Clinical Trial Number was NO. ChiCTR2000033494.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yaxin Fan and Ben Su contributed equally to this work.
Contributor Information
Zhenhui Lu, Email: Dr_luzh@shutcm.edu.cn.
Lei Qiu, Email: dr_qiulei@shutcm.edu.cn.
References
- 1.Hill AT, Sullivan AL, Chalmers JD, De Soyza A, Elborn JS, Floto RA, et al. British thoracic society guideline for bronchiectasis in adults. BMJ Open Respir Res. 2018;5:e000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron Respir Dis. 2017;14:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ringshausen FC, de Roux A, Diel R, Hohmann D, Welte T, Rademacher J. Bronchiectasis in Germany: a population-based estimation of disease prevalence. Eur Respir J. 2015;46:1805–7. [DOI] [PubMed] [Google Scholar]
- 4.Diel R, Ewig S, Blaas S, Jacob C, Juelich F, Korfmann G, et al. Incidence of patients with non-cystic fibrosis bronchiectasis in Germany - A healthcare insurance claims data analysis. Respir Med. 2019;151:121–7. [DOI] [PubMed] [Google Scholar]
- 5.Quint JK, Millett ER, Joshi M, Navaratnam V, Thomas SL, Hurst JR, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J. 2016;47:186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monteagudo M, Rodríguez-Blanco T, Barrecheguren M, Simonet P, Miravitlles M. Prevalence and incidence of bronchiectasis in Catalonia, Spain: a population-based study. Respir Med. 2016;121:26–31. [DOI] [PubMed] [Google Scholar]
- 7.Xu JF, Gao YH, Song YL, Qu JM, Guan WJ. Research advances and clinical management of bronchiectasis: Chinese perspective. ERJ Open Res. 2022;8. [DOI] [PMC free article] [PubMed]
- 8.Lin JL, Xu JF, Qu JM. Bronchiectasis in China. Ann Am Thorac Soc. 2016;13:609–16. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell AE. Bronchiectasis - A clinical review. N Engl J Med. 2022;387:533–45. [DOI] [PubMed] [Google Scholar]
- 10.Barker AF, Bronchiectasis. N Engl J Med. 2002;346:1383–93. [DOI] [PubMed]
- 11.Quittner AL, O’Donnell AE, Salathe MA, Lewis SA, Li X, Montgomery AB, et al. Quality of Life Questionnaire-Bronchiectasis: final psychometric analyses and determination of minimal important difference scores. Thorax. 2015;70:12–20. [DOI] [PubMed] [Google Scholar]
- 12.Chalmers JD, Aliberti S, Filonenko A, Shteinberg M, Goeminne PC, Hill AT, et al. Characterization of the frequent Exacerbator phenotype in Bronchiectasis. Am J Respir Crit Care Med. 2018;197:1410–20. [DOI] [PubMed] [Google Scholar]
- 13.De Angelis A, Johnson ED, Sutharsan S, Aliberti S. Exacerbations of bronchiectasis. Eur Respir Rev. 2024;33(173):240085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalmers JD, Aliberti S, Filonenko A, Shteinberg M, Goeminne PC, Hill AT, et al. Characterization of the frequent Exacerbator phenotype in Bronchiectasis. Am J Respir Crit Care Med. 2018;197(11):1410–20. [DOI] [PubMed] [Google Scholar]
- 15.Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. [DOI] [PubMed] [Google Scholar]
- 16.Haworth CS, Floto RA. Antibiotic management in Bronchiectasis. Clin Chest Med. 2022;43(1):165–77. [DOI] [PubMed] [Google Scholar]
- 17.Hill AT, Haworth CS, Aliberti S, Barker A, Blasi F, Boersma W et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J. 2017;49. [DOI] [PubMed]
- 18.Finch S, McDonnell MJ, Abo-Leyah H, Aliberti S, Chalmers JD. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in Adult Bronchiectasis. Ann Am Thorac Soc. 2015;12:1602–11. [DOI] [PubMed] [Google Scholar]
- 19.Roberts ME, Lowndes L, Milne DG, Wong CA. Socioeconomic deprivation, readmissions, mortality and acute exacerbations of bronchiectasis. Intern Med J. 2012;42:e129–36. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Chen X, He S, Li J, Ma T, Liu L et al. COPD Assessment Test and risk of readmission in patients with bronchiectasis: a prospective cohort study. ERJ Open Res. 2024;10. [DOI] [PMC free article] [PubMed]
- 21.Hill AT, Sullivan AL, Chalmers JD, De Soyza A, Elborn SJ, Floto AR, et al. British thoracic Society Guideline for bronchiectasis in adults. Thorax. 2019;74(Suppl 1):1–69. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekaran R, Mac Aogáin M, Chalmers JD, Elborn SJ, Chotirmall SH. Geographic variation in the aetiology, epidemiology and microbiology of bronchiectasis. BMC Pulm Med. 2018;18:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finklea JD, Khan G, Thomas S, Song J, Myers D, Arroliga AC. Predictors of mortality in hospitalized patients with acute exacerbation of bronchiectasis. Respir Med. 2010;104:816–21. [DOI] [PubMed] [Google Scholar]
- 24.Lee JM, Lee SA, Han CH, Lee SM, Kim CJ, Lee SC, et al. Body mass index as a predictor of mortality in bronchiectasis: a nationwide population-based study. Respir Med. 2021;180:106370. [DOI] [PubMed] [Google Scholar]
- 25.Wang XY, Li R, Wang WY, Li DS, Zhou YY, Chen XT, et al. Bronchiectasis severity assessment on predicting hospital readmission: a single-center prospective cohort study. Chin Med J. 2020;134:492–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araújo D, Shteinberg M, Aliberti S, Goeminne PC, Hill AT, Fardon TC et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J. 2018;51. [DOI] [PubMed]
- 27.Conceição M, Shteinberg M, Goeminne P, Altenburg J, Chalmers JD. Eradication treatment for Pseudomonas aeruginosa infection in adults with bronchiectasis: a systematic review and meta-analysis. Eur Respir Rev. 2024;33. [DOI] [PMC free article] [PubMed]
- 28.Tunney MM, Einarsson GG, Wei L, Drain M, Klem ER, Cardwell C, et al. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med. 2013;187:1118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dicker AJ, Lonergan M, Keir HR, Smith AH, Pollock J, Finch S, et al. The sputum microbiome and clinical outcomes in patients with bronchiectasis: a prospective observational study. Lancet Respir Med. 2021;9:885–96. [DOI] [PubMed] [Google Scholar]
- 30.Guan WJ, Gao YH, Xu G, Lin ZY, Tang Y, Li HM, et al. Aetiology of bronchiectasis in Guangzhou, southern China. Respirology. 2015;20:739–48. [DOI] [PubMed] [Google Scholar]
- 31.Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):660–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data supported this study can be looked up in our hospital’s electronic medical record system, further inquiries can be directed to the corresponding author. Datasets are not suitable to be deposited to publicly available repositories due to patient privacy.