Abstract
Background
Pineapple (Ananas comosus L.) is a major tropical fruit crop with considerable economic importance, and its growth and development are significantly impacted by low temperatures. The plant-specific GRAS gene family plays crucial roles in diverse processes, including flower and fruit development, as well as in stress responses. However, the role of the GRAS family in pineapple has not yet been systematically analyzed.
Results
In this study, 43 AcGRAS genes were identified in the pineapple genome; these genes were distributed unevenly across 19 chromosomes and 6 scaffolds and were designated as AcGRAS01 to AcGRAS43 based on their chromosomal locations. Phylogenetic analysis classified these genes into 14 subfamilies: OS19, HAM-1, HAM-2, SCL4/7, LISCL, SHR, PAT1, DLT, LAS, SCR, SCL3, OS43, OS4, and DELLA. Gene structure analysis revealed that 60.5% of the AcGRAS genes lacked introns. Expression profiling demonstrated tissue-specific expression, with most AcGRAS genes predominantly expressed in specific floral organs, fruit tissues, or during particular developmental stages, suggesting functional diversity in pineapple development. Furthermore, the majority of AcGRAS genes were induced by cold stress, but different members seemed to play distinct roles in short-term or long-term cold adaptation in pineapple. Notably, most members of the PAT1 subfamily were preferentially expressed during late petal development and were upregulated under cold stress, suggesting their special roles in petal development and the cold response. In contrast, no consistent expression patterns were observed among genes in other subfamilies, suggesting that various regulatory factors, such as miRNAs, transcription factors, and cis-regulatory elements, may contribute to the diverse functions of AcGRAS members, even within the same subfamily.
Conclusions
This study provides the first comprehensive analysis of GRAS genes in pineapple, offers valuable insights for further functional investigations of AcGRASs and provides clues for improving pineapple cold resistance breeding.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05913-9.
Keywords: Pineapple, GRAS transcription factor, Gene expression, Genome-wide analysis
Background
The plant-specific GRAS transcription factor family plays a pivotal role in transcriptional reprogramming associated with various biological processes, including development and stress responses [1]. The name "GRAS" originates from the first three identified genes: gibberellic acid-insensitive (GAI), repressor of GAI (RGA), and scarecrow (SCR) [2]. These proteins are characterized by a conserved GRAS domain at the C-terminus, while the N-terminus exhibits significant variability in sequence and length [3, 4]. As more plant genomes are sequenced, the GRAS gene family has been systematically identified across numerous species, including Arabidopsis (Arabidopsis thaliana) [5], rice (Oryza sativa) [5], muskmelon (Cucumis melo) [6], apple (Malus domestica) [7], grape (Vitis vinifera) [8], tomato (Solanum lycopersicum) [9], as well as mosses and ferns [10]. Phylogenetic analyses have grouped GRAS genes into various subfamilies on the basis of structural similarity, reflecting their evolutionary relationships and suggesting functional homology [5, 11–14]. In Arabidopsis, the GRAS family has been classified into eight subfamilies: LISCL, PAT1, SCL3, DELLA, SCR, SHR, LS, and HAM [11]. However, Cenci and Rouard [13] expanded this to 17 subfamilies in angiosperms, identifying five new subfamilies: DLT, RAD1, RAM1, SCLA, and SCLB. This finding indicates that GRAS family classification may vary across species and depend on the number of species analyzed [14].
The GRAS gene family has gained increasing attention because of its broad biological functions and wide distribution across the plant kingdom. Differential expression of GRAS genes in various plant tissues highlights their diverse roles in plant growth and development. For example, in Arabidopsis, SCR and SHR play important roles in root growth and development [15–17], whereas members of the HAM subfamily, such as LOM1 or LOM2, are crucial for maintaining the shoot apical meristem [18]. In Solanum lycopersicum, overexpression of SlGRAS24 disrupts gibberellin (GA) and auxin signaling, leading to dwarfism, shorter primary roots, fewer lateral roots, and more lateral shoots, suggesting that HAM genes regulate the GA/auxin balance in different meristems [19]. In rice, OsSCR1 and OsSCR2 act upstream of OsMUTE and OsFAMA, playing early roles in stomatal development [20]. GRAS transcription factors are also involved in flower, embryo, seed, and fruit development. In Arabidopsis, a quintuple DELLA mutant exhibits early flowering, suggesting that these transcription factors act as inhibitors of flowering [21]. In lily (Lilium longiflorum), LlSCL is expressed predominantly in anthers during pre-meiosis, indicating a role in microsporogenesis [22]. In tomato, the overexpression of SlGRAS24 reduces fruit set by 75%, whereas the silencing of SlFSR (SlGRAS38) significantly extends fruit shelf-life and reduces the activity of enzymes involved in cell wall degradation [23]. Additionally, the GRAS gene family is also a key component in signaling during responses to abiotic stresses, enhancing tolerance by regulating stress-related genes [24]. Overexpression of PeSCL7 from poplar (Populus euphratica) in transgenic Arabidopsis and poplar improved drought and salt stress tolerance by activating enzymes involved in carbohydrate metabolism and alleviating oxidative stress [25]. In S. lycopersicum, overexpression of SlGRAS4 enhances drought stress tolerance, whereas RNAi lines exhibit hypersensitivity to this stress. Expression profiles suggest that SlGRAS4 may also play a role in cold stress tolerance [9]. VaPAT1, a gene from wild Amur grape (Vitis amurensis), is induced by low temperatures, and its ectopic expression in Arabidopsis enhances cold tolerance. This gene is also involved in regulating jasmonic acid biosynthesis in response to cold stress in grapevines [26]. Similarly, the overexpression of the ZjCIGR1 gene from zoysiagrass (Zoysia japonica Steud.), which belongs to the PAT1 subfamily of the GRAS protein family, confers cold stress resistance in zoysiagrass [27].
Pineapple (Ananas comosus L.), a perennial herbaceous plant from the family Bromeliaceae, is one of the four major tropical and subtropical fruits cultivated globally [28, 29]. Currently, pineapples are cultivated in approximately 90 countries and regions worldwide, with a total cultivation area exceeding 400,000 hectares, primarily located in Asia, the Americas, and Africa. The top 10 pineapple-producing countries, including Thailand, the Philippines, China, Brazil, and India, collectively contribute about 73% of the global production. Pineapple remains one of the most active varieties in the global tropical fruit trade, with an annual trade volume surpassing 2.5 billion USD [28]. Pineapple is favored by consumers for its unique flavor, aroma, and high nutritional value, and its inflorescence is the source of the fruit [30]. However, pineapple cultivation faces a significant challenge due to its sensitivity to low temperatures, especially given its long production cycle of at least 14 months, which often includes exposure to cold stress during winter in subtropical regions and thus restricts year-round production [31, 32]. When exposed to 0 °C or below, ice formation in leaf tissues can cause significant damage, leading to symptoms similar to scalding and rapid tissue necrosis. Extended periods of cold, particularly in prolonged rainy weather with daily temperatures below 8 °C, can result in chilling injuries that severely impact the meristem and young leaves, leading to tissue rot and stunted growth, ultimately causing substantial losses in yield and quality [33]. Therefore, research on the genes related to the regulation of pineapple flower and fruit development, as well as the cold stress response, can provide important reference information for pineapple breeding and production. As GRAS transcription factors play key roles in these processes, a systematic study of the GRAS gene family in pineapple is essential. Using the high-quality pineapple genome [34], we conducted a genome-wide identification and analysis of the GRAS gene family in pineapple, including its sequence characteristics and expression profiles. These results offer valuable insights into the potential roles of this important gene family in pineapple development and the cold stress response.
Materials and methods
Identification and sequence analysis of GRAS genes in pineapple
Genomic data for pineapple was downloaded from the Phytozome database (version: Ananas comosus v3; variety: F153; https://phytozome-next.jgi.doe.gov/info/Acomosus_v3) [35]. GRAS protein sequences from Arabidopsis (33) and rice (50) were retrieved from the Plant Transcription Factor Database (http://planttfdb.gao-lab.org/index.php) [36]and used as queries for BLASTP searches. The hidden Markov model (HMM) for the GRAS domain (PF03514) was obtained from the PFAM database (http://pfam.xfam.org) [37] and employed for HMMER (v3.3) searches within pineapple protein sequences, with an E-value cutoff of 0.00001. After removing redundant sequences, the remaining candidates were further analyzed using NCBI CDD (https://www.ncbi.nlm.nih.gov/cdd/) [38] and the SMART tool (http://smart.embl-heidelberg.de/) [39] to confirm the presence of conserved GRAS domains. The identified GRAS genes were renamed as AcGRAS01 to AcGRAS43 according to their chromosomal distribution. The physical and chemical properties, including protein length, molecular weight (kDa), theoretical pI, grand average of hydropathicity (GRAVY), and instability index of the AcGRAS proteins, were calculated using the ExPASy website (https://web.expasy.org/compute_pi/) [40]. Subcellular localization of the AcGRAS proteins was predicted using Cell-PLoc 2.0 (http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc-2/) [41].
Phylogenetic analysis and classification of AcGRASs
To investigate the evolutionary relationships among the 43 identified AcGRAS genes, multiple sequence alignments were performed using ClustalW (http://www.clustal.org/clustal2/) [42] with default parameters. GRAS protein sequences from pineapple (43), Arabidopsis (34), and rice (50), as well as the cold resistance gene ZjCIGR1 from zoysiagrass [27] and VaPAT1 from wild Amur grape [26] (Additional file 1: Table S1), were used to construct an unrooted phylogenetic tree using IQ-Tree software. The maximum likelihood (ML) method was applied with 5000 bootstrap replicates. The AcGRASs were classified on the basis of their evolutionary relationships with the GRAS members in Arabidopsis. The phylogenetic tree was visualized using Evolview (http://www.evolgenius.info/evolview/) [43].
Gene structure, conserved motif, and Cis-regulatory element analyses of AcGRASs
The exon‒intron structure of the AcGRAS genes was determined via the GFF annotation file of the pineapple genome. Conserved motifs within the AcGRAS proteins were identified using the MEME tool (http://alternate.meme-suite.org) [44] with the following parameters: the number of motifs was set to 8, and the optimal width of each motif was between 6 and 50 residues. The upstream 1500 bp sequence of each AcGRAS gene was extracted using TBtools software on the basis of the full-length genomic DNA sequences of the AcGRAS genes [45]. Cis-regulatory elements in the promoter regions were predicted using the PlantCare database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) [46], and the results were visualized using TBtools.
Three-dimensional (3D) structural modeling of AcGRASs
Homologous protein models for the pineapple AcGRAS proteins were identified using the Protein Data Bank (PDB) database (http://www.rcsb.org/) [47]. The tertiary structures of the AcGRAS proteins were predicted through SWISS-MODEL (https://www.swissmodel.expasy.org/) [48] using default settings. Conserved structural elements were further analyzed with ConSurf (https://consurf.tau.ac.il/) [49]. Visualization and manipulation of the 3D protein models were performed using PyMOL v2.6.0 [50], while protein topology was assessed using Protter (http://wlab.ethz.ch/protter/start/) [51].
Chromosomal distribution, gene duplication, and collinearity analysis of AcGRASs
The chromosomal distribution of all 43 AcGRAS genes was determined by mapping them to their respective chromosomes using TBtools, based on physical location data from the pineapple genome annotation file. Whole-genome data for Arabidopsis, rice, banana, and grape were downloaded from the Phytozome database (https://phytozome-next.jgi.doe.gov) [35]. Gene duplication events among the 43 AcGRAS genes were identified through TBtools with default settings, and synteny analysis between pineapple and the other four species was also conducted.
Prediction of putative miRNA targets for AcGRASs
To predict potential miRNA interactions with AcGRAS genes, pineapple miRNA sequences were retrieved from published literature [52]. The coding sequences (CDS) of AcGRAS genes were extracted and submitted to the psRNATarget online database (https://www.zhaolab.org/psRNATarget/) [53] with default parameters. The interaction networks between AcGRAS genes and their predicted miRNA targets were visualized using Cytoscape v3.6software [54].
Transcription factor regulatory network analysis of AcGRASs
The Plant Transcriptional Regulatory Map (PTRM) tool (http://plantregmap.gao-lab.org/) [55] was employed to predict transcription factors (TFs) that regulate AcGRAS genes. The upstream 2000 bp sequences of AcGRAS genes were analyzed with a significance threshold of P ≤ 1e-7, using Arabidopsis as the reference species. The predicted TFs were visualized as a network using Cytoscape, and word clouds and bar charts were generated using the ggplot2 package in R.
Expression profiling of AcGRASs across different tissues and under cold stress based on RNA-seq data
The transcriptomic data from various pineapple floral and fruit tissues were obtained from our previously published work [34, 56]. These samples included four developmental stages of sepal tissues, three stages of petal tissues, six stages of stamen tissues, seven stages of gynoecium tissues, seven stages of ovule tissues, and six developmental stages of fruits. Transcriptomic data from pineapple subjected to cold treatment at 8 °C were generated from our unpublished work, which has been deposited in China National GeneBank DataBase (CNGBdb) with accession number CNP0006260 (https://db.cngb.org/search/project/CNP0006260/). For the cold treatment, pineapple variety Tainong 11 (TN 11) was used, which are provided by the Haixia Institute of Science and Technology, Center for Genomics and Biotechnology, Fujian Agriculture and Forestry University, Fujian, China. The suckers of TN 11 variety was grown in plastic pots containing soil mix under greenhouse conditions (30 °C, 70% humidity, and a 16 h light/8 h dark photoperiod). After three months, healthy TN11 seedlings with well-developed roots were exposed to cold treatment at 8 °C, and leaf samples were collected at 0, 1, 3, 5, 7, 9, 11, 13, 14, and 15 days post-treatment. RNA-seq was performed on samples collected at 0, 3, 7, and 15 days post-treatment with three biological replicates for each group, while the remaining samples were preserved for subsequent qRT-PCR analysis. The transcript abundance of AcGRAS genes was calculated as Transcripts Per Million (TPM), and a heatmap based on log2 (TPM + 0.01) values was generated using the heatmap package in R.
RNA extraction and qRT-PCR analysis of selected AcGRASs
Since cold stress significantly impacted pineapple growth and development, we further examined the expression pattern of 12 representative AcGRAS genes in response to cold stress using qRT-PCR. Total RNA was extracted using the TRIzol method (Invitrogen, Carlsbad, CA, USA), and reverse transcription was performed with the ThermoScript RT-PCR kit (Thermo Fisher Scientific, Carlsbad, CA, USA). qRT-PCR was conducted using the SYBR Premix Ex Taq II system (TaKaRa Perfect Real Time) on a Bio-Rad Real-Time PCR system (Foster City, CA, USA), with primers listed in Additional file 2: Table S2. The qRT-PCR program was as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 34 s, and a final step of 95 °C for 15 s. The pineapple Actin2 gene was used as the internal reference. For each analysis, three technical replicates and three biological replicates were performed, and gene expression levels were calculated using the 2^–ΔΔCT method.
Results
Identification and physicochemical properties of GRAS genes in pineapple
In this study, a total of 43 GRAS gene family members were identified in pineapple genome (Additional file 3: Table S3) and designated AcGRAS01 to AcGRAS43 according to their chromosomal localization. The proteins encoded by the AcGRAS genes exhibited a diverse range of lengths, spanning from 110 amino acids (AcGRAS16) to 782 amino acids (AcGRAS14), with an average length of 449.7 amino acids. The predicted isoelectric points (pI) of AcGRAS proteins ranged from 4.07 (AcGRAS16) to 10.86 (AcGRAS13). The minimum molecular weight was determined to be 11,876.43 Da (AcGRAS16), while the maximum molecular weight reached 82,426.32 Da (AcGRAS14). Subcellular localization predictions indicated that all AcGRAS proteins were likely located in the nucleus. Based on the instability index, only AcGRAS16 could be considered stable, while the other 42 were predicted to be unstable. Additionally, the GRAVY index ranged from − 0.87 (AcGRAS29) to 0.146 (AcGRAS13), with most AcGRAS proteins (41 of 43) showing negative values, indicating that AcGRAS proteins were generally hydrophilic. Collectively, AcGRAS proteins displayed considerable variation in their physicochemical properties, implying potential functional diversity.
Classification and phylogenetic relationships of AcGRASs
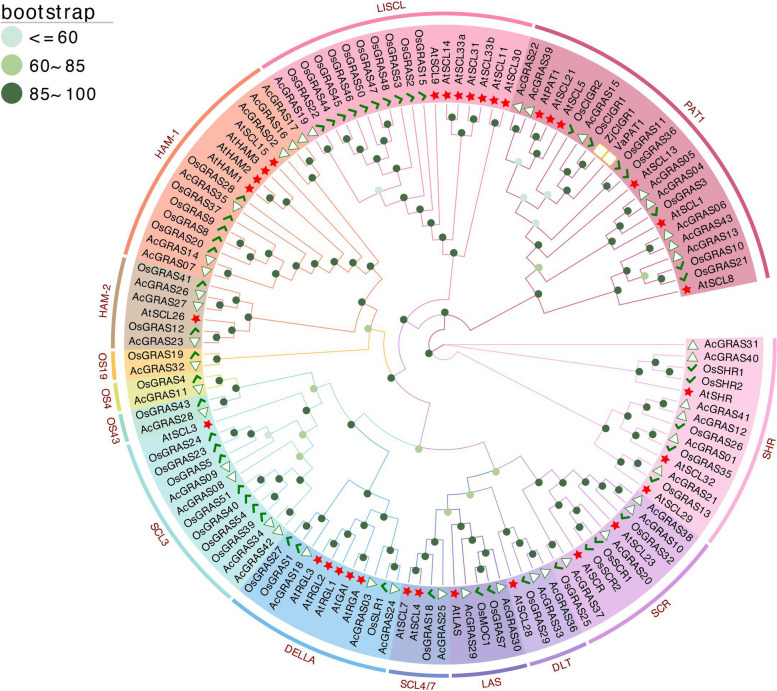
Phylogenetic analysis of the GRAS members from pineapple (AcGRASs), Arabidopsis (AtGRASs), and rice (OsGRASs) classified all the GRAS members into 14 subfamilies, including OS19, HAM-1, HAM-2, SCL4/7, LISCL, SHR, PAT1, DLT, LAS, SCR, SCL3, OS43, OS4, and DELLA (Fig. 1, Additional file 1: Table S1). Among these subfamilies, eight AcGRAS members (AcGRAS04, AcGRAS05, AcGRAS06, AcGRAS13, AcGRAS15, AcGRAS22, AcGRAS39, and AcGRAS43) clustered with the reported GRAS cold resistance genes ZjCIGR1 [27] and VaPAT1 [26], belonging to the PAT1 subfamily. Six AcGRAS genes each were classified into the SHR (AcGRAS01, AcGRAS12, AcGRAS21, AcGRAS31, AcGRAS40, and AcGRAS41) and HAM-1 (AcGRAS02, AcGRAS07, AcGRAS14, AcGRAS16, AcGRAS17, and AcGRAS35) subfamilies. Four genes each were found in the SCL3 (AcGRAS08, AcGRAS09, AcGRAS34, AcGRAS42) and SCR (AcGRAS10, AcGRAS20, AcGRAS37, AcGRAS38) subfamilies, and three genes each were found in the DELLA subfamily (AcGRAS03, AcGRAS18, AcGRAS24) and HAM-2 subfamily (AcGRAS23, AcGRAS26, AcGRAS27). The DLT (AcGRAS33, AcGRAS36) and LAS (AcGRAS29, AcGRAS30) subfamilies each contained two gene members, while LISCL (AcGRAS19), OS4 (AcGRAS11), OS43 (AcGRAS28), OS19 (AcGRAS32), and SCL4/7 (AcGRAS25) subfamilies each contained only one member. Furthermore, most pineapple GRAS proteins clustered closely with rice GRAS proteins, and some subfamilies, such as OS4 and OS19, contained gene members exclusively from rice and pineapple, indicating a close evolutionary relationship. This close clustering suggests that a shared evolutionary process occurred after the divergence of monocots and dicots, contributing to the diversity observed in the GRAS gene family.
Fig. 1.
Unrooted maximum-likelihood phylogenetic tree of GRAS proteins from Ananas comosus (Ac), Arabidopsis thaliana (At), and Oryza sativa (Os). The green triangle, green hook, and red star indicate the GRAS members from pineapple, rice, and Arabidopsis, respectively. The yellow-white squares represent the protein encoded by ZjCIGR1 from Zoysia japonica [27] and the gene VaPAT1 from Vitis vinifera [26]
Gene structure, conserved motif and domain analyses of AcGRASs
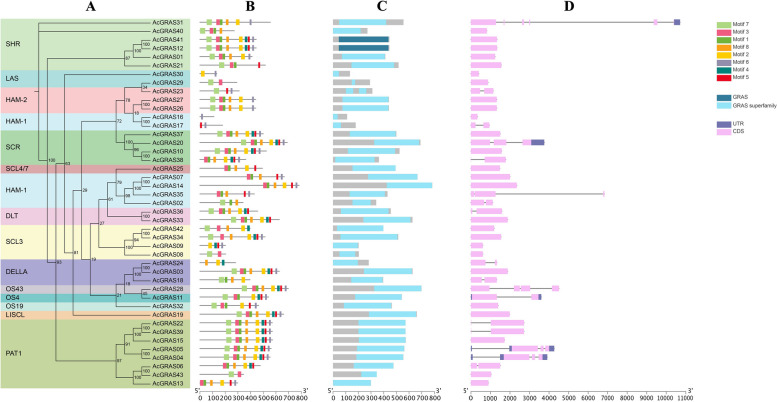
The gene structure, conserved motifs, and domains of AcGRASs were analyzed and shown according to their phylogenetic relationships (Fig. 2, Additional file 4: Table S4). Among the 43 AcGRAS proteins, eight conserved motifs (named motif 1 to motif 8) were predicted (Fig. 2B). The results revealed that most conserved motifs in the AcGRASs were situated in the C-terminal domain and were organized in the sequences of Motif 7, Motif 3, Motif 1, Motif 8, Motif 2, Motif 4, Motif 5, and Motif 6, with Motif 3 being the most highly conserved. The SCR and PAT1 subfamilies displayed a relatively stable conserved C-terminal domain with almost no motif deletions. Conversely, members of other subfamilies showed significant deletions of specific motifs, with variations in motif loss among different subfamily members. For example, the DLT subfamily members lost Motif 6, whereas Motif 4 was frequently absent in members of the HAM-1 subfamily. These variations in motif composition might be contributed to the functional diversity observed among different subfamily members. All AcGRAS-encoded proteins contained the conserved GRAS domain (Fig. 2C). Additionally, AcGRAS31 contained the extra Ribosomal_L38 domain, while AcGRAS03 and AcGRAS18, belonging to the DELLA subfamily, contained the extra DELLA domain. The genomic exon–intron structural analysis of the 43 AcGRAS genes revealed variability in the number of exons, ranging from 1 to 6 (Fig. 2D). Among them, AcGRAS31 in the SHR subfamily exhibited the highest number of exons and introns, with 6 exons and 5 introns. AcGRAS28 and AcGRAS35 had 4 exons, AcGRAS20, AcGRAS5, and AcGRAS4 had 3 exons, and the remaining genes had 1 or 2 exons. Most of these AcGRAS genes (26, 60.5%) lacked introns. Moreover, only AcGRAS31, AcGRAS20, AcGRAS11, AcGRAS5, and AcGRAS4 possessed UTRs (non-coding regions), with AcGRAS11, AcGRAS5, and AcGRAS4 having two UTRs, while AcGRAS31 and AcGRAS20 had only one. These findings suggest that variations in motif compositions and exon–intron structures occurred dynamically during the evolutionary development of the AcGRAS gene family, and that AcGRAS genes with similar features may serve similar functions.
Fig. 2.
Phylogenetic relationships, motif compositions, conserved domains and gene structures of AcGRASs. A Maximum likelihood phylogenetic tree of AcGRAS proteins; B Conserved motif distribution of AcGRAS proteins. A total of eight motifs were predicted, the scale bar indicated 100 aa, and the logo and sequence of the conserved motifs were provided in Additional file 4: Table S4. C Conserved domain distribution of AcGRAS proteins; D Gene structure of AcGRAS genes, including introns (black line), exons (pink rectangle) and untranslated regions (UTRs, purple rectangles). The scale bar represented 1 kb
Three-dimensional (3D) structural modeling of AcGRAS proteins
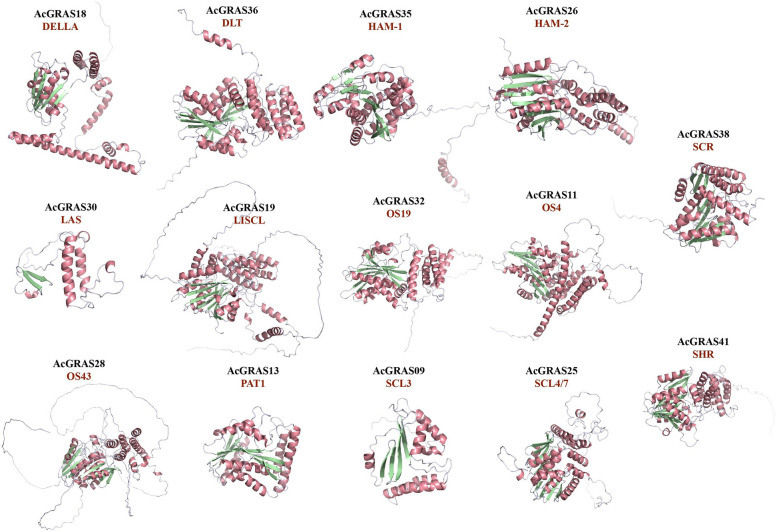
Understanding the 3D structure is essential for elucidating protein function. Homology modeling of all AcGRAS proteins was performed based on the AlphaFoldDB and SWISS-MODEL databases (Additional file 5: Fig.S1). For each subfamily, the structure with the highest GMQE and QMEAN scores (Additional file 6: Table S5) was chosen as the representative model (Fig. 3). The resulting models revealed that the protein structures in each branch could be divided into two main components: an α/β core subdomain and an α-helix domain. Additionally, some proteins, such as AcGRAS35 and AcGRAS36, were found to have an extra α-helix at the N-terminus, connected to the α-helix domain via a random coil. Within the α/β core subdomain, the β-sheets are encased by α-helices, and these β-sheets are conserved [57]. While most AcGRAS proteins contain eight β-sheets, a few, such as AcGRAS09 and AcGRAS30, have fewer. The two proteins in the LAS subfamily contain only two β-sheets, and in AcGRAS43, β-sheets were not detected. Transcription factors typically utilize α-helices to bind directly to the major groove of DNA, whereas β-sheets are crucial for maintaining the structural stability of transcription factors, forming effector domains, and facilitating protein–protein interactions [57]. The variation in the number of β-sheets among AcGRAS proteins may indicate their evolutionary adaptation and functional diversification.
Fig. 3.
Predicted 3D structural modeling of AcGRAS proteins. The structure with the highest GMQE and QMEAN scores in each subfamily was selected as the representative model
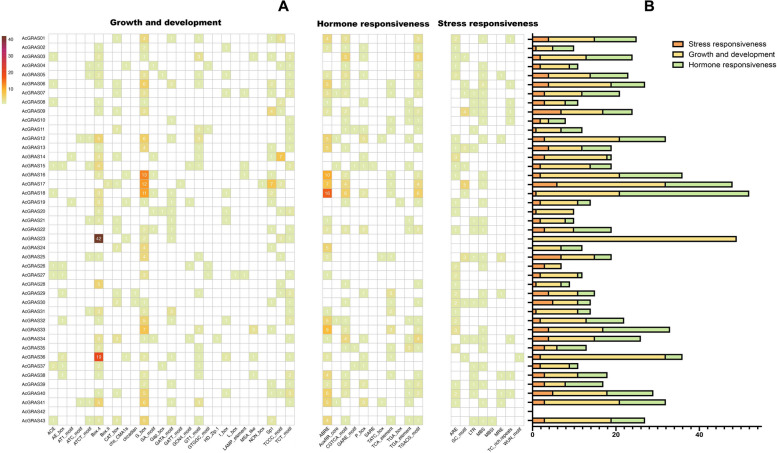
Cis-regulatory element analysis of AcGRAS genes
Cis-regulatory elements (CREs) refer to non-coding DNA sequences located in the promoter region of genes, playing a key role in regulating the transcription of associated genes [58]. The CREs in the putative promoter regions of AcGRAS genes were predicted and classified into three main types: plant growth and development CREs, phytohormone-responsive CREs, and stress-responsive CREs (Fig. 4, Additional file 7: Table S6). (1) Among the plant growth and development CREs, light-responsive elements were present in nearly all AcGRAS genes, indicating widespread regulation by light. CREs involved in meristem expression were found in the putative promoters of approximately one-third of the AcGRAS members, with multiple occurrences in the promoters of AcGRAS24, AcGRAS17, AcGRAS30, AcGRAS11, AcGRAS43, and AcGRAS34. Other plant growth and development CREs were less common, appearing only in the promoters of specific AcGRAS members. For example, CREs related to cell cycle regulation were found only in AcGRAS03, AcGRAS33, AcGRAS36, and AcGRAS38, while endosperm expression-related CREs were present only in AcGRAS26, AcGRAS19, AcGRAS15, and AcGRAS34. These growth and development-related CREs showed no consistent distribution pattern within subfamilies, suggesting that even within the same subfamily, AcGRAS members may play distinct roles in pineapple growth and development. (2) Various phytohormone-responsive CREs were predicted in the promoters of AcGRAS genes, indicating that hormones played a significant role in their regulation. Among these, abscisic acid (ABA) response-related CREs were the most abundant (114), which were present in 27 out of 43 AcGRAS members including 6 from the PAT1 subfamily (AcGRAS05, AcGRAS06, AcGRAS13, AcGRAS22, AcGRAS39, and AcGRAS43), 6 from the SHR subfamily (AcGRAS01, AcGRAS12, AcGRAS21, AcGRAS31, AcGRAS40, and AcGRAS41), and 4 from the HAM-1 subfamily (AcGRAS02, AcGRAS07, AcGRAS16, and AcGRAS17). MeJA response-related CREs were the second most abundant (104), appearing in the promoters of 26 AcGRAS members, including 7 from the PAT1 subfamily (AcGRAS04, AcGRAS05, AcGRAS06, AcGRAS13, AcGRAS22, AcGRAS39, and AcGRAS43) and 5 from the HAM-1 subfamily (AcGRAS02, AcGRAS07, AcGRAS16, AcGRAS17, and AcGRAS35). These CREs were especially abundant in two DELLA subfamily members, AcGRAS03 and AcGRAS18, with 10 and 12 CREs, respectively. Gibberellin-responsive CREs were identified in 18 AcGRAS members, including 4 from the PAT1 subfamily (AcGRAS05, AcGRAS15, AcGRAS22, and AcGRAS39), 3 from the HAM-1 subfamily (AcGRAS02, AcGRAS14, and AcGRAS35), and 2 from the DELLA subfamily (AcGRAS03 and AcGRAS18). Additionally, auxin- and salicylic acid-responsive CREs were also identified but were found only in few specific AcGRAS members. (3) Diverse stress-responsive CREs were also identified involved in low-temperature responsiveness, defense and stress responses, and MYB binding sites involved in drought inducibility. These elements presented varied distributions and combinations in the promoter region across AcGRAS members. For example, low-temperature responsive CREs were present in the promoters of 18 AcGRAS members, including 4 from the PAT1 subfamily (AcGRAS13, AcGRAS22, AcGRAS39, and AcGRAS43), 3 from the SCL3 subfamily (AcGRAS08, AcGRAS09, and AcGRAS34), and 1 from the DELLA subfamily (AcGRAS07). The variation in CRE distribution and combinations in the promoters of different AcGRAS members contributed to their diverse roles in pineapple growth, development, and stress responses.
Fig. 4.
Cis-regulatory elements in the putative promoter regions of the AcGRAS genes. A Heatmap of the number of cis-regulatory elements, the different color presented the number of cis-elements. B The sum of cis-regulatory elements in each category is shown in the histogram
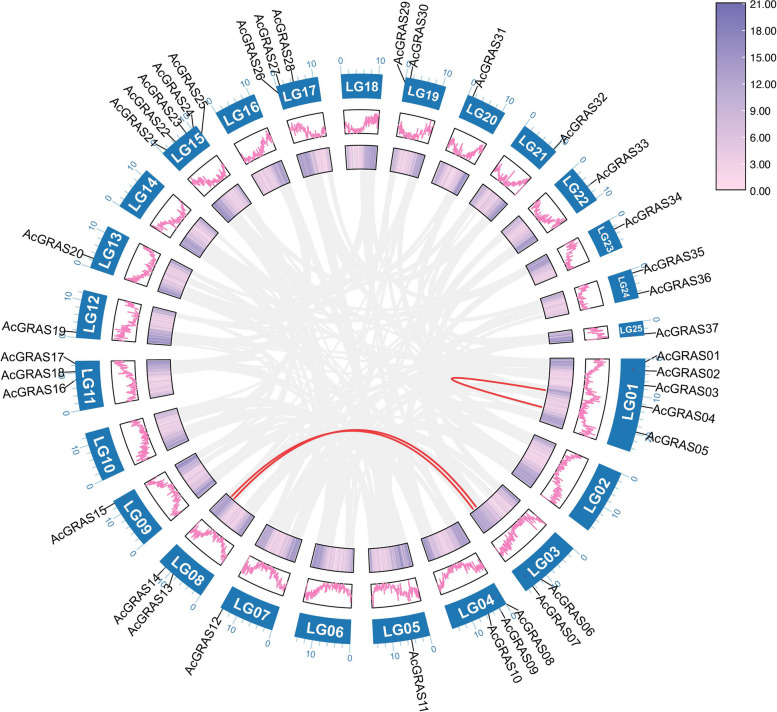
Chromosomal location and collinearity analysis
The 43 AcGRAS genes were unevenly distributed across 19 chromosomes and 6 scaffolds (Fig. 5). Within the pineapple genome (LG01-LG25), no genes were found on LG02, LG06, LG10, LG14, LG16, or LG18. There are 5 GRAS genes each on LG01 and LG15, 3 GRAS genes each on LG04, LG11, and LG17, 2 genes each on LG03, LG08, LG19, and LG24, and 1 gene each on the remaining chromosomes. Most genes were distributed in regions with high gene density and high recombination frequency, such as near the chromosome ends. The expansion of gene families is generally driven by various gene duplication patterns, which are considered to be a key force in species evolution. Gene duplication events were analyzed using the MCScanX method, revealing only three segmental duplicated gene pairs: AcGRAS04/AcGRAS05, AcGRAS14/AcGRAS07, and AcGRAS13/AcGRAS06. Combining with the phylogenetic analysis results (Fig. 1), it was found that the genes within each duplicated gene pair clustered together in the same subfamilyCombining with the phylogenetic analysis results , it was that the genes within each duplicated gene pair clustered together in the same subfamily. Specifically, one pair (AcGRAS07 and AcGRAS14) clustered within the HAM-1 subfamily, while the other two pair (AcGRAS04 and AcGRAS05, AcGRAS06 and AcGRAS13) both clustered within the PAT1 subfamily. These findings suggest that gene duplication might have played an important role in the development of the AcGRAS gene family in the pineapple genome.
Fig. 5.
Distribution and collinearity of AcGRAS genes in the pineapple genome. The background gray lines represent all the syntenic blocks in the pineapple genome, and the red lines represent duplicate AcGRAS gene pairs. Chromosome numbers are shown at the bottom of each chromosome. The two rings in the middle represent the gene density of each chromosome
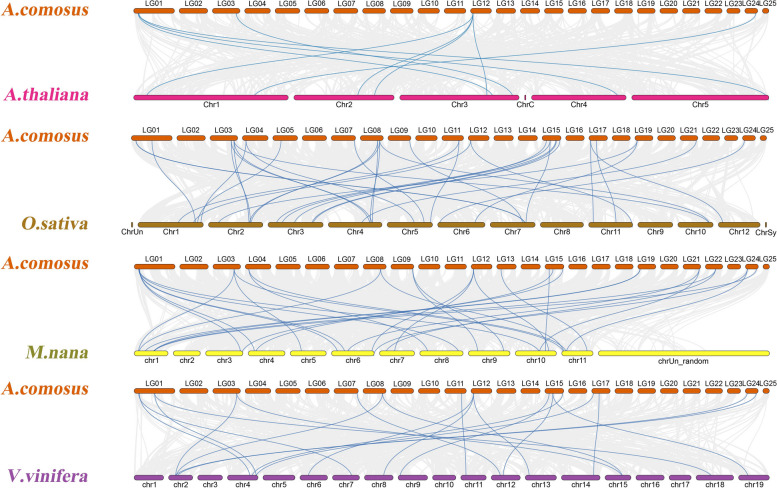
Collinearity analysis among different species is an effective method to explore their evolutionary relationships. Here, we conducted a comparative collinearity analysis between pineapple and four representative species: two dicots (Arabidopsis thaliana and Vitis vinifera) and two monocots (Oryza sativa and Musa nana) (Fig. 6, Additional file 8: Table S7). A total of 35 AcGRAS genes show collinearity with the rice genome, followed by bananas (31), grapes (21), and Arabidopsis (9) (Additional file 8: Table S7). These findings suggest that the collinearity between the pineapple and monocot genomes is greater than that between the pineapple and dicot genomes. The 34 AcGRAS genes with collinearity to rice were primarily located on chromosomes 3, 8, 15, and 17, while those with collinearity to Arabidopsis were distributed mainly on chromosomes 1 and 12. Some homologous genes exhibited one-to-many or many-to-one relationships. Notably, two AcGRAS genes (AcGRAS01 and AcGRAS19) displayed collinearity across all four selected species, indicating that these GRAS family genes may have played a significant role in evolutionary processes.
Fig. 6.
Synteny analysis of AcGRAS genes and four representative plant species. Grey lines in the background indicate collinear blocks in pineapple and other plant genomes, whereas the colored lines highlight syntenic GRAS gene pairs. Species names are prefixed with ‘A. thaliana’, ‘O. sativa’, ‘V.vinifera’ and ‘M.nana’, denote Arabidopsis thaliana, Oryza sativa, Vitis vinifera and Musa nana, respectively
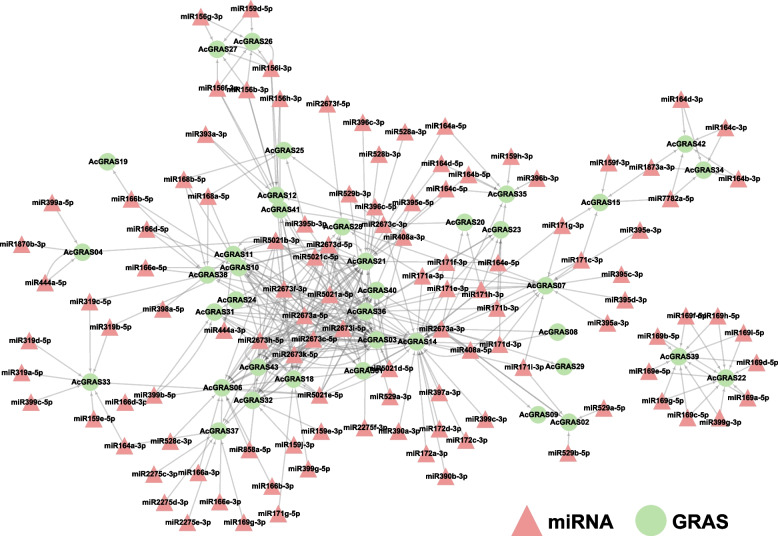
Prediction of putative miRNAs directing AcGRASs
MicroRNAs (miRNAs) played a crucial role in gene expression regulation by targeting mRNA degradation [59]. Previous studies indicated that many GRAS members were regulated by miRNAs, particularly miRNA171 [60, 61]. To investigate the potential regulatory relationships between miRNAs and AcGRAS genes in pineapple, a total of 385 miRNA-gene target pairs were found (Fig. 7). Specifically, 38 out of 43 AcGRAS genes were found to be targeted by miRNAs (excluding AcGRAS5/13/16/17/30). AcGRAS14 (HAM-1) was the one targeted by most miRNAs (36), followed by AcGRAS03 (DELLA, 30) and AcGRAS21 (SHR, 30). Among the 36 miRNAs targeting AcGRAS14, the miRNA2673 family had the most members (12). The miRNA2673 family also had the highest number of target mRNA interactions, with 158 pairs, and the gene most frequently targeted by this family was AcGRAS03 (27 times). Among all miRNAs, miR2673a-5p targeted the most AcGRAS genes (17), while its counterpart from the same precursor, miR2673a-3p, targeted only 10 AcGRAS genes, with only four genes overlapping between these two miRNAs. These findings suggest that even miRNAs derived from the same precursor can have significantly different functions depending on their processing. The interaction network results of the predicted miRNA targets for AcGRAS genes revealed that even members within the same subfamily were regulated by different types and numbers of miRNAs. For example, AcGRAS14 from the HAM-1 subfamily was primarily regulated by different members of the miRNA2673 family, while AcGRAS35, also from the HAM-1 subfamily, was mainly targeted by various members of the miRNA164 family. Similarly, AcGRAS07 from the same subfamily was predominantly regulated by members of the miRNA171 family. Previous studies have indicated that several GRAS members are regulated by miRNAs, especially miRNA171 [60, 61]. In pineapple, the miRNA171 family was predicted to target eight AcGRAS gene members from the DLT, HAM-1, HAM-2, OS43, PAT1, and SCR subfamilies (Additional file 9: Table S8). Among these, the members from the HAM-1 and HAM-2 subfamilies were targeted by the most miRNA171 family members. For instance, AcGRAS14 (HAM-1 subfamily) was predicted to be targeted by nine different miRNA171 family members, including miR171a-3p, miR171g-3p, miR171b-3p, miR171c-3p, miR171d-3p, miR171e-3p, miR171f-3p, miR171h-3p, and miR171i-3p. Additionally, AcGRAS07 (HAM-1 subfamily) and AcGRAS23 (HAM-1 subfamily) were predicted to be targeted by seven and six different miRNA171 family members, respectively. These findings suggested that the miRNA171 family may also have played a specific role in regulating the HAM subfamily members in pineapple.
Fig. 7.
Predicted miRNAs targeting AcGRAS genes. The network diagram shows the predicted miRNA targets for AcGRAS genes. Red triangular nodes represent the predicted miRNAs, and green circular nodes represent the targeted AcGRAS genes
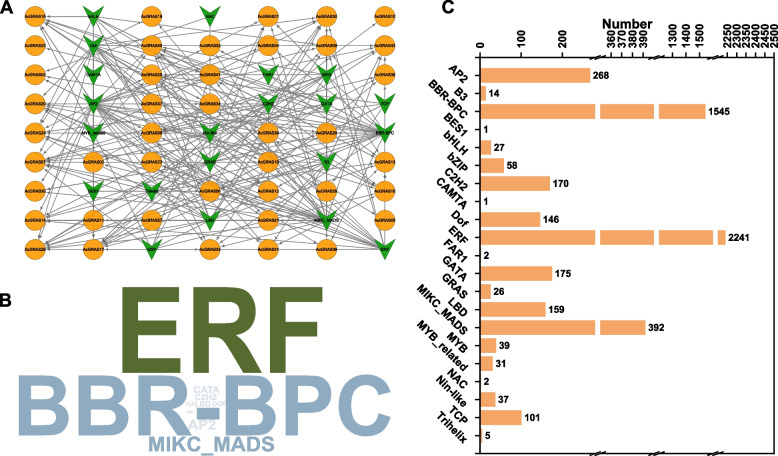
Transcription factor regulatory network of AcGRASs
To gain a more comprehensive understanding of the factors influencing AcGRAS gene expression, the potential transcription factor (TF) regulatory network for all 43 GRAS genes in pineapple was analyzed using the PTRM online database (http://plantregmap.gao-lab.org/). The analysis revealed that, except for AcGRAS42, the promoter regions of the other 42 GRAS genes were enriched with 21 types of TFs (Fig. 8, Additional file 10: Table S9). Among all TFs, ERF was the most abundant (2,241), followed by BBR-BPC (1,545) and MIKC-MADS (392). AcGRAS17 was found to be the most transcriptionally regulated gene (894), followed by AcGRAS24 (503) and AcGRAS29 (485), with AcGRAS17 and AcGRAS24 being predominantly regulated by ERF TFs (Additional file 10: Table S9). The proportion of ERF among the TFs regulating different AcGRAS gene members ranged from 89.52% to 100%, indicating a distinct preference for ERF binding motifs in the promoter regions of most AcGRAS genes. Diverse TFs involved in plant growth and development, including MIKC-MADS, LBD, bHLH, and AP2, were identified. Some stress-related TFs, such as bZIP [62] and NAC [63], have also been identified. Detailedly, the NAC TFs targeted members from the PAT1 (AcGRAS06) and DELLA (AcGRAS03) subfamilies, while various bZIP TFs primarily targeted members from the HAM-1 (AcGRAS07, AcGRAS16, AcGRAS17), SHR (AcGRAS01, AcGRAS12, AcGRAS41), and DELLA (AcGRAS18) subfamilies.
Fig. 8.
Putative TF regulatory network analysis of AcGRAS genes. A Network diagram illustration of the predicted TFs that target AcGRAS genes. Green arrow-shaped nodes represent TFs, and orange circular nodes represent AcGRAS genes. B Word cloud of TFs, where the font size is positively correlated with the number of corresponding TFs. C Statistical results of the number of TFs
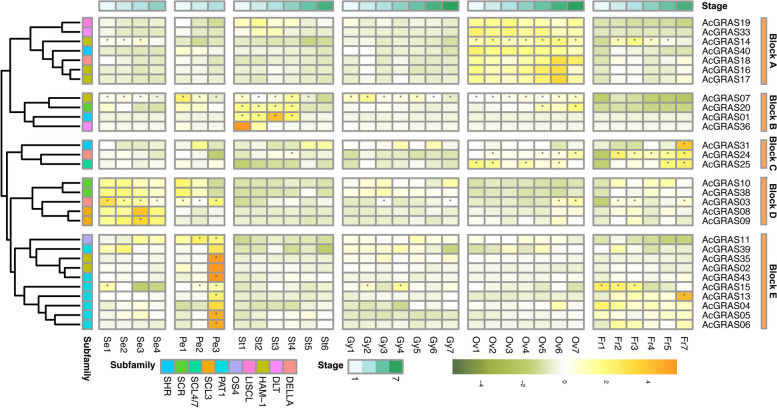
Expression patterns of AcGRAS in different tissues of pineapple
To explore the potential functions of AcGRAS genes, the expression profiles of the 43 AcGRAS genes in various pineapple tissues, including floral organs and fruit at different developmental stages, were analyzed using RNA-seq data (Fig. 9). After filtering out genes with low expression levels, 29 AcGRAS genes remained. Most of these genes exhibited preferential expression in specific tissues, suggesting that members of the GRAS gene family tend to have roles in particular tissues or at specific developmental stages. Hierarchical clustering grouped the AcGRAS genes into five clusters (blocks A-E), revealing diverse expression profiles even among genes within the same subfamilies. The AcGRAS genes in different blocks displayed distinct temporal and spatial expression patterns: (A) The AcGRAS genes in block A were preferentially expressed during ovule development, including one member each from the LISCL (AcGRAS19), DLT (AcGRAS33), SHR (AcGRAS40), and DELLA (AcGRAS18) subfamilies, as well as three members from the HAM-1 subfamily (AcGRAS16, AcGRAS17, and AcGRAS14). Among these, AcGRAS14 was also highly expressed during the early stages of sepal development and the middle stages of fruit development. (B) AcGRAS genes in block B were highly expressed during the early developmental stages of stamens, including one member each from the HAM-1 (AcGRAS07), SCR (AcGRAS20), SHR (AcGRAS01), and DLT (AcGRAS36) subfamilies. (C) The AcGRAS genes in block C tended to have higher expression levels at the late developmental stages of fruit, including one member each from the SHR (AcGRAS31), DELLA (AcGRAS24) and SCL4/7 (AcGRAS25) subfamilies. Besides, AcGRAS24 and AcGRAS25 were also showing high expression at certain stages of ovule development. (D) The AcGRAS genes in block D exhibited preferential expression during sepal development, including one member from the DELLA (AcGRAS03) subfamily and two members each from the SCR (AcGRAS10, AcGRAS38) and SCL3 (AcGRAS08, AcGRAS09) subfamilies. Among these genes, AcGRAS03 tended to decrease in expression during sepal development but was highly expressed at the later stages of gynoecium, ovule, petal, and fruit development. (E) The AcGRAS genes in block E were preferentially expressed during petal development, with an ascending trend, including one member from the OS4 (AcGRAS11), subfamilies, two members from the HAM-1 (AcGRAS35, AcGRAS02), and seven members from the PAT1 subfamily (AcGRAS43, AcGRAS15, AcGRAS13, AcGRAS04, AcGRAS05, AcGRAS06, AcGRAS39). Among these genes, AcGRAS13 was also highly expressed at the late developmental stages of fruit, while AcGRAS15 was also higher expressed at the early development stages of fruit. In summary, different AcGRAS genes displayed diverse expression profiles within or across subfamilies, with most showing tissue- or developmental stage-specific expression patterns. For example, AcGRAS35, AcGRAS43, AcGRAS02, AcGRAS05 and AcGRAS06 in block C were specifically preferentially expressed only at the late developmental stage of petals. However, a few genes, such as AcGRAS07, exhibited relatively high expression levels across all floral tissues.
Fig. 9.
Hierarchical clustering of the expression profiles of AcGRASs in floral tissues and fruits at different developmental stages. Se, sepal; Gy, gynoecium; Ov, ovule; Pe, petal; St, stamen; Fr, fruit; numbers represent developmental stages as described in Wang et al. (2020) [56]; the heatmap was created based on the log2(TPM + 0.01) value of AcGRASs and normalized by row. The TPM value higher than 50 was shown as abundant genes and marked with “*”. Differences in gene expression changes are shown in color as the scale, orange for high expression and dark green for low expression
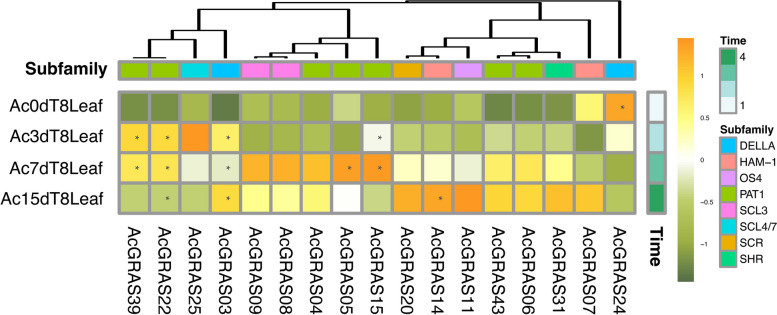
Low temperature has the most significant impact on pineapple production among various stressors, and many GRAS genes have been reported to play roles in plant cold stress responses [64]. To elucidate the response of AcGRAS genes to low-temperature stress, we analyzed their expression profiles in pineapple leaves at different time points under long-term cold treatment at 8 °C (0 d, 3 d, 7 d, and 15 d) via RNA-seq data (Fig. 10). After filtering out low-expression genes, our results indicated that, except for AcGRAS24, which was downregulated by cold stress, all other AcGRAS members were upregulated in response to low temperature. A few genes, including AcGRAS15, AcGRAS25, AcGRAS22, and AcGRAS39, exhibited peak expression during the mid-phase of cold treatment, while other members, such as AcGRAS03, AcGRAS04, AcGRAS05, AcGRAS07, AcGRAS11, AcGRAS14, AcGRAS19, and AcGRAS43, showed increased expression in the mid-to-late stages of cold treatment.
Fig. 10.
Hierarchical clustering of the expression profiles of the AcGRASs under cold treatment at 8 °C (0 d, 3 d, 7 d, and 15 d). The heatmap was created based on the log2(TPM + 0.01) value of AcGRASs and normalized by row. The TPM value higher than 50 was shown as abundant genes and marked with “*”. Differences in gene expression changes are shown in color as the scale, orange for high expression and dark green for low expression
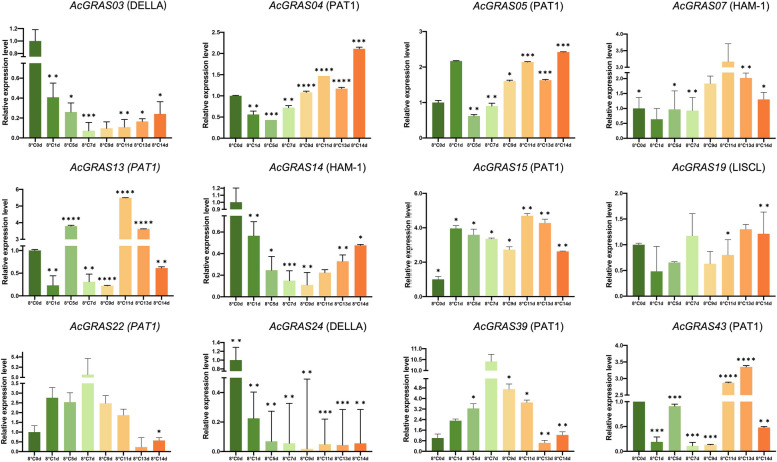
To validate the cold response trends of AcGRAS genes, we further analyzed the expression of 12 representative AcGRAS genes at additional time points (0 d, 1 d, 5 d, 7 d, 9 d, 11 d, 13 d, and 14 d) under the same batch of cold treatments as the transcriptome data using qRT-PCR (Fig. 11). These genes included seven members from the PAT1 subfamily, two from the DELLA subfamily, two from the HAM-1 subfamily, and one from the LISCL subfamily. Under cold stress conditions, the expression of AcGRAS03, AcGRAS14, and AcGRAS24 was significantly downregulated, initially decreasing and then showing some recovery with extended treatment time. Other GRAS genes were upregulated to varying degrees in response to cold stress. Specifically, AcGRAS04, AcGRAS05, and AcGRAS43 exhibited decreased expression early in the cold treatment but were significantly upregulated after 11 days, suggesting their involvement in the long-term adaptation of pineapple to cold environments. In contrast, AcGRAS07, AcGRAS22, and AcGRAS39 showed an initial increase in expression followed by a decrease, with peak expression occurring in the mid-treatment period. While expression levels of most AcGRAS genes varied throughout the cold treatment, AcGRAS15 was consistently upregulated at different time points, indicating its potential ongoing role in pineapple’s cold stress response. With a few exceptions, the results of the qRT-PCR analysis were largely consistent with the transcriptome data in revealing the response of AcGRAS genes to cold stress. Different AcGRAS members played roles in the short-term response or long-term adaptation of pineapple to low temperatures.
Fig. 11.
qRT-PCR analysis of 12 representative AcGRAS genes (AcGRAS03, AcGRAS14, AcGRAS07, AcGRAS24, AcGRAS19, AcGRAS39, AcGRAS22, AcGRAS15, AcGRAS13, AcGRAS05, AcGRAS43, and AcGRAS04) under cold (8 °C) stress in pineapple. All the experiments were conducted independently at least three times. Error bars indicate the standard deviation across three replicates. Asterisks denote significant differences in transcript levels relative to the blank control without treatment (0 d) (*P < 0.05, **P < 0.01, ***P < 0.001)
Discussion
Pineapple is an important tropical fruit crop, and the normal development of its flowers and fruits is crucial for fruit quality formation [56]. The growth and development of pineapple are significantly affected by low temperatures, with winter cold limiting year-round production [32]. Identifying gene resources involved in regulating flower and fruit development, as well as cold response, is of great significance for molecular breeding in pineapple. Members of the plant-specific GRAS gene family play essential roles in plant growth, fruit maturation, and stress responses including cold stress [1]. In this study, we identified all GRAS gene family members in the pineapple genome and systematically analyzed their structural characteristics, phylogenetic relationships, regulatory elements, and expression patterns. This research provides a reference for future functional studies of GRAS genes and molecular breeding in pineapple.
A total of 43 GRAS genes were identified in the whole pineapple genome, which were unevenly distributed across 19 chromosomes and 6 scaffolds, and named AcGRAS01 to AcGRAS43 based on their chromosomal locations (Additional file 3: Table S3, Additional file 11: Fig S2). The number of GRAS genes in pineapple was comparable to species like Arabidopsis thaliana (34), Cucumis melo (37), small gourd (Lagenaria siceraria) (37), physcomitrella moss (Physcomitrium patens) (42), cocoa (Theobroma cacao) (44), and wintersweet (Prunus mume) (46), but fewer than in Oryza sativa (50), Populus trichocarpa (106), Malus domestica (127), and winter rape (Brassica napus) (87). It seemed that the number of GRAS genes did not appear to correlate with genome size, for example, pineapple possesses a larger genome (526 Mb) [34] with fewer GRAS gene family members (43) compared to Brachypodium distachyon ( 271 Mb, 63GRASs) [65, 66]. Previous studies have shown that gene duplication events likely drive the expansion of the GRAS gene family [67] In pineapple, we identified only three segmental duplicated gene pairs: AcGRAS04/AcGRAS05, AcGRAS14/AcGRAS07, and AcGRAS13/AcGRAS06 (Fig. 5). Phylogenetic analysis revealed that each duplicate gene pair, two genes are clustered in the same subfamily, and 3 gene pairs are distributed in 3 subfamilies (Fig. 1). Expression analysis showed that AcGRAS04/AcGRAS05 and AcGRAS13/AcGRAS06 were predominantly expressed during late petal development (Fig. 9) and were induced by cold stress during the later stages of treatment (Figs. 10 and 11), suggesting possible functional redundancy. Differently, while AcGRAS14/AcGRAS07 were both highly expressed during ovule development, AcGRAS14 was predominantly expressed during mid-fruit development, whereas AcGRAS07 showed abundant expression in other flower organs (Fig. 9), with differing expression trends under cold stress (Figs. 10 and 11), indicating possible functional divergence. Gene duplication might have played role in the development of the AcGRAS gene family in pineapple, and the limited duplication events may explain the relatively smaller size of the GRAS gene family in this species.
Phylogenetic analysis indicated that GRAS members from pineapple, Arabidopsis, and rice clustered into 14 subfamilies (Fig. 1), with most subfamilies containing GRAS members from all three species. However, some subfamilies, such as OS4 and OS19, contained genes only from rice and pineapple, suggesting distinct development processes of GRAS gene family in these species. In most subfamilies, pineapple GRAS proteins clustered closely with rice GRAS proteins, indicating a close evolutionary relationship. Comparative collinearity analysis between pineapple and four representative species also showed higher collinearity between the pineapple genome and monocots (Fig. 6). The classification of GRAS genes in rice and Arabidopsis was consistent with previous reports [68], suggesting the reliability of our phylogenetic tree. However, the number of subfamilies differed from other studies; for example, 13 subfamilies were identified in the phylogenetic analysis of grapevine and Arabidopsis GRASs [8], while 10 subfamilies were identified in the phylogenetic analysis of GRASs from orchids (Dendrobium chrysotoxum), A. thaliana, and O. sativa [69]. These variations may reflect differences in the species included in the analysis. As more species undergo GRAS gene family analysis, species-specific subfamilies are being discovered [1], underscoring the importance of studying GRAS gene evolution in a broader range of species to gain a comprehensive understanding of their evolutionary history.
Gene exon–intron structural analysis of the 43 AcGRAS genes revealed variability in the number of exons, ranging from 1 to 6 (Fig. 2D). Most AcGRAS genes (26, or 60.5%) lacked introns, a phenomenon also reported in other GRAS family studies [70]. The origin of plant GRAS genes is thought to stem from horizontal gene transfer from ancient prokaryotic soil bacteria, followed by duplication events in flowering plants, which may explain the prevalence of intronless genes [71]. Over time, some GRAS genes developed different exon–intron structures, potentially acquiring new functions to adapt to specific environments. We found that most conserved motifs in AcGRAS proteins were located in the C-terminal domain and arranged in the sequences of Motif 7, Motif 3, Motif 1, Motif 8, Motif 2, Motif 4, Motif 5, and Motif 6, with Motif 3 being the most highly conserved. The SCR and PAT1 subfamilies displayed relatively stable C-terminal domains with almost no motif loss, whereas other subfamilies exhibited significant motif deletions (Fig. 2B), contributing to the functional diversity observed among subfamily members. Protein 3D structure prediction showed relatively higher similarity among proteins within the same subfamily, while there were distinct differences in proteins from different subfamilies (Fig. 3, Additional file 5: Fig. S1). This structural diversity might contribute to the functional diversity of GRAS family members.
Research has demonstrated that GRAS family members play critical roles in various aspects of plant growth and development, including root and shoot development, lateral organ formation, flower, embryo, and seed development, as well as fruit development and maturation [1]. Expression analysis of AcGRAS genes during pineapple floral organs and fruit development revealed that, after filtering out low-expression genes, most AcGRAS members were predominantly expressed in specific tissues or at certain developmental stages (Fig. 9). For instance, genes in Block A, which include members from the HAM-1, DLT, DELLA, and SHR subfamilies, were highly expressed during ovule development but showed relatively low expression in other flower organs and during fruit development. Previous studies have also shown that GRAS genes from different subfamilies are involved in flower and fruit development regulation [9, 21]. For example, GRAS proteins from the SHR subfamily have been shown to play a key role in ovule polarity establishment in Arabidopsis [72]. Genes from different subfamilies clustered into distinct blocks based on their expression patterns (Fig. 9). Nearly all members of the PAT1 subfamily clustered in Block E and were predominantly expressed during late petal development, suggesting a specialized role in this process. However, the expression patterns of other subfamily members across different tissues were less consistent. Although members of the same subfamily shared some similarities in gene and protein structure, the composition and distribution of CREs related to growth and development varied significantly among subfamily members (Fig. 4, Additional file 7: Table S6). Most AcGRAS genes were also predicted to be targeted by miRNAs (Fig. 7, Additional file 9: Table S8), indicating that the expression of these members might be regulated by miRNAs. Consistent with previous reports, we found that AcGRAS members from the HAM-1 and HAM-2 subfamilies were predominantly targeted by the miRNA171 family, reflecting conserved complementarity between HAM subfamily genes and miRNA171 across species [1]. However, even within the same subfamily, the miRNAs targeting different GRAS gene members varied considerably in type and number. Additionally, the TF predictions showed that various growth and development-related TFs were predicted to regulate different AcGRAS gene members (Fig. 8, Additional file 10: Table S9). Collectively, the diversity of these regulatory factors likely contributed to the functional diversification of GRAS gene members during the growth and development in pineapple.
Many GRAS family members have been reported to play roles in stress responses across various plants [1]. The growth and development of pineapple is particularly sensitive to cold stress. Our results showed that, except for a few AcGRAS genes downregulated under cold stress, such as AcGRAS03 and AcGRAS24 from the DELLA subfamily, the expression of most AcGRAS members were upregulated in response to cold. Some genes, such as AcGRAS22 and AcGRAS39 from the PAT1 subfamily, were predominantly expressed during early to mid-cold treatments, while most genes, including AcGRAS04, AcGRAS05, AcGRAS13, and AcGRAS43, were highly expressed during the later stages of prolonged cold exposure. These findings suggest that different AcGRAS members may play distinct roles in short-term or long-term cold adaptation in pineapple. Additionally, the expression of AcGRAS15 from the PAT1 subfamily was upregulated at all time points during cold stress, indicating its continuous involvement in cold stress adaptation. These findings highlighted the critical role of the PAT1 subfamily in pineapple’s response to cold stress. In wild Amur grape, the PAT1 subfamily member VaPAT1 has been shown to regulate jasmonic acid biosynthesis in response to cold stress [26], and in zoysiagrass, overexpression of the PAT1 subfamily gene ZjCIGR1 has been found to confer cold stress resistance [27]. CRE prediction analysis revealed that many stress-related CREs were present in the promoter region of most PAT1 subfamily members, including ABA-responsive, MeJA-responsive, and low-temperature-responsive elements (Fig. 4). These candidate genes involved in pineapple cold stress response could serve as potential targets for future research and molecular breeding efforts.
Conclusion
In summary, this study provides the first comprehensive analysis of the GRAS gene family in pineapple, identifying 43 AcGRAS genes and revealing their diverse roles in development and stress responses. The findings highlight the functional diversity of AcGRAS genes, particularly their tissue-specific expression and significant involvement in cold stress adaptation, with specific emphasis on the unique roles of the PAT1 subfamily in petal development and cold response. These insights not only deepen our understanding of the molecular mechanisms underlying pineapple development and stress tolerance but also offer potential applications in breeding programs aimed at enhancing cold resistance in this economically important crop. This research also serves as a valuable resource for future functional studies of AcGRAS genes in pineapple.
Supplementary Information
Additional file 1: Table S1. Table S1.1 shows the gene IDs and GRAS subfamily information for pineapple, Arabidopsis, rice, ZjCIGR1 and VaPAT1, and Table S1.2 shows the protein sequences used to construct the maximum likelihood (ML) phylogenetic tree.
Additional file 2: Table S2. The primer sequences for qRT‒PCR used in this study.
Additional file 3: Table S3. Physicochemical properties of AcGRAS gene family members in pineapple.
Additional file 4: Table S4. Putative motifs identified from AcGRAS proteins via MEME. The sequence logos were generated via WebLogo.
Additional file 5: Fig. S1. Homology modeling of all AcGRAS proteins. All AcGRAS proteins were homologously modeled via the AlphaFoldDB and SWISS-MODEL databases and classified by subfamily.
Additional file 6: Table S5. GMQE and QMEAN scores of AcGRAS protein 3D structure modeling.
Additional file 7: Table S6. Related information on the cis-regulatory elements in the promoter regions of AcGRASs.
Additional file 8: Table S7. Information on collinearity analysis and interpecies collinear gene repetition events.
Additional file 9: Table S8. Prediction of putative miRNAs directing AcGRASs.
Additional file 10: Table S9. Potential transcription factor (TF) prediction information for all 43 GRAS genes in pineapple.
Additional file 11: Fig. S2. Chromosomal localization of all AcGRAS genes. The colors on the chromosomes indicate gene density, with red indicating relatively high density and blue indicating relatively low density.
Acknowledgements
We thank all our colleagues for providing useful discussions and technical assistance. We are very grateful to the editor and reviewers for critically evaluating the manuscript and providing constructive comments for its improvement.
Abbreviations
- GAI
Gibberellic acid-insensitive
- RGA
Repressor of GAI
- SCR
Scarecrow
- CREs
Cis-regulatory elements
- GMQE
Global Model Quality Estimation
- QMEAN
Quality Model Energy Assessment
- GRAVY
Grand Average of hydropathicity
- miRNAs
MicroRNAs
- pI
Isoelectric point
- RNAi
RNA interference
- TF
Transcription Factor
- TPM
Transcripts Per Million
- UTR
Untranslated Region
- CDS
Coding Sequence
- ABA
Abscisic Acid
- MeJA
Methyl Jasmonate
Authors’ contributions
P.Z., Y.Q. and R.L. designed the experiments and revised the manuscript; J.L., J.W. and X.C. performed the gene family analysis; D.Z. conducted the cold treatment and collected the samples; Q.Y., S.X. and X.X. collected the different pineapple tissues; S.C. generated the expression data from RNA-seq; L.D., L.L., and C.L. performed the qRT‒PCR and constructed the vectors. P.Z., J.L. and R.L. wrote the original manuscript; P.Z., Y.Q. and X.W. reviewed and edited the manuscript.
Funding
This work was supported by the Science and Technology Major Project of Guangxi (Gui Ke AA22068096); Construction Funds for the Key Core Technology of Biological Breeding at the Institute of Future Technology, Fujian Agriculture and Forestry University (72202202307); the Research Funds on Breeding Technology Innovation for Characteristic Fruit Trees of Yunnan (KH230435A).
Data availability
The entire Ananas comosus L. genome sequence information was obtained from the Phytozome website (version: Ananas comosus v3; https://phytozome-next.jgi.doe.gov/info/Acomosus_v3). The original RNA-seq data of pineapple floral organs used in this study were obtained from the European Nucleotide Archive (ENA) under accession number PRJEB38680. Data on the different stages of pineapple fruit development were obtained from https://de.iplantcollaborative.org/de/?type=data&folder=/iplant/home/cmwai/coge_data/Pineapple_tissue_RNAseq. Transcriptomic data from pineapple subjected to cold treatment at 8 °C were generated from our unpublished work, which has been deposited in China National GeneBank DataBase (CNGBdb) with accession number CNP0006260 (https://db.cngb.org/search/project/CNP0006260/). The datasets supporting the conclusions of this article are included within the article and its additional files.
Declarations
Ethics approval and consent to participate
The experimental research and method on pineapple species comply with relevant institutional, national, and international guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jinting Lin, Jiahao Wu and Dan Zhang contributed equally to this work.
Contributor Information
Ruoyu Liu, Email: liuruoyu13@mails.ucas.ac.cn.
Yuan Qin, Email: yuanqin@fafu.edu.cn.
Ping Zheng, Email: zhengping13@mails.ucas.ac.cn.
References
- 1.Neves C, Ribeiro B, Amaro R, Expósito J, Grimplet J, Fortes AM. Network of GRAS transcription factors in plant development, fruit ripening and stress responses. Hortic Res. 2023;10(12):uhad220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pysh LD, Wysocka-Diller JW, Camilleri C, Bouchez D, Benfey PN. The GRAS gene family in Arabidopsis: sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J. 1999;18(1):111–9. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez DH. Plant transcription factors: evolutionary, structural and functional aspects. London: Academic Press; 2015.
- 4.Hirsch S, Oldroyd GE. GRAS-domain transcription factors that regulate plant development. Plant Signal Behav. 2009;4(8):698–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Widmer A. Genome-wide comparative analysis of the GRAS gene family in Populus, Arabidopsis and rice. Plant Mol Biol Repo. 2014;32:1129–45. [Google Scholar]
- 6.Bi Y, Wei B, Meng Y, Li Z, Tang Z, Yin F, Qian C. Genome-wide GRAS gene family analysis reveals the classification, expression profiles in melon (Cucumis melo L.). Phyton. 2021;90(4):1161. [Google Scholar]
- 7.Fan S, Zhang D, Gao C, Zhao M, Wu H, Li Y, Shen Y, Han M. Identification, classification, and expression analysis of GRAS gene family in Malus domestica. Front Physiol. 2017;8:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grimplet J, Agudelo-Romero P, Teixeira RT, Martinez-Zapater JM, Fortes AM. Structural and functional analysis of the GRAS gene family in grapevine indicates a role of GRAS proteins in the control of development and stress responses. Front Plant Sci. 2016;7:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang W, Xian Z, Kang X, Tang N, Li Z. Genome-wide identification, phylogeny and expression analysis of GRAS gene family in tomato. BMC Plant Biol. 2015;15:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B, Liu J, Yang ZE, Chen EY, Zhang CJ, Zhang XY, Li FG. Genome-wide analysis of GRAS transcription factor gene family in Gossypium hirsutum L. BMC Genomics. 2018;19:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian C, Wan P, Sun S, Li J, Chen M. Genome-wide analysis of the GRAS gene family in rice and Arabidopsis. Plant Mol Biol. 2004;54:519–32. [DOI] [PubMed] [Google Scholar]
- 12.Liu B, Sun Y, Xue J, Jia X, Li R. Genome-wide characterization and expression analysis of GRAS gene family in pepper (Capsicum annuum L.). PeerJ. 2018;6:e4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cenci A, Rouard M. Evolutionary analyses of GRAS transcription factors in angiosperms. Front Plant Sci. 2017;8:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Wang W. Characterization of the GRAS gene family reveals their contribution to the high adaptability of wheat. PeerJ. 2021;9:e10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallagher KL, Benfey PN. Both the conserved GRAS domain and nuclear localization are required for SHORT-ROOT movement. Plant J. 2009;57(5):785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn MG, Feldmann KA, Benfey PN. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell. 1996;86(3):423–33. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Zhao P, Peng X, Sun MX. Seed development in Arabidopsis: what we have learnt in the past 30 years. Seed Biolog. 2023;2(1):6. [Google Scholar]
- 18.Schulze S, Schäfer BN, Parizotto EA, Voinnet O, Theres K. LOST MERISTEMS genes regulate cell differentiation of central zone descendants in Arabidopsis shoot meristems. Plant J. 2010;64(4):668–78. [DOI] [PubMed] [Google Scholar]
- 19.Huang W, Peng S, Xian Z, Lin D, Hu G, Yang L, Ren M, Li Z. Overexpression of a tomato miR171 target gene SlGRAS24 impacts multiple agronomical traits via regulating gibberellin and auxin homeostasis. Plant Biotechnol J. 2017;15(4):472–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes TE, Langdale JA. SCARECROW is deployed in distinct contexts during rice and maize leaf development. Development. 2022;149(7):dev200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galvão VC, Horrer D, Küttner F, Schmid M. Spatial control of flowering by DELLA proteins in Arabidopsis thaliana. Development. 2012;139(21):4072–82. [DOI] [PubMed] [Google Scholar]
- 22.Morohashi K, Minami M, Takase H, Hotta Y, Hiratsuka K. Isolation and characterization of a novel GRAS gene that regulates meiosis-associated gene expression. J Biol Chem. 2003;278(23):20865–73. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Zhu M, Ren L, Li A, Chen G, Hu Z. The SlFSR gene controls fruit shelf-life in tomato. J Exp Bot. 2018;69(12):2897–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Li G, Sun Y, Qin Z, Feng P. Genome-wide analysis and characterization of GRAS family in switchgrass. Bioengineered. 2021;12(1):6096–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma H-S, Liang D, Shuai P, Xia X-L, Yin W-L. The salt-and drought-inducible poplar GRAS protein SCL7 confers salt and drought tolerance in Arabidopsis thaliana. J Exp Bot. 2010;61(14):4011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Wong DCJ, Wang Y, Xu G, Ren C, Liu Y, Kuang Y, Fan P, Li S, Xin H, et al. GRAS-domain transcription factor PAT1 regulates jasmonic acid biosynthesis in grape cold stress response. Plant Physiol. 2021;186(3):1660–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y-J, Yang D-H, Park M-Y, Sun H-J, Song P-S, Kang H-G, Suh S-C, Lee Y-E, Lee H-Y. Overexpression of Zoysia ZjCIGR1 gene confers cold stress resistance to zoysiagrass. Plant Biotechnol Re. 2020;14(1):21–31. [Google Scholar]
- 28.Li D, Jing M, Dai X, Chen Z, Ma C, Chen J. Current status of pineapple breeding, industrial development, and genetics in China. Euphytica. 2022;218(6):85. [Google Scholar]
- 29.Zhu Z, Johnson J, Zaman QU, Wang H. Challenges and opportunities to improve tropical fruits in Hainan, China. Trop Plants. 2022;1(1):1–10. [Google Scholar]
- 30.Purseglove JW. Tropical crops: monocotyledons. Vols. 1 and 2. London: Longman; 1972.
- 31.Hewajulige I, Wilson Wijeratnam R, Wijesundera R, Abeysekere M. Fruit calcium concentration and chilling injury during low temperature storage of pineapple. J Sci Food Agric. 2003;83(14):1451–4. [Google Scholar]
- 32.Chen C, Zhang Y, Xu Z, Luan A, Mao Q, Feng J, Xie T, Gong X, Wang X, Chen H, et al. Transcriptome profiling of the pineapple under low temperature to facilitate its breeding for cold tolerance. PLoS One. 2016;11(9):e0163315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun W, Wu Q, Dou M, Dou T, Dou G. Causes and management of pineapple freeze and chill damage. China Trop Agric. 2007;02:58–9. [Google Scholar]
- 34.Ming R, VanBuren R, Wai CM, Tang H, Schatz MC, Bowers JE, Lyons E, Wang M-L, Chen J, Biggers E, et al. The pineapple genome and the evolution of CAM photosynthesis. Nat Genet. 2015;47(12):1435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2011;40(D1):D1178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin J, Tian F, Yang D-C, Meng Y-Q, Kong L, Luo J, Gao G. PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants. Nucleic. 2016;45(D1):D1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mistry J, Chuguransky S, Williams L, Qureshi M, Salazar Gustavo A, Sonnhammer ELL, Tosatto SCE, Paladin L, Raj S, Richardson LJ, et al. Pfam: the protein families database in 2021. Nucleic Acids Res. 2020;49(D1):D412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Chitsaz F, Derbyshire MK, Gonzales NR, Gwadz M, Lu S, Marchler GH, Song JS, Thanki N, Yamashita RA, et al. The conserved domain database in 2023. Nucleic Acids Res. 2023;51(D1):D384-d388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Letunic I, Khedkar S, Bork P. SMART: recent updates, new developments and status in 2020. Nucleic Acids Res. 2020;49(D1):D458–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duvaud S, Gabella C, Lisacek F, Stockinger H, Ioannidis V, Durinx C. Expasy, the Swiss bioinformatics resource portal, as designed by its users. Nucleic Acids Res. 2021;49(W1):W216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chou KC, Shen HB. Cell-PLoc 2.0: an improved package of web-servers for predicting subcellular localization of proteins in various organisms. Nat Sci. 2010;2(10):1090. [DOI] [PubMed] [Google Scholar]
- 42.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23(21):2947–8. [DOI] [PubMed] [Google Scholar]
- 43.Subramanian B, Gao S, Lercher MJ, Hu S, Chen W-H. Evolview v3: a webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019;47(W1):W270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME Suite: tools for motif discovery and searching. Nucleic Acids Res. 2009;37(suppl_2):W202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen C, Wu Y, Li J, Wang X, Zeng Z, Xu J, Liu Y, Feng J, Chen H, He Y, et al. TBtools-II: a “one for all, all for one” bioinformatics platform for biological big-data mining. Mol Plant. 2023;16(11):1733–42. [DOI] [PubMed] [Google Scholar]
- 46.Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The protein data bank. Nucleic Acids Res. 2000;28(1):235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46(W1):W296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashkenazy H, Abadi S, Martz E, Chay O, Mayrose I, Pupko T, Ben-Tal N. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016;44(W1):W344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeLano WL. Pymol: an open-source molecular graphics tool. CCP4 Newsl Protein Crystallogr. 2002;40(1):82–92. [Google Scholar]
- 51.Omasits U, Ahrens CH, Müller S, Wollscheid B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 2014;30(6):884–6. [DOI] [PubMed] [Google Scholar]
- 52.Zheng Y, Li T, Xu Z, Wai CM, Chen K, Zhang X, Wang S, Ji B, Ming R, Sunkar R. Identification of microRNAs, phasiRNAs and their targets in pineapple. Trop Plant Biol. 2016;9(3):176–86. [Google Scholar]
- 53.Dai X, Zhuang Z, Zhao PX. psRNATarget: a plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018;46(W1):W49-w54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian F, Yang D-C, Meng Y-Q, Jin J, Gao G. PlantRegMap: charting functional regulatory maps in plants. Nucleic Acids Res. 2019;48(D1):D1104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L, Li Y, Jin X, Liu L, Dai X, Liu Y, Zhao L, Zheng P, Wang X, Liu Y, et al. Floral transcriptomes reveal gene networks in pineapple floral growth and fruit development. Commun Biol. 2020;3(1):500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hakoshima T. Structural basis of the specific interactions of GRAS family proteins. FEBS Lett. 2018;592(4):489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao J, Zhai Z, Li Y, Geng S, Song G, Guan J, Jia M, Wang F, Sun G, Feng N. Genome-wide identification and expression profiling of the TCP family genes in spike and grain development of wheat (Triticum aestivum L.). Front Plant Sci. 2018;9:1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Axtell MJ. Classification and comparison of small RNAs from plants. Annu Rev Plant Biol. 2013;64(1):137–59. [DOI] [PubMed] [Google Scholar]
- 60.Bolle C. The role of GRAS proteins in plant signal transduction and development. Planta. 2004;218:683–92. [DOI] [PubMed] [Google Scholar]
- 61.Ma Z, Hu X, Cai W, Huang W, Zhou X, Luo Q, Yang H, Wang J, Huang J. Arabidopsis miR171-targeted scarecrow-like proteins bind to GT cis-elements and mediate gibberellin-regulated chlorophyll biosynthesis under light conditions. PLoS Genet. 2014;10(8):e1004519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim SY. The role of ABF family bZIP class transcription factors in stress response. Physiol Plant. 2006;126(4):519–27. [Google Scholar]
- 63.Puranik S, Sahu PP, Srivastava PS, Prasad M. NAC proteins: regulation and role in stress tolerance. Trends Plant Sci. 2012;17(6):369–81. [DOI] [PubMed] [Google Scholar]
- 64.Tong N, Li D, Zhang S, Tang M, Chen Y, Zhang Z, Huang Y, Lin Y, Cheng Z, Lai Z. Genome-wide identification and expression analysis of the GRAS family under low-temperature stress in bananas. Front Plant Sci. 2023;14:1216070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang Z, Song N, Peng W, Yang Y, Qiu T, Huang C, Dai L, Wang B. Genome Identification and Expression Analysis of GRAS Family Related to Development, Hormone and Pathogen Stress in Brachypodium distachyon. Front Sustain Food Syst. 2021; 5. [Google Scholar]
- 66.Sreedasyam A, Plott C, Hossain MS, Lovell John T, Grimwood J, Jenkins Jerry W, Daum C, Barry K, Carlson J, Shu S, et al. JGI Plant Gene Atlas: an updateable transcriptome resource to improve functional gene descriptions across the plant kingdom. Nucleic Acids Res. 2023;51(16):8383–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu J, Wang T, Xu Z, Sun L, Zhang Q. Genome-wide analysis of the GRAS gene family in Prunus mume. Mol Genet Genomics. 2015;290:303–17. [DOI] [PubMed] [Google Scholar]
- 68.Fan Y, Yan J, Lai D, Yang H, Xue G, He A, Guo T, Chen L, Cheng XB, Xiang DB, et al. Genome-wide identification, expression analysis, and functional study of the GRAS transcription factor family and its response to abiotic stress in sorghum [Sorghum bicolor (L.) Moench]. BMC Genomics. 2021;22(1):509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao X, Liu DK, Wang QQ, Ke S, Li Y, Zhang D, Zheng Q, Zhang C, Liu ZJ, Lan S. Genome-wide identification and expression analysis of the GRAS gene family in Dendrobium chrysotoxum. Front Plant Sci. 2022;13:1058287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong X, Han B, Yin X, Mao P, Luo D, Zhou Q, Liu Z. Genome-wide identification of the GRAS transcription factor family in autotetraploid cultivated alfalfa (Medicago sativa L.) and expression analysis under drought stress. Ind Crops Prod. 2023;194:116379. [Google Scholar]
- 71.Zhang D, Iyer LM, Aravind L. Bacterial GRAS domain proteins throw new light on gibberellic acid response mechanisms. Bioinformatics. 2012;28(19):2407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui H, Levesque MP, Vernoux T, Jung JW, Paquette AJ, Gallagher KL, Wang JY, Blilou I, Scheres B, Benfey PN. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science. 2007;316(5823):421–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Table S1.1 shows the gene IDs and GRAS subfamily information for pineapple, Arabidopsis, rice, ZjCIGR1 and VaPAT1, and Table S1.2 shows the protein sequences used to construct the maximum likelihood (ML) phylogenetic tree.
Additional file 2: Table S2. The primer sequences for qRT‒PCR used in this study.
Additional file 3: Table S3. Physicochemical properties of AcGRAS gene family members in pineapple.
Additional file 4: Table S4. Putative motifs identified from AcGRAS proteins via MEME. The sequence logos were generated via WebLogo.
Additional file 5: Fig. S1. Homology modeling of all AcGRAS proteins. All AcGRAS proteins were homologously modeled via the AlphaFoldDB and SWISS-MODEL databases and classified by subfamily.
Additional file 6: Table S5. GMQE and QMEAN scores of AcGRAS protein 3D structure modeling.
Additional file 7: Table S6. Related information on the cis-regulatory elements in the promoter regions of AcGRASs.
Additional file 8: Table S7. Information on collinearity analysis and interpecies collinear gene repetition events.
Additional file 9: Table S8. Prediction of putative miRNAs directing AcGRASs.
Additional file 10: Table S9. Potential transcription factor (TF) prediction information for all 43 GRAS genes in pineapple.
Additional file 11: Fig. S2. Chromosomal localization of all AcGRAS genes. The colors on the chromosomes indicate gene density, with red indicating relatively high density and blue indicating relatively low density.
Data Availability Statement
The entire Ananas comosus L. genome sequence information was obtained from the Phytozome website (version: Ananas comosus v3; https://phytozome-next.jgi.doe.gov/info/Acomosus_v3). The original RNA-seq data of pineapple floral organs used in this study were obtained from the European Nucleotide Archive (ENA) under accession number PRJEB38680. Data on the different stages of pineapple fruit development were obtained from https://de.iplantcollaborative.org/de/?type=data&folder=/iplant/home/cmwai/coge_data/Pineapple_tissue_RNAseq. Transcriptomic data from pineapple subjected to cold treatment at 8 °C were generated from our unpublished work, which has been deposited in China National GeneBank DataBase (CNGBdb) with accession number CNP0006260 (https://db.cngb.org/search/project/CNP0006260/). The datasets supporting the conclusions of this article are included within the article and its additional files.