Abstract
Background
Drug susceptibility testing (DST) for Nocardia spp. is essential to initiate effective antibiotic therapy. Currently, the only recommended technique is the determination of minimum inhibitory concentrations (MICs) by microdilution. This method can be tedious to perform, despite the availability of ready-to-use plates. Herein, the aim was to determine the critical inhibition diameters specific to Nocardia spp.
Methods
MICs of 134 Nocardia isolates were determined by microdilution. Interpretative categories (Susceptible/Intermediate/Resistant) were determined using Clinical and Laboratory Standards Institute breakpoints. In parallel, disk diffusion DST was performed. Receiver-operating-characteristic (ROC) curves were constructed to determine the inhibition diameter value that best discriminated between susceptible and non-susceptible strains (intermediate/resistant). The category agreement (CA), the rate of major (maj) and very major (vmj) discrepancies between microdilution and disk diffusion method was calculated.
Results
For tobramycin, the critical diameter of 19 mm (diameter ≤ 19 mm = resistant strain; diameter > 19 mm = susceptible strain) provided a CA of 98.5%, 0.0% vmj, and 2.9% maj discrepancies, reaching strictly the acceptable performance criteria defined by the U.S. Food and Drug Administration (FDA). For amikacin, the critical diameter of 25 mm (diameter ≤ 25 mm = resistant strain; diameter > 25 mm = susceptible strain) provided a CA of 98.5%, 0.0% vmj, and 1.5% maj discrepancies. For imipenem, excluding N. farcinica and N. cyriacigeorgica, the critical diameter of 29 mm (diameter ≤ 29 mm = resistant strain; diameter > 29 mm = susceptible strain), provided a CA of 98.6%, 0.0% vmj, and 0.0% maj discrepancies. Despite an estimated vmj rate 0.0%, the 95%-confident-interval exceeded the FDA criteria due to an insufficient number of amikacin/imipenem-resistant strains. For other tested antibiotics (ciprofloxacin, moxifloxacin, amoxicillin-clavulanate, ceftriaxone, cotrimoxazole, linezolid), the FDA criteria were not reached.
Conclusions
Although the FDA criteria were mostly unmet, disk diffusion DST was suitable to accurately categorize Nocardia isolates into interpretative categories for the aminoglycosides and imipenem only, excluding species N. farcinica and N. cyriacigeorgica.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12941-024-00768-2.
Keywords: Nocardia spp., Drug susceptibility testing, Disk diffusion, Microdilution, Critical inhibition diameters
Background
Nocardiosis is a disease caused by telluric bacteria of the Nocardia genus; this rare but serious infection affects approximately 500 to 1000 individuals per year in the United States [1] and predominantly occurs in immunocompromised patients, such as those with solid organ and hematopoietic stem cell transplanted recipients, patients with auto-immune diseases treated by immunotherapies and/or corticosteroids, and patients with structural and functional lung impairments (cystic fibrosis, chronic obstructive pulmonary disease, and bronchiectasis) [1, 2]. Since high mortality rates due to disseminated nocardiosis are reported, reaching 50% in Europe [3], an early diagnosis leading to the initiation of a therapy using appropriate antibiotics is vitally important. Drug susceptibility testing (DST) is typically recommended for all Nocardia infections, as drug susceptibility strongly differs among Nocardia species as well as the patients’ tolerance to antibacterial agents [1, 2].
Currently, the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method is the only recommended method to perform DST on Nocardia isolates (2018 edition, 4). Although ready-to-use plates are available for microdilution susceptibility testing, such as the Sensititre™ Myco RAPMYCOI or NOCARDIA AST plates (Thermo Fischer Scientific, Waltham, MA, US), the microdilution technique can be tedious and expensive to set up for small to medium-sized laboratories. Therefore, these laboratories have to recourse to a reference laboratory, which inevitably delays DST turnaround times. Disk diffusion DST techniques are more easily implemented in this type of structure for the sake of simplicity and practicability [4]. Prior to the European harmonization recommended by the European Committee Antimicrobial Susceptibility Testing (EUCAST), the Comité de l’Antibiogramme de la Société Française de Microbiologie (CA-SFM) proposed in 2013 critical inhibition diameters for many antibiotics, they were however not related to any particular bacterial species [5]. A large French retrospective epidemiological study used these critical inhibition diameters to assess antibiotic susceptibility in Nocardia clinical isolates isolated in France (n = 793) [6]. However, Lebeaux et al. did not validate these critical inhibition diameters by comparison with the determination of the minimum inhibitory concentration (MIC) using broth microdilution ; they justified their choice by explaining that a previous study on a limited number of Nocardia strains (n = 26) had compared different DST methods on Nocardia [7]. Of note, this study reported that the disk diffusion method had a very good percentage of category agreement (95.7%) using a composite reference method, which was the same as the one found using MIC determination by broth dilution. However, the aforementioned study used the National Committee for Clinical and Laboratory Standards Institute breakpoints (edition of 1993, 1994) for microorganisms that grow aerobically, and not specifically for Nocardia [7]. Earlier studies had attempted to determine Nocardia-specific breakpoints for disks inhibition diameters and MIC [8, 9].
Since the EUCAST harmonization, critical inhibition diameters not related to a bacterial species have been removed from CA-SFM guidelines, and no critical inhibition diameter has been proposed specifically for Nocardia. At a time of laboratory accreditation, it seems difficult to use critical inhibition diameters that are 10 years old [5], present in a reference system that has not been updated. Therefore, the aim of the present study was to attempt to determine critical inhibition diameters specific to Nocardia, using broth microdilution MIC determination as the reference method [10] and a modified-EUCAST methodology for fastidious organisms disk diffusion DST [11].
Methods
Nocardia isolates
A collection of 134 Nocardia strains from different geographical locations (Provincial Laboratory for Public Health, Edmonton, AB, Canada; the Karolinska University Hospital, Stockholm, Sweden; and the Infection Control Lab [NHLS] and Department of Clinical Microbiology and Infectious Diseases, Charlotte Maxeke Hospital, Johannesburg, South Africa), collected by the EUCAST, were used in the present study. The isolates originate from clinical samples collected from patients affected by nocardiosis. Nocardia isolates were cultured on Columbia agar with 5% sheep blood (COS, bioMérieux, Marcy l’Etoile, France) and incubated at 35 °C ± 2 °C during 48 to 72 h, in an aerobic atmosphere. Then, isolated colonies were used to perform Nocardia identification by 16 S rRNA/hsp65 amplification and sequencing, as previously described [12].
Microdilution DST
Microdilution DST was performed using ready-to-use plate Sensititre™ Myco RAPMYCOI AST plates (Thermo Fischer Scientific) according to the manufacturer’s instruction. Briefly, Nocardia colonies cultured on COS agar for 48 to 72 h were emulsified using a moistened swab in demineralized water, to obtain a bacterial suspension at 0.5 McFarland turbidity standard. Then 50µL of the suspension were transferred into Muller-Hinton cation adjusted broth (Thermo Fischer Scientific). Sensititre™ Myco RAPMYCOI AST plates were inoculated using the Sensititre™ Automated Inoculation Delivery System (Thermo Fischer Scientific) with 100µL of suspension added to each well. Sensititre™ Myco RAPMYCOI AST plates were then incubated at 35 °C ± 2 °C for 48 h and 72 h, depending on the growth of the positive control well. Sensititre™ Myco RAPMYCOI AST plates were read using manual and automated reading (Sensitire™ VIZION™, Thermo Fischer Scientific) and MICs were recorded. Interpretative categories defined as Susceptible (S), Intermediate (I), and Resistant (R) were determined using the CLSI critical concentrations for Nocardia spp and other aerobic actinomycetes [13].
Disk diffusion DST
A sterile cotton swab was dipped into the bacterial suspension at 0.5 McFarland turbidity standard, and a MH-F agar plates (Mueller Hinton agar with 5% defibrinated horse blood and 20 mg/L β-Nicotinamide adenine dinucleotide, Biorad, Hercules, CA, US) was inoculated by swabbing in three directions according to the EUCAST methodology for fastidious organisms [11]. Then, the following antibiotic disks were applied: tobramycin 10 µg, amikacin 30 µg, ciprofloxacin 5 µg, moxifloxacin 5 µg, amoxicillin-clavulanate acid 20 –10 µg, ceftriaxone 30 µg, imipenem 10 µg, trimethoprim-sulfamethoxazole 1.25–23.75 µg (cotrimoxazole), linezolid 10 µg (SIRscan® Discs, i2a, Perols, France). Agar plates were incubated at 35 °C ± 2 °C, in an aerobic atmosphere, and the inhibition zones were read after 48 and 72 h of incubation using a digital caliper.
Statistical analysis
For statistical analyses, non-susceptible Nocardia strains were considered to be Nocardia strains with interpretative categories I and R determined from broth microdilution MIC and (CLSI) critical concentrations for Nocardia spp and other aerobic actinomycetes [13].
Chi2 test was performed to compare the proportions of S, I, and R strains to ceftriaxone and imipenem after 48 h and 72 h incubation using RStudio, version 1.2.1335 (RStudio Team (2009–2019), RStudio: Integrated Development for R. RStudio, Inc., Boston, MA, USA). For N. cyriacigeorgica and N. farcinica, the I and R strains were grouped together in the same category in order to comply with the validity conditions for the use of the Chi2 test.
Receiver operating characteristic (ROC) curves were used to quantify the power of the test to discriminate between S and I/R strains, and to determine the inhibition diameter value that best discriminated between them (Youden index or the point closest to the upper left-hand corner of the ROC curve referred as ROC01 herein). They were constructed and calculated using easyROC, a web-tool for ROC curve analysis (ver 1.3.1) (http://biosoft.erciyes.edu.tr/app/easyROC/) and interpreted according to the Desquilbet’s tutorial [14].
The US Food and Drug Administration (FDA) criteria were used to assess the acceptability of the disk diffusion method’s performance for each antibiotic [15]: percent category agreement (CA) > 89.9%, a major discrepancy (maj: the reference category result is S and the new device result is R) rate ≤ 3% based on the number of susceptible organisms tested, a very major discrepancy (vmj: the reference category result is R and the new device result is S) rate based on the number of resistant organisms tested for which the upper limit of the 95% confident interval (95%CI) is < 7.5% and the lower limit of the 95%CI is < 1.5%. A minor discrepancy is when the reference category is S or I and the reported category is S or I, but differs from the reference.
Results
Nocardia isolates diversity
The collection included 134 strains from 20 different Nocardia species (Table 1), with a predominant number of strains of the species most frequently found in human pathology (N. farcinica, N. cyriacigeorgica, N. nova complex, N. abscessus complex; [6, 16–18]).
Table 1.
Species identification of 134 Nocardia isolates included in the study
| Nocardia species | Number N = 134 |
|---|---|
| N. cyriacigeorgica | 31 |
| N. farcinica | 31 |
| N. nova complex | 29 |
| N. nova | 26 |
| N. veterana | 2 |
| N. elegans | 1 |
| N. abscessus complex | 14 |
| N. gamkensis | 8 |
| N. gamkensis/exalbida/arthritidis | 2 |
| N. asiatica | 4 |
| N. brasiliensis | 9 |
| N. wallacei | 6 |
| N. gipuzkoensis | 4 |
| N. puris | 2 |
| N. brevicatena/paucivorans | 1 |
| N. barduliensis | 1 |
| N. flavorosea | 1 |
| N. otitidiscavarium | 1 |
| N. pseudobrasiliensis | 1 |
| N. shimofusensis | 1 |
| N. terpenica | 1 |
| N. thailandica | 1 |
Impact of incubation time on MIC reading for imipenem and ceftriaxone
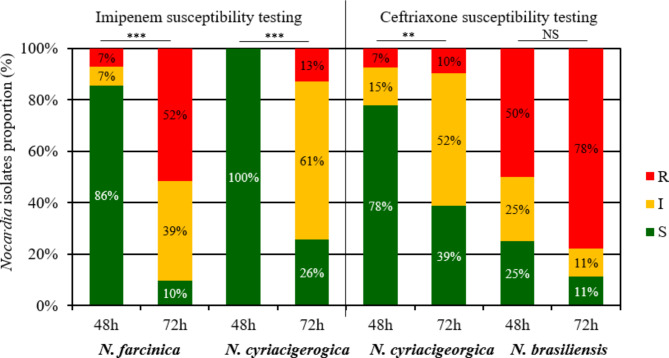
We compared the proportions of S, I and, R strains to ceftriaxone and imipenem after 48 h and 72 h incubation (Fig. 1 and Table S1), knowing that the DST microdilution of 4/31 N. farcinica strains, 5/31 N. cyriacigeorgica strains, and 1/9 N. brasiliensis strains were not interpretable after 48 h incubation, due to insufficient growth control. For these strains, the DST microdilution was only read at 72 h incubation.
Fig. 1.
N. farcinica (n = 28 at 48 h and 31 at 72 h), N. cyriacigeorgica (n = 27 at 48 h and 31 at 72 h) and N. brasiliensis (n = 8 at 48 h and 9 at 72 h) isolates proportion according to interpretative category S (susceptible, in green), I (intermediate, in orange), R (resistant, in red) for imipenem (in the left) and ceftriaxone (in the right) according to the incubation time (48–72 h). Minimal inhibitory concentrations were determined by broth microdilution using Sensititre™ Myco RAPMYCOI and interpretative categories were determined using Clinical Laboratory Standard Institute critical concentrations. *** p < 0.001; ** p < 0.01; NS: not significant
The extension of the incubation of broth microdilution DST at 72 h resulted in a significant increase in the proportion of imipenem-R strains and a significant decrease in imipenem-S strains for N. farcinica, as well as a significant increase in imipenem-I/R strains and a significant decrease in imipenem-S strains for N. cyriacigeorgica (Fig. 1, Table S1). Similarly, the extension of the incubation resulted in a significant increase in the proportion of ceftriaxone-I/R strains and a decrease in ceftriaxone-S strains for N. cyriacigeorgica. Conversely, the extension of the incubation had no significant impact on the distribution of ceftriaxone interpretative categories for N. brasiliensis.
It should be noted that incubation length also had an impact on the detection of expected resistance [13], depending on the species concerned; for example, 25.0% (2/8) of N. brasiliensis strains were categorized as S to imipenem after 48 h of incubation, and 3.6% (1/28) of N. farcinica strains were categorized as S to ceftriaxone after 48 h of incubation (Table S1).
ROC curves analysis
Cotrimoxazole and linezolid
All Nocardia strains were S to linezolid (MIC ranged from ≤ 1 to 4 mg/L; MIC50 ≤ 1 mg/L; and MIC90 = 2 mg/L) and cotrimoxazole (MIC ranged from ≤ 0.25/4.75 to 2/38 mg/L; MIC50 ≤ 0.25/4.75 mg/L; and MIC90 = 0.5/9.5 mg/L) using the DST microdilution with 72 h incubation. There was however a large distribution of inhibition diameters, ranging from 14 to ≥ 40 mm for linezolid, and from 6 (for 25% of the strains) to ≥ 40 mm for cotrimoxazole, which did not allow to determine a critical inhibition diameter (Figure S1 and S2).
Imipenem
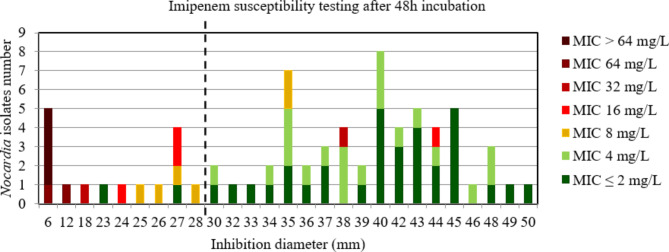
Considering all the strains after 48 h of incubation, the area under the curve (AUC) of ROC curve was > 0.9, p < 0.001 (Figure S3), indicating a high discriminatory power of disk diffusion DST to accurately categorize Nocardia strains (Fig. 2). The critical inhibition diameter calculated was 28 mm according to the Youden and ROC01 methods. Nevertheless, the FDA criteria were not reached using this critical inhibition diameter (Table 2). Among the discrepancies observed, the 2/2 strains with vmj discrepancies after 48 h incubation that would have been categorized S according to the imipenem critical diameter (diameter > 28 mm (S)), with MIC at 16 and 32 mg/L (R) both belong to the N. farcinica species, as does the strain with a minor discrepancy (diameter > 28 mm (S) with MIC at 8 mg/L (I)). Similarly, all strains of N. farcinica and N. cyriacigeorgica for which an inhibition diameter around the imipenem disk was measurable (49/49) at 48 h incubation, including these two strains with vmj discrepancies and the strain with a minor discrepancy, showed inhibition diameters around the imipenem disk > 28 mm (from 32 mm to 50 mm).
Fig. 2.
Distribution of Nocardia isolates excluding N. farcinica and N. cyriacigeorgica (n = 72) according to the inhibition diameter (mm) measured around the imipenem disk and their minimal inhibitory concentration (MIC) for imipenem determined by broth microdilution using Sensititre™ Myco RAPMYCOI after 48 h incubation. Shades of green: strains categorized as susceptible; orange: strains categorized as intermediate; shades of red: strains categorized as resistant according to Clinical Laboratory Standard Institute critical concentrations. Bold dotted line: critical inhibition diameter according to Youden index and the point closest to the upper left-hand corner of the ROC curve (ROC01) = 28 mm (inhibition diameter ≤ 28: imipenem I or R; inhibition diameter > 28: imipenem S)
Table 2.
Critical inhibition diameter determination for antibiotics and FDA criteria associated
| Antibiotic/Incubation time | Number of strains categorized S by the reference method | Number of strains categorized R by the reference method | Break point determination Method | Critical inhibition diameter value (mm; ≤ X: I/R; > X: S) |
CA % 95%CI [; ] |
vmj rate % 95%CI [; ] |
maj rate % 95%CI [; ] |
|---|---|---|---|---|---|---|---|
| Imipenem/48 h | 54 | 12 | Youden index | 28 |
93.1 [84.5; 97.7] |
16.7 [2.1; 48.4] |
3.7 [0.5; 12.7] |
| ROC01 | |||||||
| Imipenem/72 h (without N. farcinica. N. cyriacigeorgica) | 45 | 26 | Youden index | 29 |
98.6 [92.5; 99.9] |
0.0 [0.0; 13.2] |
0.0 [0.0; 7.9] |
| ROC01 | |||||||
| Amoxicillin-clavulanate/48 h | 57 | 30 | Youden index | 20 |
89.3 [82.0; 94.3] |
3.3 [0.1; 17.2] |
15.8 [7.5; 27.9] |
| ROC01 | |||||||
| Amikacin/48 h | 111 | 1 | Youden index | 22 |
97.3 [92.4. 99.4] |
0.0 [0.0; 97.5] |
1.8 [0.2; 6.4] |
| ROC01 | |||||||
| Amikacin/72 h | 130 | 4 | Youden index | 25 |
98.5 [94.7; 99.8] |
0.0 [0.0; 60.2] |
1.5 [0.2; 5.4] |
| ROC01 | |||||||
| Tobramycin/48 h | 62 | 50 | Youden index | 19 |
95.5 [89.9; 98.5] |
0.0 [0.0; 7.1] |
8.1 [2.7; 17.8] |
| ROC01 | 18 |
6.5 [17.9; 15.7] |
|||||
| Tobramycin/72 h | 69 | 65 | Youden index | 19 |
98.5 [94.7; 99.8] |
0.0 [0.0; 5.5] |
2.9 [0.4; 10.1] |
| ROC01 | |||||||
| Ciprofloxacin/48 h | 15 | 67 | Youden index | 24 |
85.6 [76.9; 91.9] |
9.0 [3.4; 18.5] |
6.7 [0.2. 31.9] |
| ROC01 | |||||||
| Ciprofloxacin/72 h | 21 | 97 | Youden index | 25 |
79.9 [72.1; 86.3] |
12.4 [6.6; 20.6] |
0.0 [0.0; 16.1] |
| ROC01 | 27 |
82.8 [75.4; 88.8] |
8.2 [3.6; 15.6] |
4.8 [0.1; 23.8] |
|||
| Moxifloxacin/48 h | 32 | 35 | Youden index | 29 |
84.9 [76.0; 91.5] |
5.7 [0.7; 19.2] |
21.9 [9.3; 40.0] |
| ROC01 | |||||||
| Moxifloxacin/72 h | 64 | 48 | Youden index | 33 |
91.0 [84.9; 95.3] |
0.0 [0.0; 5.6] |
14.6 [6.1; 27.8] |
| ROC01 | 32 |
90.3 [84.0; 94.7] |
12.5 [4.7; 25.4] |
FDA: U.S. Food and Drug Administration; CA: category agreement; vmj: very major discrepancies rate; maj: major discrepancies rate. 95%CI: 95% confident interval. In bold: conditions that reached the FDA acceptable performance criteria
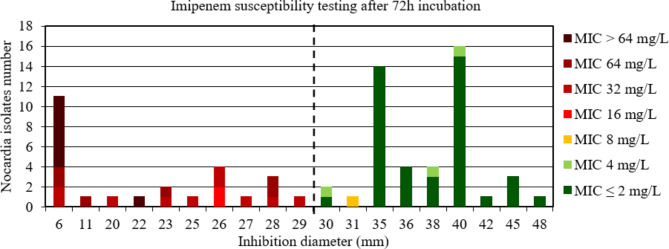
Conversely, considering all the strains after 72 h of incubation, the AUC of the ROC curve was between 0.5 and 0.7, indicating a low discriminatory power of disk diffusion DST to accurately categorize Nocardia strains (Figure S4). After 72 h incubation, 61/62 of the N. farcinica and N. cyriacigeorgica had an inhibition diameter around the imipenem disk of > 29 mm (from 30 mm to 50 mm). Since this low discriminatory power for imipenem could be due to the risk of false imipenem resistance with increased MIC due to the extended delay of 72 h for N. farcinica and N. cyriacigeorgica, the analysis was repeated by removing the data of these species. By removing the data for N. farcinica and N. cyriacigeorgica, the AUC of the ROC curve was > 0.9, p < 0.001 (Figure S5), indicating a high discriminatory power of disk diffusion DST (Fig. 3). The critical inhibition diameter calculated was 29 mm according to the Youden and ROC01 methods, but this inhibition diameter did not reach the FDA criteria (Table 2).
Fig. 3.
Distribution of Nocardia isolates excluding N. farcinica and N. cyriacigeorgica (n = 72) according to the inhibition diameter (mm) measured around the imipenem disk and their minimal inhibitory concentration (MIC) for imipenem determined by broth microdilution using Sensititre™ Myco RAPMYCOI after 72 h incubation. Shades of green: strains categorized as susceptible; orange: strains categorized as intermediate; shades of red: strains categorized as resistant according to Clinical Laboratory Standard Institute critical concentrations. Bold dotted line: critical inhibition diameter according to Youden index and the point closest to the upper left-hand corner of the ROC curve (ROC01) = 29 mm (inhibition diameter ≤ 29: imipenem I or R; inhibition diameter > 29: imipenem S)
Amoxicillin-clavulanate and ceftriaxone
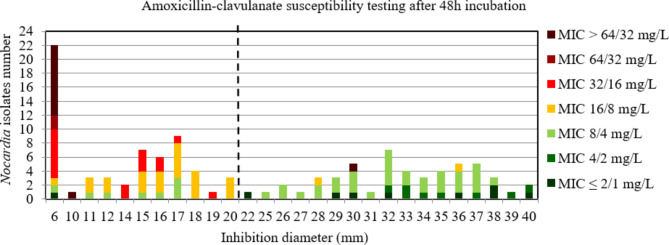
For amoxicillin-clavulanate at 48 h incubation, the AUC of the ROC curve was > 0.9, p < 0.001 (Figure S6) indicating a high discriminatory power of disk diffusion DST (Fig. 4). The critical inhibition diameter calculated was 20 mm for 48 h according to the Youden and ROC01 methods, but this inhibition diameter did not reach the FDA criteria (Table 2). It should be noted that 9/9 strains with maj discrepancies after 48 h incubation, that would have been categorized R according to the amoxicillin-clavulanate critical diameter, belong to the complex N. nova (5 strains) and to the N. cyriacigeorgica species (4 strains).
Fig. 4.
Distribution of Nocardia isolates (n = 112) according to the inhibition diameter (mm) measured around the amoxicillin-clavulanate disk and their minimal inhibitory concentration (MIC) for amoxicillin-clavulanate determined by broth microdilution using Sensititre™ Myco RAPMYCOI after 72 h incubation. Shades of green: strains categorized as susceptible; orange: strains categorized as intermediate; shades of red: strains categorized as resistant according to Clinical Laboratory Standard Institute critical concentrations. Bold dotted line: critical inhibition diameter according to Youden index and the point closest to the upper left-hand corner of the ROC curve (ROC01) = 20 mm (inhibition diameter ≤ 20: amoxicillin-clavulanate I or R; inhibition diameter > 20: amoxicillin-clavulanate S)
On the contrary, the AUC of the ROC curve was between 0.7 and 0.9 at 72 h incubation, indicating a medium discriminatory power of disk diffusion DST to accurately categorize Nocardia strains (Figure S7). For ceftriaxone at 48 h and 72 h incubation, the AUC was between 0.5 and 0.7, indicating a low discriminatory power of disk diffusion DST to accurately categorize Nocardia strains (Figure S8 and S9).
Amikacin
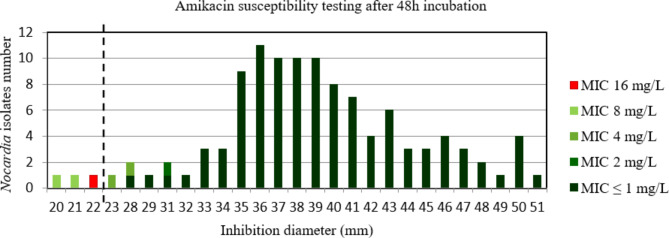
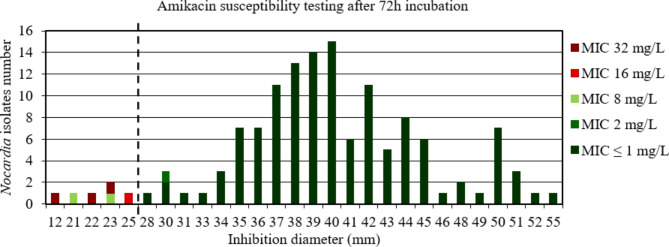
For amikacin after 48 h and 72 h incubation, the AUC of the ROC curve was > 0.9, p < 0.001 (Figure S10 et S11) indicating a high discriminatory power of disk diffusion DST (Figs. 5 and 6). The critical inhibition diameter calculated was 22 mm and 25 mm, for 48 h and 72 h incubation, respectively, according to the Youden and ROC01 methods. Nevertheless, the FDA criteria were not reached using these critical inhibition diameters (Table 2). It should be noted that 2/2 strains with maj discrepancies after 48 h incubation and 2/2 strains with maj discrepancies after 72 h incubation, that would have been categorized R according to the amikacin critical diameter, belong to the N. wallacei species. Moreover, these strains had MIC for amikacin of 8 mg/L after 48 h and 72 h incubation.
Fig. 5.
Distribution of Nocardia isolates (n = 112) according to the inhibition diameter (mm) measured around the amikacin disk and their minimal inhibitory concentration (MIC) for amikacin determined by broth microdilution using Sensititre™ Myco RAPMYCOI after 48 h incubation. Shades of green: strains categorized as susceptible; red: strain categorized as resistant according to Clinical Laboratory Standard Institute critical concentrations. Bold dotted line: critical inhibition diameter according to Youden index and the point closest to the upper left-hand corner of the ROC curve (ROC01) = 22 mm (inhibition diameter ≤ 22: amikacin I or R; inhibition diameter > 22: amikacin S)
Fig. 6.
Distribution of Nocardia isolates (n = 134) according to the inhibition diameter (mm) measured around the amikacin disk and their minimal inhibitory concentration (MIC) for amikacin determined by broth microdilution using Sensititre™ Myco RAPMYCOI after 72 h incubation. Shades of green: strains categorized as susceptible; shades of red: strains categorized as resistant according to Clinical Laboratory Standard Institute critical concentrations. Bold dotted line: critical inhibition diameter according to Youden index and the point closest to the upper left-hand corner of the ROC curve (ROC01) = 25 mm (inhibition diameter ≤ 25: amikacin I or R; inhibition diameter > 25: amikacin S)
Tobramycin
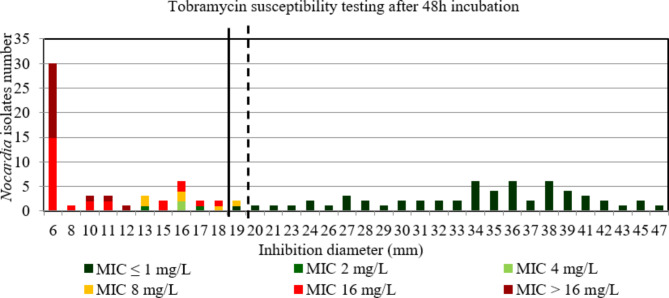
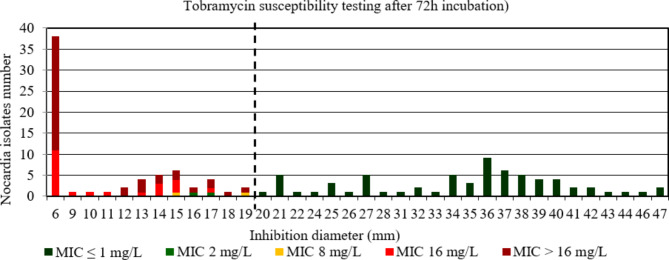
For tobramycin after 48 h and 72 h incubation, the AUC of the ROC curve was > 0.9, p < 0.001 (Figure S12 et S13) indicating a high discriminatory power of disk diffusion DST (Figs. 7 and 8). The critical inhibition diameter calculated was 19 mm and 18 mm, for 48 h according to the Youden and ROC01 methods, respectively; and 19 mm for 72 h according to both methods. The FDA criteria were not reached using the critical inhibition diameters after a 48 h incubation. By contrast, the FDA criteria were reached using the critical inhibition diameter of 19 mm after a 72 h incubation (Table 2). It should be noted that 3/4 strains with maj discrepancies after 48 h incubation, that would have been categorized R according to the tobramycin critical diameter of 18 mm, and 1/2 strains with maj discrepancies after 72 h incubation, that would have been categorized R according to the tobramycin critical diameter of 19 mm, belong to the N. nova species. Moreover, these strains had MIC for tobramycin between 2 and 4 mg/L after 48 h and MIC of 2 mg/L after 72 h incubation.
Fig. 7.
Distribution of Nocardia isolates (n = 112) according to the inhibition diameter (mm) measured around the tobramycin disk and their minimal inhibitory concentration (MIC) for tobramycin determined by broth microdilution using Sensititre™ Myco RAPMYCOI after 48 h incubation. Shades of green: strains categorized as susceptible; orange: strains categorized as intermediate; shades of red: strains categorized as resistant according to Clinical Laboratory Standard Institute critical concentrations. Bold dotted line: critical inhibition diameter according to Youden index = 19 mm (inhibition diameter ≤ 19: tobramycin I or R; inhibition diameter > 19: tobramycin S). Solid bold line: critical inhibition diameter according to the point closest to the upper left-hand corner of the ROC curve (ROC01) = 18 mm (inhibition diameter ≤ 18: tobramycin I or R; inhibition diameter > 18: tobramycin S)
Fig. 8.
Distribution of Nocardia isolates (n = 134) according to the inhibition diameter (mm) measured around the tobramycin disk and their minimal inhibitory concentration (MIC) for tobramycin determined by broth microdilution using Sensititre™ Myco RAPMYCOI after 72 h incubation. Shades of green: strains categorized as susceptible; orange: strains categorized as intermediate; shades of red: strains categorized as resistant according to Clinical Laboratory Standard Institute critical concentrations. Bold dotted line: critical inhibition diameter according to Youden index and the point closest to the upper left-hand corner of the ROC curve (ROC01) = 19 mm (inhibition diameter ≤ 19: tobramycin I or R; inhibition diameter > 19: tobramycin S)
Fluoroquinolones
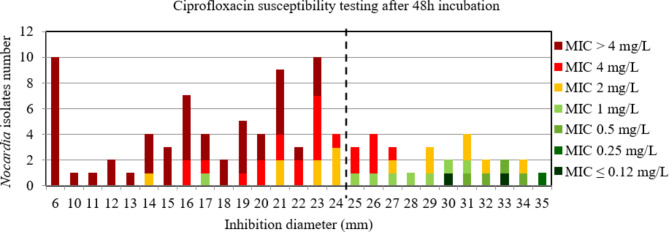
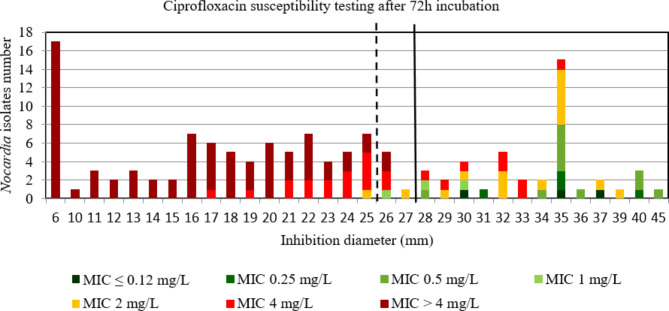
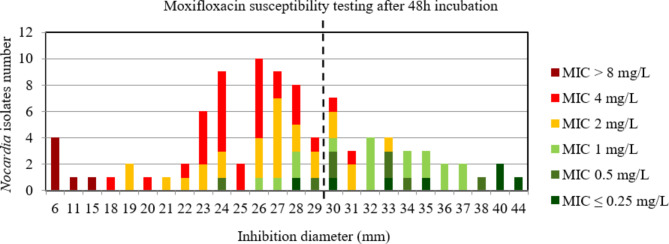
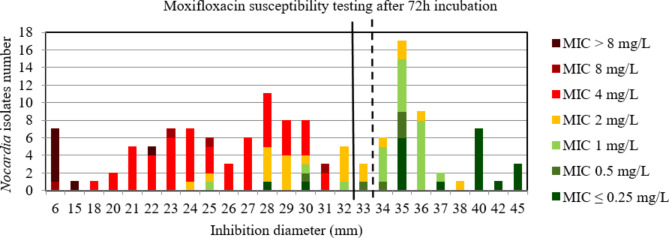
For ciprofloxacin and moxifloxacin, the AUC of the ROC curve was > 0.9, p < 0.001 using both incubation time (Figure S14, S15, S16, and S17) indicating a high discriminatory power of disk diffusion DST (Figs. 9, 10, 11 and 12). For ciprofloxacin, at 48 h the critical inhibition diameter calculated was 24 mm according to the Youden and ROC01 methods, and at 72 h it was 25 mm according to the Youden method and 27 mm according to the ROC01 method. For moxifloxacin, the critical inhibition diameter calculated was 29 mm for 48 h according to the Youden and ROC01 methods, and at 72 h it was 33 mm according to the Youden methods and 32 mm according to the ROC01 method. Nevertheless, the FDA criteria were not reached using these critical inhibition diameters (Table 2).
Fig. 9.
Distribution of Nocardia isolates (n = 97) according to the inhibition diameter (mm) measured around the ciprofloxacin disk and their minimal inhibitory concentration (MIC) for ciprofloxacin determined by broth microdilution using Sensititre™ Myco RAPMYCOI after 48 h incubation. Shades of green: strains categorized as susceptible; orange: strains categorized as intermediate; shades of red: strains categorized as resistant according to Clinical Laboratory Standard Institute critical concentrations. Bold dotted line: critical inhibition diameter according to Youden index and the point closest to the upper left-hand corner of the ROC curve (ROC01) = 24 mm (inhibition diameter ≤ 24: ciprofloxacin I or R; inhibition diameter > 24: ciprofloxacin S)
Fig. 10.
Distribution of Nocardia isolates (n = 134) according to the inhibition diameter (mm) measured around the ciprofloxacin disk and their minimal inhibitory concentration (MIC) for ciprofloxacin determined by broth microdilution using Sensititre™ Myco RAPMYCOI after 72 h incubation. Shades of green: strains categorized as susceptible; orange: strains categorized as intermediate; shades of red: strains categorized as resistant according to Clinical Laboratory Standard Institute critical concentrations. Bold dotted line: critical inhibition diameter according to Youden index = 25 mm (inhibition diameter ≤ 25: ciprofloxacin I or R; inhibition diameter > 25: ciprofloxacin S). Solid bold line: the point closest to the upper left-hand corner of the ROC curve (ROC01) = 27 mm (inhibition diameter ≤ 27: ciprofloxacin I or R; inhibition diameter > 27: ciprofloxacin S)
Fig. 11.
Distribution of Nocardia isolates (n = 93) according to the inhibition diameter (mm) measured around the moxifloxacin disk and their minimal inhibitory concentration (MIC) for moxifloxacin determined by broth microdilution using Sensititre™ Myco RAPMYCOI after 48 h incubation. Shades of green: strains categorized as susceptible; orange: strains categorized as intermediate; shades of red: strains categorized as resistant according to Clinical Laboratory Standard Institute critical concentrations. Bold dotted line: critical inhibition diameter according to Youden index and the point closest to the upper left-hand corner of the ROC curve (ROC01) = 29 mm (inhibition diameter ≤ 29: moxifloxacin I or R; inhibition diameter > 29: moxifloxacin S)
Fig. 12.
Distribution of Nocardia isolates (n = 134) according to the inhibition diameter (mm) measured around the moxifloxacin disk and their minimal inhibitory concentration (MIC) for moxifloxacin determined by broth microdilution using Sensititre™ Myco RAPMYCOI after 72 h incubation. Shades of green: strains categorized as susceptible; orange: strains categorized as intermediate; shades of red: strains categorized as resistant according to Clinical Laboratory Standard Institute critical concentrations. Bold dotted line: critical inhibition diameter according to Youden index = 33 mm (inhibition diameter ≤ 33: moxifloxacin I or R; inhibition diameter > 33: moxifloxacin S). Solid bold line: the point closest to the upper left-hand corner of the ROC curve (ROC01) = 32 mm (inhibition diameter ≤ 32: moxifloxacin I or R; inhibition diameter > 32: moxifloxacin S)
Discussion
The aim of the present study was to propose specific critical inhibition diameters for the Nocardia genus that could accurately discriminate between susceptible and non-susceptible strains to the main antibiotics used in the treatment of nocardiosis.
The CLSI warns of the risk of false resistance to ceftriaxone for N. cyriacigeorgica and N. brasiliensis species, as well as for the risk of false resistance to imipenem for N. farcinica and N. cyriacigeorgica species, notably due to an extension of the incubation period for DST to 72 h [10]. Regarding the incubation time for broth microdilution DST, the extension to 72 h had a strong impact on MIC values and consecutively on interpretative categories for imipenem for N. farcinica and N. cyriacigeorgica, as well as for ceftriaxone for N. cyriacigeorgica; with the risk of producing false R results. We therefore strongly advise laboratories performing broth microdilution DST for Nocardia to systematically read the plates for these species after a 48 h incubation. On the other hand, for N. brasiliensis, and despite our limited number of strains (n = 8), we found that a 48 h incubation did not sufficiently detect the expected resistance to imipenem (I or R were detected only in 75% of cases after 48 h incubation), whereas an extension to 72 h incubation had no significant impact on ceftriaxone results. For this reason, we would recommend to read the broth microdilution DST of N. brasiliensis after a 72 h incubation.
For disk diffusion DST, despite a high discriminatory power after the analysis of the AUC of the ROC curve for several antibiotics (imipenem and amoxicillin-clavulanate after 48 h incubation, and amikacin, tobramycin, and fluoroquinolones after 48 h and 72 h incubation), the FDA criteria to assess the performance acceptability of disk diffusion method were reached only for tobramycin after a 72 h incubation (critical inhibition diameter of 19 mm). Moreover, for tobramycin at 72 h incubation, given that 1/2 strains categorized S using MIC determination in broth microdilution with inhibition diameters < 19 mm belong to the N. nova species, expected to be resistant [13], the determination of the interpretative category for this strain would be more reliable using the disk diffusion DST than the broth microdilution DST. Considering that the true interpretative category of these strains was amikacin R (according to their species identification), then we would obtain a CA of 99.3% 95%CI [95.9%; 99.9%], a vmj rate 0% 95%CI [0.0%; 5.4%] and a maj rate 1.5% [0.0%; 7.9%] (data not shown). Interestingly, the critical inhibition diameter of 19 mm is almost exactly the median of the range that was approximately fifty years ago proposed by Wallace et al. for a 10 µg tobramycin disk load: R ≤ 15 mm, S ≥ 25 S [9].
For the other conditions, it might be interesting to investigate in details the results for which the FDA criteria were close to be reached, e.g. for amikacin 72 h and for imipenem 72 h, excluding the data for N. farcinica and N. cyriacigeorgica. For amikacin, after 72 h of incubation, where the estimated vmj rate was zero and the maj rate was < 3%, a critical diameter of 25 mm appears to accurately discriminate between susceptible and non-susceptible (I or R) Nocardia strains. This is especially true considering that the two strains categorized as susceptible based on MIC determination by broth microdilution, with inhibition diameters < 25 mm, belong to the N. wallacei species, which is expected to be resistant [13]. In this case, interpretative category determination would be more reliable using the disk diffusion DST than the broth microdilution DST. Considering that the true interpretative category of these strains was amikacin R (according to their species identification), then we would obtain a vmj rate 0% 95%CI [0.0%; 45.9%] and a maj rate 0% [0.0%; 2.8%] (data not shown). The upper limit of 95%CI of vmj rate would remain > 7.5%. Nevertheless, in order to obtain an upper limit of the 95%CI < 7.5% for an estimated vmj rate of 0%, a minimum of 48 amikacin-R Nocardia strains would have to be included [15]. Amikacin resistance in Nocardia is unusual, apart from the N. transvalensis complex [16], and this species is not the most frequently found in human pathology [6, 16–18], thus, enriching the collection with N. transvalensis complex strains would appear to be a complicated task. Interestingly, this critical inhibition diameter of 25 mm is exactly the median of the range that was proposed approximately fifty years ago by Wallace et al. for a 30 µg amikacin disk load: R ≤ 20 mm, S ≥ 30 mm [9].
For imipenem at 72 h, excluding the data for N. farcinica and N. cyriacigeorgica, for which the estimated vmj rate was 0.0% and the maj rate < 3%, a critical diameter of 29 mm seems to accurately discriminate between susceptible and non-susceptible (I or R) Nocardia strains. The upper limit of 95%CI of vmj rate was > 7.5% due to insufficient included number of strains categorized imipenem R (n = 26). In order to obtain an upper limit of the 95%CI < 7.5% for an estimated vmj rate of 0%, a minimum of 48 imipenem-R Nocardia strains would have to be included. As an imipenem resistance is more common in Nocardia strains, it would be possible to complete the available data with additional imipenem-R strains in order to confirm this result. Since the vast majority (61/62) of the N. farcinica and N. cyriacigeorgica strains retained a diameter > 29 mm around the imipenem disk even after 72 h incubation, this confirms that the low discriminating power observed at 72 h for imipenem, when considering all Nocardia strains, was due to these strains, categorized as falsely R to imipenem with higher MIC due to the extended 72 h delay. For these species, extending the incubation period from 48 h to 72 h seems to have less impact on inhibition diameters around the imipenem disk than on imipenem microdilution MIC determination. For N. farcinica and N. cyriacigeorgica, admitting the clinical categorization S, I, and R obtained through the MIC for imipenem at 48 h incubation (available for 55 strains) and considering a critical inhibition diameter of 29 mm for imipenem after 72 h incubation, the CA would be 90.9% 95%CI [80.0; 96.9] but two N. farcinica strains would remain with vmj discrepancies (strains categorized R with imipenem MIC at 48 h incubation and S with imipenem disk diffusion DST at 72 h incubation), one N. cyriacigeorgica strain with maj discrepancies (strain categorized S with imipenem MIC at 48 h incubation and R with imipenem disk diffusion DST at 72 h incubation), and two N. farcinica strains with a minor discrepancy (strains categorized I with imipenem MIC at 48 h incubation and categorized S with imipenem disk diffusion DST at 72 h incubation; data not shown). Hence, even if the critical inhibition diameter for imipenem at 72 h incubation of 29 mm was confirmed, it would only be valid for Nocardia species other than N. cyriacigeorgica and N. farcinica. Yet these two species are the most frequently found in human pathology [6, 16–18] and imipenem is one of the drugs of choice in the probabilistic treatment of Nocardiosis [2]. Consequently, although limited, the interest in having disk diffusion DST validated exclusively for Nocardia species other than N. farcinica and N. cyriacigeorgica, especially for such an important drug, must be considered.
For amoxicillin-clavulanate after a 48 h incubation, the determination of the interpretative category could be more reliable by using a critical inhibition diameter around the disk of 20 mm than the broth microdilution DST. Considering that the true interpretative category was amoxicillin-clavulanate-R for the 9 strains having MIC ≤ 8/4 mg/L and an amoxicillin-clavulanate inhibition diameters ≤ 20, and according to species identification (N. nova complex and N. cyriacigeorgica,, which were expected to be R to amoxicillin-clavulanate according to CLSI [13]), it would allow to improve the maj rate (0.0% 95%CI [0.0%; 7.4]; data not shown) and the CA (97.3% 95%CI [92.4; 99.4], data not shown) without allowing the vmj rate (2.6% 95%CI [0.0; 13.5], data not shown) to reach the FDA criteria. Herein, the determination of a zone I for amoxicillin-clavulanate diffusion DST would not enable to improve the criteria since the strain with vmj discrepancies and a MIC > 64/32 mg/L had an inhibition diameter around the amoxicillin-clavulanate disk (30 mm) too distant from the critical inhibition diameter (20 mm).
For other antibiotics with an AUC of the ROC curves > 0.9 (imipenem and fluoroquinolones), determining a zone I for disk diffusion DST did not meet the FDA criteria. This was partly due to the strains with vmj discrepancies for which the inhibition diameters were widely distributed toward higher values.
Although the composition of MH-F medium is strictly controlled, and that it is the most appropriate medium for cotrimoxazole susceptibility testing for fastidious germs such as Haemophilus influenzae [19], it seems unsuitable to test this antibiotic on Nocardia; in the present study, 25% of the strains showed a contact inhibition diameter to the cotrimoxazole disk (6 mm), while broth microdilution DST categorized all Nocardia strains as susceptible. In the study by Wallace et al. in which a critical diameter of 20 mm was proposed for cotrimoxazole 1.25–23.75 µg, they found 94% of susceptible strains [8]. This implies that they did not observe strains with contact bacterial diameters, which may indicate that MH-F medium is not suitable for cotrimoxazole disk diffusion DST on Nocardia. This finding may also be due to the difficult reading of the diameter around the cotrimoxazole disk, which often includes a zone of partial inhibition, making the 80% inhibition reading subjective. Nevertheless, the choice of MH-F medium for the study is questionable. While it provides certainty regarding the growth of all Nocardia species, even the most fastidious (e.g. N. nova complex, N. abscessus complex), it should be noted that some species are not particularly fastidious (N. farcinica and N. cyriacigeorgica) and can easily grow on conventional MH medium. It would then be interesting to see whether a change of medium could improve the performance of the disk diffusion DST and to determine specific critical diameters for the MH medium.
Regarding the impact of Nocardia species on the DST technique, the present study did not reveal any particular species with high rates of vmj and maj discrepancies except for imipenem, for which all strains with vmj discrepancies were either N. farcinica or N. cyriacigeorgica after 48 h and 72 h of incubation. Therefore there is no particular species that could not be tested for DST using disk diffusion. However, to precisely assess this point, experiments would have to be carried out on large collections containing a single species of Nocardia, which may be difficult to obtain for particularly rare species.
Conclusions
In conclusion, the present study did not find that the disk diffusion DST is suitable to accurately categorize all the susceptibilities of Nocardia spp. to antibiotics, according to the experimental conditions used. It might however be suitable to assess the resistance to aminoglycosides and imipenem, except for N. farcinica and N. cyriacigeorgica.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Shanez Haouari (DRS, Hospices Civils de Lyon, Lyon, France) for help in manuscript preparation. The authors also thank Erika Matuschek and Per Rydström (EUCAST Development Laboratory, Clinical Microbiology, Central Hospital, Växjö, Sweden), Jeffrey Fuller and Tanis Dingle (Provincial Laboratory for Public Health, Edmonton, AB, Canada), Christian Giske (Karolinska University Hospital, Stockholm, Sweden), and Teena Thomas (Infection Control Lab [NHLS] and Department Of Clinical Microbiology and Infectious Diseases, Charlotte Maxeke Hospital, Johannesburg, South Africa) for kindly granting us access to their Nocardia strain collection.
Abbreviations
- 95%CI
95% confident interval
- AUC
Area under the curve
- CA
Category agreement
- CA-SFM
Comité de l’Antibiogramme de la Société Française de Microbiologie
- CLSI
Clinical and Laboratory Standards Institute
- COS
Columbia agar with 5% sheep blood
- DST
Drug susceptibility testing
- EUCAST
European Committee Antimicrobial Susceptibility Testing
- FDA
U.S. Food and Drug Administration
- I
Intermediate
- MIC
Minimum inhibitory concentration
- MH
Mueller-Hinton
- MH-F
Mueller Hinton agar with 5% defibrinated horse blood and 20 mg/L β-Nicotinamide adenine dinucleotide
- maj
Major discrepancies
- R
Resistant
- ROC
Receiver-operating-characteristic
- ROC01
The point closest to the upper left-hand corner of the ROC curve
- S
Susceptible
- vmj
Very major discrepancies
Author contributions
Conceptualization, G.L. and E.H.; methodology, C.P., E.DL.; validation, G.L., O.D., and E.H.; formal analysis, C.P., and E.H.; investigation, E.H.; data curation, C.P., E.DL., O.D., and E.H.; writing—original draft preparation, E.H. and B.J.; writing—review and editing, B.J., G.L. and O.D.; visualization, E.H.; supervision, G.L. and E.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Traxler RM, Bell ME, Lasker B, Headd B, Shieh W-J, McQuiston JR. Updated review on Nocardia species: 2006–2021. Clin Microbiol Rev. 2022;35:e0002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Margalit I, Lebeaux D, Tishler O, Goldberg E, Bishara J, Yahav D et al. How do I manage nocardiosis? Clin Microbiol infect off publ eur soc clin Microbiol Infect Dis. 2021;27:550–8. [DOI] [PubMed]
- 3.Ott SR, Meier N, Kolditz M, Bauer TT, Rohde G, Presterl E, et al. Pulmonary nocardiosis in Western Europe-Clinical evaluation of 43 patients and population-based estimates of hospitalization rates. Int J Infect Dis IJID off Publ Int Soc Infect Dis. 2019;81:140–8. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis off Publ Infect Dis Soc Am. 2009;49:1749–55. [DOI] [PubMed] [Google Scholar]
- 5.CASFM_2013.pdf [Internet]. [cited 2024 Jan 16]. https://www.sfm-microbiologie.org/wp-content/uploads/2020/07/CASFM_2013.pdf
- 6.Lebeaux D, Bergeron E, Berthet J, Djadi-Prat J, Mouniée D, Boiron P, et al. Antibiotic susceptibility testing and species identification of Nocardia isolates: a retrospective analysis of data from a French expert laboratory, 2010–2015. Clin Microbiol Infect off Publ Eur Soc Clin Microbiol Infect Dis. 2019;25:489–95. [DOI] [PubMed] [Google Scholar]
- 7.Ambaye A, Kohner PC, Wollan PC, Roberts KL, Roberts GD, Cockerill FR. Comparison of agar dilution, broth microdilution, disk diffusion, E-test, and BACTEC radiometric methods for antimicrobial susceptibility testing of clinical isolates of the Nocardia asteroides complex. J Clin Microbiol. 1997;35:847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace RJ, Septimus EJ, Musher DM, Martin RR. Disk diffusion susceptibility testing of Nocardia species. J Infect Dis. 1977;135:568–76. [DOI] [PubMed] [Google Scholar]
- 9.Wallace RJ, Steele LC. Susceptibility testing of Nocardia species for the clinical laboratory. Diagn Microbiol Infect Dis. 1988;9:155–66. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and laboratory standards institute. M24 - Susceptibility testing of Mycobacteria, Nocardia spp., and Other aerobic actinomycetes. 3rd Edition. 2018. [PubMed]
- 11.European Comimittee on Antimicrobial Susceptibility testing. Media preparation for EUCAST disk diffusion testing and for determination of MIC values by the broth microdilution method. Version 7. 2022.
- 12.Hodille E, Prudhomme C, Dumitrescu O, Benito Y, Dauwalder O, Lina G, Rapid. Easy, and Reliable Identification of Nocardia sp. by MALDI-TOF Mass Spectrometry, VITEK®-MS IVD V3.2 database, using Direct Deposit. Int J Mol Sci. 2023;24:5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and laboratory standards institute. M62 - Performance Standards for Susceptibility testing of Mycobacetria, Nocardia spp., and other aerobic actinomycetes. 1St Edition. 2018. [PubMed]
- 14.Desquilbet L. Tutoriel sur les courbes ROC et leur création grâce au site Internet easyROC. 2022.
- 15.Health C, for D. and R. Antimicrobial Susceptibility Test (AST) Systems - Class II Special Controls Guidance for Industry and FDA. FDA [Internet]. 2023 [cited 2023 Dec 19]; https://www.fda.gov/medical-devices/guidance-documents-medical-devices-and-radiation-emitting-products/antimicrobial-susceptibility-test-ast-systems-class-ii-special-controls-guidance-industry-and-fda
- 16.Wang H, Zhu Y, Cui Q, Wu W, Li G, Chen D, et al. Epidemiology and Antimicrobial Resistance profiles of the Nocardia species in China, 2009 to 2021. Microbiol Spectr. 2022;10:e0156021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo S-F, Chen F-J, Lan I-C, Chien C-C, Lee C-H. Epidemiology of Nocardia Species at a Tertiary Hospital in Southern Taiwan, 2012 to 2020: MLSA Phylogeny and Antimicrobial susceptibility. Antibiot Basel Switz. 2022;11:1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valdezate S, Garrido N, Carrasco G, Medina-Pascual MJ, Villalón P, Navarro AM, et al. Epidemiology and susceptibility to antimicrobial agents of the main Nocardia species in Spain. J Antimicrob Chemother. 2017;72:754–61. [DOI] [PubMed] [Google Scholar]
- 19.Sierra Y, Tubau F, González-Díaz A, Carrera-Salinas A, Moleres J, Bajanca-Lavado P, et al. Assessment of trimethoprim-sulfamethoxazole susceptibility testing methods for fastidious Haemophilus spp. Clin Microbiol Infect. 2020;26:e9441–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.