Abstract
Background
There is some evidence of reduced major cardiovascular event (MACE) rates associated with moderate coffee consumption in the general population. However, there is concern about the potential risks of coffee consumption in patients with atrial fibrillation (AF). Therefore, we aimed to investigate the association between coffee consumption and MACE in AF patients.
Methods
Data of patients with documented AF enrolled in two large prospective observational multicenter cohort studies (Swiss-AF and Beat-AF) were analyzed. Follow-up information was obtained on a yearly basis. Coffee consumption was categorized into two main groups: “daily” and “not-daily” coffee consumers as well as additional subcategories. The primary endpoint was MACE, defined as a composite of stroke or systemic embolism, myocardial infarction, hospitalization for acute heart failure, and cardiovascular mortality. Secondary endpoints were the individual components of MACE and all-cause mortality. We performed time-updated multivariable adjusted Cox regression analyses to investigate the association between coffee consumption and MACE.
Results
The incidence rate for MACE was 5.09 per 100 person-years (py) in daily and 7.49 per 100 py in not-daily consumers (median follow-up duration: 4.7 years). After adjustment for pre-selected confounding variables, daily coffee consumption was associated with a 23% lower hazard for MACE compared to not-daily consumption (hazard ratio (HR) (95% confidence interval (CI)) 0.77 (0.66; 0.89)). Patients with moderate coffee consumption (2–3 cups/day) had the lowest hazard for MACE compared to patients with not-daily coffee consumption (HR (95% CI) 0.74 (0.63; 0.87)).
Conclusions
In a population of AF patients, daily coffee consumption was associated with a reduced risk for MACE, hospitalization for acute heart failure, and all-cause mortality. The results were inconclusive for stroke or systemic embolism, myocardial infarction, and cardiovascular death. In this analysis, we found no evidence of an unfavourable association of daily coffee consumption in AF Patients with adverse outcome events.
Trial registration
ClinicalTrials.gov Identifier: NCT02105844.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03817-x.
Keywords: Atrial fibrillation, Coffee, Caffeine, MACE, All-cause mortality, Outcome events
Background
Coffee is one of the most common beverages consumed worldwide [1]. Based on current data, coffee consumption is not associated with an increased risk of atrial fibrillation (AF) in the general population [2–8]. Additionally, moderate coffee consumption seems to have a protective effect on the occurrence of stroke [9, 10], the development of heart failure [11], and coronary artery disease [10]. Furthermore, different studies and meta-analyses in general populations have shown that moderate coffee consumption is associated with reduced risk of all-cause mortality [12, 13] and cardiovascular mortality [14, 15]. The role of coffee consumption in myocardial infarction is not yet fully understood. However, an increased risk with high coffee consumption was observed in men but not in women [16].
AF is strongly associated with an increased risk of stroke, systemic embolism, heart failure, myocardial infarction, and mortality [17–20]. Whether these clinically relevant complications are influenced by coffee consumption in patients with AF is still unknown. AF patients often report coffee consumption as one of the three most important factors that subjectively trigger an AF episode, although this is not objectively validated [21, 22]. Consequently, AF patients are often concerned whether they should lower or even quit consuming coffee.
The aim of this study was to examine whether coffee consumption is associated with adverse cardiovascular events in a large population of AF patients.
Methods
Study design and patient population
This analysis is based on the data of the Swiss-AF (Swiss Atrial Fibrillation Cohort Study, ClinicalTrials.gov Identifier: NCT02105844) and Beat-AF (Basel Atrial Fibrillation Study) studies. Both studies are ongoing prospective, observational, multicenter cohort studies. The Swiss-AF cohort study enrolled 2415 patients between 2014 and 2017 at 14 different study centers in Switzerland. The Beat-AF study enrolled 1553 patients between 2010 and 2014 at seven different Swiss study centers. Detailed information about the study methodology was published previously [23]. The main inclusion criterion of both studies was documented AF. Swiss-AF primarily included patients aged ≥ 65 years (with around 10% of the patients aged < 65 years included to investigate the role of AF in the working population), whereas there was no age restriction in the Beat-AF cohort. The main exclusion criteria were short and reversible forms of AF (e.g., due to cardiac surgery or sepsis), acute illness within the last 4 weeks, and inability to provide informed consent. The study protocols were approved by the ethics committee and conducted in accordance with the Helsinki Declaration.
Overall, 3968 patients were enrolled. For this analysis, 67 patients were excluded because no follow-up information was available, and 7 patients were excluded due to double inclusion in both studies. Another 59 patients were excluded due to missing information on coffee consumption (n = 16) or other relevant covariates (n = 43), resulting in 3835 patients remaining for this analysis (Swiss-AF, n = 2387; Beat-AF, n = 1507). Patient selection is presented in Supplementary Fig. S1.
Data assessment
In both studies, data were collected at baseline and at consecutive yearly follow-up visits with identical standardized case report forms (CRFs) to have comparable data. In the Swiss-AF cohort, data were collected during personal visits via physical examination. If personal visits were not possible, follow-up visits were performed by phone or by using medical records. After a personal visit at baseline, all yearly follow-up visits in Beat-AF were performed by phone. Information on lifestyle factors, comorbidities and medication was obtained at baseline and updated on a yearly basis in both studies.
Coffee consumption
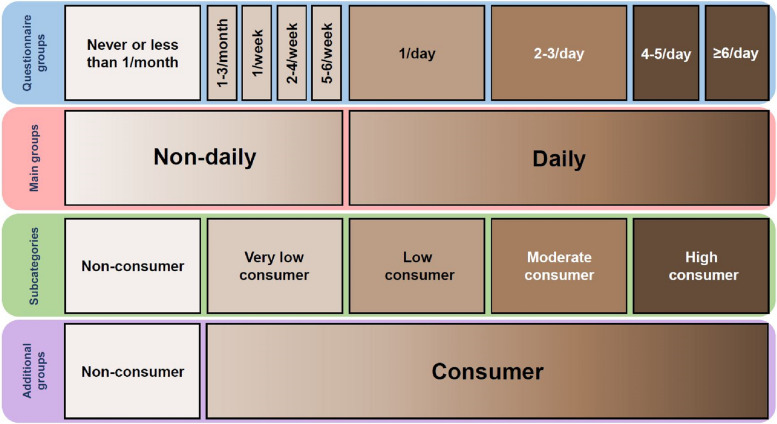
Coffee consumption was assessed using a standardized questionnaire. Information on coffee consumption was updated yearly and the same questionnaire was used in both studies. Coffee consumption was assessed using a CRF containing nine different coffee consumption categories: never or less than once a month; 1–3 cups per month; 1 cup per week; 2–4 cups per week; 5–6 cups per week; 1 cup per day; 2–3 cups per day; 4–5 cups per day; and 6 or more cups per day. We categorized coffee consumption according to the questionnaire into two groups: daily and not-daily consumption. Additionally, we constructed five subcategories according to the questionnaire, which correspond to the literature [7–10, 24]: not-daily consumption included non-consumers (never or less than once a month) and very-low consumers (1–3 cups per month; 1 cup per week; 2–4 cups per week; 5–6 cups per week). Daily consumption included low consumers (1 cup per day), moderate consumers (2–3 cups per day), and high consumers (4–5 cups per day; 6 or more cups per day). An overview about the different categories of coffee consumption is provided in Fig. 1.
Fig. 1.
Coffee consumption categorization. The categorization is based on the data presented in the first line of the figure that was assessed by questionnaire (coffee cups per month, week or day)
Adverse cardiovascular events
Documented outcome events were independently validated by two different physicians. In cases of uncertainty, a third physician validated the event. The main outcome event was major adverse cardiovascular events (MACE), a composite of myocardial infarction, stroke and systemic embolism, hospitalization for acute heart failure, and cardiovascular mortality. As secondary outcome events, we defined all-cause mortality and the individual components of MACE. The definitions of all endpoints are shown in Supplementary Table S1.
Other variables
Body mass index (BMI) was defined as body weight in kg divided by height in m2. Educational status was categorized into three different levels (basic, middle and high) according to the highest level achieved. CHA2DS2-VASc Score was calculated based on the participants’ history of the included risk factors (history of congestive heart failure, hypertension, age, diabetes mellitus, stroke or transient ischemic attack, vascular disease, sex). Systolic and diastolic blood pressure were measured three times, and the mean of all measurements was used for this analysis. AF type was determined by the available clinical patient data and categorized according to the ESC guidelines from 2010 into paroxysmal, persistent, or permanent AF [25]. Alcohol consumption was categorized into non-drinkers, > 0 to < 1 drinks per day, 1 to < 2 drinks per day, and > 2 drinks per day. Regular exercise is defined as engaging in physical activity at least once per week. A fruit and/or vegetable consumption of ≥ 5 servings per day was used as a surrogate of a healthy diet. AF-related symptoms were classified using the European Heart Rhythm Association (EHRA) Score [26]. The following AF symptoms were obtained separately: palpitations, dizziness, syncope, dyspnea, fatigue, limitation in performance, and thoracic pain.
Statistical analysis
Baseline characteristics were stratified by coffee consumption (daily vs. not-daily consumption at baseline). In addition, baseline characteristics were stratified by coffee consumption subcategories (non-consumer; very low consumer; low consumer; moderate consumer; and high consumer). Continuous data are presented as mean (± standard deviation (SD)) and categorical data as numbers (percentages). Depending on the distribution and number of strata, continuous data were compared using Student’s t-test or ANOVA. Categorical data were compared using chi-square tests.
Person years of follow-up were calculated as the difference from baseline to death, loss to follow-up, dropout, last follow-up date, or the respective event date. To investigate the association between coffee consumption and adverse outcome events, we performed multivariable adjusted time-updated Cox regression analyses. Coffee consumption and all covariates were used as time-updated variables (visit-based). In case of missing values in between, we replaced missing values by using the “last value carried forward” method. Results are presented as hazard ratios (HRs) and 95% confidence intervals (CIs). Potential competing risk due to non-cardiovascular death was taken into account, by using cause-specific hazard models. In addition, we calculated p-values for linear trend across the coffee consumption categories. The incidence rate was calculated per 100 person-years (py). In addition, the incidence stratified by daily versus not-daily coffee consumption was calculated at 1, 3, and 5 years of follow-up. Cumulative incidence curves with corresponding confidence intervals stratified by daily versus not-daily coffee consumption were (1) constructed over the whole study period and (2) truncated at 5 years of follow-up.
Different Cox regression models were constructed. Model 1 was adjusted for age and sex. Model 2 was additionally adjusted for education (basic, middle, high), hypertension, history of heart failure, history of stroke, transient ischemic attack or systemic embolism, history of coronary artery disease, history of diabetes, history of renal failure, BMI, AF type, oral anticoagulation, betablocker, and antiarrhythmic drugs (Vaughan Williams classification Ic or III). A third model was additionally adjusted for lifestyle factors, including active smoking, alcohol consumption, regular physical activity, and healthy diet.
We performed specific subgroup analyses according to AF type (paroxysmal versus non-paroxysmal), patient sex, and study (Swiss-AF versus Beat-AF) for the association between coffee consumption (daily vs not-daily) and MACE. p-value for interaction was calculated by adding a multiplicative interaction term.
Considering the exploratory nature of the analysis, we performed no correction for multiple testing and interpreted p-values as a continuous variable. Statistical analysis was performed using R version R 4.1.0 (2021–05-18, R Core Team).
Results
Of the 3835 patients included in this analysis, 80.7% (n = 3095) reported daily coffee consumption (Table 1). Mean age of the individuals in the study cohort was 71.4 (± 10) years, and 1073 (28.0%) were female. Baseline characteristics stratified by daily versus not-daily coffee consumption are presented in Table 1. Most baseline characteristics were well balanced between the two groups. Female sex (31% versus 27%, p = 0.03) and renal failure (23% versus 18%, p = 0.001) were more common in patients with not-daily consumption compared to patients with daily coffee consumption. In contrast, alcohol consumption was higher in patients with daily coffee consumption (p < 0.001). AF-related symptoms did not differ between the two groups. Baseline characteristics stratified by more detailed coffee consumption categories are presented in Supplementary Table S2.
Table 1.
Baseline characteristics stratified by daily versus not-daily coffee consumption
| Characteristic | Overall (n = 3835) | Not-daily (n = 740, 19.3%) | Daily (n = 3095, 80.7%) | p-value |
|---|---|---|---|---|
| Age, years, mean (SD) | 71.4 (10.0) | 71.7 (11.0) | 71.4 (9.7) | 0.36 |
| Female sex, n (%) | 1073 (28.0) | 231 (31.2) | 842 (27.2) | 0.03 |
| BMI, kg/m2, mean (SD) | 27.4 (4.8) | 27.3 (4.8) | 27.5 (4.8) | 0.45 |
| Education level, n (%) † | 0.25 | |||
| Basic (%) | 456 (11.9) | 96 (13.0) | 360 (11.6) | |
| Middle (%) | 1889 (49.3) | 345 (46.6) | 1544 (49.9) | |
| Advanced (%) | 1490 (38.9) | 299 (40.4) | 1191 (38.5) | |
| Blood pressure, mmHg | ||||
| Systolic (mean (SD)) | 133.8 (18.7) | 133.4 (18.6) | 133.9 (18.7) | 0.55 |
| Diastolic (mean (SD)) | 77.7 (12.0) | 77.1 (11.9) | 77.8 (12.0) | 0.16 |
| History of hypertension, n (%) | 2641 (68.9) | 510 (68.9) | 2131 (68.9) | 1.00 |
| History of diabetes, n (%) | 611 (15.9) | 106 (14.3) | 505 (16.3) | 0.20 |
| History of renal failure, n (%) | 718 (18.7) | 170 (23.0) | 548 (17.7) | 0.001 |
| History of congestive heart failure, n (%) | 914 (23.8) | 168 (22.7) | 746 (24.1) | 0.45 |
| History of coronary artery disease, n (%) | 1029 (26.8) | 204 (27.6) | 825 (26.7) | 0.65 |
| History of myocardial infarction, n (%) | 566 (14.8) | 99 (13.4) | 467 (15.1) | 0.26 |
| History of stroke or TIA, n (%) | 661 (17.2) | 143 (19.3) | 518 (16.7) | 0.11 |
| History of systemic embolism, n (%) | 192 (5.0) | 44 (5.9) | 148 (4.8) | 0.23 |
| CHA2DS2-VASC score, mean (SD) | 3.2 (1.7) | 3.3 (1.7) | 3.2 (1.8) | 0.11 |
| Atrial fibrillation type, n (%) | 0.11 | |||
| Paroxysmal | 1885 (49.2) | 348 (47.0) | 1537 (49.7) | |
| Persistent | 1076 (28.1) | 202 (27.3) | 874 (28.2) | |
| Permanent | 874 (22.8) | 190 (25.7) | 684 (22.1) | |
| Active smoking status, n (%) | 298 (7.8) | 45 (6.1) | 253 (8.2) | 0.07 |
| Alcohol consumption, n (%) | < 0.001 | |||
| Non-drinkers | 684 (17.8) | 180 (24.3) | 504 (16.3) | |
| > 0 to < 1 drink/day | 1777 (46.3) | 354 (47.8) | 1423 (46.0) | |
| 1 to < 2 drinks/day | 644 (16.8) | 99 (13.4) | 545 (17.6) | |
| ≥ 2 drinks/day | 730 (19.0) | 107 (14.5) | 623 (20.1) | |
| Regular physical activity (yes %) | 1868 (48.7) | 336 (45.4) | 1532 (49.5) | 0.05 |
| Healthy diet (yes %) | 1073 (28.6) | 212 (29.4) | 861 (28.4) | 0.63 |
| Oral anticoagulation, n (%) | 3231 (84.3) | 626 (84.6) | 2605 (84.2) | 0.82 |
| Vitamin K antagonist n (%) | 1867 (48.7) | 359 (48.5) | 1508 (48.7) | 0.95 |
| Direct oral anticoagulants n (%) | 1362 (35.5) | 266 (35.9) | 1096 (35.4) | 0.82 |
| Antiplatelet therapy, n (%) | 826 (21.5) | 143 (19.3) | 683 (22.1) | 0.11 |
| Betablocker therapy, n (%) | 2648 (69) | 508 (68.6) | 2140 (69.1) | 0.11 |
| Antiarrhythmic drugs (Vaughan Williams Classification 1c and III), n (%) | 896 (23.4) | 171 (23.1) | 725 (23.4) | 0.83 |
| PVI, n (%) | 815 (21.3) | 148 (20.0) | 667 (21.6) | 0.39 |
| Device, n (%) | 675 (17.6) | 146 (19.7) | 529 (17.1) | 0.10 |
| EHRA score, n (%) | 0.37 | |||
| I | 1380 (58.1) | 286 (59.2) | 1094 (57.9) | |
| II | 766 (32.3) | 145 (30.0) | 621 (32.8) | |
| III | 186 (7.8) | 45 (9.3) | 141 (7.5) | |
| IV | 42 (1.8) | 7 (1.4) | 35 (1.9) | |
| AF symptoms | 2540 (66.4) | 479 (64.7) | 2061 (66.8) | 0.31 |
| Palpitations | 1610 (42.0) | 315 (42.6) | 1295 (41.8) | |
| Dizziness | 577 (22.7) | 105 (14.2) | 472 (15.3) | |
| Syncope | 119 (4.7) | 29 (3.9) | 90 (2.9) | |
| Dyspnea | 960 (37.8) | 174 (23.5) | 786 (25.4) | |
| Fatigue | 680 (26.8) | 134 (18.1) | 546 (17.6) | |
| Limitation in performance | 707 (27.8) | 134 (18.1) | 573 (18.5) | |
| Thoracic pain | 401 (15.8) | 81 (11.0) | 320 (10.3) |
Data are presented as means ± standard deviation or counts (percentages). CHA2DS2-VASC score: stroke risk score in atrial fibrillation patients. EHRA score: AF symptom score classified from the European Heart Rhythm Association. Regular physical exercise: physical activity such as jogging, Nordic walking, cycling, aerobics, or ball sports for at least once per week. Healthy diet was defined as ≥ 5 servings of fruit or vegetable per day. † Basic: ≤ 6 years (less than the current compulsory education curriculum); middle: 6 to ≤ 12 years (high school or similar); advanced: ≥ 12 years (college or university degree). AF, atrial fibrillation; BMI, body mass index; EHRA, European Heart Rhythm Association; PVI, pulmonary vein isolation; SD, standard deviation; TIA, transient ischemic attack
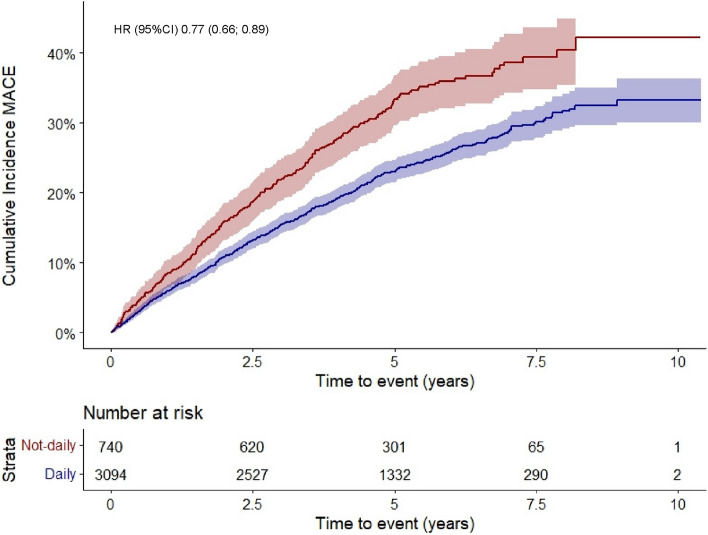
The results of the association between coffee consumption and MACE are presented in Table 2. MACE occurred in 979 patients. Of them, hospitalization for acute heart failure occurred in 543 patients, MI in 116 patients, stroke or systemic embolism in 169 patients, and cardiac death in 173 patients (some events occurred at the same day). Overall, the incidence rate for MACE was 5.55 per 100 py (7.49 and 5.09 per 100 py in patients with not-daily and daily coffee consumption, respectively). Median (interquartile range) follow-up duration for MACE was 4.7 (4.0–6.2) years. Cumulative incidence curves of MACE stratified by daily versus not-daily consumption are shown in Fig. 2. The incidence of MACE in patients with daily versus not-daily coffee consumption was 6.0 versus 8.4% after 1 year, 15.4 versus 21.8% after 3 years, and 23.0 versus 33.3% after 5 years of follow-up (Supplementary Table S3). Compared to patients with not-daily coffee consumption, daily consumers had a 23% relative risk reduction for MACE after adjustment for a broad set of covariates (HR (95% CI) 0.77 (0.66; 0.89), final model). When looking at subcategories of daily coffee consumption, the HR (95% CI) for MACE in patients with low, moderate, or high consumption was 0.83 (0.69; 1.00), 0.74 (0.63; 0.87), and 0.77 (0.60; 0.99), respectively (p-value for linear trend = 0.02).
Table 2.
Association between coffee consumption and major adverse cardiovascular events (MACE)
| MACE | No. of events | Person-years | Incidence rate per 100 person-years | Cox regression model 1 HR (95% CI) |
Cox regression model 2 HR (95% CI) |
Cox regression model 3 (lifestyle) HR (95% CI) |
|---|---|---|---|---|---|---|
| Overall | 979 | 17,636 | 5.55 | |||
|
Not-daily Non-consumer (0 cup/day) Very low consumer (< 1 cup/day) |
253 | 3377 | 7.49 | Ref | Ref | |
| Daily | 726 | 14,260 | 5.09 | 0.74 (0.64;0.85) | 0.76 (0.66;0.88) | 0.77 (0.66; 0.89) |
| Low consumer (1 cup/day) | 216 | 3591 | 6.02 | 0.80 (0.67;0.96) | 0.84 (0.70;1.01) | 0.83 (0.69; 1.00) |
| Moderate consumer (2–3 cups/day) | 418 | 8665 | 4.82 | 0.70 (0.60;0.82) | 0.72 (0.61;0.84) | 0.74 (0.63; 0.87) |
| High consumer (≥ 4 cups/day) | 92 | 2004 | 4.59 | 0.79 (0.62;1.00) | 0.80 (0.63;1.01) | 0.77 (0.60; 0.99) |
| p-value for linear trend | 0.02 | 0.03 | 0.02 | |||
| p-value for quadratic trend | 0.02 | 0.06 | 0.13 |
MACE (major adverse cardiovascular events): composite of hospitalization for acute heart failure, stroke and systemic embolism, myocardial infarction, cardiovascular mortality. Model 1: adjusted for age and sex. Model 2: additionally adjusted for education (basic, middle, high), hypertension, history of heart failure, history of stroke, transient ischemic attack or systemic embolism, history of coronary artery disease, history of diabetes, history of renal failure, body mass index, AF type, oral anticoagulation, betablocker and antiarrhythmic drugs. Model 3 (lifestyle) was additionally adjusted for active smoking, alcohol consumption, regular physical activity, and healthy diet
Fig. 2.
Cumulative incidence curves of major adverse cardiovascular events (MACE) stratified by daily versus not-daily coffee consumption
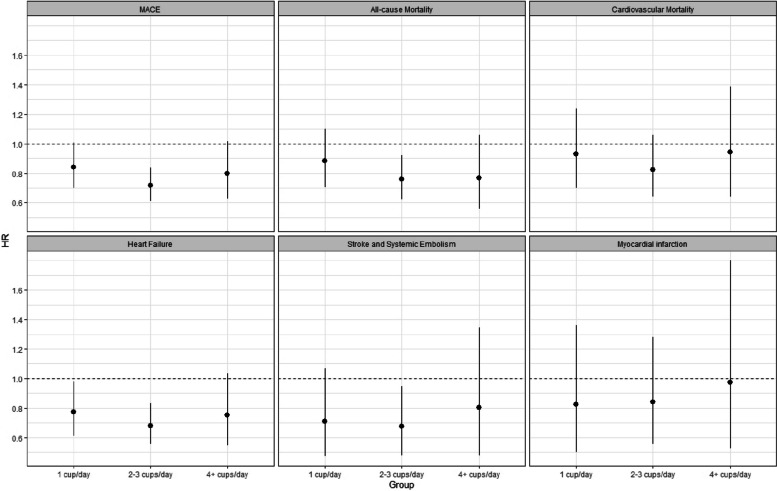
The incidence rates per 100 py for all-cause and cardiovascular mortality were 3.24 and 2.02, respectively (Supplementary Table S4). After a comprehensive adjustment of covariates, we found a significant association between daily coffee consumption and all-cause mortality (HR (95% CI) 0.79 (0.66; 0.94)) but not between daily coffee consumption and cardiovascular mortality (HR (95% CI) 0.89 (0.70; 1.27)). There was a strong association between daily coffee consumption and hospitalization for acute heart failure with an HR (95% CI) of 0.71 (0.59; 0.86), compared to patients with not-daily consumption, which was also present across the different subcategories of daily consumption (p for linear trend = 0.04). Compared to patients who consumed coffee on a not-daily basis, patients with moderate coffee consumption (2–3 cups per day) had the lowest hazard ratio (HR (95% CI) 0.69 (0.56; 0.85)) (Fig. 3). Additionally, daily coffee consumption was associated with a reduced risk of stroke and systemic embolism even after multivariable adjustment (HR (95% CI) 0.70 (0.51; 0.95), model 2) but lost level of significance when additionally adjusting the model for lifestyle factors (HR (95% CI) 0.75 (0.54; 1.04), model 3). No association was found between daily coffee consumption and the occurrence of myocardial infarction.
Fig. 3.
Hazard ratio (95% confidence intervals) stratified by categories of coffee consumption for primary and secondary outcome events. The regression model was adjusted for age, sex, education (basic, middle, high), hypertension, history of heart failure, history of stroke, TIA or systemic embolism, history of coronary artery disease, history of diabetes, history of renal failure, body mass index, AF type, oral anticoagulation, betablocker, and antiarrhythmic drugs (model 2)
The results of the subgroup analysis for AF type (paroxysmal vs. non-paroxysmal), patient sex, and study (Swiss-AF vs. Beat-AF) for the association between daily coffee consumption and MACE are presented in Supplementary Table S5. There was a significant association between daily consumption and MACE in patients with non-paroxysmal AF (HR (95% CI) 0.71 (0.59; 0.85)) but not in patients with paroxysmal AF (HR (95% CI) 0.86 (0.67; 1.09)). However, there was no evidence for an interaction between AF type and daily coffee consumption (p for interaction = 0.25). Additionally, we found an inverse association between daily coffee consumption and MACE in male patients (HR (95%CI) 0.71 (0.60; 0.84)), however without evidence of an effect modifying effect by sex (p for interaction = 0.16). Finally, there was an inverse association between coffee consumption and MACE in both studies separately with a HR (95%CI) of 0.79 (0.66; 0.95) for Swiss-AF and 0.72 (0.56; 0.92) for Beat-AF (p for interaction = 0.43).
Discussion
Several new findings emerged from our analysis which used data from two large cohorts of AF patients. First, we found no evidence for an increased risk for any of our studied adverse outcome events when comparing daily versus not-daily coffee consumption in AF patients. In contrast, compared to AF patients with not-daily coffee consumption, daily coffee consumption was associated with a decreased risk of MACE, all-cause mortality, and hospitalization for acute heart failure. Since baseline characteristics were well distributed between daily and not-daily coffee consumers, we assume that these variables had a small confounding effect. Second, AF type and patient sex had no modifying effect on the association between coffee consumption and MACE.
To the best of our knowledge, there is no evidence in the current literature of an association between coffee consumption and adverse cardiovascular events in AF patients. In contrast, there is much evidence in the general population in favor of coffee consumption [9–15, 27]. In our study with elderly AF patients, we found comparable results as in patients from the general population. For example, we found a 23% relative risk reduction for MACE in patients with daily coffee consumption compared to patients with not-daily consumption. The inverse relationship between daily coffee consumption and MACE was mainly based on strong associations with hospitalization for acute heart failure and in part stroke/systemic embolism. Cardiovascular mortality and myocardial infarction did not contribute to these findings to the same extent, even though the shape of the association was similar. The association between coffee consumption and MACE was mainly driven by a strong association in patients with moderate coffee consumption (2–3 cups/day).
According to our subgroup analysis, the incidence rate and relative risk reduction was higher in patients with non-paroxysmal AF than in those with paroxysmal AF (7.15 vs 4.06 per 100 py and 29% versus 14%). However, AF type had no modifying effect on the association between coffee consumption and MACE. The widespread individual concern in paroxysmal AF patients that an AF episode could be triggered with coffee consumption [22] did not seem to apply in this specific subgroup, as we did not find an increased number of events. In addition, the relative risk reduction was higher in men compared to women (29% versus 10%), however, without evidence of an interaction effect.
Results from a meta-analysis in a general population, which investigated the association of heart failure in coffee drinkers compared to non-drinkers, revealed a J-shaped association with the lowest risk in the group of participants consuming 3–4 cups/day (HR (95% CI) 0.89 (0.81; 0.99)) [11]. This result is in line with our findings showing that patients with moderate coffee consumption had the lowest risk (2–3 cups/day). Current evidence suggests that moderate coffee consumption as part of a normal lifestyle does not lead to an excessive fluid loss [28]. In contrast, excessive caffein consumption might induce an acute diuretic effect as compared to a moderate caffein consumption as shown in young adults [29]. However, the role of a potential diuretic effect of caffein on the association between coffee consumption and heart failure hospitalizations still remains unclear.
In another meta-analysis, the authors were able to show a U-shaped inverse association between coffee consumption and stroke, with the lowest risk again occurring in the group of patients who consumed 3–4 cups/day (HR (95% CI) 0.83 (0.74; 0.92)) [9]. In our analysis, we found similar effect sizes in all groups. However, the 95% confidence interval was the narrowest for patients with moderate coffee consumption (2–3 cups/day), and the difference was significant compared to patients with not-daily consumption (model 1 and model 2). The effect of coffee consumption on the occurrence of myocardial infarction is still not well understood. There is some evidence that regular coffee consumption might reduce the risk of coronary artery disease [10]. However, a different study showed an increased incidence of myocardial infarction in men with high coffee consumption [16]. This uncertainty resembles our findings, with no significant association between coffee consumption and the occurrence of myocardial infarction. Another meta-analysis focusing on all-cause mortality revealed a nonlinear inverse relationship with the lowest risk in patients consuming 3 cups of coffee/day (HR (95% CI) 0.83 (0.83; 0.88)) [14]. Again, our data showed similar effect sizes across the groups. However, only patients who consumed 2–3 cups/day had a significantly reduced risk compared to patients who did not consume on a daily basis (HR (95% CI) 0.77 (0.63; 0.94). Another analysis of the general population showed a favorable effect of coffee consumption on cardiac death [14, 15]. However, our study was not able to show a significant difference between patients with daily versus not-daily coffee consumption, even though the hazard ratio showed a trend toward a favorable effect of daily coffee consumption (HR (95% CI) 0.89 (0.70; 1.27)).
Based on the comparison of our results with the results of the general population, we suggest that having AF of any type has no effect on the association between coffee consumption and clinical outcome events. The dose–response relationship followed a similar pattern when we compared the results of our AF population with the results of different general populations. The reason for this shape of association is not clear. Since we found no evidence of unfavorable association of daily coffee consumption with adverse outcome events and observed comparable results as in general populations, it can be assumed that recommendations for coffee consumption in AF patients may not differ from those in the general populations when referring to adverse outcome events. However, based on the observational study design, we must be cautious when interpreting the results and overinterpretation should be avoided.
A comprehensive management of modifiable risk factors in AF patients (ABC pathway) is of high relevance to improve AF management and reduce the risk of AF-related complications [30]. Recently, the HEAD 2 TOES concept was introduced, which summarizes modifiable risk factor targets to prevent AF [31]. In our analysis, the confounding effect of lifestyle factors on the association between coffee consumption and adverse outcome events was minimal, as was the influence of other modifiable risk factors when comparing the different multivariable adjusted models. Potentially, coffee consumption per se reflects a certain pattern of lifestyle factors, even though the baseline characteristics were not completely different among patients with daily versus not-daily coffee consumption.
The biochemical active components of coffee are complex and may have harmful and beneficial effects on cardiovascular health. The most famous component of coffee is caffeine, of which up to 400 mg per day (approximately 4 cups/day) appears to be safe for cardiovascular health [32]. Several clinical studies have shown that there is no electrophysiological inducibility of arrhythmias on ECG monitoring under oral or intravenous caffeine exposure [33–36], even though it raises the level of epinephrine and norepinephrine and shortens the refractory period in the right ventricle, AV-Node, and right atrium and prolongs the one of the left atrium [35]. Other important components of coffee are antioxidants, such as p-coumaric, caffeic, and ferulic acids, which may play a protective role in atherosclerotic genesis by reducing oxidation of low-density lipoprotein [37]. Furthermore, there is evidence of an inverse relationship between coffee consumption and inflammatory markers in the bloodstream, which might also contribute to the beneficial health effects of coffee consumption [38, 39]. The risk-reducing effect of coffee consumption seems to disappear and thus to equal that of not-daily consumption if a certain low daily dose is consumed. One can only speculate whether this observation is due to unidentified confounding factors or whether there might be a dose-dependent effect of biochemicals. One theory might be that the negative effects of an increasing caffeine intake might outweigh the protective effects of antioxidant components and thus affect cardiovascular health negatively. However, this theory is not supported by a meta-analysis that compares the relationship between caffeinated and decaffeinated coffee on all-cause mortality as they both showed a similar and an approximately U-shaped relationship [27, 40]. Therefore, the reasons for the neutralization of the risk-reducing effect of coffee consumption when consuming a certain amount still needs to be better understood.
Strengths and limitations
A main strength of this study was the large sample size of well-characterized AF patients. Furthermore, information on coffee consumption and covariates was obtained on a regular basis, which allowed us to perform time-updated analyses. Nevertheless, several limitations should be taken into account when interpreting our results. First, both studies are conducted in Switzerland and the main population of our study was of European origin. Whether the results are generalizable to patients of other ethnicities is uncertain. In addition, coffee consumption habits may clearly be different in patients from other geographical regions (within Europe and globally), and the generalizability of our results in these patients is uncertain. Second, coffee consumption was self-assessed by the patients, and misclassification of coffee consumption is theoretically possible. However, we assume that misclassification would rather be non-differential, meaning that the probability of individuals being misclassified is equal across all groups. Third, male patients are overrepresented, which is common in cardiovascular studies. However, there was no effect-modifying effect of patient sex in this context. Fourth, caffeine, as an important component of coffee, is also an important ingredient of other food or beverages, such as black tea, coke, energy drinks, and chocolate. These other sources of caffeine may influence the amount of caffeine intake per person and were not taken into account in our study. This issue has been handled similarly in other studies; therefore, the comparability of the data remains unproblematic. Furthermore, daily caffeine intake is mostly driven by coffee, and other sources do not play a comparable role in an elderly population. Fifth, we did not correct for multiple comparisons due to the exploratory nature of the analysis. Finally, due to the observational nature of the study, residual confounding is still possible. However, we made a comprehensive adjustment for the regression analyses, including comorbidities, cardiovascular risk factors, and educational status.
Conclusions
In this cohort of AF patients, daily coffee consumption was associated with a reduced risk of MACE, hospitalization for acute heart failure, and all-cause mortality compared to not-daily coffee consumption. This association was mainly driven by a risk reduction in patients with moderate coffee consumption (2–3 cups/day). The observational nature of this study does not allow to draw causal inferences. Nevertheless, we found no evidence of an unfavourable association of daily coffee consumption in AF Patients with adverse outcome events.
Supplementary Information
Supplementary Material 1: Supplement: Swiss-AF investigators. Supplementary Table S1. Definition of outcome events. Supplementary Table S2. Baseline characteristics overall and stratified by coffee consumption subcategories (none, very low, low, moderate, high). Supplementary Table S3: Incidence rate at 1, 3 and 5 years, stratified by coffee consumption. Supplementary Table S4: Association between coffee consumption and secondary outcome events. Supplementary Table S5. Association between coffee consumption and major adverse cardiovascular event stratified by atrial fibrillation type. Supplementary Fig. S1. Flowchart of patient inclusion. Supplementary Fig. S2. Cumulative incidence curves for major adverse cardiovascular events (MACE) stratified by daily versus not-daily coffee consumption truncated after five years of follow-up.
Acknowledgements
We thank the participants for their involvement in the study, and the study research staff at participating centers.
All Swiss-AF investigators are listed in the supplement.
Abbreviations
- AF
Atrial fibrillation
- BMI
Body mass index
- CI
Confidence interval
- CRF
Case report form
- EHRA
European heart rhythm association
- ESC
European society of cardiology
- HR
Hazard ratio
- MACE
Major adverse cardiovascular events
- py
Person years
- SD
Standard deviation
Authors’ contributions
Design of the work: VI, SA, CMZ; Data acquisition: VI, EH, EHen, SA, REP and TS; Data analysis: VI, SA, EH, MC; Dataset preparation: MC; Interpretation of the results: VI, SA, CMZ, REP, MC, EHen, TR, NR, ASM, AS, JHB, RB, GC, EC, RK, MdV, PC, TS, LHB, MK, SO and DC; Drafted the work: VI, SA, CMZ; Substantively revised the manuscript: all authors; All authors have seen and approved the final version of the manuscript for publication.
Funding
BEAT-AF has been supported by the Swiss National Science Foundation (PP00P3_159322), the Swiss Heart Foundation, the University of Basel, Roche Diagnostics, Boehringer Ingelheim, Sanofi-Aventis, Merck Sharp & Dome, Bayer, Daiichi-Sankyo, Pfizer/Bristol-Myers Squibb, and the Foundation for Cardiovascular Research Basel. The Swiss-AF cohort study is supported by grants of the Swiss National Science Foundation (Grant numbers 33CS30_148474, 33CS30_177520, 32473B_176178, and 32003B_197524), the University of Basel, Roche Diagnostics, and the Foundation for Cardiovascular Research Basel (FCVR Basel).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocols were approved by the main ethics committee (Ethikkommission Nordwest- und Zentralschweiz EKNZ) and conducted in accordance with the Helsinki Declaration. Written informed consent to participate in this study was provided by all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Stefanie Aeschbacher and Christine S. Zuern contributed equally to this work.
Contributor Information
Stefanie Aeschbacher, Email: stefanie.aeschbacher@usb.ch.
Christine S. Zuern, Email: christinestefanie.meyer-zuern@usb.ch
References
- 1.Özen AE, Bibiloni Mdel M, Pons A, Tur JA. Fluid intake from beverages across age groups: a systematic review. J Hum Nutr Diet. 2015;28(5):417–42. [DOI] [PubMed] [Google Scholar]
- 2.Abdelfattah R, Kamran H, Lazar J, Kassotis J. Does caffeine consumption increase the risk of new-onset atrial fibrillation? Cardiology. 2018;140(2):106–14. [DOI] [PubMed] [Google Scholar]
- 3.Frost L, Vestergaard P. Caffeine and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Clin Nutr. 2005;81(3):578–82. [DOI] [PubMed] [Google Scholar]
- 4.Conen D, Chiuve SE, Everett BM, Zhang SM, Buring JE, Albert CM. Caffeine consumption and incident atrial fibrillation in women. Am J Clin Nutr. 2010;92(3):509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng M, Hu Z, Lu X, Huang J, Gu D. Caffeine intake and atrial fibrillation incidence: dose response meta-analysis of prospective cohort studies. Can J Cardiol. 2014;30(4):448–54. [DOI] [PubMed] [Google Scholar]
- 6.Caldeira D, Martins C, Alves LB, Pereira H, Ferreira JJ, Costa J. Caffeine does not increase the risk of atrial fibrillation: a systematic review and meta-analysis of observational studies. Heart. 2013;99(19):1383–9. [DOI] [PubMed] [Google Scholar]
- 7.Bodar V, Chen J, Gaziano JM, Albert C, Djoussé L. Coffee consumption and risk of atrial fibrillation in the physicians’ health study. J Am Heart Assoc. 2019;8(15):e011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsson SC, Drca N, Jensen-Urstad M, Wolk A. Coffee consumption is not associated with increased risk of atrial fibrillation: results from two prospective cohorts and a meta-analysis. BMC Med. 2015;13:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsson SC, Orsini N. Coffee consumption and risk of stroke: a dose-response meta-analysis of prospective studies. Am J Epidemiol. 2011;174(9):993–1001. [DOI] [PubMed] [Google Scholar]
- 10.Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129(6):643–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mostofsky E, Rice MS, Levitan EB, Mittleman MA. Habitual coffee consumption and risk of heart failure: a dose-response meta-analysis. Circ Heart Fail. 2012;5(4):401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunter MJ, Murphy N, Muller DC, Riboli E. Coffee drinking and mortality in 10 European countries. Ann Intern Med. 2018;168(5):380–1. [DOI] [PubMed] [Google Scholar]
- 13.Liu D, Li ZH, Shen D, Zhang PD, Song WQ, Zhang WT, Huang QM, Chen PL, Zhang XR, Mao C. Association of sugar-sweetened, artificially sweetened, and unsweetened coffee consumption with all-cause and cause-specific mortality : a large prospective cohort study. Ann Intern Med. 2022;175(7):909–17. [DOI] [PubMed] [Google Scholar]
- 14.Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ. 2017;359:j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y, Je Y, Giovannucci E. Coffee consumption and all-cause and cause-specific mortality: a meta-analysis by potential modifiers. Eur J Epidemiol. 2019;34(8):731–52. [DOI] [PubMed] [Google Scholar]
- 16.Mo L, Xie W, Pu X, Ouyang D. Coffee consumption and risk of myocardial infarction: a dose-response meta-analysis of observational studies. Oncotarget. 2018;9(30):21530–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–5. [DOI] [PubMed] [Google Scholar]
- 18.Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174(1):107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, Dietz R, Gavazzi A, Hobbs R, Korewicki J, et al. The EuroHeart Failure survey programme– a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24(5):442–63. [DOI] [PubMed] [Google Scholar]
- 20.Reiffel JA. Atrial fibrillation and stroke: epidemiology. Am J Med. 2014;127(4):e15-16. [DOI] [PubMed] [Google Scholar]
- 21.Marcus GM, Modrow MF, Schmid CH, Sigona K, Nah G, Yang J, Chu TC, Joyce S, Gettabecha S, Ogomori K, et al. Individualized studies of triggers of paroxysmal atrial fibrillation: the I-STOP-AFib randomized clinical trial. JAMA Cardiol. 2022;7(2):167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groh CA, Faulkner M, Getabecha S, Taffe V, Nah G, Sigona K, McCall D, Hills MT, Sciarappa K, Pletcher MJ, et al. Patient-reported triggers of paroxysmal atrial fibrillation. Heart Rhythm. 2019;16(7):996–1002. [DOI] [PubMed] [Google Scholar]
- 23.Conen D, Rodondi N, Mueller A, Beer J, Auricchio A, Ammann P, Hayoz D, Kobza R, Moschovitis G, Shah D, et al. Design of the Swiss Atrial Fibrillation Cohort Study (Swiss-AF): structural brain damage and cognitive decline among patients with atrial fibrillation. Swiss Med Wkly. 2017;147:w14467. [DOI] [PubMed] [Google Scholar]
- 24.Klatsky AL, Hasan AS, Armstrong MA, Udaltsova N, Morton C. Coffee, caffeine, and risk of hospitalization for arrhythmias. Perm J. 2011;15(3):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010;12(10):1360–420. [DOI] [PubMed] [Google Scholar]
- 26.Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener HC, Goette A, Hindricks G, Hohnloser S, Kappenberger L, et al. Outcome parameters for trials in atrial fibrillation: executive summary. Eur Heart J. 2007;28(22):2803–17. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Liu Y, Sun X, Yin Z, Li H, Cheng C, Liu L, Zhang R, Liu F, Zhou Q, et al. Caffeinated and decaffeinated coffee consumption and risk of all-cause mortality: a dose-response meta-analysis of cohort studies. J Hum Nutr Diet. 2019;32(3):279–87. [DOI] [PubMed] [Google Scholar]
- 28.Maughan RJ, Griffin J. Caffeine ingestion and fluid balance: a review. J Hum Nutr Diet. 2003;16(6):411–20. [DOI] [PubMed] [Google Scholar]
- 29.Seal AD, Bardis CN, Gavrieli A, Grigorakis P, Adams JD, Arnaoutis G, Yannakoulia M, Kavouras SA. Coffee with high but not low caffeine content augments fluid and electrolyte excretion at rest. Front Nutr. 2017;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proietti M, Romiti GF, Olshansky B, Lane DA, Lip GYH. Comprehensive management with the ABC (Atrial Fibrillation Better Care) pathway in clinically complex patients with atrial fibrillation: a post hoc ancillary analysis from the AFFIRM trial. J Am Heart Assoc. 2020;9(10):e014932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott AD, Middeldorp ME, Van Gelder IC, Albert CM, Sanders P. Epidemiology and modifiable risk factors for atrial fibrillation. Nat Rev Cardiol. 2023;20(6):404–17. [DOI] [PubMed] [Google Scholar]
- 32.Nawrot P, Jordan S, Eastwood J, Rotstein J, Hugenholtz A, Feeley M. Effects of caffeine on human health. Food Addit Contam. 2003;20(1):1–30. [DOI] [PubMed] [Google Scholar]
- 33.Chelsky LB, Cutler JE, Griffith K, Kron J, McClelland JH, McAnulty JH. Caffeine and ventricular arrhythmias. An electrophysiological approach. JAMA. 1990;264(17):2236–40. [PubMed] [Google Scholar]
- 34.Graboys TB, Blatt CM, Lown B. The effect of caffeine on ventricular ectopic activity in patients with malignant ventricular arrhythmia. Arch Intern Med. 1989;149(3):637–9. [PubMed] [Google Scholar]
- 35.Dobmeyer DJ, Stine RA, Leier CV, Greenberg R, Schaal SF. The arrhythmogenic effects of caffeine in human beings. N Engl J Med. 1983;308(14):814–6. [DOI] [PubMed] [Google Scholar]
- 36.Newcombe PF, Renton KW, Rautaharju PM, Spencer CA, Montague TJ. High-dose caffeine and cardiac rate and rhythm in normal subjects. Chest. 1988;94(1):90–4. [DOI] [PubMed] [Google Scholar]
- 37.Natella F, Nardini M, Belelli F, Scaccini C. Coffee drinking induces incorporation of phenolic acids into LDL and increases the resistance of LDL to ex vivo oxidation in humans. Am J Clin Nutr. 2007;86(3):604–9. [DOI] [PubMed] [Google Scholar]
- 38.Kempf K, Herder C, Erlund I, Kolb H, Martin S, Carstensen M, Koenig W, Sundvall J, Bidel S, Kuha S, et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr. 2010;91(4):950–7. [DOI] [PubMed] [Google Scholar]
- 39.Kotani K, Tsuzaki K, Sano Y, Maekawa M, Fujiwara S, Hamada T, Sakane N. The relationship between usual coffee consumption and serum C-reactive protein level in a Japanese female population. Clin Chem Lab Med. 2008;46(10):1434–7. [DOI] [PubMed] [Google Scholar]
- 40.Loftfield E, Cornelis MC, Caporaso N, Yu K, Sinha R, Freedman N. Association of coffee drinking with mortality by genetic variation in caffeine metabolism: findings from the UK Biobank. JAMA Intern Med. 2018;178(8):1086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplement: Swiss-AF investigators. Supplementary Table S1. Definition of outcome events. Supplementary Table S2. Baseline characteristics overall and stratified by coffee consumption subcategories (none, very low, low, moderate, high). Supplementary Table S3: Incidence rate at 1, 3 and 5 years, stratified by coffee consumption. Supplementary Table S4: Association between coffee consumption and secondary outcome events. Supplementary Table S5. Association between coffee consumption and major adverse cardiovascular event stratified by atrial fibrillation type. Supplementary Fig. S1. Flowchart of patient inclusion. Supplementary Fig. S2. Cumulative incidence curves for major adverse cardiovascular events (MACE) stratified by daily versus not-daily coffee consumption truncated after five years of follow-up.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.