Abstract
Background
Recognition of carbapenem-resistant gram-negative bacteria (CR-GNB) carriage is frequently delayed, which increases the risk of subsequent infection and transmission. Previously, we developed a scoring system to identify CR-GNB carriage upon intensive care unit (ICU) admission. Although the ICU-CARB score showed satisfactory performance, it has not been externally validated. In this study, therefore, we externally validated the ICU-CARB score.
Methods
In the previous article, we introduced a risk-scoring system that incorporated seven key variables: neurological disease, high-risk department history, length of stay ≥ 14 days, ICU history, invasive mechanical ventilation, gastrointestinal tube placement, and carbapenem usage. To externally validate the ICU-CARB score, we conducted a study involving patients admitted to the ICUs of four tertiary hospitals between January 2021 and December 2023. Patients from three hospitals were grouped into Cohort I (n = 815) and those from the fourth hospital into Cohort II (n = 1602). Model calibration, discrimination, and performance were then assessed.
Results
A total of 2417 patients were included, among which 289 (12%) carried CR-GNB upon ICU admission. Neurological disease, high-risk department history and length of stay ≥ 14 days were still 3 most important contributing factors in the scoring system. The ICU-CARB score exhibited high calibration, with an area under the receiver operating characteristic curve of 0.825 (95% confidence interval [CI], 0.778–0.873) for Cohort I and 0.823 (95% CI, 0.791–0.855) for Cohort II. The ICU-CARB score showed a highly positive association with CR-GNB carriage in both cohort I (C = 0.315; P < 0.001) and Cohort II (C = 0.381; P < 0.001).
Conclusions
Despite differences in patient population characteristics, the ICU-CARB score for CR-GNB carriage upon ICU admission exhibited good discrimination in external validation, supporting its potential generalizability to other ICU settings.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13756-024-01509-y.
Keywords: Carbapenem-resistant gram-negative bacteria, Intensive care unit, External validation, Precaution, Predictive analytics
Introduction
Carbapenem-resistant gram-negative bacteria (CR-GNB) are epidemic-causing pathogens in health care settings and often associated with hospital-acquired infections, sepsis, and a high mortality rate [1, 2]. The prevalence of Multidrug Resistant Organisms (MDROs) in long-term care is high, reaching 40–65% in nursing homes and 80% in long-term acute care (LTAC) hospital settings [3, 4], exceeding the typical hospital prevalence of 10–20% [5, 6]. Most cases of CR-GNB carriage are not recognized upon admission to the intensive care unit (ICU) due to resource constraints that preclude routine screening and lack of warning systems regarding CR-GNB status [7, 8]. Delayed identification in turn leads to failure to implement timely isolation, such that CR-GNB carriers can contaminate the ICU environment and increase the likelihood of cross-transmission. The development of a generalizable model that accurately identifies CR-GNB carriage irrespective of setting could guide clinical decision support and enable better and more timely recognition of CR-GNB carriers at the point of care [9, 10].
Several clinical risk models for prediction of CR-GNB or MDROs have been identified and can be roughly classified into carriage prediction model and colonization/infection development models. The majority of those proposed carriage prediction models focused on hematologic malignancies, organ transplantation, and ICU patients, and the accuracy of these models yielded C-statistics ranging from 0.77 to 0.91 [11–17]. Giannella et al.’s risk score model, yielding an area under the curve (AUC) of 0.74–0.75, is one of the most influential models [17]. However, these prediction models focus on the occurrence of infection, and to develop a risk model that uses easily calculated variables that can achieve quick applicability of CR-GNB carriage risk before patients are subjected to pathogen screening and infection control measures may be more clinically attractive. In a recent study, we developed a risk-scoring system, known as the ICU-CARB score, for the early prediction of CR-GNB carriage upon ICU admission [15]. The model exhibited an AUC of 0.82 (95% confidence interval [CI], 0.78–0.86) in the training cohort and 0.83 (95% CI, 0.77–0.89) in the internal validation cohort. However, the external applicability of the model was not assessed because the model was derived and validated at a single center [18].
The aim of the present study was to externally validate the ICU-CARB score for the prediction of CR-GNB carriage in separate, independent ICU settings. This study is significant because demonstration of accurate prediction of CR-GNB carriage in this new cohort would support the potential generalizability of our model to other patient populations and institutions.
Methods
Setting and study design
We conducted this external validation study at four hospitals in Shanghai: Shanghai Ruijin Hospital, Shanghai Sixth People’s Hospital, Shanghai Tenth People’s Hospital, and Shuguang Hospital. The study was approved by the Institutional Ethics Committee of Ruijin Hospital affiliated with Shanghai Jiao Tong University School of Medicine (no. 2022-LLS-93) and the hospital’s research review committee. The requirement for informed consent was waived as this study did not involve any intervention.
All patients admitted to the ICU during the indicated periods were consecutively screened for study eligibility. Cohort I was recruited from Shanghai Sixth People’s Hospital (surgical ICU, between November 2022, and October 2023), Shanghai Tenth People’s Hospital (surgical ICU, between June 2023, and October 2023), and Shuguang Hospital (emergency ICU, between June 2023, and August 2023). Cohort II was recruited from Shanghai Ruijin Hospital (general ICU) between January 2021, and December 2023. The research was conducted in compliance with ethical standards and adapted to clinical realities. The exclusion criteria for participation were as follows: (1) age < 18 years at the time of admission and (2) absence of active surveillance culture (ASC) within 72 h of admission.
Data collection and endpoint definition
The ICU-CARB score for prediction of CR-GNB carriage was previously described in detail [15]. Briefly, seven variables were included in the risk-scoring system: neurologic disease (100 points), history of stay in a high-risk department (95 points), length of stay (LOS) ≥ 14 days (75 points), ICU history (65 points), invasive mechanical ventilation (45 points), gastrointestinal tube placement (36 points), and carbapenem administration (29 points). After transfer to the ICU, the patient was immediately tested and scored using an online calculator (https://www.wjx.cn/vm/eSTavIv.aspx). Patients were then risk stratified according to the total risk score as follows: negligible (score 0-110), low- (111–220), medium- (221–330), and high-risk (331–450).
All relevant data were carefully recorded by trained ICU physicians from the same clinical information system. Neurologic diseases include cerebral degeneration, Parkinson’s disease, epilepsy, recurrent seizures, spinocerebellar disease, cerebellar ataxia, spinal muscular atrophy, and encephalopathy. History of stay in a high-risk department was defined to include patients with a history of hospitalization within the previous 30 days in a hospital department in which carbapenem-resistant pathogens were detected in the previous quarter. Each center quarterly updated the scope of high-risk department based on the epidemiological characteristics of its own facility. ICU history was defined as ICU hospitalization for more than 3 days within last 2 months. Assessment for invasive mechanical ventilation (tracheal intubation and tracheotomy) and gastrointestinal tube placement was performed upon admission to the ICU. History of carbapenem use was defined as intravenous administration of antibiotics within last 14 days prior to ICU admission. Data regarding other variables were also collected, including patient demographic characteristics, Acute Physiology and Chronic Health Evaluation (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, and discharge disposition.
ASCs were performed immediately upon admission then twice a week during ICU stay to detect pathogen colonization/infection. Colonization screening must be performed, including rectal and pharyngeal swabs. Clinical specimens are collected when sepsis or infection is suspected, including sputum, drainage, urine, catheter and blood [19]. ASCs were performed the same frequency and culture sites in the four ICUs participating in the study. CR-GNB carriage was defined as the detection of CR-GNB through ASCs and clinical cultures either before ICU admission or within 72 h after admission [20]. CR-GNB included Carbapenem-resistant Enterobacteriaceae (CRE), Carbapenem-resistant Acinetobacter baumannii (CRAB), and Carbapenem-resistant Pseudomonas aeruginosa (CRPA).
Microbiological procedures
All ASCs cultures obtained at the clinician’s discretion were sent to the Department of Clinical Microbiology of each center for conventional testing. Isolates were identified using matrix-assisted laser desorption ionisation-time of flight mass spectrometer (bioMérieux, Marcy l’Etoile, France). Antimicrobial susceptibilities were determined in vitro using the VITEK 2 Compact system (bioMérieux) or disk-diffusion assays. Each center followed the guidelines set by Clinical and Laboratory Standards Institute (CLSI) and carbapenem resistance was defined based on the CLSI interpretation [21].
Parameters used to evaluate model performance
The model was calibrated by plotting apparent (actual), bias-corrected (adjusted), and ideal (100% agreement) curves with 1000 bootstrap resamples, and the level of agreement between predicted probabilities versus observed outcomes was then evaluated. Model discrimination was assessed by calculating the area under the receiver operating characteristic curve (C-statistic with 95% confidence interval). The clinical utility of the model was evaluated using decision curve analysis (DCA), which quantifies the net benefit at different threshold probabilities.
Sample size and missing data
Determining sample size for observational studies is quite difficult. Typically, in multivariate regression models for binary outcomes, a well-known rule of thumb for the required sample size is to the events per variable (EPV) was 10 or greater [22]. We initially estimated that a sample size of 1000 patients, with 100 outcome events (event rate, 10%) would be adequate for our study. Ultimately, we enrolled 2417 patients, of which 289 were identified with CR-GNB carriage. The EPV was calculated at 41.3, significantly exceeding the benchmark of 10. A complete case analysis was performed with no missing data.
Statistical analysis
Statistical analyses were conducted using R statistical software, version 4.2.1 (http://cran.r-project.org). Continuous variables are reported as mean and standard deviation (SD) for normally distributed variables or as median and interquartile range (IQR) for non-normally distributed variables. Categorical variables are expressed as frequency and percentage. Characteristics between the two cohorts were compared using the Student t-test or Mann-Whitney U test for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables. Two-sided p-values less than 0.05 were considered to indicate statistical significance. This study was reported according to the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis guidelines [23].
Results
Clinical characteristics of patients
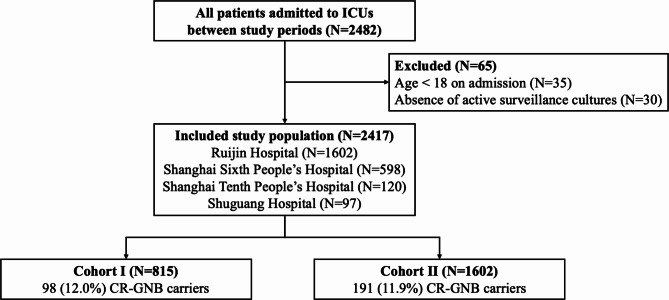
A total of 2482 patients admitted to the ICUs were initially identified for screening. After applying the exclusion criteria (Fig. 1), a final analysis was conducted on 1736 adult patients. A total of 2417 patients were identified, of which 41.1% were female, and the mean age was 65.3 ± 18.8 years. The demographic characteristics and variables for both cohorts are summarized in Table 1 for comparison. The rate of CR-GNB carriage upon ICU admission was 12.0% (289/2417) among the overall patient group, with similar incidence in Cohort I and Cohort II (12.0% vs. 11.9%, respectively). In cohort I, CR-GNB carriage rate upon ICU admission was 9.2%, 26.7% and 11.3% (Additional file: Table S1). Surgical and emergency patients comprised a larger proportion of Cohort I compared with Cohort II according to ICU type (68.3% vs. 58.1% and 27.2% vs. 14.1%, respectively).
Fig. 1.
Flow chart of study participants. CR-GNB: carbapenem-resistant gram-negative bacterial
Table 1.
Baseline characteristics of the study cohort
| All (n = 2417) |
Cohort I (n = 815) |
Cohort II (n = 1602) |
p | |
|---|---|---|---|---|
| CRGNB carrier (%) | 289 (12.0) | 98 (12.0) | 191 (11.9) | 0.942 |
| Female (%) | 975 (40.3) | 312 (38.3) | 663 (41.4) | 0.141 |
| Age (mean (SD)) | 65.3 (18.9) | 66.0 (16.7) | 65.0 (19.8) | 0.221 |
| APACHE.II (median [IQR]) | 12 [8, 18] | 12 [9, 16] | 15 [10, 20] | < 0.001 |
| SOFA (median [IQR]) | 3 [1, 6] | 3 [2, 5] | 5 [2, 8] | < 0.001 |
| Department (%) | < 0.001 | |||
| surgical | 1488 (61.6) | 557 (68.3) | 931 (58.1) | |
| medical | 476 (19.7) | 36 (4.4) | 440 (27.5) | |
| emergency | 453 (18.7) | 222 (27.2) | 231 (14.4) | |
| ICU mortality (%) | 255 (10.6) | 74 (9.1) | 181 (11.4) | 0.082 |
| Variables | ||||
| Neurological disease (%) | 167 (6.9) | 88 (10.8) | 79 (4.9) | < 0.001 |
| High-risk departments history (%) a | 1201 (49.7) | 560 (68.7) | 641 (40.0) | < 0.001 |
| LOS before ICU admission ≥ 14 (%) | 511 (21.1) | 74 (9.1) | 437 (27.3) | < 0.001 |
| Previous ICU admission (%) b | 228 (9.4) | 64 (7.9) | 164 (10.2) | 0.058 |
| Invasive mechanical ventilation (%) | 1407 (58.2) | 649 (79.6) | 758 (47.3) | < 0.001 |
| Gastrointestinal tube (%) | 1078 (44.6) | 207 (25.4) | 871 (54.3) | < 0.001 |
| Carbapenem usage (%) c | 689 (28.5) | 118 (14.5) | 571 (35.6) | < 0.001 |
CR-GNB, Carbapenem-resistant Gram-negative bacteria; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment; LOS, length of stay; ICU, intensive care unit. a Within last month prior to ICU admission. b Administration for more than 3 days within last 2 months prior to ICU admission. c Intravenous administration of antibiotics within last 14 days prior to ICU admission
Clinical characteristics and variables differed significantly between the two groups (Table 1). Compared with Cohort II, patients in Cohort I showed lower scores for both APACHE II (12 vs. 15; P < 0.001) and SOFA (3 vs. 5; P < 0.001). A higher proportion of patients in Cohort I had a neurologic disease (10.8% vs. 4.9%, respectively; P < 0.001), history of hospitalization in a high-risk department within the previous 30 days (67.8% vs. 40.0%, respectively; P < 0.001), and history of invasive mechanical ventilation upon ICU admission (79.6% vs. 47.3%, respectively; P < 0.001). A higher proportion of patients in Cohort II were hospitalized for ≥ 14 days before being transferred to the ICU (27.3% vs. 9.1%, respectively; P < 0.001), had a gastrointestinal tube placed upon ICU admission (54.3% vs. 25.4%, respectively; P < 0.001), and were administered carbapenem within 14 days (35.6% vs. 14.5%, respectively; P < 0.001). There were no significant differences between Cohorts I and II in terms of ICU mortality (9.1% vs. 11.4%, respectively; P = 0.082) and ICU history in the previous 2 months (7.9% vs. 10.2%, respectively; P = 0.058).
Model performance
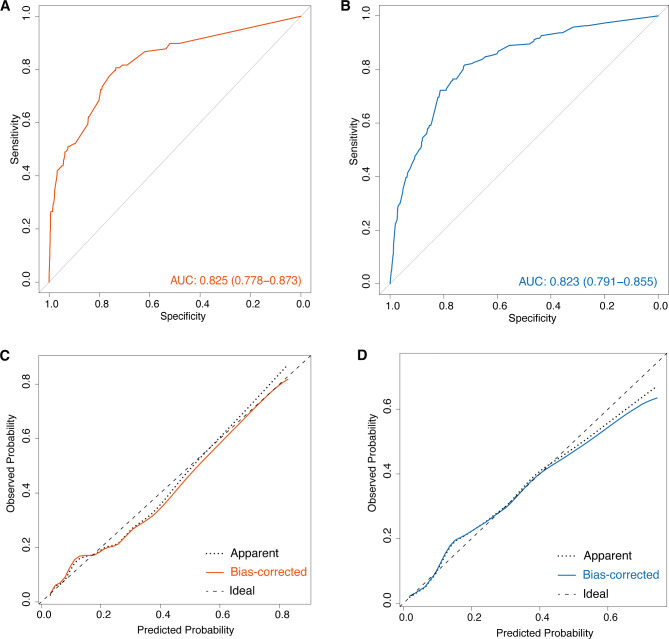
The performance of the model for the two cohorts is summarized in Fig. 2. Calibration plots showed adequate agreement between the predictive probabilities and actual observations in both cohorts, indicating a robust calibration of the ICU-CARB scoring system. Furthermore, the AUC for predicting CR-GNB carriage upon ICU admission was 0.825 (95% CI, 0.778–0.873) for Cohort I and 0.823 (95% CI, 0.791–0.855) for Cohort II, indicating acceptable discrimination. As for individual assessment of the three centers within Cohort I, the respective AUC values were 0.842 (95% CI, 0.779–0.905), 0.739 (95% CI, 0.637–0.841), and 0.761 (95% CI, 0.589–0.932) (Additional file: Fig. S1).
Fig. 2.
Area under the receiver operating characteristic curve (AUC) values for the prediction of CR-GNB carriage upon ICU admission in Cohort I (A) and Cohort II (B). Calibration curve analysis in Cohort I (C) and Cohort II (D). CR-GNB: carbapenem-resistant gram-negative bacteria; ICU: intensive care unit
Clinical implications of the scoring system
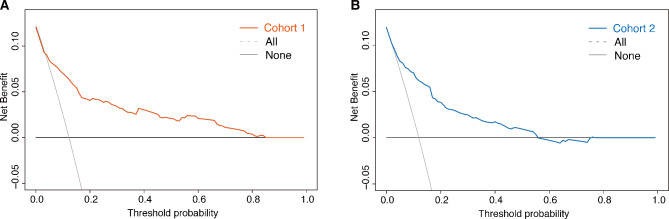
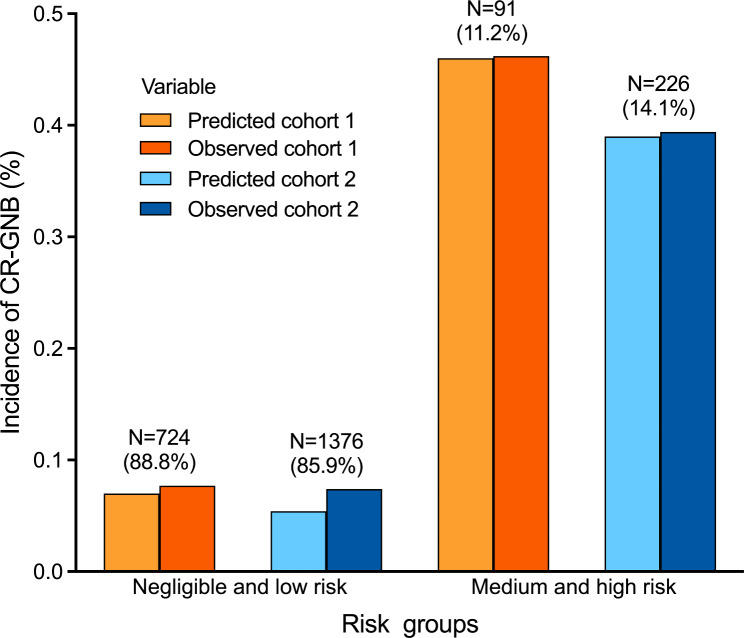
The results of the DCA demonstrated that within a threshold probability range of 1–85% in Cohort I (Fig. 3A) and 1–60% in Cohort II (Fig. 3B), our risk prediction nomogram for CR-GNB carriage would yield a net benefit for patients. As shown in Fig. 4, the risk score was highly and positively associated with the risk of CR-GNB carriage (P < 0.001 for the trend) in both cohorts. The observed incidence of CR-GNB carriage in both cohorts (46.2%, 39.4%) was consistent with the predicted incidence (46.0%, 39.0%) among patients at medium to high risk (Fig. 4; Table 2). The observed incidence of CR-GNB carriage in Cohort II (10.6%) was higher than the predicted incidence in the negligible-risk group (3.1%). As shown in Fig. 3, the risk score showed a highly positive association with the risk of CR-GNB carriage in both cohort I (Pearson’s contingency coefficient (C) = 0.315; P < 0.001) and Cohort II (C = 0.381; P < 0.001).
Fig. 3.
Decision curve analysis of the ICU-CARB score for predicting CR-GNB carriage upon ICU admission in Cohort I (A) and Cohort II (B). ICU: intensive care unit; CR-GNB: carbapenem-resistant gram-negative bacteria
Fig. 4.
Increasing risk of CR-GNB carriage upon ICU admission with increasing risk score is evident in the Cohort I and Cohort II. Observed CR-GNB carriage rates in patient groups categorized as negligible/low risk and medium/high risk by the model closely matched the predicted rates. CR-GNB: carbapenem-resistant gram-negative bacteria; ICU: intensive care unit
Table 2.
Comparison of the patients between different risk levels in the external validation sets
| Risk level | Score | Cohort I | Cohort II | ||||
|---|---|---|---|---|---|---|---|
| Patients (%) | Predicted risk (%) | Observed risk (%) | Patients (%) | Predicted risk (%) | Observed risk (%) | ||
| Negligible | 0-110 | 246 (30.2) | 3.1 | 10.6 | 818 (51.1) | 3.0 | 3.2 |
| Low | 111–220 | 478 (58.7) | 10 | 6.3 | 558 (34.8) | 12 | 13.6 |
| Medium | 221–330 | 61 (7.5) | 34 | 34.4 | 181 (11.3) | 34 | 35.4 |
| High | 331–450 | 30 (3.7) | 72 | 67.8 | 45 (2.8) | 62 | 55.6 |
Discussion
In this study, we externally validated a previously developed scoring system consisting of seven variables that predicts CR-GNB carriage upon ICU admission. The ICU-CARB score was found to be highly predictive of the observed incidence. When applied to the external validation datasets consisting of information for 2417 critically ill patients, the predictive capacity of the ICU-CARB score for Cohorts I and II (AUC = 0.825 & 0.823, respectively) was very similar to that for the development datasets (AUC = 0.819 for the training set and 0.828 for the validation set). The model also performed well in terms of predicting whether the carriage risk would be high or low.
The strength of this study is that we externally validated our model’s performance by demonstrating that the power of the scoring system was similar even when applied at different centers. The CR-GNB carriage rate was similar between two cohorts; however, the demographic characteristics, hospitalization history, and medical exposure differed between the two cohorts. Nevertheless, we showed that the accuracy of the ICU-CARB scoring system for predicting CR-GNB carriage rate and risk allocation was similar for both cohorts. A potential criticism of the study could be that accuracy is not the best metric for a skewed dataset, as it may be artificially improved by simply predicting all patients without CR-GNB carriage [24]. In the medium- and high-risk groups, which accounted for only 15% of the datasets, approximately 40–45% of patients carried CR-GNB, but the ICU-CARB score still provided good recognition.
Unsurprisingly, fluctuations in forecast performance were observed when distinguishing between the negligible- and low-risk groups. The observed incidence of CR-GNB carriage (10.6%) in the negligible-risk group was higher than the predicted incidence (3.1%) in Cohort I, but these values remained consistent in Cohort II (3.2% and 3.0%, respectively). This could have been due to differences in the composition of ICU wards and patients between the two datasets. The majority of patients in Cohort I were surgical patients, and nearly 80% of the patients were intubated and received invasive mechanical ventilation at the time of ICU transfer. This generally resulted in patients in the negligible-risk group exhibiting higher prediction scores. Critically ill patients typically exhibit lung and gut microbial dysbiosis after mechanical ventilation [25], and prolonged mechanical ventilation results in a decrease in lung microbial diversity and an increase in intestinal flora [26]. Patients in the surgical ICU receive mechanical ventilation primarily during the perioperative period. However, disease progression differs between patients who undergo scheduled extubation after surgery and those who receive mechanical ventilation for reasons such as acute respiratory distress syndrome or cardiac arrest. Underestimating the CR-GNB carriage rate in the negligible-risk group could lead to patients not adopting appropriate infection control measures; hence, ASC remained a critically important component of infection prevention and control strategy. Therefore, some particular parameter of the ICU-CARB score also may be adjusted in actual practice if the physician requires a narrower margin of error or if a wider margin of error could be tolerated.
A head-to-head comparison of the present results with those of prior studies is difficult because prediction targets differ, but our model performs well against other prediction models. For example, Zhang et al. developed a nomogram for the risk of CR-GNB infection in ICU patients using six variables, with AUC values of 0.776 and 0.723 for the training and validation sets, respectively. However, for laboratory and clinical indicators involving multiple measured values, only the worst value on the first day of ICU admission is included. Therefore, models that predict dynamic outcomes actually perform poorly [27]. The model developed by Liao et al. for predicting development of CR-GNB infection in the ICU also showed similar predictive capabilities (AUC = 0.753 and 0.718 for the experimental and validation cohorts, respectively) [28]. For greater accuracy, Liang et al. developed machine learning prediction models for early prediction of CR-GNB carriage within 7 days in ICU settings. The accuracy and AUROC for their algorithm were > 85% and > 0.91, respectively [29]. However, their model relied on a real-time database; thus, the 16 variables that require updating limit the model’s clinical application. Notably, our study specifically targeted risk factors for CR-GNB carriage upon ICU admission. The applicability of the ICU-CARB score in different ICU settings could be enhanced by incorporating simple and readily accessible indicators.
Notably, the AUC values, which range from 0.74 to 0.84 across various external validation datasets, suggest a probably considerable level of generalizability for our model. This indicates that our model may be applicable in ICUs with similar patient demographics in regions where CR-GNB is highly prevalent, such as Eastern Europe and Southeast Asia [4]. Nevertheless, in areas with lower prevalence, like Africa, or in non-ICU settings, additional validation is essential [30]. ASC continues to be the foundational method for screening in these contexts. Also, we endeavored to refine our model using machine learning techniques. However, given the linear relationship between the variables and the outcomes, we determined through further exploration that a logistic regression model maintained satisfactory performance and retained strong interpretability. Consequently, we opted to adhere to the original model for its effectiveness and clarity. To further validate and refine our model, multicenter prospective studies or the utilization of public databases could be conducted.
In-time isolation is crucial for limiting the spread of extensively drug-resistant (XDR)/CR-GNB among critically ill patients, and pre-emptive isolation based on predictive model data can indeed reduce cross-transmission and infection [31, 32]. For critically ill patients, both CR-GNB colonization (odds ratio [OR] = 1.10, 95% CI 0.87–1.40) or CR-GNB acquisition in the ICU (OR = 2.72, 95% CI 1.95–3.77, P < 0.001) are associated with significantly increased risk of mortality [33–35]. Evidence is currently insufficient to provide recommendations for any intervention in patients colonized with MDROs [17], so comprehensive infection prevention and control interventions are of high priority in clinical practice [36, 37]. Moreover, ASCs and pre-emptive isolation enable the containment of CRE bacteremia (0.554 cases per 10,000 patient-days to 0.447 cases per 10,000 patient-days, P < 0.001) and CRE infections (2.09 to 1.49, P < 0.001) [32].
Rapid and widespread increases in CR-GNB necessitate identification of risk levels to guide appropriate interventions [9]. The predictive scoring model developed by Qiao et al. found that the mortality rate of patients classified as high risk (≥ 160 points) was 8 times higher than that of the low-risk group [38]. Similarly, our model indicated that the risk of CR-GNB carriage in patients in the medium- and high-risk groups was nearly 6 times that of patients in the negligible- and low-risk groups. Therefore, pre-emptive isolation of these groups (especially high-risk patients) is strongly recommended to decrease cross-transmission risk. The use of ASCs in ICUs appears to be highly cost-effective [39], but ASCs can also cause significant cost pressures; therefore, their use should be revisited and revised as needed, especially in low-resource settings with a high CR-GNB burden [40]. The ICU-CARB scoring system can, to some extent, serve as a supplement to ASCs for selective screening and isolation. We recommend ASCs for all patients and preemptive isolation of medium- and high-risk groups pending ASC results. Alternatively, in settings of low prevalence or where routine screening may not be feasible, ASCs or rapid molecular testing is suggested to be conducted selectively on patients who are identified as being at medium- or high-risk [41].
The strengths of this study include its prospective nature, different types of ICUs as study sites, the large number of samples, and demonstration of the wide applicability of the ICU-CARB scoring system and its guiding role in clinical practice. We are aware of limitations of this study as well. First, the potential discordance in ASCs and information collection between the facilities may affect the precision of proposed models, The risk score model needs further validation and recalibration in prospective studies for more widespread use. Second, we collected relatively few patients’ clinical characteristics to generalize the model across multiple centers; therefore, some missing special clinical features of patients and potential indication bias may affect the conclusions. Third, we did not collect pathogenic bacteria uniformly for review at a central laboratory. In addition, the inability to collect CR-GNB strains also limited further microbiological analyses. Finally, we conducted external validation in different types of ICUs in four tertiary hospitals. Future research should focus on more medical institutions, such as high-risk departments or ICUs in non-teaching hospitals.
Conclusion
In conclusion, we externally validated the ICU-CARB model by testing it on patient cohorts with different population characteristics. These analyses showed that the ICU-CARB scoring system performed similarly for the different cohorts and ICUs, supporting the generalizability of the model to other ICU settings. Our model may help ICU physicians and health systems identify cases of CR-GNB carriage more expeditiously. The ICU-CARB score could therefore serve as a tool for implementing pre-emptive isolation of patients carrying CR-GNB.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Abbreviations
- APACHE II
Acute physiology and chronic health evaluation
- ASC
Active surveillance culture
- AUC
Area under the receiver operating characteristic curve
- CR-GNB
Carbapenem-resistant gram-negative bacteria
- CRAB
Carbapenem-resistant Acinetobacter baumannii
- CRE
Carbapenem-resistant enterobacteriaceae
- CRPA
Carbapenem-resistant Pseudomonas aeruginosa
- DCA
Decision curve analysis
- ICU
Intensive care units
- IQR
Interquartile range
- LOS
Length of stay
- LTAC
Long-term acute care
- MDRO
Multidrug resistant organism
- ROC
Receiver operating characteristic
Author contributions
RT, XW and HQ designed the study. TW, YD, and ZS analyzed the data. ZZ, YL, FR, QW, SW and QZ performed the surveillance. YD, TW and XW wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by National Natural Science Foundation of China [822241033], Clinical science and technology innovation project of Shanghai Hospital Development Center [SHDC22021212], and Guangci discipline group construction of public health and disaster emergency center [XKQ-09].
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Committee of Ruijin Hospital affiliated with Shanghai Jiao Tong University School of Medicine (No. 2022-LLS-93), and informed consent was waived as this study did not involve an intervention.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tong Wu and Xiaoli Wang contributed equally to this work.
Contributor Information
Hongping Qu, Email: hongpingqu0412@hotmail.com.
Yunqi Dai, Email: daiyunqi52@hotmail.com.
Ruoming Tan, Email: sandratan37@hotmail.com.
References
- 1.Nordmann P, Poirel L. Epidemiology and Diagnostics of Carbapenem Resistance in Gram-negative Bacteria. Clin Infect Dis. 2019;69(Suppl 7):S521–8. 10.1093/cid/ciz824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(2):S82–9. 10.1086/499406. [DOI] [PubMed] [Google Scholar]
- 3.Chen HY, Jean SS, Lee YL, et al. Carbapenem-Resistant Enterobacterales in Long-Term Care Facilities: A Global and Narrative Review. Front Cell Infect Microbiol. 2021;11:601968. 10.3389/fcimb.2021.601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050. Lancet, 2024;404(10459):1199–226. 10.1016/s0140-6736(24)01867-1 [DOI] [PubMed]
- 5.Gussin GM, McKinnell JA, Singh RD, et al. Reducing Hospitalizations and Multidrug-Resistant Organisms via Regional Decolonization in Hospitals and Nursing Homes. JAMA. 2024. 10.1001/jama.2024.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang S, Jeong IS. Epidemiological characteristics of carbapenem-resistant Enterobacteriaceae and carbapenem-resistant Acinetobacter baumannii in a tertiary referral hospital in Korea. Osong Public Health Res Perspect. 2022;13(3):221–9. 10.24171/j.phrp.2022.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luterbach CL, Chen L, Komarow L, et al. Transmission of Carbapenem-Resistant Klebsiella pneumoniae in US Hospitals. Clin Infect Dis. 2023;76(2):229–37. 10.1093/cid/ciac791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimasaki T, Segreti J, Tomich A, et al. Active screening and interfacility communication of carbapenem-resistant Enterobacteriaceae (CRE) in a tertiary-care hospital. Infect Control Hosp Epidemiol. 2018;39(9):1058–62. 10.1017/ice.2018.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palacios-Baena ZR, Giannella M, Manissero D, et al. Risk factors for carbapenem-resistant Gram-negative bacterial infections: a systematic review. Clin Microbiol Infect. 2021;27(2):228–35. 10.1016/j.cmi.2020.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Meschiari M, Kaleci S, Orlando G, et al. Risk factors for nosocomial rectal colonization with carbapenem-resistant Acinetobacter baumannii in hospital: a matched case-control study. Antimicrob Resist Infect Control. 2021;10(1):69. 10.1186/s13756-021-00919-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song JY, Jeong IS. Validation of a carbapenem-resistant Enterobacteriaceae colonization risk prediction model: A retrospective cohort study in Korean intensive care units. Am J Infect Control. 2019;47(12):1436–42. 10.1016/j.ajic.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Freire MP, Rinaldi M, Terrabuio DRB, et al. Prediction models for carbapenem-resistant Enterobacterales carriage at liver transplantation: A multicenter retrospective study. Transpl Infect Dis. 2022;24(6):e13920. 10.1111/tid.13920. [DOI] [PubMed] [Google Scholar]
- 13.Lu G, Zhang J, Shi T, et al. Development and application of a nomogram model for the prediction of carbapenem-resistant Klebsiella pneumoniae infection in neuro-ICU patients. Microbiol Spectr. 2024;12(1):e0309623. 10.1128/spectrum.03096-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu WQ, Zhang YQ, Xu J, et al. Risk Factors for Carbapenem-Resistant Enterobacteriaceae Colonization and the Effect on Clinical Outcomes and Prognosis in Allogeneic Hematopoietic Stem Cell Transplanted Patients. Infect Drug Resist. 2023;16:6821–31. 10.2147/idr.S424048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai Y, Zhang L, Pan T, et al. The ICU-CARB score: a novel clinical scoring system to predict carbapenem-resistant gram-negative bacteria carriage in critically ill patients upon ICU admission. Antimicrob Resist Infect Control. 2023;12(1):118. 10.1186/s13756-023-01326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Hu S, Xu D, et al. Risk Factors for Carbapenem Resistant Gram Negative Bacteria (CR-GNB) Carriage Upon Admission to the Gastroenterology Department in a Tertiary First Class Hospital of China: Development and Assessment of a New Predictive Nomogram. Infect Drug Resist. 2022;15:7761–75. 10.2147/idr.S396596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giannella M, Freire M, Rinaldi M, et al. Development of a Risk Prediction Model for Carbapenem-resistant Enterobacteriaceae Infection After Liver Transplantation: A Multinational Cohort Study. Clin Infect Dis. 2021;73(4):e955–66. 10.1093/cid/ciab109. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Zhou X, Yang R, et al. Carbapenem-resistant Gram-negative bacteria (CR-GNB) in ICUs: resistance genes, therapeutics, and prevention - a comprehensive review. Front Public Health. 2024;12:1376513. 10.3389/fpubh.2024.1376513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinese Preventive Medicine Association (CPMA) and, Diseases CSoI. Chinese Guidelines for Infection Prevention and Control of Carbapenem-Resistant Organisms (CRO). Chin J Nosocomiol. 2019;29(13):2075–80. 10.11816/cn.ni.2019-191088. [Google Scholar]
- 20.Debby BD, Ganor O, Yasmin M, et al. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur J Clin Microbiol Infect Dis. 2012;31(8):1811–7. 10.1007/s10096-011-1506-5. [DOI] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 34th Edition, CLSI M100-Ed34 2024.
- 22.Riley RD, Ensor J, Snell KIE, et al. Calculating the sample size required for developing a clinical prediction model. BMJ. 2020;368:m441. 10.1136/bmj.m441. [DOI] [PubMed] [Google Scholar]
- 23.Moons KG, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–73. 10.7326/m14-0698. [DOI] [PubMed] [Google Scholar]
- 24.Li LT, Huang T, Bernstam EV, et al. External Validation of a Laboratory Prediction Algorithm for the Reduction of Unnecessary Labs in the Critical Care Setting. Am J Med. 2022;135(6):769–74. 10.1016/j.amjmed.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou P, Zou Z, Wu W, et al. The gut-lung axis in critical illness: microbiome composition as a predictor of mortality at day 28 in mechanically ventilated patients. BMC Microbiol. 2023;23(1):399. 10.1186/s12866-023-03078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin-Loeches I, Dickson R, Torres A, et al. The importance of airway and lung microbiome in the critically ill. Crit Care. 2020;24(1):537. 10.1186/s13054-020-03219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Liu W, Shi W, et al. A Nomogram With Six Variables Is Useful to Predict the Risk of Acquiring Carbapenem-Resistant Microorganism Infection in ICU Patients. Front Cell Infect Microbiol. 2022;12:852761. 10.3389/fcimb.2022.852761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao Q, Feng Z, Lin H, et al. Carbapenem-resistant gram-negative bacterial infection in intensive care unit patients: Antibiotic resistance analysis and predictive model development. Front Cell Infect Microbiol. 2023;13:1109418. 10.3389/fcimb.2023.1109418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang Q, Zhao Q, Xu X, et al. Early prediction of carbapenem-resistant Gram-negative bacterial carriage in intensive care units using machine learning. J Glob Antimicrob Resist. 2022;29:225–31. 10.1016/j.jgar.2022.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Mitgang EA, Hartley DM, Malchione MD, et al. Review and mapping of carbapenem-resistant Enterobacteriaceae in Africa: Using diverse data to inform surveillance gaps. Int J Antimicrob Agents. 2018;52(3):372–84. 10.1016/j.ijantimicag.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Jean SS, Harnod D, Hsueh PR. Global Threat of Carbapenem-Resistant Gram-Negative Bacteria. Front Cell Infect Microbiol. 2022;12:823684. 10.3389/fcimb.2022.823684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biagetti C, Tatarelli P, Tebano G, et al. Containment of carbapenem-resistant Enterobacterales colonisations and infections: Results from an integrated infection control intervention in a large hospital trust of northern Italy. Am J Infect Control. 2024;52(1):66–72. 10.1016/j.ajic.2023.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Junior APN, Del Missier GM, Praça APA, et al. In-hospital mortality and one-year survival of critically ill patients with cancer colonized or not with carbapenem-resistant gram-negative bacteria or vancomycin-resistant enterococci: an observational study. Antimicrob Resist Infect Control. 2023;12(1):8. 10.1186/s13756-023-01214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dantas LF, Dalmas B, Andrade RM, et al. Predicting acquisition of carbapenem-resistant Gram-negative pathogens in intensive care units. J Hosp Infect. 2019;103(2):121–7. 10.1016/j.jhin.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Willems RPJ, van Dijk K, Vehreschild M, et al. Incidence of infection with multidrug-resistant Gram-negative bacteria and vancomycin-resistant enterococci in carriers: a systematic review and meta-regression analysis. Lancet Infect Dis. 2023;23(6):719–31. 10.1016/s1473-3099(22)00811-8. [DOI] [PubMed] [Google Scholar]
- 36.Tacconelli E, Mazzaferri F, de Smet AM, et al. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant Gram-negative bacteria carriers. Clin Microbiol Infect. 2019;25(7):807–17. 10.1016/j.cmi.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Chi X, Meng X, Xiong L, et al. Small wards in the ICU: a favorable measure for controlling the transmission of carbapenem-resistant Klebsiella pneumoniae. Intensive Care Med. 2022;48(11):1573–81. 10.1007/s00134-022-06881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian C, Wu Q, Ruan Z, et al. A Visualized Mortality Prediction Score Model in Hematological Malignancies Patients with Carbapenem-Resistant Organisms Bloodstream Infection. Infect Drug Resist. 2023;16:201–15. 10.2147/idr.S393932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho KW, Ng WT, Ip M, et al. Active surveillance of carbapenem-resistant Enterobacteriaceae in intensive care units: Is it cost-effective in a nonendemic region? Am J Infect Control. 2016;44(4):394–9. 10.1016/j.ajic.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 40.Zaidah AR, Mohammad NI, Suraiya S, et al. High burden of Carbapenem-resistant Enterobacteriaceae (CRE) fecal carriage at a teaching hospital: cost-effectiveness of screening in low-resource setting. Antimicrob Resist Infect Control. 2017;6:42. 10.1186/s13756-017-0200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenkins DR, Auckland C, Chadwick C, et al. A practical approach to screening for carbapenemase-producing Enterobacterales- views of a group of multidisciplinary experts from English hospitals. BMC Infect Dis. 2024;24(1):444. 10.1186/s12879-024-09307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.