Abstract
Background
Immunochemotherapy with pembrolizumab has been integrated into clinical practice as part of the standard-of-care for non-metastatic triple-negative breast cancer (TNBC) with high risk. We conducted a real-world study in TNBC patients treated with neoadjuvant chemotherapy to compare pathologic complete response (pCR) rates relative to stromal tumor-infiltrating lymphocytes (sTIL) across different regimens: non-carboplatin, carboplatin-, and pembrolizumab-chemotherapy.
Patients and methods
We analyzed a cohort of 450 patients with TNBC who underwent surgery following neoadjuvant chemotherapy between March 2007 and February 2024. Treatment groups included 247 non-carboplatin, 120 carboplatin, and 83 pembrolizumab-chemotherapy recipients. sTIL was evaluated in biopsied samples. Lymphocyte-predominant breast cancer (LPBC) was defined as tumors with high sTIL (≥ 50%).
Results
The pCR rates were 32% in the non-carboplatin-, 57% in the carboplatin-, and 64% in the pembrolizumab-chemotherapy group. Ninety-two patients (20.4%) had LPBC. In LPBC, the pCR rates did not increase with the addition of carboplatin (50.0% in the non-carboplatin and 41.7% in carboplatin) but reached 83.3% with the addition of pembrolizumab and carboplatin. Among the non-LPBC, the pCR rate increased from 26.7 to 61.1% with the addition of carboplatin, but there was no difference in the pCR rate between the carboplatin and pembrolizumab groups (61.1% and 61.2%, respectively).
Conclusions
In LPBC patients, the addition of carboplatin did not result in an elevated pCR rate; however, the addition of pembrolizumab tended to raise the pCR rate. In non-LPBC, the addition of carboplatin significantly increased the pCR rate, while the addition of pembrolizumab did not have the same effect.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-024-01944-0.
Introduction
Since neoadjuvant pembrolizumab combined with chemotherapy has been shown to increase pathologic complete response (pCR) rates [1], and subsequent administration of adjuvant pembrolizumab post-surgery has extended event-free survival (EFS) compared to neoadjuvant chemotherapy alone in patients with early triple-negative breast cancer (TNBC) [2], immunochemotherapy including pembrolizumab has become the standard of care for managing early TNBC patients at high risk.
Prior to the era of immunochemotherapy with pembrolizumab, anthracycline- and taxane-based (A-T) chemotherapy regimens were the preferred treatment approach in TNBC [3]. Furthermore, investigators endeavored to enhance clinical outcomes by incorporating carboplatin into the A-T regimen. Previous trials have commonly shown that the addition of carboplatin to A-T increases the rate of pCR [4–6], although improvements in EFS were inconsistent [6–8]. Despite the controversy surrounding the addition of carboplatin to A-T, carboplatin-containing chemotherapy has been the most preferred polychemotherapy regimen for TNBC with high risk [1].
Personalizing neoadjuvant chemotherapy with or without pembrolizumab remains a significant challenge in early-stage TNBC, largely due to the limited availability of robust biomarkers. While the KEYNOTE-522 trial demonstrated benefits of pembrolizumab in improving pathological complete response (pCR) and event-free survival (EFS) regardless of PD-L1 expression [9, 10], this does not confirm that all patients derived benefit. Given the substantial clinical burden of immunotherapy-related toxicities [11] and the associated financial costs, there is an urgent need to develop reliable biomarkers to tailor treatment regimens. Compelling evidence suggests that high levels of stromal tumor-infiltrating lymphocytes (sTILs) in pre-treatment biopsy samples are strongly correlated with higher pCR rates in TNBC patients undergoing neoadjuvant chemotherapy [12–17]. However, the role of sTILs to inform treatment decisions regarding the addition or omission of pembrolizumab in early TNBC remains inadequately explored.
In this study, we conducted a real-world study in TNBC patients treated with neoadjuvant chemotherapy, comparing pCR rates relative to sTIL levels across different neoadjuvant chemotherapy regimens: (i) non-carboplatin-chemotherapy (ii) carboplatin-chemotherapy, and (iii) pembrolizumab-chemotherapy.
Patients and methods
Study population
In this retrospective cohort study, we included 450 non-metastatic TNBC patients treated with neoadjuvant chemotherapy at Gangnam Severance Hospital, Seoul, Republic of Korea. All patients underwent curative surgery after neoadjuvant chemotherapy between March 2007 and February 2024. Chemotherapy regimen was chosen as per local guidelines at the time of diagnosis. We excluded the patients diagnosed with recurrent or metachronous BC. This study included the patients at clinical stage IIIC. Staging was performed according to the 8th edition of the American Joint Committee on Cancer system, and histologic grading was conducted using the Elston-Ellis modification of the Scarff-Bloom-Richardson grade.
TNBC was defined as estrogen receptor (ER)-negative and progesterone receptor (PR)–negative according to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists using immunohistochemistry results of tumor. HER2 negativity was determined by IHC (score 0 or 1+) or by fluorescent silver in situ hybridization for cases with an IHC score of 2+. Clinical stage information was determined through physical examination and imaging studies, including mammography, breast ultrasonography, and breast magnetic resonance imaging. To rule out distant metastasis, abdominal and chest computed tomography scans, and bone scans, with/without PET-CT, were performed. Information regarding ER, PR, HER2 status, histologic grade, and sTILs was collected using biopsied samples before neoadjuvant chemotherapy. Germline BRCA1/2 mutation status was assessed in whole blood samples. Unknown information was treated as missing values. Pathological complete response (pCR) was defined as ypT0/is N0. The study was approved by the Institutional Review Board, adhering to good clinical practice guidelines under the Declaration of Helsinki.
Chemotherapy regimen
Among the study population, 247 (54.9%) patients received the non-carboplatin-chemotherapy, 120 (26.7%) received the carboplatin-chemotherapy, and 83 (18.4%) received the pembrolizumab-chemotherapy [1].
The non-carboplatin group included anthracycline-cyclophosphamide followed by taxane regimen (AC/T), and concurrent anthracycline plus taxane regimen, or anthracycline alone or taxane alone regimen. The carboplatin-chemotherapy group included patients with anthracycline-cyclophosphamide followed by taxane-carboplatin regimen or taxane-carboplatin followed by anthracycline-cyclophosphamide regimen. Patients in the pembrolizumab-chemotherapy group were all treated with the KN-522 regimen (8 cycles of pembrolizumab 200 mg, given with 12 cycles of 1 week (W) paclitaxel and 4 cycles of 3 W carboplatin, followed by 4 cycles of 3 W doxorubicin and cyclophosphamide. Five patients were unable to complete 8 cycles of chemotherapy due to adverse effects. In this study, patients who received at least one dose of pembrolizumab were included in the pembrolizumab-chemotherapy group for analysis. Our study population was summarized in Supplementary Fig. 1.
Stromal tumor-infiltrating lymphocytes
sTILs were evaluated in biopsied samples before neoadjuvant chemotherapy based on hematoxylin and eosin-stained slides following the protocol by the TIL international working group [18]. We previously reported that sTILs of biopsied samples were correlated with that of surgical specimens, allowing evaluation in biopsied samples [19]. The average sTIL levels was counted from all available tumor area for each case, and the average percentage score was reported. In this study, lymphocyte-predominant breast cancer (LPBC) was defined as tumors having high sTIL levels (≥ 50%) [20, 21]. Non-LPBC tumors were classified into two groups; (i) low sTIL (≤ 10%) and (ii) intermediate sTILs (11-49%) [13].
Statistics
Continuous variables were compared using the Mann–Whitney U test and Student’s t-test. Kolmogorov–Smirnov tests were used to assess the normal distribution of the continuous variables. Nominal variables were compared using χ2 or Fisher’s exact tests. Multivariable binary logistic regression analysis was performed to identify predictive factors for pCR. The variables with p-value < 0.05 were included in the multivariable model, and the stepwise backward Wald method was used to arrive at the final model. All statistical analyses were performed using SPSS program version 27.0 (SPSS Inc., Chicago, USA) and R software (https://www.r-projet.org; version 3.6.1). A p-value < 0.05 was considered statistically significant.
Results
Study population
We identified 450 patients with TNBC who underwent neoadjuvant chemotherapy followed by curative surgery. Baseline demographics and tumor characteristics are described in Table 1. Among these patients, 185 (41.4%) were aged ≥ 50 years, and 188 (41.8%) had histologic grade III tumors. Of these, germline BRCA1/2 mutation testing was conducted in 264 patients, with mutations identified in 58 (22.0%) of them. Most of the patients had clinical T2 or T3 (399/450 [88.6%]) or clinically node-positive tumors (367/450 [81.6%]). Evaluation of sTILs in biopsy specimens was conducted in 369 patients (82.0%), revealing a median sTILs level of 20% (interquartile range [IQR], 3–40%).
Table 1.
Baseline characteristics of patients according to neoadjuvant chemotherapy regimen (n = 450)
| Non-carboplatin (N = 247) | Carboplatin (N = 120) | Pembrolizumab (N = 83) | Total (N = 450) | P | |
|---|---|---|---|---|---|
| Age, n (%) | 0.630 | ||||
| ≤ 50 | 143 (57.9) | 75 (62.5) | 47 (56.6) | 265 (58.9) | |
| > 50 | 104 (42.1) | 45 (37.5) | 36 (43.4) | 185 (41.4) | |
| Germline BRCA1/2 mutation* | 0.090 | ||||
| No | 86 (81.9) | 64 (70.3) | 56 (82.4) | 206 (78.0) | |
| Yes | 19 (18.1) | 27 (29.7) | 12 (17.6) | 58 (22.0) | |
| Clinical tumor stage | 0.156 | ||||
| 1 | 14 (5.7) | 7 (5.8) | 6 (7.2) | 27 (6.0) | |
| 2 | 163 (66.0) | 91 (75.8) | 62 (74.7) | 316 (70.2) | |
| 3 | 51 (20.6) | 18 (15.0) | 14 (16.9) | 83 (18.4) | |
| 4 | 19 (7.7) | 4 (3.3) | 1 (1.2) | 24 (5.3) | |
| Clinical nodal stage | 0.280 | ||||
| 0 | 47 (19.0) | 25 (20.8) | 11 (13.3) | 83 (18.4) | |
| 1 | 100 (40.5) | 51 (42.5) | 43 (51.8) | 194 (43.1) | |
| 2 | 87 (35.2) | 36 (30.0) | 21 (25.3) | 144 (32.0) | |
| 3 | 13 (5.3) | 8 (6.7) | 8 (9.6) | 29 (6.4) | |
| Clinical Stage, n (%) | 0.133 | ||||
| I, IIA | 49 (19.8) | 24 (20.0) | 13 (15.7) | 86 (19.1) | |
| IIB | 75 (30.4) | 41 (34.2) | 37 (44.6) | 153 (34.0) | |
| IIIA | 93 (37.7) | 43 (35.8) | 24 (28.9) | 160 (35.6) | |
| IIIB | 17 (6.9) | 4 (3.3) | 1 (1.2) | 22 (4.9) | |
| IIIC | 13 (5.3) | 8 (6.7) | 8 (9.6) | 29 (6.4) | |
| Histologic grade*, n (%) | 0.369† | ||||
| 1 | 4 (2.2) | 0 | 1 (1.3) | 5 (1.3) | |
| 2 | 85 (47.0) | 53 (46.5) | 44 (55.0) | 182 (48.5) | |
| 3 | 92 (50.8) | 61 (53.5) | 35 (43.8) | 188 (48.5) | |
| sTILs, median (range) | 20 (10–60) | 20 (3–40) | 10 (3–25) | 20 (3–40) | < 0.001 |
| sTILs*, % | 0.009 | ||||
| < 50 | 120 (68.2) | 90 (78.9) | 67 (84.8) | 277 (75.1) | |
| ≥ 50 | 56 (31.8) | 24 (21.1) | 12 (15.2) | 92 (24.9) | |
| Surgery type | 0.512 | ||||
| Lumpectomy | 137 (55.5) | 74 (61.7) | 49 (59.0) | 260 (57.8) | |
| Mastectomy | 110 (44.5) | 46 (38.3) | 34 (41.0) | 190 (42.2) |
*Missing values †P-values were obtained with the Fisher’s exact test. sTILs, stromal tumor-infiltrating lymphocytes
Based on the treatment regimen, the patients were divided as follows: non-carboplatin-chemotherapy group (247 patients [54.9%]), carboplatin-chemotherapy group (120 patients [26.7%]), and pembrolizumab-chemotherapy group (83 patients [18.4%]). The prevalence of LPBC (sTILs ≥ 50%) varied across three groups, with higher frequencies observed sequentially in the non-carboplatin (56/247 [31.8%]), carboplatin (24/114 [21.1%]), and pembrolizumab groups (12 /89 [15.2%]). Other clinicopathological factors did not significantly differ among the three groups. In addition, among patients with available sTILs data, baseline features were comparable across treatment regimens (Supplementary Table 1).
Pathologic complete response rates according to the regimens
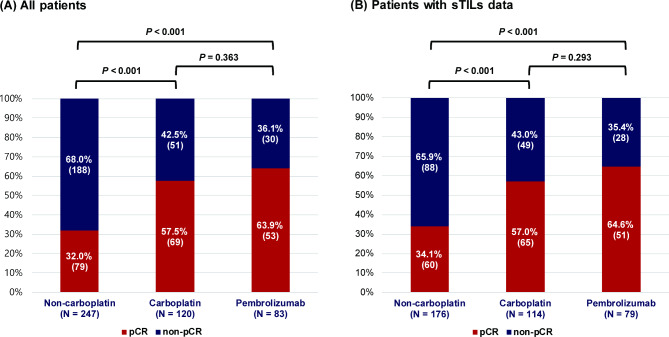
Of 450 patients, 201 (44.7%) achieved pCR after neoadjuvant systemic treatments. Specifically, pCR rates were 32.4% (79/247) in the non-carboplatin chemotherapy group, 57.5% (69/120) in the carboplatin-chemotherapy group, and 63.9% (53/83) in the pembrolizumab-chemotherapy group (Fig. 1A). The pCR rate of the non-carboplatin-chemotherapy group was significantly lower than those of the carboplatin- and pembrolizumab-chemotherapy groups, respectively. However, no statistical difference in pCR rate was noted between the carboplatin- and pembrolizumab-chemotherapy group (P = 0.363).
Fig. 1.
Pathologic complete response (pCR) rate according to neoadjuvant treatment regimens (A) in all patients and (B) patients with available stromal tumor-infiltrating lymphocytes (sTILs) data
These outcomes of pCR according to the regimens were consistent in the subset of 369 patients evaluated for sTILs: pCR rates were 34.1% (60/176) in the non-carboplatin-chemotherapy group, 57.0% (65/114) in the carboplatin regimen group, and 64.6% (51/79) in the pembrolizumab-chemotherapy group (Fig. 1B).
Multivariable analysis showed that the neoadjuvant chemotherapy regimen was an independent factor for pCR. Compared to the non-carboplatin regimen, the carboplatin (adjusted odds ratio [OR], 2.73; 95% CI, 1.64–4.57; P < 0.001) and pembrolizumab (adjusted OR, 4.00; 95% CI, 2.20–7.16; P < 0.001) regimens were significantly associated with increased odds of achieving pCR (Table 2). However, in this analysis, high TILs (≥ 50%) was not demonstrated as an independent factor for pCR (Table 2).
Table 2.
Odds ratio (OR) and 95% confidence interval (CI) for pathologic complete response in 369 patients with available sTILs
| Univariable analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | adjusted OR | 95% CI | P | ||
| Age | |||||||
| ≤ 50 | Ref. | Ref. | |||||
| > 50 | 0.69 | 0.46–1.04 | 0.079 | 0.80 | 0.51–1.26 | 0.341 | |
| Histologic grade | 0.074 | 0.175 | |||||
| 1 | Ref. | Ref. | |||||
| 2 | 3.01 | 0.33–27.48 | 0.329 | 2.17 | 0.21–22.13 | 0.512 | |
| 3 | 4.55 | 0.50–41.50 | 0.179 | 3.20 | 0.31–32.78 | 0.327 | |
| Clinical tumor stage | 0.126 | 0.578 | |||||
| 1 | Ref. | Ref. | |||||
| 2 | 0.94 | 0.41–2.13 | 0.876 | 0.83 | 0.35–1.96 | 0.666 | |
| 3 | 0.62 | 0.25–1.59 | 0.322 | 0.67 | 0.25–1.78 | 0.417 | |
| 4 | 0.25 | 0.06–1.13 | 0.071 | 0.37 | 0.08–1.81 | 0.220 | |
| Clinical nodal stage | 0.555 | ||||||
| 0 | Ref. | Ref. | |||||
| 1 | 1.15 | 0.66–2.01 | 0.612 | 0.90 | 0.50–1.64 | 0.730 | |
| 2 | 0.78 | 0.43–1.40 | 0.399 | 0.67 | 0.35–1.28 | 0.227 | |
| 3 | 0.73 | 0.30–1.77 | 0.482 | 0.64 | 0.241.72 | 0.379 | |
| sTILs | |||||||
| < 50% | Ref. | Ref. | |||||
| ≥ 50% | 1.27 | 0.79–2.04 | 0.322 | 1.60 | 0.95–2.69 | 0.079 | |
| Regimen | < 0.001 | ||||||
| Non-carboplatin | Ref. | Ref. | |||||
| Carboplatin | 2.57 | 1.58–4.16 | < 0.001 | 2.73 | 1.64–4.53 | < 0.001 | |
| Pembrolizumab | 3.52 | 2.02–6.14 | < 0.001 | 4.00 | 2.20–7.16 | < 0.001 | |
sTILs, stromal tumor-infiltrating lymphocytes
Pathologic complete response rates of the regimen groups in relation to sTILs
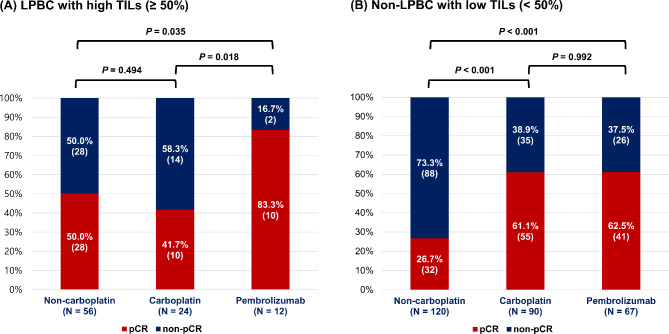
In the cohort with available sTILs data, 24.9% (92/369) were classified as LPBC (sTILs ≥ 50%), and 75.1% (277/369) were classified as non-LPBC (sTILs < 50%). Within the patients with LPBC, the pCR rates for the non-carboplatin- (28/56 [50.0%]) and carboplatin- (10/24 [41.7%]) chemotherapy groups were similar, whereas the pCR rate for the pembrolizumab-chemotherapy group (10/12 [83.3%]) was significantly higher compared to the other two groups (Fig. 2A). In the multivariable analysis (Table 3), the pembrolizumab-chemotherapy group (adjusted OR, 6.40; 95% CI, 1.09–37.47; P = 0.040) showed a significant increase in the pCR rate compared to those in the non-carboplatin group, whereas the carboplatin group showed no difference from the non-carboplatin group (adjusted OR, 1.01; 95% CI, 0.31–3.26; P = 0.986).
Fig. 2.
Pathologic complete response (pCR) rate according to neoadjuvant treatment regimens stratified by stromal tumor-infiltrating lymphocytes (sTILs): (A) in lymphocyte-predominant breast cancer (LPBC) with high sTILs (≥ 50%), and (B) in non-LPBC with low sTILs (< 50%)
Table 3.
Odds ratio (OR) and 95% confidence interval (CI) of treatment regimen for pathologic complete response stratified by stromal tumor-infiltrating lymphocytes
| Cohort | Regimen | Multivariable analysis | |||
| adjusted OR* | 95% CI | P | |||
| All patients with sTILs | LPBC | Non-carboplatin | Ref. | ||
| Carboplatin | 1.01 | 0.31–3.26 | 0.986 | ||
| Pembrolizumab | 6.40 | 1.09–37.47 | 0.040 | ||
| Non-LPBC | Non-carboplatin | Ref. | |||
| Carboplatin | 4.09 | 2.24–7.48 | < 0.001 | ||
| Pembrolizumab | 4.47 | 2.30–8.67 | < 0.001 | ||
| Cohort | Regimen | Multivariable analysis | |||
| adjusted OR† | 95% CI | P | |||
| cN + patients with sTILs | LPBC | Non-carboplatin | Ref. | ||
| Carboplatin | 0.98 | 0.30–3.23 | 0.977 | ||
| Pembrolizumab | 11.45 | 1.17-112.43 | 0.036 | ||
| Non-LPBC | Non-carboplatin | Ref. | |||
| Carboplatin | 4.36 | 2.17–8.78 | < 0.001 | ||
| Pembrolizumab | 3.94 | 1.91–8.13 | < 0.001 | ||
*Adjusted for age (≤ 50 vs. > 50), histologic grade (1 vs. 2 vs. 3), clinical tumor stage (1 vs. 2 vs. 3 vs. 4), and clinical nodal stage (0 vs. 1 vs. 2 vs. 3). †Adjusted for age (≤ 50 vs. > 50), histologic grade (1 vs. 2 vs. 3), clinical tumor stage (1 vs. 2 vs. 3 vs. 4), and clinical nodal stage (1 vs. 2 vs. 3). sTILs, stromal tumor-infiltrating lymphocytes; LPBC, lymphocyte-predominant breast cancer. Supplemental Table 1. Baseline characteristics of patients according to neoadjuvant chemotherapy regimen with baseline sTIL values (n = 369)
Among the patients with non-LPBC, the non-carboplatin-chemotherapy group (32/120 [26.7%]) had a significantly lower pCR rate compared to the other groups, while the carboplatin- (55/90 [61.1%]) and pembrolizumab- (41 of 67 [62.5%]) chemotherapy groups had similar pCR rates (Fig. 2B). The multivariable analysis showed that both the carboplatin (adjusted OR, 4.36; 95% CI, 2.17–8.78; p < 0.001) and pembrolizumab (adjusted OR, 3.94; 95% CI, 1.91–8.13; p < 0.001) groups significantly increased pCR rate compared the non-carboplatin group (Table 3).
Consistent outcomes were noted in the subset of 295 clinically node-positive disease. Among LPBC patients, the pembrolizumab-chemotherapy group demonstrated a significantly higher pCR rate (9/10 [90.0%]) than the other groups. In non-LPBC patients, the non-carboplatin-chemotherapy group exhibited a lower pCR rate compared to the carboplatin- and pembrolizumab-chemotherapy groups (Supplementary Fig. 2). The multivariable analysis confirmed these findings, indicating that the pembrolizumab-chemotherapy regimen was associated with a higher likelihood of pCR in LPBC compared to the other two groups (Table 3). However, the probability of pCR did not differ between the carboplatin- and pembrolizumab-chemotherapy groups in non-LPBC.
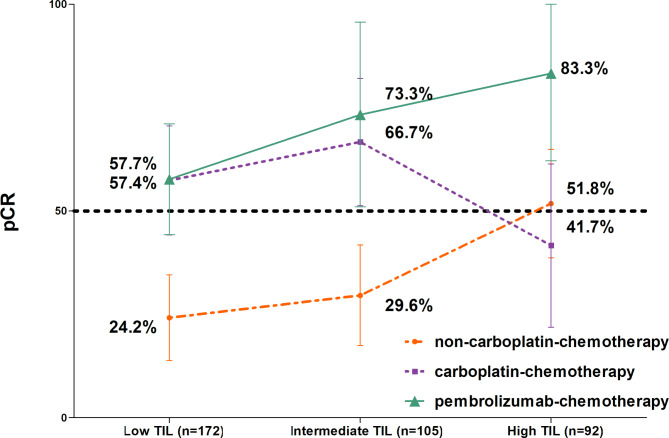
Lastly, we divided the non-LPBC group into two subgroups based on TIL levels (low [sTIL ≤ 10%] and intermediate [sTIL, 11–49%]) and compared the pCR rates of each regimen among the three groups defined by sTILs. In this analysis, the pCR rate for the pembrolizumab-chemotherapy group showed a consistent increase across the TILs subgroups: 57.7% in the low-, 73.3% in the intermediate-, and 83.3% in the high (Fig. 3). However, the pCR rates for the carboplatin-chemotherapy group were 57.4% and 66.7% in the low and intermediate groups, respectively, but dropped to 41.7% in the high group. The pCR rates for the non-carboplatin chemotherapy group were low at 24.2% and 29.6% in the low and intermediate groups, respectively, but increased to 51.8% in the high group.
Fig. 3.
Changes in pathologic complete response (pCR) rate according to stromal tumor-infiltrating lymphocytes (sTILs) level in each neoadjuvant treatment regimen
Discussion
While our real-world data represent the early experiences following the introduction of pembrolizumab, we observed a numerical increase in pCR rates across different regimens, consistent with the results of the KeyNote-522 study: non-carboplatin-chemotherapy, 32.4%; carboplatin- chemotherapy, 57.5%; pembrolizumab- chemotherapy, 67.8%. Furthermore, in TNBC cases with high sTILs, referred as to LPBC, the addition of pembrolizumab to chemotherapy resulted in a pCR rate exceeding 80%, whereas the incorporation of carboplatin into AC/T chemotherapy did not enhance the response. Conversely, in non-LPBC, the inclusion of carboplatin in AC/T significantly enhanced the pCR rate. However, the introduction of pembrolizumab did not demonstrate additional efficacy in this subgroup.
When the patients were classified into three groups by sTIL level (Fig. 3), the pembrolizumab-chemotherapy group correlated with increased pCR rates in higher TILs subgroups, whereas carboplatin-chemotherapy group did not show this positive trend. In addition, the non-carboplatin-chemotherapy group exhibited a trend of rising pCR rates corresponding to increasing TILs; however, these rates remained noticeably lower than those observed in the pembrolizumab-chemotherapy group. The analysis demonstrates that pembrolizumab-chemotherapy is particularly effective, and its efficacy appears to be related to sTIL levels, suggesting a potential immunological synergy. As supporting evidence, the KEYNOTE-086 trial showed that linear with increasing sTILs is related to the efficacy of pembrolizumab monotherapy in advanced TNBC [22].
It is well established that TNBC tumors exhibit a tumor microenvironment (TME) enriched with immune cells, characterized by high levels of sTILs, PD-L1-positivity and up-regulated immune gene signatures [16, 17, 23, 24]. These immune biomarkers exhibit correlations [16, 24]. Presently, in advanced TNBC, pembrolizumab is exclusively indicated for patients with high PD-L1 expression, as identified by the 22C3 pharmDx assay with a cutoff of a combined positive score (CPS) of 10 [25, 26]. However, in early TNBC, pembrolizumab is administered to all patients regardless of PD-L1 status [1, 2]. Nevertheless, pembrolizumab-based immunochemotherapy achieved a pCR rate of 68.9% in the PD-L1-positive group compared to 45.3% in the PD-L1-negative group [1]. Considering positive correlation between PD-L1 status and TILs level [24, 27], these findings suggest that pembrolizumab-based immunochemotherapy may enhance response in TNBC with a more immunogenic TME.
Although in the KEYNOTE-522 trial, the application of pembrolizumab is recommended for all patients with stage II-III TNBC regardless of immune status [1], our findings suggest that the application of pembrolizumab should be more actively considered in patients with high sTILs within this population. Furthermore, among non-LPBC patients, those with non-pCR remain at high risk, irrespective of the neoadjuvant chemotherapy regimen used. The application of novel antibody-drug conjugates or sequential or combination therapies with currently available agents such as capecitabine, pembrolizumab, or olaparib can be considered for these high-risk patients [28].
The pronounced effect of pembrolizumab in LPBC suggests several implications for treatment de-escalation strategies. First, it highlights the potential feasibility of omitting anthracyclines or reducing the duration of chemotherapy in neoadjuvant regimens that incorporate pembrolizumab for LPBC. In the Neo-PACT trial, which evaluated the efficacy of an anthracycline-free neoadjuvant regimen of carboplatin and docetaxel combined with pembrolizumab in early TNBC, high sTIL as biomarkers for the degree of immune enrichment was predictive of pCR [16]. Additionally, these findings support that the efficacy of pembrolizumab in TNBC is positively correlated with sTIL levels and may reach its maximum in cases classified as LPBC [20, 21, 29], as observed in our study. Ongoing trials are exploring immunochemotherapy with de-escalation of backbone chemotherapy, such as anthracyclines-free regimen or 4 cycles taxane-platinum [30, 31], based on sTIL levels.
A second consideration is the potential for de-escalation of adjuvant pembrolizumab. Based on the design of the KEYNOTE-522 trial, current guidelines recommend continuation of pembrolizumab in the adjuvant setting regardless of treatment response or immune-related biomarkers. However, the updated analysis from the KEYNOTE-522 trial demonstrated no differences in EFS or overall survival between treatment arms in patients who achieved pCR [9, 10]. Moreover, pooled data from two studies revealed excellent survival outcomes in patients with high sTILs who achieved pCR following anthracycline-free neoadjuvant chemotherapy [32]. In line with this, for stage I TNBC with high sTIL levels, omission of adjuvant chemotherapy has been proposed due to the low recurrence risk [33, 34]. These findings collectively suggest that adjuvant pembrolizumab de-escalation based on sTIL levels may be a promising approach [28], warranting further investigation.
Intriguingly, we noted that carboplatin increased pCR rate in non-LPBC (sTIL < 50%). While high sTIL levels were associated with pCR in neoadjuvant chemotherapy trials with carboplatin in TNBC, it remains unknown whether carboplatin addition can elevate pCR in non-LPBC.
Among previous trials, the GeparSixto study demonstrated a pCR rate of 74% with carboplatin compared to 43% without carboplatin in LPBC (sTIL ≥ 60%), while in non-LPBC cases, the rates were 46% with carboplatin and 34% without carboplatin, respectively [4]. In contrast, the study by Dieci MV et al. showed that carboplatin significantly increased the pCR rate in non-LPBC (sTIL < 60%) [35]. The discrepancy between the two studies may be partly explained by differences in backbone chemotherapy regimens; the GeparSixto study utilized weekly paclitaxel and liposomal doxorubicin for 18 weeks, whereas the study by Dieci MV et al. employed AC followed by weekly paclitaxel for 24 weeks. Further investigations are necessary to fully understand the distinct response patterns to incorporation of carboplatin based on TIL levels in TNBC. Additionally, ongoing trials could assess the potential of novel agents, such as TROP2-targeting antibody-drug conjugates, to enhance response rates in non-LPBC cases [36].
Our study has several limitations. First, our study represents early-phase real-world outcomes following the implementation of pembrolizumab-based immunochemotherapy for early-stage TNBC, which has led to a relatively small cohort in the pembrolizumab group, with an even smaller subset of high sTILs cases. Second, survival outcomes were not analyzed in this study, primarily because patients treated with pembrolizumab received the therapy relatively recently, with a median follow-up of only 9 months. Third, valuable clinical factors such as the PD-L1 CPS and residual cancer burden (RCB) class were not evaluated in relation to sTILs, as these indicators were not assessed in tumor samples collected a decade ago. A comprehensive analysis exploring the impact of various immune-related biomarkers on clinical outcomes, including pCR, RCB, and survival, will be necessary in future studies.
Concerns have been raised regarding intraobserver and interobserver heterogeneity in the assessment of sTILs. To address these challenges, an online educational resource (www.tilsinbreastcancer.org/pitfalls) has been developed [37], and efforts to implement automated assessment have been initiated [38]. Nevertheless, in our study, sTILs were evaluated by a specialized pathologist with eight years of experience in sTIL assessment. Furthermore, a previous study demonstrated acceptable agreement in TIL scoring when applying the currently proposed guidelines [19, 39].
Despite these limitations, our study leveraged sTILs and addressed differential responses across three different regimens: carboplatin-based and non-carboplatin-based, as well as pembrolizumab immunochemotherapy. Our findings may provide a foundation for tailoring immunochemotherapy based on stromal TILs in TNBC.
In conclusion, our real-world data consistently demonstrates an increased pCR rate with the addition of carboplatin and pembrolizumab. In LPBC, the addition of carboplatin did not result in an elevated pCR rate. However, the addition of pembrolizumab tended to boost the pCR rate, surpassing 80%. On the other hand, among patients with non-LPBC, the addition of carboplatin significantly increased the pCR rate, while the addition of pembrolizumab did not have the same effect. Further efforts should be made to improve the response to pembrolizumab-chemotherapy for patients with low baseline sTIL levels.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Author contributions
Dr. Ahn and Cha (Co-corresponding authors) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: SG Ahn, YJ Cha. Data curation: Y Kook, SH Baek, JH Kim, SE Lee, SH Moon. Acquisition, analysis, or interpretation of data: SJ Bae, JH Kim, MJ Kim. Drafting of the manuscript: SJ Bae, JH Kim, MJ Kim. Critical revision of the manuscript for important intellectual content: J Jeong, SG Ahn, YJ Cha. Statistical analysis: SJ Bae, JH Kim, MJ Kim. Supervision: SG Ahn, YJ Cha. SJ Bae, JH Kim, MJ Kim contributed to this work equally.
Funding
This work was supported by grant RS-2024-00343001 from the National Research Foundation of Korea (NRF), funded by the Korean government. Additional support was provided by the Basic Science Research Program through the NRF, funded by the Ministry of Education (2021R1I1A1A01060338), and another NRF grant funded by the Korean government (NRF-2021R1G1A1093596). This study also received funding from a faculty research grant of the Department of Surgery, Yonsei University College of Medicine.
Data availability
Ahn SG and Cha YJ, co-corresponding authors, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board, adhering to good clinical practice guidelines under the Declaration of Helsinki. Informed consent was waived because of the retrospective nature of the study and the analysis used anonymous clinical data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Soong June Bae, Jee Hung Kim and Min Ji Kim are contributed to this work equally.
Contributor Information
Yoon Jin Cha, Email: yooncha@yuhs.ac.
Sung Gwe Ahn, Email: asg2004@yuhs.ac.
References
- 1.Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al. Pembrolizumab for early triple-negative breast Cancer. N Engl J Med. 2020;382(9):810–21. [DOI] [PubMed] [Google Scholar]
- 2.Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, et al. Event-free survival with Pembrolizumab in Early Triple-negative breast Cancer. N Engl J Med. 2022;386(6):556–67. [DOI] [PubMed] [Google Scholar]
- 3.Burstein HJ, Curigliano G, Loibl S, Dubsky P, Gnant M, Poortmans P, Colleoni M, Denkert C, Piccart-Gebhart M, Regan M, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30(10):1541–57. [DOI] [PubMed] [Google Scholar]
- 4.von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, Blohmer JU, Jackisch C, Paepke S, Gerber B, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(7):747–56. [DOI] [PubMed] [Google Scholar]
- 5.Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geyer CE, Sikov WM, Huober J, Rugo HS, Wolmark N, O’Shaughnessy J, Maag D, Untch M, Golshan M, Lorenzo JP, et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann Oncol. 2022;33(4):384–94. [DOI] [PubMed] [Google Scholar]
- 7.Loibl S, Weber KE, Timms KM, Elkin EP, Hahnen E, Fasching PA, Lederer B, Denkert C, Schneeweiss A, Braun S, et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann Oncol. 2018;29(12):2341–7. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd JH, Ballman K, Polley MC, Campbell JD, Fan C, Selitsky S, Fernandez-Martinez A, Parker JS, Hoadley KA, Hu Z, et al. CALGB 40603 (Alliance): long-term outcomes and genomic correlates of response and Survival after Neoadjuvant Chemotherapy with or without carboplatin and Bevacizumab in Triple-negative breast Cancer. J Clin Oncol. 2022;40(12):1323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pusztai L, Denkert C, O’Shaughnessy J, Cortes J, Dent R, McArthur H, Kümmel S, Bergh J, Park YH, Hui R, et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: exploratory analysis from KEYNOTE-522. Ann Oncol. 2024;35(5):429–36. [DOI] [PubMed] [Google Scholar]
- 10.Schmid P, Cortes J, Dent R, McArthur H, Pusztai L, Kümmel S, Denkert C, Park YH, Hui R, Harbeck N, et al. Overall survival with Pembrolizumab in Early-Stage Triple-negative breast Cancer. N Engl J Med. 2024;391(21):1981–91. [DOI] [PubMed] [Google Scholar]
- 11.Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–80. [DOI] [PubMed] [Google Scholar]
- 12.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983–91. [DOI] [PubMed] [Google Scholar]
- 13.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. [DOI] [PubMed] [Google Scholar]
- 14.Yam C, Yen EY, Chang JT, Bassett RL, Alatrash G, Garber H, Huo L, Yang F, Philips AV, Ding QQ, et al. Immune phenotype and response to Neoadjuvant Therapy in Triple-negative breast Cancer. Clin Cancer Res. 2021;27(19):5365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolberg-Liedtke C, Feuerhake F, Garke M, Christgen M, Kates R, Grischke EM, Forstbauer H, Braun M, Warm M, Hackmann J, et al. Impact of stromal tumor-infiltrating lymphocytes (sTILs) on response to neoadjuvant chemotherapy in triple-negative early breast cancer in the WSG-ADAPT TN trial. Breast Cancer Res. 2022;24(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma P, Stecklein SR, Yoder R, Staley JM, Schwensen K, O’Dea A, Nye L, Satelli D, Crane G, Madan R, et al. Clinical and biomarker findings of Neoadjuvant Pembrolizumab and Carboplatin Plus Docetaxel in Triple-negative breast Cancer: NeoPACT Phase 2 clinical trial. JAMA Oncol. 2024;10(2):227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma P, Kimler BF, O’Dea A, Nye L, Wang YY, Yoder R, Staley JM, Prochaska L, Wagner J, Amin AL, et al. Randomized Phase II Trial of Anthracycline-free and anthracycline-containing Neoadjuvant Carboplatin Chemotherapy Regimens in Stage I-III Triple-negative breast Cancer (NeoSTOP). Clin Cancer Res. 2021;27(4):975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cha YJ, Ahn SG, Bae SJ, Yoon CI, Seo J, Jung WH, Son EJ, Jeong J. Comparison of tumor-infiltrating lymphocytes of breast cancer in core needle biopsies and resected specimens: a retrospective analysis. Breast Cancer Res Treat. 2018;171(2):295–302. [DOI] [PubMed] [Google Scholar]
- 20.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31(7):860–7. [DOI] [PubMed] [Google Scholar]
- 21.Dieci MV, Mathieu MC, Guarneri V, Conte P, Delaloge S, Andre F, Goubar A. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol. 2015;26(8):1698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loi S, Salgado R, Schmid P, Cortes J, Cescon DW, Winer EP, Toppmeyer DL, Rugo HS, De Laurentiis M, Nanda R, et al. Association between Biomarkers and Clinical Outcomes of Pembrolizumab Monotherapy in patients with metastatic triple-negative breast Cancer: KEYNOTE-086 exploratory analysis. JCO Precis Oncol. 2023;7:e2200317. [DOI] [PubMed] [Google Scholar]
- 23.Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, Sher X, Liu XQ, Lu H, Nebozhyn M et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018;362(6411). [DOI] [PMC free article] [PubMed]
- 24.Ahn SG, Kim SK, Shepherd JH, Cha YJ, Bae SJ, Kim C, Jeong J, Perou CM. Clinical and genomic assessment of PD-L1 SP142 expression in triple-negative breast cancer. Breast Cancer Res Treat. 2021;188(1):165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–28. [DOI] [PubMed] [Google Scholar]
- 26.Cortes J, Rugo HS, Cescon DW, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Perez-Garcia J, Iwata H, et al. Pembrolizumab plus Chemotherapy in Advanced Triple-negative breast Cancer. N Engl J Med. 2022;387(3):217–26. [DOI] [PubMed] [Google Scholar]
- 27.Emens LA, Molinero L, Loi S, Rugo HS, Schneeweiss A, Diéras V, Iwata H, Barrios CH, Nechaeva M, Nguyen-Duc A, et al. Atezolizumab and nab-Paclitaxel in Advanced Triple-negative breast Cancer: biomarker evaluation of the IMpassion130 study. J Natl Cancer Inst. 2021;113(8):1005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kok M, Gielen RJ, Adams S, Lennerz JK, Sharma P, Loibl S, Reardon E, Sonke G, Linn S, Delaloge S, et al. Academic Uphill Battle to personalize treatment for patients with stage II/III triple-negative breast Cancer. J Clin Oncol. 2024;42(30):3523–9. [DOI] [PubMed] [Google Scholar]
- 29.Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of Tumor-infiltrating lymphocytes in breast Cancer subtypes: a systematic review. JAMA Oncol. 2016;2(10):1354–60. [DOI] [PubMed] [Google Scholar]
- 30.Study Details |. Shorter chemo-immunotherapy without anthracycline drugs for early-stage Triple Negative Breast Cancer | ClinicalTrials.gov. 2023.
- 31.Study Details | Neoadjuvant TIL-. and Response-Adapted Chemoimmunotherapy for TNBC | ClinicalTrials.gov. 2022.
- 32.Martín M, Yoder R, Salgado R, Del Monte-Millán M, Álvarez EL, Echavarría I, Staley JM, O’Dea AP, Nye LE, Stecklein SR, et al. Tumor-infiltrating lymphocytes refine outcomes in Triple-negative breast Cancer treated with Anthracycline-Free Neoadjuvant Chemotherapy. Clin Cancer Res. 2024;30(10):2160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leon-Ferre RA, Jonas SF, Salgado R, Loi S, de Jong V, Carter JM, Nielsen TO, Leung S, Riaz N, Chia S, et al. Tumor-infiltrating lymphocytes in Triple-negative breast Cancer. JAMA. 2024;331(13):1135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geurts VCM, Balduzzi S, Steenbruggen TG, Linn SC, Siesling S, Badve SS, DeMichele A, Ignatiadis M, Leon-Ferre RA, Goetz MP, et al. Tumor-infiltrating lymphocytes in patients with Stage I triple-negative breast Cancer untreated with chemotherapy. JAMA Oncol. 2024;10(8):1077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dieci MV, Carbognin L, Miglietta F, Canino F, Giorgi CA, Cumerlato E, Amato O, Massa D, Griguolo G, Genovesi E, et al. Incorporating weekly carboplatin in anthracycline and paclitaxel-containing neoadjuvant chemotherapy for triple-negative breast cancer: propensity-score matching analysis and TIL evaluation. Br J Cancer. 2023;128(2):266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Study Details | A Phase III Randomised Study to Evaluate. Dato-DXd and Durvalumab for Neoadjuvant/Adjuvant Treatment of Triple-Negative or hormone Receptor-low/HER2-negative breast Cancer | ClinicalTrials.gov. 2023.
- 37.Kos Z, Roblin E, Kim RS, Michiels S, Gallas BD, Chen W, van de Vijver KK, Goel S, Adams S, Demaria S, et al. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer. 2020;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vidal JM, Tsiknakis N, Staaf J, Bosch A, Ehinger A, Nimeus E, Salgado R, Bai Y, Rimm DL, Hartman J et al. The analytical and clinical validity of AI algorithms to score TILs in TNBC: can we use different machine learning models interchangeably? eClinicalMedicine 2024;78. [DOI] [PMC free article] [PubMed]
- 39.Swisher SK, Wu Y, Castaneda CA, Lyons GR, Yang F, Tapia C, Wang X, Casavilca SA, Bassett R, Castillo M, et al. Interobserver Agreement between pathologists assessing tumor-infiltrating lymphocytes (TILs) in breast Cancer using methodology proposed by the International TILs Working Group. Ann Surg Oncol. 2016;23(7):2242–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Ahn SG and Cha YJ, co-corresponding authors, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The data that support the findings of this study are available from the corresponding author, upon reasonable request.