Abstract
Background
As the population ages, chronic diseases, frailty, and physical-psychological multimorbidity (PP-MM) increase. However, the association between frailty and PP-MM remains unclear. This study aimed to investigate this relationship in middle-aged and elderly Chinese individuals.
Methods
This study used four waves of data from the Chinese Longitudinal Study of Health and Aging. The main measures included frailty by the frailty index(FI) constructed using 40 indicators. PP-MM was defined as the concurrent presence of two kinds of diseases (physical illness and psychological disorders). The relationship between FI and PP-MM was evaluated using COX risk regression models and restricted cubic spline (RCS) curves and P < 0.05 was considered statistically significant.
Results
This study included 10,707 subjects, and after adjusting for potential confounders, the HR was 3.01 (95% confidence interval (CI) = 2.05–4.23) for pre-frail and 6.11 (95% CI = 3.79–9.84) for frail. COX regression analysis indicated a potential association between FI and PP-MM progression. RCS analysis revealed that the risk of PP-MM prevalence increased faster with an FI between 0.10 and 0.25.
Conclusion
Our study suggests that FI is positively associated with the prevalence of PP-MM and that the pre-frail phase may be a better opportunity to implement interventions for PP-MM prevention, with early monitoring of FI to identify patients at high risk for PP-MM and to provide direction and rationale for preventing PP-MM.
Keywords: Frailty, Chronic illness, Depressive symptoms, Multimorbidity, Middle-aged and people
Introduction
As the population ages and lives longer, chronic diseases have become a significant global public health challenge [1]. Many people also experience multimorbidity, characterised by multiple health conditions [2]. Patients with chronic diseases may experience negative emotions, including depression. Due to the long-term nature of chronic diseases and their impact on quality of life, many patients with chronic diseases suffer from depression [3, 4], and the prevalence of depression in patients with chronic diseases has been estimated to be 9.3%–25% [5]. Chronic diseases are associated with a significantly higher risk of depression in middle-aged and elderly Chinese [6].
Multimorbidity has been associated with frailty [7]. Frailty—an emerging global health concern—is characterised by a decline in the functioning of multiple physiological systems and increased vulnerability to stressors [8]. It is commonly related to various adverse outcomes, including falls, disability, hospitalisation, and even death [9]. The National Institute for Health and Care Excellence and the British Geriatrics Society have emphasised the importance of identifying frail and multimorbid patients at higher risk of adverse outcomes and more likely to benefit from treatment [10]. These patients are at greater risk of adverse outcomes and are more likely to benefit from treatment optimisation.
The prevalence of multimorbidity and frailty increases with age. Research on frailty and multimorbidity has primarily focused on the elderly population [10–12]. However, as there are more middle-aged individuals than older individuals, there are more adults under 65 years of age with multiple long-term conditions than those aged 65 years [13]. To date, no study has assessed the correlation between frailty and the occurrence of physical-psychological multimorbidity (PP-MM) in Chinese middle-aged and older adults. This longitudinal study aimed to explore the concurrent trajectories of frailty and physical-mental multimorbidity in a national cohort of middle-aged and older adults in China and to quantify the dose–response relationship between frailty and PP-MM.
Materials and methods
Population and data sources
We used data from the China Health and Retirement Longitudinal Study (CHARLS). CHARLS was established in 2011 as a national longitudinal cohort survey on ageing. It was designed based on the US Health and Retirement Study [14] and used a multistage cluster sampling method to select participants and conduct a series of data collections. The Ethical Application for Collecting Human Subject Data in CHARLS was approved by the Biomedical Ethics Review Board of Peking University (IRB00001052-11015), and all participants provided written informed consent. Detailed information regarding the CHARLS data is available on their website at http://charls.pku.edu.cn/en.
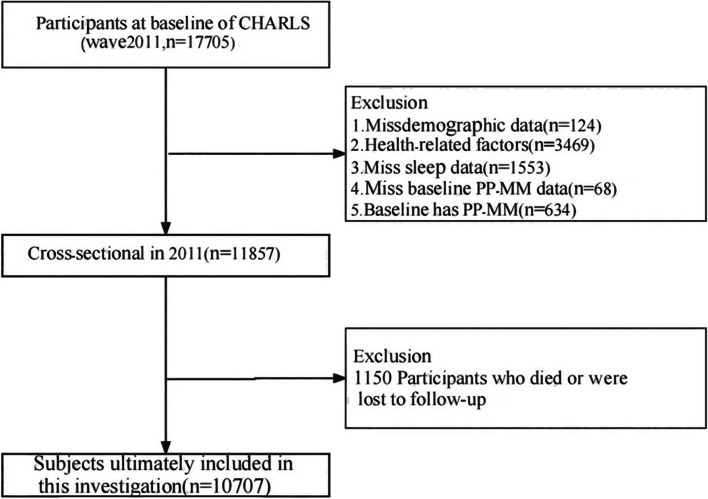
We conducted four rounds of the CHARLS core survey [2011 (baseline), 2013, 2015, and 2018], encompassing 17,708 participants. Of these, 7001 participants were excluded for the following reasons: having PP-MM at baseline (n = 634), missing baseline PP-MM data (n = 68), missing demographic data (n = 124), health-related factors (n = 3469), missing sleep data (n = 1553), and missing lifestyle data follow-up data (n = 1153). Ultimately, 10,707 participants were included in this study. Details of the selection process are displayed in Fig. 1.
Fig. 1.
The flow chart of participants selection process
Data collection
Data was methodically gathered through organised household interviews carried out by skilled staff. These interviews included demographic data, including age, gender, residential address, marital status, educational level and medical history, with a particular emphasis on chronic illnesses. In addition, comprehensive data were collected on health behaviours such as smoking status, alcohol consumption and body mass index (BMI). The inclusion of these behaviours in our study provides a more comprehensive understanding of the direct and indirect factors that contribute to PP-MM and frailty. Data on health behaviours, particularly BMI, were assessed by measuring an individual's weight and height (kg/m2), while information on smoking and drinking habits was obtained through self-reporting.The CHARLS data collection procedure adhered to the code of ethics established by the Research Ethics Committee of Peking University, which ensured that participant privacy and autonomy were preserved throughout the course of the study.
Assessment of frailty
The frailty index (FI) was constructed using the deficit accumulation model developed by Searle et al. This methodology takes advantage of the extensive data available in the CHARLS dataset and is in line with accepted research standards [15]. Indexes containing 30–40 variables are effective in predicting unfavourable health outcomes, according to previous research [16]. To construct the FI, we selected 40 items, including activities of daily living (11 items), functional limitations (9 items), diagnosis (14 items), disabilities (5 items), and self-reported health status. In order to create this index, we used a binary coding system where ‘0’ indicates no deficit and ‘1’ indicates a deficit. In cases where the variable contains intermediate response options (e.g. ‘sometimes’ or ‘maybe’), the value ‘0.5’ is assigned to reflect partial deficits. Using this approach, a more complex picture of an individual's health can be created. We defined the following three frailty state categories: non-frail (FI ≤ 0.10), pre-frail (FI > 0.10 to < 0.25), and frail (FI ≥ 0.25) [17].
Assessment of PP-MM
The occurrence of physical-psychological multimorbidity (PP-MM) was the outcome of this research. PP-MM was defined as the presence of two kinds of diseases (including physical illness, psychological disorders) [18].
Physical Illness: As in previous studies [19], a physical illness was identified if a physician informed the participant that they had at least one of the following twelve chronic diseases: hypertension, diabetes, dyslipidemia, heart disease, stroke, chronic lung disease, asthma, liver disease, cancer, digestive disease, kidney disease, and arthritis.
Psychological disorders: As in previous studies, a psychological condition was determined by the following two methods: assessment based on study-specific symptoms and self-reported diagnosis. Using the 10-item Centre for Epidemiological Studies Depression Scale (CES-D; 0–30 on the CHALS), participants were defined as having depression when they exhibited a CES-D score of ≥ 10 [20]. Alternatively, a physician informed participants that they were experiencing affective, emotional, nervous, or psychiatric issues.
Accompanying factors
The covariates included information on socio-demographics, lifestyle behaviours, and health-related factors.
The sociodemographic variables encompassed age(under 65,65 and over), sex(male, female), marital status (married and other), Hukou (Agricultual Hukou and other), and educational level (Primary school below, Primary school, and Middle school or above).
The behavioral characteristics included smoking (yes or no) and alcohol consumption (yes or no), sleep (average hours per night).
Health factors: The BMI was determined by measuring the weight and height of individuals (kg/m2) and categorised into three categories based on the guidelines set by the WHO: underweight/normal (<25), overweight (25 to 30) and obese (≥30). Hypertension(yes or no) was identified by self-reported diagnosis or medication use, as well as by clinical criteria: a systolic blood pressure of ≥140 mm Hg or a diastolic blood pressure of ≥90 mm Hg, based on the average of three consecutive measurements obtained by proficient medical staff throughout the interview.
Statistical methods
Baseline characteristics are presented by the following three frailty status categories: non-frail, pre-frailty, and frail. Data are expressed as mean ± standard deviation (SD) for continuous variables and as a percentage for categorical variables. Categorical and continuous variables were tested using the chi-squared and t-tests, respectively. We recorded the number of individuals who were followed up from the baseline date to the date of diagnosis or December 31, 2018, whichever occurred first. We employed COX proportional risk models to estimate the association between frailty indices (included as continuous and categorical variables in the models, respectively) and PP-MM. We built model 1 (the crude model) for the initial analyses, with model 2 incorporating confounders, including age, gender, residence, marriage, education level, drinking, smoking, sleep, and BMI. We used the restricted cubic spline (RCS) to further assess the potential nonlinear association between FI and PP-MM progression. For subgroup analyses, the samples were stratified by various factors, including age, gender, residence, hukou, education, marriage, alcohol consumption, sleep, smoking, and BMI. A P < 0.05 was considered statistically significant in each analysis. R 4.4.1 was used for the analysis.
Results
Descriptive statistics
The characteristics of the 10,707 subjects in this study according to PP-MM subgroups are summarised in Table 1. There were 8213 (76.7%) subjects younger than 65 years of age and 2494 (23.3%) subjects 65 years of age and older, of which 5760 (53.80%) were female participants.179 (1.67%) participants had new onset of PP-MM during the follow-up period.Analyses showed that older age, being a female, being of rural origin, having a lower level of education, being a non-smoker, consuming alcohol, and having a short period of sleep were were associated with the occurrence of PP-MM.
Table 1.
Baseline characteristics of the longitudinal study population by PP-MM
| Characteristic | Overall (n = 10,707) | Non-PP-MM (n = 10,528) | PP-MM (n = 179) | P value |
|---|---|---|---|---|
| Age_group (%) | 56.4 (8.64) | 59.3 (9.24) | 58.2 (9.18) | 0.032 |
| Under 65 | 8213 (76.7) | 8061 (76.6) | 152 (84.9) | |
| 65 and over | 2494 (23.3) | 2467 (23.4) | 27 (15.1) | |
| Gender (%) | ||||
| Men | 4947 (46.2) | 4896 (46.5) | 51 (28.5) | < 0.001 |
| Women | 5760 (53.8) | 5632 (53.5) | 128 (71.5) | |
| Hukou (%) | ||||
| Agricultual Hukou | 8771 (81.9) | 8606 (81.7) | 165 (92.2) | 0.002 |
| Other | 1936 (18.1) | 1922 (18.3) | 14 (7.8) | |
| Marriage (%) | ||||
| Married and living with spouse | 9166 (85.6) | 9019 (85.7) | 147 (82.1) | 0.408 |
| Other | 1541 (14.4) | 1509 (14.3) | 32 (17.9) | |
| Education (%) | ||||
| Primary school below | 4863 (45.4) | 1923 ( 35.9) | 562 ( 65.8) | < 0.001 |
| Primary school | 2390 (22.3) | 1223 ( 22.9) | 154 ( 18.0) | |
| Middle school or above | 3454 (32.3) | 2205 ( 41.2) | 138 ( 16.2) | |
| Smoking (%) | ||||
| Smoker | 4081 (38.1) | 4031 (38.3) | 50 (27.9) | 0.018 |
| Non-smoker | 6626 (61.9) | 6497 (61.7) | 129 (72.1) | |
| Drinking (%) | ||||
| Drinker | 3522 (32.9) | 7049 (67.0) | 136 (76.0) | < 0.001 |
| Non-drinker | 7185 (67.1) | 3479 (33.0) | 43 (24.0) | |
| Sleep_group (Mean (SD)) | 6.42 (1.85) | 6.43 (1.85) | 5.83 (2.05) | < 0.001 |
| BMI_group (%) | ||||
| Under 25 | 7294 (68.1) | 7177 (68.2) | 117 (65.4) | 0.935 |
| 25–30.0 | 2851 (26.6) | 2798 (26.6) | 53 (29.6) | |
| 30.0 and over | 562 ( 5.2) | 553 (5.3) | 9 (5.0) | |
| Hypertension (%) | ||||
| Yes | 3635 (33.9) | 6961 (66.1) | 111 (62.0) | 0.516 |
| No | 7072 (66.1) | 3567 (33.9) | 68 (38.0) | |
Association between FI and PP-MM
In univariate analyses, the risk of physical-psychological multimorbidity (PP-MM) increased by 1.57 (95% confidence interval (CI) = 1.40–1.76) for each 0.1 increase in FI (Table 2). After adjusting for confounders including age, sex, race, education, household income, marital status, social security, smoking, alcohol consumption, and BMI, the HR (Hazard Ratio,HR) was 1.53 (95% CI = 1.34–1.74). After categorising FI into non-frail (FI ≤ 0.10), pre-frail (0.10 < FI < 0.25), and frail (FI ≥ 0.25), the results of the univariate analyses revealed that, compared to non-frail, the HR was 3.33 (95% CI = 2.28–4.84) for pre-frail and 6.50 (95% CI = 4.15–10.16) for frail. After adjusting for the confounders of age, sex, race, education, household income, marital status, social security, smoking, alcohol consumption, and BMI, the HR was 3.01 (95% CI = 2.05–4.23) for pre-frail and 6.11 (95% CI = 3.79–9.84) for frail.
Table 2.
Hazard ratios and 95% CI of frailty status for PP-MM
| Unadjusted model | Adjusted model | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Continuous | 1.57(1.40, 1.76) | < 0.001 | 1.53(1.34, 1.74) | < 0.001 |
| Categories | ||||
| Healthy | Ref | Ref | ||
| Pro-frailty | 3.33(2.28, 4.84) | < 0.001 | 3.01(2.05, 4.23) | < 0.001 |
| Frailty | 6.50(4.15,10.16) | < 0.001 | 6.11(3.79, 9.84) | < 0.001 |
Notes: Model adjusted for age, gender, hukou, marriage, education level, drinking, smoking, sleep, BMI and Hypertension
Moreover, the RCS model demonstrated a nonlinear relationship between frailty (FI) and PP-MM incidence in all subjects (overall, P < 0.001; nonlinear, P = 0.001) (Fig. 2). The incidence of PP-MM increased as FI increased. The 95% CI for the HR of PP-MM were all above 1 at an FI of approximately 0.1, and the rate of increase in the incidence of PP-MM was faster when the FI was between 0.10 and 0.25 and decreased after an FI of 0.25. These findings indicate that the association between increased FI and the risk of developing PP-MM in subjects was more significant in the pre-frailty period.
Fig. 2.
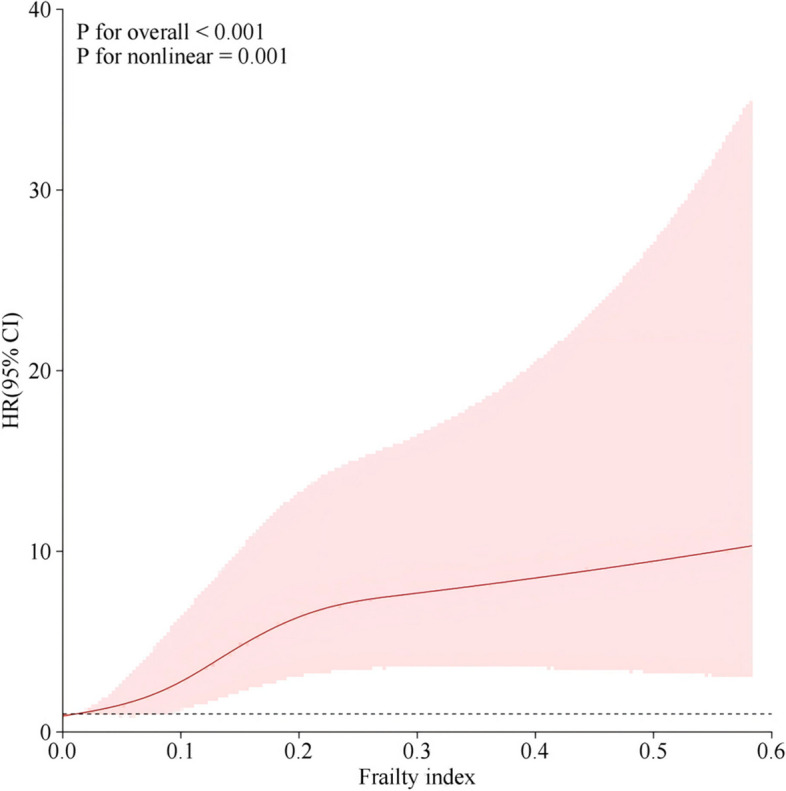
RCS between frailty and PP-MM
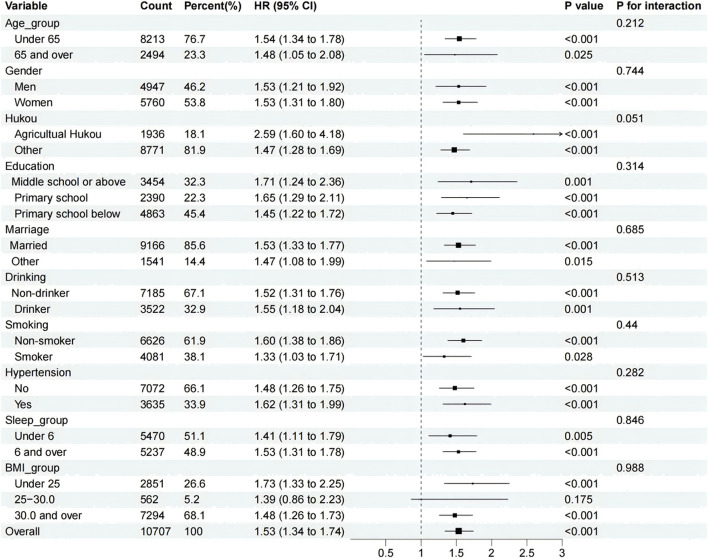
Subgroup analyses and interactions
Subgroup and interaction analyses were performed to further evaluate the relationship between FI and PP-MM incidence. These analyses were based on various characteristics, including age, gender, household, education, smoking, alcohol consumption, and sleep. The effects of FI and PP-MM were consistent across subgroups, testing the robustness of the results (Fig. 3).
Fig. 3.
Subgroup and interaction analyses
Discussion
This prospective nationwide study utilised a 4-node RCS curve and a COX regression model for the first time to evaluate the relationship between FI and PP-MM. This study identified 4,502 cases of pre-frail and 854 cases of frailty. The COX regression model indicated a correlation between FI and PP-MM prevalence in middle-aged and older Chinese individuals. The RCS findings revealed that the prevalence risk of PP-MM increased as FI increased, and the rate of increase was more rapid in the pre-frail.
Positive associations between frailty and the risk of a single chronic condition have been studied, including heart failure, coronary artery disease [21], chronic obstructive pulmonary disease, and asthma [22]. Several studies have also explored frailty and multimorbidity, demonstrating that frailty is strongly associated with chronic disease multimorbidity [23, 24]. This is consistent with our findings. A study discovered that depression partially mediated the association between prior frailty and subsequent multimorbidity [25], possibly because most frail patients have chronic inflammatory conditions, including elevated levels of interleukin-6, which is a significant risk factor for various chronic illnesses [26]. Frail older adults with multiple chronic diseases experience an accumulation of inflammatory conditions that may prolong the pro-inflammatory state, increase cortisol levels, and reduce muscle mass, physiological reserve, and immunocompetence, creating a vicious cycle [27]. Frail older adults may experience disability or functional dependence, pain due to chronic conditions, activity limitations, and poor endurance, leading to depression and psychological problems [28]. Therefore, we suggest incorporating frailty assessment into the routine monitoring and evaluation of patients with chronic conditions, leading to the identification of those at greater risk of PP-MM and facilitating targeted clinical care and health management.
This study determined that the HR and 95% CI for PP-MM were greater than 1 at FI of 0.10 and that the risk of developing PP-MM increased more rapidly as FI increased between 0.10 and 0.25 and slowed down after FI of 0.25. This revealed a significant relationship between the increase in FI and the risk of PP-MM prevalence in the pre-frail period. Moreover, the pre-frail period may present a better opportunity to implement interventions to prevent PP-MM, providing new evidence for PP-MM prevention. A comprehensive longitudinal study on frailty transitions in community-dwelling adults revealed that over 50% of participants transitioned between frail states. Moreover, although most of these transitions were to more frail states, some made transitions to less frail states, suggesting that frailty is reversible [29] and can be reversed through Exercise and nutritional improvement [30]. A study shows that pre-frail people are more likely to transition to strength than those who are frail. So we should pay more attention to the early identification of pre-frail.It has been shown that Lifestyle risk factors including alcohol consumption [31], poor sleep [32] and social determinants including low educational attainment [32] are associated with the development of frailty. This is consistent with our study and provides some basis for identifying early frailty in key populations.
In this study, we found that the prevalence risk of PP-MM rose faster with increasing FI between 0.10 and 0.25 and slowed down after FI of 0.25. This suggests that the relationship between rising FI and PP-MM prevalence risk is meaningful in the pre-frail period and the pre-frail period may be a better opportunity to implement interventions for PP-MM prevention. This provides new evidence for preventing PP-MM.
This is the first time that the association between FI and PP-MM has been explored longitudinally using a large sample from a nationally representative sample. This study used the COX regression and RCS models to estimate the association between FI and PP-MM in middle-aged and older adults. A series of subgroup analyses and interactions were conducted to confirm the robustness of our results.
This study has several limitations. First, the respondents self-reported physical, psychological, and cognitive impairments, which may have introduced recall bias and neglected undiagnosed disorders. Second, despite adjusting for many potential confounders, the possibility of residual confounding factors could not be eliminated. Finally, although longitudinal studies have yielded stronger correlations between FI and PP-MM than cross-sectional studies, we remain unable to establish a causal relationship or elucidate the underlying biological mechanisms. Further experimental studies are required to confirm this association.
Conclusions
In summary, the prevalence of PP-MM increased with increasing FI. Pre-frail may represent a better opportunity to implement interventions to prevent PP-MM. Early identification of pre-frailty and frailty can assist in identifying individuals at a greater risk of PP-MM and facilitate the provision of targeted clinical treatment and health management.
Acknowledgements
There is no acknowledgement.
Authors’ contributions
ZhiYing Fei: Data curation, Formal analysis, and Writing—original draft; Qian Yin:Writing- Reviewing and Editing; YingYing Tu: Writing- Reviewing and Editing; ChunQiao Wu: Conceptualization, Writing—original draft, Writing—review & editing, and Validation.
Funding
This research received no external funding.
Data availability
The CHARLS dataset is publicly available online, accessible at http://charls.pku.edu.cn/en
Declarations
Ethics approval and consent to participate
The data comes from a public database and the project was approved by the Biomedical Ethics Committee of Peking University (Beijing, China). All of the participants signed a written informed consent form.
The authors of this article consent.
Consent for publication
NA.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bauer UE, et al. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384(9937):45–52. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, et al. Healthcare for older adults with multimorbidity: a scoping review of reviews. Clin Interv Aging. 2023;18:1723–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(8):1–45. [PMC free article] [PubMed]
- 4.Miao YF, et al. Chronic conditions and depressive symptoms in middle-aged and older Chinese adults: Roles of perceived social support and area of residence. J Affect Disord. 2023;340:290–8. [DOI] [PubMed] [Google Scholar]
- 5.Ingle VK, et al. Screening of patients with chronic medical disorders in the outpatient department for depression using handheld computers as interface and patient health questionnaire-9 as a tool. Int J Appl Basic Med Res. 2017;7(2):129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bi YH, et al. The relationship between chronic diseases and depression in middle-aged and older adults: A 4-year follow-up study from the China Health and Retirement Longitudinal Study. J Affect Disord. 2021;289:160–6. [DOI] [PubMed] [Google Scholar]
- 7.Vetrano DL, et al. Frailty and multimorbidity: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2019;74(5):659–66. [DOI] [PubMed] [Google Scholar]
- 8.Clegg A, et al. Frailty in elderly people. Lancet. 2013;381(9868):752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrasco-Ribelles LA, et al. Dynamics of multimorbidity and frailty, and their contribution to mortality, nursing home and home care need: A primary care cohort of 1 456 052 ageing people. EClinicalMedicine. 2022;52:101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zazzara MB, et al. Frailty and chronic disease. Panminerva Med. 2019;61(4):486–92. [DOI] [PubMed] [Google Scholar]
- 11.Tazzeo C, et al. Multimorbidity patterns and risk of frailty in older community-dwelling adults: a population-based cohort study. Age Ageing. 2021;50(6):2183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong CH, et al. Frailty and its association with disability and comorbidity in a community-dwelling sample of seniors in Montreal: a cross-sectional study. Aging Clin Exp Res. 2010;22(1):54–62. [DOI] [PubMed] [Google Scholar]
- 13.Arakelyan S, et al. Effectiveness of holistic assessment-based interventions for adults with multiple long-term conditions and frailty: an umbrella review of systematic reviews. Lancet Healthy Longev. 2023;4(11):e629–44. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, et al. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Searle SD, et al. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriat Soc. 2004;52:625–34. 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 17.Cao X, et al. Aging metrics incorporating cognitive and physical function capture mortality risk: results from two prospective cohort studies. BMC Geriatr. 2022;22(1):378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, et al. Associations between socioeconomic inequalities and progression to psychological and cognitive multimorbidities after onset of a physical condition: a multicohort study. eClinicalMedicine. 2024;74:102739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo C, et al. Associations of healthy lifestyle and three latent socioeconomic status patterns with physical multimorbidity among middle-aged and older adults in China. Prev Med. 2023;175:107693. [DOI] [PubMed] [Google Scholar]
- 20.Ni Y, et al. Socioeconomic inequalities in physical, psychological, and cognitive multimorbidity in middle-aged and older adults in 33 countries: a cross-sectional study. Lancet Healthy Longevity. 2023;4(11):e618–28. [DOI] [PubMed] [Google Scholar]
- 21.Ijaz N, et al. Interventions for frailty among older adults with cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(5):482–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu J, et al. Causal relationship between frailty and chronic obstructive pulmonary disease or asthma: a two sample bidirectional Mendelian randomization study. Arch Gerontol Geriatr. 2024;118:105310. [DOI] [PubMed] [Google Scholar]
- 23.Hanlon P, et al. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3(7):e323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Y, et al. Associations between multimorbidity and frailty transitions among older Americans. J Cachexia Sarcopenia Muscle. 2023;14(2):1075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Z, et al. Bidirectional association between multimorbidity and frailty and the role of depression in older Europeans. J Gerontol A Biol Sci Med Sci. 2023;78(11):2162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang SS, et al. Patterns of comorbid inflammatory diseases in frail older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2010;65(4):407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woods NF, et al. Frailty: emergence and consequences in women aged 65 and older in the women’s health initiative observational study. J Am Geriatr Soc. 2005;53(8):1321–30. [DOI] [PubMed] [Google Scholar]
- 29.Gill TM, et al. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166(4):418–23. [DOI] [PubMed] [Google Scholar]
- 30.Shi SM, et al. Changes in a frailty index and association with mortality. J Am Geriatr Soc. 2021;69(4):1057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemoto Y, Sato S, Kitabatake Y, Nakamura M, Takeda N, Maruo K, Arao T. Bidirectional relationship between insomnia and frailty in older adults: A 2-year longitudinal study. Arch Gerontol Geriatr. 2021;97:104519. [DOI] [PubMed] [Google Scholar]
- 32.Jung Y, Lyu J, Kim G. Multi-group frailty trajectories among older Koreans: Results from the Korean Longitudinal Study of Aging. Arch Gerontol Geriatr. 2022;98:104533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The CHARLS dataset is publicly available online, accessible at http://charls.pku.edu.cn/en