Abstract
Background
Zoster-associated neuralgia refers to neuropathic pain from herpes zoster, which can persist as postherpetic neuralgia (PHN). Preventing the progression to chronic PHN is crucial, yet optimal interventions is still not clear.
Objectives
This study evaluates the efficacy of short-term spinal cord stimulation (tSCS) in patients with subacute and chronic PHN.
Methods
A clinical study involved 135 patients with herpes zoster-associated pain (HZAP), divided into two groups: Experimental group which received short-term spinal cord stimulation therapy, and Control group which received conventional medical treatment and nerve block therapy. Pain intensity, sleep quality, anxiety and depression and quality of life were assessed at baseline and at 2 weeks, 1-, 3-, 6-, and 12-month post-treatment. Univariate and multivariate analyses identified factors associated with treatment efficacy.
Results
At 1-month follow-up, the experimental group showed significantly higher efficacy in pain reduction (P < 0.01). Higher Pittsburgh Sleep Quality Index (β = 0.093, P = 0.004) and PHQ-9 scores (β = 0.065, P = 0.031) before treatment were associated with better outcomes. At 3 months, longer disease duration (β = 0.103, P = 0.008) and higher Pittsburgh Sleep Quality Index scores (β = 0.114, P = 0.002) correlated with better efficacy, while higher Patient Health Questionnaire-9 scores were negatively correlated (β = − 0.023, P = 0.036). Although as follow-up time increases, the significant superiority of efficacy gradually shrinks compared with nerve block therapy at 6–12 months, the tSCS group still had better effects in improving sleep quality, anxiety and depression symptoms, and quality of life.
Conclusions
Short-term spinal cord stimulation is a safe and effective short-term treatment for HZAP, offering faster and more effective pain relief and quality of life improvement compared to nerve block therapy. However, there are challenges in maintaining the long-term effects of tSCS. Further studies with larger samples are needed to confirm these findings.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-024-02196-6.
Keywords: Herpes zoster, Short-term spinal cord stimulation, Neuromodulation
Introduction
Herpes zoster (HZ) is a skin condition also known as shingles caused by the reactivation of varicella zoster virus (VZV) after being latent in the sensory ganglia [1]. Their characteristics are blister eruption in the skin and also neuropathic pain [2]. In a lifetime at least 3% of population develop Herpes Zoster and this risk of incidence increases with the advanced age of over 50 years and its immunosuppression [3]. When HZ becomes advanced it is considered as postherpetic neuralgia (PHN). This PHN is the long-lasting pain after the healing of blisters and rashes on the person affected [4]. A recent systematic review of 130 studies from 26 countries reported that the risk of developing PHN in patients with herpes zoster ranges from 5% to 30% [5].
Current researches are still controversial regarding the staging of herpes zoster. Some scholars believe that PHN should be defined as sustained pain for more than 90 days. Another research by Liu et al. [6, 24] claimed that the right time to cluster it as PHN if the pain persists for more than 1-month long acute HZ and they also termed the pain which is resolved within a period of 90 days is defined as subacute herpetic neuralgia after a 30-day acute phase of PHN [6, 7]. As noted by Chen et al. [8] the incidences of PHN range between 8% and 19% according to the variables classified and the pain lasts for a period of over 12 months in many patients which is over 6% [8].This PHN is critical as it affects the quality of life of a patient by causing sleep order and other psychological distresses resulting in demand for higher and quality health care and other social amenities. Many factors contribute to the risk of developing PHN and they include age, acute and sever pain and rash [8, 9]. However, effective management for patient’s pains is still obscure and brings a burden to the kidneys and liver due to long-term medication dependencies which may lead to drug addiction [10]. Other interventions like the nerve blocks and epidural blocks, which are classic treatment methods, have a certain efficacy in clinical practice [11]. However, some patients do not experience long-term improvement during extended follow-ups preventing sustainable pain relief which could allow them carry on with their daily lives properly, therefore, to prevent this, management and treatment of HZ is crucial to avoid the transition to chronic PHN [12]. This is in accordance to the global perception which concerns management of chronic pains. The epidural injection can reduce HZ acute pain but does not prevent the transitioning to chronic PHN after a period of 3 months [13]. Spinal cord stimulation has proved to be an advantageous and effective treatment mode for the chronic neuropathic pains from different sources [14]. The advantage of SCS treatment is that it is a green therapy with no related adverse reactions, high safety, and it promotes the repair of nerve structure and functioning and very cost-effective procedure [15]. The exact mechanism underlying Spinal cord stimulation analgesia for neuropathic pain is not well covered [16]. Short-term spinal cord stimulation which involves placing the spinal cord stimulation electrodes within the patient's body for less than 2 weeks [17] is more cost-effective and effective as it treats pain which resists other forms of treatments within a short period [18].
This hypothesis of this study is that short-term spinal cord stimulation (SCS) is an effective and viable method meant for relieving pain in patients with both subacute herpes zoster-associated pain and PHN and can also prevent the transition of subacute PHN to chronic PHN. Therefore, this research aims to assess the efficacy of short-term SCS in the management of pain at different stages of herpes zoster, by evaluating its potential in relieving pain in patients with subacute PHN and chronic PHN, as well as to determine its effectiveness in preventing progression from subacute PHN to chronic PHN.
Methods
Retrospective review
Human Ethics Committee of The First Affiliated Hospital of Chongqing Medical University (K2023-200) approved this case study. After the approval from the ethics committee medical records of patients who received short-term spinal cord stimulation treatment from the years 2016 to 2023 were examined. Documentation was done for medical records of the patients with herpes zoster-associated pain and follow-up done 2 weeks after being discharged from the hospital through a 12-month questionnaire forms.
Inclusion and exclusion
A total of 150 patients were included in the study, and 15 patients failed to follow-up, resulting in a final total of 135 patients with herpes zoster-associated pain were included and divided into two groups; The experimental group who went through the short-term spinal cord stimulation treatment and drugs and the control group that received conventional medical treatment and nerve block therapy (epidural nerve block).
Inclusion criteria
(1) Patients diagnosed with postherpetic neuralgia, (2) disease duration of 1–15 months, (3) patients have undergone standardized medication and nerve block therapy or short-term spinal cord stimulation therapy in our hospital, (4) complete clinical information, treatment details, and follow-up information and lastly (5) the patients had persistent severe pain (NRS score ≥ 4) after receiving standardized treatment like the Oral NSAIDs/tramadol/opioid analgesics at regular doses, combined with gabapentin/pregabalin, and tricyclic antidepressants such as amitriptyline for analgesic treatment.
Exclusion criteria
The patients excluded were due to the following reasons: (1) patients with significant organ diseases, (2) patients with abnormal coagulation function, (3) thoracolumbar MRI indicating intrathecal pathology, (4) history of cardiac pacemaker implantation, (5) incomplete clinical information, treatment details, or follow-up information, and (6) severe mental illness.
Evaluation of treatment effectiveness
The treatment efficacy was evaluated based on the reduction in the pain intensity using the Numerical Rating Scale (NRS) scores at different postoperative time points compared to the preoperative scores. To define the efficacy: (1) patients with NRS reduced by > 75% = significant effect, (2) reduction between 50 and 75% = good effect, (3) reduction between 25 and 50% = moderate effect, and (4) reduction of < 25% = No effect.
In this study, the patients who received effective treatment are the patients who had either significant or good effect treatment.
Description of short-term SCS
tSCS is mainly a therapy intervention which is used in pain management, particularly in subacute PHN and chronic PHN. In this study, we utilized tSCS in assessing its effectiveness in pain relieve from herpes zoster-related discomfort and to prevent the progression of the pain into chronic PHN.
Herpes zoster-affected dermatome was used to determine therapeutic target areas a combination done with either allodynia or hyperalgesia. Then, 1 × 8 electrodes test stimulation lead implanted in adherence to the implantation manual under X-ray guidance in the theatre when patient is awake, with local anesthesia around the puncture site. Shortly the patient was placed in a prone pose and the segment to be punctured is determined under the X-ray guidance. A spinal cord stimulation electrode (model 3777, produced by Medtronic, USA) was them inserted in the epidural space which is slightly ABOVE the spinal cord and this was done through a paramedial approach at an appropriate angle. Once in the epidural space, the inserted lead electrode is positioned till the tip is at an appropriate anatomical and physiological level that is able to execute the stimulation according to the statement of the patient. After the operation, stimulation parameters and contacted points were adjusted and the pain which the patients earlier felt was replaced by a "numbing feeling". The patients accepted this feeling, because it was comfortable to them.
Statistical methods
This study used SPSS 27.0 for statistical analysis and GraphPad Prism 10 for statistical graph plotting. Statistical description and univariate analysis were conducted on the data. For measurement data that met the normal distribution, the mean ± standard deviation (x ± SD) was used, and group comparisons were made using t tests or one-way ANOVA. For data that did not meet the normal distribution, the median and interquartile range M (IQR) were used, and group comparisons were made using the Mann–Whitney U test or Kruskal–Wallis test. Count data were expressed as numbers (n) and percentages (%), and group comparisons were made using the χ2 test. Multivariate analysis in this study was conducted using multiple linear regression, with variable selection by stepwise regression (αin = 0.05, αout = 0.10). A two-sided α = 0.05 was used as the statistical significance level.
Results
This study conducted univariate and multivariate analyses on the treatment efficacy at 1-, 3-, 6-, and 12-month post-treatment. Table 1 compares the experimental group and the control group, showing no statistically significant differences in gender, age, segment, and side, indicating that the two groups are comparable. However, the variables of age, duration of disease, and comorbidities were significantly higher in the experimental group compared to the control group. The two variables which emerged as statistically significant are complication and course of disease. The presence of complications was markedly higher in the experimental group, where 70.5% of participants had complications compared to only 21.1% in the control group. This significant difference (P < 0.001) indicates that the experimental group patients were dealing with more severe or complex health conditions, which could influence the treatment outcomes and the overall effectiveness of the interventions.
Table 1.
Comparison of general data between the two groups
| Variables | Experimental group (SCS) | Control group (Nerve block) | Statistical value | P | |
|---|---|---|---|---|---|
| Gender | Male | 39 (50.0) | 29 (50.9) | 0.01 | 0.92 |
| Female | 39 (50.0) | 28 (49.1) | |||
| Age | 72.00 (67.00, 78.25) | 65.00 (59.50, 75.50) | − 2.655 | 0.01 | |
| Disease duration | 2.00 (1.00, 4.00) | 2.00 (1.00, 2.00) | − 2.271 | 0.02 | |
| Segment | 1 | 11 (14.1) | 10 (17.5) | 4.664 | 0.20 |
| 2 | 56 (71.8) | 37 (64.9) | |||
| 3 | 11 (14.1) | 7 (12.3) | |||
| 4 | 0 (0.0) | 3 (5.3) | |||
| Side | 1 | 41 (52.6) | 26 (45.6) | 4.445 | 0.11 |
| 2 | 37 (47.4) | 28 (49.1) | |||
| 3 | 0 (0.0) | 3 (5.3) | |||
| Complication | No | 23 (29.5) | 45 (78.9) | 32.23 | < 0.001 |
| Yes | 55 (70.5) | 12 (21.1) |
In addition, the course of disease, measured by the duration of the condition, showed a significant difference between the groups. The median course of disease in the experimental group was 2.00 months (Interquartile Range (IQR: 1.00, 4.00), compared to 2.00 months (IQR: 1.00, 2.00) in the control group, with a P value of 0.023. This suggests that patients in the experimental group had a longer duration of disease, which may reflect more chronic or persistent cases of herpes zoster-associated pain. This longer disease duration could potentially affect the responsiveness to treatment and the overall prognosis.
Analysis of treatment efficacy at 1-month follow-up
During the 1-month follow-up, univariate analysis showed significant differences in treatment efficacy. On treatment, the experimental group demonstrated a significantly higher efficacy compared to the control group. Specifically, 75.7% of patients in the experimental group experienced a significant effect, and 67.3% had a good curative effect, compared to control group which had the highest on ineffective of effect of 85.7%. This difference is statistically significant with a P < 0.001, indicating that tSCS is more effective than nerve block therapy in the short term. There were no significant differences in treatment efficacy based on gender, as indicated by a P value of 0.337. Both male and female patients had similar outcomes. The efficacy of treatment did not significantly differ based on the number of affected spinal segments, with a P value of 0.254. Patients treated on the left side showed significantly better efficacy compared to those treated on the right side, with a P value of 0.015. Age showed a trend towards significance with a P value of 0.091. Older patients in the experimental group tended to have better treatment outcomes. The duration of the disease showed a significant effect on treatment efficacy. Patients with a shorter course of disease (median 2.00 years) responded better to treatment compared to those with a longer duration (median 3.00 years), with a P value of 0.041. The Numerical Rating Scale (NRS) for pain before treatment showed a trend towards significance with a P value of 0.059. Patients with higher initial pain levels tended to have better treatment outcomes. The PSQI scores before treatment were significantly associated with treatment efficacy. Patients with better sleep quality before treatment (lower PSQI scores) had better outcomes, with a P value of 0.003. Physiological function before treatment was highly significant, with a P < 0.001. Patients with better physiological function before treatment had significantly better treatment outcomes. Energy levels before treatment did not show a significant impact on treatment efficacy, with a P value of 0.295. Social function before treatment was significantly associated with treatment outcomes, with a P value of 0.003. Patients with better social function before treatment had better outcomes. Mental health before treatment was also significant, with a P value of 0.015. Better mental health status before treatment was associated with better treatment efficacy. The PHQ-9 scores before treatment were significantly associated with treatment outcomes, with a P value of 0.046. Lower depression scores before treatment predicted better efficacy, as shown in Table 2.
Table 2.
Univariate analysis results of treatment efficacy at 1-month follow-up
| Variable | Significant efficacy | Good efficacy | Moderate efficacy | Ineffective | Statistical Value | P | |
|---|---|---|---|---|---|---|---|
| Group | Experimental | 28 (75.7) | 33 (67.3) | 16 (38.1) | 1 (14.3) | 18.795 | < 0.001 |
| Control | 9 (24.3) | 16 (32.7) | 26 (61.9) | 6 (85.7) | |||
| Gender | Male | 20 (54.1) | 28 (57.1) | 18 (42.9) | 2 (28.6) | 3.379 | 0.337 |
| Female | 17 (45.9) | 21 (42.9) | 24 (57.1) | 5 (71.4) | |||
| Segment | 1 | 11 (29.7) | 4 (8.2) | 6 (14.3) | 0 (0.0) | 11.326 | 0.254 |
| 2 | 22 (59.5) | 36 (73.5) | 29 (69.0) | 6 (85.7) | |||
| 3 | 4 (10.8) | 8 (16.3) | 5 (11.9) | 1 (14.3) | |||
| 4 | 0 (0.0) | 1 (2.0) | 2 (4.8) | 0 (0.0) | |||
| Side | 1 | 23 (62.2) | 28 (57.1) | 14 (33.3) | 2 (28.6) | 15.788 | 0.015 |
| 2 | 14 (37.8) | 18 (36.7) | 28 (66.7) | 5 (71.4) | |||
| 3 | 0 (0.0) | 3 (6.1) | 0 (0.0) | 0 (0.0) | |||
| Age | 70.00 (59.50, 77.00) | 73.00 (65.00, 79.00) | 69.00 (59.75, 75.50) | 62.00 (59.00, 69.00) | 6.470 | 0.091 | |
| Disease duration | 2.00 (1.00, 2.00) | 2.00 (1.00, 3.50) | 3.00 (1.00, 5.25) | 2.00 (1.00, 2.00) | 8.262 | 0.041 | |
| NRS score | 7.00 (6.00, 8.00) | 7.00 (7.00, 8.00) | 7.00 (6.00, 8.00) | 6.00 (5.00, 7.00) | 7.438 | 0.059 | |
| PSQI | 13.00 (9.00, 16.50) | 15.00 (12.00, 18.00) | 16.00 (14.00, 18.25) | 17.00 (16.00, 20.00) | 13.924 | 0.003 | |
| Physiological function | 65.00 (40.00, 80.00) | 35.00 (27.50, 55.00) | 30.00 (25.00, 50.00) | 55.00 (40.00, 95.00) | 18.550 | 0.000 | |
| Activities of daily living | 25.00 (12.50, 50.00) | 25.00 (25.00, 40.00) | 25.00 (0.00, 25.00) | 25.00 (25.00, 50.00) | 3.710 | 0.295 | |
| Bodily pain | 32.00 (22.00, 41.00) | 31.00 (22.00, 41.00) | 22.00 (22.00, 40.00) | 31.00 (22.00, 41.00) | 4.303 | 0.231 | |
| Energy levels | 50.00 (37.50, 60.00) | 35.00 (25.00, 50.00) | 35.00 (30.00, 50.00) | 60.00 (45.00, 60.00) | 16.331 | 0.001 | |
| Social functioning | 50.00 (40.95, 62.50) | 37.50 (33.30, 50.00) | 37.50 (33.30, 50.00) | 50.00 (37.50, 62.50) | 13.913 | 0.003 | |
| Emotional functioning | 33.30 (0.00, 33.30) | 0.00 (0.00, 33.30) | 33.30 (0.00, 33.30) | 33.30 (0.00, 66.60) | 4.232 | 0.237 | |
| Mental health | 52.00 (40.00, 64.00) | 40.00 (37.00, 56.00) | 40.00 (36.00, 48.00) | 48.00 (40.00, 56.00) | 10.463 | 0.015 | |
| PHQ-9 score | 10.00 (8.00, 12.50) | 12.00 (8.00, 15.50) | 14.00 (8.00, 16.50) | 14.00 (8.00, 18.00) | 8.004 | 0.046 | |
| GAD-7 score | 8.00 (5.50, 9.50) | 8.00 (6.00, 11.00) | 9.00 (8.00, 12.25) | 8.00 (7.00, 16.00) | 7.324 | 0.062 | |
| IDPAIN | 3.00 (2.50, 3.00) | 3.00 (3.00, 3.00) | 3.00 (2.75, 3.00) | 3.00 (2.00, 4.00) | 1.007 | 0.799 | |
| General health status | 45.00 (37.00, 52.00) | 42.00 (35.00, 50.00) | 41.00 (34.25, 45.00) | 45.00 (37.00, 50.00) | 8.679 | 0.034 |
Multivariate analysis
Findings show that the experimental group had a significant impact (β = 0.914, P < 0.001), PSQI (β = 0.093, P = 0.004), PHQ9 (β = 0.065, P = 0.031). All are positively correlated with treatment outcomes, as shown in Table 3.
Table 3.
Multivariate analysis results of treatment efficacy at 1-month follow-up
| Variables | β value | β Standard value | Standardized β value | t | P value | 95.0% Confidence Interval of B | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| Disease duration | 0.044 | 0.033 | 0.107 | 1.348 | 0.180 | − 0.021 | 0.109 |
| Grouping | 0.914 | 0.242 | 0.290 | 3.778 | 0.000 | 0.435 | 1.393 |
| Side | 0.295 | 0.214 | 0.103 | 1.377 | 0.171 | − 0.129 | 0.718 |
| PSQI score | 0.093 | 0.032 | 0.241 | 2.924 | 0.004 | 0.030 | 0.156 |
| Physiological function | − 0.008 | 0.006 | − 0.124 | − 1.349 | 0.180 | − 0.020 | 0.004 |
| General health status | − 0.023 | 0.015 | − 0.136 | − 1.497 | 0.137 | − 0.052 | 0.007 |
| Energy levels | 0.005 | 0.012 | 0.047 | 0.409 | 0.684 | − 0.019 | 0.029 |
| Social Functioning | 0.008 | 0.010 | 0.088 | 0.822 | 0.413 | − 0.011 | 0.27 |
| Mental health | − 0.013 | 0.011 | − 0.114 | − 1.212 | 0.228 | − 0.034 | 0.008 |
| PHQ-9 | 0.065 | 0.030 | 0.173 | 2.181 | 0.031 | 0.006 | 0.125 |
Analysis of treatment efficacy at 3-month follow-up
Univariate analysis
At 3-month follow-up univariate analysis results in Table S1 showed that the duration of the disease significantly affects efficacy, with patients having a longer duration of the disease experiencing higher efficacy rates (P < 0.001). Pre-treatment sleep quality, as measured by PSQI scores, significantly impacts efficacy (P value 0.002). Patients with better sleep quality before treatment tend to have better treatment outcomes. Pre-treatment depression levels, assessed using PHQ9 scores, significantly influence efficacy (P value 0.016). Patients with lower depression scores before treatment tend to have better treatment outcomes. Other variables such as NRS before treatment, physical function before treatment, life function before treatment, energy before treatment, physical pain before treatment, social function before treatment, and mental health before treatment also showed significant differences in their effect on efficacy 3 months after treatment. An additional univariate file shows this in more detail [see Additional file 1].
Multivariate analysis
In the multivariate analysis, significant variables from the univariate analysis of treatment efficacy at 3 months were included, as shown in Table 4. It was found that disease duration (β = 0.103, P = 0.008) and PSQI score before treatment (β = 0.114, P = 0.002) were positively correlated with treatment outcomes, indicating that longer disease duration and poorer sleep quality before treatment may lead to better treatment efficacy. On the other hand, PHQ9 score before treatment (β = − 0.023, P = 0.036) was negatively correlated with treatment outcomes, suggesting that higher levels of depression symptoms before treatment may lead to worse treatment efficacy.
Table 4.
Multivariate analysis results of treatment efficacy at 3-month follow-up
| Variables | β Value | β Standard value | Standardized β value | t | P Value | 95.0% Confidence Interval of B | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| Disease duration | 0.103 | 0.038 | 0.222 | 2.697 | 0.008 | 0.027 | 0.178 |
| NRS score | 0.073 | 0.118 | 0.052 | 0.623 | 0.535 | − 0.160 | 0.307 |
| PSQI score | 0.114 | 0.037 | 0.265 | 3.088 | 0.002 | 0.041 | 0.187 |
| Physiological function | 0.000 | 0.007 | − 0.005 | − 0.049 | 0.961 | − 0.014 | 0.014 |
| Activities of daily living | 0.000 | 0.008 | − 0.002 | − 0.025 | 0.980 | − 0.016 | 0.016 |
| Bodily pain | − 0.023 | 0.011 | − 0.206 | − 2.117 | 0.036 | − 0.045 | − 0.002 |
| General health status | 0.011 | 0.019 | 0.061 | 0.610 | 0.543 | − 0.025 | 0.048 |
| Energy levels | − 0.007 | 0.014 | − 0.058 | − 0.472 | 0.638 | − 0.035 | 0.021 |
| Social functioning | 0.004 | 0.011 | 0.035 | 0.319 | 0.750 | − 0.018 | 0.025 |
| Mental health | − 0.001 | 0.012 | − 0.008 | − 0.078 | 0.938 | − 0.025 | 0.023 |
| PHQ9 score | 0.068 | 0.035 | 0.161 | 1.954 | 0.053 | − 0.001 | 0.137 |
Analysis of treatment efficacy at 6-month follow-up
Univariate analysis
At 6 months after treatment there was no major differences from 3-month follow-up, however, significant difference in efficacy between different treatment groups (P value 0.306) was seen on the two groups. Pre-treatment depression levels, as assessed by PHQ9 scores, demonstrated a trend towards significance in influencing efficacy (P value 0.002). An additional univariate file shows this in more detail [see Additional file 1] as shown in Table S2.
Multivariate analysis
In the multivariate analysis, significant variables from the univariate analysis of treatment efficacy at 6 months were included, as shown in Table 5. It was found that disease duration (β = 0.104, P = 0.009) and PSQI score before treatment (β = 0.086, P = 0.024) were positively correlated with treatment outcomes. This suggests that longer disease duration and poorer sleep quality before treatment may lead to better treatment efficacy.
Table 5.
Multivariate analysis results of treatment efficacy at 6-month follow-up
| Variables | β Value | β Standard value | Standardized β value | t | P Value | 95.0% Confidence Interval of β | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| Disease duration | 0.104 | 0.039 | 0.219 | 2.672 | 0.009 | 0.027 | 0.181 |
| PSQI score before treatment | 0.086 | 0.038 | 0.194 | 2.283 | 0.024 | 0.011 | 0.161 |
| Physiological function | − 0.002 | 0.007 | − 0.029 | − 0.288 | 0.774 | − 0.017 | 0.012 |
| Activities of daily living | − 0.005 | 0.008 | 0.056 | − 0.642 | 0.522 | − 0.02 | 0.01 |
| Energy levels | − 0.011 | 0.014 | − 0.088 | 0.786 | 0.433 | − 0.037 | 0.016 |
| Social functioning | − 0.004 | 0.011 | − 0.039 | − 0.364 | 0.716 | − 0.026 | 0.018 |
| Mental health | − 0.01 | 0.012 | − 0.079 | − 0.812 | 0.418 | − 0.035 | 0.014 |
| PHQ9 score | 0.067 | 0.036 | 0.154 | 1.872 | 0.063 | − 0.004 | 0.138 |
The other factors such as physiological function before treatment, occupational therapy, spiritual treatment, social function treatment, mental health treatment, and PHQ9 before treatment did not show significant correlations with efficacy in the multivariate analysis. These findings underscore the importance of considering the duration of the disease and pre-treatment sleep quality when evaluating and predicting treatment outcomes for patients undergoing treatment for herpes zoster-associated pain over a longer period.
Analysis of treatment efficacy at 12-month follow-up
Univariate analysis
At 12-month follow-up similar to previous time points, the duration of the disease (β = 0.104, P = 0.012) remained a significant factor influencing treatment efficacy at the 12-month follow-up. Patients with a longer disease course tended to have better treatment outcomes. The pre-treatment assessment of sleep quality (β = 0.086, P = 0.012) continued to show a significant positive correlation with treatment efficacy at the 12-month mark. This suggests that patients with better sleep quality before treatment tended to experience better treatment outcomes at the long-term follow-up. Before treatment, patients' physiological function, life occupation, and energy levels significantly influenced treatment efficacy at the 12-month follow-up. However, the direction of these effects and their implications for treatment efficacy require further investigation. The efficacy before mental health treatment (β = − 0.010, P = 0.036) remained a significant factor affecting treatment efficacy at the 12-month follow-up. This implies that patients with poorer mental health status before treatment may experience less favorable treatment outcomes in the long term. The efficacy before PHQ9 and GAD7 treatment also showed significant associations with treatment efficacy at the 12-month follow-up, suggesting that baseline levels of depression and anxiety may influence treatment outcomes over time. An additional univariate file shows this in more detail [see Additional file 1] as shown in Table S3.
Multivariate analysis
At 12 months after treatment revealed that none of the variables included in the analysis showed a significant correlation with treatment efficacy, as indicated in Table 6. This indicates that factors such as disease course, pre-treatment PSQI, pre-treatment physiological function, pre-treatment life occupation, pre-treatment mental health, efficacy before PHQ9 treatment, and efficacy before GAD7 treatment did not have a meaningful impact on treatment outcomes at the 12-month follow-up.
Table 6.
Multivariate analysis results of treatment efficacy at 12-month follow-up
| Variables | β Value | β standard value | Standardized β value | t | P Value | 95.0% Confidence Interval of β | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| Disease duration | 0.079 | 0.045 | 0.154 | 1.777 | 0.078 | − 0.009 | 0.167 |
| PSQI score | 0.045 | 0.044 | 0.094 | 1.019 | 0.31 | − 0.043 | 0.133 |
| Physiological function | 0.003 | 0.008 | 0.04 | 0.391 | 0.696 | − 0.013 | 0.02 |
| Activities of daily living | − 0.02 | 0.009 | − 0.164 | − 1.765 | 0.08 | − 0.033 | 0.002 |
| Energy levels | − 0.02 | 0.015 | − 0.124 | − 1.108 | 0.27 | − 0.045 | 0.013 |
| Mental health | 0.006 | 0.014 | 0.043 | 0.43 | 0.668 | − 0.021 | 0.033 |
| PHQ9 score | 0.058 | 0.047 | 0.122 | 1.229 | 0.221 | − 0.035 | 0.15 |
| GAD7 score | 0.068 | 0.056 | 0.118 | 1.207 | 0.23 | − 0.043 | 0.179 |
In summary, regardless of the treatment method employed, there was no significant effect on long-term efficacy. This suggests that the optimal benefit of the treatment is observed at the 6-month mark. Based on the analysis tSCS group was more effective for short-term efficacy.
The analysis of scale scores at various time points post-treatment reveals significant differences between the control and experimental groups across multiple dimensions of health. Notably, the NRS scores indicate a significant reduction in pain perception in the experimental group compared to the control group over the 12-month period (P < 0.05; Fig. 1). Similarly, the PHQ-9 scores demonstrate a substantial improvement in depressive symptoms in the experimental group, reflecting enhanced mental health outcomes (P < 0.05; Fig. 2). Sleep quality, assessed via PSQI scores, also shows showed improvement in the experimental group, indicating better sleep patterns post-treatment (P < 0.05; Fig. 3),and GAD-7 scores reveal reduced anxiety levels in the experimental group compared to the control group (P < 0.05; Fig. 4).
Fig. 1.
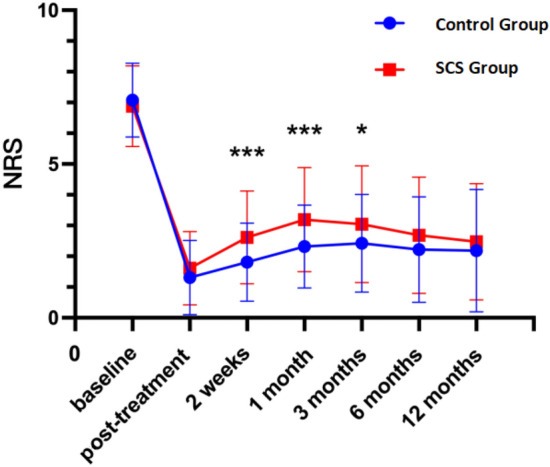
NRS at different periods
Fig. 2.
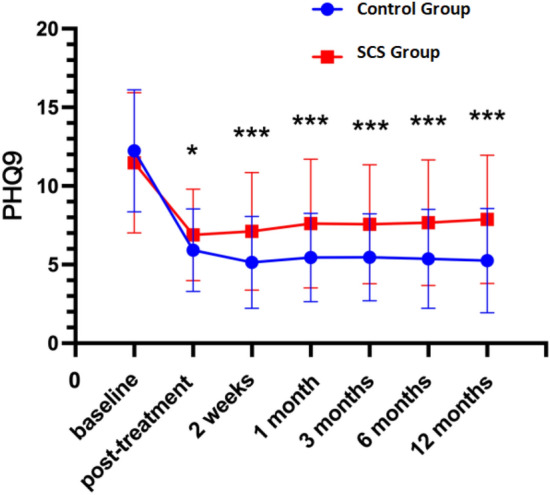
PHQ9 at different periods
Fig. 3.
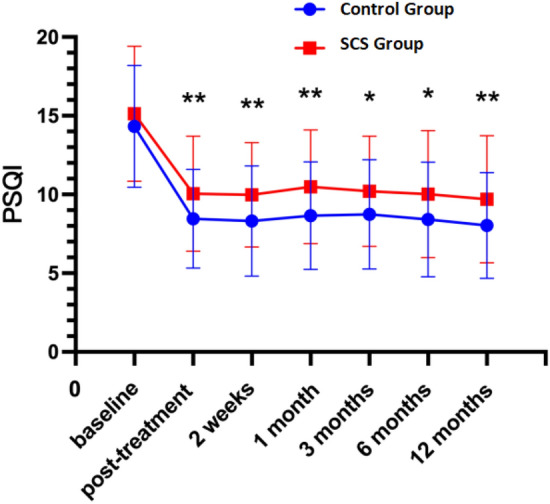
PSQI at different periods
Fig. 4.
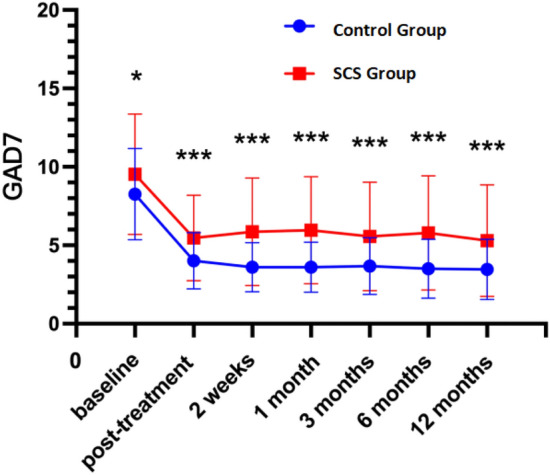
GAD7 at different periods
SF-36
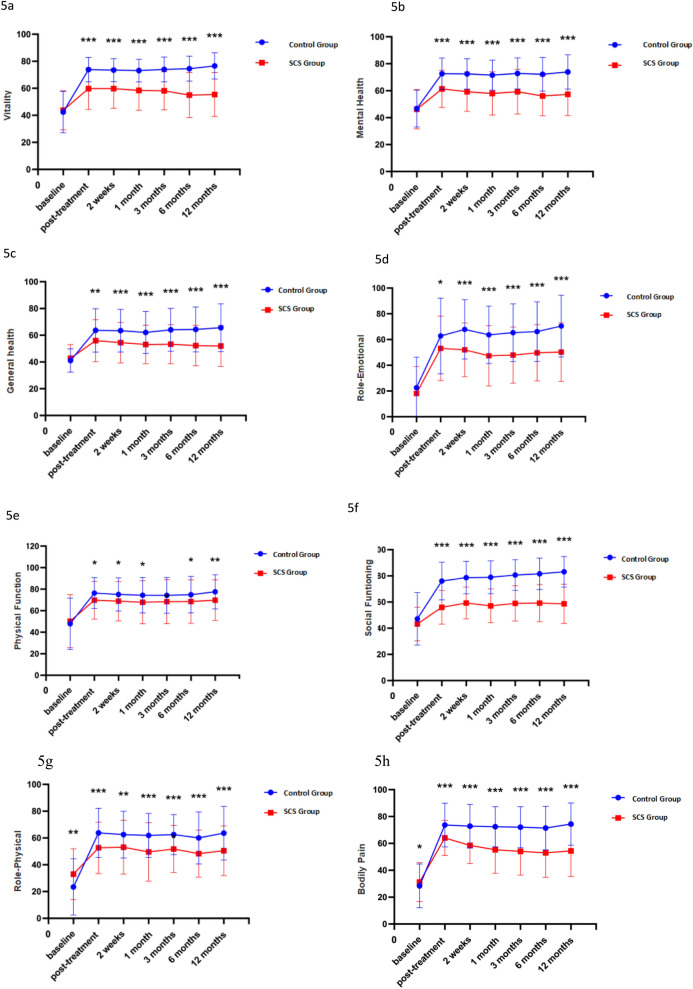
Vitality levels, measured by vitality scores, are significantly higher in the experimental group, suggesting increased overall vitality (P < 0.05; Fig. 5a). Mental health scores, another critical component of the SF-36, illustrate significant enhancements in the experimental group, underscoring the psychological benefits of the treatment (P < 0.05; Fig. 5b). Furthermore, the experimental group exhibits better Role-emotional and general health scores, pointing to improved emotional well-being and overall health perceptions (P < 0.05; Fig. 5c, d). Physiological function and role-physical scores are also notably higher in the experimental group, indicating better physical functioning and life satisfaction (P < 0.05; Fig. 5e, f).Social function scores reflect significant improvements in the experimental group, suggesting enhanced social interactions and community involvement (P < 0.05; Fig. 5g). Lastly, Bodily pain scores are significantly lower (P > 0.05; Fig. 5h).
Fig. 5.
a–h SF-36 at different periods
These results collectively highlight the comprehensive benefits of the experimental treatment across various health domains measured by the SF-36, emphasizing its efficacy in improving both physical and mental health outcomes over a year.
Discussion
When acute HZ infection is not managed early, it transitions into PHN, a chronic neuropathic pain that affects quality of life physically and psychologically, leading to a huge demand for better healthcare services [19]. Currently, no medications have been proven to cure chronic PHN [20]. This study investigates the efficacy of tSCS in treating sub-acute herpes zoster-associated pain and preventing progression to PHN lasting more than 3 months [21]. Previous research has not clearly identified the neuropathic pain mechanism of SCS-induced analgesia, with only animal models showing that it involves the release of antinociceptive factors in the spinal cord dorsal horn, affecting tSCS and neuropathic pain development [22, 23].
This study compared the efficacy of short-term spinal cord stimulation therapy (SCS group) and epidural nerve block therapy (N group) in patients with PHN. It focused on analyzing the differences in efficacy at various time points (1 day, 2 weeks, 1 month, 3 months, 6 months, and 12 months after surgery) and explored scoring indicators related to efficacy, such as sleep quality (PSQI), depression level (PHQ-9), and anxiety level (GAD-7). The application of short-term spinal cord stimulation (SCS) and epidural nerve block (N) therapy in the treatment of herpes zoster-related pain each has its unique advantages and limitations. Comparing the most commonly used clinical treatments in real-world studies can not only help optimize treatment plans in clinical practice but also provide a research foundation for further exploring the differences between different programming modes of SCS. At the beginning of the study, although there were no significant differences between the two groups in terms of gender, segment distribution, and left and right sides, the median age of patients in the SCS group was significantly higher than that in the N group, and the duration of disease was longer. This baseline difference may have a certain impact on subsequent efficacy analysis, and the advantages of SCS therapy may be obscured.
SCS treatment has a particularly significant impact on improving the sleep quality of PHN patients. Within 1 month, the PSQI scores of patients in the SCS group were significantly lower than those in the N group, indicating that SCS treatment can significantly improve patients' sleep quality. This phenomenon is consistent with previous research conclusions [24], possibly by inhibiting the transmission of pain signals through SCS, eliminating the interference of pain on sleep disorders, and indirectly improving patients' sleep.
At 3 months, when comparing the SCS group with the N group, the difference in efficacy of the SCS group remained significant. Not only did the SCS group continue to outperform the N group in terms of PSQI scores, but it also demonstrated better improvement in PHQ-9 scores. This result further supports the effectiveness of SCS in improving the mood of PHN patients during mid-term efficacy evaluation. In contrast, although the N group showed better pain relief in the short term, its improvement in mood was relatively limited. This may be because SCS has a more significant regulatory effect on the central nervous system [25], while the role of nerve block therapy is more focused on the peripheral nervous system.
At 6 months, the difference in long-term efficacy of SCS began to diminish. Although the SCS group still outperformed the N group in terms of sleep quality and improvement of anxiety and depression, the gap in pain relief between the two groups began to narrow. In contrast, although the long-term efficacy of N group treatment is not as significant as SCS, due to its simpler clinical procedure, repeated implementation can maintain pain control to a certain extent, making it a more suitable alternative treatment for patients with shorter disease duration who cannot undergo SCS treatment.
At 12 months, the difference in treatment effects between Group S and Group N was no longer significant, indicating that tSCS faces certain challenges in maintaining long-term efficacy. This also suggests that there may be a self-limitation inherent to the disease itself in the treatment of PHN. Furthermore, it implies that SCS may need to be combined with other treatment methods or longer-term stimulation (implantation of a long-term spinal cord electrical stimulation system) intervention to achieve more durable efficacy.
Previous studies align with our findings. Ahn et al. [26] highlighted the effectiveness of SCS in treating post-zoster neuralgia, and Xue et al. [27] acknowledged the safety and efficacy of SCS for zoster-related pain. However, small sample sizes and lack of follow-up led to underestimations of SCS’s clinical value, especially during acute/sub-acute stages. Wan et al. and Zhang et al. also showed significant analgesic effects, with similar limitations in their studies [28, 29]. Söreskog [30] noted that SCS efficacy was relatively weak, and this study agrees, finding weak efficacy in PHN and the high cost of permanent implants. Yan et al. [31] reported that 72% of patients with sacral nerve stimulation achieved > 50% pain relief when treated within 6 months of rash onset. Motov et al. [32] found that SCS relieved pain in patients who did not respond to epidural block therapy.
Goudman et al. [33] found that over 50% of patients achieved favorable outcomes with SCS compared to other treatments. Their findings, along with this study, emphasize the importance of early intervention to achieve higher pain reduction rates. Based on these studies, patients were grouped by gender, age, segment, side, and complications, with pain reduction calculated using NRS scores. Short-term SCS showed better efficacy than nerve block treatment, confirming previous studies by Szok et al. [34, 35].
The patients in this study had severe subacute herpes zoster pain unresponsive to conventional treatments, resulting in lower immediate pain relief possibilities. The results showed effective long-term benefits of tSCS, with pain relief of 57.65% at 3 months, 61.18% at 6 months, 62.35% at 9 months, and 63.53% at 12 months (NRS < 2.4). A few patients had poor improvement at different stages, with 5 patients at 3 months, 4 at 6 months, 3 at 9 months, and 2 at 12 months.
Univariate analysis at the first month showed 75.7% of tSCS patients with significant pain relief, compared to 24.3% for nerve block patients (P < 0.001), highlighting the effectiveness of SCS. Side of treatment was significant, with left-side treatments showing better outcomes (P value 0.015). Age was also significant (P value 0.091), suggesting older patients benefit more from SCS. Pre-treatment conditions, such as shorter disease duration (P value 0.041), higher pre-treatment pain levels (P value 0.059), better sleep quality (P value 0.003), better physiological function (P < 0.001), and better mental health (P value 0.015), significantly impacted pain reduction, emphasizing the importance of comprehensive pre-treatment assessments and lastly multivariate analysis confirmed that SCS treatment and pre-treatment PSQI and PHQ-9 scores were strongly correlated with better efficacy (β = 0.914, P < 0.001), with improved sleep quality and lower depression levels also positively correlated with better outcomes throughout the treatment period.
In addition, this study conducted a systematic analysis of the relevant influencing factors on treatment outcomes. Although there were no significant differences between the two groups in terms of gender, segment distribution, and left and right sides, the median age of patients in the SCS group was significantly higher than that in the N group, and their disease duration was longer. This baseline difference may have a certain impact on subsequent efficacy analysis. Patients who are older and have a longer disease duration may require more attention to individualized treatment plans in the management of PHN. Treatment plans, including drug selection and minimally invasive intervention options, need to be tailored to the specific circumstances of each patient. For some patients with psychological anxiety and depression, psychological intervention may be necessary to achieve optimal efficacy. Furthermore, the duration of illness, age, psychological state, and other health conditions of patients are also key factors to consider when developing treatment plans. Therefore, this study delves deeper into the factors affecting the efficacy of PHN treatment by incorporating comprehensive assessments of sleep, mood, and quality of life. Isagulyan et al. pointed out that in the treatment of post-herpetic neuralgia, individualized neuromodulation therapy can significantly improve patients' quality of life [17, 36]. Similarly, in the treatment of PHN, selecting appropriate treatment methods based on the characteristics of patients' conditions can help improve treatment efficacy and reduce adverse reactions.
Although existing research has provided abundant evidence for the treatment of PHN with SCS [20–23], many issues still remain unresolved. Future research can focus on the following aspects: first, further exploring the long-term stable efficacy of SCS and other neuromodulation therapies and developing new stimulation modes; second, developing new non-invasive treatment methods to reduce treatment-related risks; third, studying the combined use of multiple treatment methods to improve overall efficacy. In addition, with the development of neuroscience and technology, new treatment methods such as gene therapy and nerve growth factor therapy may provide new ideas for the management of PHN. These methods, by regulating the growth and function of neurons, are expected to fundamentally treat PHN, rather than merely alleviating symptoms.
Conclusion
tSCS can provide persistent long-term pain relief and improvement in the quality of life in patients with zoster-associated pain and PHN. Results from this study proves that tSCS is safe, effective, and less invasive analgesic method which should be used by patients. However, there are still challenges in long-term efficacy maintenance. And in addition, tSCS may have a curative effect at early stages of neuropathic pain as compared to the long term.
Limitations and future direction
The limitation of this study was the sample size of as well as from one specific hospital, and therefore, generalizability of the results can be improper. Second, our follow-up period was only up to 12 months and thereafter we could not how the patients coped. Therefore, future researchers should focus on large sample sizes and also extend follow-up periods to even 3 years which will give better evidence of the long-term benefits of SCS for herpes zoster-associated pain.
Supplementary Information
Author contributions
L.Z. and Y.C.X. did conceptualization and wrote first draft. X.Q.Y. and A.S. did analysis and prepared final draft. All the authors approved final manuscript.
Funding
The authors confirm and declare that no funding was received for this manuscript.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Ethical approval for this study was obtained from the Human Ethics Committee of The First Affiliated Hospital of Chongqing Medical University (K2023-200). Patient privacy and confidentiality were strictly maintained throughout the study by anonymizing personal identifiers. This study has ensured that all CARE guidelines and reports are fully conformed.
Consent for publication
The patients were informed and we obtained written consent before writing this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parkar S, Kegade P, Gade A, Sawant R. An overview of herpes zoster: Aetiology, pathogenesis and treatment.
- 2.Park JM, Kim SE, Yang HC. Clinical characteristics of herpes zoster laryngitis. Eur Arch Otorhinolaryngol. 2020;277:2907–12. [DOI] [PubMed] [Google Scholar]
- 3.van Oorschot D, Vroling H, Bunge E, Diaz-Decaro J, Curran D, Yawn B. A systematic literature review of herpes zoster incidence worldwide. Hum Vaccin Immunother. 2021;17(6):1714–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Money S, Gharib M, Aiyer R. Post herpetic neuralgia: recent advancements. Curr Emerg Hosp Med Rep. 2020;8:45–9. [Google Scholar]
- 5.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6): e004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu DY, Chen JS, Lin CY, Gong QJ, Zhao Q, Wan L. Subcutaneous peripheral nerve stimulation for treatment of acute/subacute herpes zoster-related trigeminal neuralgia: a retrospective research. Clin J Pain. 2021;37(12):867–71. [DOI] [PubMed] [Google Scholar]
- 7.Fontaine D. Spinal cord stimulation for neuropathic pain. Revue neurologique. 2021;177(7):838–42. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Li J, Liu H, Yang P, Zuo Y, Ye L. Interventions for zoster-associated pain: a retrospective study based on the clinical database. Front Neurol. 2022;13:1056171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du, J., Sun, G., Ma, H., Xiang, P., Guo, Y., Deng, Y., ... & Li, X. (2021). Prevalence and risk factors of anxiety and depression in patients with postherpetic neuralgia: a retrospective study. Dermatology, 237(6), 891–895. [DOI] [PubMed]
- 10.Zhou H, Wang Z, Jin H, Chen X, Lei L. A systematic review and meta-analysis of independent risk factors for postherpetic neuralgia. Annals of Palliative Medicine. 2021;10(12):121812189–121812189. [DOI] [PubMed] [Google Scholar]
- 11.Stern JI, Chiang CC, Kissoon NR, Robertson CE. Narrative review of peripheral nerve blocks for the management of headache. Headache: J Head Face Pain. 2022;62(9):1077–92. [DOI] [PubMed]
- 12.Dworkin RH, O’Connor AB, Kent J, Mackey SC, Raja SN, Stacey BR, Levy RM, Backonja M, Baron R, Harke H, Loeser JD. Interventional management of neuropathic pain: NeuPSIG Recommend. PAIN®. 2013;154(11):2249–61. [DOI] [PMC free article] [PubMed]
- 13.Lee CH, Choi SS, Lee MK, Lee YJ, Park JS. Efficacy of continuous epidural infusion with epidural electric stimulation compared to that of conventional continuous epidural infusion for acute herpes zoster management: a retrospective study. BMC Anesthesiol. 2020;20:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt GL. The use of spinal cord stimulation/neuromodulation in the management of chronic pain. JAAOS-J Am Acad Orthop Surg. 2019;27(9):e401–7. [DOI] [PubMed] [Google Scholar]
- 15.Pérez JT. Spinal cord stimulation: beyond pain management. Neurología (English Edition). 2022;37(7):586–95. [DOI] [PubMed] [Google Scholar]
- 16.Joosten EA, Franken G. Spinal cord stimulation in chronic neuropathic pain: mechanisms of action, new locations, new paradigms. Pain. 2020;161:S104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong DS, Yu X, Wan CF, Liu Y, Zhao L, Xi Q, Cui WY, Wang QS, Song T. Efficacy of short-term spinal cord stimulation in acute/subacute zoster-related pain: a retrospective study. Pain Phys. 2017;20(5):E633–45. [PubMed]
- 18.Isagulyan E, Slavin K, Konovalov N, Dorochov E, Tomsky A, Dekopov A, Makashova E, Isagulyan D, Genov P. Spinal cord stimulation in chronic pain: technical advances. Kor J Pain. 2020;33(2):99–107. [DOI] [PMC free article] [PubMed]
- 19.Lang PO, Aspinall R. Vaccination for quality of life: herpes–zoster vaccines. Aging Clin Exp Res. 2021;33(4):1113–22. [DOI] [PubMed] [Google Scholar]
- 20.Helmers RN. Postherpetic Neuralgia management. 2020.
- 21.Huang J, Yang S, Yang J, Sun W, Jiang C, Zhou J, Li D, Xiong D, Bates D, Xiao L. Early treatment with temporary spinal cord stimulation effectively prevents development of postherpetic neuralgia. Pain Phys. 2020;23(2):E219. [PubMed]
- 22.Sun L, Peng C, Joosten E, Cheung CW, Tan F, Jiang W, Shen X. Spinal cord stimulation and treatment of peripheral or central neuropathic pain: mechanisms and clinical application. Neural Plast. 2021;2021(1):5607898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhongyi Z, Lei S, Botao L, et al. Clinical study on spinal cord electrical stimulation and pulsed radiofrequency in the treatment of herpetic neuralgia in the elderly. Chin J Neurol. 2019;18(10):1025–30. [Google Scholar]
- 24.Liu J, Zhang A, Ye X, et al. The effect of short-term spinal cord electrical stimulation on patients with postherpetic neuralgia and its effect on sleep quality. Neuro Endocrinol Lett. 2021;42(2):81–86. [PubMed]
- 25.Iseki M, Morita Y, Nakamura Y, Ifuku M, Komatsu S. Efficacy of limited-duration spinal cord stimulation for subacute postherpetic neuralgia. Ann Acad Med Singap. 2009;38(11):1004–6. [PubMed] [Google Scholar]
- 26.Ahn JH, Jang SR, Lee SB, Kim YW, Lee TK. Effect of spinal cord stimulation for patients with post-zoster neuralgia on the trunk: a case report. Asian Journal of Pain. 2019;5(1):17–9. [Google Scholar]
- 27.Xue S, Yang WJ, Cao ZX, Sun T. Comparing the efficacy and safety of short-term spinal cord stimulation and pulsed radiofrequency for zoster-related pain: a systematic review and meta-analysis. Medicine. 2022;101(11): e29073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan CF, Song T. Efficacy of pulsed radiofrequency or short-term spinal cord stimulation for acute/subacute zoster-related pain: a randomized, double-blinded, controlled trial. Pain Phys. 2021;24(3):215–22. [PubMed] [Google Scholar]
- 29.Rui W, Sun Mingjie Yu, Yang CF. Comparison of the efficacy of pulsed radiofrequency and short-term spinal cord electrical stimulation in the treatment of postherpetic neuralgia. Chin J Pain Med. 2019;25(11):831–6. [Google Scholar]
- 30.Söreskog, E. Spinal cord stimulation in chronic pain: a study of health outcomes and costs (Doctoral dissertation, Karolinska Institutet (Sweden). 2021.
- 31.Yan Liu MD, Han Yan BS, Yanwei Wang BS, Liang Qi BS, Jingmei Zhang BS. Short-Term supraorbital nerve stimulation and pain relief for acute and subacute ophthalmic herpetic neuralgia: a randomized controlled crossover trial. Pain Physician. 2024;27:203–12. [PubMed] [Google Scholar]
- 32.Motov S, Aftahy K, Jörger AK, Wagner A, Meyer B, Shiban E. High-frequency spinal cord stimulation in failed back surgery syndrome patients with predominant low back pain—single-center experience. Neurosurg Rev. 2021;44:2809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goudman L, Billot M, Duarte RV, Eldabe S, Rigoard P, Moens M. Gradation of clinical holistic response as new composite outcome to evaluate success in spinal cord stimulation studies for pain. Neuromodul Technol Neural Interface. 2023;26(1):139x46. [DOI] [PubMed]
- 34.Makharita MY, Amr YM. In response to comments on role of repeated paravertebral injection in the prevention of post herpetic neuralgia. Pain Physician. 2021;24(2):E258. [PubMed]
- 35.Szok D, Tajti J, Nyári A, Vécsei L. Therapeutic approaches for peripheral and central neuropathic pain. Behav Neurol. 2019;2019(1):8685954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isagulyan E, Tkachenko V, Semenov D, et al. The effectiveness of various types of electrical stimulation of the spinal cord for chronic pain in patients with postherpetic neuralgia: a literature review. Pain Res Manag. 2023;2023:6015680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.