Abstract
Background
The COVID-19 pandemic marked a unique period characterised by an extraordinary global virus spread. The collective effort to halt the transmission of the virus led to various public health initiatives, including a variety of COVID-19 vaccine trials. Many of these trials used adaptive methods to address the pandemic’s challenges, such as the need for rapid recruitment. These adaptive methods allow for modifications to the trial procedures without undermining the trial’s integrity, making the research process more flexible and efficient. However, recruiting participants for vaccine trials remains a considerable challenge. The aim of this qualitative evidence synthesis (QES) is to explore the factors that influence a person’s decision to participate in a COVID-19 vaccine trial. Lessons learned from this could help shape future trials’ design and conduct, particularly those conducted within a pandemic.
Methods
We conducted a systematic search for qualitative studies and mixed methods studies with a qualitative component in the WHO COVID-19 Research Database, MEDLINE, CINAHL, PsycINFO, Epistemomikos, Online Resource for Research in Clinical Trials (ORCCA), and the Cochrane COVID-19 Study Register. We used the best-fit framework synthesis approach and the Social Ecological Model as an a priori framework. We used the GRADE-CERQual approach to assess our confidence in the review findings.
Results
Five studies involving 539 participants were included. One of these studies included participants in a COVID-19 vaccine trial. In three of the studies, participants were asked hypothetically about their attitudes. Another study included people who had either not responded to or declined an invitation to participate in a COVID-19 vaccine trial.
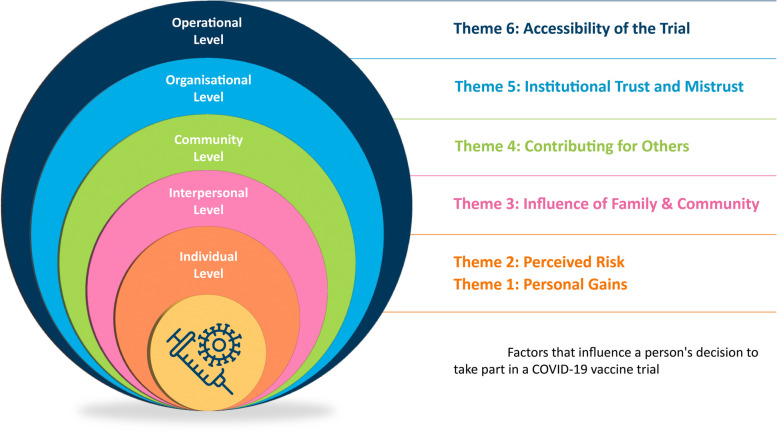
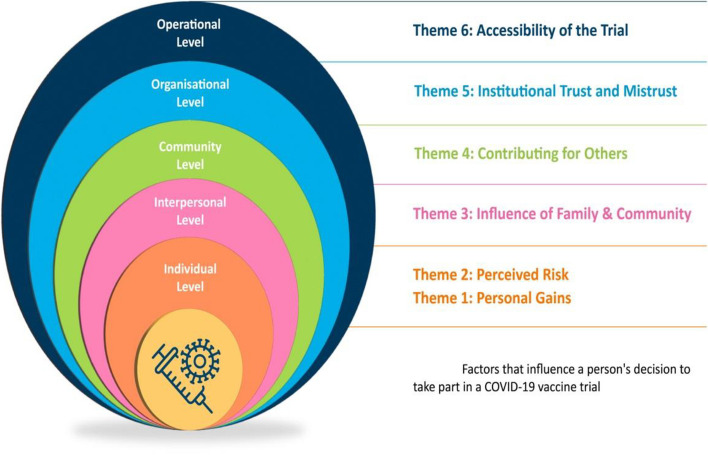
We developed six themes outlining the factors that influence a person’s decision to participate in a COVID-19 vaccine trial: (1) personal gains, (2) perceived risk, (3) influence of family and community, (4) contributing for others, (5) institutional trust and mistrust, and (6) accessibility of the trial.
Conclusion
This review sheds light on how people perceive the potential personal, family, and community advantages of trial participation and how these perceptions may be weighed against concerns about vaccine safety. The findings also point toward specific aspects of trial methodology to consider when designing COVID-19 vaccine trials.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-024-08670-0.
Keywords: Qualitative evidence synthesis, Recruitment, RCTs, COVID-19, Vaccination trials
Background
The World Health Organisation (WHO) defines COVID-19 as an infectious respiratory disease caused by the severe acute respiratory coronavirus 2 (SARS-CoV-2) [1]. Initially detected in Wuhan, China [2], the spread of the disease was declared a public health emergency of international concern (PHEIC) by the WHO on the 30 January 2020. On 11 March 2020, the director general of the WHO referred to the global outbreak of COVID-19 as a pandemic [3]. Whilst the WHO ended the PHEIC declaration on the 5 May 2023, the organisation continues to view COVID-19 as a global threat [4]. As of October 2024, over 700 million cases have been confirmed since COVID-19 was first detected, and nearly seven million deaths were reported [5].
Governments and global agencies/struggled to provide immediate and effective responses to identify and restrain COVID-19 amid uncertainties about the disease’s severity, the efficacy of interventions, and the unparalleled magnitude of its spread [6]. In some settings, this struggle was further impacted by underdeveloped public health systems and insufficient social support [6]. The pandemic was also accompanied by what the WHO has called an ‘infodemic’—an over-abundance of information (some accurate, some not), making it challenging for people to find trustworthy information when they need it [7].
Alongside the implementation and testing of a range of nonpharmaceutical interventions (NPIs) (e.g. social distancing, school closures, lockdown measures, mask-wearing) [8], the development of a vaccine against COVID-19 was prioritised by researchers, governments, and the pharmaceutical industry [9] in an attempt to limit the pandemic [2]. Despite unprecedented financial investment [10], streamlined development processes, and worldwide scientific collaboration [11] in developing COVID-19 vaccines, rigorous clinical trials remain an essential step in validating both the safety and efficacy of vaccine candidates [12]. The concerted effort to ensure the prompt testing of COVID-19 vaccine candidates [10] resulted in numerous vaccine trials. In response to the difficulties posed by the pandemic, including the need for quick participant enrolment, many trials employed adaptive trial designs. These designs allow for pre-specified modifications to trial parameters based on interim data analysis, including sample size re-estimation, treatment arm allocation ratios, and participant eligibility criteria, whilst preserving statistical rigor and trial validity [13]. The unprecedented scale and accelerated timeline of COVID-19 vaccine trials presents a unique opportunity to evaluate methodological innovations and challenges, providing valuable insights to enhance future clinical trial designs [14] and advance ‘vaccine science’ [10:16].
Achieving representative recruitment targets remains one of the ongoing major challenges in clinical trials, with inadequate recruitment cited as the most common cause for early termination of trials [15]. Although some improvement in trial recruitment has been noted in recent years [16], up to 40% of trials still fall short of achieving their intended recruitment numbers [17]. A systematic review exploring the methodological design and reporting practices of 35 COVID-19 trials (not exclusively COVID-19 vaccine trials) highlighted that 28.57% did not achieve their recruitment intentions [18]. In addition to concerns regarding participant numbers, trialists must also be cognisant of recruiting participants from all relevant demographic and ethnic groups [19]. Researchers designing trials must, therefore, consider strategies that include participants who are representative of those who may derive advantages from the outcome of the trial [20].
Recruitment to vaccine trials in general
Recruitment to vaccine trials is considered difficult for many reasons, not least because recruitment typically focuses on healthy volunteers [21]. In contrast to trial participants with pre-existing health conditions, there may be no therapeutic gains for healthy trial participants. Indeed, they may potentially be exposing themselves to adverse events [22]. A survey conducted in France during the early stages of the pandemic (March–April 2020) showed that 48% of the participants would certainly or probably take part in a COVID-19 vaccine trial [23]. The likelihood of agreeing to participate was linked to older age, male gender, and the individual’s perceived risk and occupation as a healthcare professional.
In contrast, disinterest in participating was linked to vaccine hesitancy [23]. In September–October 2020, an online cross-sectional survey in the UK noted that 41% of the participants were interested in participating in a COVID-19 vaccine trial, whilst 31% were unsure [2]. Male respondents, graduates, those without health issues, and those in the 40–59 age group were interested in participation [2]. The groups of people least interested in participating in a COVID-19 vaccine trial were aged 70 or older, from ‘villages’, or were members of the Black, Asian, and minority ethnic communities [2]. The use of qualitative research methodologies can help us explore the issues of trial recruitment further and has the potential to offer more in-depth insights into the experiences of potential participants of trials, including those who agree or decline to participate [24].
Recruitment to vaccine trials in the context of a pandemic or epidemic
A recently published Cochrane Qualitative Evidence Synthesis (QES) [25] explored the factors that influence a person’s decision to participate in a vaccine trial in the context of a pandemic or epidemic. Thirty-five qualitative studies were included in this review. One of these studies explored participation in a COVID-19 vaccine trial. The other studies examined participation in vaccine trials for the human immunodeficiency virus (HIV) (n = 25), tuberculosis (TB) (n = 1), the Ebola virus (n = 5), the Zika virus (n = 1), and participation in vaccine trials in general (n = 1). The Cochrane QES [25] identified several factors specific to vaccine trial participation during pandemics and epidemics. These factors include the potential stigma of participation, vaccine side effects, the role of community leaders in trial dissemination, and levels of trust in vaccine-developing entities [25]. However, given that most included studies focused on HIV vaccine trials (n = 25), with only one COVID-19 vaccine trial study, there remains uncertainty about whether these factors apply equally to COVID-19 vaccine trials.
Recruitment to vaccine trials in the context of COVID-19
Recruitment to COVID-19 vaccine trials may differ from recruitment to other trials, including trials done in the context of other pandemics. COVID-19 vaccine trials occurred rapidly, employing rapid recruitment strategies amidst an unparalleled time of uncertainty and misinformation [26]. To understand the factors influencing recruitment to a COVID-19 vaccine trial, it is essential to explore the factors influencing the decision-making of individuals considering trial participation. A QES, a systematic synthesis of qualitative research studies [27], can facilitate a comprehensive understanding.
This QES explores the factors that influence a person’s decision to participate in a COVID-19 vaccine trial.
Methods
The protocol for this review was registered with PROSPERO (Registration Number CRD42022382028). Methodologically, the team was guided by the chapter on Qualitative Evidence Synthesis in the Cochrane Handbook [28] and the Cochrane EPOC QES Protocol and Review Template [29]. The review is reported per the ENTREQ Statement [30] and the PRISMA Guidelines [31].
Eligibility criteria
We used the SPIDER (Sample, Phenomenon of Interest, Design, Evaluation and Research Type) Framework [32] to help refine our research question, identify the main concepts of our review, and generate search terms (Table 1).
Table 1.
SPIDER framework
| Sample | Adults (age > 18) who have been invited to participate in a COVID-19 vaccine trail and adults who have not received an invitation but who were asked about their attitudes towards participating |
|---|---|
| Phenomenon of interest | Factors that influence recruitment to COVID-19 vaccine trials |
| Design | Primary qualitative studies (e.g. ethnography, phenomenology, case studies, grounded theory studies) that use recognised methods of qualitative data collection (e.g. focus groups, interviews, observations) and analysis (e.g. thematic analysis, framework analysis). Mixed-methods studies, if data were collected and analysed using qualitative methods, and qualitative data can be extracted |
| Evaluation | The synthesis focused on people’s attitudes, motivations, barriers to participating in a COVID-19 vaccine trial |
| Research type | Qualitative or mixed methods |
The eligibility criteria are noted in Appendix 1. In summary:
Phenomenon of Interest: we aimed to synthesise the factors influencing recruitment to COVID-19 vaccine trials by exploring people’s attitudes, motivation and barriers to participating in a COVID-19 vaccine trial
Sample: we included all adults (age 18 years or older) who had been invited to participate in a COVID-19 vaccine trial and adults who had not received an invitation but were asked about their attitudes towards participating
Types of studies: we included primary research studies (qualitative and mixed methods) without date restriction. We included primary qualitative studies (e.g. ethnography, phenomenology, case studies, grounded theory studies) that use recognised methods of qualitative data collection (e.g. focus groups, interviews, observations) and analysis (e.g. thematic analysis, framework analysis). We included mixed-methods studies if data were collected and analysed using qualitative methods, and we could extract qualitative data
Information sources
We conducted an expansive search of the literature [33] for qualitative studies and mixed methods studies with a qualitative component using the following electronic databases: WHO COVID-19 Research Database, MEDLINE, CINAHL, Scopus, PsycINFO, Epistemomikos, ORCCA. We searched grey literature via ProQuest Dissertations and Theses Global and UK & Ireland. We searched the Cochrane COVID-19 Study Register for qualitative research on COVID-19 vaccine trials. In addition, we manually searched reference lists of included studies.
Search strategy
We developed pre-planned searches using search terms derived from the different concepts in the formulated review question [33]. Keywords and Medical Subject Headings (MeSH) terms were used for the concepts of the SPIDER framework (Table 1). An initial scoping search helped us to formulate our research question and identify key search terms. The search strategy was developed by SS and LB and peer-reviewed by the team, including a review author who is a health information specialist. The search was refined after the team’s feedback and adapted for each database searched. The final search strategy employed for MEDLINE is noted in Appendix 2. No language, geographic, or publication date restrictions were applied to our search. The final list of citations of the studies identified through the search was saved to Endnote, and duplicates were removed. The references were then imported to the data management tool Rayyan for screening. The search was conducted on 22 November 2022. Given the focus of this review and the rapid increase of studies related to COVID-19, an additional search, following the methods outlined in our original search, was conducted on 14 March 2023 to capture any new papers. Both searches were combined and are presented in Appendix 3.
Study selection
Two review authors (LB, SS) independently screened the studies using a two-step screening process. Title and abstract screening were first conducted in line with the eligibility criteria (Appendix 1); the two review authors piloted the process. The full text of papers potentially meeting the inclusion criteria was sourced, and full-text screening was conducted independently by the two review authors. This double-screening, independent approach was employed to ensure the inclusion criteria were used consistently and minimise errors. A third review author (CH) resolved any disagreements. The findings of the identification and screening phases of this QES are presented using a PRISMA Flow Diagram (Appendix 3).
Data extraction
Two review authors (LB, SS) extracted data from all included studies using a pre-designed data extraction form. Information extracted included characteristics of the study (design, data collection and analysis methods), population description, sampling procedures, and sample size. We extracted whether participants were invited to participate in a COVID-19 vaccine trial, the type of vaccine offered, and whether the scenario presented was real or hypothetical. In both the hypothetical and real situations, we extracted data to note if the participants agreed/would agree or declined/would decline to participate in the trial. The Characteristics of Included Studies Table is documented in Appendix 4.
We extracted data relating to the aim of this QES, such as themes and verbatim text extracts included in the individual studies relating to factors that influence a person’s decision to participate (or not) in COVID-19 vaccine trials. The data were extracted in line with the steps of best-fit framework synthesis [34] in that the data were extracted and aligned to the domains of an a priori framework (see the ‘Data synthesis’ section). Data extraction from one study, reported in two papers [35, 36], was linked and compared for completeness and to identify any contradictions.
Assessing the methodological limitations of included studies
An assessment of the methodological limitations of each included study was undertaken by SS and LB and verified by MD. To conduct this assessment, we used eight guiding questions utilised in other QESs [25, 37, 38]. The questions are based on the adaptation of the Critical Appraisal Skills Programme Checklist for Qualitative Research (CASP) tool and have undergone various iterations in line with the methodological progress of QES. The questions we used to assess methodological limitations are noted below; we omitted inquiries regarding the suitability of qualitative methodology, or the particular research design employed, as these aspects were already addressed in our inclusion criteria.
Are the setting(s) and context described adequately?
Is the sample strategy described, and is this appropriate?
Is the data collection strategy described, and is this appropriate?
Is the data analysis described, and is this appropriate?
Are the claims made/findings supported by sufficient evidence?
Is there evidence of reflexivity?
Does the study demonstrate sensitivity to ethical concerns?
Any other concerns?
The summary of methodological limitations is included in Appendix 5. The quality assessment was not used as an exclusion criterion; this appraisal allowed the team to explore the methodological quality of the studies contributing to the findings of this QES.
Data synthesis
The ‘best-fit framework’ synthesis [39, 40] guided our data analysis and synthesis approach. The ‘best-fit framework’ is a dual inductive and deductive synthesis approach [40]. It is used to build the synthesis of data onto an a priori framework whilst supporting the generation of new theory [34].
The social ecological model
The Social Ecological Model [41] was identified early in the process as a ‘good enough’ a priori framework, as defined by Booth and Carroll [34]. Here, ‘good enough’ refers to the guidance that the a priori framework selected need not be a perfect match for the data, as the framework will undergo modification(s) as required during the process of synthesis [27].
Aligned primarily with the psychologist Urie Broffenbrenner, the Social Ecological Model provides a framework to help understand human development [41]. The model has been used to conceptualise health and health behaviours broadly and suggests that health behaviour is influenced by several influencing and interacting factors in a person’s environment and the broader social context in which they exist [42]. The Social Ecological Model attributes multilevel influences on a person’s health and behaviours beyond their characteristics [43]. Bronfenbrenner’s original model placed an individual at the centre of four interrelated systems—microsystem, mesosystem, exosystem and macrosystem. Adapting Bronfenbrenner’s theory, McLeroy et al. [44] introduced five levels aligned to these systems. The model has undergone several iterations, but we were guided by the five-level model operationalised by the United National Children’s Fund (UNICEF) [45]. This model is based on the adaptations introduced by McLeroy and colleagues [44], which encompass individual, interpersonal, organisational/institutional, community, and societal/policy [46].
The Social Ecological Model has been used across a variety of research spaces, including health literacy [47], public health [48], risk management, and safe healthcare practices [49]. However, its use as a theoretical framework in the context of research focusing on vaccination behaviours and health behaviours during COVID-19 drew our attention. In brief, the model has been used to conceptualise vaccine acceptance [50, 51], to help understand the use of technology to promote vaccinations [43], and to explore health behaviours related to COVID-19, including vaccine intention [52–54] and the use of mask-wearing in public spaces [55]. Because of the Social Ecological Model’s focus on multilevel influences, we decided it would be a valuable model to communicate the multiple stimuli that may affect people’s attitudes, motivations, and barriers to participating in a COVID-19 vaccine trial.
The team used the stages of the ‘best-fit framework’ synthesis process described by Booth and Carroll [34]. As previously noted, whilst undertaking data extraction, one of the review team (SS) extracted the data from included studies according to the levels of the Social Ecological Model. Extracted data, when possible, were organised under the corresponding headings of the model (e.g. all data highlighting the perceived personal advantages of trial participation were noted under the Individual level of the Social Ecological Model). This was reviewed and discussed with another team member (LB). Both review authors were mindful of the deductive nature of this step and were careful not to force the data to align, as cautioned by Flemming and Noyes [27]. We planned that any data that did not relate to the a priori levels of the Social Ecological Model would be captured under new, additional headings that would add to our conceptualisation of the model. The two review authors independently coded the data using an inductive thematic synthesis approach. Coding was conducted in Microsoft Word using highlighting and labelling functions (see example in Appendix 6). One review author (LB) developed the themes by translating the codes and concepts between studies and rearranging data according to the relationship between and within themes. The initial draft of the themes was then presented to three other team members (PM, MD, CH), and during discussions, codes and descriptive themes were revisited, refined when needed, and agreed upon. During these meetings, we decided and agreed our conceptualisation of the Social Ecological Model. We also agreed the headings of the model to present our findings, in the context of this QES and the analytical themes (please see Appendix 6 for an example of Theme Development). Draft findings, including interpretations, were reviewed by the broader review team. Findings were organised according to levels of the Social Ecological Model, where the 5th level was renamed ‘Operational level’ (from the original ‘Policy Level’) to reflect trial design and other methodological factors that impacted recruitment to COVID-19 vaccine trials (see Appendix 6).
Assessment of confidence in the findings of this review
The Grading of Recommendations Assessment, Development and Evaluation-Confidence in the Evidence from Reviews of Qualitative Research (Grade-CERQual) approach [56] was used to assess our confidence in each review’s findings. This approach considers four components: methodological limitations, coherence, adequacy of data, and relevance [56]. Three review team members (LB, PM, CH) assessed the confidence of the findings using the four components, starting with an assumption of ‘high confidence’ in all findings, and we downgraded as we deemed appropriate in line with the methodological guidance offered by the Grade-CERQual Coordinating Team for applying the GRADE-CERQual Approach (https://www.cerqual.org/). The assessment of each finding was carried out independently by two review authors. The final assessments, judged as high, low, or moderate confidence in review findings, were presented and agreed upon with the broader team (Appendix 7).
Reflexive note
We offer a brief reflexive note to highlight any positions that may influence the process we have undertaken and the findings and discussion we present in this QES. Some of the teams are healthcare professionals; all are researchers in healthcare.
Most of this team have worked together previously. Except for one person, all team members are authors on a recently published Cochrane QES exploring the factors that influence a person’s decision to participate in a vaccine trial in the context of a pandemic or epidemic [25]. One study in that QES focused on participating in a COVID-19 vaccine trial. Some of the teams are authors of a Cochrane QES exploring factors influencing people’s decisions on trial participation [57].
The teams hold diverse perspectives regarding vaccination programmes and vaccine development. Their perspectives are informed by a variety of professional and personal experiences, including, but not limited to, working as a doctor specialising in infectious diseases during the COVID-19 pandemic (XHC), engaging with research within the domains of pandemic vaccine trials (RC), infectious diseases (XHC), trial recruitment (LB, PM, MD, DD, CH), and population health and healthcare services (SS, CG, AB).
The consensus among all team members is that participation in trials, whether during a pandemic or under normal circumstances, should be based on voluntary informed choice. The team also believes that vaccine trial participation decisions should be informed by readily available evidence concerning the potential advantages and disadvantages of trial involvement. This information should also include details about a vaccine’s potential side effects and areas of uncertainty.
The core methods of this review were conducted primarily by five review authors (LB, SS, PM, MD, CH), who contributed feedback on their interpretations to the broader research team. All the teams provided commentary on interpretations and synthesis. The diverse perspectives brought by the various review authors enabled a deeper and more nuanced understanding of the multifaceted situation of vaccine trial participation. It also provided an opportunity to identify and discuss any preconceptions, values, or beliefs held by individual review authors. LB and SS kept reflective notes throughout the lifespan of this review to document, e.g. the stages of synthesis, the team’s discussions, and the rationale for decisions made.
Results
Search and study selection
The database searches conducted on 22 November 2022 yielded 4292 records. Duplicates (n = 2095) were removed using Endnote, and 2197 remaining papers underwent title and abstract screening against the inclusion criteria; 2172 were excluded at this stage. The remaining 25 papers progressed to the full-text screening stage. Of these, five papers reporting four studies were included in the review. Two papers [35, 36] report the same study. The second database search on 14 March 2023 identified 486 new records. One hundred and seven duplicates were removed using Endnote, and the same 2-step screening process was undertaken as applied to the first search. Three hundred and seventy-eight studies were excluded at title and abstract screening; one study was included for full-text review; however, this study did not meet the inclusion criteria.
A commentary paper [58], excluded at full-text screening, discussed a qualitative study funded by the National Institute for Health Research (NIHR) through the Applied Research Collaboration (ARC) that met the inclusion criteria of this review. We could access the NIHR Report on the East Midland ARC website. The NIHR Report [59] was included in this review. Although the commentary paper was excluded [58], it did provide some methodological information (e.g. recruitment, when the data were collected, and data analysis method) not documented in the NIHR Report.
Five studies, reported in five published papers and one NIHR Report, were included in this QES, four qualitative studies and one mixed method study.
Description of included studies
The summary of the descriptive characteristics of the studies included in this QES is presented in Appendix 4. This summary reflects how the study authors described the participants of their studies and gives more detail about the communities contributing to the research.
In total, 539 people participated in the five studies included in this QES. Participants’ gender was often not reported. Where this was reported, 92 people identified as female and 44 as male. Study participants were pregnant women (n = 31) [60], ethnic minorities (as described by the study authors) (n = 442 in 2 studies) [59, 61] and vulnerable communities (as described by the study authors, please see Appendix 4 for more detail) (n = 15) [59], and Vietnamese-Americans (n = 20), a group of people reported to be overrepresented in the cases of COVID-19 reported [62] (see Appendix 4 for more detail). Participants’ age was also often not reported. Where this was reported, 82 participants ranged from 18 to 75 years old (see Appendix 4 for more detail).
In one study, participants [35, 36] participated in a COVID-19 vaccine trial. In three studies, the participants were hypothetically asked about their attitudes to participating in the COVID-19 vaccine [59, 60, 62]. One study included people who had either not responded to or declined an invitation to join a COVID-19 vaccine trial [61].
Two studies were conducted in the UK [59, 60] and three in the USA [35, 36, 61]. All studies were published between 2021 and 2022, but only three of the studies noted the timeframe of their data collection [35, 36, 60, 62]. In these studies, data were collected between April 2020 and October 2021 (the WHO referred to the global outbreak of COVID-19 as a pandemic in March 2020; the first COVID-19 vaccines were administrated in December 2020).
The data collection methods were individual interviews [35, 36, 59–62] and focus groups [59, 62]. Data collection was conducted via telephone, teleconference, or online videoconference platforms. One study team also offered in-person interviews [62]. However, it is not clear how many participants availed of that option. Thematic analysis or a thematic approach was identified as the analysis method used in all studies.
Assessment of the methodological limitations of included studies
The methodological limitations were assessed. We had no concerns in relation to the methodological quality of three of the included studies, minor concerns with regard to one study, moderate concerns about one study, and major concerns about one study (see Appendix 5 for more detail).
Confidence in the review findings
We developed six main themes outlining factors influencing a person’s decision to participate in a COVID-19 vaccine trial. Thirteen key findings are presented within these themes. We conducted a GRADE-CERQual assessment on the 13 findings. The summary is shown in Table 2, and a detailed evidence profile, including the rational for our assessments, is presented in Appendix 7.
Table 2.
Summary of findings
| Summary of review finding | Studies contributing to review finding | GRADE-CERQual assessment of confidence in the evidence | Explanation of GRADE-CERQual assessment |
|---|---|---|---|
| Individual level: Theme 1 Personal gains | |||
| Finding 1: People’s participation in a COVID-19 vaccine trial can be motivated by the potential for both financial and health benefits | Wentzell and Racila 2021 [35]; Ekezie 2020 [59]; Nguyen 2022 [62], Yuh 2022 [61] | Moderate confidence | Minor concerns regarding coherence and adequacy; moderate concerns regarding methodological limitations and relevance |
| Individual level: Theme 2 Perceived risk | |||
| Finding 2: People are hesitant to participate in COVID-19 vaccine trials and consider them risky and experimental if they are concerned about the speed of vaccine development, the unknown vaccine side-effects, and if they view trial participants as experimental subjects akin to ‘guinea pigs’ | Anderson 2021 [60], Ekezie 2020 [59], Yuh 2022 [61] | Moderate confidence | No/very minor concerns regarding coherence; minor concerns regarding adequacy; moderate concerns regarding methodological limitations and relevance |
| Finding 3: People may consider participating in COVID-19 vaccine trials in the future when the vaccine has undergone additional testing and a greater understating of vaccine side effects is established | Anderson 2021 [60], Ekezie 2020 [59] | Moderate confidence | Minor concerns regarding methodological limitations, no/very minor concerns regarding coherence, moderate concerns regarding adequacy and relevance |
| Interpersonal level: Theme 3 Influence of family and community | |||
| Finding 4: People’s decisions about participating in COVID-19 vaccine trials can be influenced by the perspectives of their family and the norms within their culture | Ekezie 2020 [59], Nguyen 2022 [62] | Moderate confidence | No/very minor concerns regarding coherence, moderate concerns regarding methodological limitations, adequacy and relevance |
| Finding 5: People’s perceptions of whether their participation in trials would be advantageous or disadvantageous for their family can influence their decision-making when considering COVID-19 vaccine trial participation | Ekezie 2020 [59]; Wentzell and Racila 2021, 2022 [35, 36] | Moderate confidence | No/very minor concerns regarding coherence, minor concerns regarding methodological limitations and adequacy; moderate concerns regarding relevance |
| Community level: Theme 4 Contributing for others | |||
| Finding 6: People’s desire to help others can serve as a motivation for participating in a COVID-19 vaccine trial | Ekezie 2020 [59], Nguyen 2022 [62], Wentzell and Racila 2021 and 2022 [35, 36] | Moderate confidence | No/very minor concerns regarding coherence. minor concerns regarding methodological limitations and adequacy, and moderate concerns regarding relevance |
| Finding 7: People’s desire to help communities return to pre-pandemic life can motivate participation in a COVID-19 vaccine trial | Ekezie 2020 [59], Wentzell and Racila 2021 and 2022 [35, 36] | Moderate confidence | No/very minor concerns regarding coherence; minor concerns regarding methodological limitations and adequacy; and moderate concerns regarding relevance |
| Finding 8: People’s perception of whether participating in COVID-19 vaccine trial would be beneficial or detrimental for their specific demographic group, along with their sense of responsibility to act as an exemplar for vaccination behaviour, can influence their consideration of COVID-19 vaccine trial participation | Anderson 2021 [60], Ekezie 2020 [59], Wentzell and Racila 2021 and 2022 [35, 36] | Moderate confidence | Minor concerns regarding methodological limitations, coherence and adequacy; and moderate concerns regarding relevance |
| Organisational level: Theme 5 Institutional trust and mistrust | |||
| Finding 9: People’s trust or mistrust of science can influence their willingness to participate in a COVID-19 vaccine trial | Ekezie 2020 [59]; Wentzell and Racila 2021, 2022 [35, 36] | Moderate confidence | No/very minor concerns regarding coherence; minor concerns regarding methodological limitations, adequacy, moderate concern regarding relevance |
| Finding 10: People’s lack of trust in the government can influence their willingness to participate in a COVID-19 vaccine trial | Ekezie 2020 [59], Nguyen 2022 [62], Yuh 2022 [61] | Moderate confidence | No/minor concerns regarding coherence; minor concerns regarding adequacy; moderate concerns regarding methodological limitations and relevance |
| Operational level: Theme 6 Accessibility of the trial | |||
| Finding 11: Some people from ethnic minorities and vulnerable communities suggest trial designs which address their cultural and community-specific requirements facilitate their consideration of COVID-19 vaccine trial participation | Ekezie 2020 [59], Nguyen 2022 [62], Yuh 2022 [61] | Moderate confidence | No/minor concerns regarding coherence; minor concerns regarding adequacy; and moderate concerns regarding methodological limitations and relevance |
| Finding 12: Some people from ethnic minorities and vulnerable communities view COVID-19 vaccine trials that prioritise logistical accessibility and minimise discomfort for participants as facilitators to COVID-19 vaccine trial participation | Ekezie 2020 [59], Yuh 2022 [61] | Low confidence | No/minor concerns regarding coherence; moderate concerns regarding methodological limitations, adequacy and relevance |
| Finding 13: For some people from ethnic minorities and vulnerable communities communicating trial information, transparently and understandably, involving individuals known within their own community in trial processes (such as recruitment) can serve as a facilitator to COVID-19 vaccine trial participation | Ekezie 2020 [59], Nguyen 2022 [62] | Low confidence | No/minor concerns regarding coherence; moderate concerns regarding methodological limitations, adequacy and relevance |
Our confidence in the findings was downgraded because of concerns about methodological limitations, relevance, and/or adequacy issues. Some of the studies had methodological limitations, including lack of evidence about research reflexivity, insufficient evidence to support the findings presented, and a lack of description about data analysis. We also had concerns about findings that were based on studies from only a limited number of types of settings and participants or that were based on studies that used hypothetical scenarios to explore people’s attitudes to trial participation. Finally, we had concerns about findings based only on a few studies or thin data.
Synthesis and findings
Our review findings aligned to the five levels presented in the Social Ecological Model. At the individual level, our findings suggest that the personal impact of participation guides attitudes to trial participation and that this impact could be viewed either positively or negatively. The intrapersonal level speaks to the influence of family and cultural background on a person’s decision. Here, our findings suggest that individuals’ perceptions of how their participation in a COVID-19 vaccine trial could impact their family may play a role in their decision-making process. At the community level, our findings describe how people’s perceptions of contributing to their wider community may impact on their attitudes to trial participation. At the organisation level, our findings describe how trust (and mistrust) in institutions such as governments and ‘science’ can determine trial participation. The policy level of the Social Ecological Model is often conceptualised as factors at the broad societal level that influence health decisions and behaviours. In the context of this review, we have titled that ‘Operational level’ relating to the specifics of the COVID-19 vaccine trial methods and processes the have the potential to influence people’s decisions whether or not to participate in a COVID-19 vaccine trial (e.g. aspects of the trial design and conduct—how the trial is planned, if the methodology proposed is inclusive, if practical supports exist for trial participants and if all aspects of trial information are communicated clearly).
Alongside each finding, we present our confidence in this finding by documenting our GRADE-CERQual assessment. A detailed evidence profile, including the studies contributing to each finding and the rational for our assessments, is presented in Appendix 7 Fig.1.
Fig. 1.
Factors that influence a person’s decision to take part in a COVID-19 vaccine trial
Individual level
Theme 1: Personal gains
This theme highlights the personal advantages of trial participation. Perceived benefits to health and finances served as determinants for peoples’ decisions concerning trial participation.
Finding 1: People’s participation in a COVID-19 vaccine trial can be motivated by the potential for both financial and health benefits (we have moderate confidence in this finding).
Financial gain and compensation could increase interest in trial participation [35, 59, 61, 62]. Focus groups of Vietnamese Americans identified the offer of money for trial participation as a facilitator to increase interest [62], whilst a large U.S. survey of Black, White, and Hispanic/Latinx identified lack of compensation as a barrier to participation [61]. The participants in [59] provided additional justifications for their views and suggested that financial incentives were part of a suite of considerations that could ‘minimise the discomfort of participation’ [59: 4]; the study authors suggested that providing financial support was one aspect necessary to tailor trial participation to the reality of people’s lives [59]. In Wentzell and Racila (2021) [35], some participants recalled monetary incentives as a reason for participating in a COVID-19 vaccine trial. This financial need as a motivating factor was primarily observed among younger participants (five of the six were noted to be aged in their twenties). One participant stated she was ‘broke’ and participated in the trial because of the money. Conversely, except for one person, the older participants of Wentzell and Racila (2021) dismissed the influence of payment and suggested that ‘well that’s not why I’m doing it’ [36: 2449].
One study’s participants [35, 36] suggested that personal health gains and the ‘desire to protect themselves from COVID-19’ aligned with their decision to participate in a Pfizer/BioNTech COVID-19 vaccine trial. The opportunity to receive a ‘potentially effective vaccine … early’ acted as motivators for participation [36: 2448].
Theme 2: Perceived risk
Participants also perceived trial participation as risky. This potential barrier to trial participation was influenced by people’s perceptions of the early phases of COVID-19 vaccine trials, the speed of vaccine development, and concerns about the limited knowledge regarding the vaccinations’ potential side effects.
Finding 2: People are hesitant to participate in COVID-19 vaccine trials and consider them risky and experimental if they are concerned about the speed of vaccine development, the unknown vaccine side-effects, and if they view trial participants as experimental subjects akin to ‘guinea pigs’ (we have moderate confidence in this finding).
Participants of Anderson 2021 [60], Ekezie 2020 [59], and Yuh 2022 [61] noted how their perception of risk can hinder participation in COVID-19 vaccine trials. Risk was associated with the rapid speed of COVID-19 vaccination development, leading to apprehension about the vaccine’s potential, yet unknown, side effects. Participants [59, 60] used the phrase guinea pig to describe trial participants—‘I am not going to be a guinea pig’ (ID79) [60:5], indicating their reluctance to be involved in a process perceived as experimental. Pregnant women in one study (Anderson 2021) [60] emphasised their focus on avoiding risks during pregnancy. They cited the newness of the COVID-19 vaccine and the potential unknown risks to their baby as reasons for their unwillingness to participate in a COVID-19 vaccine trial. The study authors report that three pregnant women held definite views that the vaccine should be tested on the non-pregnant population before considering its use during pregnancy, other study participants expressed uncertainty, even those who considered it a positive idea indicated they would not personal participate [60:4].
The perception of trial participants as guinea pigs being experimented on was also noted among participants from ethnic minorities and vulnerable communities (as described by study authors). A participant in Ekezie (2020) [59] noted that ‘everything would worry me, it is the law of average for these companies, someone will be used as guinea pigs; and it is my belief that this could be detrimental to Black People’ (African Caribbean participant) [59: 6]. Participants of Yuh (2022) [61] also expressed their reluctance to participate in trials, included ‘not wanting to be experimented upon within a research study’ [62: 3].
Finding 3: People may contemplate participating in COVID-19 vaccine trials in the future when the vaccine has undergone additional testing and a greater understating of vaccine side effects is established (we have moderate confidence in this finding).
Whilst people associated risks with the early stages of vaccine development, it did not necessarily mean they would completely rule out trial participation in the future [59, 60]. Participants expressed a willingness to consider trial participation once further testing was completed, and more knowledge was available about the vaccine’s safety and efficacy—‘One participant commented ‘maybe at a later stage after tests had shown the vaccine was safe in health people and those with different conditions first’’ (ID13) [60: 5]. For participants in the Ekezie study, ‘outcomes from people who previously participated in the trials, at different phases, was highlighted as a criterion to be satisfied before participant could be considered’ [59: 7].
Interpersonal level
Theme 3: Influence of family and community
This theme presents the influence of family and people’s regard for family on COVID-19 vaccine trial participation. At the interpersonal level, the normative influences from family and culture regarding the acceptability of vaccines were noted in trial participation decisions. The findings also highlight that the perceptions of benefits or risks associated with trial participation extend beyond the individual and can also encompass the potential impact on family members. The potential impact on the family, both in terms of benefits and risks, has the potential to act as a prompt or deterrent to trial participation.
Finding 4: People’s decisions about participating in COVID-19 vaccine trials can be influenced by the perspectives of their family and the norms within their culture (we have moderate confidence in this finding).
The views on vaccines within one’s family and social group can influence positions regarding trial participation. Study authors suggest that these views often align with cultural norms and beliefs regarding health, reflecting the cultural context in which individuals make decisions about COVID-19 vaccine trials. For example, study authors argued that participants from the Gypsy, Roma and Traveller Communities held ‘a fatalistic approach to health issues’ [59:7], believing that health outcomes are predetermined and cannot be prevented by interventions. Somali participants of the Ekezie study expressed ‘strong cultural and religious views against the vaccine, which included the possibility of ostracising those involved in vaccine trials, as they would be considered to be infected with COVID-19’ [59:7]. Additionally, concerns were raised about vaccine ingredients potentially conflicting with religious practices. It was also noted that trial could invoke ‘cultural unfamiliarity …’ potentially creating hesitancy to trial participation [59, 62]. The reluctance, influenced by cultural factors, was often considered at odds with scientific knowledge—‘I live in a multicultural society where often cultural views distort scientific facts so people would not trust science and data even if it shows that there is minimal risk’ (South Asian Participant) [59:6].
Finding 5: People’s perceptions of whether their participation in trials would be advantageous or disadvantageous for their family can influence their decision-making when considering COVID-19 vaccine trial participation (we have moderate confidence in this finding).
Protecting one’s family also influenced people’s views of COVID-19 vaccine trial participation. People in two studies [35, 36, 59] were particularly concerned about those they considered vulnerable and older. The concept of protection was informed by the perceived risks associated with vaccines versus the perceived risks associated with contracting COVID-19. Individuals who chose to participate in a COVID-19 vaccine were often motivated by a belief that this could protect their family [35, 36]—‘if I get the vaccine and if the vaccine worked, that would give me protection. But the bigger part of that point is that if it gave me protection, that I felt like if it kept me from getting it, I wouldn’t be able to give it to my wife or my children, that’s a bigger part of it’ [37: 6]. The sense of protection extended to their caregiving roles, it ‘place[ed] them in a position where they could engage with elderly or vulnerable family members and not worry that they were going to expose them to COVID 19’ [36: 2449]. Conversely, Ekezie (2020) [59] participants expressed concerns about the perceived risks they associated with the trials, and their wish to protect their families discouraged potential engagement with COVID-19 vaccine trials. Those with children and elderly family members were ‘sceptical about participating due to assumed higher risk’ [59: 5].
Community level
Theme 4: Contributing for others
The theme relates to helping others, especially those within one’s demographic group or community. By participating in COVID-19 vaccine trials, people felt that they could contribute towards ending the pandemic and assist in developing a vaccine that could benefit their demographic and ethnic group, acknowledging the importance of diverse participation in trials to ensure the effectiveness and safety of vaccination for all. Furthermore, people suggested their involvement in vaccine trials could influence future COVID-19 vaccine acceptance within their community.
Finding 6: People’s desire to help others can serve as a motivation for participating in a COVID-19 vaccine trial (we have moderate confidence in this finding).
A desire to help others motivated trial participation—‘individuals consistently stressed the potential of clinical trials to help the community’ [61: 490]. The appeal to help the wider community—a ‘collectivist approach and perspective’ [61: 492]—was considered, by Vietnamese Americans participants of focus groups, to be part of who they were; they possessed an intrinsic desire to be a ‘helper’—‘I’m just one of those people, I want to help people if I can’ (Participant 3) [36: 2448]. Others, participants of a phase 3 clinical trial, felt that it was their duty as ‘good citizen’ and that they should participate in a COVID-19 vaccine trial because ‘it should just be in mankind’s nature to want to help’ (Participant 4) [36: 2447].
Finding 7: People’s desire to help communities return to pre-pandemic life can motivate participation in a COVID-19 vaccine trial (we have moderate confidence in this finding).
Altruistic motives were presented as a way to help the community return to pre-pandemic existence to ‘restore normalcy’ for the future [36: 2449] by helping to end the pandemic—‘the more people who participate the quicker the studies proceed and we can get a vaccine that’s safe and effective out there for the people in general that would want or need it’ [37: 8]. The altruistic motivations for trial participation, for example—‘identification of a solution for overcoming the pandemic, supporting all of humanity and getting life back to normal’ [59: 5]—were not limited to those who were already participants of a trial but were also observed as a potential motivation to trial participation among individuals from ethnic minority and vulnerable communities [59].
Finding 8: People’s perception of whether participating in COVID-19 vaccine trial would be beneficial or detrimental for their specific demographic group, along with their sense of responsibility to act as an exemplar for vaccination behaviour, can influence their consideration of COVID-19 vaccine trial participation (we have moderate confidence in this finding).
In addition to helping the wider community, decisions around trial participation were also aligned with an opportunity to represent one’s community in testing COVID-19 vaccines. A participant in Anderson (2021) spoke of her willingness to ‘… have the test and be part of that [trial] if it helped other pregnant women, definitely’ (ID 24) [60: 6]. Others stated their ‘… hope of protecting groups characterized by shared demographic characteristics like race/ethnicity, age or sex to which they belonged’ [36: 2448]. Participants of Wentzell and Racila 2021, 2022 [35, 36] (all participants of a COVID-19 vaccine trial), ‘hoped by representing their groups in the trial, they would help ensure that the vaccine efficacy for members of that group’ [37: 7]. A different orientation to representing their ethnic group in a trial was held by participants of Ekezie (2020) [59]—‘a few people wanted non-BAME ethnicities to first start on the trials, and BAME communities join in later, as an assurance of equal participation’ [59: 5]. In keeping with the fears of being a research guinea pig (noted in theme 2), participants of the Ekezie study from the African and African Caribbean Communities were suspicious of trials and ‘proof’ was required to ensure that their contribution to research would be valued, and that trial participants included all ethnicities ‘so this group do not feel like they are the only ones participating in vaccine trials’ [59: 7].
Trial participants recognised the opportunity to be self-declared ‘role models’ within their communities and contribute to the vaccination effort. By actively participating in a COVID-19 vaccine trial, they aimed to instil vaccine confidence in others and inspire them to consider vaccination (participants of a phase 3 clinical trial). There was a sense of wanting to alleviate vaccination hesitancy by example—‘I posted it on Facebook because both my husband and I are in the trial, and hey we are doing this. I feel like it’s important for people to see. To inspire them into getting the vaccine when it comes out’ (P21) [36: 2448].
Organisational level
Theme 5: Institutional trust and mistrust
The concepts of trust and mistrust are noted in this theme. Trust in science and a wish to engage in activities that would support the advancement of science to address the COVID-19 pandemic played a role in motivating individuals to consider trial participation. Alternatively, mistrust in science, both in a broader sense and specifically related to the development of COVID-19 vaccines, people’s perceived suspicions about invitations to trial participation, and mistrust in governments and leadership were noted as potential deterrents to trial participation.
Finding 9: People’s trust or mistrust of science can influence their willingness to participate in a COVID-19 vaccine trial (we have moderate confidence in this finding).
People’s perceptions regarding science can influence people’s willingness to engage with COVID-19 vaccine trials [35, 36, 59]. The participants of one study [35] ‘overwhelmingly viewed their participation [in a COVID-19 vaccine trial] as support for the enterprise of science’ [36: 2448]. They ‘supported’ science, ‘believed in’ science and viewed their participation in a COVID-19 vaccine trial as their opportunity to contribute to ‘ground-breaking science’. Participants of Wentzell and Racila (2021) [35] described how they felt safe participating in a COVID-19 vaccine trial; they trusted science and the guidelines that were in place to ensure their safety within the trial. It was noted that eight of the participants in Wentzell and Racila (2021) [35] were healthcare professionals; others worked within scientific fields; they suggested that participating in a COVID-19 vaccine trial enabled them to ‘… practice what they preach’ and demonstrate their trust of medicine and science. Contrary opinions of science, but equally influential views on trial participation, were held by participants in Ekezie (2020) [59], with ‘the majority of people feeling anxious and scared of getting involved’ [59: 4]. Research distrust among people with mental health illnesses was noted as a potential barrier to trial participation and was often based on previous negative experiences. Participants from the African and African Caribbean communities in Ekezie (2020) [59] were suspicious of the invitations to include them in COVID-19 vaccine trials, stating that ‘they believed that since Black people were previously always placed ‘at the back of the line’ for everything, especially in healthcare and research, current insistence on their involvement was worrisome’ [59: 6]. This mistrust stemmed from various factors, including historical experiences and the perception that the development of vaccines was rushed without sufficient long-term data.
Finding 10: People’s lack of trust in the government can influence their willingness to participate in a COVID-19 vaccine trial (we have moderate confidence in this finding).
Whilst the participants of Wentzell and Racila (2021) [35], all participants of a COVID-19 vaccine trial, noted their ‘trust in science and its institutions’, mistrust in institutions, particularly in government institutions, was noted by participants in other studies [59, 61, 62]. Participants in three studies proposed that their lack of trust in the government influenced [61] or would influence [59, 62] their engagement with COVID-19 trials. This mistrust was viewed as a barrier to participation.
Operational level
Theme 6: Accessibility of the trial
This theme highlights the influence trial processes may have on individuals’ decision-making regarding COVID-19 vaccine trial participation. Participants emphasised key aspects related to trial processes, such as how the trial is planned, if the methodology proposed is inclusive, if practical supports exist for trial participants and if all aspects of trial information are communicated clearly and appropriately.
Finding 11: Some people from ethnic minorities and vulnerable communities suggest trial designs which address their cultural and community-specific requirements facilitate their consideration of COVID-19 vaccine trial participation (we have moderate confidence in this finding).
Participants from ethnic minorities and vulnerable communities [as noted in studies 59, 61, 62] highlighted that trials should be ‘tailored to people’s different circumstances and vulnerabilities’ [59: 4]. Planning trials with contributions from patients, public, and specific community groups was viewed by participants as a way to ensure that trials were sensitive to cultural and community-specific needs. Cultural examples were related to the vaccine used in the trial: ‘… needs to make sure what is included in the vaccine and its halal and certified’ (South Asian Participant) [59: 6] as well as to organisational factors: ‘they [participants from South Asian Communities] also advocated for the research to not fall during Ramadan or fasting festivals’ [59: 6].
Finding 12: Some people from ethnic minorities and vulnerable communities view COVID-19 vaccine trials that prioritise logistical accessibility and minimise discomfort for participants as facilitators to COVID-19 vaccine trial participation (we have low confidence in this finding).
Logistical factors about the trial, such as selecting easily accessible locations for its execution, transportation options, car parking facilities, and childcare arrangements, were highlighted as concerns for people from ethnic minorities and vulnerable communities. Gender-specific trial appointments, the availability of remote participation alternatives, and the selection of venues that were not environments requiring social distancing were also raised as issues that had the potential to minimise discomfort for trial participants. These considerations could positively influence individuals’ willingness to participate in a COVID-19 vaccine trial [59, 61].
Finding 13: For some people from ethnic minorities and vulnerable communities communicating trial information transparently and understandably, involving individuals known within their community in trial processes (such as recruitment) can serve as a facilitator to COVID-19 vaccine trial participation (we have low confidence in this finding).
Communication of trial information in a manner that made it understandable and useful to potential trial participants was raised by study participants as a potential facilitator to influence trial participation [59, 62]. Information about the vaccine, including its ingredients, side effects, and outcomes, the requirements of trial participation and COVID-19 more generally, was not always available in an appropriate and understandable format or a language potential participants understood [59, 62]. Given the scarcity of translated materials and people’s lack of knowledge about COVID-19, this could lead to a mistrust of COVID-19 trials [59, 62]. In addition to information materials in relevant languages, people with underlying medical conditions called for information addressing the impact of trial participation on their well-being [62]. Study authors suggest that information reflecting the religious and cultural appropriateness of the trial and the vaccination is also required [59]. A ‘trusted messenger’ from within one’s community was a suggestion offered by study authors to help effectively communicate trial information [61: 491]. The potential benefits of including ‘third sector organisations’ with people known to the members of ethnic minority and vulnerable communities (as described by the study authors) (e.g. outreach workers) to act as ‘intermediaries’ to support trial processes and to offer further support and advice to potential trial participants, was also noted by study authors [59: 7].
Discussion
This review has identified personal, social, cultural, and structural factors that can influence people’s participation in COVID-19 vaccine trials. To our knowledge, this is the first qualitative evidence synthesis that uses the multilevel Social Ecological Model to frame the multiple stimuli that may influence people’s attitudes, motivations, and barriers to participating in a COVID-19 vaccine trial. The findings demonstrate the intricate interaction between people’s perceptions of the personal, family, and community benefits to be gained by trial participation and how these perspectives may be counterbalanced by people’s uncertainty about the safety of a vaccine and the vaccine development process and their trust and mistrust of the stakeholders involved. The review findings also indicate trial methodology considerations in designing a COVID-19 vaccine trial, particularly ensuring trials are inclusive and accessible. Similar findings in relation to vaccine trial participation (unrelated to the public health urgency linked with pandemics or epidemics) were identified in a mixed methods narrative synthesis of 32 studies covering a broad spectrum of target diseases [63]. This synthesis explored factors influencing trial participation to identify strategies to improve recruitment for vaccine research [63] and generated findings concerning altruism, personal benefit, risk, trust, practical impactions, and stigma [ 63: 5]. The theme of ‘research vanguard’ (a sense of responsibility, setting an example) was identified by Dean and colleagues as a new emergent theme in the space of vaccine trial recruitment that has synergies with the findings we present in theme 4 of our QES.
Our review synthesised qualitative studies of people’s views and perceptions of participating in COVID-19 vaccine trials. Other reviews have explored people’s views and behaviour outside of the trial context and through quantitative studies. A recently published umbrella review of reviews identified elements influencing decision-making concerning COVID-19 vaccines in the general population [64]. The review explored factors contributing to COVID-19 vaccine hesitancy and summarised the findings of included reviews using a content-based structure. Thirty-one reviews were included by Kafadar and colleagues, including systematic reviews (n = 18), scoping reviews (n = 8), rapid reviews (n = 4), and a living review (n = 1) [64]; no QES was included. Study participants were from the general population, pregnant women, minority ethnic groups, the LGBTQ + community, older people, and healthcare professionals [64]. The review authors categorised the most noted reasons connected to COVID-19 vaccine hesitancy into four domains (contextual, individual, group and vaccine-specific factors) informed by the framework developed by the SAGE Working Group on Vaccine Hesitancy [65]. The contextual factors described by the review authors as most important to an increase in COVID-19 vaccine hesitancy included being female, younger, experiencing social inequalities, being pregnant, and having a ‘conservative’ religious belief [64]. Some social factors (e.g. being a healthcare professional, working in the private sector, living with others) and having a liberal orientation to political views were found to heighten people’s inclination to receive a COVID-19 vaccination. The most commonly observed individual and group factors related to information sources, trust, and personal experiences. Negative encounters with vaccines among family and friends, conflicting public health messages about vaccinations, lack of trust in public health authorities and health systems, and lack of trust in the government and vaccine developers were described as personal and trust factors associated with increased vaccine hesitancy [64]. Perceiving COVID-19 vaccination as a collective duty, bound to altruistic characteristics, was identified as drivers for vaccination. The most prominent vaccine-specific determinants that influenced people’s willingness to receive a vaccine were worries regarding vaccine safety and effectiveness, concerns about the rapid development, and inadequate knowledge about the vaccine [63]. Individuals’ knowledge of the COVID-19 virus and their perceptions of the disease’s severity, in comparison to perceived risks linked to the vaccine, influenced both vaccine hesitancy and acceptance [64]. Some of the findings of our QES align with concepts noted in the findings of the umbrella review [64].
To date, no QES synthesising individuals’ views and experiences about COVID-19 vaccines, exploring people’s COVID-19 vaccine acceptance and hesitancy, has been published. However, a published Cochrane QES Protocol highlights such a qualitative review is currently underway by J Maria and colleagues [66]. Juxtaposing the findings of the QES presented in this paper and the QES when published by J Maria et al. may provide further interesting insights about the connections between individuals’ views of COVID-19 vaccine acceptance and hesitancy and COVID-19 vaccine trial participation. However, some qualitative data available at the individual study level about people’s views of COVID-19 vaccines and vaccination initiatives show similarities to the thematic findings of the QES presented in this paper [67–72]. Some qualitative studies published in 2022 and 2023 across diverse geographical regions, including participants from various demographic backgrounds, highlight that people’s acceptance of COVID-19 vaccines and vaccination initiatives can be influenced by their concerns for their safety [67, 68] protecting themselves and their family and friends [69, 70]; a social obligation [71, 72]; the vaccination choices of their family and friends [69]; their trust in medical science, vaccine efficiency, and medical advice [68, 71, 72]; and a desire to adhere to pandemic public health guidelines whilst also engaging in travel and social activities, made possible by being vaccinated [67, 71].
Some qualitative data highlighting people’s hesitancy about COVID-19 vaccines and vaccine initiatives aligns with the thematic findings of this QES. For example, people’s hesitation about COVID-19 vaccination was associated with concerns about the safety of COVID-19 vaccines [67–69, 73], often exacerbated by lack of confidence in either science or governments [67, 68, 72] and apprehensions regarding the rapidity of vaccine development [67, 69]. Individuals expressing acceptance and hesitancy to COVID-19 vaccines expressed feeling overwhelmed by the abundance of information about COVID-19 [70] and fear or mistrust of COVID-19 messaging and messengers often linked to vaccine hesitancy [68, 71, 72]. The diverse narratives about COVID-19 vaccine safety can also cause uncertainty for some people, with Chandok and colleagues referring to these people as inbetweeners, where they are neither explicitly pro nor against vaccination [74]. This hesitancy to vaccinations, as opposed to the opposition [67], aligns with a ‘wait and see approach’ [75], also evident in finding 3 of our QES.
Comparing the findings of three qualitative evidence syntheses focusing on trial recruitment
Exploring the findings of this review alongside other qualitative reviews in the space of trial recruitment helps inform and advance trial methodology. This is the third recently conducted QES exploring the factors that impact the decision to participate in a trial. Houghton and colleagues [57] explored the factors that influence people’s decision whether to participate in a randomised trial, which encompassed a broad range of trials, including cancer, pregnancy and childbirth, medicine and surgery, mental health, and health promotion. The review by Meskell et al. [25] focused on the factors influencing a person’s decision to participate in a vaccine trial during a pandemic or epidemic. In the latter review [25], most of the studies included were related to HIV vaccine trials. The other studies are associated with Ebola, tuberculosis, Zika, and COVID-19. In the review reported in this paper, we focused on COVID-19 vaccine trials exclusively. The key findings from the three reviews are presented in a matrix (Appendix 8) for comparison.
The matrix table illuminates some interesting similarities and differences in how people are influenced when considering trial participation. For instance, in terms of reasons for taking part in a trial, contribution to society is evident across the three reviews, but personal benefit becomes less important when considering taking part in a vaccine trial for a pandemic or epidemic, specifically in the context of COVID-19. Whilst the concept of feeling like a ‘guinea pig’ was shared across the three reviews, for those being invited to a trial that may improve a healthcare condition that the individual may be living with, there was a process of weighing up ‘what have I got to lose?’. In the vaccine trials, because personal benefits were less evident, the decision around risk was more directly linked to the vaccine’s potential side effects.
Across the three reviews, family, friends, and healthcare professionals influence a person’s decision, and healthcare professionals influence a person’s decision on whether to participate in a trial. However, for vaccine trials, specifically COVID-19 vaccine trials, the influence of society more broadly becomes more of an influencing factor, such as people’s level of trust in their government being an influential factor.
A final comparison relates to considerations of accessibility, communication, and trial design. In the review conducted by Houghton and colleagues [57], communication of trial information was a significant factor: how the data was presented, at what time, and by whom. It was also important that the trial was not overly burdensome or disruptive. These were also important factors identified in Meskell and colleagues QES [25]. In the studies included in this review, whilst communication of trial information was influential, there was a greater emphasis on the trial being accessible for ethnic minorities and vulnerable groups. In terms of financial incentive, it was welcome but not overly influential for those considering healthcare trials more generally [57]. Financial incentives were more influential when considering participation in vaccine trials, as evident in this review and the QES conducted by Meskell et al. [25]. This could be attributed to the fact that healthy volunteers were being asked to consider participation, and as noted earlier, there were fewer personal benefits for taking part in a vaccine trial, whereas the types of trials included in the Houghton review [57], may mean improved healthcare and symptom relief.
The studies included in this review were conducted in the UK and USA only. It is important to acknowledge that the confidence in our review findings is primarily moderate (with two findings in which we have low confidence). However, there was an explicit representation of ethnic minorities and vulnerable groups in the included studies (as described by the study authors, please see Appendix 4), which may mean our review findings are more inclusive of these groups of people. A limitation of the Houghton et al. review [57] was the sampling technique used. It is acknowledged that the sampling approach favoured data richness and that if a sampling approach had been used that incorporated geographical spread and maximum representation from different participant groups, including ethnic minority groups and lower socioeconomic groups, there might have been differing perspectives. A limitation of the review by Meskell et al. [25] was the significant heterogeneity within the primary studies, with many studies including participants irrespective of their gender/sexual identity and socioeconomic backgrounds. This made conducting any sub-group analysis or comparisons across different populations complex. The review authors involved in the three reviews reflect and acknowledge their learning on the importance of inclusivity as a key lens through which to examine recruitment in trials and trials methodology research. The impact of the COVID-19 pandemic has been unevenly experienced by communities of Black, Asian, and minority ethnic backgrounds and vulnerable populations [76, 77]. Yet, these groups and communities have been insufficiently included in COVID-19 vaccine trials [20]. The findings of this QES presented in theme 6, ‘Accessibility of the trial’, highlight considerations in designing a COVID-19 vaccine trial, concerning making trials inclusive and accessible.
Strengths and limitations of this review
The strength of this review is the attention given to conducting a rigorous QES underpinned by a systematic and transparent approach. The findings considered the views of 539 people’s attitudes, motivations, and barriers to participating in a COVID-19 vaccine trial. The participants included pregnant women and people from ethnic minorities and vulnerable communities.
Overall, our confidence in the review findings was either moderate or low. Our confidence in most of the findings is limited by the need for more detail about the findings presented in the included studies and the confined geographical location in which the studies were conducted. The number of studies and the limited richness of some data restricted our ability to advance to a more analytical level. We must also acknowledge that in three of the included studies, the participants were hypothetically asked about their attitudes to participating in the COVID-19 vaccine. Whilst hypothetical scenarios can support study participants in highlighting the factors that could influence their decisions, we must also acknowledge the limitations of not capturing real experiences. In future updates of this review, when additional data are available, the team will conduct a sensitivity analysis and make a distinction between attitudes and experiences.
Conclusion
In conclusion, this QES provides an understanding of factors influencing a person’s decision to participate in a COVID-19 vaccine trial. The findings presented are more nuanced than a straightforward dichotomy, and peoples’ decisions are shaped by various influencing factors within their environment and the broader social context they inhabit. The matrix we present in Appendix 8, comparing the findings of three qualitative evidence syntheses focusing on trial recruitment, suggests parallels and distinctions in the factors that affect individuals’ decisions regarding COVID-19 vaccine trial participation compared to decisions related to general trial recruitment.
Supplementary Information
Additional file 1: Appendix 1. Eligibility Criteria.
Additional file 2: Appendix 2. Search Strategy.
Additional file 3: Appendix 3. PRISMA Flow Diagram.
Additional file 4: Appendix 4. Table of Characteristics of Included Studies.
Additional file 5: Appendix 5. Assessment of Methodological Limitations of Included Studies.
Additional file 6: Appendix 6. Example of Theme Development.
Additional file 7: Appendix 7. GRADE-CERQual Assessment.
Additional file 8: Appendix 8. Matrix Comparing the findings of Three QES focusing on Trial Recruitment.
Authors’ contributions
All authors conceptualised this review and developed the methodology. SS performed the searches. SS and LB reviewed the abstracts, selected the included studies, extracted the data, and conducted the initial data synthesis. LB, PM, MD, and CH refined the data synthesis. LB and MD assessed the methodological limitations of the included studies. LB, PM, and CH conducted the GRADE-CERQual assessment. LB, SS, and CH drafted the initial manuscript, and all review authors refined and reviewed the final manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme (grant agreement 101037867; VACCELERATE).
Data availability
All relevant data are provided within this paper and appendices.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organisation. Coronavirus disease (COVID 19). Available at https://www.who.int/health-topics/coronavirus#tab=tab_1. Accessed 5 Aug 2023.
- 2.Sethi S, Kumar A, Mandal A, Shaikh M, Hall CA, Kirk JMW, Moss P, Brookes MJ, Basu S. The UPTAKE study: implications for the future of COVID-19 vaccination trial recruitment in UK and beyond. Trials. 2021;22(1):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation (WHO) WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. 2020. Available at: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed 5 Aug 2023.
- 4.United Nations, UN News. WHO chief declares end of COVID-19 as a global health emergency. 2023. Available at https://news.un.org/en/story/2023/05/1136367. Accessed 28 Oct 2024.
- 5.World Health Organisation, COVID-19 pandemic. 2023. Available at https://covid19.who.int. Accessed 21 Nov 2023.
- 6.Haldane V, De Foo C, Abdalla SM, Jung AS, Tan M, Wu S, Chua A, Verma M, Shrestha P, Singh S, Perez T, Tan SM, Bartos M, Mabuchi S, Bonk M, McNab C, Werner GK, Panjabi R, Nordström A, Legido-Quigley H. Health systems resilience in managing the COVID-19 pandemic: lessons from 28 countries. Nat Med. 2021;27(6):964–80. [DOI] [PubMed] [Google Scholar]
- 7.Zarocostas J. How to fight an infodemic. Lancet. 2020;395(10225):676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talic S, Shah S, Wild H, Gasevic D, Maharaj A, Ademi Z, Li X, Xu W, Mesa-Eguiagaray I, Rostron J, Theodoratou E, Zhang X, Motee A, Liew D, Ilic D. Effectiveness of public health measures in reducing the incidence of COVID-19, SARS-CoV-2 transmission, and COVID-19 mortality: systematic review and meta-analysis. BMJ. 2021;375:e068302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munzert S, Ramirez-Ruiz S, Çalı B, Stoetzer LF, Gohdes A, Lowe W. Prioritization preferences for COVID-19 vaccination are consistent across five countries. Humanit Soc Sci Commun. 2022;9(1):439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ball P. The lightning-fast quest for COVID vaccines - and what it means for other diseases. Nature. 2021;589(7840):16–8. [DOI] [PubMed] [Google Scholar]
- 11.Kalinke U, Barouch DH, Rizzi R, Lagkadinou E, Türeci Ö, Pather S, Neels P. Clinical development and approval of COVID-19 vaccines. Expert Rev Vaccines. 2022;21(5):609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Plas JL, Roestenberg M, Cohen AF, Kamerling IMC. How to expedite early-phase SARS-CoV-2 vaccine trials in pandemic setting-a practical perspective. Br J Clin Pharmacol. 2021;87(5):2167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janiaud P, Hemkens LG, Ioannidis JPA. Challenges and lessons learned from COVID-19 trials: should we be doing clinical trials differently? Can J Cardiol. 2021;37(9):1353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawson DO, Wang MK, Kim K, Eikelboom R, Rodrigues M, Trapsa D, Thabane L, Moher D. Lessons from the COVID-19 pandemic and recent developments on the communication of clinical trials, publishing practices, and research integrity: in conversation with Dr. David Moher Trials. 2022;23(1):671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briel M, Elger BS, McLennan S, Schandelmaier S, von Elm E, Satalkar P. Exploring reasons for recruitment failure in clinical trials: a qualitative study with clinical trial stakeholders in Switzerland, Germany, and Canada. Trials. 2021;22(1):844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frampton GK, Shepherd J, Pickett K, Griffiths G, Wyatt JC. Digital tools for the recruitment and retention of participants in randomised controlled trials: a systematic map. Trials. 2020;21(1):478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walters SJ, Bonacho Dos Anjos Henriques-Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, Knox C, Nadin B, Rothwell J, Surtees M, Julious SA. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open. 2017;7(3):e015276 [DOI] [PMC free article] [PubMed]
- 18.Dillman A, Park JJH, Zoratti MJ, Zannat NE, Lee Z, Dron L, Hsu G, Smith G, Khakabimamaghani S, Harari O, Thorlund K, Mills EJ. Reporting and design of randomized controlled trials for COVID-19: a systematic review. Contemp Clin Trials. 2021;101:106239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witham MD, Anderson E, Carroll C, Dark PM, Down K, Hall AS, Knee J, Maier RH, Mountain GA, Nestor G, Oliva L, Prowse SR, Tortice A, Wason J, Rochester L. INCLUDE writing group. Developing a roadmap to improve trial delivery for under-served groups: results from a UK multi-stakeholder process. Trials. 2020;21(1):694. [DOI] [PMC free article] [PubMed]
- 20.Treweek S, Banister K, Bower P, Cotton S, Devane D, Gardner HR, Isaacs T, Nestor G, Oshisanya A, Parker A, Rochester L, Soulsby I, Williams H, Witham MD. Developing the INCLUDE Ethnicity Framework-a tool to help trialists design trials that better reflect the communities they serve. Trials. 2021;22(1):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasqualetti G, Gori G, Blandizzi C, Del Tacca M. Healthy volunteers and early phases of clinical experimentation. Eur J Clin Pharmacol. 2010;66(7):647–53. [DOI] [PubMed] [Google Scholar]
- 22.Cattapan A, Browne K, Halperin DM, Di Castri A, Fullsack P, Graham J, Langley JM, Taylor BA, McNeil SA, Halperin SA. Motivation for participating in phase 1 vaccine trials: Comparison of an influenza and an Ebola randomized controlled trial. Vaccine. 2019;37(2):289–95. [DOI] [PubMed] [Google Scholar]
- 23.Detoc M, Bruel S, Frappe P, Tardy B, Botelho-Nevers E, Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine. 2020;38(45):7002–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hennessy M, Hunter A, Healy P, Galvin S, Houghton C. Improving trial recruitment processes: how qualitative methodologies can be used to address the top 10 research priorities identified within the PRioRiTy study. Trials. 2018;19(1):584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meskell P, Biesty LM, Dowling M, Roche K, Meehan E, Glenton C, Devane D, Shepperd S, Booth A, Cox R, Chan XHS, Houghton C. Factors that impact on recruitment to vaccine trials in the context of a pandemic or epidemic: a qualitative evidence synthesis. Cochrane Database Syst Rev. 2023;9(9):MR000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langford AT. Health communication and decision making about vaccine clinical trials during a pandemic. J Health Commun. 2020;25(10):780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flemming K, Noyes J. Qualitative Evidence Synthesis: Where Are We at?International Journal of Qualitative Methods. 2021:20. 10.1177/1609406921993276.
- 28.Noyes J, Booth A, Cargo M, Flemming K, Harden A, Harris J, Garside R, Hannes R, Pantoja T, Thomas J. Chapter 22: Qualitative Evidence. In Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T. Page MJ, Welch VA (eds) Cochrane Handbook for Systematic Reviews of Interventions (version 6.3 updated Feb 2022): Cochrane.
- 29.Glenton C, Bohren MA, Downe S, Paulsen EJ, Lewin S, on behalf of Effective Practice and Organisation of Care (EPOC). EPOC Qualitative Evidence Synthesis: protocol and review template. Version 1.3. EPOC Resources for review authors. Oslo: Norwegian Institute of Public Health; 2022. Available at: http://epoc.cochrane.org/epoc-specific-resources-review-authors. Accessed 5 Aug 2023.
- 30.Tong A, Flemming K, McInnes E, Oliver S, Craig J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol. 2012;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooke A, Smith D, Booth A. Beyond PICO: the SPIDER tool for qualitative evidence synthesis. Qual Health Res. 2012;22(10):1435–43. [DOI] [PubMed] [Google Scholar]
- 33.Booth A. Searching for qualitative research for inclusion in systematic reviews: a structured methodological review. Syst Rev. 2016;5:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Booth A, Carroll C. How to build up the actionable knowledge base: the role of ‘best fit’ framework synthesis for studies of improvement in healthcare. BMJ Qual Saf. 2015;24(11):700–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wentzell E, Racila AM. The social experience of participation in a COVID-19 vaccine trial: subjects’ motivations, others’ concerns, and insights for vaccine promotion. Vaccine. 2021;39(17):2445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wentzell E, Racila AM. Collective care amid US individualism through COVID-19 vaccine trial participation. Med Anthropol. 2022;41(1):34–48. [DOI] [PubMed] [Google Scholar]
- 37.Ames HM, Glenton C, Lewin S. Parents’ and informal caregivers’ views and experiences of communication about routine childhood vaccination: a synthesis of qualitative evidence. Cochrane Database Syst Rev. 2017;2(2):CD011787. [DOI] [PMC free article] [PubMed]
- 38.Hanrahan V, Gillies K, Biesty L. Recruiters’ perspectives of recruiting women during pregnancy and childbirth to clinical trials: a qualitative evidence synthesis. PLoS ONE. 2020;15(6):e0234783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carroll C, Booth A, Cooper K. A worked example of “best fit” framework synthesis: a systematic review of views concerning the taking of some potential chemopreventive agents. BMC Med Res Methodol. 2011;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carroll C, Booth A, Leaviss J, Rick J. “Best fit” framework synthesis: refining the method. BMC Med Res Methodol. 2013;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broffenbrenner U. The ecology of human development: experiments by nature and design. Harvard: Harvard University Press; 1979. [Google Scholar]
- 42.Kilanowski JF. Breadth of the socio-ecological model. J Agromedicine. 2017;22(4):295–7. [DOI] [PubMed] [Google Scholar]
- 43.Kolff CA, Scott VP, Stockwell MS. The use of technology to promote vaccination: a social ecological model based framework. Hum Vaccin Immunother. 2018;14(7):1636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15(4):351–77. [DOI] [PubMed] [Google Scholar]
- 45.UNICEF. A social and behavioural changes agenda for inclusion and equity in education. Programme Brief. 2016. Available at https://www.unicef.org/esa/media/1756/file/UNICEF-ESA-2016-Program-Brief-Education-Inclusion.pdf%20UNICEF%20(2016). Accessed 5 Aug 2023.
- 46.Janzen S, Orr C, Terp SJ. Cognitive security and resilience: a social ecological model of disinformation and other harms with applications to COVID-19 vaccine information behaviours. 2022. 2nd Workshop Reducing Online Misinformation through Credible Information Retrieval, available via https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1871119. Accessed 5 Aug 2023.
- 47.McCormack L, Thomas V, Lewis MA, Rudd R. Improving low health literacy and patient engagement: a social ecological approach. Patient Educ Couns. 2017;100(1):8–13. [DOI] [PubMed] [Google Scholar]
- 48.Wold B, Mittelmark MB. Health-promotion research over three decades: the social-ecological model and challenges in implementation of interventions. Scand J Public Health. 2018;46(20_suppl):20–26. [DOI] [PubMed]
- 49.Litchfield I, Perryman K, Avery A, Campbell S, Gill P, Greenfield S. From policy to patient: Using a socio-ecological framework to explore the factors influencing safe practice in UK primary care. Soc Sci Med. 2021;277:113906. [DOI] [PubMed] [Google Scholar]
- 50.Bell J, Lartey B, Spickernell G, Darrell N, Salt F, Gardner C, Richards E, Fasakin L, Egbeniyi S, Odongo E, Ssenkungu J, Kouadio RK, Cissé M, Rérambyah ABAI, Adou M, West R, Sharma S. Applying a social-ecological model to understand factors impacting demand for childhood vaccinations in Nigeria, Uganda, and Guinea. SSM Qual Res Health. 2022. 10.1016/j.ssmqr.2022.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryan G, Avdic L, Daly E, Askelson N, Farris PE, Shannon J, McRee AL, Hanson J, Kenyon DB, Seegmiller L. Influences on HPV vaccination across levels of the social ecological model: perspectives from state level stakeholders. Hum Vaccin Immunother. 2021;17(4):1006–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Jayyousi GF, Sherbash MAM, Ali LAM, El-Heneidy A, Alhussaini NWZ, Elhassan MEA, Nazzal MA. Factors influencing public attitudes towards COVID-19 vaccination: a scoping review informed by the socio-ecological model. Vaccines (Basel). 2021;9(6):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Latkin CA, Dayton L, Yi G, Konstantopoulos A, Boodram B. Trust in a COVID-19 vaccine in the U.S.: a social-ecological perspective. Soc Sci Med. 2021;270:113684. [DOI] [PMC free article] [PubMed]
- 54.Lun P, Gao J, Tang B, Yu CC, Jabbar KA, Low JA, George PP. A social ecological approach to identify the barriers and facilitators to COVID-19 vaccination acceptance: a scoping review. PLoS One. 2022. 17(10):e0272642. [DOI] [PMC free article] [PubMed]
- 55.Casola AR, Kunes B, Cunningham A, Motley RJ. Mask use during COVID-19: a social-ecological analysis. Health Promot Pract. 2021;22(2):152–5. [DOI] [PubMed] [Google Scholar]
- 56.Lewin S, Booth A, Glenton C, Munthe-Kaas H, Rashidian A, Wainwright M, Bohren MA, Tunçalp Ö, Colvin CJ, Garside R, Carlsen B, Langlois EV, Noyes J. Applying GRADE-CERQual to qualitative evidence synthesis findings: introduction to the series. Implement Sci. 2018;13(Suppl 1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Houghton C, Dowling M, Meskell P, Hunter A, Gardner H, Conway A, Treweek S, Sutcliffe K, Noyes J, Devane D, Nicholas JR, Biesty LM. Factors that impact on recruitment to randomised trials in health care: a qualitative evidence synthesis. Cochrane Database Syst Rev. 2020;10(10):MR000045. [DOI] [PMC free article] [PubMed]
- 58.Ekezie W, Czyznikowska BM, Rohit S, Harrison J, Miah N, Campbell-Morris P, Khunti K. The views of ethnic minority and vulnerable communities towards participation in COVID-19 vaccine trials. J Public Health (Oxf). 2021;43(2):e258–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ekezie W, Czyznikowska B, Campbell-Morris P, Rohit S, Miah N, Harrison J, Hulley K, Khunti K, Akroyd C, Farooqi A. Public perceptions towards vaccine trial within ethnic minority and vulnerable communities’. UK: The National Institute for Health Research; 2020.
- 60.Anderson E, Brigden A, Davies A, Shepherd E, Ingram J. Maternal vaccines during the COVID-19 pandemic: a qualitative interview study with UK pregnant women. Midwifery. 2021;100:103062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuh T, Srivastava T, Fiore D, Schmidt H, Frank I, Metzger D, Momplaisir F. Using a patient portal as a recruitment tool to diversify the pool of participants in COVID-19 vaccine clinical trials. JAMIA Open. 2022;5(4):ooac091. [DOI] [PMC free article] [PubMed]
- 62.Nguyen C, Gilbert L, Diep J, Nguyen BM. Identifying facilitators and barriers to increasing COVID-19 vaccination and trial participation in vaccinated Vietnamese Americans. Health Equity. 2022;6(1):485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dean A, Rose F, Jones K, Scantlebury A, Adamson J, Knapp P. Why do people take part in vaccine trials? A mixed methods narrative synthesis. Patient Educ Couns. 2023;114: 107861. 10.1016/j.pec.2023.107861. [DOI] [PubMed] [Google Scholar]
- 64.Kafadar AH, Tekeli GG, Jones KA, Stephan B, Dening T. Determinants for COVID-19 vaccine hesitancy in the general population: a systematic review of reviews. Z Gesundh Wiss. 2022. 10.1007/s10389-022-01753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.The SAGE Working Group on Vaccine Hesitancy 2014. Available at https://www.asset-scienceinsociety.eu/sites/default/files/sage_working_group_revised_report_vaccine_hesitancy.pdf. Accessed 5 Aug 2023.
- 66.J Maria AR, Cooper S, Glenton C, Lewin S, Meskell P, Suleman M, Shepperd S. Adults’ views and experiences of vaccines developed in response to the COVID-19 pandemic: a qualitative evidence synthesis (Protocol), Cochrane Database of Systematic Reviews. 2022. Issue 4 Art. No.: CD015291.
- 67.Denford S, Mowbray F, Towler L, Wehling H, Lasseter G, Amlôt R, Oliver I, Yardley L, Hickman M. Exploration of attitudes regarding uptake of COVID-19 vaccines among vaccine hesitant adults in the UK: a qualitative analysis. BMC Infect Dis. 2022;22(1):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams SN, Armitage CJ, Dienes K, Drury J, Tampe T. Public decisions about COVID-19 vaccines: a UK-based qualitative study. PLoS ONE. 2023;18(3):e0277360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khorasani LN, Bastani A, Shen T, Kaur G, Shah ND, Juarez L, Heyman M, Grassian J, Cho AC, Hotez E. A qualitative investigation on COVID-19 vaccine hesitancy in neurodivergent communities. Vaccines (Basel). 2023;11(5):895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lockyer B, Moss RH, Endacott C, Islam S, Sheard L. Bradford Institute for Health Research COVID-19 Scientific Advisory Group. Compliant citizens, defiant rebels or neither? Exploring change and complexity in COVID-19 vaccine attitudes and decisions in Bradford, UK: Findings from a follow-up qualitative study. Health Expect. 2023;26(1):376–387. [DOI] [PMC free article] [PubMed]
- 71.Herry AM, Greaves D, Smith P, Toledo NA, Wildman A, Wildman T, Browne R, Chen M, Jones M, Aymat S. Facilitators of and barriers to COVID-19 vaccination in Grenada: a qualitative study. Rev Panam Salud Publica. 2023;47:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siu JY, Cao Y, Shum DHK. Perceptions of and hesitancy toward COVID-19 vaccination in older Chinese adults in Hong Kong: a qualitative study. BMC Geriatr. 2022;22(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abad N, Messinger SD, Huang Q, Hendrich MA, Johanson N, Fisun H, Lewis Z, Wilhelm E, Baack B, Bonner KE, Kobau R, Brewer NT. A qualitative study of behavioral and social drivers of COVID-19 vaccine confidence and uptake among unvaccinated Americans in the US April-May 2021. PLoS ONE. 2023;18(2):e0281497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chandok RS, Madar P, Majeed A. A qualitative study of factors influencing COVID-19 vaccine hesitancy among South Asians in London. JRSM Open. 2022;13(10):20542704221123430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dong L, Bogart LM, Gandhi P, Aboagye JB, Ryan S, Serwanga R, Ojikutu BO. A qualitative study of COVID-19 vaccine intentions and mistrust in Black Americans: recommendations for vaccine dissemination and uptake. PLoS ONE. 2022;17(5):e0268020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raisi-Estabragh Z, McCracken C, Bethell MS, Cooper J, Cooper C, Caulfield MJ, Munroe PB, Harvey NC, Petersen SE. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK Biobank. J Public Health (Oxf). 2020;42(3):451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yao H, Chen JH, Xu YF. Patients with mental health disorders in the COVID-19 epidemic. Lancet Psychiatry. 2020;7(4):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Appendix 1. Eligibility Criteria.
Additional file 2: Appendix 2. Search Strategy.
Additional file 3: Appendix 3. PRISMA Flow Diagram.
Additional file 4: Appendix 4. Table of Characteristics of Included Studies.
Additional file 5: Appendix 5. Assessment of Methodological Limitations of Included Studies.
Additional file 6: Appendix 6. Example of Theme Development.
Additional file 7: Appendix 7. GRADE-CERQual Assessment.
Additional file 8: Appendix 8. Matrix Comparing the findings of Three QES focusing on Trial Recruitment.
Data Availability Statement
All relevant data are provided within this paper and appendices.