Abstract
Background
Acute pulmonary embolism (APE) is a critical disease with a high mortality rate, some of the surviving patients may develop chronic thromboembolic pulmonary disease (CTEPD), which affects the patient’s prognosis. However, the research on the early diagnosis of CTEPD is limited. This study aimed to establish a prediction model for earlier identification of CTEPD.
Methods
This prospective study included 464 consecutive patients with APE confirmed between January 2020 and September 2023, at 7 centers from China. After follow-up for at least 3 months, the patients were divided into the CTEPD and non-CTEPD groups based on symptoms and computed tomography pulmonary angiography (CTPA) or pulmonary ventilation perfusion (V/Q) scans showing residual thrombosis. The independent risk factors for CTEPD were identified via univariate and multivariate logistic regression analyses. Next, a nomogram of predictive model was established, and validation was completed via decision curve analysis (DCA) and receiver operating characteristic curve analysis.
Result
In total, 130 (28%) patients presented with CTEPD, 17% (22/130) of CTEPD patients developed chronic thromboembolic pulmonary hypertension (CTEPH). Based on the multivariate analysis, a time interval from symptoms onset to diagnosis (time-to-diagnosis) ≥ 15 days (95% confidence interval [CI]: 3.392–14.972, p < 0.001), recurrent pulmonary embolism (RPE) (95%CI: 1.560–17.300, p = 0.007), right ventricular dysfunction (RVD) (95%CI: 1.042–6.437, p = 0.040), central embolus (95%CI: 1.776–7.383, p < 0.001) and residual pulmonary vascular obstruction (RPVO) > 10% (95%CI: 4.884–21.449, p < 0.001) were identified as the independent predictors of CTEPD. Then, A prediction model with a C-index of 0.895 (95% CI 0.863–0.927) was established for high-risk patients. The nomogram had an excellent predictive performance for earlier identification of CTEPD, with an area under the curve of 0.908 (95%CI: 0.875–0.941) in the training cohort and 0.875 (95%CI: 0.803–0.947) in the validation cohort.
Conclusion
The current study established and validated a reliable nomogram for predicting CTEPD, which would assist clinicians identify the high-risk patients for CTEPD earlier.
Keywords: Acute pulmonary embolism, Risk factors, Chronic thromboembolic pulmonary disease, Prediction model
Introduction
Acute pulmonary embolism (APE) is a group of clinical syndromes caused by endogenous or exogenous emboli blocking the pulmonary artery (PA) or its branches [1]. It is associated with significant mortality and morbidity and is the third most common cause of cardiovascular-related mortality after myocardial infarction and stroke [2].Anticoagulation, and reperfusion of the PA are the main treatments for APE [3].
With the significant advancements in treatment strategies, the overall short-term mortality of patients with APE has decreased. However, up to 50% of patients present with persistent perfusion defects after an APE [4, 5]. All patients with symptoms that can be attributed to post-thromboembolic fibrotic obstructions within the PA have chronic thromboembolic pulmonary disease (CTEPD), which can be accompanied by resting pulmonary hypertension (PH) or not [6–8]. CTEPD is a disease entity belonging to a broader group of diseases affecting the pulmonary arterial vasculature caused by pulmonary embolism (PE). Further, it is a progressive, life-threatening and irreversible disease [9]. The clinical manifestations of CTEPD are not specific. Hence, the condition is often misdiagnosed or missed. Current studies have commonly focused on the progression and risk factors of APE, and research on the early and timely detection of CTEPD is limited [10–12]. Hence, a simple and effective tool that can be used to assess the individualized risk and progression of CTEPD should be urgently developed to facilitate early intervention.
The current study aimed to analyze the risk factors associated with CTEPD during follow-up in patients with APE using a clinical prediction model for the early identification of high-risk patients.
Methods
Participants
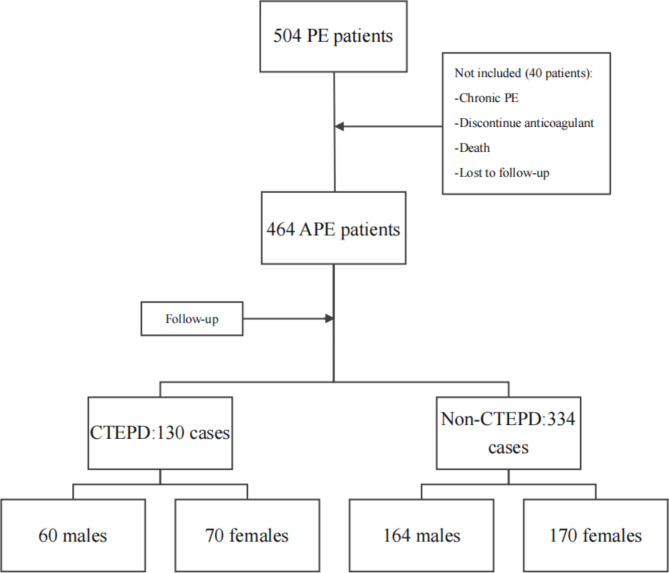
Consecutive patients with APE confirmed between January 2020 and September 2023, at 7 centers from China, were prospectively recruited. The inclusion criteria were as follows: (1) all patients who were diagnosed with APE on computed tomography pulmonary angiography (CTPA) or pulmonary ventilation perfusion (V/Q) scan [13]; (2) those aged > 18 years; (3) and those with complete imaging and clinical data and informed consent. The exclusion criteria were as follows: (1) patients with chronic pulmonary embolism; (2) those with PA filling defect due to vasculitis and other reasons; (3) and those who discontinued treatment with anticoagulants within 3 months and who were loss to follow-up [14]. Figure 1 shows the flowchart of participant selection.
Fig. 1.
The participants selected flowchart
This study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committees of Shandong Provincial Hospital (SWYX: no.2019-070). All patients provided informed consents.
Data collection
Data on demographic characteristics, symptoms, comorbidities conditions, risk factors, laboratory test, electrocardiogram, transthoracic echocardiography (TTE), lower extremity doppler ultrasonography, CTPA, and V/Q scan results were recorded. All patients were enrolled with TTE parameters, and PE was diagnosed by CTPA or V/Q scan. The images are partially interpreted by 2 experienced specialists.
CTEPD criteria: Patients with APE who received adequate anticoagulant therapy for at least 3 months and those who presented with similar symptoms such as dyspnea, perfusion defects, and organized fibrotic obstructions in patients with or without PH at rest [6]. In this study, according to the 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension, the diagnosis of CTEPD was considered for all APE patients who exhibited symptoms after at least 3 months of regular anticoagulation, and had residual thrombosis confirmed by CTPA or V/Q scan.
Visualization of right ventricular dysfunction (RVD) on TTE: dilated right ventricular (RV) with basal RV/left ventricular (LV) > 1.0, hypokinesis of the right ventricular free wall or abnormal motion of the interventricular septum, peak systolic gradient at the tricuspid valve > 30 mmHg, and tricuspid annular plane systolic excursion (TAPSE) <16 mm [13, 15, 16].
Residual pulmonary vascular obstruction (RPVO) is defined as incomplete repermeabilization of the pulmonary arteries after APE [4]. The anatomic severity of RPVO was quantified according to the Qanadli and Meyer score [17, 18].
All patients were randomly divided into the training and validation cohorts in a 7:3 ratio using R with RStudio.
Primary outcomes
Patients were followed up for at least 3 months via hospital visits or telephone interview, and the primary outcomes were about the diagnosis of CTEPD.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences software (version 25.0, IBM Inc, Chicago, IL, the USA). Continuous variables had a normal distribution and were expressed as mean ± standard deviation (SD). Meanwhile, categorical variables were expressed as frequency and percentages. The t-test was used to compare the continuous variables with the research results, and the 𝜒2 test was utilized to compare the categorized variables.
Univariate and multivariate analyses using logistic regression models were used to test the significance of independent risk factors. Variables with p values of < 0.05 in the univariate analysis were entered into the multivariate analysis to estimate the significance of each variable. A nomogram for predicting CTEPD was established based on the independent predictors, which were constructed using R (version 4.3.2; R Foundation for Statistical Computing) with RStudio (version 2023.12.0; RStudio). The performance of the nomogram to discriminate and calibrate was measured in the training cohort using Harrell concordance index (C-index), the area under the curve (AUC) of the receiver operating characteristic curve (ROC), calibration graphs, and decision curve analysis (DCA). Then the nomogram was validated in the validation cohort. P values of < 0.05 were considered significant.
Results
Baseline characteristics of the patients
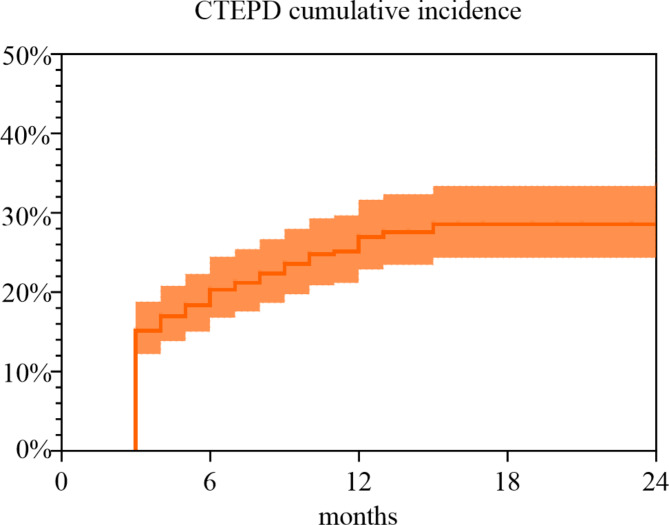
In total, 464 patients with APE were included in this study, and 70 (53.85%) patients were women. The average ages of CTEPD and non-CTEPD groups were 61.71(SD ± 14.24) and 64.39 (SD ± 13.85) years, respectively. Data on the basic characteristics listed in Table 1 were obtained from the hospital electronic medical records. The median follow-up time was 12 (interquartile range: 3–24) months. The overall incidence of CTEPD was 28%, 17% (22/130) of CTEPD patients developed chronic thromboembolic pulmonary hypertension (CTEPH). Figure 2 shows the cumulative incidence of CTEPD. Dyspnea, chest pain, time interval from symptom onset to diagnosis (time-to-diagnosis) ≥ 15 days, recurrent PE (RPE), cancer, connective tissue diseases (CTD), RVD, central embolus, RPVO > 10%, risk stratification and simplified pulmonary embolism severity index (sPESI) score were found to be important clinical indicators of CTEPD (all p values < 0.05). The remaining indicators did not significantly differ.
Table 1.
Baseline characteristics on patients of APE
| Total (n = 464) | CTEPD Group (n = 130) | Non-CTEPD Group (n = 334) | p value | |
|---|---|---|---|---|
| Male (n, %) | 224 (48.28%) | 60 (46.15%) | 164 (49.10%) | 0.568 |
| Age (years) | 63.12 ± 14.00 | 61.71 ± 14.24 | 64.39 ± 13.85 | 0.065 |
| BMI (kg·m− 2) | 24.58 ± 3.90 | 24.59 ± 3.90 | 24.60 ± 3.90 | 0.982 |
| Temperature (℃) | 37.17 ± 13.91 | 38.82 ± 26.27 | 36.53 ± 0.42 | 0.623 |
| SBP (mmHg) | 128.24 ± 18.82 | 126.88 ± 15.91 | 128.77 ± 19.83 | 0.332 |
| DBP (mmHg) | 82.23 ± 40.31 | 79.70 ± 11.41 | 83.22 ± 46.96 | 0.384 |
| Heart rate (beats/min) | 83.95 ± 13.94 | 85.75 ± 13.80 | 83.26 ± 13.96 | 0.085 |
| Respiratory rate (breaths/min) | 19.92 ± 5.75 | 20.29 ± 6.27 | 19.78 ± 5.54 | 0.407 |
| Symptoms | ||||
| Dyspnea (n, %) | 272 (58.62%) | 98 (75.38%) | 174 (52.10%) | < 0.001 |
| Chest pain (n, %) | 40 (8.62%) | 5 (3.85%) | 35 (10.48%) | 0.028 |
| Hemoptysis (n, %) | 28 (6.03%) | 7 (5.38%) | 21 (6.29%) | 0.714 |
| Syncope (n, %) | 23 (4.96%) | 10 (7.69%) | 13 (3.89%) | 0.096 |
| Time-to-diagnosis ≥ 15 days (n, %) | 150 (32.33%) | 83 (63.85%) | 67 (20.06%) | < 0.001 |
| RPE (n, %) | 31 (6.68%) | 23 (17.69%) | 8 (2.40%) | < 0.001 |
| Comorbidities | ||||
| Hypertension (n, %) | 153 (32.97%) | 37 (28.46%) | 116 (34.73%) | 0.198 |
| CHD (n, %) | 92 (19.83%) | 27 (20.77%) | 65 (19.46%) | 0.751 |
| CLD (n, %) | 49 (10.56%) | 19 (14.62%) | 30 (8.98%) | 0.079 |
| Diabetes (n, %) | 54 (11.64%) | 13 (10.00%) | 41 (12.28%) | 0.493 |
| Nervous system disease (n, %) | 51 (10.99%) | 13 (10.00%) | 38 (11.38%) | 0.663 |
| Venous thrombus (n, %) | 253 (54.53%) | 78 (60.00%) | 175 (52.40%) | 0.140 |
| Cancer (n, %) | 139 (29.96%) | 15 (11.54%) | 124 (37.13%) | < 0.001 |
| CTD (n, %) | 42 (9.05%) | 18 (13.85%) | 24 (7.19%) | 0.027 |
| Risk factors | ||||
| Smoking history (n, %) | 135 (29.09%) | 38 (29.23%) | 97 (29.04%) | 0.968 |
| Surgery (n, %) | 47 (10.13%) | 10 (7.69%) | 37 (11.08%) | 0.280 |
| Trauma (n, %) | 16 (3.45%) | 3 (2.31%) | 13 (3.89%) | 0.403 |
| RVD (n, %) | 67 (14.44%) | 44 (33.85%) | 23 (6.89%) | < 0.001 |
| Central embolus | 145 (31.25%) | 66 (50.77%) | 79 (23.65%) | < 0.001 |
| RPVO > 10% (n, %) | 115 (24.78%) | 78 (60.00%) | 37 (11.08%) | < 0.001 |
| Risk stratification | < 0.001 | |||
| Low-risk (n, %) | 243 (52.37%) | 50 (38.46%) | 193 (57.78%) | |
| Moderate-risk (n, %) | 217 (46.77%) | 77 (59.23%) | 140 (41.92%) | |
| High risk (n, %) | 4(0.86%) | 3(2.31%) | 1 (0.30%) | |
| sPESI | 0.003 | |||
| 1 (n, %) | 163 (35.13%) | 36 (27.69%) | 127 (38.02%) | |
| 2 (n, %) | 33 (7.11%) | 4 (3.08%) | 29 (8.68%) | |
| 3 (n, %) | 2 (0.43%) | 1 (0.77%) | 1 (0.30%) | |
| > 3 (n, %) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Initial anticoagulant | 0.402 | |||
| LMH (n, %) | 430 (92.67%) | 118 (90.77%) | 312 (93.41%) | |
| DOACs (n, %) | 27 (5.82%) | 9 (6.92%) | 18 (5.39%) | |
| LMH and DOACs (n, %) | 7 (1.51%) | 3 (2.31%) | 4 (1.20%) | |
| Time-to-anticoagulant ≥ 6 h (n, %) | 172 (37.07%) | 49 (37.69%) | 123 (36.83%) | 0.862 |
Data are presented as mean ± standard deviation (SD) or n (%). BMI, body mass index; CHD, coronary heart disease; CLD, chronic lung disease; CTD, connective tissue diseases; DBP, diastolic blood pressure; DOACs, direct oral anticoagulants; LMH, low molecular heparin; RPE, recurrent pulmonary embolism; RPVO, residual pulmonary vascular obstruction; RVD, right ventricular dysfunction; sPESI, simplified pulmonary embolism severity index; SBP, systolic blood pressure
Fig. 2.
Cumulative incidence of CTEPD in 464 patients followed after APE
Selection of clinical risk factors
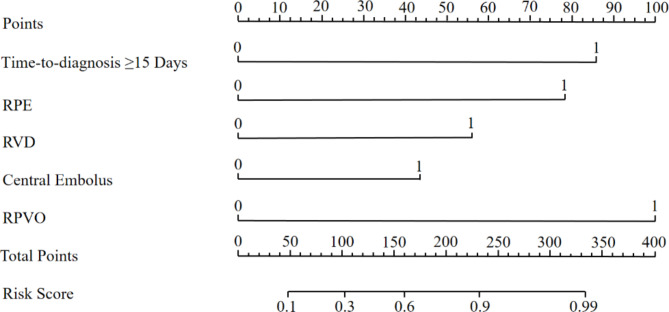
Table 2 shows the clinical data of the patients in the training cohort (n = 324) and validation cohort (n = 140). The independent predictors of CTEPD were screened based on univariate and multivariate analyses, as shown in Table 3. Dyspnea, chest pain, time-to-diagnosis ≥ 15 days, RPE, cancer, CTD, RVD, central embolus, RPVO > 10%, risk stratification, sPESI, platelet (PLT) count, hemoglobin levels, platelet-to-lymphocytes ratio (PLR), prothrombin time (PT), international normalized ratio of prothrombin time (PT-INR), D-dimer, total bilirubin (TBIL), blood urea nitrogen (BUN), uric acid (UA) and N-terminal-pro-B-type natriuretic peptide levels (NT-pro-BNP) ≥ 600 pg/mL (all p values < 0.05) were possible indicators of CTEPD by the univariate logistic regression analysis. Based on the multiple logistic regression analysis time-to-diagnosis ≥ 15 days (OR: 7.126, 95%CI: 3.392–14.972), RPE (OR: 5.195, 95%CI: 1.560–17.300), RVD (OR: 2.590, 95%CI: 1.042–6.437), central embolus (OR: 3.621, 95%CI: 1.776–7.383) and RPVO > 10% (OR: 10.235, 95%CI: 4.884–21.449) were independent risk factors of CTEPD, with p values < 0.05. Finally, time-to-diagnosis ≥ 15 days, RPE, RVD, central embolus, and RPVO > 10% were incorporated into the establishment of the clinical factor model.
Table 2.
Comparison of clinical indicators in training and validation cohorts
| Training cohort (n = 324) | Validation cohort (n = 140) | |||||
|---|---|---|---|---|---|---|
| CTEPD (n = 89) | Non-CTEPD (n = 235) | p value | CTEPD (n = 41) | Non-CTEPD (n = 99) | p value | |
| Male (n, %) | 42 (47.19%) | 115 (48.94%) | 0.779 | 18 (43.90%) | 49 (49.49%) | 0.547 |
| Age (years) | 61.25 ± 14.36 | 64.25 ± 13.30 | 0.078 | 62.71 ± 14.10 | 64.72 ± 15.15 | 0.465 |
| BMI (kg·m− 2) | 24.21 ± 3.89 | 24.81 ± 3.94 | 0.287 | 25.44 ± 3.85 | 23.99 ± 3.78 | 0.086 |
| Temperature (℃) | 39.89 ± 31.75 | 36.55 ± 0.40 | 0.613 | 36.50 ± 0.39 | 36.48 ± 0.46 | 0.857 |
| SBP (mmHg) | 127.80 ± 15.73 | 128.29 ± 19.55 | 0.846 | 124.90 ± 16.31 | 130.02 ± 20.53 | 0.159 |
| DBP (mmHg) | 79.54 ± 11.37 | 83.97 ± 55.50 | 0.508 | 80.05 ± 11.63 | 81.42 ± 11.55 | 0.52 |
| Heart rate (beats/min) | 86.13 ± 14.08 | 83.43 ± 14.62 | 0.134 | 84.90 ± 13.28 | 82.86 ± 12.29 | 0.381 |
| Respiratory rate (breaths/min) | 19.67 ± 2.30 | 19.52 ± 2.66 | 0.627 | 21.63 ± 10.60 | 20.39 ± 9.33 | 0.505 |
| Symptoms | ||||||
| Dyspnea (n, %) | 65 (73.03%) | 121 (51.49%) | 0.001 | 33 (80.49%) | 53 (53.54%) | 0.004 |
| Chest pain (n, %) | 5 (5.62%) | 26 (11.06%) | 0.144 | 0 (0.00%) | 9 (9.09%) | 0.999 |
| Hemoptysis (n, %) | 5 (5.62%) | 14 (5.96%) | 0.908 | 2 (4.88%) | 7 (7.07%) | 0.632 |
| Syncope (n, %) | 8 (8.99%) | 8 (3.40%) | 0.046 | 2 (4.88%) | 5 (5.05%) | 0.966 |
| Time-to-diagnosis ≥ 15 days (n, %) | 61 (68.54%) | 51 (21.70%) | < 0.001 | 22 (53.66%) | 16 (16.16%) | < 0.001 |
| RPE (n, %) | 16 (17.98%) | 5 (2.13%) | < 0.001 | 7 (17.07%) | 3 (3.03%) | 0.009 |
| Comorbidities | ||||||
| Hypertension (n, %) | 23 (25.84%) | 86 (36.60%) | 0.069 | 14 (34.15%) | 30 (30.30%) | 0.656 |
| CHD (n, %) | 20 (22.47%) | 47 (20.00%) | 0.624 | 7 (17.07%) | 18 (18.18%) | 0.876 |
| CLD (n, %) | 14 (15.73%) | 22 (9.36%) | 0.107 | 5 (12.20%) | 8 (8.08%) | 0.448 |
| Diabetes (n, %) | 8 (8.99%) | 32 (13.62%) | 0.262 | 5 (12.20%) | 9 (9.09%) | 0.579 |
| Nervous system disease (n, %) | 10 (11.24%) | 28 (11.91%) | 0.865 | 3 (7.32%) | 10 (10.10%) | 0.596 |
| Venous thrombus (n, %) | 50 (56.18%) | 121 (51.49%) | 0.451 | 28 (68.29%) | 54 (54.55%) | 0.135 |
| Cancer (n, %) | 8 (8.99%) | 94 (40.00%) | < 0.001 | 7 (17.07%) | 30 (30.30%) | 0.111 |
| CTD (n, %) | 16 (17.98%) | 20 (8.51%) | 0.018 | 2 (4.88%) | 4 (4.04%) | 0.824 |
| Risk factors | ||||||
| Smoking history (n, %) | 28 (31.46%) | 63 (26.81%) | 0.406 | 10 (24.39%) | 34 (34.34%) | 0.251 |
| Surgery (n, %) | 7 (7.87%) | 26 (11.06%) | 0.398 | 3 (7.32%) | 11 (11.11%) | 0.499 |
| Trauma (n, %) | 2 (2.25%) | 5 (2.13%) | 0.951 | 1 (2.44%) | 8 (8.08%) | 0.243 |
| RVD (n, %) | 28 (31.46%) | 16 (6.81%) | < 0.001 | 16 (39.02%) | 7 (7.07%) | < 0.001 |
| Central embolus | 47 (52.81%) | 61 (25.96%) | < 0.001 | 19 (46.34%) | 18 (18.18%) | 0.001 |
| RPVO > 10% (n, %) | 55 (61.80%) | 23 (9.79%) | < 0.001 | 23 (56.10%) | 14 (14.14%) | < 0.001 |
| Risk stratification | 0.003 | 0.009 | ||||
| Low-risk (n, %) | 34 (38.20%) | 130 (55.32%) | 16 (39.02%) | 63 (63.64%) | ||
| Moderate-risk (n, %) | 53 (59.55%) | 105 (44.68%) | 24 (58.54%) | 35 (35.35%) | ||
| High risk (n, %) | 2 (2.25%) | 0 (0.00%) | 1 (2.44%) | 1 (1.01%) | ||
| sPESI | 0.001 | 0.776 | ||||
| 1 (n, %) | 19 (21.35%) | 93(39.57%) | 17 (41.46%) | 34 (34.34%) | ||
| 2 (n, %) | 4 (4.49%) | 24 (10.21%) | 0 (0.00%) | 5 (5.05%) | ||
| 3 (n, %) | 1 (1.12%) | 1 (0.43%) | 0 (0.00%) | 0 (0.00%) | ||
| > 3 (n, %) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||
| Initial anticoagulant | 0.524 | 0.581 | ||||
| LMH (n, %) | 81 (91.01%) | 220 (93.62%) | 37 (90.24%) | 92 (92.93%) | ||
| DOACs (n, %) | 6 (6.74%) | 13 (5.53%) | 3 (7.32%) | 5 (5.05%) | ||
| LMH and DOACs (n, %) | 2 (2.25%) | 2 (0.85%) | 1 (2.44%) | 2 (2.02%) | ||
| Time-to-anticoagulant ≥ 6 h (n, %) | 35 (39.33%) | 95 (40.43%) | 0.857 | 14 (34.15%) | 28 (28.28%) | 0.491 |
| Blood test | ||||||
| WBC count (10^9/L) | 6.73 ± 2.51 | 6.92 ± 2.74 | 0.569 | 6.77 ± 2.93 | 6.33 ± 2.05 | 0.307 |
| PLT count (10^9/L) | 224.12 ± 68.18 | 248.03 ± 95.63 | 0.033 | 221.39 ± 68.29 | 227.69 ± 70.18 | 0.626 |
| Hemoglobin levels (g/L) | 133.18 ± 21.81 | 124.59 ± 21.51 | 0.002 | 134.15 ± 20.92 | 125.91 ± 20.81 | 0.038 |
| RDW (%) | 14.14 ± 3.97 | 14.46 ± 4.51 | 0.557 | 13.53 ± 1.45 | 14.80 ± 5.43 | 0.182 |
| Lymphocytes count (10^9/L) | 1.7 ± 0.64 | 1.66 ± 0.90 | 0.654 | 1.57 ± 0.48 | 1.65 ± 0.57 | 0.466 |
| Monocytes count (10^9/L) | 0.65 ± 1.10 | 0.58 ± 0.96 | 0.589 | 0.47 ± 0.19 | 0.51 ± 0.26 | 0.394 |
| Neutrophils count (10^9/L) | 5.25 ± 6.67 | 4.89 ± 5.24 | 0.616 | 4.51 ± 2.39 | 4.09 ± 1.72 | 0.248 |
| NLR | 3.48 ± 4.81 | 3.36 ± 2.59 | 0.772 | 3.18 ± 2.05 | 2.81 ± 1.74 | 0.284 |
| PLR | 144.84 ± 64.79 | 173.76 ± 92.70 | 0.008 | 157.26 ± 85.2 | 151.20 ± 67.32 | 0.654 |
| MLR | 0.42 ± 0.67 | 0.37 ± 0.36 | 0.433 | 0.32 ± 0.15 | 0.33 ± 0.18 | 0.701 |
| PT(s) | 15.42 ± 8.10 | 13.72 ± 4.48 | 0.04 | 14.98 ± 6.55 | 13.66 ± 3.51 | 0.151 |
| PT-INR (INR) | 1.33 ± 0.72 | 1.14 ± 0.29 | 0.005 | 1.28 ± 0.58 | 1.16 ± 0.32 | 0.152 |
| TT (s) | 21.00 ± 31.14 | 17.67 ± 21.81 | 0.302 | 15.42 ± 3.98 | 17.75 ± 11.11 | 0.173 |
| FIB (g/L) | 3.57 ± 1.54 | 3.79 ± 1.58 | 0.259 | 3.33 ± 0.96 | 3.45 ± 1.05 | 0.551 |
| APTT (s) | 36.10 ± 10.81 | 33.29 ± 8.81 | 0.022 | 30.68 ± 7.65 | 32.27 ± 10.91 | 0.399 |
| D-dimer (mg/L) | 3.53 ± 6.78 | 6.18 ± 9.18 | 0.017 | 4.56 ± 5.37 | 4.34 ± 4.46 | 0.807 |
| CHOL (mmol/L) | 5.08 ± 1.54 | 5.04 ± 1.60 | 0.891 | 4.54 ± 1.26 | 4.94 ± 1.06 | 0.158 |
| TG (mmol/L) | 1.61 ± 0.87 | 1.52 ± 0.61 | 0.459 | 1.22 ± 0.43 | 1.51 ± 0.63 | 0.06 |
| LDL-C (mmol/L) | 3.31 ± 1.23 | 3.21 ± 1.27 | 0.669 | 2.83 ± 0.89 | 3.16 ± 0.78 | 0.102 |
| ALT (U/L) | 30.95 ± 28.38 | 34.77 ± 62.82 | 0.586 | 43.00 ± 61.47 | 37.14 ± 77.11 | 0.668 |
| AST (U/L) | 29.19 ± 18.46 | 31.16 ± 38.06 | 0.645 | 36.22 ± 40.37 | 36.20 ± 72.17 | 0.998 |
| TBIL (umol/L) | 16.03 ± 10.84 | 13.63 ± 9.30 | 0.081 | 15.51 ± 7.71 | 13.00 ± 5.16 | 0.032 |
| DBIL (umol/L) | 3.68 ± 3.87 | 3.17 ± 4.50 | 0.362 | 2.99 ± 1.81 | 2.60 ± 1.20 | 0.147 |
| ALB (g/L) | 36.98 ± 4.05 | 36.16 ± 5.45 | 0.2 | 37.83 ± 5.44 | 37.28 ± 5.07 | 0.568 |
| BG (mmol/L) | 5.73 ± 1.64 | 5.87 ± 1.70 | 0.495 | 5.93 ± 1.80 | 5.83 ± 1.84 | 0.783 |
| HCY (umol/L) | 15.66 ± 11.44 | 13.05 ± 6.41 | 0.129 | 13.15 ± 5.25 | 16.31 ± 21.23 | 0.536 |
| FAR | 0.10 ± 0.04 | 0.11 ± 0.07 | 0.094 | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.396 |
| BUN (mmol/L) | 5.37 ± 1.78 | 4.98 ± 2.21 | 0.151 | 6.46 ± 3.49 | 4.85 ± 2.09 | 0.005 |
| Cr (umol/L) | 68.82 ± 17.63 | 67.30 ± 24.54 | 0.598 | 74.30 ± 25.77 | 65.99 ± 16.89 | 0.036 |
| UA (umol/L) | 353.43 ± 118.75 | 321.08 ± 117.87 | 0.31 | 381.02 ± 140.18 | 293.84 ± 80.65 | < 0.001 |
| PC (%) | 96.74 ± 29.18 | 100.17 ± 28.60 | 0.473 | 98.21 ± 34.05 | 95.88 ± 25.57 | 0.732 |
| PS (%) | 100.01 ± 37.34 | 97.22 ± 36.06 | 0.647 | 103.50 ± 46.38 | 91.73 ± 35.43 | 0.22 |
| AT-III (%) | 89.97 ± 14.75 | 91.35 ± 17.14 | 0.718 | 93.24 ± 15.06 | 100.12 ± 17.03 | 0.272 |
| cTNT (pg/ml) | 18.00 ± 22.36 | 26.53 ± 47.08 | 0.160 | 27.23 ± 32.02 | 21.69 ± 60.58 | 0.631 |
| NT-pro-BNP ≥ 600 pg/ml | 29 (32.58%) | 34 (14.47%) | 0.045 | 17 (41.46%) | 11 (11.11%) | 0.002 |
ALB, albumin; ALT, glutamic-pyruvic transaminase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; AT-III, antithrombin-III; BG, blood glucose; BMI, body mass index; BUN, blood urea nitrogen; CHD, coronary heart disease; CHOL, cholesterol; CLD, chronic lung disease; Cr, Creatinine; CTD, connective tissue diseases; cTNT, cardiac troponin T; DBIL, direct bilirubin; DBP, diastolic blood pressure; DOACs, direct oral anticoagulants; FAR, fibrinogen to albumin ratio; FIB, fibrinogen; HCY, homocysteine; LDL-C, low density lipoprotein cholesterol; LMH, low molecular heparin; MLR, monocytes to lymphocytes ratio; NLR, neutrophils to lymphocytes ratio; NT-pro-BNP, N-terminal-pro-B-type natriuretic peptide; PC, Protein C; PLR, platelet to lymphocytes ratio; PLT, platelet; PS, Protein S; PT, prothrombin time; PT-INR, international normalized ratio of prothrombin time; RDW, red cell distribution width; RPE, recurrent pulmonary embolism; RPVO, residual pulmonary vascular obstruction; RVD, right ventricular dysfunction; SBP, systolic blood pressure; sPESI, simplified pulmonary embolism severity index; TBIL, total bilirubin; TG, triacylglycerol; TT, thromboplastin time; UA, uric acid; WBC, white blood cell
Table 3.
Univariable and multivariable logistic regression analyses for identification of CTEPD
| factor | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95%CI) | p value | OR (95%CI) | p value | |
| Gender | 0.889 (0.592–1.334) | 0.568 | – | – |
| Age (years) | 0.987 (0.973–1.001) | 0.065 | – | – |
| BMI (kg·m–2) | 1.001 (0.943–1.062) | 0.982 | – | – |
| Temperature (℃) | 1.020 (0.942–1.104) | 0.623 | – | – |
| SBP (mmHg) | 0.995 (0.984–1.006) | 0.332 | – | – |
| DBP (mmHg) | 0.992 (0.976–1.009) | 0.384 | – | – |
| Heart rate (beats/min) | 1.013 (0.998–1.027) | 0.085 | – | – |
| Respiratory rate (breaths/min) | 1.014 (0.981–1.047) | 0.407 | – | – |
| Symptoms | ||||
| Dyspnea (n, %) | 2.816 (1.790–4.430) | < 0.001 | 1.127 (0.530–2.393) | 0.756 |
| Chest pain (n, %) | 0.342 (0.131–0.892) | 0.028 | 0.527 (0.131–2.115) | 0.366 |
| Hemoptysis (n, %) | 0.848 (0.352–2.046) | 0.714 | ||
| Syncope (n, %) | 2.058 (0.879–4.817) | 0.096 | ||
| Time-to-diagnosis ≥ 15 days (n, %) | 7.037 (4.501–11.003) | < 0.001 | 7.126 (3.392–14.972) | < 0.001 |
| RPE (n, %) | 8.759 (3.806–20.160) | < 0.001 | 5.195 (1.560–17.300) | 0.007 |
| Comorbidities | ||||
| Hypertension (n, %) | 0.748 (0.480–1.164) | 0.198 | – | – |
| CHD (n, %) | 1.085 (0.656–1.794) | 0.751 | – | – |
| CLD (n, %) | 1.735 (0.938–3.206) | 0.079 | – | – |
| Diabetes (n, %) | 0.794 (0.411–1.536) | 0.493 | – | – |
| Nervous system disease (n, %) | 0.863 (0.444–1.678) | 0.663 | – | – |
| Venous thrombus (n, %) | 1.363 (0.903–2.056) | 0.140 | – | – |
| Cancer (n, %) | 0.221 (0.123–0.395) | < 0.001 | 0.453 (0.127–1.616) | 0.222 |
| CTD (n, %) | 2.076 (1.086–3.969) | 0.027 | 1.881 (0.630–5.615) | 0.258 |
| Risk factors | ||||
| Smoking history (n, %) | 1.009 (0.646–1.576) | 0.968 | – | – |
| Surgery (n, %) | 0.669 (0.322–1.388) | 0.280 | – | – |
| Trauma (n, %) | 0.581 (0.163–2.075) | 0.403 | – | – |
| RVD (n, %) | 6.918 (3.960–12.087) | < 0.001 | 2.590 (1.042–6.437) | 0.040 |
| Central embolus | 3.329 (2.173–5.099) | < 0.001 | 3.621 (1.776–7.383) | < 0.001 |
| RPVO > 10% (n, %) | 12.041 (7.378–19.65) | < 0.001 | 10.235 (4.884–21.449) | < 0.001 |
| Risk stratification | 2.232 (1.496–3.329) | < 0.001 | 0.814 (0.372–1.782) | 0.606 |
| sPESI | 0.585 (0.412–0.831) | 0.003 | 1.847 (0.876–3.895) | 0.107 |
| Initial anticoagulant | 1.187 (0.795–1.770) | 0.402 | – | – |
| Time-to-anticoagulant ≥ 6 h (n, %) | 1.038 (0.683–1.577) | 0.862 | – | – |
| Blood test | ||||
| WBC count (10^9/L) | 1.000 (0.924–1.082) | 1.000 | – | – |
| PLT count (10^9/L) | 0.997 (0.995–1.000) | 0.032 | 1.001 (0.996–1.006) | 0.715 |
| Hemoglobin levels (g/L) | 1.020 (1.009–1.030) | < 0.001 | 0.999 (0.983–1.016) | 0.953 |
| RDW (%) | 0.962 (0.908–1.020) | 0.193 | – | – |
| Lymphocytes count (10^9/L) | 1.015 (0.780–1.321) | 0.911 | – | – |
| Monocytes count (10^9/L) | 1.044 (0.834–1.307) | 0.708 | – | – |
| Neutrophils count (10^9/L) | 1.014 (0.976–1.054) | 0.480 | – | – |
| NLR | 1.020 (0.957–1.088) | 0.544 | – | – |
| PLR | 0.997 (0.994–1.000) | 0.034 | 0.999 (0.994–1.004) | 0.649 |
| MLR | 1.167 (0.730–1.866) | 0.519 | – | – |
| PT (s) | 1.052 (1.010–1.095) | 0.014 | 0.949 (0.705–1.277) | 0.728 |
| PT-INR (INR) | 2.399 (1.387–4.152) | 0.002 | 1.976 (0.072–54.049) | 0.687 |
| TT (s) | 1.003 (0.994–1.012) | 0.497 | – | – |
| FIB (g/L) | 0.898 (0.765–1.055) | 0.190 | – | – |
| APTT (s) | 1.014 (0.994–1.035) | 0.172 | – | – |
| D-dimer (mg/L) | 0.955 (0.916–0.995) | 0.029 | 0.976 (0.935–1.019) | 0.275 |
| CHOL (mmol/L) | 0.948 (0.772–1.164) | 0.612 | – | – |
| TG (mmol/L) | 0.921 (0.594–1.429) | 0.714 | – | – |
| LDL-C (mmol/L) | 0.962 (0.743–1.245) | 0.767 | – | – |
| ALT (U/L) | 1.000 (0.996–1.003) | 0.909 | – | – |
| AST (U/L) | 0.999 (0.995–1.004) | 0.793 | – | – |
| TBIL (umol/L) | 1.031 (1.005–1.058) | 0.021 | 1.017 (0.968–1.068) | 0.506 |
| DBIL (umol/L) | 1.031 (0.978–1.086) | 0.256 | – | – |
| ALB (g/L) | 1.030 (0.989–1.073) | 0.158 | – | – |
| BG (mmol/L) | 0.976 (0.863–1.103) | 0.694 | – | – |
| HCY (umol/L) | 1.004 (0.980–1.028) | 0.768 | – | – |
| FAR | 0.008 (0–1.180) | 0.058 | – | – |
| BUN (mmol/L) | 1.151 (1.051–1.260) | 0.003 | 1.149 (0.963–1.371) | 0.123 |
| Cr (umol/L) | 1.007 (0.998–1.016) | 0.128 | – | – |
| UA (umol/L) | 1.004 (1.002–1.005) | < 0.001 | 1.000 (0.997–1.003) | 0.794 |
| PC (%) | 0.998 (0.989–1.007) | 0.691 | – | – |
| PS (%) | 1.004 (0.997–1.011) | 0.266 | – | – |
| AT-III (%) | 0.987 (0.962–1.012) | 0.314 | – | – |
| cTNT (pg/ml) | 0.997 (0.991–1.004) | 0.421 | – | – |
| NT-pro-BNP ≥ 600 pg/ml | 2.390 (1.460–3.912) | 0.001 | 2.037 (0.848–4.895) | 0.112 |
Data are presented as mean ± standard deviation or n (%). ALB, albumin; ALT, glutamic–pyruvic transaminase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; AT–III, antithrombin-III; BG, blood glucose; BMI, body mass index; BUN, blood urea nitrogen; CHD, coronary heart disease; CHOL, cholesterol; CLD, chronic lung disease; CHOL, cholesterol; Cr, Creatinine; CTD, connective tissue diseases; cTNT, cardiac troponin T; DBIL, direct bilirubin; DBP, diastolic blood pressure; DOACs, direct oral anticoagulants; FAR, fibrinogen to albumin ratio; FIB, fibrinogen; HCY, homocysteine; LDL-C, low density lipoprotein cholesterol; LMH, low molecular heparin; MLR, monocytes to lymphocytes ratio; NLR, neutrophils to lymphocytes ratio; NT-pro-BNP, N-terminal-pro-B-type natriuretic peptide; PC, Protein C; PLR, platelet to lymphocytes ratio; PLT, platelet; PS, Protein S; PT, prothrombin time; PT-INR, international normalized ratio of prothrombin time; RDW, red cell distribution width; RPE, recurrent pulmonary embolism; RPVO, residual pulmonary vascular obstruction; RVD, right ventricular dysfunction; SBP, systolic blood pressure; sPESI, simplified pulmonary embolism severity index; TBIL, total bilirubin; TG, triacylglycerol; TT, thromboplastin time; UA, uric acid; WBC, white blood cell
Model development and validation
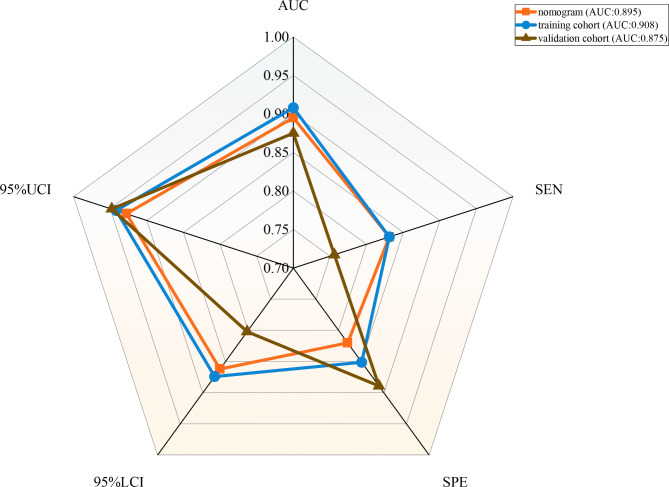
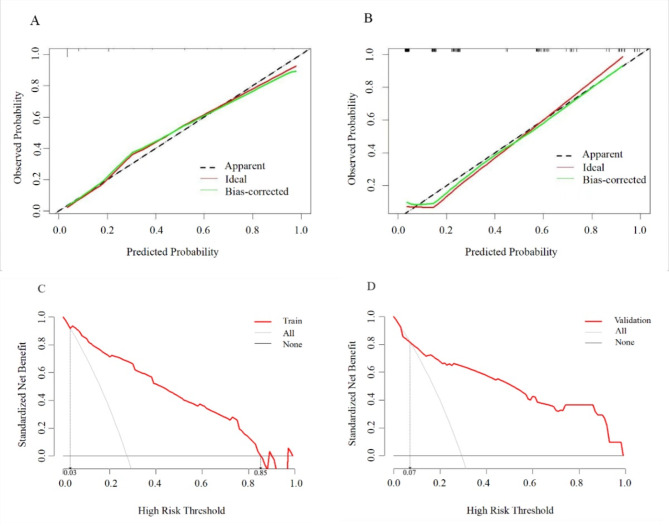
A nomogram with a C-index of 0.895 (95% CI 0.863–0.927) was established based on the independent risk factors of the early identification of CTEPD among the patients with APE (Figs. 3 and 4). As measured by the AUC of the operating characteristic curve, the discriminability values of the model were 0.908 (95%CI: 0.875–0.941) in the training cohort and 0.875 (95%CI: 0.803–0.947) in the validation cohort (Fig. 4). Hence, the prediction model could effectively identify CTEPD. The calibration graphs of the training and validation cohorts showed that the CTEPD identified by the model had a good agreement with the actual CTEPD (Fig. 5A, B). The clinical application of the nomogram was evaluated via DCA. The DCA of the training and validation cohorts indicated that the nomogram had a good net benefit for the early prediction of CTEPD if the risk threshold probabilities were 0.03–0.85 and 0.07–1.00 for the training and internal validation cohorts, respectively (Fig. 5C, D).
Fig. 3.
The radiological nomogram for prediction of CTEPD among the patients with APE
Fig. 4.
The ROC curves of nomogram, the training cohort and the validation cohort. AUC, area under the subject operating characteristic curve; SEN, sensitivity; SPE, specificity; 95%UCI, 95% upper confidence limit; 95% LCI, 95% lower confidence limit
Fig. 5.
The calibration graphs of nomogram in the training cohort (A) and validation cohort (B). The decision curve analysis of nomogram in the training cohort (C) and validation cohort (D)
Discussion
At present, the incidence of CTEPD is not clear, and previous research on this notion is limited. In this study, 464 patients with APE were followed up for at least 3 months, and the incidence rate of CTEPD was 28%. We focused on developing a prediction model that combines the noninvasive imaging tests and the clinical risk factors for the early identification of CTEPD.
The model integrates five principal predictors: time-to-diagnosis ≥ 15 days, RPE, RVD, central embolus, and RPVO > 10%. Consistent with a recent study, the current study including 71 consecutive patients with a previous episode of PE showed an association between prolonged time to diagnosis and incomplete perfusion recovery after APE [19]. Further, the CTEPD group had a higher proportion of patients with a time-to-diagnosis of ≥ 15 days than the control group. RPE, one of the most serious prognostic adverse events in patients with APE, can lead to recurrent or persistent clinical symptoms and progressive hemodynamic deterioration [20]. Notably, patients with APE who present with RPE were more likely to develop CTEPD than those without RPE. Our results are consistent with those of recent studies, which had a strong correlation between the development of RPE and CTEPH [21, 22].
RVD, defined as a combination of findings of right ventricular overload at echocardiography, is a predictor of short-term mortality in all-comers with APE [12, 22]. Based on our previous research, which included 520 consecutive patients with APE, RVD was a discriminator for a poor prognosis in normotensive patients [16]. This study further validated that patients with RVD were at a higher risk of developing CTEPD, and RVD was a significant predictor of CTEPD in the multivariate analysis. Some studies have shown that RVD is closely associated with incomplete pulmonary thromboembolic recanalization [4, 23, 24].
Blood clots can resolve over time after the first occurrence, and clots commonly resolve completely within 3 months after APE [25]. However, the thrombus in the right or left PA or pulmonary trunk may not resolve completely, some studies have shown that the rate of complete resolution of APE ranged from 32–85% [25–27]. Pulmonary emboli located in the right or left PA or pulmonary trunk were referred to as central embolism [25]. Recent studies showed that central embolus was associated with a poor prognosis in patients with APE [28, 29]. In this study, central embolus was an independent predictor of CTEPD. In addition, some studies further indicated that an RPVO > 10%, according to the Qanadli and Meyer score, was associated with a significantly higher risk of unfavorable outcomes in patients with APE [4, 30]. Therefore, patients with an RPVO > 10% at follow-up should be highly valued.
The nomogram established in this study provides a convenient tool for the early identification of CTEPD and to prevent CTEPH. Moreover, it had a good prediction effect. The timely identification of CTEPD risk factors and early intervention can be an effective tool for guiding medical staff in immediately confirming CTEPD, which may be helpful in making timely treatment decision. In addition, in contrast to previous single-center studies [31], our study included a larger sample of patients, and used a multicenter study design and internal validation. These processes contributed to improving the reliability of our research findings. Hence, our prediction model may help physicians screen high-risk patients for CTEPD in a timely manner, thereby enhancing management and optimizing follow-up strategies to guide clinical strategies, reducing the incidence of adverse events in these patients.
The current study had several limitations. First, although this study is a multicenter prospective study, the sample size may limit the comprehensive analysis of risk factors. Future work should focus on expanding the sample size. Second, some auxiliary tests, such as the pulmonary function tests and cardiopulmonary exercise testing (CPET), are incomplete. We will make supplements according to the patient’s condition in the subsequent follow-up to more accurately assess the patient’s cardiopulmonary function. Third, even though the internal validation cohort showed excellent discriminative ability with a satisfactory agreement for the early identification of CTEPD by the calibration graphs and DCA results, we did not perform external validation. Hence, the prediction model should be further validated and assessed.
Conclusions
Our study established a clinical prediction model for the early identification of CTEPD. The clinical parameters included in the nomogram are easily and simply accessible, exhibit an excellent predictive potential, and possess substantial clinical utility. The model can be incorporated into clinical programs to help physicians facilitate the early identification of high-risk patients for CTEPD and probably prevent CTEPH as early as possible.
Acknowledgements
Not applicable.
Abbreviations
- APE
Acute pulmonary embolism
- CPET
Cardiopulmonary exercise testing
- PA
Pulmonary artery
- CTEPD
Chronic thromboembolic pulmonary disease
- CTEPH
Chronic thromboembolic pulmonary hypertension
- PH
Pulmonary hypertension
- PE
Pulmonary embolism
- CTPA
Computed tomography pulmonary angiography
- V/Q
Ventilation perfusion
- TTE
Transthoracic echocardiography
- RVD
Right ventricular dysfunction
- RV
Right ventricular
- LV
Left ventricular
- TAPSE
Tricuspid annular plane systolic excursion
- RPVO
Residual pulmonary vascular obstruction
- SD
Standard deviation
- C-index
Concordance index
- AUC
The area under the curve
- DCA
Decision curve analysis
- RPE
Recurrent pulmonary embolism
- CTD
Connective tissue diseases
- sPESI
Simplified pulmonary embolism severity index
- PLT
Platelet
- PLR
Platelet-to-lymphocytes ratio
- PT
Prothrombin time
- PT-INR
International normalized ratio of prothrombin time
- TBIL
Total bilirubin
- BUN
Blood urea nitrogen
- UA
Uric acid
- NT-pro-BNP
N-terminal-pro-B-type natriuretic peptide levels
Author contributions
The research concept of the study was developed by Ling Zhu and Yuanyuan Sun. All authors undertook the study. Ling Zhu, Yuanyuan Sun and Mingjie Liu contributed to study design and to development of the proposal. Guixiang Liu and Jing Wen participated in drafting the manuscript, and submission of the protocol manuscript. Mingjie Liu conceptualised the statistical analyses and calculated the sample size. Chunyi Lv and Min Li did the statistical analysis. Huarui Wang accessed and verified the data. Kexia Fang, Jianwen Fei, Nannan Zhang and Xuehua Li participated in data collection and were in charge of the recruitment. All authors read and approved the final manuscript.
Funding
This research was funded by the Natural Science Foundation of Shandong Province (No. ZR2023QH200 and No. ZR2022MH138), clinical registration study from 2022 (No. ChiCTR2200064675), and the National Project (No. 2021-I2M-1-049).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committees of Shandong Provincial Hospital (SWYX: no.2019-070). All patients provided informed consents.
Consent for publication
No individual participant data is reported that would require consent to publish from the participant (or legal parent or guardian for children).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guixiang Liu and Jing Wen contributed equally to this work.
Contributor Information
Yuanyuan Sun, Email: sunyuanyuan422@163.com.
Ling Zhu, Email: zhuling7103@163.com.
References
- 1.Kulka HC, Zeller A, Fornaro J, Wuillemin WA, Konstantinides S, Christ M. Acute Pulmonary embolism–its diagnosis and treatment from a multidisciplinary viewpoint. Dtsch Arztebl Int. 2021;118:618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nasser MF, Jabri A, Limaye S, Sharma S, Hamade H, Mhanna M, Aneja A, Gandhi S. Echocardiographic evaluation of pulmonary embolism: a review. J Am Soc Echocardiogr. 2023;36:906–12. [DOI] [PubMed] [Google Scholar]
- 3.Freund Y, Cohen-Aubart F, Bloom B. Acute Pulmonary Embolism: a review. JAMA. 2022;328:1336–45. [DOI] [PubMed] [Google Scholar]
- 4.Bonnefoy PB, Margelidon-Cozzolino V, Catella-Chatron J, Ayoub E, Guichard JB, Murgier M, Bertoletti L. What’s next after the clot? Residual pulmonary vascular obstruction after pulmonary embolism: from imaging finding to clinical consequences. Thromb Res. 2019;184:67–76. [DOI] [PubMed] [Google Scholar]
- 5.Cimini LA, Luijten D, Barco S, Ghanima W, Jervan Ø, Kahn SR, Konstantinides S, Lachant D, Nakano Y, Ninaber M et al. Pulmonary perfusion defects or residual vascular obstruction and persistent symptoms after pulmonary embolism: a systematic review and meta-analysis. ERJ Open Res. 2024; 10. [DOI] [PMC free article] [PubMed]
- 6.Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano-Subias P, Ferrari P et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023; 61. [DOI] [PubMed]
- 7.Delcroix M, Torbicki A, Gopalan D, Sitbon O, Klok FA, Lang I, Jenkins D, Kim NH, Humbert M, Jais X et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J. 2021; 57. [DOI] [PubMed]
- 8.Lang IM, Campean IA, Sadushi-Kolici R, Badr-Eslam R, Gerges C, Skoro-Sajer N. Chronic Thromboembolic Disease and Chronic Thromboembolic Pulmonary Hypertension. Clin Chest Med. 2021;42:81–90. [DOI] [PubMed] [Google Scholar]
- 9.Luijten D, Talerico R, Barco S, Cannegieter SC, Delcroix M, Ende-Verhaar YM, Huisman MV, Konstantinidis S, Mairuhu ATA, van Mens TE et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: an updated systematic review and meta-analysis. Eur Respir J. 2023; 62. [DOI] [PubMed]
- 10.Aramberri M, González-Olmedo J, García-Villa A, Villanueva A, Maza CC, García-Gutiérrez S, Diaz-Pedroche C. Prediction of mortality in acute pulmonary embolism in cancer-associated thrombosis (MAUPE-C): derivation and validation of a multivariable model. J Thromb Thrombolysis. 2024;57:668–76. [DOI] [PubMed] [Google Scholar]
- 11.Xia W, Yu H, Chen W, Chen B, Huang Y. A Radiological Nomogram to predict 30-day mortality in patients with Acute Pulmonary Embolism. Acad Radiol. 2022;29:1169–77. [DOI] [PubMed] [Google Scholar]
- 12.Hobohm L, Paschke LM, Farmakis IT, Barco S, Partovi S, Münzel T, Konstantinides S, Keller K, Below M. Diagnosis of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: data from a practice-based longitudinal cohort. J Thromb Haemost. 2024;22:2203–10. [DOI] [PubMed] [Google Scholar]
- 13.Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543–603. [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Qiu X, Sun Y, Li Q, Wen J, Liu G, Yao Z, Zhu L. Intensify standardized anticoagulation for Cancer-associated pulmonary embolism: from single-center real-world data. Clin Ther. 2023;45:1236–43. [DOI] [PubMed] [Google Scholar]
- 15.Barco S, Mahmoudpour SH, Planquette B, Sanchez O, Konstantinides SV, Meyer G. Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2019;40:902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu L, Yang Y, Wu Y, Zhai Z, Wang C. Value of right ventricular dysfunction for prognosis in pulmonary embolism. Int J Cardiol. 2008;127:40–5. [DOI] [PubMed] [Google Scholar]
- 17.Qanadli SD, El Hajjam M, Vieillard-Baron A, Joseph T, Mesurolle B, Oliva VL, Barré O, Bruckert F, Dubourg O, Lacombe P. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001;176:1415–20. [DOI] [PubMed] [Google Scholar]
- 18.Meyer G, Collignon MA, Guinet F, Jeffrey AA, Barritault L, Sors H. Comparison of perfusion lung scanning and angiography in the estimation of vascular obstruction in acute pulmonary embolism. Eur J Nucl Med. 1990;17:315–9. [DOI] [PubMed] [Google Scholar]
- 19.Lami D, Cellai AP, Antonucci E, Fiorillo C, Becatti M, Grifoni E, Cenci C, Marcucci R, Mannini L, Miniati M, et al. Residual perfusion defects in patients with pulmonary embolism are related to impaired fibrinolytic capacity. Thromb Res. 2014;134:737–41. [DOI] [PubMed] [Google Scholar]
- 20.Kong J, Hardwick A, Jiang SF, Sun K, Vinson DR, McGlothlin DP, Goh CH. CTEPH: a Kaiser Permanente Northern California experience. Thromb Res. 2023;221:130–6. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Wang N, Zhai Z, Zhang M, Zhou R, Liu Y, Yang Y. Incidence and risk factors of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a systematic review and meta-analysis of cohort studies. J Thorac Dis. 2018;10:4751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, Delcroix M, Pruszczyk P, Mairuhu AT, Huisman MV, Klok FA. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J. 2017; 49. [DOI] [PubMed]
- 23.Caguana-Vélez OA, Khilzi K, Piccari L, Rodríguez-Sevilla JJ, Badenes-Bonet D, Gonzalez-Garcia J, Chalela R, Arita M, Rodó-Pin A, Herranz A, et al. Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Embolism in SARS-CoV-2. Respiration. 2024;103:79–87. [DOI] [PubMed] [Google Scholar]
- 24.Hsu CH, Lin CC, Li WT, Chang HY, Chang WT. Right ventricular dysfunction is associated with the development of chronic thromboembolic pulmonary hypertension but not with mortality post-acute pulmonary embolism. Med (Baltim). 2019;98:e17953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi KJ, Cha SI, Shin KM, Lim JK, Yoo SS, Lee J, Lee SY, Kim CH, Park JY, Lee WK. Factors determining clot resolution in patients with acute pulmonary embolism. Blood Coagul Fibrinolysis. 2016;27:294–300. [DOI] [PubMed] [Google Scholar]
- 26.Moradi F, Morris TA, Hoh CK. Perfusion scintigraphy in diagnosis and management of Thromboembolic Pulmonary Hypertension. Radiographics. 2019;39:169–85. [DOI] [PubMed] [Google Scholar]
- 27.Haramati A, Haramati LB. Imaging of chronic Thromboembolic Disease. Lung. 2020;198:245–55. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Liu G, Wang S, Du W, Lv P, Guo H, Sun Q, Liu Y, Qi X. The electrocardiographic characteristics of an acute embolism in the pulmonary trunk and the main pulmonary arteries. Am J Emerg Med. 2016;34:212–7. [DOI] [PubMed] [Google Scholar]
- 29.Sukhija R, Aronow WS, Yalamanchili K, Lee J, McClung JA, Levy JA, Belkin RN. Association of right ventricular dilatation with bilateral pulmonary embolism, pulmonary embolism in a main pulmonary artery and lobar, segmental and subsegmental pulmonary embolism in 190 patients with acute pulmonary embolism. Cardiology. 2005;103:156–7. [DOI] [PubMed] [Google Scholar]
- 30.Fauché A, Presles E, Sanchez O, Jaïs X, Le Mao R, Robin P, Pernod G, Bertoletti L, Jego P, Parent F, et al. Frequency and predictors for chronic thromboembolic pulmonary hypertension after a first unprovoked pulmonary embolism: results from PADIS studies. J Thromb Haemost. 2022;20:2850–61. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Xu M, Sun N, Cheng Z, Sui J. Factors associating with the presence of residual thrombosis after 3-month treatment of acute pulmonary embolism. J Thromb Thrombolysis. 2018;45:27–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.