Abstract
Background context
As lumbar degenerative diseases become more prevalent in an aging population, there is an increasing demand for surgical interventions, such as posterior lumbar interbody fusion (PLIF). However, cage subsidence (CS), observed in 23.9–54% of cases postoperatively, remains a significant complication. Several factors, including age, bone quality, and endplate damage, contribute to the risk of CS, with bone quality being among the most critical determinants. Although DEXA and QCT are widely employed to assess bone density, their routine use in preoperative evaluations is restricted by cost considerations and radiation exposure. Recent studies suggest that MRI-based vertebral body quality (VBQ) and endplate bone quality (EBQ) score offer a viable, non-invasive alternative for evaluating bone quality; however, there is limited research comparing their predictive value for CS.
Methods
In this retrospective study, 165 patients undergoing single-level PLIF surgery were included. MRI-based VBQ and EBQ score were calculated using T1-weighted images, and preoperative QCT was employed as a clinical standard. Cage subsidence was assessed based on postoperative imaging at 12-month follow-up. Statistical analyses, including t-tests, chi-square tests, and ROC curve analyses, were used to evaluate the predictive accuracy of VBQ and EBQ for CS.
Results
The study’s findings demonstrated that both VBQ and EBQ scores were significantly correlated with QCT measurements, thereby validating their utility as indicators of bone quality. ROC analysis revealed that VBQ had superior predictive value for CS (AUC = 0.814) compared to EBQ (AUC = 0.719), with both scores demonstrating significant clinical utility in identifying patients at risk for CS. Notably, VBQ exhibited a stronger correlation with preoperative clinical outcomes compared to EBQ, underscoring its greater reliability as a predictor.
Conclusions
This study highlights the effectiveness of MRI-based VBQ and EBQ score as practical, non-invasive tools for assessing bone quality preoperatively, with VBQ demonstrating superior predictive performance for CS risk. The findings underscore the potential of integrating these MRI-based assessments into routine preoperative planning to improve patient outcomes and minimize complications associated with PLIF surgery.
Keywords: Posterior lumbar interbody fusion, Cage subsidence, Vertebral body quality, Endplate bone quality, Quantitative computed tomography
Introduction
As the population ages, the prevalence and incidence of lumbar degenerative diseases continue to rise [1]. In managing lumbar degenerative diseases, surgical intervention is frequently required when conservative treatments fail. Posterior lumbar interbody fusion (PLIF) is commonly performed as it provides robust spinal stability and sufficient nerve root decompression [2]. However, the incidence of cage subsidence (CS) after surgery ranges between 23.9 and 54% [3, 4].
The occurrence of CS is influenced by various factors, including age, bone quality, endplate damage, and cage height [5–7]. Low bone density, in particular, remains one of the most common risk factors for CS [8, 9], which may result in fusion failure, decreased lumbar stability, nerve root invasion, and even revision surgery [10], thereby significantly impacting surgical outcomes and patient prognosis. Therefore, accurate prediction of CS risk is crucial for improving surgical outcomes and guiding clinical decisions. Identifying high-risk patients can help surgeons adjust surgical techniques, choose appropriate cage designs, and implement preventive measures, such as optimization of preoperative bone quality.
Dual-energy X-ray absorptiometry (DEXA) and quantitative computed tomography (QCT) are currently recognized as the standard methods for assessing bone quality [11, 12]. However, due to the additional economic burden and radiation exposure, they are usually not used as routine preoperative assessments for spinal surgery. DEXA mainly assesses areal bone density, which does not accurately represent the actual volumetric bone quality of localized regions such as the vertebral endplates and trabecular bone. In comparison, QCT is better at predicting postoperative spinal complications and is therefore superior to DEXA in clinical applications [13–15]. However, it is important to note that the measurements of both DEXA and QCT can be influenced by spinal degeneration and microstructural bone changes, thereby reducing the accuracy of vertebral bone density assessments [16, 17]. In recent years, MRI has been widely employed for preoperative spinal assessments, particularly for non-invasive bone quality evaluations. MRI-based endplate bone quality (EBQ) and vertebral body quality (VBQ) score provide effective alternative methods for assessing bone quality. The EBQ score primarily assesses the bone structure and signal characteristics of the vertebral endplate, while the VBQ score evaluates the overall bone quality of the vertebral body. Both MRI-based scoring methods have demonstrated distinct value in predicting postoperative cage subsidence (CS) risk. Research indicates that EBQ and VBQ score correlate with traditional DEXA T-score and QCT measurements, and demonstrate significant predictive power in differentiating healthy from osteoporotic bone [18, 19].
Few studies have evaluated the predictive performance of VBQ and EBQ score for predicting cage subsidence after PLIF. Although MRI-based bone quality score provide valuable clinical information, there is a lack of direct comparison studies that evaluate the predictive efficacy of VBQ and EBQ score for CS after PLIF [18–20]. Particularly among Chinese patients, this research gap is especially prominent due to delayed medical consultations and unique bone density characteristics, which may influence the predictive accuracy of these score. Previous studies have demonstrated the independent predictive value of VBQ and EBQ score, but the results have been inconsistent. Certain studies indicate that the VBQ surpasses the EBQ score in terms of predictive power, as it offers a more thorough evaluation of vertebral bone quality. Other research, however, highlights the importance of endplate integrity for predicting CS, especially in patients with pronounced endplate degeneration or inflammation.
Therefore, the purpose of this study is to use spinal QCT density as a clinical standard to validate the accuracy of VBQ and EBQ score for bone density measurement in the Chinese population and to evaluate the predictive efficacy of VBQ and EBQ score for cage subsidence after PLIF.
Methods
Patient population
This study included patients who underwent single-level PLIF surgery at our center from 2020 to 2023, which was performed by a spinal surgery team and approved by the Institutional Review Board of our hospital. In light of the retrospective design of the study, the requirement for informed consent was waived.
Inclusion criteria: ① Patients diagnosed with lumbar degenerative diseases by outpatient doctors, including lumbar disc herniation, lumbar spinal stenosis, and lumbar spondylolisthesis; ② Symptoms and signs consistent with imaging results; ③ Failed conservative treatment for at least 3 months or had recurrent symptoms and underwent surgery for the first time; ④ No significant surgical contraindications; ⑤ Surgery was successfully completed; ⑥ Follow-up time exceeded 12 months with complete follow-up data.
Exclusion criteria: ① History of prior spinal surgery; ② Presence of spinal deformities or severe spinal instability; ③ Combined with lumbar tuberculosis, infection, tumors, or severe bone destruction; ④ Incomplete follow-up data or patients lost to follow-up; ⑤ Postoperative fusion failure; ⑥ Long-term use of steroids or presence of immune system diseases.
Patients underwent single-level PLIF using polyetheretherketone (PEEK) cages, with at least 12 ml of autologous bone graft placed in the intervertebral space and bilateral pedicle screw fixation.
Data collection
The collected data include demographic information, perioperative imaging data, and patient surgical outcome assessments. Patient demographic data include age, sex, body mass index (BMI), diabetes, hypertension, and surgical level. Perioperative imaging data include preoperative disc height, VBQ and EBQ score, and subsidence measurements. These data are derived from preoperative lumbar CT or X-ray images, T1-weighted MRI images, and follow-up X-ray or CT scans. Surgical outcome assessments for patients include the Visual Analog Scale (VAS) for low back pain and the Oswestry Disability Index (ODI), which were measured preoperatively and at 1 year postoperatively.
MRI measurements and VBQ score calculation
Following the original study by Ehresman [21], we employed T1-weighted MRI mid-sagittal images to define regions of interest (ROIs) on the lumbar vertebral bodies from L1 to L4 and at the L3 level of the cerebrospinal fluid (CSF) to calculate the VBQ score. Each elliptical ROI was positioned 3 mm from the vertebral edge to ensure exclusion of cortical bone. The average signal intensity (SI) of the L1 to L4 vertebral bodies was recorded, and the VBQ score was determined by dividing the average SI of the L1–L4 vertebrae by the average SI of the CSF. The ROI was selected to exclude any focal lesions, including the posterior venous plexus. In cases where ROI placement was hindered by anatomic obstructions, parasagittal slices were used as an alternative. If ROI placement at the L3 level of the CSF was obstructed, the ROI was repositioned to the L2 or L4 level of the CSF (Fig. 1).
Fig. 1.
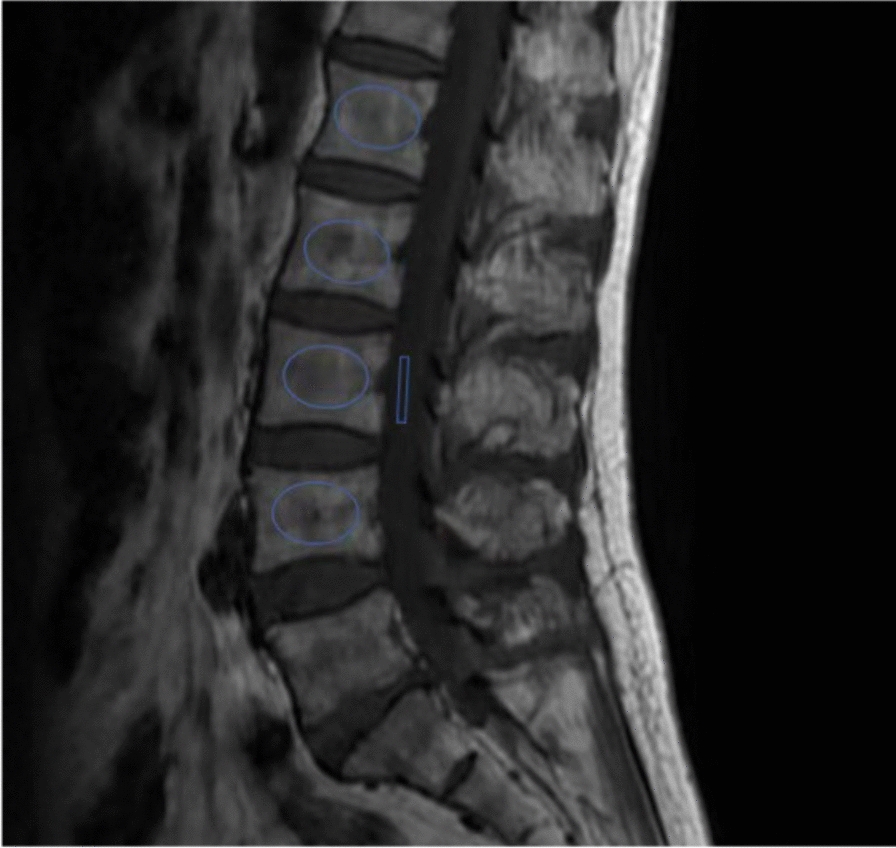
An example of a signal intensity measurement for the vertebral body quality (VBQ) score
MRI measurements and EBQ score calculation
Based on the EBQ predictive model proposed by Jones [19], we used axial T1-weighted MRI images to define ROIs 3 mm below the cartilage of the upper and lower endplates at the operative level, as well as on the CSF at the L3 level, to calculate the EBQ score. The EBQ score was calculated by dividing the average signal intensity of the upper and lower endplates at the operative level by the average signal intensity of CSF. If Schmorl’s nodes were identified at the target level, the EBQ score was measured while carefully excluding the nodes, and any obstructions in the CSF at the L3 level were treated in the same manner (Fig. 2).
Fig. 2.
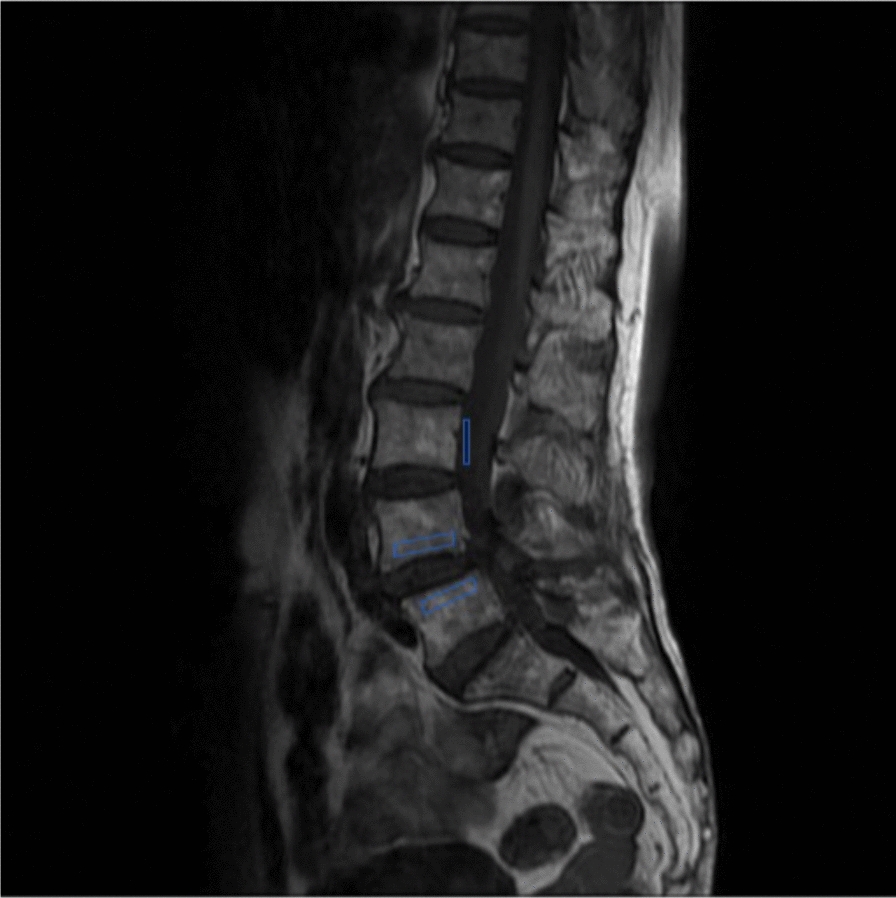
An example of a signal intensity measurement for the endplate bone quality (EBQ) score
QCT measurements
Preoperative lumbar spine CT images obtained within one month prior to surgery were utilized. For L1/2-vBMD measurements, we used the Mindways QCT Pro software, applying an asynchronous calibrated QCT method to convert Hounsfield units (HU) into volumetric bone mineral density (vBMD) [22]. An ROI was selected on the mid-sagittal CT scan of the lumbar spine and positioned at the center of the vertebral body. The ROI was selected to exclude the cortical bone and posterior venous plexus of the vertebra. Vertebral bone abnormalities such as bone islands or sclerotic areas were excluded from the ROI. If the abnormalities were too large to define a measurable ROI, the corresponding level was excluded. Based on the American College of Radiology’s QCT diagnostic criteria for osteoporosis, patients were classified as having normal bone density (> 120 mg/cm3), osteopenia (≤ 120 mg/cm3), or osteoporosis (< 80 mg/cm3) [23].
cage subsidence measurements
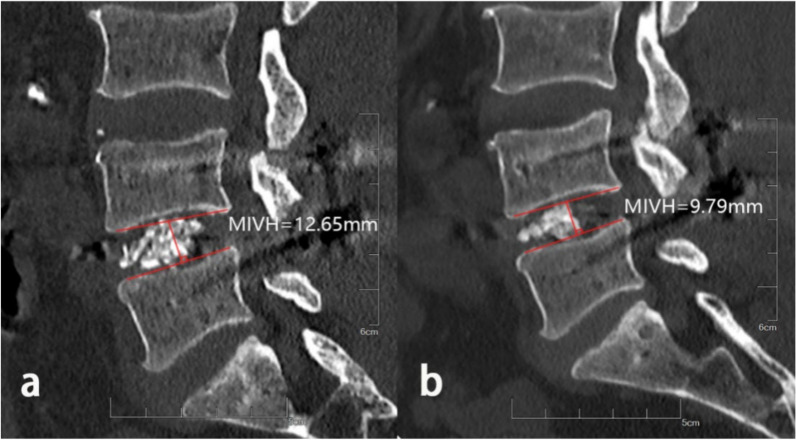
In the assessment of lumbar fusion outcomes, CT scans are considered to offer greater accuracy and sensitivity than X-rays for detecting cage subsidence and bone quality changes [24] (Fig. 3). Therefore, in this study, we performed postoperative CT scans on each patient according to our institution’s standard follow-up protocol to monitor changes in mid-vertebral body height (MIVH). Specifically, we collected imaging data using CT scans immediately after surgery and at the 12-month follow-up. CS was defined as a reduction in MIVH of more than 2 mm on CT scans taken at least 12 months postoperatively compared to the immediate postoperative scan [25]. Patients were categorized into a CS group and a non-CS group based on the presence of CS. Measurements were independently performed by two authors who were blinded to patients’ baseline characteristics and preoperative bone density.
Fig. 3.
Measurement of mid-vertebral body height. a. In the immediate postoperative CT images, the vertical distance between the upper endplate and the lower endplate was taken at the midpoint. b. The mid-vertebral body height was measured by the same method 12 months after operation
Statistical analysis
Statistical analyses were performed using SPSS 26.0 (IBM Corp., USA). Continuous variables (age, subsidence height, BMI, QCT, EBQ, VBQ, VAS, ODI) that followed a normal distribution were expressed as mean ± standard deviation, and intergroup comparisons were conducted using the t-test. For variables that did not follow a normal distribution, the rank-sum test was used. For categorical variables and frequencies, the chi-square test was used. Each predictive model was subjected to receiver operating characteristic (ROC) analysis, with the area under the curve (AUC) and the optimal cut-off point being calculated. Pearson’s coefficient was used for correlation analysis between two variables. The significance level (α) was set at 0.05 (two-sided).
Results
A total of 165 patients met the inclusion criteria, of whom 45.5% were female. Patient characteristics are shown in Table 1. Subsidence occurred in 45 cases, with an average subsidence height of 3.08 ± 1.03 mm and a subsidence rate of 27.3%, comparable to the findings reported in "Epidemiology of Osteoporotic Fractures in the Chinese Population [26]. Significant differences in age and sex were observed when comparing the characteristics between the CS and non-CS groups. There were no significant differences between the CS and non-CS groups regarding BMI (p = 0.627), hypertension (p = 0.834), diabetes (p = 0.808), Diagnosis (p = 0.932), or surgical level (p = 0.104) (Table 1).
Table 1.
The patients’ baseline data
| CS(N = 45) | Non-CS(N = 120) | P | |
|---|---|---|---|
| Age (y) | 70.4 ± 6.99 | 63.02 ± 8.24 | < 0.001 |
| Sex (female) | 14 (31) | 61 (59) | 0.023 |
| BMI ≤ 25 (kg/m2) | 24.74 ± 3.82 | 24.48 ± 2.78 | 0.627 |
| Basic disease | |||
| Diabetes | 5 (40) | 12 (108) | 0.834 |
| Hypertension | 5 (40) | 15 (105) | 0.808 |
| Diagnosis | 0.932 | ||
| Herniated intervertebral disc | 19 | 48 | |
| Lumbar stenosis | 12 | 31 | |
| Lumbar spondylolisthesis | 14 | 41 | |
| Subsidence height(mm) | 3.08 ± 1.03 | 0.89 ± 0.55 | < 0.001 |
| Surgical level | 0.104 | ||
| L3–L4 | 1 | 12 | |
| L4–L5 | 38 | 83 | |
| L5–S1 | 6 | 25 | |
Data presented as mean ± standard deviation. N, number of patients
The QCT in the CS group was 101.4 ± 13.4, whereas it was 145.5 ± 34.5 in the non-CS group (p < 0.001). The average VBQ score (L1–L4) for the CS group was 3.79 ± 0.89, compared to 2.89 ± 0.58 for the non-CS group (p < 0.001). The mean EBQ score in the CS group was 4.99 ± 0.97, whereas it was 4.29 ± 0.80 in the non-CS group, with both showing statistical significance (Table 2). All clinical and radiological factors that exhibited statistically significant differences in the univariate analysis were included in the logistic regression model. The results of the multivariate analysis (Table 3) demonstrated that age, gender, QCT, and VBQ were independent predictors of cage subsidence. The PLIF procedure significantly improved both VAS and ODI score for patients; however, the improvement was less pronounced in the subsidence group compared to the non-subsidence group (Table 4). Additionally, both VBQ and EBQ score were strongly correlated with QCT values (VBQ: R = − 0.473, p = 0.001; EBQ: R = − 0.439, p = 0.001).
Table 2.
Radiographic parameters of patients
| Parameters | CS | Non-CS | P |
|---|---|---|---|
| QCT | 101.4 ± 13.4 | 145.5 ± 34.5 | < 0.001 |
| VBQ | 3.79 ± 0.89 | 2.89 ± 0.58 | < 0.001 |
| EBQ | 4.99 ± 0.97 | 4.29 ± 0.80 | < 0.001 |
Table 3.
Multivariate analysis
| Odds ratio | 95% CI | P | |
|---|---|---|---|
| Age | 1.115 | 1.031–1.205 | 0.006 |
| Gender | 6.700 | 2.170–20.681 | 0.001 |
| QCT | 0.959 | 0.940–0.979 | 0.001 |
| VBQ | 3.771 | 1.706–8.335 | 0.001 |
| EBQ | 1.264 | 0.660–2.431 | 0.479 |
Table 4.
Pain and functional
| CS | Non-CS | P | |
|---|---|---|---|
| VAS (Lumbar) Pre-op | 5.22 ± 0.79 | 4.93 ± 0.74 | 0.025 |
| Post-op 12 month | 2.82 ± 0.58 | 1.61 ± 0.96 | < 0.001 |
| Delta change | 2.40 ± 0.91 | 3.32 ± 1.20 | < 0.001 |
| ODI Pre-op | 64.49 ± 7.75 | 58.63 ± 6.41 | < 0.001 |
| ODI Post-op 12 month | 22.53 ± 8.32 | 14.87 ± 5.91 | < 0.001 |
| Delta change | 41.96 ± 11.31 | 43.77 ± 8.96 | 0.285 |
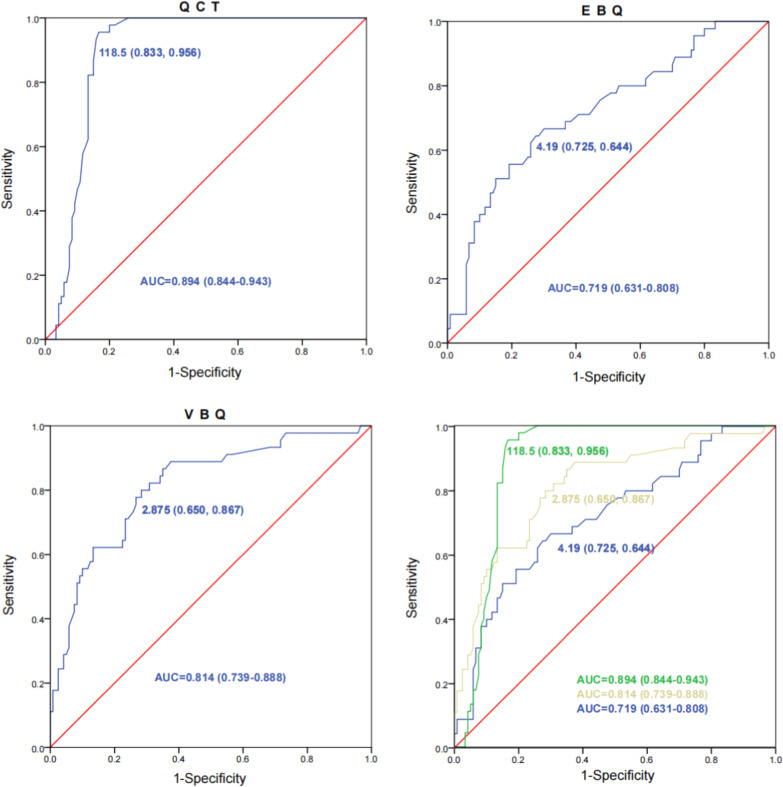
ROC curves for predicting CS were constructed. The area under the curve (AUC) for QCT was 0.894 (95% CI 0.844–0.943), with the optimal cutoff value being 118.5 (sensitivity: 83.3%, specificity: 95.6%). The AUC for VBQ score was 0.814 (95% CI 0.739–0.888), with the optimal cutoff value being 2.875 (sensitivity: 65.0%, specificity: 86.7%). The AUC for the EBQ score was 0.719 (95% CI 0.631–0.808), with the optimal cutoff value being 4.19 (sensitivity: 72.5%, specificity: 64.4%) (Fig. 4).
Fig. 4.
ROC curve of QCT, VBQ and EBQ
Discussion
In recent years, there has been increasing interest in the study of cage subsidence (CS) after PLIF, particularly regarding the role of preoperative bone quality assessment in predicting CS [18, 20, 27]. This study evaluated the predictive value of MRI-based VBQ and EBQ score for CS following PLIF, showing that both score possess a certain level of predictive efficacy. However, compared to QCT (sensitivity: 83.3%, specificity: 95.6%), the sensitivity and specificity of VBQ and EBQ were slightly lower [18, 19]. Unlike MRI-based bone quality assessments, QCT can provide more accurate information on bone structure by directly measuring volumetric bone mineral density, which has a close relationship with CS occurrence. Furthermore, QCT allows for selective measurement of trabecular bone density, an area with higher metabolic activity than cortical bone, making it more sensitive to changes that indicate disease progression and treatment response [2, 28–30]. Nevertheless, QCT demonstrates relatively low sensitivity in detecting lumbar degenerative changes, which are common in elderly populations, potentially reducing its accuracy in clinical applications [19]. Additionally, QCT involves significant radiation exposure, increasing health risks for patients [31].
Due to limitations in examination methods and concerns regarding radiation exposure, QCT and DEXA are typically not used as routine preoperative evaluation methods [32]. Consequently, numerous researchers have proposed MRI-based alternatives to evaluate lumbar spine bone density, specifically the VBQ and EBQ score. However, there is currently a lack of comparative studies assessing the relative effectiveness of these two methods for predicting CS after lumbar fusion surgery. In this study, we directly compared the predictive value of the MRI-based VBQ and EBQ score for predicting CS after PLIF surgery. The results indicated that both score demonstrated good performance in predicting CS, with effectiveness values of 0.814 and 0.719, respectively. In contrast, the study by Salzmann and Jones, reported that the effectiveness of VBQ and EBQ in predicting CS was as follows: VBQ showed an AUC of 0.71, sensitivity of 74.3%, specificity of 57.0%, and an optimal cutoff value of 2.388; EBQ had an AUC of 0.61, sensitivity of 40.0%, specificity of 84.5%, and an optimal cutoff value of 5.1 [18, 19]. The results of this study differ from previous research, which may be attributed to differences in surgical techniques, sample characteristics, or variations in diagnostic equipment parameters, potentially impacting study efficacy.
MRI, with its high resolution and multiplanar imaging capabilities, is widely used for the evaluation of spinal soft tissues and bone structures. The vertebral bone quality (VBQ) and endplate bone quality (EBQ) scoring methods based on MRI assess bone quality by evaluating differences in signal characteristics between water and fat molecules in MRI. These signal differences can be used for the quantitative analysis of bone quality in the vertebrae and endplates. Both EBQ and VBQ score are closely related to postoperative cage subsidence (CS), but their predictive performance differs in focus. VBQ scoring emphasizes assessing the overall bone condition of the vertebral body, including the signal intensity, distribution, and structural integrity of the trabeculae. EBQ scoring mainly relies on the signal characteristics, morphological changes, and structural integrity of the endplate observed on MRI for evaluation. We found that the VBQ and EBQ score of the subsidence group were significantly higher than those of the non-subsidence group (p < 0.05). However, in the multivariate regression analysis, EBQ did not emerge as an independent predictor of CS, which is somewhat inconsistent with previous studies [33]. The reason for this result may be related to the phenomenon of delayed medical consultation among Chinese patients, especially for chronic conditions such as cervical spondylosis and lumbar disc herniation. Patients often mistake early symptoms for general muscle strain or common discomfort due to mild pain, opting for self-treatment rather than seeking immediate medical attention [30, 34]. This leads to the occurrence or worsening of endplate inflammation. Bone marrow edema and inflammatory responses in the endplate and adjacent vertebral bodies are represented on MRI as low T1 signals and high T2 signals, which may result in a lower EBQ score, thus explaining the inconsistency with previous research findings. Similarly, the predictive efficacy of EBQ is inferior to that of VBQ (0.814; 0.714), which may also be due to this phenomenon. We also found that the VBQ score of the subsidence group obtained in this study was higher than that reported by Jones. (3.79 ± 0.89; 2.67 ± 1.08) [19]. This may be due to degenerative changes in the intervertebral disc, which can alter the mechanical load between adjacent vertebrae. When the intervertebral disc degenerates, its load-bearing and cushioning capacity are reduced, resulting in more mechanical stress being transmitted directly to the vertebral bodies. This uneven mechanical load can lead to trabecular bone degeneration and microstructural damage, ultimately resulting in an increased VBQ score [35]. Interestingly, some studies suggest that severe and prolonged disc degeneration can lead to reduced bone marrow fat content, potentially making the bone stronger and thereby resulting in a lower VBQ score [36]. This appears to be a contradiction. Therefore, in clinical practice, the EBQ score is more suitable for a detailed assessment of the implantation site for the cage before surgery, providing important reference information, especially when selecting patients with weaker endplates. In contrast, the VBQ score is more suitable for assessing the overall bone quality of the patient, to guide preoperative medication and surgical strategy planning [37, 38]. Combining these two score can provide a more precise assessment for individualized treatment plans, helping surgeons to better evaluate preoperative bone quality status and potential postoperative risks.
We found differences in VAS and ODI score between the preoperative subsidence group and the non-subsidence group. Studies have shown that the VBQ score can reveal the state of vertebral osteoporosis and trabecular degeneration, and these bone changes are closely related to patients’ pain and functional impairment. In patients with lumbar degenerative changes, worsening osteoporosis may lead to increased pain sensitivity, thereby raising VAS score [39]. Furthermore, the degeneration of bone trabeculae weakens the support of the vertebral body, leading to localized mechanical low back pain, which is also an important factor contributing to higher VAS score [40]. With respect to the higher ODI score, some researchers believe that trabecular bone degeneration makes the vertebral body more fragile, increasing the risk of microdamage and functional instability. This instability can cause pain and discomfort during movement, restricting patients’ ability to perform daily activities, ultimately leading to increased ODI score [41]. Other studies have pointed out that a compromised trabecular bone support structure in patients with lumbar degenerative changes can affect spinal load transmission and overall motor function. As a major load-bearing structure, the degeneration of vertebral bone directly impacts patient mobility, thereby increasing the difficulty for the lower back to bear weight or carry out daily tasks, which is a key factor contributing to the increase in ODI score [42].
Although this study evaluated the value of MRI-based VBQ and EBQ score in predicting CS after PLIF, these two score still show deficiencies in sensitivity and specificity compared to QCT. QCT, by directly measuring volumetric bone mineral density, can provide more accurate information on bone structure. In contrast, VBQ and EBQ score mainly rely on variations in MRI signal intensity, which have certain limitations in reflecting bone density and bone microstructure, particularly when predicting lumbar degenerative changes, potentially leading to biases. Therefore, additional comprehensive studies are needed to assess the relative effectiveness of these three methods in different patient populations. Moreover, we hypothesize that delayed medical consultation may lead to endplate inflammation and bone marrow edema, thereby affecting EBQ and VBQ score. However, this hypothesis is primarily based on observational data and lacks robust causal validation. This study did not conduct a detailed quantitative analysis of patients’ delays in seeking medical attention, nor did it provide specific statistical support; therefore, the scientific foundation of this hypothesis necessitates further validation. Finally, cage subsidence is not only related to bone loss in patients but is also influenced by the parameters of the cage itself (e.g., cage size, cross-sectional area, and hardness) [5, 7, 43]. These factors were not considered in this study, and future research should allocate additional resources to these aspects to enhance the comprehensiveness of our evaluations.
In conclusion, we suggest that the MRI-based VBQ score exhibits superior predictive value in forecasting cage subsidence (CS) following PLIF surgery, compared to the EBQ score, the VBQ score has better adaptability in assessing the overall bone quality of the Chinese population.
Acknowledgements
No applicable
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by FZ, JL and YW, ZY. The first draft of the manuscript was written by ZY and FZ, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Availability of data and materials
The datasets used or analysis during the current study are available from corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This retrospective study used completely anonymized data from the Yichang Central People’s Hospital. The study protocol was reviewed by the Medical Ethics Committee of Yichang Central People’s Hospital, which determined that formal ethics approval was not required, as the study utilized previously collected data that could not identify individuals.
Consent for publication
All authors had reviewed the final manuscript and give consent for submission and publication.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yu Wu, Email: 13972565385@163.com.
Zijian Yang, Email: 13886997194@163.com.
References
- 1.Alentado VJ, Caldwell S, Gould HP, Steinmetz MP, Benzel EC, Mroz TE. Independent predictors of a clinically significant improvement after lumbar fusion surgery. Spine J. 2017;17:236–43. [DOI] [PubMed] [Google Scholar]
- 2.Fenton-White HA. Trailblazing: the historical development of the posterior lumbar interbody fusion (PLIF). Spine J. 2021;21:1528–41. [DOI] [PubMed] [Google Scholar]
- 3.Pu HY, Chen Q, Huang K, Zeng R, Wei P. Forearm T-score as a predictor of cage subsidence in patients with degenerative lumbar spine disease following posterior single-segment lumbar interbody fusion. BMC Musculoskelet Disord. 2022;23:1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JH, Jeon DW, Lee SJ, Chang BS, Lee CK. Fusion rates and subsidence of morselized local bone grafted in titanium cages in posterior lumbar interbody fusion using quantitative three-dimensional computed tomography scans. Spine. 2010;35(15):1460–5. [DOI] [PubMed] [Google Scholar]
- 5.Amorim-Barbosa T, Pereira C, Catelas D, et al. Risk factors for cage subsidence and clinical outcomes after transforaminal and posterior lumbar interbody fusion. Eur J Orthop Surg Traumatol. 2022;32:1291–9. [DOI] [PubMed] [Google Scholar]
- 6.Park MK, Kim KT, Bang WS, et al. Risk factors for cage migration and cage retropulsion following transforaminal lumbar interbody fusion. Spine J. 2019;19:437–47. [DOI] [PubMed] [Google Scholar]
- 7.Shen S, You X, Ren Y, Ye S. Risk factors of cage subsidence following oblique lumbar interbody fusion: a meta-analysis and systematic review. World Neurosurg. 2024;183:180–6. [DOI] [PubMed] [Google Scholar]
- 8.Yao YC, Chou PH, Lin HH, Wang ST, Liu CL, Chang MC Risk Factors of Cage Subsidence in Patients Received Minimally Invasive Transforaminal Lumbar Interbody Fusion. Spine 2020; (Phila Pa 1976) 45:E1279–e1285 [DOI] [PubMed]
- 9.Zhao L, Xie T, Wang X, et al. Clinical and radiological evaluation of cage subsidence following oblique lumbar interbody fusion combined with anterolateral fixation. BMC Musculoskelet Disord. 2022;23:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parisien A, Wai EK, ElSayed MSA, Frei H. Subsidence of spinal fusion cages: a systematic review. Int J Spine Surg. 2022;16:1103–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan SL, Prater GL. Quality in dual-energy X-ray absorptiometry scans. Bone. 2017;104:13–28. [DOI] [PubMed] [Google Scholar]
- 12.Kim KJ, Kim DH, Lee JI, Choi BK, Han IH, Nam KH. Hounsfield units on lumbar computed tomography for predicting regional bone mineral density. Open Med (Wars). 2019;14:545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin W, He C, Xie F, et al. Discordance in lumbar bone mineral density measurements by quantitative computed tomography and dual-energy X-ray absorptiometry in postmenopausal women: a prospective comparative study. Spine J. 2023;23:295–304. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Dash A, Cunningham M, et al. Patients with abnormal microarchitecture have an increased risk of early complications after spinal fusion surgery. Bone. 2021;143: 115731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dall’Ara E, Pahr D, Varga P, Kainberger F, Zysset P. QCT-based finite element models predict human vertebral strength in vitro significantly better than simulated DEXA. Osteoporos Int. 2012;23:563–72. [DOI] [PubMed] [Google Scholar]
- 16.Choi MK, Kim SM, Lim JK. Diagnostic efficacy of Hounsfield units in spine CT for the assessment of real bone mineral density of degenerative spine: correlation study between T-score determined by DEXA scan and Hounsfield units from CT. Acta Neurochir (Wien). 2016;158:1421–7. [DOI] [PubMed] [Google Scholar]
- 17.Klingberg E, Lorentzon M, Mellström D, et al. Osteoporosis in ankylosing spondylitis—prevalence, risk factors and methods of assessment. Arthritis Res Ther. 2012;14:R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salzmann SN, Okano I, Jones C, et al. Preoperative MRI-based vertebral bone quality (VBQ) score assessment in patients undergoing lumbar spinal fusion. Spine J. 2022;22:1301–8. [DOI] [PubMed] [Google Scholar]
- 19.Jones C, Okano I, Arzani A, et al. The predictive value of a novel site-specific MRI-based bone quality assessment, endplate bone quality (EBQ), for severe cage subsidence among patients undergoing standalone lateral lumbar interbody fusion. Spine J. 2022;22:1875–83. [DOI] [PubMed] [Google Scholar]
- 20.Ran L, Xie T, Zhao L, et al. MRI-based endplate bone quality score predicts cage subsidence following oblique lumbar interbody fusion. Spine J. 2024. 10.1016/j.spinee.2024.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Ehresman J, Schilling A, Pennington Z, et al. A novel MRI-based score assessing trabecular bone quality to predict vertebral compression fractures in patients with spinal metastasis. J Neurosurg Spine. 2020;32:499–506. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Su Y, Wang Q, et al. Validation of asynchronous quantitative bone densitometry of the spine: Accuracy, short-term reproducibility, and a comparison with conventional quantitative computed tomography. Sci Rep. 2017;7:6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu ZJ, Zhang C, Ma C, et al. Automatic phantom-less QCT system with high precision of BMD measurement for osteoporosis screening: technique optimisation and clinical validation. J Orthop Translat. 2022;33:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wangaryattawanich P, Kale HA, Kanter AS, Agarwal V. Lateral lumbar interbody fusion: review of surgical technique and postoperative multimodality imaging findings. AJR Am J Roentgenol. 2021;217:480–94. [DOI] [PubMed] [Google Scholar]
- 25.Xi Z, Mummaneni PV, Wang M, et al. The association between lower Hounsfield units on computed tomography and cage subsidence after lateral lumbar interbody fusion. Neurosurg Focus. 2020;49:E8. [DOI] [PubMed] [Google Scholar]
- 26.Yu F, Xia W. The epidemiology of osteoporosis, associated fragility fractures, and management gap in China. Arch Osteoporos. 2019;14:32. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Wang L, Li Q, Deng Z, Wang L, Song Y. A novel MRI-based cervical-endplate bone quality score independently predicts cage subsidence after anterior cervical discectomy and fusion. Eur Spine J. 2024;33:2277–86. [DOI] [PubMed] [Google Scholar]
- 28.Poilliot A, Gay-Dujak MH, Müller-Gerbl M. The quantification of 3D-trabecular architecture of the fourth cervical vertebra using CT osteoabsorptiometry and micro-CT. J Orthop Surg Res. 2023;18:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ai Y, Chen Q, Huang Y, et al. MRI-based vertebral bone quality score for predicting cage subsidence by assessing bone mineral density following transforaminal lumbar interbody fusion: a retrospective analysis. Eur Spine J. 2023;32:3167–75. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Peng L, Wang Y, Yang Y, Wang Z. Risk factors for low back pain in the Chinese population: a systematic review and meta-analysis. BMC Public Health. 2024;24:1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Açikgöz G, Bora A, Nur S. Comparison of QCT and DEXA findings for lumbar vertebra in young adults and the elderly. Acta Radiol. 2024;65:759–64. [DOI] [PubMed] [Google Scholar]
- 33.Chen Q, Ai Y, Huang Y, et al. MRI-based endplate bone quality score independently predicts cage subsidence following transforaminal lumbar interbody fusion. Spine J. 2023;23:1652–8. [DOI] [PubMed] [Google Scholar]
- 34.Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:968–74. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Lei F, Ye F, Yuan H, Li S, Feng D. MRI-based vertebral bone quality score for the assessment of osteoporosis in patients undergoing surgery for lumbar degenerative diseases. J Orthop Surg Res. 2023;18:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moser M, Adl Amini D, Albertini Sanchez L et al. The Reciprocal Relationship Between Lumbar Intervertebral Disk Degeneration and the MRI-based Vertebral Bone Quality Score. Spine (Phila Pa 1976) 2024;49:1227–1234. [DOI] [PubMed]
- 37.Jones C, Okano I, Salzmann SN, et al. Endplate volumetric bone mineral density is a predictor for cage subsidence following lateral lumbar interbody fusion: a risk factor analysis. Spine J. 2021;21:1729–37. [DOI] [PubMed] [Google Scholar]
- 38.Ai Y, Zhu C, Chen Q, et al. Comparison of predictive value for cage subsidence between MRI-based endplate bone quality and vertebral bone quality score following transforaminal lumbar interbody fusion: a retrospective propensity-matched study. Spine J. 2024;24:1046–55. [DOI] [PubMed] [Google Scholar]
- 39.Wong CK, Mak RY, Kwok TS, et al. Prevalence, incidence, and factors associated with non-specific chronic low back pain in community-dwelling older adults aged 60 years and older: a systematic review and meta-analysis. J Pain. 2022;23:509–34. [DOI] [PubMed] [Google Scholar]
- 40.Schmeel FC, Enkirch SJ, Luetkens JA, et al. Diagnostic accuracy of quantitative imaging biomarkers in the differentiation of benign and malignant vertebral lesions : combination of diffusion-weighted and proton density fat fraction Spine MRI. Clin Neuroradiol. 2021;31:1059–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang YH, Zhao CQ, Jiang LS, Chen XD, Dai LY. Modic changes: a systematic review of the literature. Eur Spine J. 2008;17:1289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gopal D, Ho AL, Shah A, Chi JH. Molecular basis of intervertebral disc degeneration. Adv Exp Med Biol. 2012;760:114–33. [DOI] [PubMed] [Google Scholar]
- 43.Singhatanadgige W, Sukthuayat A, Tanaviriyachai T, et al. Risk factors for polyetheretherketone cage subsidence following minimally invasive transforaminal lumbar interbody fusion. Acta Neurochir (Wien). 2021;163:2557–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analysis during the current study are available from corresponding author on reasonable request.