Abstract
Background
Right atrial (RA) dysfunction and atrial arrhythmias are relatively common in adults with repaired tetralogy of Fallot. The purpose of this study was to determine whether RA function improved after surgical pulmonary valve replacement (PVR), and the association between postoperative RA reverse remodeling and late postoperative atrial arrhythmias.
Method
RA reverse remodeling (ΔRA reservoir strain based speckle tracking echocardiography) was calculated as: ([postoperative RA reservoir strain – preoperative RA reservoir strain]/preoperative RA reservoir strain)x100. Optimal RA reverse remodeling was defined as ΔRA reservoir strain >15%.
Results
Of 411 patients (age 36 ± 13 years), preoperative RA reservoir strain was 31 ± 13%, postoperative RA reserve remodeling was 13 ± 9%, and 171 (42%) had optimal RA reserve remodeling. Preoperative RA reservoir strain (β±SE 1.12 ± 0.09, p < 0.001) was associated with postoperative RA reverse remodeling on multivariable analysis. Preoperative RA reservoir strain ≥33% predicted optimal postoperative RA reverse remodeling (area under the curve 0.792).
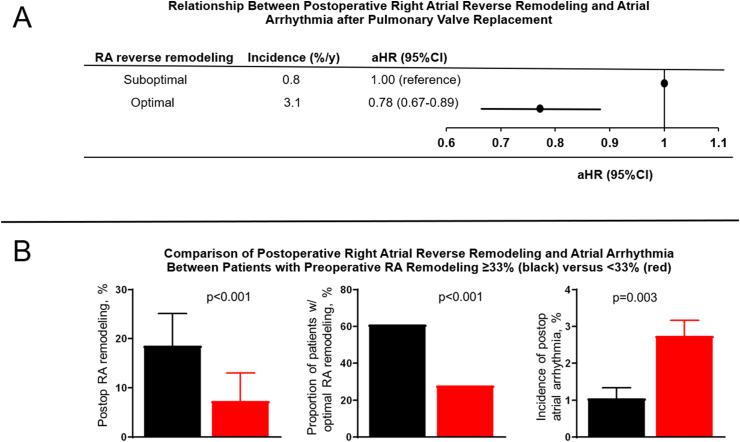
ΔRA reservoir strain was associated with postoperative atrial arrhythmias (HR 0.91, 95%CI 0.86–0.96, p = 0.004), on multivariable analysis. Compared to patients with preoperative RA reservoir strain <33% (n = 242, 59%), those with RA reservoir strain ≥33% (n = 169, 41%) had more robust RA reverse remodeling (ΔRA reverse remodeling 19 ± 11% versus 7 ± 10%, p < 0.001), and lower incidence of atrial arrhythmias (1.1% versus 2.9%, p = 0.003).
Conclusions
Preoperative RA reservoir strain was associated with RA reverse remodeling after PVR, and in turn, postoperative atrial arrhythmia. These results suggest that RA strain indices could be used to determine optimal timing for PVR in order to reduce the risk of atrial arrhythmia.
Keywords: Right atrial dysfunction, Pulmonary valve replacement, Atrial arrhythmias
Highlights
-
•
RA reverse remodeling occurred after PVR.
-
•
RA reverse remodeling after PVR was associated with lower risk of atrial arrhythmias.
-
•
Preoperative RA reservoir strain was associated with RA reverse remodeling, and atrial arrhythmiaS.
Abbreviations
- CMRI
Cardiac magnetic resonance imaging
- CI
Confidence interval
- HR
Hazard ratio
- LA
Left atrium
- LV
Left ventricle
- PVR
Pulmonary valve replacement
- RA
Right atrium
- RV
Right ventricle
- ROC
Receiver operating characteristic
- SE
Standard error
- TOF
Tetralogy of Fallot
1. Introduction
Patients with repaired tetralogy of Fallot (TOF) develop recurrent hemodynamic lesions over time, the most common lesion being pulmonary regurgitation and right ventricular (RV) outflow tract stenosis [[1], [2], [3]]. Surgical and transcatheter pulmonary valve replacement (PVR) are effective in relieving the hemodynamic stress secondary to RV outflow tract lesions, and have been shown to promote RV reverse remodeling (changes in RV structure and function) over time [4,5]. Previous studies have proposed optimal cut-off points for RV volumetric indices beyond which the normalization of RV structure and function were unlikely to occur after PVR [2,[4], [5], [6], [7], [8], [9]]. These studies provide the empirical basis for the guideline recommendations for PVR in this population [[9], [10], [11]].
Although RV volumetric indices provide robust framework for the timing of PVR in order to achieve the normalization of RV structure and function, the use of these RV volumetric cut-off points for clinical decision-making regarding timing of PVR have not been shown to provide survival benefits across different studies [1,3,8,12,13]. Bokma et al. demonstrated a survival benefit after PVR only in patients with preoperative RV end-systolic volume index >80 ml/m2, but not in patients that underwent PVR prior to this threshold [14]. These inconsistent results may be related to the fact that RV volumetric indices only provide an assessment of RV preload and systolic function, but not an assessment of the other aspects of right heart hemodynamic performance such as RV diastolic function and right atrial (RA) function [2,9,[15], [16], [17], [18]]. These other aspects of right heart function (RV diastolic function and RA function) play critical roles in the pathogenesis of atrial arrhythmias, right heart failure, and cardiovascular death based on studies conducted in patients with acquired heart disease [[19], [20], [21], [22]].
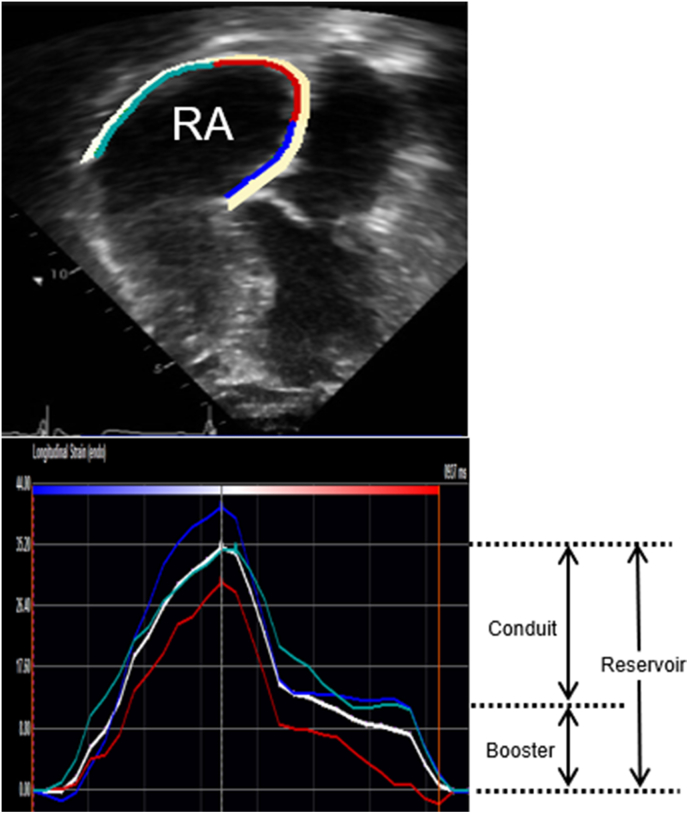
Similar data are lacking in adults with repaired TOF, even though RA dysfunction, atrial arrhythmias, heart failure, and cardiovascular death are relatively common in this population [16,[23], [24], [25], [26], [27]]. Atrial strain imaging provides an assessment of atrial function at different phases of the cardiac cycle (reservoir strain, conduit strain, and booster strain) [[28], [29], [30], [31]]. Atrial reservoir strain measures atrial compliance; atrial conduit strain measures ventricular relaxation and chamber stiffness, and atrial booster strain measures intrinsic atrial contractility and ventricular end-diastolic compliance [[28], [29], [30], [31]]. However, the role of atrial strain imaging for risk stratification in patients with TOF has not been studied. As the next step towards addressing this knowledge gap, the current study would answer two clinical questions. (1) Does RA function (RA reverse remodeling) improve after PVR? (2) Is postoperative improvement in RA function (extent of RA reverse remodeling) associated with a lower risk of atrial arrhythmias, an important link in the pathogenesis of heart failure and cardiovascular death in this population?
2. Methods
2.1. Study population
The Mayo Clinic Institutional Review Board approved this retrospective cohort study of adults (age ≥18 years) with repaired TOF that underwent surgical PVR and had at least one year of imaging follow-up after PVR at Mayo Clinic Rochester from January 1, 2003 and December 31, 2020. We restricted to inclusion criteria to only patients that underwent surgical PVR (i.e., excluding patients that underwent transcatheter PVR) since myocardial insult and recovery may differ significantly between patients that underwent surgical PVR versus transcatheter PVR because of the use of cardiopulmonary bypass in the surgical group. From this cohort, we excluded patients with the following conditions: (1) prior tricuspid valve replacement or requiring tricuspid valve replacement at the time of PVR. (2) inadequate echocardiographic images for offline assessment of RA strain.
2.2. Study objectives
The study objectives were to determine the clinical and hemodynamic correlates of postoperative RA reverse remodeling after PVR, and the relationship between postoperative RA reverse remodeling and late postoperative atrial arrhythmias. The exploratory objective was to determine the relationship between restrictive RV physiology on preoperative echocardiogram, and the risk of late postoperative atrial arrhythmias. Similar to previous studies from our group, we defined restrictive RV physiology as the combination of dilated inferior vena cava with <50% collapsibility on inspiration and the presence hepatic vein diastolic flow reversal or pulmonary artery forward flow during atrial systole [18].
2.3. Data collection and echocardiography
All patients underwent comprehensive transthoracic echocardiograms at 3 different time points. (1) Preoperative echocardiogram (Echo #1) which was the last comprehensive echocardiogram performed within 3 months prior to PVR. The echocardiographic and clinical indices obtained within this time frame were used to define the baseline clinical characteristics of the cohort. (2) Immediate postoperative echocardiogram (Echo #2) which was the echocardiogram performed at the time of hospital dismissal after PVR. The purpose of this echocardiogram was to determine the new baseline hemodynamic characteristics after PVR, and we limited the indices retrieved from this study to only pulmonary valve gradient, estimated RV systolic pressure, estimated RA pressure, and residual tricuspid regurgitation since these variables can influence long-term right heart remodeling. We also retrieved data on heart rate and hemoglobin at the time of echocardiogram since these variables influence echocardiographic indices. (3) Late postoperative echocardiogram (Echo #3) which was the echocardiogram performed at the time of routine annual outpatient cardiac evaluation, within 12–18 months after PVR.
2.4. Echocardiography
RA function was assessed using RA reservoir strain imaging derived from offline analysis of echocardiographic images. RA reverse remodeling (Δ RA reservoir strain) was defined as a relative increase (or decrease) in RA reservoir strain at Echo #3 from preoperative levels, and was calculated as follows: Δ RA reservoir strain = ([RA reservoir strain from Echo #3 - Echo #1]/RA reservoir strain from Echo #1) x100. There are no defined criteria for optimal RA reverse remodeling. However, a previous study by Tops et al. [32] showed that >15% reduction in left atrial (LA) volume after catheter ablation for atrial fibrillation was associated with lower risk of recurrence of atrial fibrillation, hence signifying clinical meaningful change in LA reverse remodeling post intervention. Based on these data, we defined optimal RA reverse remodeling as Δ RA reservoir strain >15% from preoperative levels, and suboptimal RA reverse remodeling as Δ RA reservoir strain ≤15% from preoperative level.
The procedural details for speckle tracking strain imaging in our laboratory have been described [33,34]. In brief, imaging was acquired using Vivid E9 and E95 (General Electric Co, Fairfield, Connecticut) with M5S and M5Sc-D transducers (1.5–4.6 MHz) at frame rate of 40–80 Hz, and these images were exported (DICOM) and then analyzed offline using TomTec (TomTec Imaging Systems, Unterschleissheim, Germany). The offline assessment of RA reservoir strain involved manual endocardial tracing of a single frame at end-systole by a point-click approach, starting from the lateral tricuspid annulus to the septal tricuspid annulus using images from an RV focused view (Supplementary Fig. 1). RA reservoir strain, RA conduit strain, and RA booster strain were assessed using the QRS as the fiduciary point. LA reservoir strain was assessed using similar techniques. Other indices of right and left heart structure, function, and hemodynamics were assessed as per guidelines [35,36].
2.5. Atrial arrhythmias
We defined atrial arrhythmia as a diagnosis of atrial fibrillation, atrial flutter, or atrial tachycardia lasting longer that 30 s, based on electrocardiogram, Holter monitor, rhythm strip or device interrogation reports [37]. Similar to previous studies [37], we defined atrial fibrillation as the absence of a constant atrial activity/p-wave on surface ECG, atrial flutter as a macro-reentrant atrial arrhythmia that is typically characterized by sudden onset and termination clinically, and atrial tachycardia has atrial arrhythmia originating from a focal source. We grouped atrial flutter and atrial tachycardia together because of difficulty to reliably differentiate between these arrhythmias on surface electrocardiogram.
Preoperative atrial arrhythmia was considered to be present if a patient had documented atrial arrhythmia prior to the time of PVR. Postoperative atrial arrhythmia was defined as documented atrial arrhythmia from 30 days after PVR until the last clinical encounter within study period. We excluded atrial arrhythmias occurring within the first 30 days after surgery since atrial arrhythmias are common within this period based on previous studies [38].
2.6. Statistical analysis
Data were presented as mean ± standard deviation, median (interquartile range), and count (%). Between-group comparisons were performed using unpaired t-test, Wilcoxon rank sum test and chi-square test, as appropriate. The relationships between continuous variables were assessed using Pearson correlation.
Linear regression analysis was used to identify the correlates of preoperative RA reservoir strain, and postoperative RA reverse remodeling. These models were adjusted for demographic indices (age, sex), surgical history (age at time of TOF repair, transannular patch repair, prior systemic to pulmonary shunt, PVR prior to baseline), preoperative right heart indices (RA volume, RA pressure, RV global longitudinal strain, moderate tricuspid regurgitation, RV systolic pressure, pulmonary valve gradient), preoperative left heart indices (LA volume, LA reservoir strain, left ventricular [LV] global longitudinal strain), and comorbidities (hypertension, diabetes, coronary artery disease). Of note, LV and RV global longitudinal strain were modeled as absolute values (i.e., without the negative sign). Prior to creating the multivariable models, we first created univariable linear regression models assessing the correlates of preoperative RA reservoir strain and postoperative RA reverse remodeling. The covariables used in these univariable models (demographic indices, surgical history, echocardiographic indices, and comorbidities) were selected based on clinical relevance, and known association with outcomes. The covariates with p < 0.1 on univariable analysis were entered into the multivariable models, and final selection of covariates in multivariable models were based on stepwise backwards selection of covariates with a p < 0.05 required for a covariate to remain in the model. Age and sex were forced into all the models. The strength of association was measured using β and standard error (SE). In an exploratory analysis, we used receiver operating characteristic (ROC) curve to determine the ideal cut-off point for preoperative RA reservoir strain that predicts optimal RA reverse remodeling (Δ RA reverse remodeling >15%) based on Youden index.
Cox regression analysis was used to assess the relationship between RA reverse remodeling and postoperative atrial arrhythmias (new-onset and recurrent atrial arrhythmias), and the Cox models were adjusted for the same covariates listed above. Postoperative atrial arrhythmia was analyzed as a time-to-event outcome, and patients were censored at the time of first episode of postoperative arrhythmia or last clinical encounter in patients without postoperative atrial arrhythmias. The strength of association was measured using hazard ratio (HR) and 95% confidence interval (CI). Subgroup analyses were performed to assess the relationship between RA reverse remodeling and postoperative atrial arrhythmias in the following patient subgroups: (1) Patients with history of preoperative atrial arrhythmias (n = 112); (2) Patients without history of preoperative atrial arrhythmias (n = 299); (3) Patients with ≥moderate tricuspid regurgitation prior to PVR (n = 90); (4) Patients with <moderate tricuspid regurgitation prior to PVR (n = 321); (5) Patients with complete echocardiographic and cardiac magnetic resonance imaging (CMRI) data (n = 331); (6) Patients without atrial arrhythmias within the first 12 months after PVR (n = 397).
The single conditional imputation method was used to correct for missing data. The imputation for continuous variable was performed by replacing the missing variables with the group mean for each covariate. For the categorical variables, we created two sets of covariates, and missing data were coded as positive and negative responses in the different sets. We performed sensitivity analyses using the subset of patients with complete echocardiographic data (complete case analysis). All statistical analyses were performed with BlueSky Statistics software (version. 7.10; BlueSky Statistics LLC, Chicago, IL, USA), and p value < 0.05 was considered to be statistically significant for all analyses.
3. Results
3.1. Baseline characteristics
There were 411 patients that met the study inclusion criteria, and the mean age at the time of preoperative echocardiogram (Echo #1) was 36 ± 13 years, and 212 (52%) were male patients. Of the 411 patients, 112 (27%) patients had a history of preoperative atrial arrhythmias, of which 81 had atrial flutter/tachycardia while 72 had atrial fibrillation (41 patients had both types of arrhythmias at different times). Compared to patients without preoperative atrial arrhythmias (n = 299, 73%), those with preoperative atrial arrhythmias (n = 112, 27%) were older, more likely to have cardiac implantable electronic devices and PVR at baseline, comorbidities, and end-organ dysfunction, and also had worse echocardiographic indices of right and left heart function (Table 1). Of the 411 patients, 66 (16%) had restrictive RV physiology, and there was no significant between group difference in the prevalence of restrictive RV physiology between the patients with preoperative atrial arrhythmia (n = 21, 19%) versus those without preoperative arrhythmias (n = 45, 15%), p = 0.8 (Table 1).
Table 1.
Preoperative clinical and imaging data (n = 411).
| All (n = 411) | Preop AA (n = 112, 27%) | No Preop AA (n = 299, 73%) | p | |
|---|---|---|---|---|
| Age, years | 36 ± 13 | 45 ± 13 | 32 ± 12 | <0.001 |
| Male sex | 212 (52%) | 53 (47%) | 159 (53%) | 0.3 |
| Body mass index, kg/m2 | 27 ± 6 | 28 ± 6 | 26 ± 5 | 0.06 |
| Anatomic/surgical history | ||||
| Age at time of TOF repair, years | 3 (0.5–7) | 4 (1–7) | 1 (0.6–2) | 0.08 |
| PVR prior to baseline | 202 (49%) | 73 (65%) | 129 (43%) | <0.001 |
| CIED prior to baseline | 56 (14%) | 38 (34%) | 18 (6%) | <0.001 |
| Comorbidities | ||||
| Hypertension | 79 (19%) | 41 (37%) | 38 (13%) | <0.001 |
| Diabetes | 53 (13%) | 21 (19%) | 32 (11%) | 0.03 |
| Coronary artery disease | 24 (6%) | 14 (13%) | 10 (3%) | <0.001 |
| Medications | ||||
| Beta blockers | 89 (21%) | 39 (35%) | 50 (17%) | <0.001 |
| ACEI/ARB | 72 (18%) | 35 (31%) | 37 (12%) | <0.001 |
| MRA | 17 (4%) | 7 (6%) | 10 (3%) | 0.2 |
| Loop diuretics | 69 (17%) | 38 (34%) | 31 (10%) | <0.001 |
| Laboratory data | ||||
| GFR, ml/min/1.73 m [2] | 102 ± 18 | 96 ± 17 | 105 ± 18 | 0.006 |
| NT-proBNP, ng/l |
210 (95–463) |
395 (225–1056) |
164 (84–329) |
0.003 |
| Right heart indices (Echo #1) | ||||
| RA reservoir strain, % | 31 ± 13 | 24 ± 11 | 34 ± 13 | <0.001 |
| RA conduit strain, % | 18 ± 9 | 15 ± 7 | 19 ± 9 | 0.01 |
| RA booster strain, % | 13 ± 8 | 10 ± 6 | 15 ± 7 | <0.001 |
| RA volume index, ml/m2 | 42 ± 19 | 44 ± 17 | 35 ± 13 | 0.02 |
| Estimated RA pressure, mmHg | 8 ± 4 | 10 ± 4 | 7 ± 3 | <0.001 |
| Estimated RV systolic pressure, mmHg | 43 (34–54) | 47 (36–63) | 41 (33–51) | 0.03 |
| ≥Moderate tricuspid regurgitation | 110 (27%) | 49 (44%) | 61 (20%) | <0.001 |
| RV end-diastolic area, cm2 | 38 ± 10 | 41 ± 13 | 36 ± 8 | 0.2 |
| RV end-systolic area, cm2 | 25 ± 8 | 26 ± 11 | 24 ± 7 | 0.5 |
| RV fractional area change, % | 37 ± 9 | 36 ± 9 | 37 ± 8 | 0.6 |
| RV global longitudinal strain, % | −19 ± 4 | −18 ± 4 | −19 ± 3 | 0.4 |
| ≥Moderate pulmonary regurgitation | 385 (94%) | 102 (91%) | 283 (95%) | 0.6 |
| Pulmonary valve mean gradient, mmHg | 32 ± 19 | 35 ± 23 | 30 ± 16 | 0.4 |
| Restrictive RV physiology | 66 (16%) | 21 (19%) | 45 (15%) | 0.8 |
| Left heart indices (Echo #1) | ||||
| LA reservoir strain, % | 40 ± 15 | 35 ± 12 | 43 ± 15 | 0.001 |
| LA conduit strain, % | 26 ± 10 | 21 ± 8 | 29 ± 11 | <0.001 |
| LA booster strain, % | 14 ± 8 | 11 ± 6 | 15 ± 7 | 0.006 |
| LA volume index, ml/m2 | 27 ± 11 | 33 ± 15 | 24 ± 9 | <0.001 |
| LV end-diastolic volume index, ml/m2 | 54 ± 9 | 56 ± 10 | 52 ± 11 | 0.2 |
| LV end-systolic volume index, ml/m2 | 25 ± 7 | 27 ± 8 | 24 ± 6 | 0.1 |
| LV ejection fraction, % | 58 ± 7 | 56 ± 9 | 58 ± 10 | 0.2 |
| LV global longitudinal strain, % | −20 ± 5 | −18 ± 5 | −22 ± 6 | <0.001 |
| Cardiac MRI (n = 331) | ||||
| RV end-diastolic volume index, ml/m2 | 161 ± 40 | 166 ± 41 | 158 ± 3 | 0.6 |
| RV end-systolic volume index, ml/m2 | 85 ± 29 | 87 ± 26 | 84 ± 23 | 0.8 |
| RV stroke volume index, ml/m2 | 86 ± 21 | 81 ± 22 | 88 ± 25 | 0.7 |
| RV ejection fraction, % | 44 ± 10 | 43 ± 10 | 44 ± 9 | 0.8 |
| Pulmonary regurgitant volume index, ml/m2 | 45 ± 19 | 42 ± 17 | 47 ± 20 | 0.2 |
| Pulmonary regurgitant fraction, % | 52 ± 24 | 51 ± 26 | 53 ± 21 | 0.4 |
| LV stroke volume index, ml/m2 | 39 ± 11 | 35 ± 10 | 43 ± 11 | 0.1 |
| LV ejection fraction, ml/m2 | 58 ± 9 | 56 ± 11 | 59 ± 10 | 0.3 |
Abbreviations: AA: atrial arrhythmia; ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; CIED: cardiac implantable electronic devices; GFR: glomerular filtration rate; LA: left atrium; LV: left ventricle; MRI: magnetic resonance imaging; MRA: mineralocorticoid receptor antagonist; NT-proBNP: N-terminal pro-brain natriuretic peptide; Preop: preoperative; PVR: pulmonary valve replacement; Preop: preoperative; RA: right atrium; RV: right ventricle TOF: Tetralogy of Fallot.
3.2. Preoperative RA function
The mean RA reservoir strain in Echo #1 was 31 ± 13% for the entire cohort. RA reservoir strain had a modest correlation with estimated RA pressure (r = −0.66, p < 0.001), LA reservoir strain (r = 0.61, p < 0.001), ≥moderate tricuspid regurgitation (r = −0.57, p < 0.001), but a poor correlation with RA volume index (r = −0.48, p < 0.001), estimated RV systolic pressure (r = −0.45, p = 0.006), RV global longitudinal strain (r = 0.41, p = 0.01), restrictive RV physiology (r = −0.38, p = 0.02), and LV global longitudinal strain (r = 0.33, p = 0.03).
Of the 411 patients, 331 (81%) underwent CMRI, and the median interval between preoperative echocardiogram and CMRI was 2 (1–3) days. There was a weak correlation between preoperative RA reservoir strain and CMRI-derived RV ejection fraction (r = 0.41, p = 0.003), but no correlation between preoperative RA reservoir strain and RV end-diastolic volume index (r = 0.19, p = 0.2) or RV end systolic volume index (r = 0.28, p = 0.1).
Table 2 show the clinical and hemodynamic correlates of preoperative RA reservoir strain. Age, RA volume index, RA pressure, and LA reservoir strain were associated with preoperative RA strain after adjustment for demographic indices, surgical history, comorbidities, and imaging indices.
Table 2.
Linear regression model showing clinical and hemodynamic correlates of preoperative RA reservoir strain.
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| Variables | β±SE | p | β±SE | p |
| Demographics | ||||
| Age, years | −0.28 ± 0.11 | 0.005 | −0.21 ± 0.09 | 0.008 |
| Male sex | 0.05 ± 0.16 | 0.5 | 0.08 ± 0.22 | 0.4 |
| Surgical History | ||||
| Age at TOF repair, years | −0.14 ± 0.29 | 0.2 | ||
| Transannular patch repair | −1.07 ± 1.04 | 0.3 | ||
| Prior systemic to pulmonary shunt | −0.26 ± 0.51 | 0.1 | ||
| PVR prior to baseline | −0.17 ± 0.21 | 0.4 | ||
| Comorbidities | ||||
| Hypertension | −4.66 ± 2.92 | 0.004 | ||
| Diabetes | −2.43 ± 1.87 | 0.07 | ||
| Coronary artery disease |
−4.76 ± 3.08 |
0.1 |
||
| Preoperative Echo Indices (Echo #1) | ||||
| RA volume index, ml/m2 | −0.39 ± 0.13 | <0.001 | −0.26 ± 0.14 | 0.02 |
| RA pressure, mmHg | −0.62 ± 0.27 | <0.001 | −0.53 ± 0.24 | <0.001 |
| RV global longitudinal strain*, % | 0.28 ± 0.13 | 0.01 | ||
| RV systolic pressure, mmHg | −0.37 ± 0.15 | 0.006 | ||
| Restrictive RV physiology | 0.26 ± 0.26 | 0.1 | ||
| ≥Moderate tricuspid regurgitation | −4.13 ± 1.87 | <0.001 | ||
| Pulmonary valve mean gradient, mmHg | −0.08 ± 0.95 | 0.3 | ||
| LA volume index, ml/m2 | −0.16 ± 0.11 | 0.08 | ||
| LA reservoir strain, % | 0.39 ± 0.14 | <0.001 | 0.31 ± 0.12 | 0.002 |
| LV global longitudinal strain*, % |
0.35 ± 0.21 |
0.03 |
||
| Preoperative CMRI Indices | ||||
| RV end-diastolic volume index, ml/m2 | −0.08 ± 0.11 | 0.2 | ||
| RV end-systolic volume index, ml/m2 | −0.16 ± 0.14 | 0.1 | ||
| RV ejection fraction, % | 0.22 ± 0.09 | 0.003 | ||
Abbreviations: CMRI: cardiac magnetic resonance imaging; LA: left atrium; LV: left ventricle; PVR: pulmonary valve replacement; RA: right atrium; RV: right ventricle; TOF: tetralogy of Fallot.
Footnote: * LV and RV global longitudinal strain were modeled as absolute values (i.e., without the negative sign). Note that only the covariates with statistically significant association with outcome are displayed in the multivariable model.
3.3. Surgical data
Of the 411 patients, 382 (93%) and 29 (7%) received bioprosthetic valves and mechanical prosthetic valves, respectively. Of the 411 patients, 73 (18%) and 16 (19%) had concomitant tricuspid valve repair and RA Maze procedure at the time of PVR, respectively. The average size of the pulmonary valve prosthesis was 27 ± 3 mm. The median hospital stay was 5 (4–8) days, and all patients survived to hospital discharge (inclusion criteria).
All patients had echocardiogram at the time of hospital discharge after PVR (Echo #2). At time of Echo #2, the mean RA pressure was 7 ± 3 mmHg, RV systolic pressure was 33 ± 8 mmHg, pulmonary prosthetic valve mean gradient was 14 ± 6 mmHg, and none of the patient had ≥ moderate tricuspid regurgitation. The mean hemoglobin was 8 ± 2 g/dl, and heart rate was 79 ± 14 beat per minute at the time of the hospital discharge echocardiogram.
3.4. Postoperative RA reverse remodeling
The mean RA reservoir strain in Echo #1 and Echo #3 were 31 ± 13% and 35 ± 15%, respectively, and Δ RA reservoir strain (RA reverse remodeling) was 13 ± 9%. Of the 411 patients, 171 (42%) had optimal RA reserve remodeling (>15% increase from preoperative RA reservoir strain), while 240 (58%) had suboptimal RA reverse remodeling. Table 3 shows a comparison of clinical and imaging indices between patients with optimal versus suboptimal RA reverse remodeling.
Table 3.
Comparison of clinical and imaging indices between patients with optimal versus suboptimal RA reverse remodeling.
| Optimal RA reverse remodeling (n = 171, 42%) | Sub-optimal RA reverse remodeling (n = 240, 58%) | p | |
|---|---|---|---|
| Age, years | 31 ± 11 | 39 ± 10 | 0.007 |
| Male sex | 85 (50%) | 127 (53%) | 0.4 |
| Body mass index, kg/m2 | 27 ± 5 | 26 ± 5 | 0.2 |
| Age at time of TOF repair, years | 2 (0.7–5) | 3 (1–7) | 0.3 |
| Laboratory data | |||
| GFR, ml/min/1.73 m [2] | 98 ± 14 | 103 ± 16 | 0.2 |
| NT-proBNP, ng/l |
185 (141–383) |
231 (182–511) |
0.09 |
| Right heart indices (Echo #1) | |||
| RA reservoir strain, % | 36 ± 10 | 28 ± 12 | 0.007 |
| RA volume index, ml/m2 | 37 ± 15 | 46 ± 18 | 0.01 |
| Estimated RA pressure, mmHg | 7 ± 4 | 9 ± 5 | 0.006 |
| Estimated RV systolic pressure, mmHg | 38 (32–49) | 47 (36–60) | 0.02 |
| RV end-diastolic area, cm2 | 35 ± 12 | 41 ± 12 | 0.1 |
| RV end-systolic area, cm2 | 24 ± 10 | 26 ± 9 | 0.6 |
| RV fractional area change, % | 38 ± 9 | 36 ± 10 | 0.6 |
| RV global longitudinal strain, % | −20 ± 4 | −18 ± 5 | 0.1 |
| Left heart indices (Echo #1) | |||
| LA reservoir strain, % | 43 ± 11 | 38 ± 12 | 0.006 |
| LA volume index, ml/m2 | 25 ± 12 | 29 ± 13 | 0.2 |
| LV global longitudinal strain, % | −21 ± 5 | −18 ± 4 | 0.07 |
| Cardiac MRI | |||
| RV end-diastolic volume index, ml/m2 | 156 ± 33 | 168 ± 31 | 0.4 |
| RV end-systolic volume index, ml/m2 | 83 ± 21 | 87 ± 22 | 0.7 |
| RV ejection fraction, % | 45 ± 11 | 43 ± 9 | 0.6 |
Abbreviations: GFR: glomerular filtration rate; LA: left atrium; LV: left ventricle; MRI: magnetic resonance imaging; NT-proBNP: N-terminal pro-brain natriuretic peptide; PVR: pulmonary valve replacement; RA: right atrium; RV: right ventricle TOF: Tetralogy of Fallot.
The clinical and hemodynamic indices associated with RA reverse remodeling were postoperative RA pressure (beta coefficient [β] ± SE -0.47 ± 0.12, p = 0.006), age (β±SE -0.19 ± 0.07, p = 0.01), preoperative RA reservoir strain (β±SE 1.12 ± 0.09, p < 0.001), and preoperative LA reservoir strain (β±SE 0.22 ± 0.03, p = 0.004), Table 4.
Table 4.
Linear regression model showing clinical and hemodynamic correlates of RA reverse remodeling.
|
Variables |
Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| β±SE | p | β±SE | p | |
| Postoperative Echo Indices (Echo #2) | ||||
| Postop pulmonary valve mean gradient | −0.05 ± 0.19 | 0.1 | ||
| Pulmonary valve size, mm | 0.26 ± 0.43 | 0.6 | ||
| Concomitant tricuspid valve repair | −0.17 ± 0.22 | 0.4 | ||
| Concomitant RA maze operation | −0.52 ± 0.34 | 0.02 | ||
| Postop RA pressure, mmHg | −0.84 ± 0.31 | 0.007 | −0.47 ± 0.12 | 0.006 |
| Postop RV systolic pressure, mmHg | −0.37 ± 0.14 | 0.01 | ||
| Hemoglobin, g/dl | 0.09 ± 17 | 0.3 | ||
| Heart rate, bpm |
−0.03 ± 0.21 |
0.5 |
||
|
Preoperative Clinical Indices Demographics | ||||
| Age, years | −0.23 ± 0.09 | 0.008 | −0.19 ± 0.07 | 0.01 |
| Male sex | 0.08 ± 0.13 | 0.4 | 0.11 ± 0.19 | 0.2 |
| Surgical History | ||||
| Age at TOF repair, year | −0.16 ± 0.34 | 0.3 | ||
| Transannular patch repair | −1.55 ± 1.32 | 0.5 | ||
| Prior systemic to pulmonary shunt | −0.16 ± 0.48 | 0.2 | ||
| PVR prior to baseline | −0.14 ± 0.29 | 0.3 | ||
| Comorbidities | ||||
| Hypertension | −7.51 ± 2.41 | 0.002 | ||
| Diabetes | −4.84 ± 3.31 | 0.5 | ||
| Coronary artery disease |
−6.22 ± 3.77 |
0.2 |
||
| Preoperative Echo Indices (Echo #1) | ||||
| RA reservoir strain, % | 0.53 ± 0.07 | <0.001 | 1.12 ± 0.09 | <0.001 |
| RA volume index, ml/m2 | −0.24 ± 0.04 | <0.001 | ||
| RA pressure, mmHg | −0.54 ± 0.28 | 0.004 | ||
| RV global longitudinal strain*, % | 0.28 ± 0.21 | 0.2 | ||
| RV systolic pressure, mmHg | −0.32 ± 0.12 | 0.01 | ||
| ≥Moderate tricuspid regurgitation | −6.46 ± 2.91 | 0.03 | ||
| Pulmonary valve mean gradient, mmHg | −0.03 ± 0.26 | 0.2 | ||
| LA volume index, ml/m2 | −0.28 ± 0.16 | 0.04 | ||
| LA reservoir strain, % | 0.24 ± 0.05 | 0.01 | 0.22 ± 0.10 | 0.004 |
| LV global longitudinal strain*, % |
0.27 ± 0.22 |
0.4 |
||
| Preoperative CMRI Indices | ||||
| RV end-diastolic volume index, ml/m2 | −0.09 ± 0.14 | 0.3 | ||
| RV end-systolic volume index, ml/m2 | −0.13 ± 0.11 | 0.1 | ||
| RV ejection fraction, % | 0.16 ± 0.18 | 0.3 | ||
Abbreviations: CMRI: cardiac magnetic resonance imaging; LA: left atrium; LV: left ventricle; PVR: pulmonary valve replacement; RA: right atrium; RV: right ventricle; TOF: tetralogy of Fallot.
Footnote: * LV and RV global longitudinal strain were modeled as absolute values (i.e., without the negative sign). Note that only the covariates with statistically significant association with outcome are displayed in the multivariable model.
3.5. Postoperative RA reverse remodeling and atrial arrhythmias
Of the 411 patients, 65 (16%) developed atrial arrhythmias (atrial flutter/tachycardia 36, and atrial fibrillation 29) during a median follow-up of 7.1 (4.4–7.3) years, yielding an annual incidence of 2.2% per year. Of the 65 patients, 14 had atrial arrhythmias within the first year after PVR (from postoperative day 30 to the end of 12 months postoperative period) while 51 had atrial arrhythmias after 12 months post PVR. Among the 397 patients without atrial arrhythmias within the first year after PVR, the incidence of late atrial arrhythmias was 2.1% per year after the first year. RA reverse remodeling was associated with postoperative atrial arrhythmias (hazard ratio [HR] 0.91, 95% confidence interval [CI] 0.86–0.96, p = 0.004), after adjustment for preoperative RA reservoir strain, preoperative arrhythmia history, demographic indices, comorbidities, and echocardiographic indices (Table 5).
Table 5.
Cox model showing clinical and hemodynamic correlates of postoperative atrial arrhythmia.
| Variables | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Δ RA reservoir strain, % | 0.93 (0.91–0.95) | <0.001 | 0.91 (0.86–0.96) | 0.004 |
| Postoperative Echo Indices (Echo #2) | ||||
| Postop pulmonary valve mean gradient | 1.04 (0.95–1.12) | 0.1 | ||
| Pulmonary valve size, mm | 0.96 (0.85–1.08) | 0.4 | ||
| Concomitant tricuspid valve repair | 1.14 (0.93–1.25) | 0.3 | ||
| Concomitant RA maze operation | 1.28 (1.03–1.59) | 0.02 | ||
| Postop RA pressure, mmHg | 1.06 (1.03–1.09) | <0.001 | 1.03 (1.01–1.06) | 0.03 |
| Postop RV systolic pressure, mmHg |
1.04 (1.01–1.07) |
0.008 |
||
| Preoperative Clinical Indices Demographics | ||||
| Age, years | 1.06 (1.05–1.08) | <0.001 | 1.05 (0.98–1.12) | 0.2 |
| Male sex | 0.65 (0.41–1.03) | 0.08 | 1.46 (0.94–1.76) | 0.4 |
| Arrhythmia History | ||||
| Preoperative atrial arrhythmias | 3.11 (2.04–4.75) | <0.001 | 2.76 (1.55–3.98) | 0.006 |
| Surgical History | ||||
| Age at TOF repair, years | 1.65 (0.86–2.04) | 0.3 | ||
| Transannular patch repair | 1.97 (1.07–3.61) | 0.03 | ||
| Prior systemic to pulmonary shun | 1.34 (0.73–2.18) | 0.5 | ||
| PVR replacement prior to baseline | 1.12 (0.86–1.55) | 0.2 | ||
| Comorbidities | ||||
| Hypertension | 1.93 (1.21–3.08) | 0.005 | ||
| Diabetes | 1.42 (0.82–1.46) | 0.2 | ||
| Coronary artery diseas |
1.92 (1.01–3.65) |
0.04 |
||
| Preoperative Echo Indices (Echo #1) | ||||
| RA reservoir strain, % | 0.97 (0.95–0.99) | 0.02 | 0.96 (0.93–0.99) | 0.04 |
| RA volume index, ml/m2 | 1.03 (1.02–1.04) | 0.003 | 1.02 (1.01–1.03) | 0.03 |
| RA pressure, mmHg | 1.15 (1.09–1.21) | <0.001 | ||
| RV global longitudinal strain*, % | 0.93 (0.84–1.03) | 0.2 | ||
| RV systolic pressure, mmHg | 1.02 (1.02–1.03) | 0.004 | ||
| ≥Moderate tricuspid regurgitation | 2.32 (1.46–3.68) | <0.001 | ||
| ≥Moderate pulmonary regurgitation | 2.04 (0.83–4.11) | 0.4 | ||
| Pulmonary valve mean gradient, mmHg | 1.01 (0.99–1.03) | 0.3 | ||
| LA volume index, ml/m2 | 1.02 (1.00–1.04) | 0.08 | ||
| LA reservoir strain, % | 0.96 (0.93–0.99) | 0.02 | ||
| LV global longitudinal strain*, % |
0.97 (0.93–1.01) |
0.3 |
||
| Preoperative CMRI Indices | ||||
| RV end-diastolic volume index, ml/m2 | 0.96 (0.91–1.01) | 0.1 | ||
| RV end-systolic volume index, ml/m2 | 0.95 (0.91–0.99) | 0.09 | ||
| RV ejection fraction, % | 0.96 (0.92–1.00) | 0.1 | ||
Abbreviations: CMRI: cardiac magnetic resonance imaging; CI: confidence interval; HR: hazard ratio; LA: left atrium; LV: left ventricle; PVR: pulmonary valve replacement; RA: right atrium; RV: right ventricle; TOF: tetralogy of Fallot.
Footnote: * LV and RV global longitudinal strain were modeled as absolute values (i.e., without the negative sign). Note that only the covariates with statistically significant association with outcome are displayed in the multivariable model.
Compared to the patients with suboptimal RA reverse remodeling, those with optimal RA reverse remodeling had lower annual incidence of atrial arrhythmias 0.8% versus 3.1% per year, p < 0.001. Having optimal RA reverse remodeling was associated 22% reduction in the risk of postoperative atrial arrhythmia (adjusted HR 0.78, 95% CI 0.67–0.89, p < 0.001), after adjustment for preoperative RA reservoir strain, preoperative arrhythmia history, demographic indices, comorbidities, and echocardiographic indices (Fig. 1).
Fig. 1.
(A) Title: Association between postoperative right atrial (RA) remodeling and atrial arrhythmia. Caption: Forrest plot showing the relationship between postoperative RA reverse remodeling and atrial arrhythmia after pulmonary valve replacement. (B) Title: Postoperative RA reverse remodeling and atrial arrhythmias. Caption: Bar graphs comparing postoperative RA reverse remodeling and atrial arrhythmia based on preoperative RA reservoir strain..
Prespecified subgroup analyses were performed assessing the prognostic performance of RA reverse remodeling in the different groups. The association between RA reverse remodeling and postoperative atrial arrhythmia was consistent across all subgroups (Table 6).
Table 6.
Multivariable Cox Regression Models Assessing the Relationship Between RA Reverse Remodeling (Δ RA Reservoir strain) and Postoperative Atrial Arrhythmias.
| HR (95%CI) for Δ RA Reservoir strain modeled as continuous variable | ||||||
|
Model A |
Model B |
Model C |
Model D |
Model E |
Model F |
|
|
aHR |
0.93 (0.87–0.99) |
0.89 (0.86–0.93) |
0.93 (0.87–0.99) |
0.90 (0.87–0.93) |
0.91 (0.85–0.97) |
0.94 (0.91–0.97) |
| HR (95%CI) for Δ RA Reservoir strain modeled as binary variable (>15% vs ≤ 15%) | ||||||
|
Model A |
Model B |
Model C |
Model D |
Model E |
Model F |
|
| aHR | 0.79 (0.64–0.83) | 0.74 (0.61–0.69) | 0.81 (0.68–0.95) | 0.75 (0.69–0.81) | 0.77 (0.71–0.83) | 0.78 (0.72–0.84) |
Abbreviations: RA: right atrium; aHR: adjusted hazard ratio; CI: confidence interval.
Footnote: Adjusted signifies HR adjusted for demographic indices (age, sex), surgical history (age at time of TOF repair, transannular patch repair, prior systemic to pulmonary shunt, and PVR prior to baseline), preoperative right heart indices (RA volume, RA pressure, RV global longitudinal strain, moderate tricuspid regurgitation, RV systolic pressure, pulmonary valve gradient), preoperative left heart indices (LA volume, LA reservoir strain, LV global longitudinal strain), and comorbidities (hypertension, diabetes, coronary artery disease).
Model A: Analysis restricted patients with history of preoperative atrial arrhythmias (n = 112).
Model B: Analysis restricted to patients without history of preoperative atrial arrhythmias (n = 299).
Model C: Analysis restricted to patients with ≥moderate tricuspid prior to PVR (n = 110).
Model D: Analysis restricted to patients with <moderate tricuspid prior to PVR (n = 301).
Model E: Analysis restricted to patients with complete echocardiographic and cardiac MRI data (n = 331).
Model E: Analysis restricted to patients without atrial arrhythmias within the first year postoperatively (n = 397).
3.6. Exploratory analyses
Exploratory analysis was performed to determine the optimal cut-off point for preoperative RA reservoir strain that predicts optimal postoperative RA reverse remodeling. Based on ROC analysis, we observed that preoperative RA reservoir strain ≥33% as the ideal cut-off point to predict optimal postoperative RA reverse remodeling (area under the curve 0.792). Compared to patients with preoperative RA reservoir strain <33% (n = 242, 59%), those with RA reservoir strain ≥33% (n = 169, 41%) had more robust RA reverse remodeling (Δ RA reverse remodeling 19 ± 11% versus 7 ± 10%, p < 0.001; proportion of patients with optimal RA reverse remodeling 61% [103/169], versus 28% [68/242], p < 001), and lower incidence of postoperative atrial arrhythmias (1.1% versus 2.9%, p = 0.003), Fig. 1.
4. Discussion
In this study, we assessed the relationship between postoperative improvement in RA function (RA reverse remodeling) after PVR and the risk of postoperative atrial arrhythmias during follow-up. The main findings are: (1) About 42% of adults with TOF undergoing PVR had optimal postoperative RA reverse remodeling defined as a relative postoperative increase in preoperative RA reservoir strain >15%, and postoperative RA reverse remodeling was associated with lower risk of postoperative atrial arrhythmia. This relationship was independent of preoperative RA reservoir strain and baseline characteristics. (2) Preoperative RA reservoir strain was associated with postoperative RA reverse remodeling, and patients with preoperative RA reservoir strain ≥33% were more likely to have optimal postoperative RA reverse remodeling and less likely to have postoperative atrial arrhythmias.
Residual/recurrent hemodynamic lesions such as RV outflow tract stenosis, pulmonary regurgitation, and tricuspid regurgitation are common in adults with repaired TOF [[1], [2], [3]]. These lesions create right heart pressure and/or volume overload, and over time lead to RV remodeling (RV enlargement, hypertrophy, systolic dysfunction, and diastolic dysfunction) [2,[15], [16], [17], [18]]. However, the hemodynamic impact of right heart pressure and/or volume overload is not limited to the RV but can affect the RA and other cardiac chambers [16,17,39,40]. The RA modulates the function of the RV by providing reservoir function in systole which in turn determines RV preload and systemic venous pressure; by acting as a passive conduit to enable RV filling in early diastole; and by augmenting RV preload in late diastolic through atrial contraction [19,20]. Since the RA function is intricately linked to all phases of the cardiac cycle, changes in RA function (dysfunction), can potentially be used as a barometer of right heart function [19,20]. RA strain imaging provides an assessment of RA and RV function throughout the cardiac cycle, with RA reservoir strain providing an assessment RA compliance [[28], [29], [30], [31]]. Based on this premise, the clinical and hemodynamic benefits (or lack thereof) of right heart interventions such as PVR should result in measurable changes in RA function, but this has not been empirically tested. The current study fills this knowledge gap by demonstrating that RA function improved after PVR, and that the extent of improvement in RA function was dependent on preoperative RA function. Furthermore, it also showed that postoperative improvement in RA function after PVR was associated with a lower risk of atrial arrhythmias which is an important prognostic marker in this population.
Several studies have demonstrated the feasibility and prognostic importance of RA function indices in patients with acquired heart disease [[19], [20], [21], [22]]. Hasselberg et al. and Alenezi et al. reported that RA reservoir strain was associated with heart failure hospitalization and all-cause mortality in patients with precapillary pulmonary hypertension [19,20]. Other studies have shown that RA reservoir strain can identify patients at risk for postprocedural atrial arrhythmias after transcatheter closure of atrial septal defect and other cardiac surgeries [21,22]. To the best of our knowledge, only two previous studies have described the clinical application of RA strain in adults with repaired TOF [23,24]. In these studies, Kutty el demonstrated that RA reservoir strain as measured by CMRI was lower in TOF patients as compared to controls, and that CMRI-derived RA strain indices had poor correlations with CMRI-derived RV volumetric indices similar to the results of the current study [23]. In a different study, Timoteo et al. observed that RA strain, as measured by speckle tracking echocardiography, was associated with atrial arrhythmia in the subset of TOF patients without prior history of PVR [24]. Both studies were based on smaller samples and relied on cross-sectional analysis [23,24]. Our study builds on these previous studies, by demonstrating that RA function improved after PVR, especially when PVR was performed prior to significant deterioration in RA function, and that improvement in RA function was associated with lower risk of atrial arrhythmias during follow-up. These findings have potential clinical applications.
5. Clinical implications and future directions
The ability of the RV to remodel after PVR has been described in several studies, and these studies form the basis for the guideline recommendations regarding timing of PVR in this population [2,[4], [5], [6], [7], [8],10,11]. The current study extends our understanding of right heart remodeling after PVR by demonstrating that the RA can also remodel after PVR, and that the extent of postoperative RA remodeling was related to the risk of postoperative atrial arrhythmias. More importantly, it identifies the preoperative RA reservoir strain threshold associated with greater odds of achieving optimal RA reverse remodeling, and in turn, a lower risk of postoperative atrial arrhythmias after PVR. These data could potentially be integrated into the current clinical risk models (that are based mostly on RV volumetric indices) regarding timing of PVR, and potentially improve risk stratification in this population. Of note, the absence of correlations between RA reservoir strain and RV volumetric indices observed in this study, suggest that both sets of indices measure different aspects of right heart function. Hence, integrating both sets of indices into a single risk model would likely provide a more comprehensive assessment of right heart hemodynamic performance. Further studies are required to empirically validated these postulates.
6. Limitations
The results presented in this study were derived from retrospective analysis of data from patients that received care at a single tertiary center. It is therefore prone to selection and ascertainment bias, which may limit generalizability of the results. The criterion for optimal RA reverse remodeling used in this study was arbitrary due to lack of data. However, the results of the current study suggests that this threshold (>15% change in RA reservoir strain) may be clinically meaningful. Although we used robust statistical methods to adjust for confounders, it is possible that some of our findings could have been influenced by residual confounders. We limited the inclusion criteria to patients that underwent surgical PVR, excluding those that underwent transcatheter PVR, since myocardial injury and recovery may differ between the 2 groups because of the use of cardiopulmonary bypass in the surgical group. This would likely limit the applicability of the results to patients undergoing transcatheter PVR. Finally, we did not have CMRI data in all patients. However, the findings were consistent in the subgroup of patients with CMRI data, as well as across all subgroups analyzed.
7. Conclusions
RA reverse remodeling occurred after PVR, and the extent of RA reverse remodeling was associated with lower risk of atrial arrhythmias. The preoperative RA reservoir strain was associated with RA reverse remodeling, and in turn, postoperative atrial arrhythmia. These results suggest that RA strain indices could be used to determine optimal timing for PVR in order to reduce the risk of atrial arrhythmia, which is an important risk factor for heart failure and mortality in this population. Further studies are required to validate the results of this study in a different population, and to determine whether the inclusion of RA function indices to the current risk models for determining the timing of PVR would lead to improved clinical outcomes.
Funding
Dr. Egbe is supported by National Heart, Lung, and Blood Institute (NHLBI) grants (R01 HL158517 and R01 HL160761). The MACHD Registry is supported by the Al-Bahar Research grant.
CRediT authorship contribution statement
Abhishek J. Deshmukh: Writing – original draft, Writing – review & editing. Ahmed Younis: Writing – original draft, Writing – review & editing. Marwan Ahmed: Writing – original draft, Writing – review & editing. Luke Burchill: Writing – original draft, Writing – review & editing. C. Charles Jain: Writing – original draft, Writing – review & editing. William R. Miranda: Writing – original draft, Writing – review & editing. Malini Madhavan: Writing – original draft, Writing – review & editing. Heidi M. Connolly: Writing – original draft, Writing – review & editing. Alexander C. Egbe: Conceptualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcchd.2024.100497.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
figs1.
References
- 1.Egbe A.C., Miranda W.R., Said S.M., Pislaru S.V., Pellikka P.A., Borlaug B.A., Kothapalli S., Connolly H.M. Risk stratification and clinical outcomes after surgical pulmonary valve replacement. Am Heart J. 2018;206:105–112. doi: 10.1016/j.ahj.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson : official journal of the Society for Cardiovascular Magnetic Resonance. 2011;13:9. doi: 10.1186/1532-429X-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valente A.M., Gauvreau K., Assenza G.E., Babu-Narayan S.V., Schreier J., Gatzoulis M.A., Groenink M., Inuzuka R., Kilner P.J., Koyak Z., Landzberg M.J., Mulder B., Powell A.J., Wald R., Geva T. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart. 2014;100:247–253. doi: 10.1136/heartjnl-2013-304958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oosterhof T., van Straten A., Vliegen H.W., Meijboom F.J., van Dijk A.P., Spijkerboer A.M., Bouma B.J., Zwinderman A.H., Hazekamp M.G., de Roos A., Mulder B.J. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116:545–551. doi: 10.1161/CIRCULATIONAHA.106.659664. [DOI] [PubMed] [Google Scholar]
- 5.Bokma J.P., Winter M.M., Oosterhof T., Vliegen H.W., van Dijk A.P., Hazekamp M.G., Koolbergen D.R., Groenink M., Mulder B.J., Bouma B.J. Preoperative thresholds for mid-to-late haemodynamic and clinical outcomes after pulmonary valve replacement in tetralogy of Fallot. Eur Heart J. 2016;37:829–835. doi: 10.1093/eurheartj/ehv550. [DOI] [PubMed] [Google Scholar]
- 6.Wald R.M., Valente A.M., Gauvreau K., Babu-Narayan S.V., Assenza G.E., Schreier J., Gatzoulis M.A., Kilner P.J., Koyak Z., Mulder B., Powell A.J., Geva T. Cardiac magnetic resonance markers of progressive RV dilation and dysfunction after tetralogy of Fallot repair. Heart. 2015;101:1724–1730. doi: 10.1136/heartjnl-2015-308014. [DOI] [PubMed] [Google Scholar]
- 7.Knauth A.L., Gauvreau K., Powell A.J., Landzberg M.J., Walsh E.P., Lock J.E., del Nido P.J., Geva T. Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart. 2008;94:211–216. doi: 10.1136/hrt.2006.104745. [DOI] [PubMed] [Google Scholar]
- 8.Geva T., Mulder B., Gauvreau K., Babu-Narayan S.V., Wald R.M., Hickey K., Powell A.J., Gatzoulis M.A., Valente A.M. Preoperative predictors of death and sustained ventricular tachycardia after pulmonary valve replacement in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Circulation. 2018;138:2106–2115. doi: 10.1161/CIRCULATIONAHA.118.034740. [DOI] [PubMed] [Google Scholar]
- 9.Heng E.L., Gatzoulis M.A., Uebing A., Sethia B., Uemura H., Smith G.C., Diller G.P., McCarthy K.P., Ho S.Y., Li W., Wright P., Spadotto V., Kilner P.J., Oldershaw P., Pennell D.J., Shore D.F., Babu-Narayan S.V. Immediate and midterm cardiac remodeling after surgical pulmonary valve replacement in adults with repaired tetralogy of Fallot: a prospective cardiovascular magnetic resonance and clinical study. Circulation. 2017;136:1703–1713. doi: 10.1161/CIRCULATIONAHA.117.027402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stout K.K., Daniels C.J., Aboulhosn J.A., Bozkurt B., Broberg C.S., Colman J.M., Crumb S.R., Dearani J.A., Fuller S., Gurvitz M., Khairy P., Landzberg M.J., Saidi A., Valente A.M., Van Hare G.F. AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;73:e81–e192. doi: 10.1016/j.jacc.2018.08.1029. 2019. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner H., De Backer J. The ESC clinical practice guidelines for the management of adult congenital heart disease 2020. Eur Heart J. 2020;41:4153–4154. doi: 10.1093/eurheartj/ehaa701. [DOI] [PubMed] [Google Scholar]
- 12.Ferraz Cavalcanti P.E., Sa M.P., Santos C.A., Esmeraldo I.M., de Escobar R.R., de Menezes A.M., de Azevedo O.M., Jr., de Vasconcelos Silva F.P., Lins R.F., Lima Rde C. Pulmonary valve replacement after operative repair of tetralogy of Fallot: meta-analysis and meta-regression of 3,118 patients from 48 studies. J Am Coll Cardiol. 2013;62:2227–2243. doi: 10.1016/j.jacc.2013.04.107. [DOI] [PubMed] [Google Scholar]
- 13.Bokma J.P., Geva T., Sleeper L.A., Babu Narayan S.V., Wald R., Hickey K., Jansen K., Wassall R., Lu M., Gatzoulis M.A., Mulder B.J., Valente A.M. A propensity score-adjusted analysis of clinical outcomes after pulmonary valve replacement in tetralogy of Fallot. Heart. 2018;104:738–744. doi: 10.1136/heartjnl-2017-312048. [DOI] [PubMed] [Google Scholar]
- 14.Bokma J.P., Geva T., Sleeper L.A., Lee J.H., Lu M., Sompolinsky T., Babu-Narayan S.V., Wald R.M., Mulder B.J.M., Valente A.M. Improved outcomes after pulmonary valve replacement in repaired tetralogy of Fallot. J Am Coll Cardiol. 2023;81:2075–2085. doi: 10.1016/j.jacc.2023.02.052. [DOI] [PubMed] [Google Scholar]
- 15.Chen C.A., Dusenbery S.M., Valente A.M., Powell A.J., Geva T. Myocardial ECV fraction assessed by CMR is associated with type of hemodynamic load and arrhythmia in repaired tetralogy of Fallot. JACC Cardiovascular imaging. 2016;9:1–10. doi: 10.1016/j.jcmg.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Egbe A.C., Bonnichsen C., Reddy Y.N.V., Anderson J.H., Borlaug B.A. Pathophysiologic and prognostic implications of right atrial hypertension in adults with tetralogy of Fallot. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.014148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egbe A.C., Connolly H.M., Miranda W.R., Scott C.G., Borlaug B.A. Prognostic implications of inferior vena cava haemodynamics in ambulatory patients with tetralogy of Fallot. ESC Heart Fail. 2020;7:2589–2596. doi: 10.1002/ehf2.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egbe A.C., Pellikka P.A., Miranda W.R., Bonnichsen C., Reddy Y.N.V., Borlaug B.A., Connolly H.M. Echocardiographic predictors of severe right ventricular diastolic dysfunction in tetralogy of Fallot: relations to patient outcomes. Int J Cardiol. 2020;306:49–55. doi: 10.1016/j.ijcard.2020.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasselberg N.E., Kagiyama N., Soyama Y., Sugahara M., Goda A., Ryo-Koriyama K., Batel O., Chakinala M., Simon M.A., Gorcsan J., 3rd The prognostic value of right atrial strain imaging in patients with precapillary pulmonary hypertension. J Am Soc Echocardiogr. 2021;34:851–861 e1. doi: 10.1016/j.echo.2021.03.007. official publication of the American Society of Echocardiography. [DOI] [PubMed] [Google Scholar]
- 20.Alenezi F., Mandawat A., Il'Giovine Z.J., Shaw L.K., Siddiqui I., Tapson V.F., Arges K., Rivera D., Romano M.M.D., Velazquez E.J., Douglas P.S., Samad Z., Rajagopal S. Clinical utility and prognostic value of right atrial function in pulmonary hypertension. Circulation Cardiovascular imaging. 2018;11 doi: 10.1161/CIRCIMAGING.117.006984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aksu U., Kalkan K., Gulcu O., Aksakal E., Ozturk M., Topcu S. The role of the right atrium in development of postoperative atrial fibrillation: a speckle tracking echocardiography study. J Clin Ultrasound. 2019;47:470–476. doi: 10.1002/jcu.22736. [DOI] [PubMed] [Google Scholar]
- 22.Vitarelli A., Mangieri E., Gaudio C., Tanzilli G., Miraldi F., Capotosto L. Right atrial function by speckle tracking echocardiography in atrial septal defect: prediction of atrial fibrillation. Clin Cardiol. 2018;41:1341–1347. doi: 10.1002/clc.23051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kutty S., Shang Q., Joseph N., Kowallick J.T., Schuster A., Steinmetz M., Danford D.A., Beerbaum P., Sarikouch S. Abnormal right atrial performance in repaired tetralogy of Fallot: a CMR feature tracking analysis. Int J Cardiol. 2017;248:136–142. doi: 10.1016/j.ijcard.2017.06.121. [DOI] [PubMed] [Google Scholar]
- 24.Timoteo A.T., Branco L.M., Rosa S.A., Ramos R., Agapito A.F., Sousa L., Galrinho A., Oliveira J.A., Oliveira M.M., Ferreira R.C. Usefulness of right ventricular and right atrial two-dimensional speckle tracking strain to predict late arrhythmic events in adult patients with repaired Tetralogy of Fallot. Rev Port Cardiol : orgao oficial da Sociedade Portuguesa de Cardiologia = Portuguese journal of cardiology : an official journal of the Portuguese Society of Cardiology. 2017;36:21–29. doi: 10.1016/j.repc.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Egbe A.C., Miranda W.R., Ammash N.M., Ananthaneni S., Sandhyavenu H., Farouk Abdelsamid M., Yogeswaran V., Kapa S., Fatola A., Kothapalli S., Connolly H.M. Atrial fibrillation therapy and heart failure hospitalization in adults with tetralogy of Fallot. JACC Clin Electrophysiol. 2019;5:618–625. doi: 10.1016/j.jacep.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Egbe A.C., Kothapalli S., Borlaug B.A., Ammash N.M., Najam M., Bajwa N., Tarek K., Mathew J., Connolly H.M. Mechanism and risk factors for death in adults with tetralogy of Fallot. Am J Cardiol. 2019;124:803–807. doi: 10.1016/j.amjcard.2019.05.048. [DOI] [PubMed] [Google Scholar]
- 27.Egbe A.C., Najam M., Banala K., Vojjini R., Bonnichsen C., Ammash N.M., Faizee F., Khalil F., Deshmukh A.J., Connolly H.M. Impact of atrial arrhythmia on survival in adults with tetralogy of Fallot. Am Heart J. 2019;218:1–7. doi: 10.1016/j.ahj.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Singh A., Addetia K., Maffessanti F., Mor-Avi V., Lang R.M. LA strain for categorization of LV diastolic dysfunction. JACC Cardiovascular imaging. 2017;10:735–743. doi: 10.1016/j.jcmg.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas L., Marwick T.H., Popescu B.A., Donal E., Badano L.P. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:1961–1977. doi: 10.1016/j.jacc.2019.01.059. [DOI] [PubMed] [Google Scholar]
- 30.Tello K., Dalmer A., Vanderpool R., Ghofrani H.A., Naeije R., Roller F., Seeger W., Wiegand M., Gall H., Richter M.J. Right ventricular function correlates of right atrial strain in pulmonary hypertension: a combined cardiac magnetic resonance and conductance catheter study. Am J Physiol Heart Circ Physiol. 2020;318:H156–H164. doi: 10.1152/ajpheart.00485.2019. [DOI] [PubMed] [Google Scholar]
- 31.Richter M.J., Fortuni F., Wiegand M.A., Dalmer A., Vanderpool R., Ghofrani H.A., Naeije R., Roller F., Seeger W., Sommer N., Gall H., Ghio S., Tello K. Association of right atrial conduit phase with right ventricular lusitropic function in pulmonary hypertension. Int J Cardiovasc Imag. 2020;36:633–642. doi: 10.1007/s10554-019-01763-x. [DOI] [PubMed] [Google Scholar]
- 32.Tops L.F., Delgado V., Bertini M., Marsan N.A., Den Uijl D.W., Trines S.A., Zeppenfeld K., Holman E., Schalij M.J., Bax J.J. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2011;57:324–331. doi: 10.1016/j.jacc.2010.05.063. [DOI] [PubMed] [Google Scholar]
- 33.Egbe A.C., Miranda W.R., Connolly H.M. Role of echocardiography for assessment of cardiac remodeling in congenitally corrected transposition of great arteries. Circulation Cardiovascular imaging. 2022;15 doi: 10.1161/CIRCIMAGING.121.013477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egbe A., Miranda W., Connolly H., Dearani J. Haemodynamic determinants of improved aerobic capacity after tricuspid valve surgery in Ebstein anomaly. Heart. 2021;107:1138–1144. doi: 10.1136/heartjnl-2020-317756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., Lancellotti P., Muraru D., Picard M.H., Rietzschel E.R., Rudski L., Spencer K.T., Tsang W., Voigt J.U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. doi: 10.1016/j.echo.2014.10.003. official publication of the American Society of Echocardiography. [DOI] [PubMed] [Google Scholar]
- 36.Badano L.P., Kolias T.J., Muraru D., Abraham T.P., Aurigemma G., Edvardsen T., D'Hooge J., Donal E., Fraser A.G., Marwick T., Mertens L., Popescu B.A., Sengupta P.P., Lancellotti P., Thomas J.D. Voigt JU, Industry r and Reviewers: this document was reviewed by members of the ESDC. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. European heart journal cardiovascular Imaging. 2018;19:591–600. doi: 10.1093/ehjci/jey042. [DOI] [PubMed] [Google Scholar]
- 37.Egbe A.C., Connolly H.M., Khan A.R., Niaz T., Said S.S., Dearani J.A., Warnes C.A., Deshmukh A.J., Kapa S., McLeod C.J. Outcomes in adult Fontan patients with atrial tachyarrhythmias. Am Heart J. 2017;186:12–20. doi: 10.1016/j.ahj.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 38.Brock M.A., Coppola J.A., Reid J., Moguillansky D. Atrial fibrillation in adults with congenital heart disease following cardiac surgery in a single center: analysis of incidence and risk factors. Congenit Heart Dis. 2019;14:924–930. doi: 10.1111/chd.12857. [DOI] [PubMed] [Google Scholar]
- 39.Egbe A.C., Adigun R., Anand V., West C.P., Montori V.M., Murad M.H., Akintoye E., Osman K., Connolly H.M. Left ventricular systolic dysfunction and cardiovascular outcomes in tetralogy of Fallot: systematic review and meta-analysis. Can J Cardiol. 2019;35:1784–1790. doi: 10.1016/j.cjca.2019.07.634. [DOI] [PubMed] [Google Scholar]
- 40.Egbe A.C., Banala K., Vojjini R., Jadav R., Sufian M., Pellikka P.A., Ammash N.M. Left ventricular filling pressure in Tetralogy of Fallot: correlation between invasive and noninvasive indices. Int J Cardiol Heart Vasc. 2020;26 doi: 10.1016/j.ijcha.2019.100457. [DOI] [PMC free article] [PubMed] [Google Scholar]