Abstract
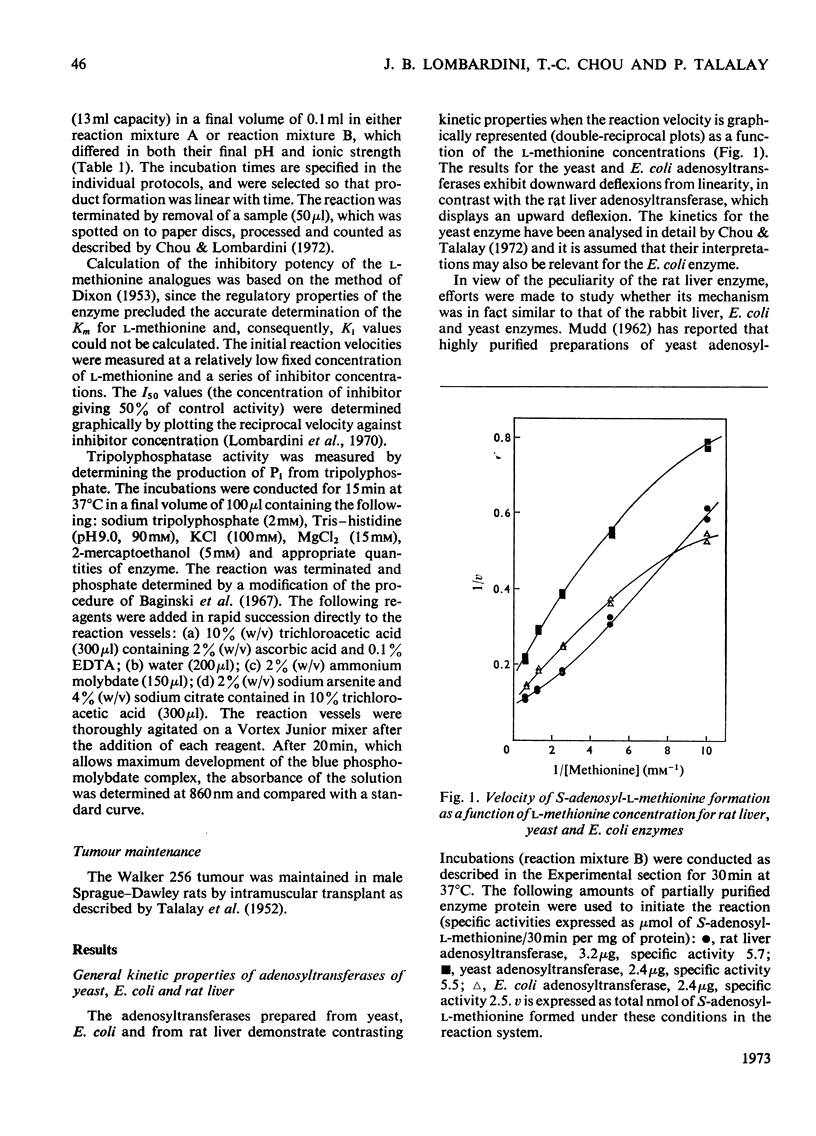
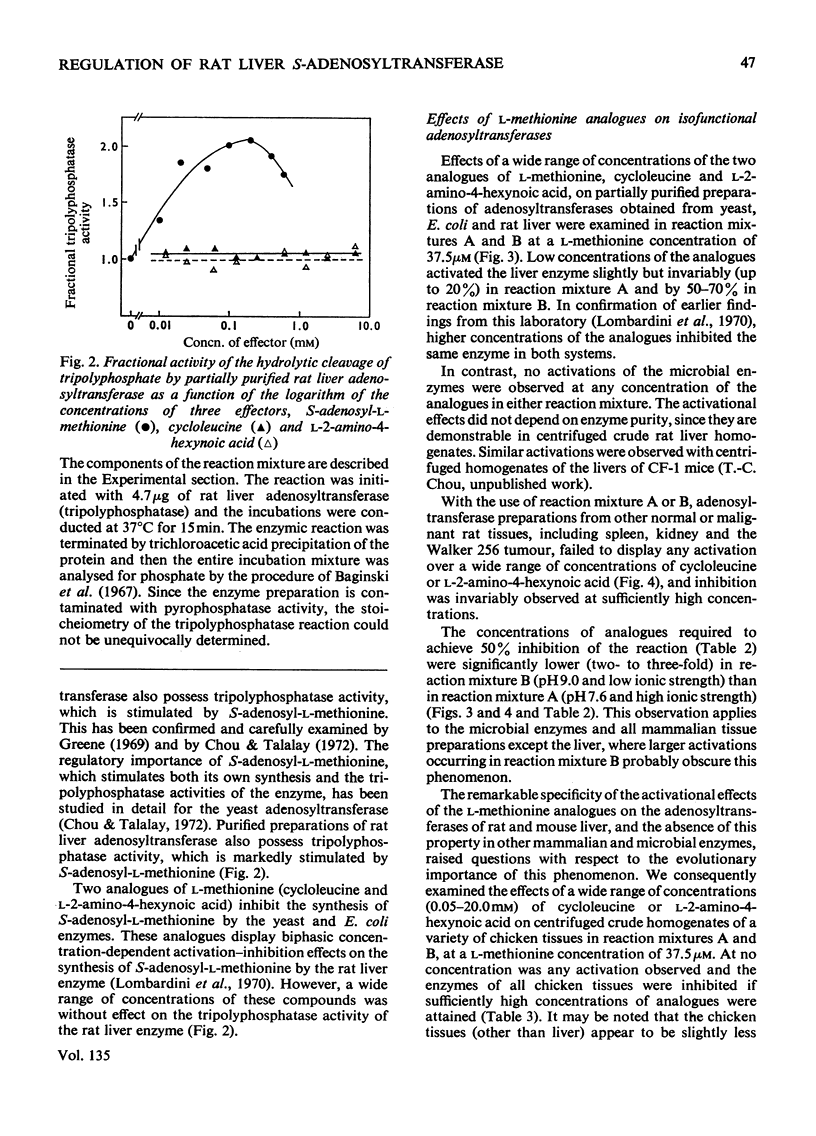
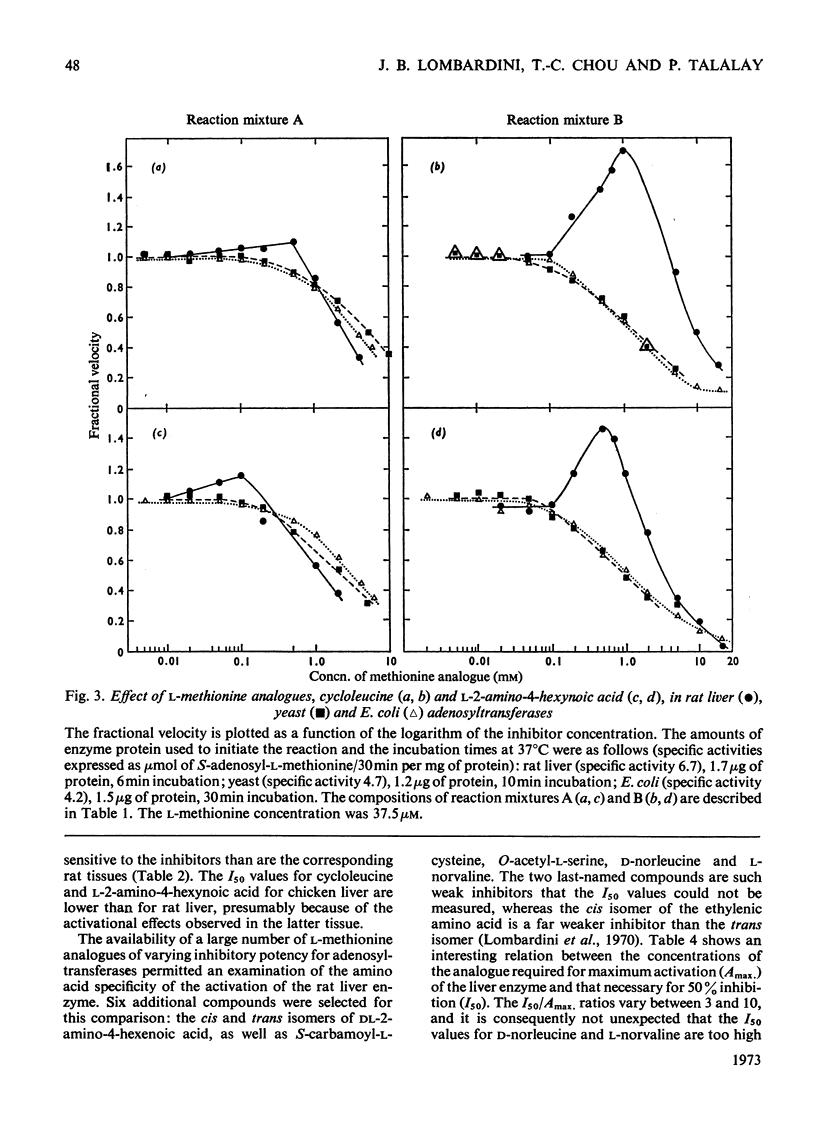
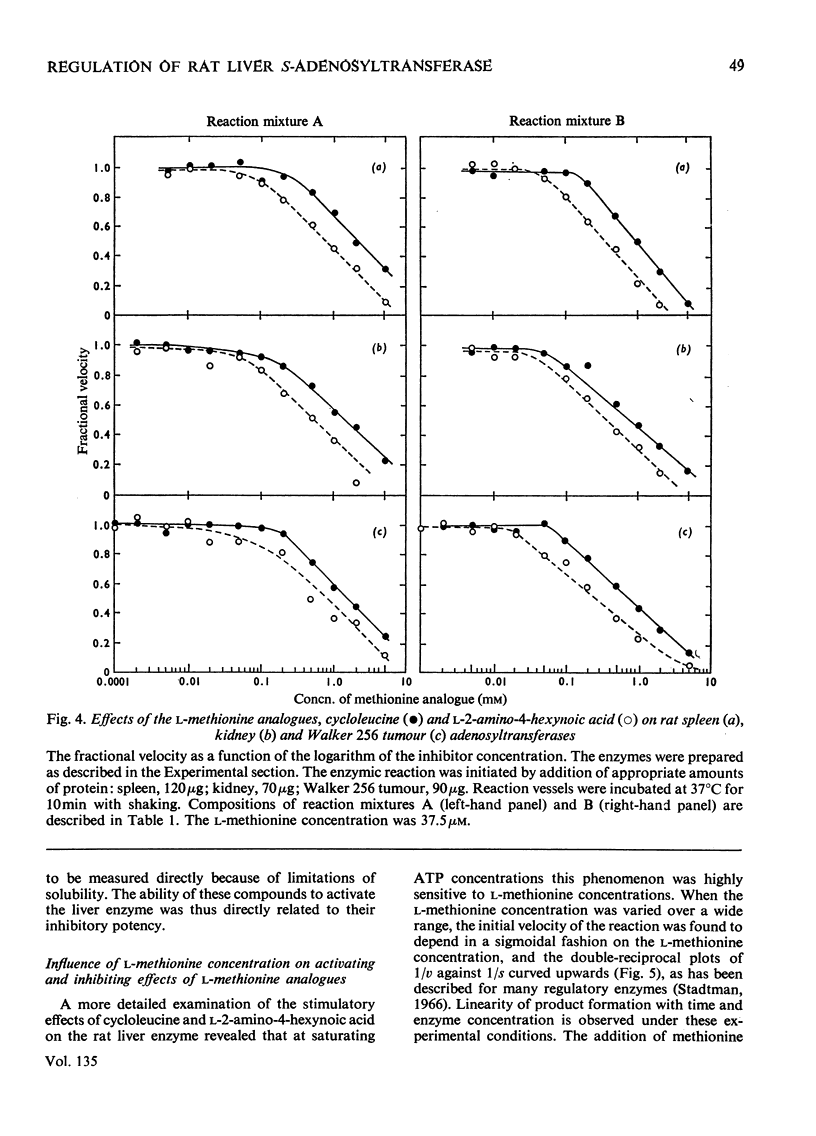
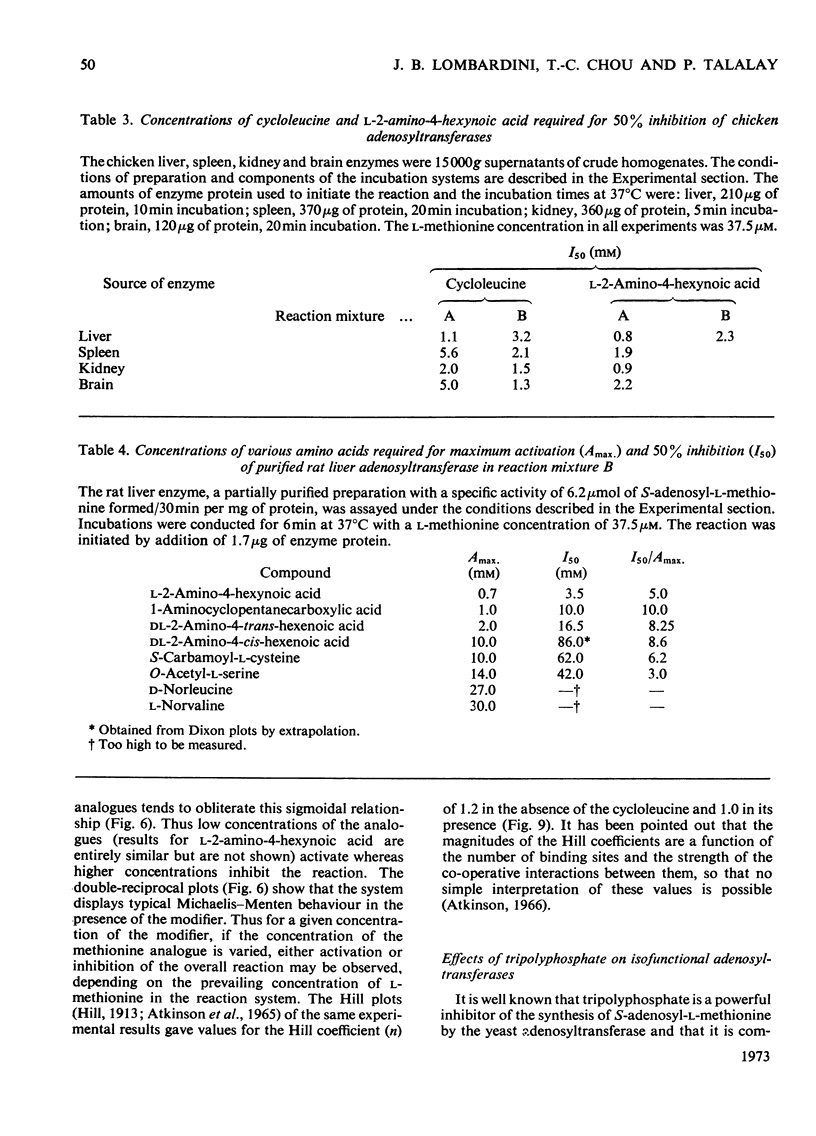
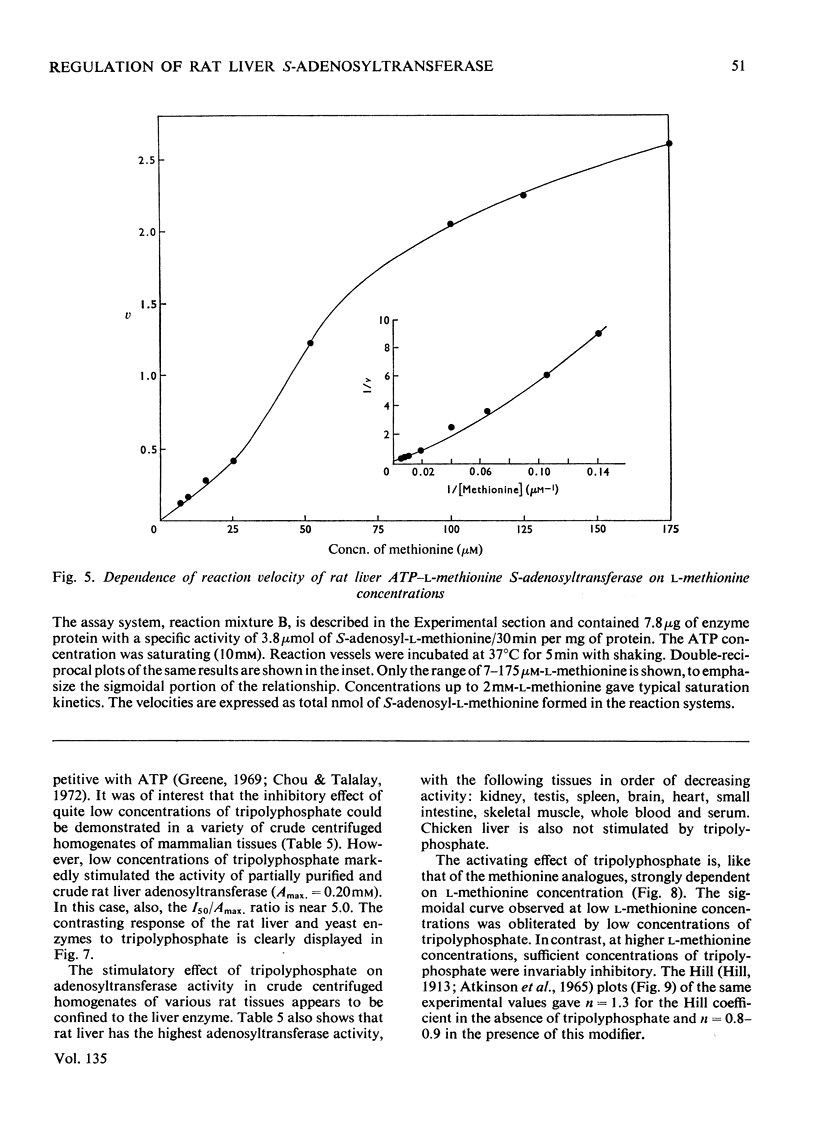
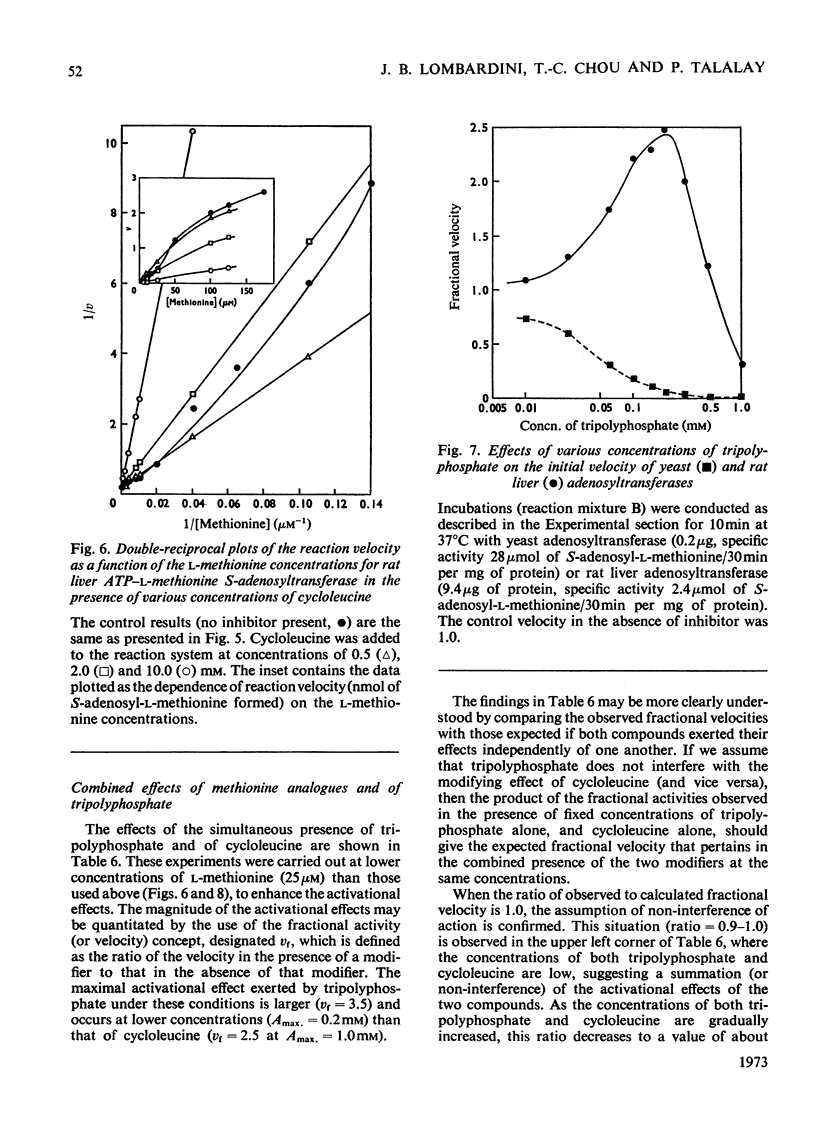
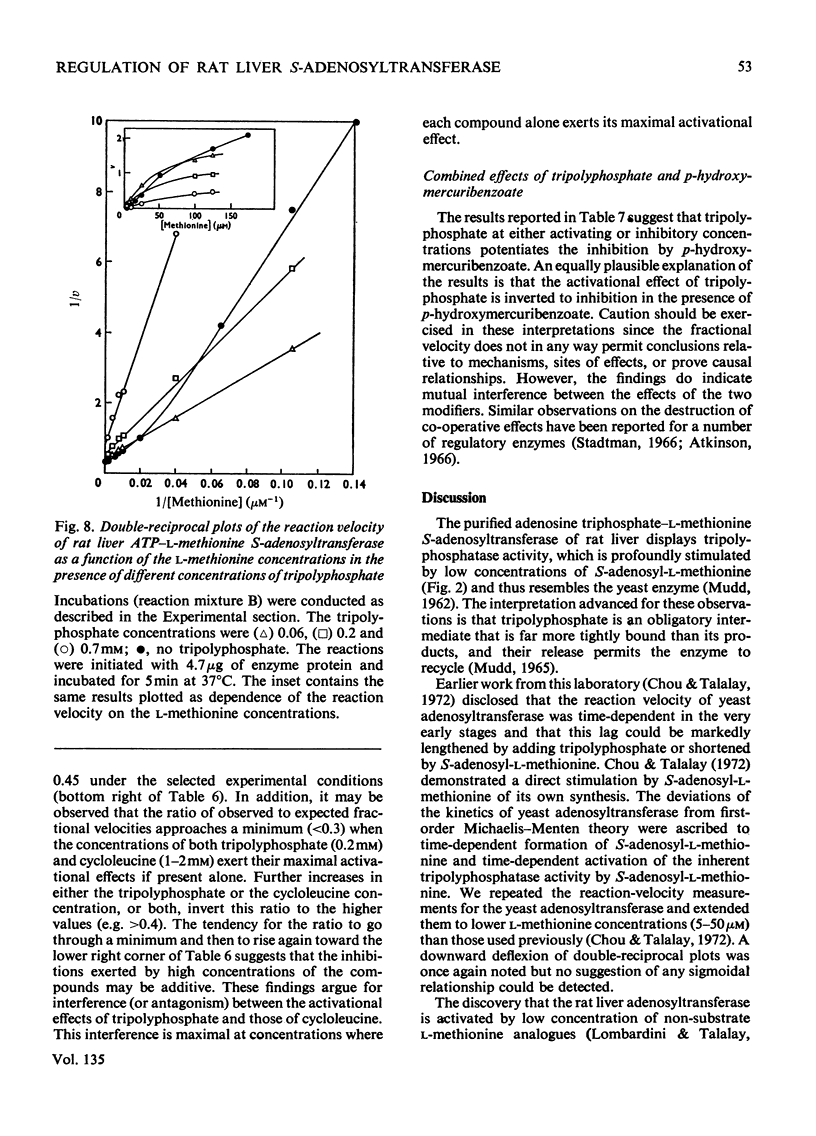
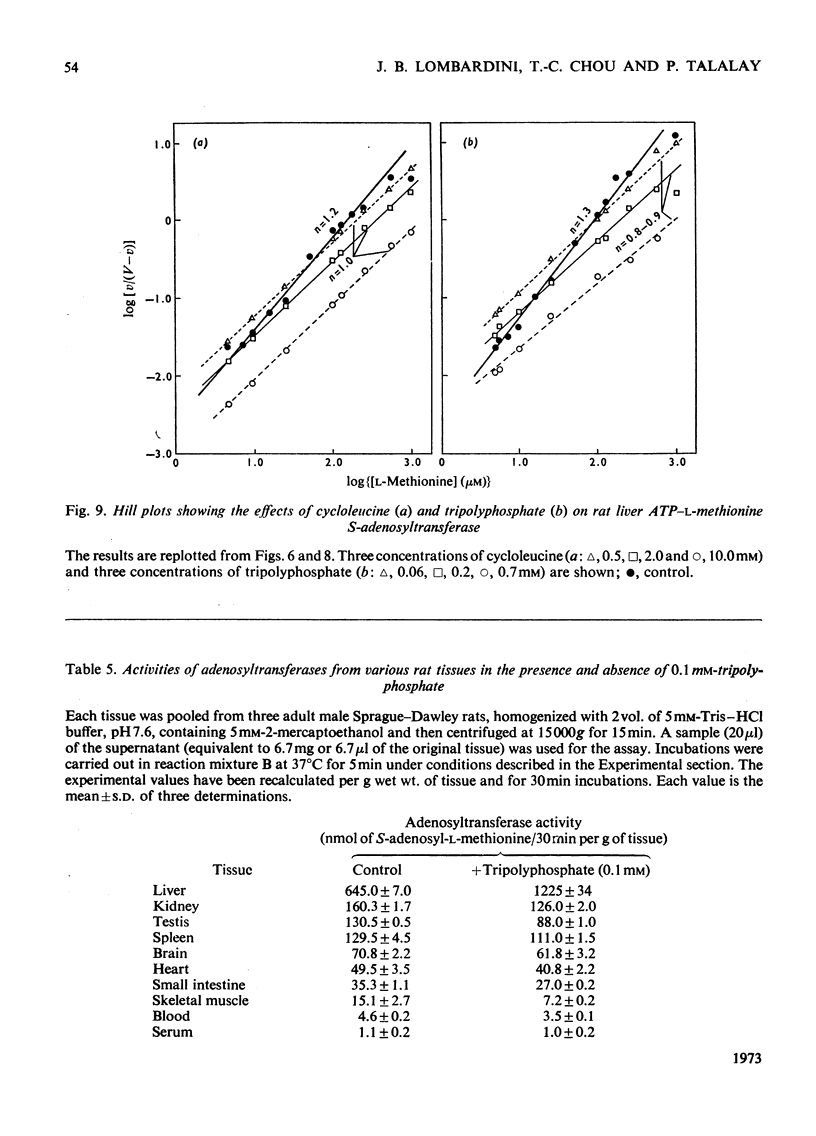
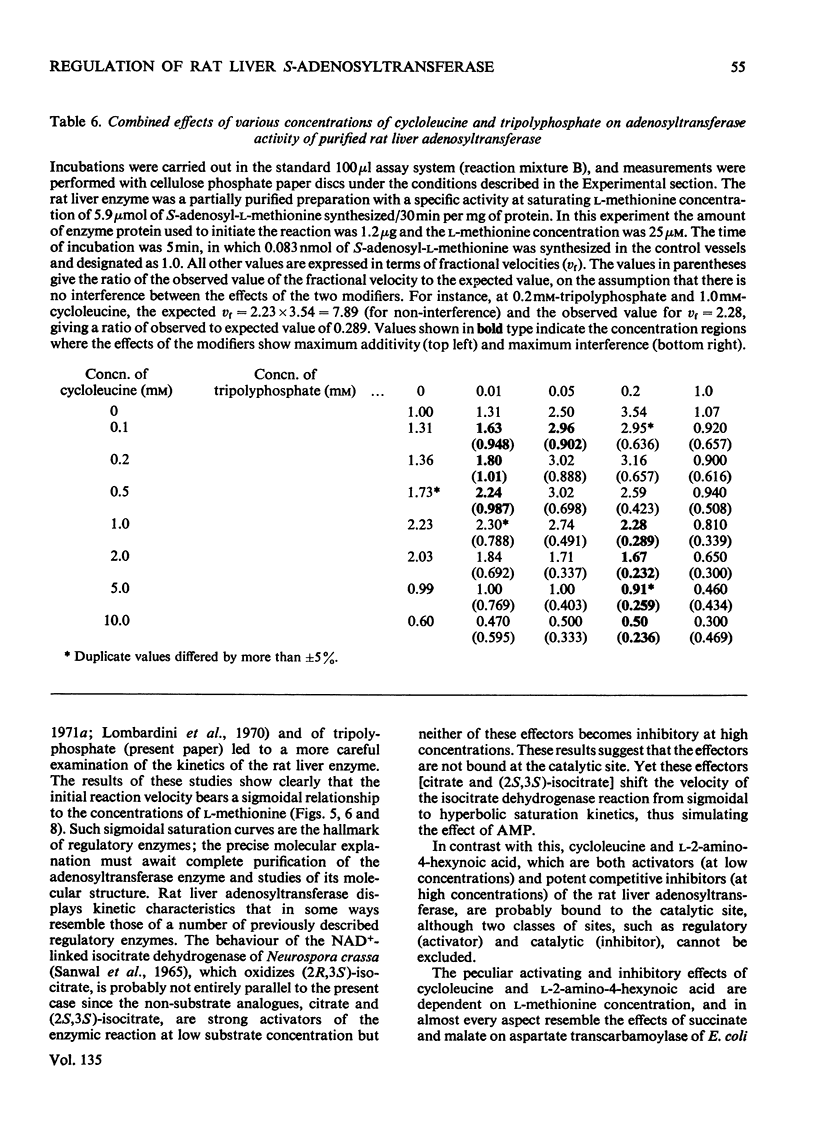
1. Double-reciprocal plots of the reaction velocity of yeast, rat liver and Escherichia coli ATP-l-methionine S-adenosyltransferases (EC 2.5.1.6) as a function of the l-methionine concentrations (under saturating ATP conditions) demonstrate downward deflexions from linearity for the yeast and E. coli adenosyltransferases and an upward deflexion for the rat liver enzyme. 2. The activities of partially purified preparations of rat liver ATP-l-methionine S-adenosyltransferase are enhanced by low concentrations of non-substrate analogues of l-methionine [e.g. 1-aminocyclopentanecarboxylic acid (cycloleucine) and l-2-amino-4-hexynoic acid], or by inorganic tripolyphosphate, an ATP analogue. When the concentrations of these analogues were raised further, the activity decreased. Double-reciprocal plots became linear in the presence of these modifier analogues. The inhibitions are common to all the l-methionine adenosyltransferases examined, but the activation(s) were only found with rat and mouse liver enzymes and not with enzymes obtained from several other tissues of these or other species. 3. The rate of formation of S-adenosyl-l-methionine bears a sigmoidal relation to the l-methionine concentrations when ATP is saturating. The activating effects of the l-methionine analogues and of tripolyphosphate are observed at low l-methionine concentrations, and become obliterated as the l-methionine concentration is raised. These findings are analysed in terms of various regulatory enzyme models.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., HATHAWAY J. A., SMITH E. C. KINETICS OF REGULATORY ENZYMES. KINETIC ORDER OF THE YEAST DIPHOSPHOPYRIDINE NUCLEOTIDE ISOCITRATE DEHYDROGENASE REACTION AND A MODEL FOR THE REACTION. J Biol Chem. 1965 Jun;240:2682–2690. [PubMed] [Google Scholar]
- CANTONI G. L., DURELL J. Activation of methionine for transmethylation. II. The methionine-activating enzyme; studies on the mechanism of the reaction. J Biol Chem. 1957 Apr;225(2):1033–1048. [PubMed] [Google Scholar]
- CATONI G. L. S-Adenosylmethionine; a new intermediate formed enzymatically from L-methionine and adenosinetriphosphate. J Biol Chem. 1953 Sep;204(1):403–416. [PubMed] [Google Scholar]
- Chou T. C., Lombardini J. B. A rapid assay procedure for ATP:L-methionine adenosyltransferase. Biochim Biophys Acta. 1972 Aug 28;276(2):399–406. doi: 10.1016/0005-2744(72)91000-5. [DOI] [PubMed] [Google Scholar]
- Chou T. C., Talalay P. The mechanism of S-adenosyl-L-methionine synthesis by purified preparations of bakers' yeast. Biochemistry. 1972 Mar 14;11(6):1065–1073. doi: 10.1021/bi00756a019. [DOI] [PubMed] [Google Scholar]
- Coulter A. W., Talalay P. Studies on the microbiological degradation of steroid ring A. J Biol Chem. 1968 Jun 25;243(12):3238–3247. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C. Kinetic studies of the mechanism of S-adenosylmethionine synthetase from yeast. Biochemistry. 1969 Jun;8(6):2255–2265. doi: 10.1021/bi00834a004. [DOI] [PubMed] [Google Scholar]
- Hill A. V. The Combinations of Haemoglobin with Oxygen and with Carbon Monoxide. I. Biochem J. 1913 Oct;7(5):471–480. doi: 10.1042/bj0070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne J., Schenone A., Williams-Ashman H. G. Separation of two proteins required for synthesis of spermidine from S-adenosyl-L-methionine and putrescine in rat prostate. Biochem Biophys Res Commun. 1971 Feb 19;42(4):758–764. doi: 10.1016/0006-291x(71)90552-3. [DOI] [PubMed] [Google Scholar]
- Lombardini J. B., Coulter A. W., Talalay P. Analogues of methionine as substrates and inhibitors of the methionine adenosyltransferase reaction. Deductions concerning the conformation of methionine. Mol Pharmacol. 1970 Sep;6(5):481–499. [PubMed] [Google Scholar]
- Lombardini J. B., Talalay P. Formation, functions and regulatory importance of S-adenosyl-L-methionine. Adv Enzyme Regul. 1970;9:349–384. doi: 10.1016/s0065-2571(71)80054-7. [DOI] [PubMed] [Google Scholar]
- MUDD S. H. Activation of methionine for transmethylation. VI. Enzyme-bound tripolyphosphate as an intermediate in the reaction catalyzed by the methionine-activating enzyme of Baker's yeast. J Biol Chem. 1963 Jun;238:2156–2163. [PubMed] [Google Scholar]
- MUDD S. H., CANTONI G. L. Activation of methionine for transmethylation. III. The methionine-activating enzyme of Bakers' yeast. J Biol Chem. 1958 Mar;231(1):481–492. [PubMed] [Google Scholar]
- Pan F., Tarver H. Comparative studies on methionine, selenomethionine, and their ethyl analogues as substrates for methionine adenosyltransferase from rat liver. Arch Biochem Biophys. 1967 Mar;119(1):429–434. doi: 10.1016/0003-9861(67)90474-2. [DOI] [PubMed] [Google Scholar]
- SANWAL B. D., STACHOW C. S., COOK R. A. A KINETIC MODEL FOR THE MECHANISM OF ALLOSTERIC ACTIVATION OF NICOTINAMIDE-ADENINE DINUCLEOTIDE-SPECIFIC ISOCRITIC DEHYDROGENASE. Biochemistry. 1965 Mar;4:410–421. doi: 10.1021/bi00879a006. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. Allosteric regulation of enzyme activity. Adv Enzymol Relat Areas Mol Biol. 1966;28:41–154. doi: 10.1002/9780470122730.ch2. [DOI] [PubMed] [Google Scholar]
- TABOR H., ROSENTHAL S. M., TABOR C. W. The biosynthesis of spermidine and spermine from putrescine and methionine. J Biol Chem. 1958 Oct;233(4):907–914. [PubMed] [Google Scholar]
- TALALAY P., TAKANO G. M. V., HUGGINS C. Studies on the Walker tumor. I. Standardization of the growth of a transplantable tumor. Cancer Res. 1952 Nov;12(11):834–837. [PubMed] [Google Scholar]


