To the editor:
Haemoptysis is the most common bleeding complication across the lifespan of patients with pulmonary arterial hypertension associated with congenital heart disease (PAH-CHD) [1]. Its incidence ranges from 6% to 49% among patients with Eisenmenger syndrome (ES) [2]. Ranging from mild to massive and life-threatening episodes, haemoptysis increases the health burden and necessitates a multidisciplinary approach for optimal treatment [2]. Bronchial artery embolization (BAE) has become a promising option over the last years; however, its efficacy across the spectrum of PAH-CHD has not been adequately investigated so far [3,4]. Moreover, the coexistence of haemoptysis and pulmonary artery (PA) thrombosis is a rare scenario, which complicates further the therapeutic approach [1].
Herein, we highlight a case of a patient with ES presenting with recurrent episodes of haemoptysis and concurrent PA thrombosis in the context of a large secundum atrial septal defect (ASD) that has been successfully treated with BAE in our centre.
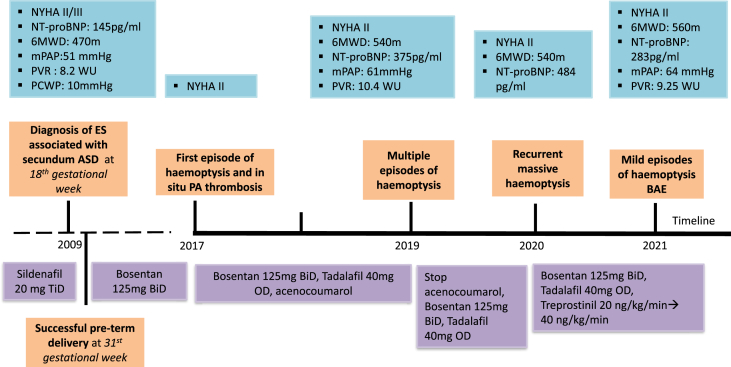
Α 40-year-old woman with ES on the background of a large secundum ASD has been followed up at our tertiary centre. Τhe diagnosis was first made at 18th gestational week, after the patient had been complaining about dyspnea in mild exertion. Sildenafil 20 mg TiD had been prescribed to the patient. Recently after until early delivery at 31 weeks of gestation, it was switched to bosentan 125mg BiD. Since then, she had been stable and mildly symptomatic until the first episode of haemoptysis 8 years after the diagnosis. In situ pulmonary thrombosis was diagnosed with computed tomography pulmonary angiogram (CTPA) and localized in the left pulmonary artery (LPA). Haemoptysis was characterized of mild to moderate severity and was treated conservatively (blood transfusion, tranexamic acid). Following haemoptysis control, she had been escalated on dual combination therapy with bosentan 125mg BiD and tadalafil 40mg OD, and was started on acenocoumarol due to pulmonary thrombosis. Τhe next multiple episodes of haemoptysis occurred 2 years later when she was readmitted to the hospital for conservative management. Anticoagulation was discontinued despite the existence of pulmonary thrombus, and the patient did not relapse for a year (Fig. 1).
Fig. 1.
Patient's timeline. ASD, atrial septal defect; BAE, bronchial artery embolization; BiD, twice a day; ES, Eisenmenger syndrome; mPAP, mean pulmonary arterial pressure; NT-proBNP, N-terminal proB-type Natriuretic Peptide; NYHA, New York Heart Association; OD, once daily; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; TiD, three times a day; WU, Wood units; 6MWD, 6-min walk distance.
The patient experienced recurrent massive haemoptysis 1 year later. Upon admission, she presented pink with dyspnea (functional class WHO III). On clinical examination, the patient's temperature was 36.6 °C, her blood pressure was 110/70 mmHg, her heart rate 100 beats/min and her oxygen saturation 89% under room-air. Cardiac auscultation revealed a pansystolic murmur in the subxiphoid area and a wide fixed splitting of the second heart sound (S2) with a loud pulmonary component. Lung auscultation was unremarkable. Physical examination of the remaining organs was unremarkable. Laboratory analysis revealed low haemoglobin levels of 8 mg/dl and increased NT-proBNP levels of 563 pg/ml.
Electrocardiogram (ECG) showed sinus rhythm with right QRS axis deviation and signs of right ventricular hypertrophy. Her transthoracic echocardiogram demonstrated severe dilation of the right atrium (RA) and right ventricle (RV) with preserved systolic function. There was moderate tricuspid regurgitation. PA was severely dilated. Intravenous blood transfusion and iron supplementation were administered.
One year later, multiple mild episodes of haemoptysis occurred, for which the patient was not hospitalized. Thorax computed tomography after intravenous contrast medium injection showed aneurysmatic dilatation of the LPA with intraluminal thrombus and adjacent post-bleeding infiltrations in the lung parenchyma (Fig. 2). A multidisciplinary team meeting took place to discuss the patient's optimal therapeutic approach. Considering her previous history of recurrent episodes of haemoptysis, catheterization of the bronchial arteries with a 5Fr Cobra catheter and subsequent embolization was opted for. Angiogram revealed the tortuous origin of the left bronchial artery (Fig. 2e). In the delayed parenchymal phase, pathologic imaging of the left posterior lung parenchyma adjacent to the descending aorta, corresponding to the bleeding area, was depicted. The left bronchial artery could not be catheterized, and therefore a 2.6Fr microcatheter was advanced in the right bronchial artery in order to inject 100–300 μm particles without any backflow in the descending aorta. Injection of particles was performed in both bronchial arteries until complete stasis was achieved. The final angiographic result was satisfactory (Fig. 2F). The patient's postoperative course was uncomplicated, and at 2-year follow-up after discharge, no bleeding recurrences occurred. She remains currently on triple PAH medication, including bosentan 125mg BiD, tadalafil 40mg OD, and subcutaneous treprostinil 40 ng/kg/min.
Fig. 2.
Sagittal (A) and axial (B) view of thorax computed tomography after intravenous contrast medium injection, showing aneurysmatic dilatation of the left pulmonary artery with intraluminal thrombus (yellow arrows). Sagittal (C) and axial (D) view of thorax computed tomography CT view in lung parenchyma window showing intraparenchymal bleeding infiltrations (yellow arrows). Catheterization of the bronchial arteries with a 5Fr Cobra catheter (E), showing the tortuous origin of the left bronchial artery (yellow arrow). Angiographic image after embolization with 100–300 μ particles through a 2.6Fr microcatheter (F) shows complete stasis, especially in the left side (yellow arrow).
In patients with PAH-CHD, haemoptysis may indicate disease progression that warrants treatment escalation. BAE has evolved into the treatment of choice for patients with recurrent episodes of haemoptysis [5]. However, there is scanty information about its safety and efficacy in patients with PAH-CHD. Only two small cohort studies have reported successful BAE in 12 and 6 PAH‐CHD patients presenting with haemoptysis [3,4]. Furthermore, the recurrence rate after BAE has been estimated at 50% in this patient population [4].
Existing literature fails to reach a consensus on the use of oral anticoagulants in patients with PAH-CHD [6,7]. Current guidelines recommend the use of anticoagulation treatment in patients with ES and low bleeding risk in the presence of atrial arrhythmias or PA thrombosis or embolism [1]. In patients with acute haemoptysis, discontinuation of the existing anticoagulation therapy should be considered [6]. The restart of oral anticoagulation after the haemoptysis termination remains a challenging issue, especially in patients with both episodes of haemoptysis and PA thrombosis. Each patient presenting with haemoptysis should be evaluated on an individual basis taking into consideration the thrombotic and bleeding risk [8]. In our case, acenocoumarol was ceased after the re-occurrence of the haemoptysis episodes.
We report a rare case of successful implementation of BAE in a patient with ES presenting with haemoptysis and concurrent PA thrombosis. As long-term data about the efficacy of BAE in PAH-CHD patients are lacking, larger and longitudinal studies are warranted to validate its use in this specific patient population.
Informed consent
The authors would like to state that informed patient consent was obtained for publication.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Given the role of the corresponding author as Associate Editor of this Journal, we confirm he had no involvement in the peer-review of this article and has no access to information regarding its peer-review process. Full responsibility for the editorial process for this article was delegated to an independent Editor."
References
- 1.Baumgartner H., De Backer J., Babu-Narayan S.V., Budts W., Chessa M., Diller G.P., et al. ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2020;42(6):563–645. doi: 10.1093/eurheartj/ehaa554. 2021. [DOI] [PubMed] [Google Scholar]
- 2.Baroutidou A., Arvanitaki A., Hatzidakis A., Pitsiou G., Ziakas A., Karvounis H., et al. Haemoptysis in pulmonary arterial hypertension associated with congenital heart disease: insights on pathophysiology, diagnosis and management. J Clin Med. 2022;11(3) doi: 10.3390/jcm11030633. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasciti E., Sverzellati N., Silva M., Casadei A., Attinà D., Palazzini M., et al. Bronchial artery embolization for the treatment of haemoptysis in pulmonary hypertension. Radiol Medica. 2017;122(4):257–264. doi: 10.1007/s11547-016-0714-6. [DOI] [PubMed] [Google Scholar]
- 4.Cantu J., Wang D., Safdar Z. Clinical implications of haemoptysis in patients with pulmonary arterial hypertension. Int J Clin Pract. 2012;66(SUPPL. 177):5–12. doi: 10.1111/ijcp.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panda A., Bhalla A.S., Goyal A. Bronchial artery embolization in hemoptysis: a systematic review. Diagnostic Interv Radiol. 2017;23(4):307–317. doi: 10.5152/dir.2017.16454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaemmerer H., Apitz C., Brockmeier K., Eicken A., Gorenflo M., Hager A., et al. Pulmonary hypertension in adults with congenital heart disease: updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol. 2018;272:79–88. doi: 10.1016/j.ijcard.2018.08.078. [DOI] [PubMed] [Google Scholar]
- 7.Diller G.P., Körten M.A., Bauer U.M.M., Miera O., Tutarel O., Kaemmerer H., et al. Current therapy and outcome of Eisenmenger syndrome: data of the German National Register for congenital heart defects. Eur Heart J. 2016;37(18):1449–1455. doi: 10.1093/eurheartj/ehv743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arvanitaki A., Gatzoulis M.A., Opotowsky A.R., Khairy P., Dimopoulos K., Diller G.-P., et al. Eisenmenger syndrome: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(12):1183–1198. doi: 10.1016/j.jacc.2022.01.022. Mar. [DOI] [PubMed] [Google Scholar]