Abstract
α2δ proteins serve as auxiliary subunits of voltage‐gated calcium channels and regulate channel membrane expression and current properties. Besides their channel function, α2δ proteins regulate synapse formation, differentiation, and synaptic wiring. Considering these important functions, it is not surprising that CACNA2D1‐4, the genes encoding for α2δ‐1 to ‐4 isoforms, have been implicated in neurological, neurodevelopmental, and neuropsychiatric disorders. Mutations in CACNA2D2 have been associated with developmental and epileptic encephalopathy (DEE) and cerebellar atrophy. In our present study, we performed a detailed functional characterization of the p.R593P mutation in α2δ‐2, a homozygous mutation previously identified in two siblings with DEE. Importantly, we analyzed both calcium channel‐dependent as well as synaptic functions of α2δ‐2. Our data show that the corresponding p.R596P mutation in mouse α2δ‐2 drastically decreases membrane expression and synaptic targeting of α2δ‐2. This defect correlates with altered biophysical properties of postsynaptic CaV1.3 channel but has no effect on presynaptic CaV2.1 channels upon heterologous expression in tsA201 cells. However, homologous expression of α2δ‐2_R596P in primary cultures of hippocampal neurons affects the ability of α2δ‐2 to induce a statistically significant increase in the presynaptic abundance of endogenous CaV2.1 channels and presynaptic calcium transients. Moreover, our data demonstrate that in addition to lowering membrane expression, the p.R596P mutation reduces the trans‐synaptic recruitment of GABAA receptors and presynaptic synapsin clustering in glutamatergic synapses. Lastly, the α2δ‐2_R596P reduces the amplitudes of glutamatergic miniature postsynaptic currents in transduced hippocampal neurons. Taken together, our data strongly link the human biallelic p.R593P mutation to the underlying severe neurodevelopmental disorder and highlight the importance of studying α2δ mutations not only in the context of channelopathies but also synaptopathies.
Keywords: auxiliary subunit, calcium current, epilepsy, neurodevelopmental disorders, trans‐synaptic function, voltage‐gated calcium channels
α2δ auxiliary subunits of voltage‐gated calcium channels modulate the calcium currents and have emerged as regulators of synaptic functions. Considering these important functions, it is not surprising that mutations of α2δ‐2 are associated with developmental and epileptic encephalopathy (DEE). However, whether an altered calcium channel modulation, synaptic mechanism, or both underlie the disease phenotype is not known. Here, we functionally characterized a homozygous missense mutation identified in two siblings with DEE. Our study strongly links the mutation to the underlying disorder and demonstrates that disruption in both channel‐dependent as well as pre‐ and postsynaptic functions of α2δ‐2 can contribute to the pathophysiological mechanism.
Abbreviations
- Ca2+
calcium
- CACNA2D1–4
calcium channel auxiliary subunit alpha2delta‐coding genes
- CaV
voltage‐gated calcium channels
- CNS
central nervous system
- DEE
developmental and epileptic encephalopathy
- DIV
days in vitro
- GABAAR
GABAA receptor
- HA
hemagglutinin
- mEPSCs
miniature excitatory postsynaptic currents
- MIDAS
metal ion‐dependent adhesion site
- RRID
Research Resource Identifier
- VGCC
voltage‐gated calcium channel
- vGLUT1
vesicular glutamate transporter 1
- VWA
von Willebrand factor type A domain
- WT
wild type
1. INTRODUCTION
Calcium (Ca2+) entry into excitable cells is tightly regulated by voltage‐gated Ca2+ channels (VGCCs, CaV). Neuronal channels of the CaV1 and CaV2 families are multimeric complexes consisting of pore‐forming α1 and two auxiliary subunits, β and α2δ. Four genes encode for α2δ subunits (CACNA2D1‐4) out of which three isoforms (α2δ‐1‐3) are highly expressed in the central nervous system (CNS) (Schlick et al., 2010). The classical roles of α2δ proteins as auxiliary subunits of VGCCs, regulating the functional membrane expression and modulating the Ca2+ currents, are widely studied (reviewed in Dolphin, 2016). Recently, all four α2δ isoforms were recognized as important regulators of synaptic functions and these functions may be partially or entirely independent of the Ca2+ channel complex (reviewed in Dolphin & Obermair, 2022; Geisler et al., 2015). For example, loss of presynaptic α2δ subunits in murine cultured hippocampal neurons disrupted both pre‐ and postsynaptic differentiation, indicating that α2δ isoforms are necessary for glutamatergic synapse formation and trans‐synaptic differentiation (Schopf et al., 2021). Moreover, altered expression levels of presynaptic α2δ‐1 and α2δ‐2 splice variants induce aberrant synaptic wiring and change the postsynaptic molecular composition by a trans‐synaptic mechanism (Ablinger et al., 2022; Geisler et al., 2019). This emphasizes the importance of α2δ proteins in brain connectivity and suggests that aberrant expression of α2δ proteins may affect the excitatory–inhibitory balance. Therefore, it is not surprising that α2δ proteins have been implicated in various neurological disorders (reviewed in Ablinger et al., 2020; Ablinger et al., 2024; Hessenberger et al., 2023).
However, whether aberrant α2δ protein functions cause such defects by altered Ca2+ channel modulation, synaptic mechanism, or both is largely unknown. Several genes encoding VGCC subunits have been associated with epileptic encephalopathy, including CACNA1A (Epi, 2016; Epi et al., 2013; Niu et al., 2022), CACNA1C (Bozarth et al., 2018), CACNA1E (Helbig et al., 2018), CACNA2D1 (Dahimene et al., 2022), and CACNA2D2 (Table 1). The α2δ‐2 isoform is highly expressed in the cerebellum and is relevant for the structure and function of cerebellar synapses (Beeson et al., 2020; Beeson et al., 2021). Besides α2δ‐1, it is one of the targets of the highly prescribed anti‐epileptic drugs pregabalin and gabapentin (Gong et al., 2001). To date, five cases of infantile‐onset epilepsy and cerebellar atrophy linked to bi‐allelic mutations in CACNA2D2 have been reported in unrelated families (Table 1; Butler et al., 2018; Edvardson et al., 2013; Pippucci et al., 2013; Punetha et al., 2019). The clinical presentation of all patients is strikingly similar to the phenotypes of ducky mutant mice, carrying a CACNA2D2 loss‐of‐function mutation, and α2δ‐2 knockout mice (Barclay et al., 2001; Brodbeck et al., 2002; Donato et al., 2006; Ivanov et al., 2004), strengthening the hypothesis that abnormal α2δ‐2 expression or function may underlie the epileptic phenotype for these patients. However, as of today, in‐depth mechanistic analysis of these mutations is still missing and, if studied, limited to the so‐called “channelopathies,” essentially describing the consequences of α2δ mutations on Ca2+ channel functions (Edvardson et al., 2013).
TABLE 1.
Previously reported cases of infantile‐onset epilepsy with bi‐allelic mutations in CACNA2D2.
| Publication | Genetic alteration | Patient | Common main symptoms | Seizure onset (month) | Functional characterization |
|---|---|---|---|---|---|
| Edvardson et al., 2013 |
Homozygous c.3199A > g (p.L1040P) |
Three affected siblings (2 Female, 1 Male) |
|
1–2 | Failed to increase current density of both N (CaV2.2) and L (CaV1.2) Type Ca2+ channels when expressed in Xenopus oocytes |
| Pippucci et al., 2013 |
Homozygous c.1295delA (p.Asn432fs) |
Male | 5 | Abolished α2δ − 2 protein expression in patient | |
| Butler et al., 2018 |
Compound Heterozygous c.782C > T (p.Pro261Leu) c.3137 T > C (p.Leu1046Pro) |
Male | 7 | Not available | |
| Punetha et al., 2019 | Homozygous c.485_486del (p.Tyr162Ter) | Male | 7 | ||
| Homozygous c.1778G > C (p.Arg593Pro) |
Two affected siblings (1 female, 1 male) |
1–2 | The present study |
To overcome this limitation, in this study, we aimed to characterize a previously reported rare homozygous missense variant (p.R593P) found in two siblings with developmental and epileptic encephalopathy (DEE) (Punetha et al., 2019) by studying the effects on the biophysical properties of Ca2+ channels, as well as potential consequences on synaptic functions. Our study identified a strongly reduced surface expression and presynaptic localization of mouse α2δ‐2 containing the p.R596P mutation, homologous to human p.R593P. Upon heterologous co‐expression, this resulted in altered CaV1.3 current properties, whereas the current density of CaV2.1 was not affected. Nevertheless, homologous expression of p.R596P in hippocampal neurons affected the ability of α2δ‐2 to induce a statistically significant increase in the presynaptic abundance of endogenous CaV2.1 and, consequentially, Ca2+ transients. Most importantly, our study identified three consequences of the p.R596P mutation on synaptic functions: firstly, reduced, albeit still functional trans‐synaptic coupling to postsynaptic receptors, secondly, reduced presynaptic synapsin clustering in glutamatergic nerve terminals, and thirdly, reduced amplitudes of glutamatergic miniature postsynaptic currents (mEPSCs). Taken together, our data strengthen the hypothesis that the human p.R593P mutation in α2δ‐2 is causal for the clinical symptoms of the siblings and demonstrate that disease‐associated α2δ mutations can alter channel‐dependent as well as synaptic functions of α2δ proteins, both of which may contribute to the pathophysiological mechanism.
2. METHODS
2.1. Animal and ethical approval
Animal procedures for wild‐type BALB/c mice were performed at the Medical University Innsbruck in compliance with EU and national regulations. Original wild‐type BALB/c mice for starting the breeding colony were bought from Charles River (Germany). The animal facility of the Medical University of Innsbruck was approved as user by the Austrian Federal Ministry of Science, Research and Economy in accordance with §16 TVG 2012, license numbers BMWF‐66.011/0017‐II/3b/2014 and BMWF‐66.011/0067‐II/3b/2014. Mice were maintained in groups of 3–5 per cage at the central animal facility in Innsbruck under standard housing conditions with food and water available ad libitum on a 12 h light/dark cycle. Mice used for breeding were 2 to 14 months old. According to the RRR principle, the number of mice used was kept to the minimum necessary for a statistical representative analysis, which was comparable to numbers reported in previous studies. In total, 13 pregnant mice were used for hippocampal culture preparations. No ethics vote was required, and anonymized information about the patients was quoted from a published study (Punetha et al., 2019).
2.2. Cell culture and transfection procedures
2.2.1. Primary cultured hippocampal neurons
Low‐density hippocampal cultures were generated from 16.5‐ to 18‐day‐old embryonic BALB/c mice of either sex as described previously (Geisler et al., 2019; Kaech & Banker, 2006; Obermair et al., 2004). Briefly, pregnant mice were killed by cervical dislocation, embryos were immediately extracted and decapitated. For each culture preparation, 2–8 hippocampi were dissected in cold Hank's balanced salt solution (HBSS, Gibco, cat. no. 14180‐046), pooled, and dissociated by 2.5% trypsin (Gibco, cat. no. 15090‐046) treatment and subsequent trituration. Dissociated neurons were plated at a density of ~3500 cells/cm2 (immunolabeling experiments) or 7000 cells/cm2 (whole‐cell patch‐clamp recordings), on five 18 mm glass coverslips (Marienfeld Superior, cat. no. 0111580) coated with poly‐l‐lysine (Sigma‐Aldrich, cat. no. P2636) in neuronal plating medium [minimum essential medium (MEM, Gibco, cat. no. 41090‐028), supplemented with 1 mM pyruvic acid (Sigma, cat. no. P2256), 0.6% glucose (Carl Roth, cat. no. HN06.3), and 10% horse serum (Gibco, cat. no. 16050‐122)]. After attachment of neurons for 3–4 h, coverslips were transferred neuron‐side down into a 60 mm culture dish containing a feeder monolayer of glia. Three days after plating, glial proliferation was inhibited with 5 μM Ara‐C (Sigma, cat. no. C6645). Neurons and glia were maintained in NBKO [serum‐free neurobasal medium (Gibco, cat. no. 21103‐049) supplemented with Glutamax (Gibco, cat. no. 35050‐038) and B‐27 (Gibco, cat. no. 17504‐044)] that was changed weekly by replacing one‐third of the volume with fresh maintenance medium. On day in vitro (DIV) six, neurons were transfected with plasmids using Lipofectamine 2000 (Thermo Fisher Scientific, cat. no. 11668019) as described previously (Obermair et al., 2004). 1.5 μg of total DNA was used for co‐transfections at equimolar ratios. For presynaptic Ca2+ measurements and whole‐cell patch‐clamp recordings, neurons were used between DIV14 and 17, whereas for immunolabeling experiments, neurons were processed between DIV21 and 25.
2.2.2. tsA201 cells
Human embryonic kidney (HEK)‐293 subclone stably expressing SV40 temperature‐sensitive T antigen (tsA201) cells [ECACC cat. No. 96121229, RRID:CVCL_2737, not listed as a commonly misidentified cell line by the International Cell Line Authentication Committee (ICLAC; http://iclac.org/databases/cross‐contaminations/)] was cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, cat. no. 11995065) completed with 10% FBS (Gibco, cat. no. 10270106), 0.1 U/mL penicillin, and 0.1 μg/mL streptomycin (PenStrep, Gibco, cat. no. 15140‐122), and were maintained at 37°C in a humidified incubator with 5% CO2. Cells were split when they reached ~80% of confluence using 0.5% trypsin–EDTA (Gibco, cat. no. 15400‐054) for detaching adherent cells. The cell's passage number did not exceed 20 passages. For whole‐cell patch‐clamp recordings, tsA201 cells were transiently transfected with α1 and β subunit as a control condition or together with WT or mutated α2δ‐2 at equimolar ratios using FuGeneHD transfection reagent (Promega, cat. no. E2311) according to the manufacturer protocol. eGFP was always included as a marker for transfected cells. One day after transfection, cells were detached and replated at very low density on poly‐l‐lysine‐coated 35 mm Petri dishes and kept at 30°C and 5% CO2 to increase protein expression and inhibit cell proliferation. Cells were used for electrophysiology experiments 48–72 h after transfection. For live‐cell Immunolabeling experiment, cells were plated on 13 mm glass coverslips (Marienfeld Superior, cat. no. 0111530) coated with poly‐l‐lysine. For western blot and live‐cell immunolabeling experiments, tsA201 cells were transfected with 1 μg 2HA‐tagged α2δ‐2 together with 0.5 μg eGFP. Experiments were performed 48 hours after transfection.
2.2.3. Lentiviral production
Lentiviruses were produced by transient transfection of confluent Lenti‐X 293 T cells (Takara cat. no. 632180), with the lentiviral expression vectors containing pHR‐βA‐eGFP, pHR‐βA‐eGFP*α2δ‐2, or pHR‐βA‐eGFP*α2δ‐2_R596P together with psPAX2 (packaging plasmid) and pVSV (envelope plasmid) using Metafectene (Biontex Laboratories, cat. no. T020‐1.0). The following day, sodium butyrate was added (5 mM final concentration) to cells early in the morning to enhance viral production. Six hours later, medium was changed to neuronal plating medium (NPM; consisting of MEM, 10% horse serum, 0.6% glucose, and 1 mM sodium pyruvate), and after 24 h, supernatants containing the viruses were harvested, sterile filtered (0.45 μm syringe filter, Sarstedt, cat. no. 83.1826), aliquoted, and stored at −20°C. Cultured hippocampal neurons were infected immediately after plating with the lentiviral medium supernatant diluted 1:2 in NPM with 3 μg/mL polybrene (Millipore Sigma, cat. no. TR‐1003‐G) and incubated for 4 h in a humidified incubator (5% CO2) at 37°C.
2.3. Expression vectors and cloning procedures
All plasmids used to transfect primary cultured hippocampal neurons were cloned into an eukaryotic expression plasmid containing a neuronal chicken β‐actin promoter (pβA) to improve neuronal expression. All newly generated constructs were verified by Sanger sequencing (Eurofins Genomics or Microsynth). The cloning procedures to generate the following plasmids were described previously: pβA‐α2δ‐2‐v1 (Geisler et al., 2019), pβA‐2HA‐α2δ‐2‐v1 (Geisler et al., 2019), pSyn‐GCaMP6f (Brockhaus et al., 2019), pβA‐α2δ‐2‐ΔMIDAS (Schopf et al., 2021), pHR‐pβA‐eGFP*α2δ‐2‐v1 (Geisler et al., 2019), pβA‐eGFP (Obermair et al., 2004), pHR‐pβA‐mCherry (Geisler et al., 2019), pGFP−‐CaV1.3 (Koschak et al., 2001), pCMV‐CaV2.1 (Mullner et al., 2004), β3 (Castellano et al., 1993), and β4 (Etemad et al., 2014).
pβA‐α 2 δ‐2‐v1_R596P: The p.R596P mutation was introduced by PCR with mutagenesis primers with overlapping extensions. Briefly, α2δ‐2‐v1 cDNA sequence was amplified with mutagenesis primers having 21 overlapping bases in two separate PCR reactions using pβA‐α2δ‐2‐v1 as template (mouse α2δ‐2‐v1 cDNA, GenBank accession number MK327277). For the first PCR reaction, the forward mutagenic primer sequence was 5′‐gaggagatccctcgcagcatgattgacggc‐3′ and the reverse primer sequence was 5′‐gacgacctagactgagctcc‐3′. For the second PCR reaction, the forward primer sequence was 5′‐gacgctgcagagaatttcca‐3′ and the reverse mutagenic primer sequence was 5′‐ catgctgcgagggatctcctccttgttctc‐3′. The two separate PCR products were then used as templates for a final PCR reaction with the two flanking primers to connect the nucleotide sequences. The resulting fragment was then EcoRI/BglII digested and ligated into the corresponding site of pβA‐α2δ‐2‐v1 yielding pβA‐α2δ‐2‐v1_R596P.
pβA‐2HA‐α 2 δ‐2‐v1_R596P: pβA‐α2δ‐2‐v1_R596P was EcoRI/BglII digested and the fragment containing the p.R596P mutation was ligated into the corresponding site of pβA‐2HA‐α2δ‐2‐v1 (mouse α2δ‐2‐v1 cDNA sequence with a double hemagglutinin (2HA) tag at the N‐terminus after the predicted signal peptide cleavage site) yielding pβA‐2HA‐α2δ‐2‐v1_R596P.
pHR‐pβA‐eGFP*α 2 δ‐2‐v1_R596P: pβA‐α2δ‐2‐v1_R596P was NheI/RsrII digested and the fragment containing the p.R596P mutation was ligated into the corresponding site of pHR‐pβA‐eGFP*α2δ‐2‐v1 yielding pHR‐pβA‐eGFP*α2δ‐2‐v1_R596P.
2.4. Immunocytochemistry and high‐resolution fluorescence microscopy
Permeabilized or live‐cell immunolabeling of neurons was performed as described previously (Folci et al., 2018; Geisler et al., 2019; Obermair et al., 2004; Schopf et al., 2021; Stanika et al., 2016), and information on primary and secondary antibodies is summarized in Table 2. For permeabilized staining, neurons were fixed with 4% paraformaldehyde and 4% sucrose in PBS (pF) for 20 min at room temperature, washed, and incubated for 30 min in 5% normal goat serum in PBS‐containing 0.2% bovine serum albumin (BSA) and 0.2% Triton X‐100 (PBS/BSA/Triton) to enable membrane permeabilization. Primary antibodies (Table 2) diluted in PBS/BSA/Triton were applied overnight at 4°C and detected by fluorochrome‐conjugated Alexa secondary antibodies incubated for 1 h at room temperature. For live‐cell surface staining of HA‐tagged α2δ proteins, transfected neurons were incubated with rat‐anti‐HA antibody diluted in glia‐conditioned neurobasal medium for 10 min at 37°C following quick rinsing in warm HBSS and fixation with pF for 10 min at room temperature. Subsequent washing and blocking steps as well as 1 h incubation with fluorochrome‐conjugated secondary goat anti‐rat Alexa Fluor 594 antibody were conducted with PBS and PBS/BSA, respectively. After washing and fixing cells in pF for 5 min, neurons were permeabilized by blocking solution (PBS/BSA/Triton) and incubated with primary mouse‐anti‐synapsin antibody overnight at 4°C and detected with goat‐anti‐mouse Alexa Fluor 350 antibody. Coverslips were mounted on microscopy slides neuron‐side down in DABCO glycerol solution (Carl Roth, cat. no. 0718.1) to retard photobleaching. Hippocampal neurons were imaged with a BX53 microscope (Olympus) using a 60 X 1.42 numerical aperture (NA) oil immersion objective lens. Fourteen‐bit grayscale images were recorded with a cooled CCD camera (XM10; Olympus) using cellSens Dimension software (Olympus) and further analyzed in MetaMorph software (Molecular Devices). To analyze presynaptic and postsynaptic protein composition, images of randomly selected well‐differentiated and positively transfected cells were acquired with the same exposure and gain settings for all conditions within an experiment. Only cells with medium eGFP expression were selected for the analysis, and overly saturated neurons (based on eGFP levels) were excluded from analysis. Figures were assembled in Adobe Photoshop CS6 using linear adjustments to correct black level and contrast.
TABLE 2.
List of antibodies used in this study.
| Antibody | Species | Dilution | Source |
|---|---|---|---|
| Anti‐HA | Rat, clone 3F10 | 1:100 (Live/A594) | Roche (cat. no. 11867423001, RRID:AB_390918) |
| Anti‐HA | Mouse, clone 5B1D10 | 1:1000 (WB) | Thermo Fisher Scientific (cat. no. 32–6700, RRID:AB_2533092) |
| Anti‐GABAARβ2/3 | Mouse, clone bd17 | 1:500 (A594) | Millipore (cat. no. MAB341, RRID:AB_2109419) |
| Anti‐synapsin1 | Mouse, clone 46.1 | 1:500 (A350) | Synaptic Systems (cat. no. 106011, RRID:AB_2619772) |
| Anti‐vGLUT1 | Rabbit, polyclonal | 1:2000 (A350) | Synaptic Systems (cat. no. 135002, RRID:AB_2315546) |
| Anti‐CaV2.1 | Rabbit, polyclonal | 1:2000 (A594) | Synaptic Systems (cat. no. 152203, RRID:AB_2619841) |
| Anti‐GAPDH | Mouse, clone 6C5 | 1:10000 | Santa Cruz Biotechnology (cat. no. sc‐32 233, PRID:AB_627679) |
| Alexa Fluor 350 | Goat anti‐rabbit | 1:500 | Thermo Fisher Scientific (cat. no. A‐21068, RRID:AB_2535729) |
| Goat anti‐mouse | 1:500 | Thermo Fisher Scientific (cat. no. A‐21049, RRID:AB_2535717) | |
| Alexa Fluor 594 | Goat anti‐rabbit | 1:4000 | Thermo Fisher Scientific (cat. no. A‐11037, RRID:AB_2534095) |
| Goat anti‐mouse | 1:4000 | Thermo Fisher Scientific (catalog #A‐11032, RRID:AB_2534091) | |
| Goat anti‐rat | 1:4000 | Thermo Fisher Scientific (catalog #A‐11007, RRID:AB_10561522) | |
| Secondary antibody coupled to HRP | Goat anti‐mouse IgG [H + L] | 1:20 000 | Thermo Fisher Scientific (catalog #G21040, RRID:AB_2536527) |
Note: Summary of primary and secondary antibodies used in immunocytochemistry and western blot.
2.5. Antibodies
Information on primary and secondary antibodies used in this study is summarized in Table 2.
2.6. Image analysis and quantification
2.6.1. Colocalization of synaptic proteins
To visualize the synaptic localization of HA‐tagged α2δ subunits, as well as presynaptic (synapsin, vGLUT1, CaV2.1) and postsynaptic proteins (GABAAR) of representative images (Figures 6, 7 and 9), lines were manually placed across the single‐/double‐/triple‐labeled synapses and intensities were recorded using the “line scan” function of MetaMorph (Molecular Devices) (Di Biase et al., 2009). Average fluorescence intensities of respective signals were measured along a 3 μm length line, followed by background subtraction, and plotted in Microsoft Excel.
FIGURE 6.
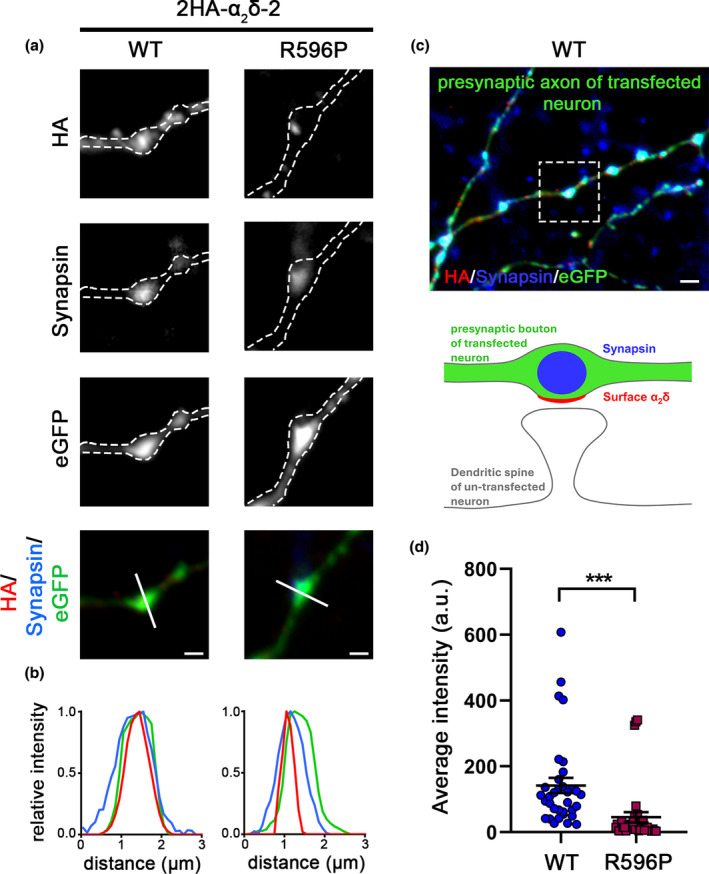
Reduced presynaptic targeting of α2δ‐2_R596P. (a) Representative presynaptic boutons of transfected cultured hippocampal neurons, identified by the eGFP expression and synapsin clustering, are outlined by a dashed line. Live‐cell staining shows that α2δ‐2_R596P is expressed at the surface of presynaptic boutons. Scale bars, 1 μm. (b) Line scan analysis of 2HA‐α2δ‐2 (red), synapsin (blue), and eGFP (green) staining pattern of representative images supports a presynaptic localization by the overlapping peaks of HA‐label (red) in relation to synapsin (blue) and eGFP (green). (c) The surrounding region of the selected WT synapse in (a) and a sketch summarizing the observed labeling pattern. Scale bar, 2 μm. (d) Average fluorescence intensity measurements of the HA signal in positively transfected boutons revealed a strong reduction in presynaptic localization of α2δ‐2_R596P compared to WT α2δ‐2. Statistics: Graph shows mean values of minimally five synapses for individual cells (dots) and means ± SEM (lines). Data were obtained from three independent culture preparations and 34 and 35 cells expressing WT or mutated HA‐tagged α2δ‐2 were analyzed, respectively. Statistics: unpaired two‐tailed t‐test, t (67) = 3.0; ***p = 0.0037.
FIGURE 7.
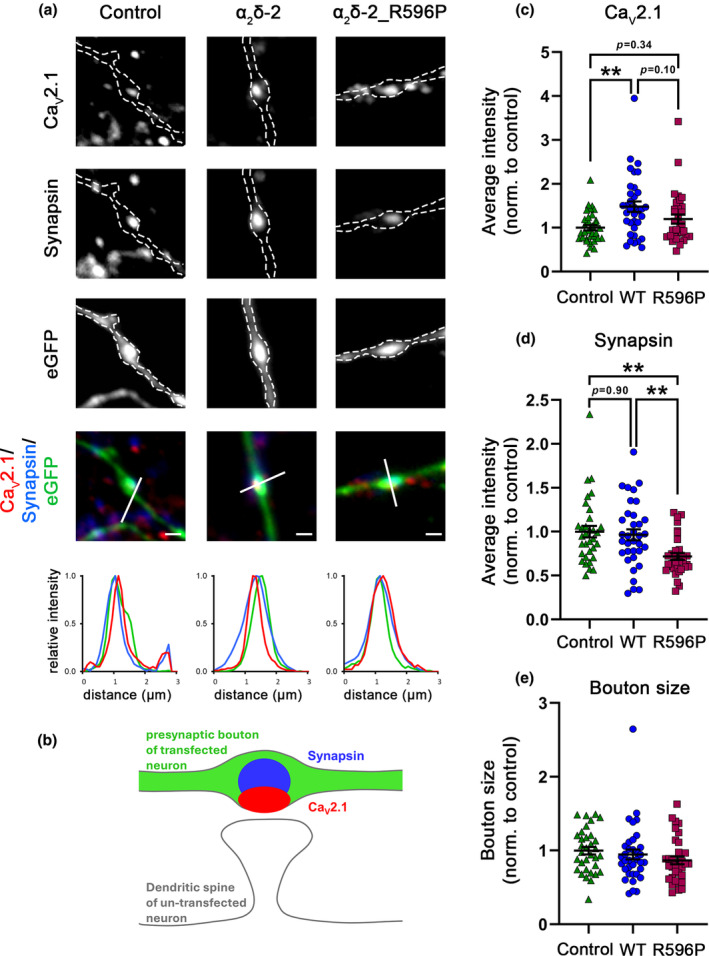
Homologous expression of α2δ‐2_R596P in hippocampal neurons fails to induce a statistically significant increase in presynaptic CaV2.1 clustering and decreases presynaptic synapsin abundance. (a) Representative micrographs of presynaptic boutons of cultured hippocampal neurons transfected with eGFP alone (control) or co‐transfected together with either WT (α2δ‐2) or mutated (α2δ‐2_R596P) α2δ‐2. Immunofluorescent signals of CaV2.1 channels colocalize with presynaptic synapsin clusters (see also co‐localizing peaks of fluorescence signals in line scan analysis). (b) Sketch depicting the expected staining pattern in (a). (c) Quantification of presynaptic CaV2.1 cluster intensity shows that contrary to WT, presynaptic expression of the p.R596P mutant fails to induce a significant increase in presynaptic CaV2.1 clustering. (d) Quantification of presynaptic synapsin abundance shows a strong reduction in synaptic synapsin intensity upon expression of the p.R596P mutant. (e) Quantification of boutons size, as identified by the eGFP fluorescence area, shows no difference between the different experimental conditions. Statistics: Graphs of CaV2.1 and synapsin average intensities and bouton size show values for individual cells (dots) and means ± SEM (lines). Cells were obtained from three independent culture preparations. One‐way ANOVA with Tukey's multiple‐comparison test. A total of 33–35 cells per condition, (c) F (2,98) = 6.1, **p = 0.0031. P‐values of the post hoc test for the respective pairwise comparisons: P = 0.002 for control versus WT, p = 0.34 for control versus R596P, and p = 0.12 for WT versus R596P. (d) F (2,98) = 7.0, **p = 0.0013. P‐values of the post hoc test for the respective pairwise comparisons: P = 0.89 for control versus WT, p = 0.002 for control versus R596P, and p = 0.01 for WT versus R596P. (e) F (2,98) = 1.2, p = 0.29. P‐values of the post hoc test for the respective pairwise comparisons: P = 0.81 for control versus WT, p = 0.27 for control versus R596P, and p = 0.61 for WT versus R596P. Significances of post hoc tests between conditions are indicated in the graphs by asterisks (**p < 0.01). Scale bar, 1 μm.
FIGURE 9.
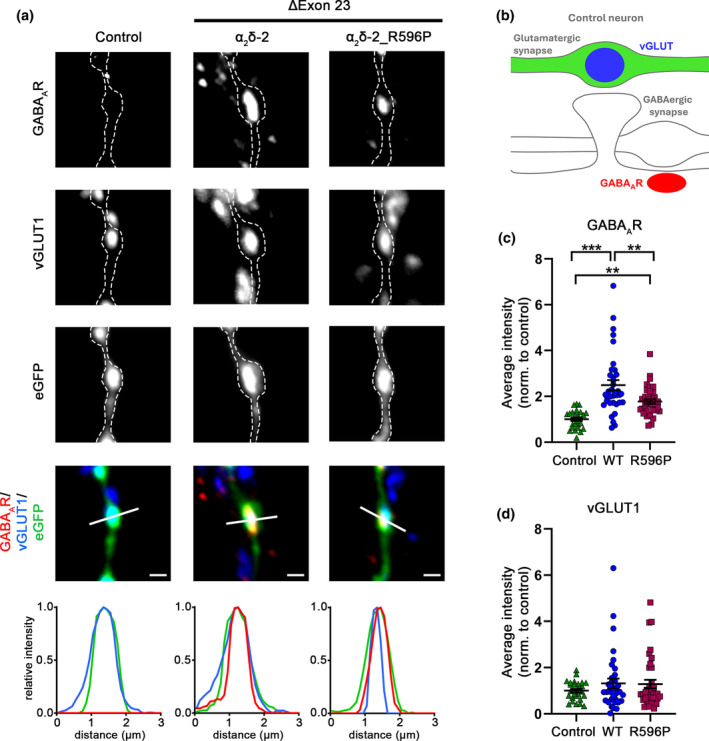
Reduced trans‐synaptic coupling to postsynaptic GABAAR of the α2δ‐2_R596P mutant. immunofluorescent labeling of presynaptic vGLUT1 and postsynaptic GABAAR was used to identify the formation of mismatched synapses in hippocampal neurons transfected with soluble eGFP as control or together with either WT or mutated α2δ‐2_ΔE23. (a) Both, homologous over‐expression of WT and mutated α2δ‐2 lead to the formation of mismatched synapses. However, compared to WT α2δ‐2, neurons transfected with α2δ‐2_R596P mutant showed a reduced postsynaptic GABAARs recruitment, as detected by postsynaptic GABAAR clusters opposite vGLUT1‐positive glutamatergic terminals (a, α2δ‐2, α2δ‐2_R596P). Colocalization of the fluorescence signals of representative images was analyzed using line scans. (b) Sketch summarizing the observed labeling pattern of a control bouton expressing eGFP alone. In control boutons, potential glutamatergic synapses are positive for presynaptic vGLUT1 (blue) but negative for postsynaptic GABAARs (red); in contrast to glutamatergic synapses, GABAergic synapses are typically located along the dendritic shaft. Quantifications of immunofluorescence intensities of GABAAR (c) and vGLUT1 (d) labeling show values for individual cells (dots) and means ± SEM (lines). Values were normalized to the fluorescent intensities of the control condition within each culture preparation. Cells were obtained from three independent culture preparations. Statistics: ANOVA with Tukey's multiple‐comparison test was performed on 26–37 cells per condition. GABAAR: F (2,96) = 20.06; p < 0.0001. P‐values of the post hoc test for the respective pairwise comparisons: P < 0.0001 for control versus WT, p = 0.003 for control versus R596P, and p = 0.003 for WT versus R596P. vGLUT1: F (2,96) = 0.81; p = 0.45. P‐values of the post hoc test for the respective pairwise comparisons: P = 0.47 for control versus WT, p = 0.53 for control versus R596P, and p = 0.99 for WT versus R596P. Significances of post hoc tests between conditions are indicated in the graphs by asterisks (***p < 0.001, **p < 0.01). Scale bars, 1 μm.
2.6.2. Quantification of fluorescent clusters in single boutons
To analyze the effects of homologous presynaptic expression of WT or mutated α2δ‐2 subunit on synapse composition of cultured hippocampal neurons, images from triple‐fluorescence labeling were acquired from the eGFP (green), GABAARβ2/3 (red), and vGLUT1 (blue) channels. Images were analyzed with a custom‐programmed and semi‐automated MetaMorph journal (Molecular Devices), as described previously (Geisler et al., 2019; Schopf et al., 2021). Briefly, corresponding eGFP and vGLUT1 images were superimposed, eGFP/vGLUT1‐positive varicosities (putative glutamatergic synapses) were randomly chosen as regions of interest (ROIs), and a background region was selected for background subtraction. Axonal varicosities were defined as prominent swellings with higher fluorescence signals compared to the adjacent axonal shaft. Subsequently, GABAAR and vGLUT1 grayscale images were measured without thresholding to avoid potential cut‐off of the fluorescent signal. By applying the “shrink region to fit” tool, automatic boundaries were drawn according to the threshold enabling only colocalized clusters to be analyzed, and selected ROIs were then transferred from the eGFP image to the GABAAR and vGLUT1 images to measure fluorescent intensities. For the individual synapses in each of the channels, the following parameters were detected in a blinded manner: eGFP threshold area as a measure for bouton size and average and integrated fluorescence intensities providing information on the size and intensity of clusters. In the same manner, the quantification of CaV2.1 together with synapsin signals was performed.
2.6.3. Quantification of live cell surface expression
To analyze presynaptic targeting of HA‐tagged α2δ, a slightly modified protocol was used because this staining pattern was not directly co‐localizing with the corresponding presynaptic eGFP signal. Therefore, the presynaptic ROI was dilated by 0.5 μm, in order to avoid false‐positive or false‐negative staining patterns, as described in Schopf et al. (2021). The fluorescent intensity of live‐cell‐stained HA‐tagged α2δ in the main three compartments of neurons was determined by measuring the fluorescent intensity along an outlined axon, dendrite, and cell soma.
2.6.4. Quantification analysis
Analyses of all experiments were conducted with Microsoft Excel. For each neuron, an average fluorescence value from minimum of 10 presynaptic varicosities was calculated and plotted in GraphPad Prism 8. For each condition, minimum of 30 cells were considered from three to four independent culture preparations. All values were additionally normalized to the control condition.
2.7. Calcium imaging
To examine presynaptic Ca2+ influx, GCaMP6f coupled to synaptophysin driven by a synapsin promotor (Brockhaus et al., 2019) was used to conduct Ca2+ imaging as described previously (Ablinger et al., 2022). Briefly, primary neurons were transfected at DIV6 with pβA‐SynGCaMP6f and pβA‐mCherry, as a control condition, or together with WT or mutated α2δ‐2 subunit. Then, at DIV14‐17, coverslips were mounted in a recording chamber, placed on an inverted microscope (Olympus IX71, 60×, 1.42 NA PlanApo objective), and superfused at 1.0–1.5 mL/min rate with bath solution (32°C), containing (in mM): 145 NaCl, 3 KCl, 1.5 MgCl2, 2 CaCl2, 11 glucose, and 10 HEPES. To suppress postsynaptic signaling, the following blockers were added to the bath solution (in μM): 10 6‐cyano‐7‐nitroquinoxaline‐2,3‐dione (CNQX, Tocris, cat. no. 1045), 25 DL‐2‐amino‐5‐phosphonovaleric acid (DL‐AP5, Tocris, cat. no. 3693), and 10 bicuculline (Tocris, cat. no. 0109); pH 7.3 adjusted with NaOH. A custom‐built stimulation electrode was positioned with a micromanipulator (MPC‐200, Sutter Instrument) and neurons were stimulated with 50 Hz trains of 1 and 10 current pulses (1 ms, 55 mA) as previously described (Ablinger et al., 2022; Schopf et al., 2021). Ca2+ transients were visualized and recorded (20 ms exposure time, frame rate 50 Hz, 200 frames, binning 2, and pixel size: 0.215 μm per pixel) with a CMOS camera (Orca Flash4.0, Hamamatsu), using a halogen lamp light source (HXP 120) in the green channel (excitation: 470/40 nm, emission: 525/50 nm). Recordings were controlled by Micromanager software (Vale Lab, UCSF). As a standard reference, 50 frames were recorded before the stimulus train was triggered and used to calculate the fluorescent baseline and to analyze differences in GCaMP6f presynaptic expression among experimental conditions.
2.7.1. Data analysis
Recordings were analyzed with FIJI/ImageJ software (National Institute of Health) as described previously (Ablinger et al., 2022). Twenty regions of interest (ROIs) per recorded cell were drawn around active presynaptic boutons using the plugin “Time Series Analyzer V3” with an AutoROI diameter of 10 pixels and the “Subtract Background” tool of ImageJ (employing a “rolling ball” algorithm with a radius of 20 pixels ≈ 4.3 μm) was used to remove the background signal. The mean of the four highest fluorescence pixels was calculated for each ROI at each frame by applying a custom‐made macro (Brockhaus et al., 2019) and further analysis was done in Microsoft Excel. To obtain mean sample traces, the changes in fluorescence as ΔF/F0 were calculated for each ROI, and 20 synapses per cell were averaged. The peak fluorescence amplitude for each ROI was obtained by averaging ΔF/F0 values from five frames after the pulse onset. Statistical analysis was performed on the mean peak amplitude of cells in GraphPad Prism 8.
2.8. Electrophysiology
2.8.1. tsA201 cells
CaV2.1 and CaV1.3 currents were measured using the whole‐cell patch‐clamp technique in voltage‐clamp mode. Borosilicate glass capillaries (Sutter Instrument, model: BF150‐75‐10) were pulled with a micropipette puller (P‐97, Sutter Instrument) and fire polished using a MF‐830 microforge (Narishige Co). Patch pipettes had a resistance of 2–3.5 MΩ when filled with internal solution containing (in mM): 135 cesium chloride (CsCl, Sigma, cat. no. C3309), 10 Cs‐EGTA (Carl Roth, cat. no. 3054.2), 1 magnesium chloride (MgCl2, Sigma, cat. no. M0250), 10 HEPES (Carl Roth, cat. no. 6763.1), and 4 Na2ATP (Sigma, cat. no. A1852‐1VL) adjusted to pH 7.3 with 1 M cesium hydroxide (CsOH, Sigma, cat. no. 232068). For measuring Ca2+ current of CaV1.3, bath solution with 15 mM Ca2+ was used, containing (in mM): 15 calcium chloride (CaCl2, Carl Roth, cat. no. 5239.2), 150 Choline‐Cl (Sigma, cat. no. C1879), 1 MgCl2, and 10 HEPES, adjusted to pH 7.3 with 1 M CsOH. For measuring Ca2+, current through CaV2.1 bath solution with 2 mM Ca2+ was used containing (in mM): 2 CaCl2, 170 Choline‐Cl, 1 MgCl2, 10 HEPES, adjusted to pH 7.3 with 1 M CsOH. Whole‐cell patch‐clamp recordings were performed at room temperature (20–23°C) using an EPC 10 amplifier controlled by Patch Master Software (HEKA Elektronik). Linear leak and capacitive currents were digitally subtracted with a P/4online protocol. Current–voltage (I‐V) relationships were measured by applying 50 ms depolarizing square pulses to various test potentials (from −80 mV to +90 mV) in steps of 5 mV, and holding potential (HP) was set to −80 mV. I‐V curves were fitted according to a Boltzmann equation:
where I is the peak current, G MAX is the maximum conductance, V is the test potential, V rev is the extrapolated reversal potential, V 0.5 is the half‐maximal activation voltage, and k is the slope factor. The voltage dependence of activation was obtained from the I–V relationship by calculating the conductance followed by normalization and plotting as a function of voltage. The G‐V curve was fitted using the following Boltzmann relationship:
Series resistance was compensated by 80%–95% and recordings were accepted for analysis when the I peak was greater than 100 pA and smaller than 3 nA in size. Voltages were not corrected for the liquid junction potential (−9 mV).
2.8.2. Primary cultured hippocampal neurons
Spontaneous miniature excitatory postsynaptic currents (mEPSCs) of primary cultured hippocampal neurons were recorded at DIV 14 and 15 using the whole‐cell patch‐clamp technique at a holding potential of −70 mV as described previously (Schopf et al., 2021). Patch pipettes were pulled from Borosilicate glass capillaries (Sutter Instrument, model: BF150‐75‐10), fire polished, and had resistances of 2.5–4 MΩ when filled with the following internal solution (in mM): 130 Cs‐methane sulfonate, 10 CsCl, 1 MgCl2, 0.1 CaCl2, 10 HEPES, 2 EGTA, 4 Mg‐ATP (Sigma, cat. no. G8877), and 0.3 Na‐GTP (Sigma, cat. no. A9187), pH 7.2 with KOH (Carl Roth, cat. no. 6751.1). The bath solution contained the following (in mM): 137 sodium chloride (NaCl, Carl Roth, cat. no. 3957.1), 3 potassium chloride (KCl, Carl Roth, cat. no. 6781.3), 10 HEPES, 2 MgCl2, 1.8 CaCl2, 10 glucose, 0.05 DL‐AP5, 0.05 bicuculline, and 0.001 tetrodotoxin (TTX, Tocris, cat. no. 1078) pH 7.4 with NaOH. Currents were recorded with an EPC 10 amplifier, controlled by PatchMaster software (HEKA Elektronik Dr. Schulze GmbH, Germany), and analyzed using Mini analysis software (Symaptosoft, USA). Frequency and average amplitude of mEPSC of each cell were normalized to the corresponding mean value of the control condition for each experiment separately.
2.9. Western blotting
To determine total protein levels of WT and mutated α2δ‐2, whole‐cell lysates (WCL) from tsA201 cells transfected with 2HA‐tagged α2δ‐2 were prepared and immunoblotted. At 48 h after transfection, cells were rinsed with phosphate‐buffered saline (PBS) harvested and resuspended in 200 μL ice‐cold RIPA buffer containing: 50 mM Tris–HCl pH 8.0, 150 mM NaCl, 0.1% SDS, 10 mM NaF, 0.5 mM EDTA, 10% Glycerol, and 1% Igepal, supplemented with protease inhibitor cocktail (Thermo Scientific, cat. no. 87785) and lysed on ice for 30 min. Lysates were then cleared by centrifugation at 13 000 × g for 15 min and assayed for total protein concentration using Pierce BCA protein assay kit (Thermo Scientific, cat. no. 23227). Fifty microgram of proteins were then resuspended in NuPAGE™ LDS Sample Buffer (Invitrogen, cat. no. NP0008), with 50 mM dithiothreitol (DTT, Invitrogen, cat. no. NP0004), and separated on precast gradient polyacrylamide gels (NuPAGE™ 4 to 12%, Bis‐Tris, Thermo Scientific, cat. no. NP0336BOX) and transferred to polyvinylidene fluoride (PVDF) membranes with 0.45 μm pore size (Immobilon, cat. no. IPVH00010). Membranes were blocked with 5% milk in Tris‐buffered saline (10 mM Tris‐Base and 0.85% NaCl) with 0.3% Tween (TBS‐T) for 30 min at RT, followed by incubation with the indicated primary antibody overnight at 4°C. The following day membranes were washed three times with TBS‐T and incubated with secondary antibodies coupled to HRP for 60 min and washed three times for 10 min with TBST‐T. The signal was obtained by HRP reaction with SuperSignal™ West Pico PLUS Chemiluminescent Substrate (Thermo Scientific, cat. no. 34580) and membranes were scanned for protein detection with an Intas NEW‐Line ECL ChemoStar Touch Imager HR 9.0 (for HRP) and subsequent protein quantification was performed with Image Studio Light and Microsoft Excel. The signal intensity of 2HA‐α2δ‐2 was normalized to the signal intensity of the loading internal control GAPDH. Then, the relative signal intensities were normalized to the corresponding WT 2HA‐α2δ‐2 relative signal intensity for each experiment separately.
2.10. Homology modeling
The structural homology model of mouse α2δ‐2 protein was obtained by superposing the predicted AlphaFold human α2δ‐2 (Jumper et al., 2021; Varadi et al., 2022) onto the published cryo‐EM structure of human α2δ‐1 subunit within the CaV2.2 complex (PDB code: 7MIY) using PyMOL (The PyMOL Molecular Graphics System, Version 2.3.2. Schrödinger, LLC, New York, NY, USA). PyMOL was used to prepare the figure.
2.11. Experimental design and statistical analysis
Number of mice used in this study was kept to the minimum necessary for a statistical representative analysis, according to the RRR principle. Where possible, investigators were blinded during experiments and analyses. Three to four independent hippocampal culture preparations were analyzed per experiment and details on cell or bouton numbers are given in the respective figure legends. Electrophysiological recordings in tsA201 cells were obtained from three to four independent experiments (i.e., cell passage and transfection). Details on cell, bouton, or recording numbers are given in the respective figure legends. The distribution of all acquired data was visually assessed using the frequency distribution function of GraphPadPrism 9. In cases where a systematic influence (e.g., tendency to values approaching 0) prevented a normal distribution (e.g., calcium imaging, surface expression analysis in tsA201 cells), statistical analyses were performed on log‐transformed data. All data are shown as mean ± SEM. No test for outliers was conducted and all data points were included in the analysis. Significance levels (p‐values) of statistical tests and post hoc analysis are presented in the respective figure legends. The model in Figure 1 was generated with PyMOL (The PyMOL Molecular Graphics System, Version 2.3.2.). Data, graphs, and figures were organized, analyzed, and assembled using MicrosoftExcel, GraphPadPrism 6, SigmaPlot (Systat Software), Adobe PhotoshopCS6, and Affinity Photo. Data contained within the article, and raw data presented in this study are available upon request.
FIGURE 1.
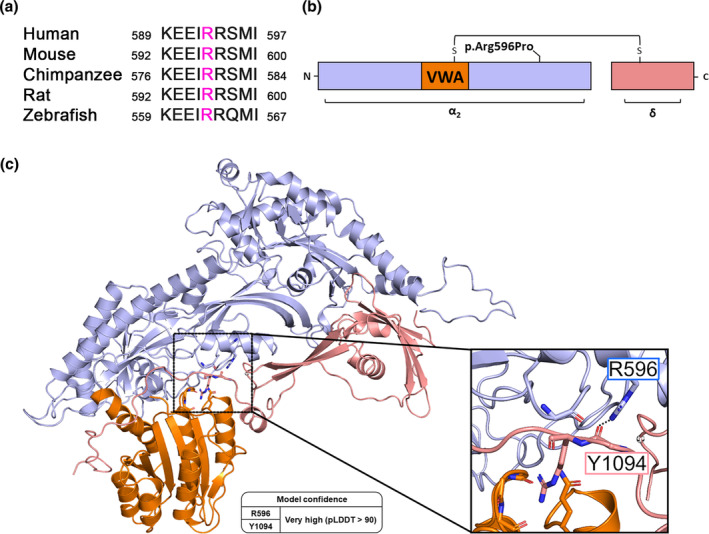
The conserved R596 residue is predicted to have a critical role in stabilizing the interaction between α2 and δ peptides. (a) Sequence alignment between α2δ‐2 proteins from different species. Position corresponding to human arginine 593 (R596 in mouse α2δ‐2) is highlighted in purple. (b) Schematic overview of α2δ‐2 protein illustrating the positions of the von Willebrand factor type A domain (VWA) and the p.R596P mutation. (c) Structural homology modeling of mouse α2δ‐2 protein based on the published Cryo‐EM structure of the human CaV2.2 channel complex (PDB code: 7MIY) predicts a critical interaction of the side chain of R596 (α2 peptide) with the backbone of Y1094 residue (δ peptide) through a hydrogen bond. (Color code: α2 peptide in purple, VWA domain in orange, and δ peptide in pink.) AlphaFold per‐residue model confidence scores (pLDDT) for R596 and Y1094 residues are very high (97.38 and 94.25, respectively).
3. RESULTS
3.1. The evolutionarily conserved arginine 596 (R596) is predicted to be critical for the protein structure of α2δ‐2
Amino acid sequence alignment of α2δ‐2 from different species shows that human arginine 593 (R593) is an evolutionary conserved amino acid (Figure 1a). Human R593 corresponds to mouse R596 and because we used mouse cDNA in our studies, the residue numbering hereafter refers to mouse α2δ‐2. α2δ‐2 is post‐translationally cleaved into α2 and δ peptides that are linked to each other by disulfide bonds and R596 is located within the α2 peptide (Figure 1b). Structural homology modeling predicts an interaction of R596 with tyrosine 1094 (Y1094) within the δ peptide (Figure 1c), suggesting an important role of R596 in maintaining and stabilizing the interaction between the two peptides. Therefore, we hypothesize that R593 is critical for the structural integrity of α2δ‐2 and that mutations at this position may alter protein functions.
3.2. Reduced membrane expression of epitope‐tagged α2δ‐2_R596P upon heterologous expression in tsA201 cells
To study the consequences of the human p.R593P mutation on the protein membrane expression, we introduced the corresponding mutation into the mouse‐coding sequence of α2δ‐2 (p.R596P). HA epitope‐tagged wild‐type (WT) or mutated α2δ‐2 were expressed together with soluble eGFP in tsA201 cells. Anti‐HA live‐cell labeling of WT α2δ‐2 revealed a striking membrane expression identified by a fine‐dotted pattern along the surface of tsA201 cells (Figure 2a, left panel). In comparison to WT α2δ‐2, surface expression of the mutated α2δ‐2_R596P was strongly reduced (Figure 2a,b). Generally, the p.R596P mutation may impair protein folding resulting in accelerated protein degradation. To test this hypothesis, we performed western blot analysis of whole‐cell lysates from tsA201 cells transfected with WT or mutated HA‐tagged α2δ‐2. Overall, α2δ‐2 protein levels were comparable between tsA201 cells transfected with WT or mutated proteins, showing that the p.R596P mutation does not alter protein expression levels (Figure 2c,d). Taken together, these data show a strongly reduced membrane expression of HA‐tagged α2δ‐2_R596P compared to WT α2δ‐2, which is not caused by an altered protein expression level.
FIGURE 2.
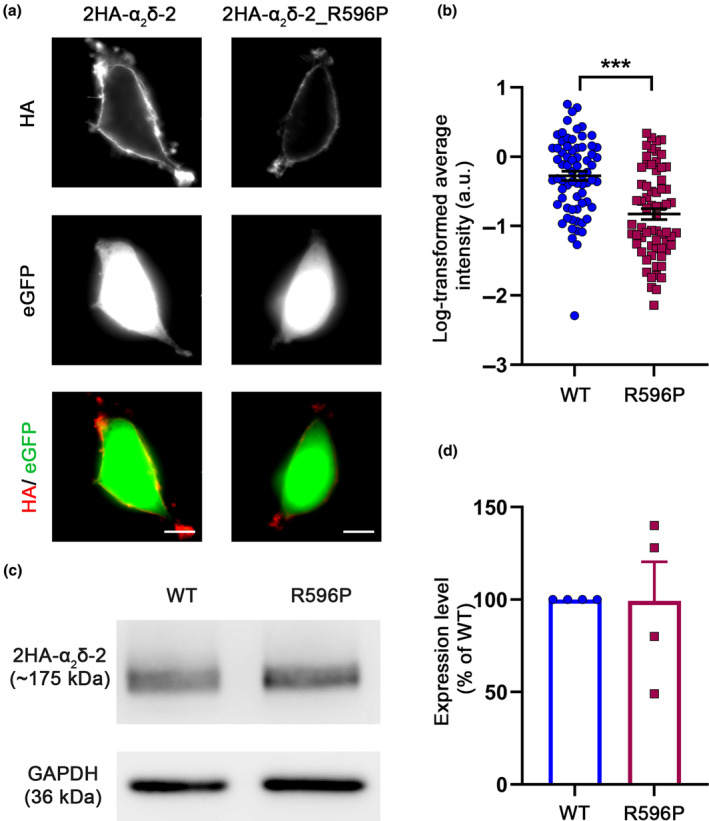
Reduced membrane expression of α2δ‐2_R596P compared to WT α2δ‐2 in tsA201 cells. tsA201 cells were transfected with soluble eGFP together with either HA‐tagged WT or mutated α2δ‐2 (R596P) and were live‐cell labeled with an antibody against the HA epitope. (a) Representative images of anti‐HA live‐cell‐labeled tsA201 cells transfected with 2HA‐α2δ‐2 or 2HA‐α2δ‐2_R596P. Membrane expression of HA‐tagged WT α2δ‐2 is identified by a fine‐dotted pattern along the surface of tsA201 cells. In contrast, dots of 2HA‐α2δ‐2_R596P labeling are sparsely localized on the cell surface, and overall fluorescence intensity is lower. (b) Quantification of α2δ‐2 surface expression. Log‐transformed anti‐HA live‐cell‐staining intensities (arbitrary units) are shown for individual cells (dots) and means ± SEM (lines). Data were obtained from three independent experiments and 69 and 66 cells transfected with WT or mutated HA‐tagged α2δ‐2 were analyzed, respectively. (c) Immunoblot of whole‐cell lysates obtained from tsA201 cells transfected with 2HA‐tagged WT or mutated α2δ‐2. α2δ‐2 protein was detected with an anti‐HA antibody (upper panel), and anti‐GAPDH labeling was used as loading control (lower panel). (d) Quantification of the total protein expression levels of WT and mutated α2δ‐2. Relative total protein expression levels of 2HA‐tagged α2δ‐2_R596P were normalized to WT 2HA‐tagged α2δ‐2 expression levels for each individual experiment (culture preparation). Data were obtained from four independent cell transfections, and values of individual experiments (dots) and mean bars ± SEM (lines) are shown. Statistics: (b) unpaired two‐tailed t‐test, t (133) = 5.4; ***p < 0.0001; (d) unpaired two‐tailed t‐test performed on raw data, t (6) = 0.42; p = 0.69. Scale bars, 10 μm.
3.3. The p.R596P mutation alters calcium current properties of the L‐type channel CaV1.3
Various heterologous co‐expression studies revealed that α2δ subunits increase the current density of CaV1 and CaV2 channels when expressed in combination with a β subunit (reviewed in Davies et al., 2007; Dolphin, 2016). Because the single‐channel conductance was not altered by α2δ subunits (Barclay et al., 2001; Brodbeck et al., 2002), the proposed mechanism underlying the increase in current density is an increase in the number of functionally expressed channels. In addition to the increase in current density, α2δ proteins have been shown to modulate the kinetics and voltage‐dependent properties of Ca2+ currents depending on the α1 subunit isoform (Obermair et al., 2005; Tuluc et al., 2007). Hence, we next analyzed whether and how the p.R596P mutant with its strongly reduced membrane expression affects the channel‐dependent functions of α2δ‐2. Because α2δ‐2 is predominantly expressed in cerebellar Purkinje cells and inner hair cells of the cochlea, we focused on the most likely CaV subunit partners of α2δ‐2 in these cells, CaV2.1 (cerebellum) and CaV1.3 (inner hair cells) (Fell et al., 2016; Schlick et al., 2010). Moreover, these two CaV channel isoforms have a distinct subcellular distribution in neurons of the CNS. While CaV1.3 channels are predominantly localized at postsynaptic sites (Stanika et al., 2016), CaV2.1 channels are located at presynaptic terminals (Etemad et al., 2014). Hence, studying the consequences of the p.R596P mutation on these CaV channel isoforms provides insight into the involvement of pre‐ and postsynaptic compartments in the pathophysiology of p.R596P variant. We first studied the current properties of the L‐type channel CaV1.3 by performing whole‐cell patch‐clamp recordings in tsA201 cells transfected with CaV1.3 and β3 without (control) or together with WT or mutated α2δ‐2. As previously reported, the presence of α2δ‐2 significantly increased CaV1.3 current amplitudes and left shifted the voltage dependence of activation (Figure 3a–e). More importantly, co‐expression of α2δ‐2_R596P failed to increase the current density of CaV1.3 to WT levels and prevented the left shift in the current–voltage relationship (Figure 3d; Table 3).
FIGURE 3.
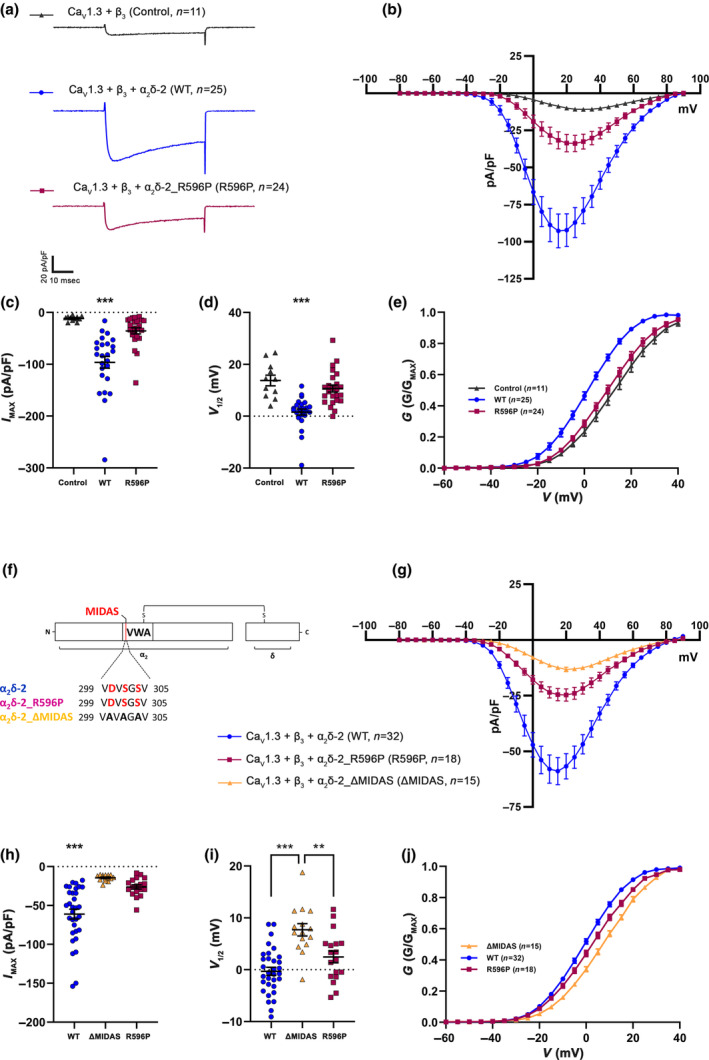
p.R596P reduces current density and alters voltage dependence of activation of the L‐type Ca2+ channel CaV1.3. (a–e) Ca2+ current properties of CaV1.3 channels recorded from tsA201 cells transfected with CaV1.3 and β3 alone (control, gray triangles) or together with wild‐type (WT, blue circles) or mutated α2δ‐2 (R596P, pink rectangles). Fifty msec test pulses from a holding potential of −80 mV to +90 mV were applied in 5 mV increments. Representative whole‐cell Ca2+ current traces at V MAX (a), current–voltage relationships (b), peak current densities (c), and half‐maximal activation potentials (d) of the respective experimental conditions. (e) Fractional activation of the total CaV1.3 channel populations of the respective experimental conditions. Statistics: One‐way ANOVA with Tukey's multiple‐comparison test was performed on 11–25 recordings per condition; (c) maximal current density: F (2,57) = 21.3; p < 0.0001. P‐values of the post hoc test for the respective pairwise comparisons: P < 0.0001 for control versus WT, p = 0.26 for control versus R596P, and p < 0.0001 for WT versus R596P. (d) Half‐maximal activation potential: F (2,57) = 19.1; p < 0.0001. P‐values of the post hoc test for the respective pairwise comparisons: P < 0.0001 for control versus WT, p = 0.38 for control versus R596P, and p < 0.0001 for WT versus R596P. Recordings were obtained from three independent experiments. (f) Schematic representation of the different α2δ‐2 constructs used in this experiment. The MIDAS motif (highlighted in red) in the α2δ‐2_ΔMIDAS construct is mutated to alanines. (g–j) Current properties of CaV1.3 channels recorded from tsA201 cells co‐transfected with CaV1.3 and β3 together with α2δ‐2_ΔMIDAS (ΔMIDAS, orange triangles), wild‐type α2δ‐2 (WT, blue circles), and α2δ‐2_R596P (R596P, pink rectangles). Current–voltage relationships (g), peak current densities (h), and half‐maximal activation potentials (i) of the respective experimental conditions. (j) Fractional activation of the total CaV1.3 channel population of the respective experimental conditions. Statistics: One‐way ANOVA with Tukey's multiple‐comparison test was performed on 15–32 recordings per condition. (h) Maximal current density: F (2,62) = 20.1; p < 0.0001. P‐values of the post hoc test for the respective pairwise comparisons: P < 0.0001 for ΔMIDAS versus WT, p = 0.40 for ΔMIDAS versus R596P, and p < 0.0001 for WT versus R596P. (i) Half‐maximal activation potential: F (2,62) = 15.8; p < 0.0001. P‐values of the post hoc test for the respective pairwise comparisons: P < 0.0001 for ΔMIDAS versus WT, p = 0.005 for ΔMIDAS versus R596P, and p = 0.10 for WT versus R596P. Recordings were obtained from four independent experiments. Significances of post hoc tests between conditions are indicated in the graphs by asterisks (***p < 0.001, **p < 0.01).
TABLE 3.
Current properties of CaV1.3 in tsA201 cells.
| CaV1.3/β3 | CaV1.3/β3 | |||||
|---|---|---|---|---|---|---|
| Control | WT | R596P | ΔMIDAS | WT | R596P | |
| Current density (pA/pF) | −11.7 ± 1.5 | −96.2 ± 11.3 | −35.5 ± 5.8 | −14.3 ± 1.0 | −61.0 ± 6.3 | −26.2 ± 2.7 |
| V 1/2 (mV) | 13.7 ± 2.0 | 1.5 ± 1.1 | 10.6 ± 1.3 | 7.7 ± 1.1 | −0.3 ± 0.7 | 2.4 ± 1.1 |
| V rev (mV) | 80.0 ± 1.4 | 70.3 ± 0.8 | 75.8 ± 1.0 | 71.1 ± 1.5 | 66.7 ± 0.6 | 68.2 ± 0.8 |
| n | 11 | 25 | 24 | 15 | 32 | 18 |
Note: All values are presented as mean ± SEM and were obtained from >3 independent experiments. V 1/2 and V rev parameters were obtained by fitting the I–V curves to a Boltzmann function. V 1/2, Half‐maximal activation potential; V rev, extrapolated reversal potential; and n, number of recordings. For statistics, see Figure 3.
In theory, two mechanisms can contribute to the observed reduction in current density obtained when α2δ‐2_R596P was co‐expressed with CaV1.3. Firstly, the p.R596P mutant may fail to support membrane trafficking of the channel complex. Secondly, the right shift in the current–voltage relationship may reduce the driving force at more positive potential, which is a known mechanism of α2δ protein action on L‐type calcium channels (Obermair et al., 2008; Tuluc et al., 2007). Similar half‐maximal activation potential obtained for cells expressing α2δ‐2_R596P or no α2δ (control) supports a failure in membrane trafficking. Hence, to test if CaV1.3 channels are still partly modulated by α2δ‐2_R596P, we compared the current properties with CaV1.3 channels co‐expressed with an α2δ‐2_ΔMIDAS (DxSxS motif is mutated to alanines) deletion construct. The metal ion‐dependent adhesion site (MIDAS) within the VWA domain of α2δ‐1 and α2δ‐2 has been previously shown to play a key role in channel trafficking. More precisely, mutating the MIDAS motif results in ER retention of α2δ proteins and thereby completely abolishes their ability to enhance membrane expression (Canti et al., 2005; Hoppa et al., 2012). Mean current density of channels co‐expressed with p.R596P was not significantly different from that of channels co‐expressed with α2δ‐2_ΔMIDAS (Figure 3h; Table 3). However, there was a statistically significant difference in the half‐maximal activation potential (Figure 3i; Table 3). Moreover, in 62% of cells co‐transfected with α2δ‐2_ΔMIDAS, no current could be detected. In contrast, only 35% and 13% of cells co‐transfected with p.R596P and WT α2δ‐2, respectively, showed no current [n numbers: α2δ‐2_ΔMIDAS (45), α2δ‐2_ R596P (40), and WT (53)]. Together, these data suggest that in contrast to α2δ‐2_ΔMIDAS, α2δ‐2_R596P proteins, despite showing a strongly reduced membrane expression, can still interact and modulate CaV1.3 channels at a very basic level.
3.4. The p.R596P mutation does not compromise current properties of the P/Q‐type channel CaV2.1
We next tested the consequences of the p.R596P mutation on the biophysical properties of Ca2+ currents mediated by the P/Q‐type channel CaV2.1. Compared to the control condition without α2δ‐2, co‐expression of CaV2.1 and β4 together with the p.R596P mutant resulted in a fivefold increased current density. This increase was basically indistinguishable from the positive control group (co‐expression of WT α2δ‐2, Figure 4a–e; Table 4), suggesting that CaV2.1 function is not compromised by the mutation. However, because CaV2.1 channels showed an upper limit of membrane expression in superior cervical ganglion neurons (Scott & Kammermeier, 2017), small changes in current densities may be masked. This prompted us to repeat the analysis of P/Q‐type currents in tsA201 cells transfected with a molar ratio of 1:0.2 (α1:α2δ‐2) instead of 1:1. However, also the low amount of α2δ‐2 cDNA used in these transfections neither resulted in a reduced CaV2.1 current density upon co‐expression of WT α2δ‐2, or upon co‐expression of mutated α2δ‐2 (Figure 4f–j; Table 4). Together this shows that α2δ‐2_R596P, despite showing an extremely low membrane expression, is still able to increase the functional membrane expression of CaV2.1 channels.
FIGURE 4.
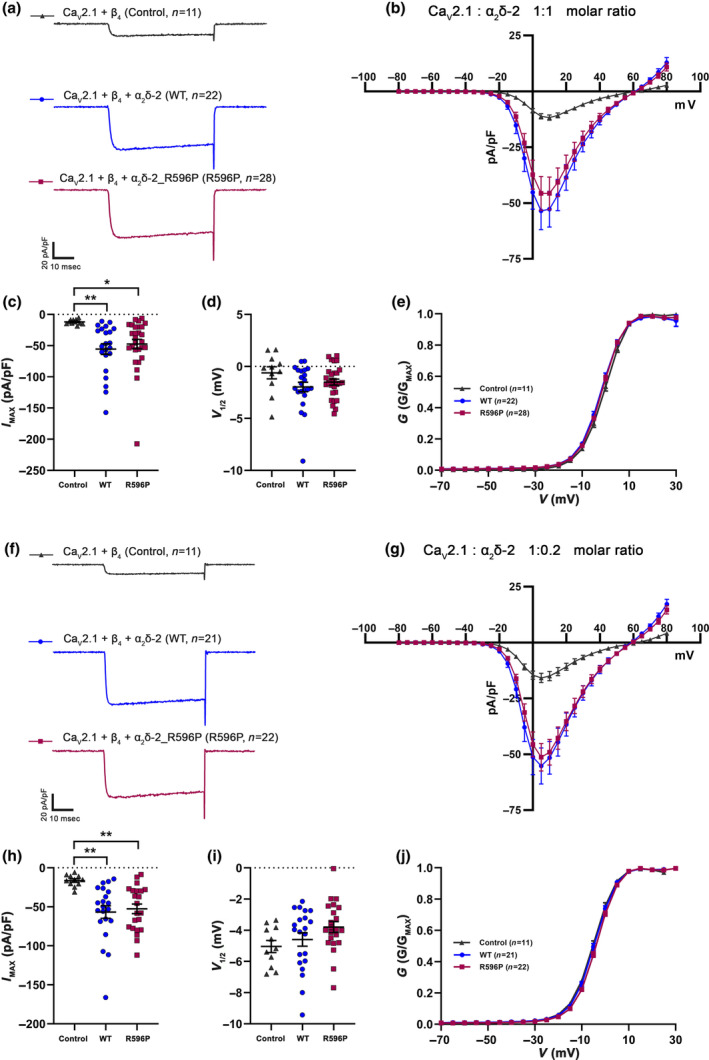
The p.R596P mutation does not compromise the current properties of the P/Q‐type channel CaV2.1. (a–j) Current properties of CaV2.1 recorded from tsA201 cells transfected with CaV2.1 and β4 alone as a control (control, gray triangles), together with WT (WT, blue circles), or mutated α2δ‐2 (R596P, pink rectangles). (a–e) Recordings obtained from tsA201 cells transfected with the three subunits CaV2.1/β4/α2δ‐2 in a molar ratio of 1:1:1, while (f–j) are recordings obtained from tsA201 cells transfected with the three subunits in a molar ratio of 1:1:0.2. Fifty msec test pulses from a holding potential of −80 mV to +80 mV were applied in 5 mV increments. (a, f) Representative whole‐cell Ca2+ current traces at VMAX. Current–voltage relationships (b, g), mean peak current densities (c, h), and mean half‐maximal activation potentials (d, i). Statistics: (c, d) One‐way ANOVA with Tukey's multiple‐comparison test was performed on 11–28 recordings per condition obtained from three independent experiments. (c) Maximal current density, F (2,58) = 5.5; p = 0.0067. P‐values of the post hoc test for the respective comparisons: P = 0.01 for control versus WT, p = 0.02 for control versus R596P, and p = 0.72 for WT versus R596P. (d) Half‐maximal activation potential, F (2,58) = 2.0; p = 0.15. P‐values of the post hoc test for the respective comparisons: P = 0.12 for control versus WT, p = 0.37 for control versus R596P, and p = 0.65 for WT versus R596P. (h, i) One‐way ANOVA with Tukey's multiple‐comparison test was performed on 11–22 recordings per condition obtained from three independent experiments. (h) Maximal current density, F (2,51) = 7.4; p = 0.0016. P‐values of the post hoc test for the respective comparisons: P = 0.002 for control versus WT, p = 0.005 for control versus R596P, and p = 0.89 for WT versus R596P. (i) Half‐maximal activation potential, F (2,51) = 2.2; p = 0.117. P‐values of the post hoc test for the respective comparisons: P = 0.76 for control versus WT, p = 0.13 for control versus R596P, and p = 0.29 for WT versus R596P. Significances of post hoc tests between conditions are indicated in the graphs by asterisks (**p < 0.01, *p = 0.02).
TABLE 4.
Current properties of CaV2.1 in tsA201 cells.
| CaV 2.1/β4/α2δ‐2 (1:1:1 molar ratio) | CaV 2.1/β4/α2δ‐2 (1:1:0.2 molar ratio) | |||||
|---|---|---|---|---|---|---|
| Control | WT | R596P | Control | WT | R596P | |
| Current density (pA/pF) | −11.9 ± 1.1 | −55.5 ± 8.5 | −47.4 ± 7.6 | −16.3 ± 2.2 | −56.6 ± 8.0 | −52.5 ± 6.1 |
| V 1/2 (mV) | −0.6 ± 0.5 | −1.9 ± 0.4 | −1.5 ± 0.2 | −5.0 ± 0.3 | −4.5 ± 0.4 | −3.8 ± 0.3 |
| V rev (mV) | 47.0 ± 0.5 | 44.7 ± 0.3 | 46.0 ± 0.3 | 44.4 ± 1.0 | 44.8 ± 0.4 | 45.7 ± 0.4 |
| n | 11 | 22 | 28 | 11 | 21 | 22 |
Note: All values are presented as mean ± SEM and were obtained from three independent experiments. V 1/2 and V rev parameters were obtained from fitting the I–V curves to a Boltzmann function. V 1/2, half maximal activation potential; V rev, extrapolated reversal potential; n, number of recordings. For statistics see Figure 4.
3.5. Strongly reduced neuronal surface expression and presynaptic targeting of epitope‐tagged α2δ‐2_R596P
So far, we studied the functional consequences of the p.R596P mutation in α2δ‐2 on the membrane expression and Ca2+‐channel properties upon heterologous expression in tsA201 cells. While this expression system is ideally suited to study the consequences of the mutated α2δ‐2 protein on distinct α1 subunits and in the absence of endogenously expressed auxiliary subunits, it is essential to investigate the behavior and the consequences of the mutated protein in its native environment, namely neurons of the CNS. Therefore, we homologously expressed HA epitope‐tagged WT or mutated α2δ‐2 together with soluble eGFP in mouse cultured hippocampal neurons. Live‐cell immunostaining revealed that, similar to heterologous expression, α2δ–2_R596P showed a strongly reduced surface expression compared to WT α2δ‐2, in all three main neuronal compartments, the soma, dendrites, and axons (Figure 5a).
FIGURE 5.
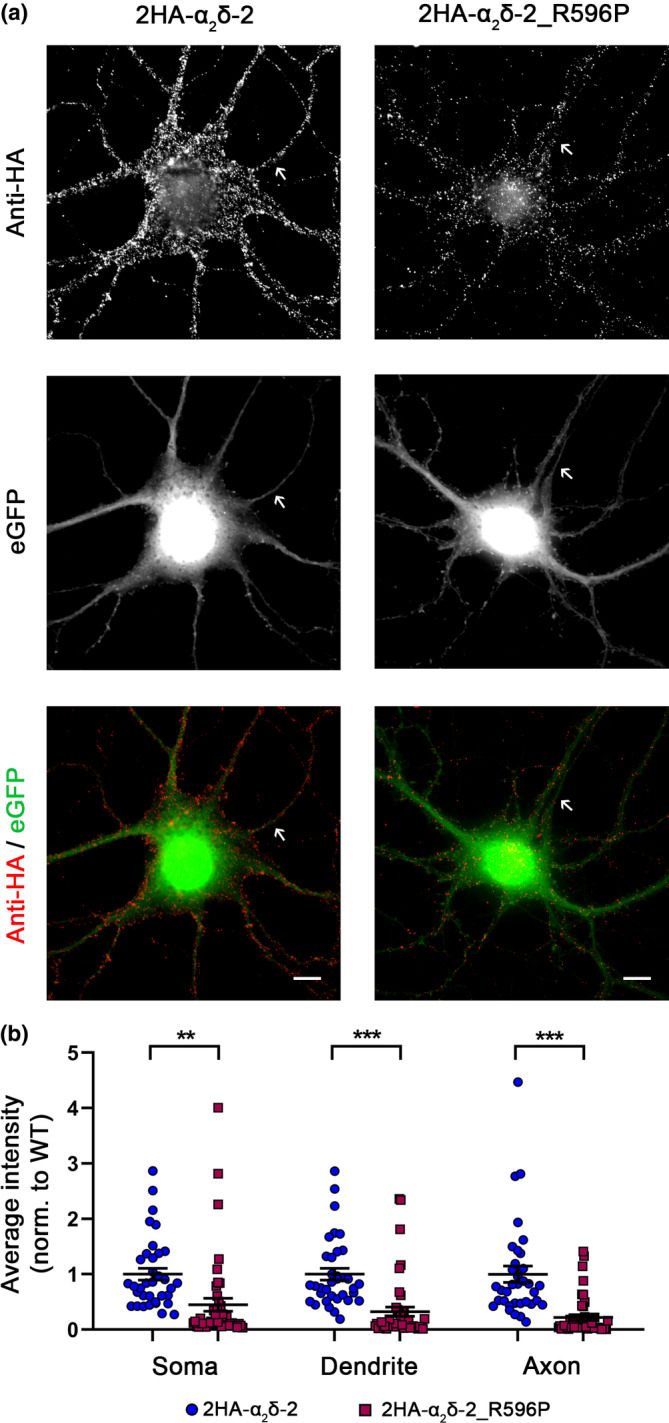
Strongly reduced membrane expression of α2δ‐2_R596P in differentiated cultured hippocampal neurons. (a) Representative examples of primary cultured hippocampal neurons transfected with soluble eGFP together with either HA‐tagged WT (2HA‐α2δ‐2) or mutated (2HA‐α2δ‐2_R596P) α2δ‐2. Anti‐HA live‐cell labeling demonstrates a reduced staining intensity of α2δ‐2_R596P in the soma, dendrites, and the axon (indicated by an arrow) compared to WT α2δ‐2. (b) Quantification of the average HA fluorescent intensities in the three compartments shows that the surface expression of mutated α2δ‐2 is strongly reduced compared to WT α2δ‐2. Graphs show values for individual cells (dots) and mean ± SEM (lines). All values were normalized to the mean of the WT 2HA‐α2δ‐2 fluorescence intensity within each culture preparation. Data were obtained from three independent culture preparations; 35 and 45 cells expressing WT or mutated HA‐tagged α2δ‐2 were analyzed, respectively. Statistics: Unpaired t‐test, soma: T (78) = 3.4; **p = 0.0011, dendrite: T (78) = 5.1; ***p < 0.0001, axon: T (78) = 5.5; ***p < 0.0001. Scale bars, 10 μm.
As α2δ‐2 is particularly expressed in presynaptic terminals (Geisler et al., 2019), we next analyzed the presynaptic localization of α2δ‐2_R596P. To this end, presynaptic boutons were identified by the clustering of synapsin protein within axonal varicosities as visualized by the eGFP fluorescence. Analysis of presynaptic boutons expressing HA‐tagged α2δ‐2 proteins revealed a presynaptic localization of α2δ‐2_R596P, similar to WT α2δ‐2 (Figure 6a), as indicated by the colocalization and the line scan analysis of the 2HA‐α2δ‐2 (red), synapsin (blue), and eGFP (green) staining pattern of representative images (Figure 6b). However, quantitative analysis revealed that, compared to WT α2δ‐2, overall presynaptic targeting of α2δ‐2_R596P was strongly reduced (Figure 6d).
3.6. Homologous expression of WT or R596P‐mutated α2δ‐2 differentially affects presynaptic differentiation
The unaltered CaV2.1 current observed when p.R596P was co‐expressed with the channel in tsA201 cells (Figure 4) suggests that despite the strong reduction in membrane expression, the p.R596P mutant was still able to traffic CaV2.1 channels to the plasma membrane. However, and as discussed above, CaV2.1 channel membrane expression ceiling may obscure small differences in channel modulation. It has previously been shown that homologous expression of α2δ‐1 in cultured neurons increases synaptic CaV2.1 channel clustering (Ablinger et al., 2022; Hoppa et al., 2012). Here, we show that WT α2δ‐2 similarly induces an increase in endogenous CaV2.1 clustering at presynaptic terminals compared to control neurons expressing eGFP alone (Figure 7a,c). This observation allowed us to test, whether this property of α2δ‐2 is compromised by the mutation. In contrast to WT α2δ‐2, α2δ‐2_R596P expression resulted only in a slight but not significant increase in presynaptic CaV2.1 expression (Figure 7c), which is neither different from control (eGFP alone) nor from WT α2δ‐2 (WT). Surprisingly, however, homologous expression of α2δ‐2_R596P significantly reduced presynaptic synapsin clustering, which was not observed in the control groups (eGFP or WT α2δ‐2; Figure 7d). The reduction in synapsin expression was not the consequence of an altered bouton size, as evidenced by quantitative analysis of the size of eGFP‐positive varicosities (Figure 7e). Moreover, the decrease in presynaptic synapsin clustering suggests that the p.R596P mutant, despite its low membrane expression, competes with endogenously expressed α2δ proteins (α2δ‐1 to ‐3), thereby mediating a dominant‐negative effect resulting in defective presynaptic differentiation.
3.7. The p.R596P mutation affects the ability of α2δ‐2 to increase presynaptic calcium transients
Homologous expression of α2δ‐2_R596P fails to induce a significant increase in presynaptic CaV2.1 channel abundance. This is in contrast to the unaltered biophysical current properties (confer Figure 4) and hence suggests a mild disruption in the interaction of α2δ‐2 with CaV2.1. Moreover, the decrease in presynaptic synapsin clustering is a sign of defective presynaptic differentiation, which, in theory, can also affect the expression of other presynaptic CaV channel isoforms. Therefore, we next tested whether and to what extent presynaptic Ca2+ transients were affected by WT or R596P α2δ‐2. To this end, cultured hippocampal neurons were transfected with the genetically encoded GCaMP6f Ca2+ indicator coupled to synaptophysin [SynGCaMPF6f; Brockhaus et al., 2019)], together with soluble mCherry (Figure 8a). Comparable synaptic expression of GCaMP6f across conditions was confirmed by analyzing the fluorescent signal intensity in synaptic boutons at baseline (control: 40.37 ± 2.59, WT: 35.31 ± 0.86, R596P: 38.35 ± 1.54; one‐way ANOVA with Tukey's multiple‐comparison test: F (2,65) = 2.14, p = 0.13). Consistent with previous findings, WT α2δ‐2 increased presynaptic peak Ca2+ amplitudes in response to 1 and 10 action potentials (AP) triggered by field stimulation at a frequency of 50 Hz (Figure 8b–e). This was evident when plotting the mean traces (Figure 8b,d) and by analyzing the peak Ca2+ signals of all individual cells (Figure 8c,e). Surprisingly, the p.R596P mutant also increased presynaptic peak Ca2+ amplitudes similar to WT α2δ‐2 in response to 1 AP (Figure 8b,c). However, in response to 10 Aps, the mean peak Ca2+ amplitude of the p.R596P condition is lower, albeit not significantly different, compared to the WT α2δ‐2 condition. Moreover, and in contrast to WT α2δ‐2, the mean peak amplitude was only approaching statistically significant difference from control neurons (Figure 8b–e). This suggests that homologous expression of α2δ‐2_R596P less efficiently enhances presynaptic Ca2+ transients than expression of WT α2δ‐2.
FIGURE 8.
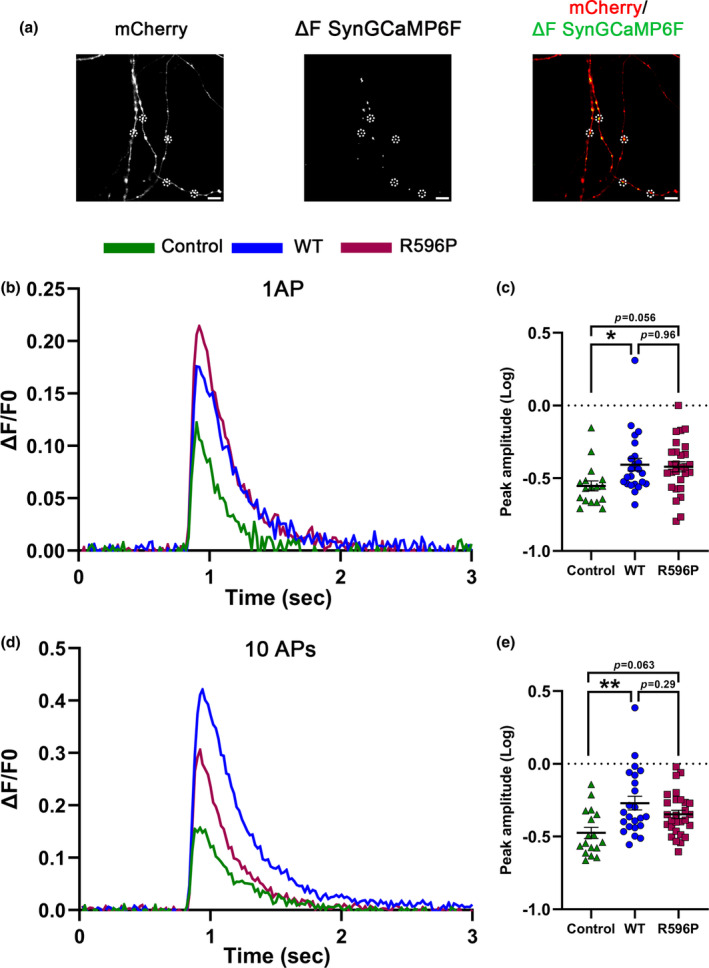
Presynaptic Ca2+ transients of neurons expressing WT α2δ‐2 and α2δ‐2_R596P in comparison with control neurons. (a) Fluorescent micrographs of representative presynaptic varicosities identified by the mCherry fluorescence. The presynaptic Ca2+ signal (ΔSynGCaMP6f) was calculated by subtracting the control SynGCaMP6f fluorescence at baseline from the SynGCaMP6f fluorescence at the maximal response. Scale bar 5 μm. Mean fluorescence traces (b, d), and quantification of the peak fluorescence (c, e) of presynaptic Ca2+ signals (ΔF/F of SynGCaMP6f) elicited by 1 action potential (AP), or 10 APs at 50 Hz stimulations, in cultured hippocampal neurons co‐transfected with SynGCaMP6f and mCherry alone (control, green, triangles) or together with either WT (WT, blue, circles) or mutated α2δ‐2 (R596P, pink, rectangles). (b, d) Lines show the mean fluorescence traces from 17 to 28 cells per condition from four independent culture preparations. Quantification of peak fluorescent amplitudes (log) in response to stimulations with 1 (c) or 10 APs (e) shows values for individual cells (symbols) and means ± SEM (lines). Each symbol represents the mean peak fluorescence signal of 20 synapses measured from one neuron. Statistics: One‐way ANOVA with Tukey's multiple‐comparison test: 1 AP: F (2,65) = 3.6, p = 0.038. P‐values of the post hoc test for the respective pairwise comparisons: P = 0.03 for control versus WT, p = 0.06 for control versus R596P, and p = 0.96 for WT versus R596P. 10 AP: F (2,65) = 6.2, p = 0.003. P‐values of the post hoc test for the respective pairwise comparisons: P = 0.002 for control versus WT, p = 0.06 for control versus R596P, and p = 0.28 for WT versus R596P. Significances of post hoc tests between conditions are indicated in the graphs by asterisks (**p < 0.01, *p = 0.038).
3.8. Trans‐synaptic signaling of α2δ‐2_R596P
Presynaptic over‐expression of a splice variant of α2δ‐2 lacking exon 23 (E23) in hippocampal neurons has previously been shown to induce aberrant axonal wiring resulting in a mismatched localization of postsynaptic GABAA receptors (GABAAR) opposite glutamatergic nerve terminals (Geisler et al., 2019). E23 is located downstream of R596 and codes for eight amino acids following residue 664. Structural homology modeling suggested that exclusion of this exon leads to the formation of an α‐helix (Geisler et al., 2019). To test whether introduction of the p.R596P mutation alters the trans‐synaptic function of α2δ‐2, we quantified the expression levels of postsynaptic GABAAR opposite mismatched vGLUT1‐containing presynaptic boutons upon homologous over‐expression of WT or mutated α2δ‐2_ΔE23 compared to neurons expressing eGFP alone as control. As shown previously (Ablinger et al., 2022; Geisler et al., 2019), WT α2δ‐2_ΔE23 induces clustering of postsynaptic GABAAR opposite vGLUT1‐positive terminals (Figure 9a,c, α2δ‐2 and WT, respectively). Neurons transfected with α2δ‐2_ΔE23_R596P were able to recruit GABAARs opposite glutamatergic nerve terminals (Figure 9a,c), however, GABAARs clustering was significantly reduced compared to WT α2δ‐2. This was particularly evident when quantifying the immunofluorescent signal of average fluorescence intensity of postsynaptic GABAARs. Because of the strong reduction in presynaptic expression of the R596P mutant (see above), a reduced trans‐synaptic action was to be expected. However, it is important to note that presynaptic expression is strongly reduced to 31% (confer Figure 6), while the trans‐synaptic recruitment of postsynaptic GABAARs is reduced to only 73% (confer Figure 9). Together this suggests that even small amounts and/or less stably expressed α2δ‐2 proteins can mediate a trans‐synaptic function.
The aberrant wiring and postsynaptic GABAAR recruitment by presynaptic α2δ‐2_ΔE23 was associated with reduced glutamatergic synaptic transmission (Geisler et al., 2019). Hence, to further assess the trans‐synaptic consequences of α2δ‐2_R596P compared to WT α2δ‐2, we recorded and analyzed spontaneous miniature excitatory postsynaptic currents (mEPSCs). To ensure a uniform expression of the transfected α2δ‐2_ΔE23 cDNAs in all neurons (Figure 10a), we here used lentivirus‐mediated transfection. This is in contrast to the above‐mentioned experiments, in which liposomal‐mediated transfection was employed to obtain a low transfection efficiency of isolated presynaptic neurons. As expected for aberrantly wired synapses, both WT and mutated α2δ‐2 decreased the frequency of mEPSCs compared to neurons infected with eGFP‐expressing lentiviral particles only (control) (Figure 10b,d). However, consistent with the observed differences in postsynaptic GABAAR recruitment (confer Figure 9c), WT α2δ‐2 resulted in a larger decrease in mEPSCs frequency (on average reduction by ~50%; mean ± SEM: 0.51 ± 0.05) compared to the mutated α2δ‐2 (on average reduction by ~30%; mean ± SEM: 0.73 ± 0.08). An efficient and synchronized synaptic transmission requires precisely matched and aligned pre‐ and postsynaptic proteins. Hence, our data suggest that the strong reduction in mEPSC frequency is the result of a reduced number of functional synapses caused by the aberrant wiring of glutamatergic boutons with GABAergic postsynaptic sites. Interestingly, however, we also observed differences between the experimental conditions in the amplitude of the mEPSC, which is an indirect measure of postsynaptic AMPA receptor expression. The mean amplitude of mEPSCs recorded in neurons expressing mutated α2δ‐2 was significantly reduced by ~24%. In contrast, we did not observe a reduction in mEPSC amplitudes in neurons infected with WT α2δ‐2. Cumulative frequency distribution of mEPSC amplitudes (Figure 10e) and analysis of all individual mEPSC events (Figure 10f) show an even more prominent reduction of the mEPSC amplitude of neurons with R596P in comparison with control and WT α2δ‐2. Together, this shows that the decrease in mEPSC amplitude was not solely caused by altered wiring, but in the case of the mutated α2δ‐2 protein, it rather reflects an independent underlying mechanism.
FIGURE 10.
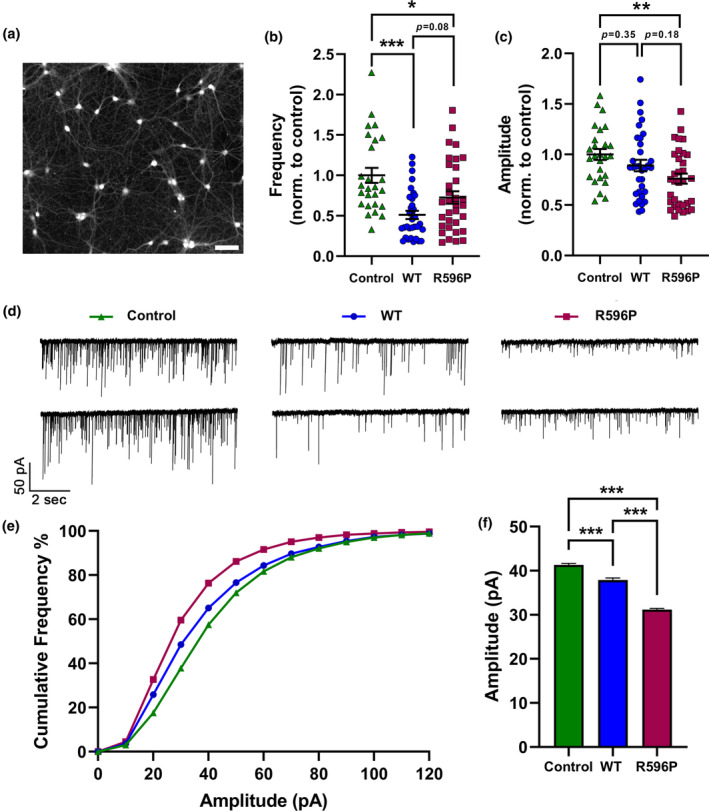
Homologous expression of α2δ‐2_R596P resulted in a greater reduction in spontaneous glutamate‐mediated synaptic transmission. Recordings of mEPSCs in 14–15 DIV cultured hippocampal neurons expressing soluble eGFP alone (control, green triangles), and either WT (WT, blue circles) or mutated (R596P, pink rectangles) α2δ‐2_ΔE23. (a) Representative image of hippocampal neurons at DIV 15 infected with α2δ‐2_R596P showing a ~ 100% infection efficiency. Scale bar, 200 μm. Quantification of mEPSC frequencies (b) and average amplitudes (c). Amplitudes and frequencies of mEPSCs were normalized to the mean value of control condition for each individual experiment. (d) Two representative traces of mEPSCs from independent recordings for all three conditions. (e) Cumulative frequency distribution histograms of all mEPSC amplitudes recorded from neurons shown in the previous panels. Number of analyzed events per condition: Control, n = 6012; WT, n = 3374; R596P, n = 5384. (f) Comparison of mean mEPSC amplitudes of all recorded events. Expression of the R596P mutant was associated with larger reduction in mEPSC amplitude compared to WT α2δ‐2. Statistics: (b, c) One‐way ANOVA with Tukey's multiple‐comparison test was performed on 26–33 recordings per condition from three independent culture preparations. (b) Frequency: F (2,89) = 10.5; p < 0.0001. P‐values of the post hoc test for the respective pairwise comparisons: P < 0.001 for control versus WT, p = 0.03 for control versus R596P, and p = 0.08 for WT versus R596P. (c) Amplitude: F (2,89) = 4.7; p = 0.01. P‐values of the post hoc test for the respective pairwise comparisons: P = 0.35 for control versus WT, p = 0.008 for control versus R596P, and p = 0.18 for WT versus R596P (f) One‐way ANOVA with Tukey's multiple‐comparison test was performed on 3374–6012 mEPSC events, F (2,14767) = 273.6, p < 0.0001. P‐values of the post hoc test for the respective pairwise comparisons: P < 0.0001 for control versus WT, control versus R596P, and WT versus R596P. Significances of post hoc tests between conditions are indicated in the graphs by asterisks (***p < 0.001, **p < 0.01, *p = 0.031).
4. DISCUSSION
Here, we provide a functional characterization of a potential disease‐causing CACNA2D2 mutation, which focuses not only on the Ca2+ channel‐dependent functions of α2δ‐2 but also on synaptic and trans‐synaptic functions of α2δ proteins. The p.R596P mutation decreases membrane expression and synaptic targeting of α2δ‐2. This defect in membrane expression differentially affects L‐type and non‐L‐type Ca2+ channels: while the p.R596P mutation alters current densities and voltage dependence of activation of the postsynaptic L‐type channel CaV1.3 upon heterologous co‐expression, it has no effect on current properties of the presynaptic P/Q‐type channel CaV2.1. However, in comparison with WT α2δ‐2 expression, α2δ‐2_R596P in hippocampal neurons did not significantly increase the presynaptic abundance of endogenously expressed CaV2.1 channels and limited its capacity to increase presynaptic Ca2+ transients, even though there was no difference between the means of WT and R596P α2δ‐2. Despite the low membrane expression, α2δ‐2_R596P could still mediate trans‐synaptic signaling to postsynaptic GABAARs. Finally, homologous expression of mutated α2δ‐2 results in a defective presynaptic differentiation indicated by decreased presynaptic synapsin clustering, and altered postsynaptic differentiation indicated by reduced mEPSCs amplitudes.
4.1. R596 is critical for membrane expression of α2δ‐2
α2δ proteins, in their capacity as Ca2+ channel subunits, play an important role in membrane trafficking of the channel complex. However, α2δ proteins can also reach the cell surface independently of the VGCC complex (Canti et al., 2005; Cassidy et al., 2014; Schredelseker et al., 2005). Structural homology modeling of α2δ‐2 predicts one critical interaction of the highly conserved R596 with Y1094 within the δ peptide. Hence, R596 is predicted to maintain the interaction between α2 and δ peptides and thereby stabilize the whole structure of α2δ‐2. Indeed, α2δ‐2_R596P shows a reduced membrane expression both upon heterologous and homologous expression. Correct folding of membrane proteins in the endoplasmic reticulum (ER) following translation is crucial for the forward trafficking to the cell surface and aberrantly folded proteins are retained in the ER and may be subject to degradation (Vembar & Brodsky, 2008). However, this seems not to be the case for α2δ‐2_R596P as total protein expression was not compromised by the mutation. Hence, the reduced membrane expression suggests that the p.R596P mutation either affects forward trafficking from the ER and/or increases the internalization rate caused by destabilization of the protein on the cell surface.
4.2. The p.R596P mutation uncovers isoform‐ and cell‐type‐specific differences in α2δ‐2‐mediated functions
All α2δ isoforms augment the Ca2+ currents of all CaV1 and CaV2 channels with little effect on the single‐channel conductance (Barclay et al., 2001; Tuluc et al., 2007), implying that α2δ proteins mediate the current enhancement by mainly increasing the functional membrane expression of the channel complexes. While the exact underlying mechanisms remain elusive, a crucial role of the MIDAS motif within the VWA domain in membrane trafficking of the α1 subunit seems likely (Canti et al., 2005). Our present data show that p.R596P differently affects the ability of α2δ‐2 to enhance Ca2+ currents of L‐type (CaV1) and non‐L‐type (CaV2) channels upon heterologous co‐expression. While the p.R596P mutation significantly reduced the current density and shifted the voltage dependence of activation to more depolarized potentials of CaV1.3 channels, it did not affect the biophysical properties of CaV2.1 channels. This differential effect could be attributed to two mechanisms: differences in the affinity between α2δ‐2 and α1 isoforms (Voigt et al., 2016) or membrane expression ceiling effects of CaV2 channels (Scott & Kammermeier, 2017). The fact that CaV2.1 α1, α2δ‐2, and β4 are the dominating isoforms expressed in the cerebellum and that their expression levels co‐increase during neuronal differentiation (Schlick et al., 2010) indicates endogenous complexing of these subunits. We can speculate that in contrast to CaV1.3, the affinity between CaV2.1 and α2δ‐2 could be strong enough to tolerate the p.R596P mutation as well as its reduced membrane expression. As CaV2 channels possess an upper limit of membrane expression (Cao et al., 2004; Scott & Kammermeier, 2017), it seems more likely that slight changes in current densities may be masked by this property. To test this, we reduced the amount of transfected α2δ‐2 WT and mutated cDNA by fivefold. However, also under this experimental paradigm, co‐expression of α2δ‐2_R596P did not reduce the current density of CaV2.1 channels. This finding suggests that the mutation does not impede membrane trafficking of CaV2.1 channels in a heterologous expression system, and hence, it differentially affects L‐type and non‐L‐type Ca2+ channels.
Because surface expression of α2δ‐2_R596P was also strongly reduced in hippocampal neurons, we next tested the consequence of the mutation on endogenous CaV2.1 channels in their native neuronal environment. An increase in presynaptic CaV2.1 clustering and presynaptic Ca2+ transients is a hallmark of homologous expression of α2δ isoforms in wild‐type hippocampal neurons (Ablinger et al., 2022; Schopf et al., 2021). In contrast to heterologous co‐expression, α2δ‐2_R596P did not increase presynaptic clustering of endogenous CaV2.1 channels and presynaptic Ca2+ transients to the same extent as WT α2δ‐2, whereby the means of WT and mutated α2δ‐2 were not significantly different. Altered modulation of endogenous CaV2.1 by mutated α2δ‐2 in synapses of differentiated hippocampal neurons uncovers cell‐type‐specific modulation by α2δ proteins which cannot be reconstituted in a heterologous expression system. Together, this suggests that modulation of Ca2+ channels by α2δ‐2 proteins is not limited to α1/α2δ‐2 protein–protein interactions but also involves the local macromolecular environment of a specialized cellular compartment such as the presynaptic terminal. Moreover, this finding highlights the importance of studying the mechanistic consequences of disease‐associated mutations within their specific and native cellular environment.
4.3. The p.R596P mutation affects pre‐ and postsynaptic differentiation by distinct mechanisms
α2δ‐2_R596P showed a reduced, albeit still functional, trans‐synaptic recruitment of postsynaptic GABAAR, a recently identified trans‐synaptic function of α2δ‐1 and α2δ‐2 splice variants in both GABAergic and glutamatergic synapses (Ablinger et al., 2022; Geisler et al., 2019). It is still elusive whether this trans‐synaptic function of α2δ is mediated by a direct trans‐synaptic interaction or indirectly by the interaction with other synaptic organizers. On the one hand, the reduced trans‐synaptic function of α2δ‐2_R596P together with its reduced surface expression point toward a direct trans‐synaptic interaction. On the other hand, the fact that the effect on trans‐synaptic GABAAR recruitment is much smaller than the reduction in presynaptic membrane expression of α2δ‐2_R596P indicates that even small amounts and/or less stably expressed α2δ‐2 proteins are sufficient for trans‐synaptic signaling. Even though the trans‐synaptic recruitment of GABAARs and its consequence on synaptic transmission were observed in a non‐physiologic experimental paradigm, namely the over‐expression in glutamatergic neurons, it may provide important insights into potential physiological roles of α2δ‐2. Purkinje cells have a single long axon that forms an inhibitory projection to the cerebellar nuclei (Paul & Limaiem, 2023) and they strongly express α2δ‐2 mRNA (Barclay et al., 2001). Hence, modulating the trans‐synaptic signaling with postsynaptic GABAAR, for example, by the p.R596P mutation of α2δ‐2, may lead to a decreased functionality of this inhibitory GABAergic projection. Subsequently, this can lead to an excitatory–inhibitory imbalance which may ultimately underlie the epileptic phenotype observed in the patients.
Recent findings suggest a pivotal and highly redundant role of presynaptic α2δ proteins in the formation and differentiation of glutamatergic synapses in CNS neurons (Ablinger et al., 2022; Schopf et al., 2021). Our present data show that expression of α2δ‐2_R596P in WT neurons endogenously expressing three α2δ isoforms (α2δ‐1‐3) affects pre‐ and postsynaptic differentiation as indicated by reduced presynaptic synapsin clustering and smaller mEPSC amplitudes. This phenotype, although milder, resembles the defect in synaptic differentiation observed in α2δ triple‐knockout/knockdown neurons (Schopf et al., 2021). α2δ proteins homologously expressed in hippocampal neurons must compete with endogenously expressed α2δ isoforms. Hence, the altered consequences of expressing mutated α2δ‐2 in comparison with WT α2δ‐2, such as defective pre‐ and subsequently postsynaptic differentiation, indicate a dominant‐negative effect. Even more so as total neuronal surface expression is strongly affected by the p.R596P mutation. A similar situation may occur in the patients harboring the recessive p.R596P mutation: since α2δ‐2 is the dominating cerebellar isoform and both patients were found to be homozygous for the mutation, synapse differentiation may be affected by the dominant negative behavior of the mutated α2δ‐2 protein in competition with the remaining endogenous α2δ isoforms 1 and 3.
The negative effect of homologous α2δ‐2_R596P expression on the amplitudes of mEPSCs is an interesting observation by itself. The reduced synapsin content upon expression of mutated α2δ‐2 may indicate a defect in presynaptic vesicle recruitment and hence may contribute to a smaller amount of neurotransmitter release in response to spontaneous Ca2+ influx, which leads to a reduced response of postsynaptic AMPA receptor. Alternatively and given the pivotal role of presynaptic α2δ for both pre‐ and postsynaptic differentiation and the identified trans‐synaptic functions of α2δ isoforms (Ablinger et al., 2022; Fell et al., 2016; Geisler et al., 2019), p.R596P may exert its deleterious effect via defective trans‐synaptic signaling thereby changing the abundance of postsynaptic AMPA receptors.
Taken together, our data demonstrate three consequences of the p.R596P mutation on synaptic functions, which may only be partially related: first, reduced trans‐synaptic recruitment of GABAAR; second, reduced presynaptic synapsin clustering in glutamatergic nerve terminals; and third, reduced amplitudes of mEPSCs.
4.4. Potential disease mechanisms
The functional characterization of the bi‐allelic mutation p.R596P in α2δ‐2 provides three possible mechanistic explanations for how a defective α2δ‐2 protein could be linked to DEE. Firstly, mutations in α2δ‐2 can affect its role as a VGCC subunit and disrupt channel‐dependent functions in an isoform‐ (differential effects on CaV1.3 L‐type and CaV2.1 P/Q‐type channels) and tissue‐specific (presynaptic abundance of CaV2.1) manner. Either way, normal Ca2+ handling in pre‐ and postsynaptic compartments will be affected. Secondly, the reduced trans‐synaptic coupling to postsynaptic GABAARs accompanying the low membrane expression of α2δ‐2_R596P may reduce the functionality of GABAergic inhibitory synapses, which is of relevance for the predominant expression of α2δ‐2 in the cerebellum. Thirdly, the reduction in presynaptic clustering of synapsin and the amplitude of mEPSCs are indications of aberrant synaptic differentiation which decreases the probability of neurotransmitter release and weakens synaptic connectivity. All these proposed pathophysiological mechanisms may ultimately lead to an aberrant excitatory–inhibitory balance that underlies DEEs and, more broadly, neurodevelopmental disorders. Moreover, our study emphasizes the importance of studying the consequences of disease‐associated α2δ protein mutations on both the classical functions as VGCC subunits and synaptic functions.
AUTHOR CONTRIBUTIONS
Sabrin Haddad: Funding acquisition; investigation; conceptualization; writing – original draft; methodology; writing – review and editing. Cornelia Ablinger: Software; conceptualization; methodology; writing – review and editing; investigation. Ruslan Stanika: Conceptualization; investigation; methodology; writing – review and editing; supervision. Manuel Hessenberger: Conceptualization; investigation; methodology; visualization; writing – review and editing; supervision. Marta Campiglio: Investigation; methodology; writing – review and editing; supervision. Nadine J. Ortner: Supervision; methodology; conceptualization; writing – review and editing. Petronel Tuluc: Conceptualization; supervision; writing – review and editing; methodology. Gerald J. Obermair: Conceptualization; funding acquisition; writing – review and editing; writing – original draft; methodology; project administration; supervision; resources.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1111/jnc.16197.
ACKNOWLEDGMENTS
This work was supported by grants from the Austrian Science Fund (DOC30 to G.J.O., P35087 to N.J.O., P31434, P36053, and DOC178 to P.T.) and the Gesellschaft füsr Forschungsförderung Niederösterreich (LSC19‐017 to G.J.O. and FTI22‐D‐013 to S.H.). We thank Anja Beierfuß and her team for animal care. We acknowledge support by the Open Access Publishing Fund of Karl Landsteiner University of Health Sciences, Krems, Austria. This work is part of the doctoral thesis of S.H.
Haddad, S. , Ablinger, C. , Stanika, R. , Hessenberger, M. , Campiglio, M. , Ortner, N. J. , Tuluc, P. , & Obermair, G. J. (2025). A biallelic mutation in CACNA2D2 associated with developmental and epileptic encephalopathy affects calcium channel‐dependent as well as synaptic functions of α2δ‐2. Journal of Neurochemistry, 169, e16197. 10.1111/jnc.16197
DATA AVAILABILITY STATEMENT
Not applicable or already included in the manuscript.
REFERENCES
- Ablinger, C. , Eibl, C. , Geisler, S. M. , Campiglio, M. , Stephens, G. J. , Missler, M. , & Obermair, G. J. (2022). α2δ‐4 and Cachd1 proteins are regulators of presynaptic functions. International Journal of Molecular Sciences, 23, 9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablinger, C. , Eibl, C. , Roznovcova, M. , Cottrell, G. S. , Stephens, G. J. , & Obermair, G. J. (2024). The presynaptic α2δ protein family and their therapeutic potential. In Stephens G. & Stevens E. (Eds.), Ion channels as targets in drug discovery (pp. 57–89). Springer. 10.1007/978-3-031-52197-3_3 [DOI] [Google Scholar]
- Ablinger, C. , Geisler, S. M. , Stanika, R. I. , Klein, C. T. , & Obermair, G. J. (2020). Neuronal α2δ proteins and brain disorders. Pflügers Archiv, 472, 845–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay, J. , Balaguero, N. , Mione, M. , Ackerman, S. L. , Letts, V. A. , Brodbeck, J. , Canti, C. , Meir, A. , Page, K. M. , Kusumi, K. , Perez‐Reyes, E. , Lander, E. S. , Frankel, W. N. , Gardiner, R. M. , Dolphin, A. C. , & Rees, M. (2001). Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. The Journal of Neuroscience, 21, 6095–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson, K. A. , Beeson, R. , Westbrook, G. L. , & Schnell, E. (2020). α2δ‐2 Protein controls structure and function at the cerebellar climbing fiber synapse. The Journal of Neuroscience, 40(12), 2403–2415. 10.1523/jneurosci.1514-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson, K. A. , Westbrook, G. L. , & Schnell, E. (2021). α2δ‐2 is required for depolarization‐induced suppression of excitation in Purkinje cells. The Journal of Physiology, 600(1), 111–122. 10.1113/jp282438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth, X. , Dines, J. N. , Cong, Q. , Mirzaa, G. M. , Foss, K. , Lawrence Merritt, J. , Thies, J. , Mefford, H. C. , & Novotny, E. (2018). Expanding clinical phenotype in CACNA1C related disorders: From neonatal onset severe epileptic encephalopathy to late‐onset epilepsy. American Journal of Medical Genetics. Part A, 176, 2733–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus, J. , Bruggen, B. , & Missler, M. (2019). Imaging and analysis of presynaptic calcium influx in cultured neurons using synGCaMP6f. Frontiers in Synaptic Neuroscience, 11, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck, J. , Davies, A. , Courtney, J. M. , Meir, A. , Balaguero, N. , Canti, C. , Moss, F. J. , Page, K. M. , Pratt, W. S. , Hunt, S. P. , Barclay, J. , Rees, M. , & Dolphin, A. C. (2002). The ducky mutation in Cacna2d2 results in altered Purkinje cell morphology and is associated with the expression of a truncated α2δ‐2 protein with abnormal function. The Journal of Biological Chemistry, 277, 7684–7693. [DOI] [PubMed] [Google Scholar]
- Butler, K. M. , Holt, P. J. , Milla, S. S. , da Silva, C. , Alexander, J. J. , & Escayg, A. (2018). Epileptic encephalopathy and cerebellar atrophy resulting from compound heterozygous CACNA2D2 variants. Case Reports in Genetics, 2018, 6308283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canti, C. , Nieto‐Rostro, M. , Foucault, I. , Heblich, F. , Wratten, J. , Richards, M. W. , Hendrich, J. , Douglas, L. , Page, K. M. , Davies, A. , & Dolphin, A. C. (2005). The metal‐ion‐dependent adhesion site in the Von Willebrand factor‐a domain of α2δ subunits is key to trafficking voltage‐gated Ca2+ channels. Proceedings of the National Academy of Sciences of the United States of America, 102, 11230–11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y. Q. , Piedras‐Renteria, E. S. , Smith, G. B. , Chen, G. , Harata, N. C. , & Tsien, R. W. (2004). Presynaptic Ca2+ channels compete for channel type‐preferring slots in altered neurotransmission arising from Ca2+ channelopathy. Neuron, 43, 387–400. [DOI] [PubMed] [Google Scholar]
- Cassidy, J. S. , Ferron, L. , Kadurin, I. , Pratt, W. S. , & Dolphin, A. C. (2014). Functional exofacially tagged N‐type calcium channels elucidate the interaction with auxiliary α2δ‐1 subunits. Proceedings of the National Academy of Sciences of the United States of America, 111, 8979–8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano, A. , Wei, X. , Birnbaumer, L. , & Perez‐Reyes, E. (1993). Cloning and expression of a third calcium channel beta subunit. The Journal of Biological Chemistry, 268, 3450–3455. [PubMed] [Google Scholar]
- Dahimene, S. , von Elsner, L. , Holling, T. , Mattas, L. S. , Pickard, J. , Lessel, D. , Pilch, K. S. , Kadurin, I. , Pratt, W. S. , Zhulin, I. B. , Dai, H. , Hempel, M. , Ruzhnikov, M. R. Z. , Kutsche, K. , & Dolphin, A. C. (2022). Biallelic CACNA2D1 loss‐of‐function variants cause early‐onset developmental epileptic encephalopathy. Brain, 145, 2721–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, A. , Hendrich, J. , Van Minh, A. T. , Wratten, J. , Douglas, L. , & Dolphin, A. C. (2007). Functional biology of the α2δ subunits of voltage‐gated calcium channels. Trends in Pharmacological Sciences, 28, 220–228. [DOI] [PubMed] [Google Scholar]
- Di Biase, V. , Flucher, B. E. , & Obermair, G. J. (2009). Resolving sub‐synaptic compartments with double immunofluorescence labeling in hippocampal neurons. Journal of Neuroscience Methods, 176, 78–84. [DOI] [PubMed] [Google Scholar]
- Dolphin, A. C. (2016). Voltage‐gated calcium channels and their auxiliary subunits: Physiology and pathophysiology and pharmacology. The Journal of Physiology, 594, 5369–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin, A. C. , & Obermair, G. J. (2022). Regulation of calcium channels and synaptic function by auxiliary α2δ subunits. In Zamponi G. W. & Weiss N. (Eds.), Voltage‐Gated Calcium Channels (pp. 93–114). Springer International Publishing. [Google Scholar]
- Donato, R. , Page, K. M. , Koch, D. , Nieto‐Rostro, M. , Foucault, I. , Davies, A. , Wilkinson, T. , Rees, M. , Edwards, F. A. , & Dolphin, A. C. (2006). The ducky(2J) mutation in Cacna2d2 results in reduced spontaneous Purkinje cell activity and altered gene expression. The Journal of Neuroscience, 26, 12576–12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardson, S. , Oz, S. , Abulhijaa, F. A. , Taher, F. B. , Shaag, A. , Zenvirt, S. , Dascal, N. , & Elpeleg, O. (2013). Early infantile epileptic encephalopathy associated with a high voltage gated calcium channelopathy. Journal of Medical Genetics, 50, 118–123. [DOI] [PubMed] [Google Scholar]
- Epi, K. C. (2016). De novo mutations in SLC1A2 and CACNA1A are important causes of epileptic encephalopathies. American Journal of Human Genetics, 99, 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epi, K. C. , Epilepsy Phenome/Genome Project , Allen, A. S. , Berkovic, S. F. , Cossette, P. , Delanty, N. , Dlugos, D. , Eichler, E. E. , Epstein, M. P. , Glauser, T. , Goldstein, D. B. , Han, Y. , Heinzen, E. L. , Hitomi, Y. , Howell, K. B. , Johnson, M. R. , Kuzniecky, R. , Lowenstein, D. H. , Lu, Y. F. , … Winawer, M. R. (2013). De novo mutations in epileptic encephalopathies. Nature, 501, 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemad, S. , Obermair, G. J. , Bindreither, D. , Benedetti, A. , Stanika, R. , di Biase, V. , Burtscher, V. , Koschak, A. , Kofler, R. , Geley, S. , Wille, A. , Lusser, A. , Flockerzi, V. , & Flucher, B. E. (2014). Differential neuronal targeting of a new and two known calcium channel β4 subunit splice variants correlates with their regulation of gene expression. The Journal of Neuroscience, 34, 1446–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell, B. , Eckrich, S. , Blum, K. , Eckrich, T. , Hecker, D. , Obermair, G. J. , Münkner, S. , Flockerzi, V. , Schick, B. , & Engel, J. (2016). α2δ2 controls the function and trans‐synaptic coupling of Cav1.3 channels in mouse inner hair cells and is essential for Normal hearing. The Journal of Neuroscience, 36, 11024–11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folci, A. , Steinberger, A. , Lee, B. , Stanika, R. , Scheruebel, S. , Campiglio, M. , Ramprecht, C. , Pelzmann, B. , Hell, J. W. , Obermair, G. J. , Heine, M. , & di Biase, V. (2018). Molecular mimicking of C‐terminal phosphorylation tunes the surface dynamics of Ca(V)1.2 calcium channels in hippocampal neurons. The Journal of Biological Chemistry, 293, 1040–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler, S. , Schopf, C. L. , & Obermair, G. J. (2015). Emerging evidence for specific neuronal functions of auxiliary calcium channel α2δ subunits. General Physiology and Biophysics, 34, 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler, S. , Schopf, C. L. , Stanika, R. , Kalb, M. , Campiglio, M. , Repetto, D. , Traxler, L. , Missler, M. , & Obermair, G. J. (2019). Presynaptic α2δ‐2 Calcium Channel subunits regulate postsynaptic GABA(a) receptor abundance and axonal wiring. The Journal of Neuroscience, 39, 2581–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, H. C. , Hang, J. , Kohler, W. , Li, L. , & Su, T. Z. (2001). Tissue‐specific expression and gabapentin‐binding properties of calcium channel α2δ subunit subtypes. The Journal of Membrane Biology, 184, 35–43. [DOI] [PubMed] [Google Scholar]
- Helbig, K. L. , Lauerer, R. J. , Bahr, J. C. , Souza, I. A. , Myers, C. T. , Uysal, B. , Schwarz, N. , Gandini, M. A. , Huang, S. , Keren, B. , Mignot, C. , Afenjar, A. , Billette de Villemeur, T. , Héron, D. , Nava, C. , Valence, S. , Buratti, J. , Fagerberg, C. R. , Soerensen, K. P. , … Wiener, J. (2018). De novo pathogenic variants in CACNA1E cause developmental and epileptic encephalopathy with contractures, macrocephaly, and Dyskinesias. American Journal of Human Genetics, 103, 666–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessenberger, M. , Haddad, S. , & Obermair, G. J. (2023). Pathophysiological roles of auxiliary calcium channel α2δ subunits. In Striessnig J. (Ed.), Voltage‐gated Ca2+ channels: Pharmacology, modulation and their role in human disease. Handbook of Experimental Pharmacology, volume 279 (pp. 289–316). Springer. [DOI] [PubMed] [Google Scholar]
- Hoppa, M. B. , Lana, B. , Margas, W. , Dolphin, A. C. , & Ryan, T. A. (2012). α2δ expression sets presynaptic calcium channel abundance and release probability. Nature, 486, 122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, S. V. , Ward, J. M. , Tessarollo, L. , McAreavey, D. , Sachdev, V. , Fananapazir, L. , Banks, M. K. , Morris, N. , Djurickovic, D. , Devor‐Henneman, D. E. , Wei, M. H. , Alvord, G. W. , Gao, B. , Richardson, J. A. , Minna, J. D. , Rogawski, M. A. , & Lerman, M. I. (2004). Cerebellar ataxia, seizures, premature death, and cardiac abnormalities in mice with targeted disruption of the Cacna2d2 gene. The American Journal of Pathology, 165, 1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper, J. , Evans, R. , Pritzel, A. , Green, T. , Figurnov, M. , Ronneberger, O. , Tunyasuvunakool, K. , Bates, R. , Žídek, A. , Potapenko, A. , Bridgland, A. , Meyer, C. , Kohl, S. A. A. , Ballard, A. J. , Cowie, A. , Romera‐Paredes, B. , Nikolov, S. , Jain, R. , Adler, J. , … Hassabis, D. (2021). Highly accurate protein structure prediction with AlphaFold. Nature, 596, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech, S. , & Banker, G. (2006). Culturing hippocampal neurons. Nature Protocols, 1, 2406–2415. [DOI] [PubMed] [Google Scholar]
- Koschak, A. , Reimer, D. , Huber, I. , Grabner, M. , Glossmann, H. , Engel, J. , & Striessnig, J. (2001). Alpha 1D (Cav1.3) subunits can form l‐type Ca2+ channels activating at negative voltages. The Journal of Biological Chemistry, 276, 22100–22106. [DOI] [PubMed] [Google Scholar]
- Mullner, C. , Broos, L. A. , van den Maagdenberg, A. M. , & Striessnig, J. (2004). Familial hemiplegic migraine type 1 mutations K1336E, W1684R, and V1696I alter Cav2.1 Ca2+ channel gating: Evidence for beta‐subunit isoform‐specific effects. The Journal of Biological Chemistry, 279, 51844–51850. [DOI] [PubMed] [Google Scholar]
- Niu, X. , Yang, Y. , Chen, Y. , Cheng, M. , Liu, M. , Ding, C. , Tian, X. , Yang, Z. , Jiang, Y. , & Zhang, Y. (2022). Genotype‐phenotype correlation of CACNA1A variants in children with epilepsy. Developmental Medicine and Child Neurology, 64, 105–111. [DOI] [PubMed] [Google Scholar]
- Obermair, G. J. , Kugler, G. , Baumgartner, S. , Tuluc, P. , Grabner, M. , & Flucher, B. E. (2005). The Ca2+ channel α2δ‐1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of α1S or excitation‐contraction coupling. The Journal of Biological Chemistry, 280, 2229–2237. [DOI] [PubMed] [Google Scholar]
- Obermair, G. J. , Szabo, Z. , Bourinet, E. , & Flucher, B. E. (2004). Differential targeting of the L‐type Ca2+ channel alpha 1C (CaV1.2) to synaptic and extrasynaptic compartments in hippocampal neurons. The European Journal of Neuroscience, 19, 2109–2122. [DOI] [PubMed] [Google Scholar]
- Obermair, G. J. , Tuluc, P. , & Flucher, B. E. (2008). Auxiliary Ca2+ channel subunits: Lessons learned from muscle. Current Opinion in Pharmacology, 8, 311–318. [DOI] [PubMed] [Google Scholar]
- Paul, M. S. , & Limaiem, F. (2023). Histology, Purkinje cells. In: StatPearls. Treasure Island (FL). [PubMed]
- Pippucci, T. , Parmeggiani, A. , Palombo, F. , Maresca, A. , Angius, A. , Crisponi, L. , Cucca, F. , Liguori, R. , Valentino, M. L. , Seri, M. , & Carelli, V. (2013). A novel null homozygous mutation confirms CACNA2D2 as a gene mutated in epileptic encephalopathy. PLoS One, 8, e82154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punetha, J. , Karaca, E. , Gezdirici, A. , Lamont, R. E. , Pehlivan, D. , Marafi, D. , Appendino, J. P. , Hunter, J. V. , Akdemir, Z. C. , Fatih, J. M. , Jhangiani, S. N. , Gibbs, R. A. , Innes, A. M. , Posey, J. E. , & Lupski, J. R. (2019). Biallelic CACNA2D2 variants in epileptic encephalopathy and cerebellar atrophy. Annals of Clinical Translational Neurology, 6, 1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlick, B. , Flucher, B. E. , & Obermair, G. J. (2010). Voltage‐activated calcium channel expression profiles in mouse brain and cultured hippocampal neurons. Neuroscience, 167, 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf, C. L. , Ablinger, C. , Geisler, S. M. , Stanika, R. I. , Campiglio, M. , Kaufmann, W. A. , Nimmervoll, B. , Schlick, B. , Brockhaus, J. , Missler, M. , Shigemoto, R. , & Obermair, G. J. (2021). Presynaptic α2δ subunits are key organizers of glutamatergic synapses. Proceedings of the National Academy of Sciences of the United States of America, 118(14), e1920827118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schredelseker, J. , Di Biase, V. , Obermair, G. J. , Felder, E. T. , Flucher, B. E. , Franzini‐Armstrong, C. , & Grabner, M. (2005). The β1a subunit is essential for the assembly of dihydropyridine‐receptor arrays in skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America, 102, 17219–17224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, M. B. , & Kammermeier, P. J. (2017). Ca(V)2 channel subtype expression in rat sympathetic neurons is selectively regulated by α2δ subunits. Channels, 11, 555–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanika, R. , Campiglio, M. , Pinggera, A. , Lee, A. , Striessnig, J. , Flucher, B. E. , & Obermair, G. J. (2016). Splice variants of the Ca(V)1.3 L‐type calcium channel regulate dendritic spine morphology. Scientific Reports, 6, 34528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuluc, P. , Kern, G. , Obermair, G. J. , & Flucher, B. E. (2007). Computer modeling of siRNA knockdown effects indicates an essential role of the Ca2+ channel α2δ‐1 subunit in cardiac excitation‐contraction coupling. Proceedings of the National Academy of Sciences of the United States of America, 104, 11091–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi, M. , Anyango, S. , Deshpande, M. , Nair, S. , Natassia, C. , Yordanova, G. , Yuan, D. , Stroe, O. , Wood, G. , Laydon, A. , Žídek, A. , Green, T. , Tunyasuvunakool, K. , Petersen, S. , Jumper, J. , Clancy, E. , Green, R. , Vora, A. , Lutfi, M. , … Velankar, S. (2022). AlphaFold protein structure database: Massively expanding the structural coverage of protein‐sequence space with high‐accuracy models. Nucleic Acids Research, 50, D439–D444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vembar, S. S. , & Brodsky, J. L. (2008). One step at a time: Endoplasmic reticulum‐associated degradation. Nature Reviews. Molecular Cell Biology, 9, 944–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt, A. , Freund, R. , Heck, J. , Missler, M. , Obermair, G. J. , Thomas, U. , & Heine, M. (2016). Dynamic association of calcium channel subunits at the cellular membrane. Neurophotonics, 3, 041809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable or already included in the manuscript.


