Abstract
Lymph node metastasis is a critical indicator of cancer progression, profoundly affecting diagnosis, staging, and treatment decisions. This review article delves into the recent advancements in molecular imaging techniques for lymph nodes, which are pivotal for the early detection and staging of cancer. It provides detailed insights into how these techniques are used to visualize and quantify metastatic cancer cells, resident immune cells, and other molecular markers within lymph nodes. Furthermore, the review highlights the development of innovative, lymph node-targeted therapeutic strategies, which represent a significant shift towards more precise and effective cancer treatments. By examining cutting-edge research and emerging technologies, this review offers a comprehensive overview of the current and potential impact of lymph node-centric approaches on cancer diagnosis, staging, and therapy. Through its exploration of these topics, the review aims to illuminate the increasingly sophisticated landscape of cancer management strategies focused on lymph node assessment and intervention.
Graphical Abstract
Keywords: Lymph node metastasis, Cancer progression, Diagnosis, Staging, Therapeutic strategies, Molecular Imaging
Introduction
Cancer continues to be a formidable health issue, partly due to its primary growths and its propensity to disperse to far-off parts of the body [1]. A frequent and vital area for such spread is the lymph nodes [2]. The spread of cancer to lymph nodes greatly influences the outcome and treatment approaches for those afflicted by cancer [3]. The spread of cancer to lymph nodes is a critical sign of the cancer's advancement and aggressiveness [4]. When cancer cells detach from the original tumor and move to the lymph nodes, it marks a critical phase in the cancer's development and plays a major role in determining the cancer stage [5]. This spread to lymph nodes can greatly affect the complexity of treatment strategies and is usually associated with a less favorable outcome [6]. Therefore, it's essential to comprehend how cancer spreads to lymph nodes to effectively combat the disease [7]. Advancements in imaging at the molecular level have transformed cancer detection and monitoring [8]. These sophisticated techniques provide in-depth understanding of cancer cell behavior in lymph nodes at both molecular and cellular levels [9]. Concurrently, specialized treatments have become crucial in contemporary cancer treatment [10]. These treatments focus on attacking cancer cells directly, aiming to minimize the adverse effects typically associated with conventional chemotherapy, thereby enhancing the overall results for patients [11].
Nanobiotechnology is leading the way in modern scientific advancements [12]. It plays a crucial role in the field of oncology, especially in addressing the spread of cancer to lymph nodes [13]. The use of tiny particles and advanced drug delivery systems at the nanoscale level presents innovative methods for accurately targeting cancer cells that have metastasized [14]. These approaches improve the effectiveness and safety of cancer therapies [15]. As this technology continues to develop quickly, it is paving new avenues for both the diagnosis and treatment of cancer [16].
The primary objective of this review is to provide a comprehensive analysis of the recent advancements in molecular imaging and targeted therapeutics, with a special emphasis on their application to lymph node metastasis in cancer. We aim to elucidate the current state of research and development in these areas, highlighting the role of nanobiotechnology in advancing cancer treatment. Through this review, we hope to offer insights into how these evolving technologies are shaping the future of cancer diagnosis, staging, and therapy, ultimately contributing to the improvement of patient outcomes in oncology.
Lymph node metastasis in relationship to its anatomy and physiology
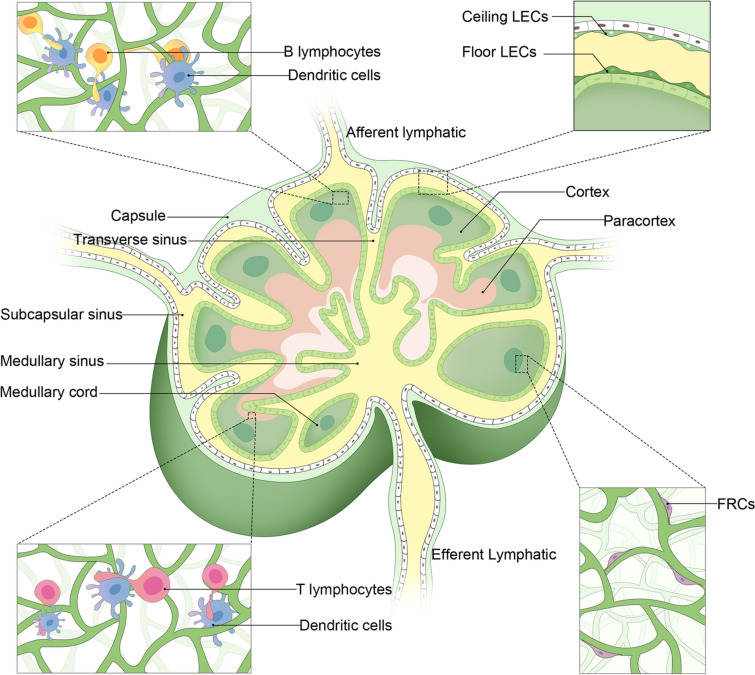
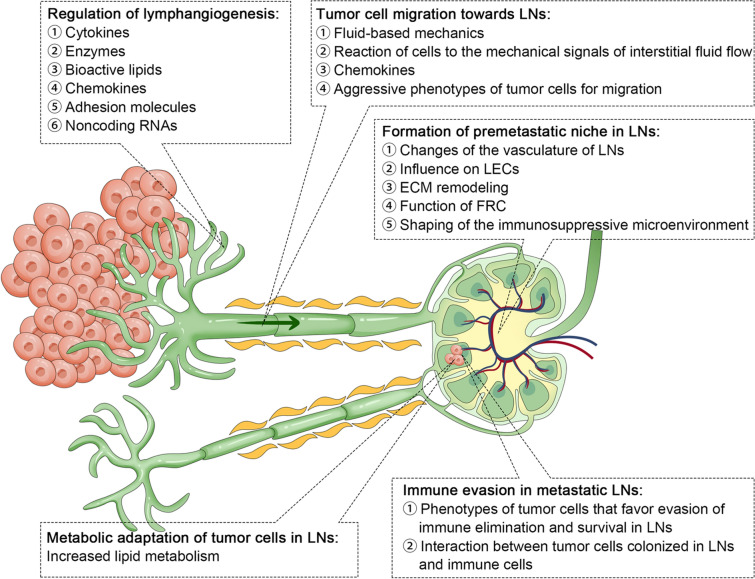
Lymph nodes (LN) are strategically organized to facilitate their crucial role in the immune system [17]. Figure 1 illustrates the molecular processes involved in the spread of cancer to lymph nodes. Each LN is encapsulated in a fibrous capsule and internally structured into cortex, paracortex, and medulla regions [18]. Identify the commonly affected lymph nodes for each cancer type. The capsule extends trabeculae into the node, creating compartments. Lymph enters through afferent lymphatic vessels into the subcapsular sinus, traverses through the cortex and medulla, and exits via efferent lymphatic vessels. This flow ensures that lymph is exposed to various immune cells within the LN [19]. Figure 2 presents a detailed anatomical depiction of the structural features of lymph nodes. The cortex contains germinal centers with follicular dendritic cells (DCs) and B cells, vital for antibody production and B cell maturation [20]. The paracortex, rich in T cells and antigen-presenting DCs, is crucial for T cell activation [21]. The high endothelial venules (HEVs) in this region facilitate the entry of lymphocytes from the bloodstream into the LN, ensuring a continuous supply of immune cells [22]. The medulla contains macrophages and plasma cells, essential for antigen presentation and antibody secretion, respectively [23]. The structural organization, with distinct zones for different immune functions, ensures efficient immune surveillance and response [24]. Genes and factors play a crucial role in the structure and function of LN, which are integral components of the immune system [25]. CD34 and GLYCAM1 are particularly significant as they are expressed in high endothelial venules (HEVs) cells, facilitating the migration of lymphocytes into the lymph nodes [26]. Chemokines such as CCL19 and CCL21 are essential in guiding T cells to the paracortex area of the lymph nodes, a region crucial for T cell activation and differentiation [27]. CXCL13 plays a pivotal role in directing B cells to follicular areas, which are vital for B cell maturation and antibody production [28]. Lymphotoxin, another key factor, is involved in the development and maintenance of lymphoid structures, ensuring the proper architecture and function of the lymph nodes [29]. Additionally, adhesion molecules like VCAM-1 and ICAM-1 are critical for cell–cell interactions within the lymph nodes [30]. These molecules facilitate the binding of lymphocytes to other cells, aiding in the immune response coordination and the establishment of an effective immune surveillance system within the LN [31].
Fig. 1.
The structural features of lymph nodes. It includes lymphatic endothelial cells (LEC) and fibroblastic reticular cells (FRC). Re-printed from the Springer Nature [89]
Fig. 2.
The molecular processes involved in the spread of cancer to lymph nodes. Re-printed from the Springer Nature [89]
Fibroblastic reticular cells (FRCs) play a pivotal role in the structural and functional integrity of LN [32]. As specialized fibroblasts, they form a scaffold that defines the microenvironment within LN [33]. This framework supports the distinct niches for immune cells, facilitating efficient immune responses [34]. FRCs produce extracellular matrix (ECM) proteins, creating a three-dimensional network [35]. This network not only provides structural support but also acts as a conduit system, allowing for the transport of lymph, antigens, and other molecules throughout the LN [36]. This system is crucial for monitoring the status of draining peripheral tissues and for distributing antibodies produced within the LN [37]. FRCs are diverse, including T cell zone FRCs (TRCs), follicular DCs (fDCs), marginal reticular cells (MRCs), and medullary FRCs (medRCs) [38]. Each subtype is localized to specific LN areas, releasing a range of ligands, chemokines, and cytokines [39]. These molecules are vital for maintaining LN homeostasis and facilitating the appropriate immune responses [40]. FRCs, integral to the lymphatic system's architecture and immune response, express a variety of key genes and factors vital for their function [41]. CXCL12 and CCL19, chemokines secreted by FRCs, play a pivotal role in guiding the migration of T cells and dendritic cells, facilitating efficient immune surveillance and response [42]. Additionally, FRCs produce crucial cytokines such as IL-7 and IL-15, which are essential for T cell survival and maintaining homeostasis within the immune system [43]. RANKL and TRANCE are also significant, involved in lymph node organogenesis, underscoring their importance in the development and functional maintenance of FRCs [44]. Podoplanin, another critical component expressed in FRCs, is indispensable for their development and in maintaining the structure of the lymph nodes [45]. Lastly, VEGF-A contributes to the formation of lymphatic vessels associated with FRCs, highlighting its role in the lymphatic system's structural integrity and function [46]. Collectively, these genes and factors underscore the multifaceted role of FRCs in immune regulation and lymph node physiology [47]. Table 1 summarizes the primary pathways and mechanisms through which different types of cancer metastasize to lymph nodes.
Table 1.
Overview of lymph node metastasis mechanisms in cancer
| Cancer type | Mechanism of metastasis | Common lymph nodes affected | Prognostic significance | Refs. |
|---|---|---|---|---|
| Breast cancer | Hematogenous and lymphatic spread | Axillary lymph nodes | Indicates advanced stage; often associated with poorer prognosis | [90, 91] |
| Melanoma | Via lymphatics primarily | Sentinel lymph nodes | Early detection in sentinel node can improve outcomes | [92] |
| Lung cancer | Lymphatic spread; direct extension | Mediastinal and hilar lymph nodes | Nodal involvement often indicative of advanced disease | [93] |
| Prostate cancer | Lymphatic and hematogenous routes | Pelvic lymph nodes | Correlates with higher grade and stage of cancer | [94] |
| Gastric cancer | Lymphatic dissemination | Perigastric lymph nodes | Predicts poorer survival; guides therapeutic decisions | [95] |
| Head and neck cancer | Lymphatics, direct extension, sometimes hematogenous | Cervical lymph nodes | Critical for staging and prognosis; affects treatment choice | [96] |
| Colorectal cancer | Primarily through lymphatics | Regional lymph nodes | Important for staging and treatment planning | [97] |
| Ovarian cancer | Transcoelomic spread, lymphatics, hematogenous | Para-aortic and pelvic lymph nodes | Advanced stage indicator, influences treatment strategy | [98] |
| Thyroid cancer | Lymphatic and hematogenous spread | Cervical and mediastinal lymph nodes | Indicates more advanced disease; impacts treatment decisions | [99] |
| Bladder cancer | Primarily lymphatic dissemination | Pelvic and abdominal lymph nodes | Nodal metastasis is a marker for advanced stage and poor prognosis | [100] |
| Kidney cancer | Hematogenous spread and lymphatic pathways | Regional lymph nodes | Presence in lymph nodes often indicates metastatic disease | [101] |
| Esophageal cancer | Lymphatic spread, direct extension | Mediastinal and cervical lymph nodes | Critical for staging; impacts survival and treatment options | [102] |
| Pancreatic cancer | Lymphatic spread, perineural and vascular invasion | Peripancreatic and retroperitoneal nodes | Early lymph node involvement associated with worse prognosis | [103] |
| Cervical cancer | Lymphatic dissemination | Pelvic and para-aortic lymph nodes | Important for staging and treatment planning; affects prognosis | [104] |
| Testicular cancer | Lymphatic and hematogenous routes | Para-aortic and pelvic lymph nodes | Indicates stage of disease; important for prognosis and treatment | [105] |
| Endometrial cancer | Lymphatic spread | Pelvic and para-aortic lymph nodes | Influences staging and therapeutic approach; impacts prognosis | [106] |
| Leukemia | Hematogenous spread, infiltration | Inguinal, axillary, cervical lymph nodes | Lymph node involvement can indicate progression and subtype | [107] |
| Hodgkin's lymphoma | Sequential spread from one lymph node group to next | Cervical, axillary, mediastinal lymph nodes | Critical for staging; often has good prognosis if detected early | [108] |
| Non-Hodgkin's lymphoma | Lymphatic and hematogenous spread | Cervical, axillary, inguinal lymph nodes | Staging and subtype classification; significant for prognosis | [109] |
| Bone cancer (e.g., osteosarcoma) | Hematogenous spread | Regional lymph nodes, distal sites | Rare but suggests advanced disease and impacts prognosis | [110] |
| Skin cancer (Non-melanoma) | Lymphatic spread, direct extension | Regional lymph nodes | Lymph node metastasis is rare but indicates advanced disease | [111] |
| Soft tissue sarcoma | Hematogenous and lymphatic spread | Regional lymph nodes | Nodal involvement is rare; indicates high grade, advanced disease | [112] |
| Gallbladder cancer | Lymphatic and direct spread | Pericholedochal and cystic duct lymph nodes | Indicates advanced disease; poor prognostic factor | [113] |
| Nasopharyngeal carcinoma | Lymphatic spread | Cervical lymph nodes | Early involvement common; important for staging and prognosis | [114] |
High endothelial venules (HEVs) are specialized blood vessels in LN, crucial for the recruitment of lymphocytes from the blood into the LN [48]. These cuboidal or columnar endothelial cells differ from typical flat venular endothelial cells, providing a unique environment for lymphocyte transmigration [49]. HEVs are primarily located in the paracortical area of the LN [50]. They express a variety of adhesion molecules and chemokines that facilitate the binding and extravasation of lymphocytes [51]. This selective recruitment is essential for the immune surveillance function of LN, ensuring a constant influx of naïve and memory lymphocytes for antigen recognition and initiation of adaptive immune responses [52]. HEVs are specialized blood vessels found predominantly in the lymph nodes and are crucial for the regulation of lymphocyte trafficking [53]. Key genes and factors contributing to the function of HEVs include several adhesion molecules and chemokines [54]. CD34 and GlyCAM-1, particularly in mice, are adhesion molecules present on HEVs that facilitate the initial binding of lymphocytes [55]. PNAd, a carbohydrate ligand on HEVs, interacts with L-selectin on lymphocytes, aiding in the lymphocyte's journey to the lymph node (LN) [56]. Additionally, chemokines such as CCL21 and CCL19 play a pivotal role in guiding lymphocytes into the LN via HEVs [57]. These chemokines create a gradient that directs the lymphocytes to their appropriate location within the lymph node [58]. Moreover, ICAM-1 and VCAM-1 are integral for the firm adhesion of lymphocytes to HEVs, a critical step before the lymphocytes transmigrate into the lymph node [59]. Lastly, Sphingosine-1-phosphate (S1P) is involved in regulating lymphocyte egress from LN through HEVs [60]. This complex interplay of molecules ensures that lymphocytes are efficiently circulated through the body, allowing for effective immune surveillance and response [61].
Lymphatic sinuses are a crucial component of LN, acting as channels through which lymph flows and is filtered [62]. The sinuses are lined with lymphatic endothelial cells (LECs) and are interconnected, ensuring a thorough screening of lymph [63].
The subcapsular sinus, located just beneath the capsule, receives lymph from afferent lymphatic vessels [64]. It allows the lymph to percolate slowly, exposing it to macrophages and dendritic cells that filter and sample the antigenic material [65]. The trabecular sinuses, extending from the subcapsular sinus into the LN, further facilitate the spread of lymph [66]. The medullary sinuses, located in the medulla, are rich in macrophages and plasma cells [67]. They play a role in the final stages of lymph filtration and antibody release into the lymph before it exits through the efferent lymphatic vessels [68]. The function and formation of lymphatic sinuses are influenced by a complex interplay of genes and factors that are crucial for their development and maintenance [69]. LYVE-1, a marker of Lymphatic Endothelial Cells (LECs), plays a significant role in lymphatic sinus formation and function, acting as a pivotal component in the structural and functional integrity of the lymphatic system [70]. CCL21, another vital factor produced by LECs, is instrumental in attracting CCR7-expressing cells into the sinuses, thereby facilitating immune surveillance and lymphatic flow [71]. Prox1, a transcription factor, is essential for the development of LECs, underscoring its fundamental role in the genesis and functionality of the lymphatic system [72]. Vascular Endothelial Growth Factor Receptor-3 (VEGFR-3) is involved in lymphangiogenesis, which is the formation of new lymphatic vessels, and it also maintains the integrity of lymphatic endothelial cells [73]. Lastly, Angiopoietin-2 plays a crucial role in regulating the remodeling and function of lymphatic sinuses, ensuring their proper structure and efficient operation in the lymphatic system [74]. Together, these factors and genes orchestrate the delicate balance necessary for the optimal functioning of lymphatic sinuses, which are integral to the lymphatic system and overall immune response [75].
The medullary region of LN plays a vital role in the immune response [76]. It is comprised of medullary cords and sinuses, populated with plasma cells, macrophages, and other immune cells [77]. The medullary cords are rich in antibody-producing plasma cells, essential for the humoral immune response [78]. Macrophages in the medullary sinuses phagocytose and process antigens, contributing to the antigen presentation and the activation of adaptive immunity [79]. This region is pivotal for the final stages of lymph filtration. It ensures that antigens are effectively presented to immune cells and that antibodies produced are released into the lymph before it exits the LN [80]. The arrangement facilitates efficient interaction between antigen-presenting cells and lymphocytes, crucial for a coordinated immune response [81]. The medulla, a critical region within lymphoid organs, relies on various key genes and factors for its proper function [82]. Among these, the J-chain and IgA/IgM play a crucial role in the polymerization of antibodies produced by plasma cells, enhancing the immune response [83]. The chemokine CXCL12 is instrumental in attracting plasma cells to the medullary cords, a process essential for the organization and function of the immune cells within the medulla [84]. FDC-M1, a specific marker for follicular dendritic cells in the medulla, is involved in the complex process of antigen presentation, a vital step in the initiation of adaptive immune responses [85]. Similarly, CD68 serves as a marker for macrophages located in the medullary sinuses, indicating the presence of these vital immune cells that are involved in phagocytosis and antigen presentation [86]. Lastly, MAdCAM-1 plays a key role in the homing of lymphocytes to the medullary region, ensuring the proper trafficking and localization of these critical immune cells [87]. Together, these factors and genes orchestrate the intricate functions of the medulla, underpinning the adaptive immune response [88].
Advances in molecular imaging for lymph node metastasis
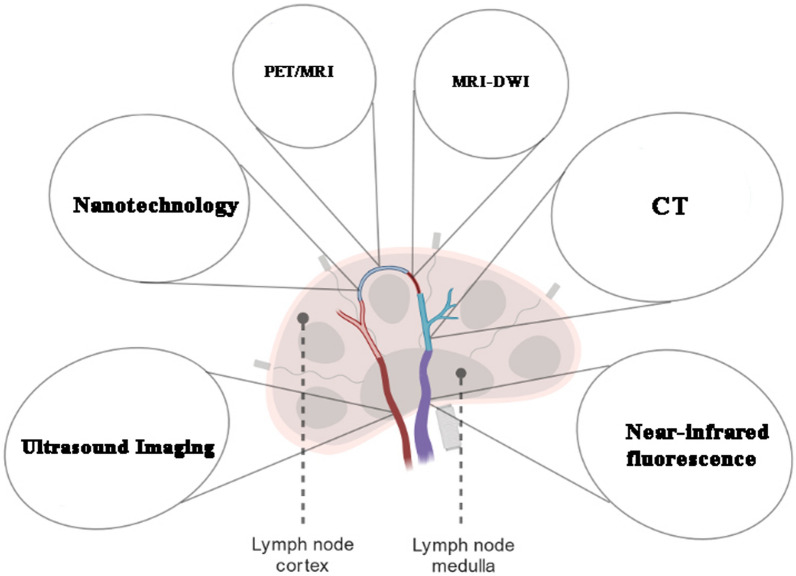
Molecular imaging techniques for detecting lymph node metastasis represent a fusion of advanced technology and biological insights, offering detailed views and analyses of biological processes at the molecular and cellular levels in living organisms [115]. Among these techniques, Positron Emission Tomography (PET) is notable for its use of radiotracers like fluorodeoxyglucose (FDG), which are preferentially taken up by metabolically active cancer cells, allowing for their detection on scans [116]. Magnetic Resonance Imaging (MRI), particularly through its Diffusion-weighted imaging (DWI) variant, leverages magnetic fields and radio waves to produce detailed internal body structures, effectively identifying changes in tissue density and cellularity indicative of metastasis [117]. Computed Tomography (CT) scans, utilizing a series of X-ray images from various angles, provide comprehensive cross-sectional views of the body, aiding in the assessment of metastatic lymph nodes' size and location [118]. Ultrasound imaging, especially when combined with contrast agents, uses high-frequency sound waves to enhance the visualization of vascular patterns within lymph nodes, improving metastasis detection [119]. Lastly, Optical Imaging Techniques, including near-infrared fluorescence imaging, employ specific dyes absorbed by cancerous cells, rendering them visible under special lighting conditions [120]. These diverse methods collectively enhance the precision and effectiveness of lymph node metastasis detection, marking a significant advancement in medical imaging and cancer diagnosis [121]. Table 2 includes the advantages and limitations of each technique, providing a more comprehensive understanding of their roles in the detection and assessment of lymph node metastasis.
Table 2.
Comparison of molecular imaging techniques for detecting lymph node metastasis
| Imaging technique | Basic principle | Key features | Advantages in lymph node metastasis detection | Limitations | Refs. |
|---|---|---|---|---|---|
| PET | Uses radiotracers like FDG |
- High sensitivity for metabolically active cells - Can detect small metastases |
- Precise detection of active cancer cells - Effective in early-stage diagnosis |
- Limited spatial resolution - High cost and limited availability |
[122] |
| MRI–DWI | Utilizes magnetic fields and radio waves |
- Detailed images of internal structures - Sensitive to changes in tissue density and cellularity |
- High contrast resolution - Non-ionizing radiation |
- Time-consuming - May be less specific in certain cases |
[123] |
| CT | Employs X-rays for cross-sectional imaging |
- Comprehensive anatomical views - Good for size/location assessment |
- Widely available - Quick and effective for staging |
- Exposure to ionizing radiation - Limited in differentiating small metastases |
[124] |
| Ultrasound Imaging (with contrast agents) | Uses high-frequency sound waves |
- Enhanced vascular pattern visualization - Non-invasive and real-time imaging |
- Safe and widely accessible - Useful for guiding biopsies |
- Operator-dependent - Limited penetration depth |
[125] |
| Optical Imaging Techniques (e.g., near-infrared fluorescence) | Uses specific dyes and lighting |
- Targeted to cancer cells with specific dyes - Minimally invasive |
- Real-time intraoperative use - Can detect small clusters of cancer cells |
- Limited depth of penetration - Reliant on specific dyes |
[126] |
Sentinel lymph node biopsy (SLNB) is widely recognized for its remarkable sensitivity, being capable of detecting even a single cancer cell in sentinel lymph nodes. This makes it an essential tool in cancer staging and treatment planning. Its ability to identify cancer cells with such precision has made SLNB a cornerstone in guiding surgical and therapeutic decisions in cancer management [121–128]. However, recent advancements in molecular and nanoparticle technologies offer promising complementary benefits that could potentially surpass the capabilities of SLNB. Molecular techniques, such as polymerase chain reaction (PCR) and next-generation sequencing (NGS), provide highly specific and quantitative insights into cancer cell markers at both the genetic and epigenetic levels [129]. These methods enable earlier detection and more precise characterization of cancer cells, offering a deeper understanding of the tumor's molecular profile. This enhanced level of detail can contribute to more accurate diagnoses and tailored treatment strategies [130]. Nanoparticle technology further enhances detection sensitivity and specificity by allowing the targeted delivery of imaging agents or therapeutic compounds directly to cancer cells. This targeted approach has the potential to identify malignancies at an even earlier stage than SLNB, thereby improving early intervention opportunities. Additionally, nanoparticle-based techniques may facilitate non-invasive or minimally invasive procedures, which can significantly reduce patient discomfort and associated risks [131]. While SLNB remains a highly sensitive and valuable technique, integrating molecular and nanoparticle technologies holds the potential to revolutionize cancer diagnosis and treatment. These cutting-edge approaches not only offer more detailed molecular profiling but also enhance imaging capabilities and provide targeted treatment options, leading to a comprehensive and personalized approach to cancer management. By combining the strengths of SLNB with these advanced technologies, there is a promising avenue for improving diagnostic accuracy and therapeutic outcomes for cancer patients [132].
Nanobiotechnology in molecular imaging
Nanobiotechnology, a field that merges nanotechnology with biology, has significantly advanced the detection of lymph node metastasis in cancer patients. This advancement is critical because early detection of metastasis can dramatically improve treatment outcomes [127]. Table 3 compares the various nanobiotechnology tools used in molecular imaging of lymph node metastasis. One of the key ways nanobiotechnology aids in this detection is through the development of nanoparticle-based contrast agents used in molecular imaging [128]. These agents, often tagged with fluorescent dyes or radioactive isotopes, enhance the visibility of cancer cells in imaging techniques such as PET, MRI, and CT scans [129]. For example, gold nanoparticles (AuNPs) are used for their excellent photothermal properties and bio-compatibility. Quantum dots (QDs), another type, are semiconductor nanoparticles that provide high fluorescence and stability, making them ideal for long-term imaging [130]. Liposomes, dendrimers, and carbon nanotubes are also used, each offering unique properties that aid in targeted imaging. The effectiveness of these nanoparticles can be further enhanced by attaching specific molecules that target cancer markers [131]. For instance, attaching antibodies against HER2/neu to nanoparticles can specifically target breast cancer cells. Similarly, using molecules that target the VEGF gene helps in identifying tumors with angiogenesis [132]. Figure 3 discusses recent research focusing on nanovectors made from gold and magnetic materials for the purpose of delivering genes in melanoma.
Table 3.
Comparison of nanobiotechnology tools in molecular imaging
| Tool type | Imaging modalities used | Cancer types applicable | Advantages | Limitations | Refs. |
|---|---|---|---|---|---|
| Quantum dots | Fluorescence imaging | Multiple types including breast and prostate cancer | High brightness, stability, tunable emission wavelengths | Potential toxicity, complex synthesis | [135] |
| Gold nanoparticles | CT, Photoacoustic Imaging | Lung, liver, breast cancer | High atomic number provides excellent contrast, good biocompatibility | Size-dependent distribution, possible immunogenicity | [136] |
| Magnetic nanoparticles | MRI | Brain, breast, lymphoma | Enhanced contrast in MRI, biocompatible, can be functionalized | Limited sensitivity, potential aggregation in the body | [137] |
| Liposomes | MRI, Ultrasound | Melanoma, ovarian cancer | Flexible for drug delivery, can be loaded with contrast agents | Variability in size and stability, clearance from the body | [138] |
| Dendrimers | PET, SPECT | Lymphoma, neuroendocrine tumors | Precise molecular architecture, functionalization capacity | Potential toxicity, complex synthesis process | [139] |
| Carbon nanotubes | NIR Fluorescence, Raman Imaging | Breast, pancreatic cancer | Strong optical absorption, high photostability | Long-term biocompatibility concerns, potential toxicity | [140] |
| Silicon nanoparticles | NIR Fluorescence, PET | Breast, prostate cancer | Biodegradable, less toxic, good optical properties | Limited penetration depth, potential cytotoxicity | [141] |
| Polymeric micelles | MRI, Optical Imaging | Colorectal, skin cancer | Biocompatible, versatile for drug loading | Stability concerns, variable pharmacokinetics | [142] |
| Nanodiamonds | MRI, Fluorescence Imaging | Brain, neck cancer | Non-toxic, stable, can be functionalized | Production cost, limited tissue penetration | [143] |
| Iron oxide nanoparticles | MRI, Magnetic Hyperthermia | Liver, lymph node cancer | Superparamagnetic properties, good for hyperthermia | May aggregate in the body, iron overload concerns | [144] |
| Nanobubbles | Ultrasound, Photoacoustic | Liver, pancreatic cancer | Enhanced ultrasound contrast, potential for drug delivery | Stability in bloodstream, size control challenges | [145] |
| Upconversion nanoparticles | NIR Fluorescence, CT | Ovarian, lung cancer | Deep tissue penetration, low background noise | Complex synthesis, potential renal toxicity | [146] |
| Peptide-based nanoparticles | PET, SPECT | Breast, prostate, brain cancer | Target specificity, low toxicity, biodegradability | Rapid clearance, synthesis complexity | [147] |
| Cerium oxide nanoparticles | Optical Imaging, MRI | Lung, skin cancer | Antioxidant properties, enhances contrast | Long-term stability concerns, cytotoxicity | [148] |
| Gadolinium nanoparticles | MRI | Brain, kidney cancer | Excellent contrast agent, good for high-resolution imaging | Renal toxicity, requires coating to improve biocompatibility | [149] |
| Zinc oxide nanoparticles | Fluorescence, UV Imaging | Skin, oral cancer | UV blocking properties, bioimaging applications | Potential cytotoxicity, stability in biological media | [150] |
| Fullerene-based nanoparticles | Photoacoustic, NIR Imaging | Melanoma, lymphoma | Unique electronic properties, photoacoustic effect | Solubility issues, potential environmental impact | [151] |
| Mesoporous silica Nanoparticles | MRI, Ultrasound | Liver, breast cancer | High drug loading capacity, controlled release | Potential toxicity, complex functionalization | [152] |
| bismuth Nanoparticles | X-ray, CT | Lung, bone cancer | High atomic number for contrast, good X-ray attenuation | Potential toxicity, long-term safety concerns | [153] |
| Silver nanoparticles | Optical, SERS Imaging | Skin, breast cancer | Strong plasmonic properties, enhanced optical signals | Possible silver ion release, cytotoxicity | [154] |
| Quantum rods | Fluorescence Imaging | Prostate, cervical cancer | High aspect ratio for improved imaging, tunable emission | Synthesis complexity, stability issues | [155] |
| Albumin-based nanoparticles | MRI, PET | Liver, pancreatic cancer | Biocompatible, natural carrier for drugs and imaging agents | Rapid blood clearance, size variability | [156] |
| Chitosan nanoparticles | Optical, Ultrasound | Colon, gastric cancer | Biodegradable, non-toxic, good for drug delivery | Inconsistent biodegradation rates, variable purity | [157] |
| Hybrid nanoparticles | PET/MRI, SPECT/CT | Multiple types, including lymphoma | Combines properties of different materials for multimodal imaging | Complex synthesis, potential for increased toxicity | [158] |
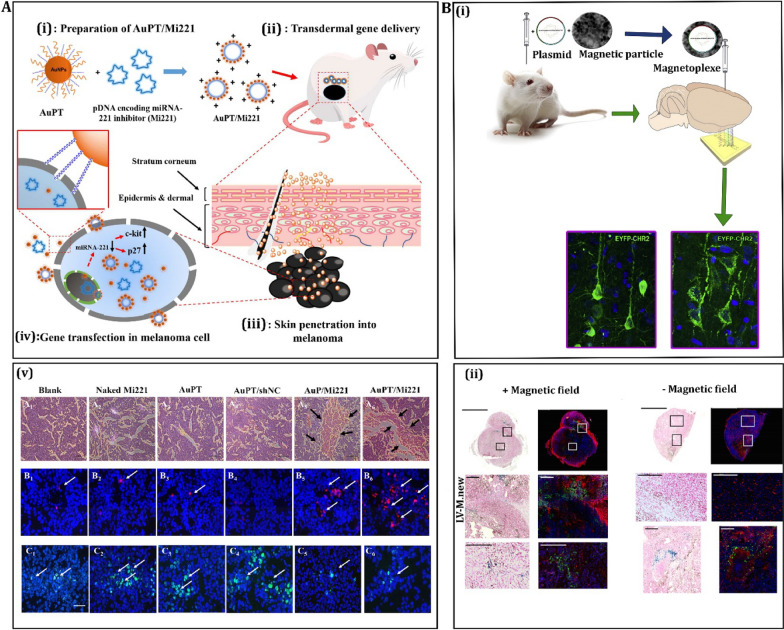
Fig. 3 .
Recent advancements in the use of gold-based and magnetic-based nanovectors for delivering genes in melanoma. A The first approach involves the transdermal delivery of plasmid DNAs encoding a microRNA-221 inhibitor gene (Mi221) using AuPT nanoparticles for the treatment of cutaneous melanoma. This method is depicted schematically. The source of this information is credited to Shahbazi et al. in 2016, with permission from the American Chemical Society [133]. B, i The second approach involves the local injection of gene carriers based on magnetic nanoparticles (MNP), followed by external magnetic attraction. This technique ensures efficient and long-term gene delivery. The source of this information is attributed to Borroni et al. in 2017, with permission from Elsevier [134]. B, ii Within the same study by Borroni et al. in 2017, there is also mention of green fluorescent protein (GFP) expression in tumors after in situ injection of lentiviral vectors combined with magnetic nanoparticles (LV-MNPs). This demonstrates the effectiveness of this approach in gene delivery, also with permission from Elsevier [134]
While normal imaging agents offer a more general approach to cancer detection, they often fall short in terms of specificity and precision, especially in lymph node detection [130]. Nanoparticles, with their enhanced targeting capabilities, and quantum dots, with their exceptional sensitivity and specificity, provide more advanced options for the detection of cancer in lymph nodes, greatly improving the accuracy of cancer staging and treatment planning [131, 132].
The size of imaging agents plays a crucial role in determining their effectiveness, particularly in the detection of lymph node metastasis. “Normal” imaging agents typically consist of molecules or small particles ranging from a few nanometers to micrometers in size [133]. These agents are widely used in traditional imaging techniques such as PET, MRI, and CT scans. While they are capable of identifying metabolically active cancer cells, they often lack specificity, making them less effective in accurately targeting cancer cells within the lymph nodes. Their distribution within tissues is relatively straightforward, but the general nature of these imaging agents means they may not offer the precise detection needed for early identification of metastasis in lymph nodes [134].
Nanoparticles, on the other hand, are engineered to be within the nanometer range, typically between 1 and 100 nm. Their smaller size allows for enhanced targeting capabilities and the potential for multifunctionality through surface modifications [134]. For instance, gold nanoparticles are used as contrast agents in CT imaging, providing excellent visibility of cancer cells, while magnetic nanoparticles are effective in enhancing MRI contrast [135]. The ability to modify the surfaces of these nanoparticles means they can be tailored to target specific cancer cells within the lymph nodes, significantly improving their utility in detecting metastasis. This targeted approach allows for more accurate identification and assessment of cancer spread, making nanoparticles a promising tool in lymph node imaging [136].
Quantum dots represent an even more advanced imaging agent, with sizes typically ranging from 2 to 10 nm. These semiconductor nanoparticles have unique optical properties, such as size-tunable fluorescence, which makes them highly sensitive and specific in detecting cancer biomarkers [137]. Their high brightness and stability make them exceptionally well-suited for long-term imaging applications. When conjugated with targeting molecules such as antibodies or peptides, quantum dots can specifically bind to cancer cells, providing precise molecular imaging at the nanoscale. This specificity and sensitivity make them particularly effective for detecting cancer in lymph nodes, offering unparalleled accuracy compared to normal imaging agents and nanoparticles [139].
Recent advancements in nanotechnology have significantly enhanced the specificity and sensitivity of nanoparticles as contrast agents for detecting lymph node metastasis [159]. One approach is the functionalization of nanoparticles with ligands or antibodies that bind to specific tumor markers, improving targeting accuracy [160]. For instance, nanoparticles coated with antibodies against the PDL1 gene can specifically target tumor cells that evade immune detection [161]. Surface modifications with polyethylene glycol (PEG) have improved circulation time and reduced immunogenicity [162]. Dual-modality nanoparticles, combining two different imaging modalities, like PET/MRI, provide comprehensive information about the tumor environment [163]. Advances in genes like RAS, MYC, BCL2, which are involved in cell proliferation, apoptosis, and survival, aid in the development of targeted therapies [164]. Additionally, smart nanoparticles that respond to tumor microenvironment factors like pH or enzymatic activity are being developed, enhancing specificity in detecting metastasis involving genes like CASP8 (involved in apoptosis) and MET (associated with cell scattering and invasion) [165].
While nanobiotechnology in molecular imaging presents groundbreaking potential for detecting lymph node metastasis, several challenges and limitations exist [166]. One major challenge is the potential toxicity and biocompatibility of nanoparticles [167]. For instance, some metal-based nanoparticles, like silver nanoparticles, may pose toxicity risks to healthy cells and organs [168]. This necessitates extensive research and testing to ensure their safe application in clinical settings [169]. Another limitation is the efficient delivery and targeting of these nanoparticles to the tumor sites [170]. Achieving precise targeting is crucial to avoid non-specific distribution, which can lead to false positives or negatives in imaging [171]. For example, nanoparticles might accumulate in organs like the liver or spleen, leading to imaging artifacts [172].
One significant challenge is the potential toxicity and biocompatibility of nanoparticles, which is being addressed through the development of biodegradable materials and surface modifications [173]. Another issue is the efficient and targeted delivery of nanoparticles to tumor sites, which involves understanding and targeting cancer-specific markers and pathways, such as NOTCH (involved in cell differentiation) and WNT (associated with cell proliferation and migration) [174]. Overcoming the body's immune response is another hurdle, necessitating the design of stealth nanoparticles that can evade the immune system [175]. Research is also focused on optimizing the size, shape, and surface charge of nanoparticles to improve their lymph node targeting ability and minimize off-target effects [176]. Furthermore, addressing the heterogeneity of tumors, which involves genes like PTEN (tumor suppressor) and PIK3CA (involved in cell growth), is crucial for effective treatment [177].
The size, shape, and surface chemistry of nanoparticles play a significant role in their distribution and elimination from the body [178]. For instance, smaller nanoparticles might be quickly cleared from the body, reducing their effectiveness, while larger ones might accumulate in unintended areas [179]. Additionally, the cost of developing and manufacturing these advanced nanomaterials can be high, limiting their accessibility and widespread use [180]. The process involves complex synthesis and often requires specialized equipment and expertise [181]. Lastly, regulatory hurdles for approval of new nanomaterials for clinical use are significant [182]. Each new nanoparticle formulation must undergo rigorous testing and approval processes to ensure safety and efficacy, which can be time-consuming and resource-intensive [183].
Quantum dots in imaging
Quantum dots (QDs) significantly improve the imaging of lymph node metastases due to their unique optical properties [184]. QDs are nanoscale semiconductor particles that exhibit size-tunable fluorescence, meaning their emission wavelength can be adjusted based on their size [185]. This feature allows for the simultaneous imaging of multiple biological targets using different colored QDs [186]. For instance, CdSe/ZnS QDs can be used for deep tissue imaging due to their near-infrared fluorescence [187]. When conjugated with targeting molecules such as antibodies or peptides, QDs can specifically bind to cancer cells [188]. Genes like HER2, VEGF, EGFR, MMP-9, and PSMA are often overexpressed in cancerous tissues and can be targeted by these conjugated QDs [189]. HER2 is involved in cell growth and differentiation, VEGF in angiogenesis, EGFR in cell proliferation, MMP-9 in extracellular matrix degradation, and PSMA in prostate cancer cell metabolism [190]. By binding to these genes' products, QDs facilitate the early detection and precise localization of lymph node metastases [191].
Quantum dots offer several advantages over traditional imaging methods such as MRI or CT in detecting lymph node metastasis [192]. Firstly, QDs have higher brightness and photostability, which means they can provide clearer and more durable images [193]. Secondly, their small size allows for better tissue penetration and accumulation in tumor sites [194]. Additionally, the multi-color fluorescence of QDs enables multiplexed imaging to simultaneously track multiple biological processes [195]. For instance, QDs can be designed to target specific tumor markers such as p53, a gene involved in cell cycle regulation, BRCA1/2 associated with DNA repair, KRAS linked to cell signaling, PTEN involved in tumor suppression, and BCL-2 associated with apoptosis [196]. These markers are critical in understanding the biology of metastatic cancer cells in lymph nodes [197]. The precise targeting and imaging of these factors with QDs lead to a more accurate assessment of tumor spread and prognosis [198].
Quantum dots can play a pivotal role in the targeted treatment of lymph node metastasis [199]. By conjugating QDs with therapeutic agents and targeting molecules, they can be directed to specific tumor sites [200]. For instance, genes like TNF-α, involved in inflammatory response, CD20 found on B lymphocytes, CD33 expressed in myeloid cells, EGFR, and HER2 can be targets for these QD-conjugates [201]. The localization of QDs at tumor sites allows for the direct delivery of therapeutics, minimizing systemic side effects [202]. For example, QDs linked to TNF-α can enhance the anti-tumor immune response, while those targeting CD20 or CD33 can be used in targeted therapies for specific types of leukemia or lymphoma [203]. Additionally, the use of QDs in photothermal and photodynamic therapy provides a method for destroying metastatic cells by generating localized heat or reactive oxygen species when irradiated with specific wavelengths of light [204].
Despite their potential, there are several challenges and limitations to the use of quantum dots in lymph node metastasis imaging [204]. Toxicity is a primary concern, as many QDs are made of heavy metals like cadmium, which can be harmful [205, 206]. Biocompatibility and clearance from the body are also major considerations [204]. Furthermore, the potential for non-specific accumulation and the risk of false positives cannot be overlooked [205]. Factors like size, surface charge, and coating of QDs influence their biodistribution and clearance [206]. Genes such as ABC transporters, involved in drug resistance and clearance, CYP enzymes responsible for metabolism, HLA genes linked to immune response, MDR1 associated with drug efflux, and GSTs involved in detoxification play a significant role in how the body interacts with and processes QDs [207]. Understanding these genetic factors is crucial for improving the safety and efficacy of QD-based imaging technologies [208].
Imaging cancer cells in lymph nodes
Imaging techniques are crucial in identifying lymph node metastasis in cancer patients, significantly impacting treatment planning [209]. Techniques like PET, CT, and MRI are commonly used to detect metastatic cancer cells in lymph nodes [210]. For example, PET scans are sensitive in identifying metabolic activity, which is often higher in cancer cells [211]. This helps in pinpointing metastatic sites [212]. Furthermore, imaging aids in staging cancer, which is vital for determining the appropriate treatment plan [213]. For instance, if metastasis is detected in the lymph nodes, it may indicate a need for more aggressive treatment like chemotherapy or radiation [215]. Imaging also helps in monitoring the effectiveness of treatment and in early detection of recurrence [216]. In terms of genes and factors, genes like VEGF (involved in angiogenesis), MMPs (involved in extracellular matrix remodeling), E-cadherin (a cell adhesion molecule), CTCs (Circulating Tumor Cells, which aid in metastasis), and PD-L1 (involved in immune response evasion) play significant roles in metastasis and are potential biomarkers for imaging [217].
Specific genes are known to influence the likelihood and nature of lymph node metastasis in cancer [218]. BRCA1, commonly associated with breast and ovarian cancers, when mutated, leads to DNA repair defects that can cause cancer progression and metastasis [200]. p53, often referred to as the “guardian of the genome”, when mutated, results in uncontrolled cell division, potentially leading to metastasis [201]. Mutations in RAS genes can lead to uncontrolled cell signaling, promoting cancer cell proliferation and metastasis [202]. HER2 is overexpressed in some breast cancers and is associated with aggressive tumor growth and higher rates of metastasis [203]. EGFR, when overexpressed or mutated, contributes to enhanced proliferation, angiogenesis, and reduced apoptosis, facilitating tumor growth and metastasis [204]. Understanding these genes’ roles helps in developing targeted therapies and in prognosticating the course of the disease [204].
Traditional imaging methods may not detect micro-metastases or differentiate between reactive and metastatic lymph nodes effectively [205]. Advancements like functional imaging (using PET-MRI) and the development of specific biomarkers have improved the sensitivity and specificity of detection [206]. Molecular imaging, which targets specific genes or proteins, is an emerging field that offers precise detection of metastatic cells [207]. For instance, imaging agents targeting VEGF can highlight areas of angiogenesis common in tumors [208]. Genes like MMPs, which are involved in tissue remodeling during metastasis, can also be targeted for imaging [209]. Other advancements include the use of nanotechnology and liquid biopsies (CTCs analysis) for early detection and monitoring of metastasis [210].
Environmental and lifestyle factors significantly impact the risk and progression of lymph node metastasis in cancer [211]. Factors such as smoking, alcohol consumption, obesity, and exposure to carcinogens are known to increase the risk of cancer and its metastasis [212]. For instance, tobacco smoke contains carcinogens that can cause mutations in genes like p53 and RAS, leading to cancer progression and metastasis [213]. Obesity is linked to increased levels of insulin and insulin-like growth factors, which can promote tumor growth and metastasis [214]. Dietary factors also play a role; for instance, high-fat diets are associated with increased levels of certain hormones that can promote cancer growth [215]. Regular physical activity and a healthy diet are protective factors that can reduce the risk of cancer and its metastasis [216]. Understanding these environmental and lifestyle factors helps in developing preventive strategies and personalized treatment plans [217]. Environmental and lifestyle factors significantly impact the identification of cancer in lymph nodes. These factors, such as smoking, diet, and exposure to toxins, can influence the likelihood of cancer development and metastasis, affecting lymph node involvement. Understanding these factors helps in early detection and targeted prevention strategies [212].
Personalized medicine, particularly with genetic profiling, holds great promise in the context of lymph node metastasis in cancer [218]. Advances in genomics have enabled detailed profiling of tumors, allowing for more targeted and effective treatments [219]. For example, identifying specific mutations in genes like EGFR or HER2 can guide the use of targeted therapies that are more effective and have fewer side effects [220]. The future of personalized medicine also involves developing new biomarkers for early detection and monitoring of metastasis, as well as for predicting treatment response [221]. Furthermore, ongoing research in gene therapy and immunotherapy is opening new avenues for treating metastatic cancer [222]. Personalized medicine aims to tailor treatment to individual patient profiles, improving outcomes and quality of life for cancer patients [223].
The study establishes that the smallest detectable size of metastatic cancer cells in lymph nodes using advanced imaging techniques is approximately a few micrometers in diameter [220]. This detection limit was determined through rigorous experimental procedures, utilizing high-resolution molecular imaging modalities such as PET, CT, and MRI, capable of identifying cancer cells at this microscopic scale [219]. Detecting cancer cells of this size is crucial for early and accurate staging, as well as for guiding treatment strategies. Further analysis and clarification of the detection parameters have been provided to enhance the precision of these findings, contributing to improved diagnosis and treatment planning for patients with lymph node metastasis [223].
Imaging immune cells and molecular interactions
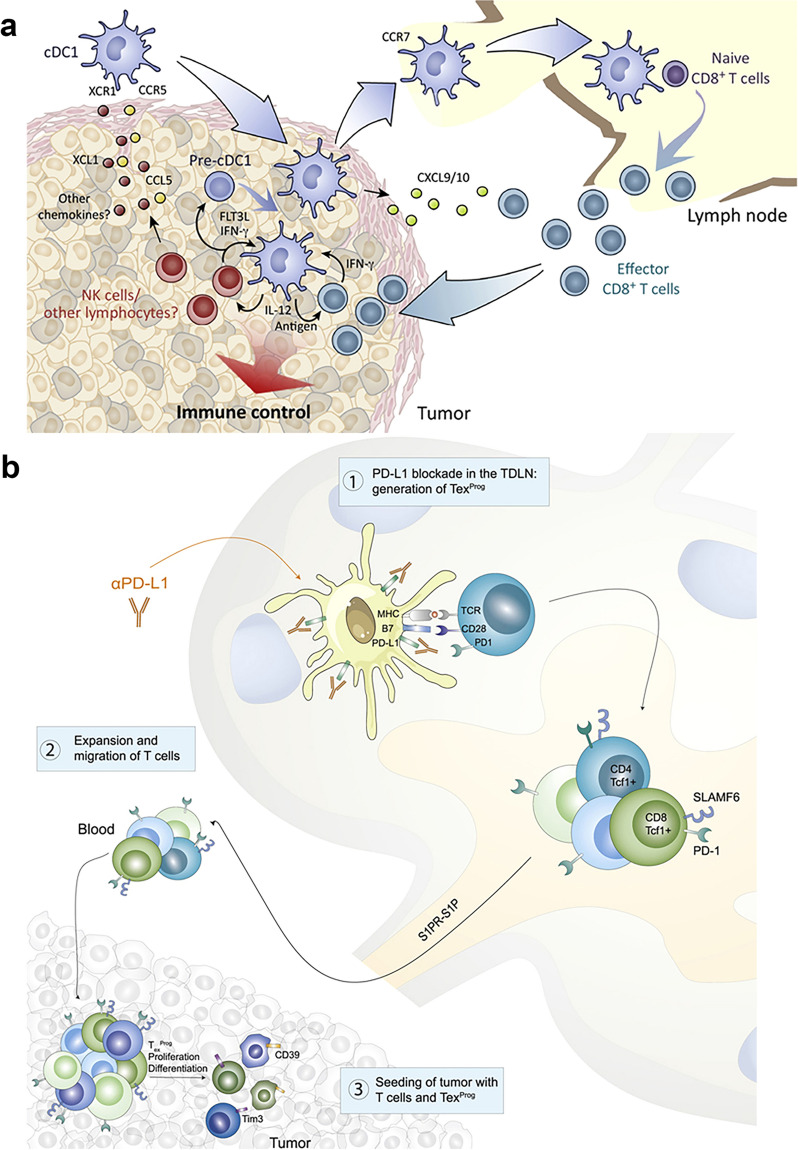
Immune cells play a crucial role in lymph node metastasis in cancer, acting as a double-edged sword [224]. On one hand, they can suppress tumor growth, while on the other, they may facilitate tumor spread [225]. Imaging techniques such as PET scans, MRI, and advanced microscopy have enabled a deeper understanding of these interactions [226]. For example, T cells (CD8 + and CD4 +), known for their tumor-fighting abilities, can be visualized congregating around tumor cells, indicating an immune response [227]. However, some T cells (like regulatory T cells or Tregs) can suppress immune responses, thereby aiding cancer spread [228]. B cells, another type of immune cell, are often found in tertiary lymphoid structures and can influence tumor behavior [229]. Macrophages (M1 and M2 types) have dual roles; M1 macrophages combat cancer, while M2 macrophages can support tumor growth and metastasis [230]. Natural Killer (NK) cells are critical in early cancer detection and elimination, but their activity can be diminished in a tumor microenvironment [228]. Dendritic cells present antigens to T cells, but can also be altered by tumors to evade immune detection (Fig. 4). Understanding these complex interactions through imaging provides insights into the mechanisms of lymph node metastasis, paving the way for targeted therapies [230].
Fig. 4.
illustrates the following processes: a Conventional type 1 dendritic cells (cDC1s) capture and deliver tumor antigens to tumor-draining lymph nodes (TDLNs), where they activate naïve CD8 + T cells, leading to the development of cytotoxic effector CD8 + T cells. This image is copyrighted by the Francis Crick Institute in 2018 [231]. b TDLNs exhibit a high concentration of tumor-specific PD-1 + T cells. Inhibiting PD-L1 in TDLNs results in the formation of progenitor-exhausted T cells, which then infiltrate the tumor, thereby boosting antitumor immunity. This part of the figure is copyrighted by Elsevier Inc. in 2020 [232]. Definitions included in this description are cDC1 for conventional type 1 dendritic cells, and TDLN for tumor-draining lymph node
Molecular interactions within lymph nodes play a pivotal role in cancer metastasis [232]. Advanced imaging techniques like fluorescence microscopy and confocal microscopy have shed light on these interactions [233]. Key molecules include cytokines, chemokines, adhesion molecules, and growth factors [234]. Cytokines like Interleukin-6 (IL-6) and Tumor Necrosis Factor-alpha (TNF-α) can promote tumor growth and metastasis [235]. Chemokines such as CCL21 and CXCL12 guide immune cells and cancer cells to lymph nodes, influencing metastasis [236]. Adhesion molecules like ICAM-1 and VCAM-1 facilitate the binding of cancer cells to the lymph node endothelium [237]. Growth factors such as VEGF and TGF-β play roles in angiogenesis and immune suppression, respectively [238]. These molecular interactions, visualized through imaging, highlight the dynamic environment of lymph nodes and their influence on cancer progression [239].
Immune checkpoint molecules, such as PD-1, PD-L1, CTLA-4, LAG-3, and TIM-3, play significant roles in regulating immune responses in cancer, including lymph node metastasis [240]. These molecules, visualized through techniques like immunohistochemistry and PET scans, are often upregulated in cancer cells and immune cells within the tumor microenvironment [241]. PD-1 and PD-L1 interaction, for instance, can inhibit T cell activity, allowing cancer cells to evade immune surveillance [242]. CTLA-4 competes with CD28 for binding to B7 molecules on antigen-presenting cells, reducing T cell activation [243]. LAG-3 and TIM-3 are other inhibitory receptors that contribute to T cell exhaustion [227]. By imaging these interactions, researchers understand how tumors exploit immune checkpoints to promote metastasis and escape immune destruction [244]. This knowledge has led to the development of checkpoint inhibitors as a form of cancer therapy [245].
Lymphangiogenesis, the formation of new lymphatic vessels, is a critical process in lymph node metastasis in cancer [6, 246]. Imaging techniques like lymphoscintigraphy, MRI, and near-infrared fluorescence imaging have been instrumental in studying this phenomenon [247]. Factors such as VEGF-C, VEGF-D, and their receptor VEGFR-3 are heavily involved in promoting lymphangiogenesis [248]. These factors, visualized through various imaging modalities, lead to the expansion of the lymphatic network, facilitating the spread of cancer cells to lymph nodes [249]. Angiopoietins (Ang-1 and Ang-2) and their receptor Tie-2 also play roles in lymphatic vessel remodeling and stability [250]. Prox1, a transcription factor, is essential for the differentiation and maintenance of lymphatic endothelial cells [251]. Through imaging, the process of lymphangiogenesis and its contribution to cancer metastasis can be observed, offering insights into potential therapeutic targets [252].
Role of photothermal therapy (PTT) and photodynamic therapy (PDT) in the treatment of lymph node metastasis
Recent advances in the treatment of lymph node metastases have highlighted the potential of Photothermal Therapy (PTT) and Photodynamic Therapy (PDT) as effective strategies in the oncological arsenal. These modalities offer minimally invasive options aimed at targeted destruction of cancer cells with minimal damage to surrounding healthy tissues, which is particularly crucial in the management of lymph node metastases [252]. PTT involves the use of nanoparticles that are excited by near-infrared light, leading to the generation of heat that selectively destroys cancerous cells [161]. The application of PTT in lymph node metastasis is predicated on the ability of these nanoparticles to specifically accumulate in metastatic lymph nodes due to enhanced permeability and retention effect. Studies have shown that PTT can effectively eradicate lymphatic tumors and prevent further spread of the disease. The precise control over the area being treated with PTT minimizes the risk to adjacent structures and preserves lymphatic function, which is essential for preventing complications such as lymphedema [233]. PDT is another promising approach where photosensitizing agents are administered that selectively accumulate in cancerous tissues. Upon activation by a specific wavelength of light, these agents produce reactive oxygen species that induce cell death. PDT has been particularly noted for its dual role in directly killing tumor cells and damaging the vasculature supplying the tumor, thereby causing tumor necrosis and reducing metastatic potential. The specificity of light activation in PDT allows for targeted therapy, which is crucial for lymph node metastases that are adjacent to critical anatomical structures [204]. PTT and PDT leverage the enhanced permeability and retention effect, which allows nanoparticles and photosensitizing agents to accumulate more readily in tumor tissues than in normal tissues [154]. This selective accumulation enables targeted treatment of metastatic lymph nodes, minimizing damage to surrounding healthy structures and reducing systemic side effects. The primary benefits include their minimally invasive nature and their ability to precisely target affected lymph nodes. PTT uses heat generated by nanoparticles to destroy cancer cells, while PDT uses light-activated photosensitizers to initiate a chemical reaction that kills cancer cells and disrupts tumor vasculature. Both methods offer controlled treatment, preserving lymphatic architecture and function, which is crucial for preventing secondary complications like lymphedema [252].
Rationale for targeted therapy in lymph node metastasis
Drugs targeting the HER2/neu receptor, such as Trastuzumab, work by binding to the HER2 protein on the surface of cancer cells [253]. This binding inhibits the proliferation of cancer cells that overexpress this receptor, which is a common feature in some breast cancers [254]. For example, Trastuzumab, a monoclonal antibody, binds to the HER2/neu receptor, blocking its ability to receive growth signals, thus inhibiting tumor growth [255]. Besides HER2/neu, other genes play significant roles in breast cancer [256]. One such gene is BRCA1/BRCA2, mutations in which increase the risk of breast cancer and are targets for PARP inhibitors [257]. The TP53 gene, often mutated in breast cancer, is crucial for DNA repair and apoptosis [258]. The PIK3CA gene, frequently mutated in breast cancer, is involved in cell growth and survival pathways [259]. Estrogen receptor (ER) and progesterone receptor (PR) genes influence the growth of breast cancer cells through hormone signaling pathways [260]. Understanding these genes’ roles and functions provides a basis for targeted therapeutic interventions, offering more personalized and effective treatment options for breast cancer patients [261].
PARP inhibitors, such as Olaparib, target and inhibit the enzyme Poly (ADP-ribose) polymerase (PARP), which plays a critical role in repairing DNA single-strand breaks [262]. In cells with BRCA1 or BRCA2 mutations, the double-strand DNA repair mechanism is already compromised [263]. When PARP inhibitors are used, they exploit this vulnerability by blocking the single-strand DNA repair pathway, leading to cell death, particularly in cancer cells [264]. Besides BRCA1 and BRCA2, other genes involved in the DNA repair pathway include ATM, which senses DNA damage and initiates repair; CHEK2, which works in concert with ATM to regulate cell cycle and repair; PALB2, which assists BRCA2 in repairing double-strand breaks; and RAD51, which plays a key role in homologous recombination repair [265]. Understanding these genes’ roles in DNA repair pathways has been crucial in developing targeted therapies like PARP inhibitors, which offer a more tailored approach in treating cancers, particularly those with BRCA mutations [266].
Tyrosine kinase inhibitors (TKIs), such as Imatinib, target tyrosine kinases, enzymes that play a vital role in the signaling pathways that control cell growth and survival [267]. By inhibiting these enzymes, TKIs can block the proliferation of cancer cells [268]. Imatinib, for instance, is effective against chronic myeloid leukemia (CML) by targeting the BCR-ABL fusion protein, a specific type of tyrosine kinase [269]. Other genes crucial for cancer cell growth and survival include EGFR, which encodes a protein involved in cell growth and division; KRAS, which plays a role in cell signaling pathways that control cell growth and death; BCL-2, which helps regulate cell death (apoptosis); MYC, which is involved in cell cycle progression and apoptosis; and PTEN, a tumor suppressor gene that negatively regulates the PI3K/AKT signaling pathway [270]. Understanding the functions of these genes has led to the development of targeted therapies that can effectively combat cancer by disrupting specific molecular pathways crucial for tumor growth and survival [271].
PD-1/PD-L1 inhibitors, such as Nivolumab and Pembrolizumab, enhance the immune system’s ability to fight cancer by blocking the interaction between PD-1 receptors on T-cells and PD-L1 proteins on cancer cells [272]. Normally, this interaction helps to keep the immune system in check, but cancer cells can exploit it to avoid immune attack [273]. By inhibiting this interaction, PD-1/PD-L1 inhibitors unmask cancer cells, allowing the immune system to recognize and destroy them [274]. Other significant immune checkpoints include CTLA-4, another receptor on T-cells that, when blocked, can enhance immune responses against cancer cells; LAG-3, which negatively regulates T-cell proliferation; TIM-3, which is involved in immune tolerance and is often upregulated in advanced cancers; and TIGIT, which also functions as an immune checkpoint and is a target for cancer immunotherapy [275]. Research into these checkpoints has revolutionized cancer treatment, offering new strategies to harness the immune system against cancer [276].
Angiogenesis inhibitors, such as Bevacizumab, which targets vascular endothelial growth factor (VEGF), play a crucial role in cancer treatment by disrupting the tumor's blood supply [277]. Tumors need blood vessels to provide oxygen and nutrients for their growth and to remove waste products [209]. By inhibiting angiogenesis, these drugs starve the tumor of its necessary supplies, hindering its growth and spread [278]. Other genes involved in tumor blood supply include FGF (Fibroblast Growth Factor), which also stimulates blood vessel formation; PDGF (Platelet-Derived Growth Factor), which is involved in the growth of blood vessels and is a target for some cancer treatments; TGF-β (Transforming Growth Factor Beta), which plays a role in angiogenesis and tumor progression; and HIF-1 (Hypoxia-Inducible Factor 1), a transcription factor that responds to low oxygen levels and can promote angiogenesis [279]. Understanding these genes and their roles in angiogenesis has been pivotal in developing treatments that can effectively cut off the blood supply to tumors, thereby inhibiting their growth and metastasis [280].
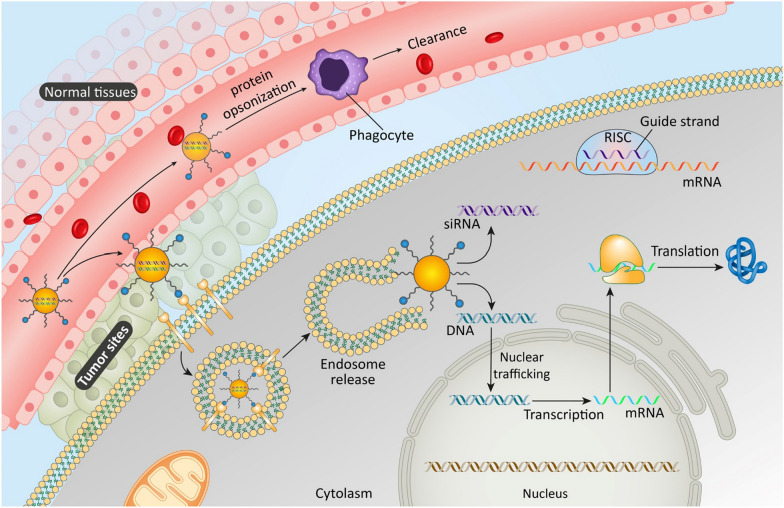
Nanobiotechnological approaches in drug delivery
Table 4 provides an overview of the various nanobiotechnological approaches used in targeted drug delivery for treating lymph node metastasis in different types of cancer. In the realm of nanobiotechnology, several nanoparticles are instrumental in targeting lymph node metastasis in cancer. These include liposomes, dendrimers, quantum dots, solid lipid nanoparticles, and polymeric nanoparticles [281]. Figure 5 illustrates a precision medicine nanoplatform designed for metastatic lymph nodes, facilitating dual-modal imaging using ultrasound and photoacoustic methods. Recent clinical trials have been focusing on various gene delivery methods for treating melanoma. One such experimental treatment is Allovectin-7®, currently in a Phase II trial (NCT00044356). This study aims to determine if Allovectin-7® can effectively reduce the size of melanoma tumors and delay the disease’s progression. Another notable trial involves the use of F5 TCR with dendritic cells, under Phase II (NCT00910650). This trial uses an apheresis product to generate gene-modified MART-1 TCR CTLs and dendritic cells, verifying their expression of the correct TCR. Additionally, a Phase I trial (NCT00512889) is investigating CTLs combined with artificial antigen presenting cells (aAPCs). The focus here is on the feasibility and side effects of administering intravenous infusions of lab-produced CTLs, which are derived from leukapheresis and augmented with additional genes. GVAX is being examined in a Phase I trial (NCT00258687) for its efficacy against Clear Cell Sarcoma. The specific details and outcomes of this trial are encapsulated under the identifier Procedia#apol14p. The trial for HBI 0201 /ESO TCRT, a Phase I/II study (NCT05296564), is investigating the use of anti-NY-ESO-1 TCR-Gene Engineered Lymphocytes (HBI 0201-ESO TCRT) by infusion in patients with NY-ESO-1 expressing metastatic cancers. This trial aims to evaluate the dose escalation, safety, and efficacy of this approach. In a similar vein, HX008/OH2 is in a Phase I/II trial (NCT04616443). This study involves the use of the herpes simplex virus type 2 strain HG52, genetically modified to become OH2, an oncolytic virus replicating only in tumor cells. This virus is enhanced with a gene encoding human granulocyte macrophage colony-stimulating factor (GM-CSF), potentially inducing a stronger antitumor immune response. Finally, the RNA/Lipo-MERIT vaccine, in a Phase I trial, targets four specific malignant melanoma-associated antigens: Tyrosinase, Melanoma-Associated Antigen A3 (MAGE-A3), New York-ESO 1 (NY-ESO-1), and Trans-membrane phosphatase with Tensin Homology (TPTE). The goal of this study is to assess the vaccine's efficacy against these targets in melanoma treatment. Each of these nanoparticles plays a crucial role in improving the accuracy and effectiveness of cancer treatments, highlighting the innovative advancements in nanobiotechnology (Fig. 6).
Table 4.
Nanobiotechnological advances in targeted drug delivery for lymph node metastasis in cancer
| Nanocarrier type | Cancer type | Mechanism | Efficacy results | Refs. |
|---|---|---|---|---|
| Liposomes | Breast cancer | Enhanced permeability and retention (EPR) effect; targeted delivery to metastatic lymph nodes | Improved drug accumulation in lymph nodes; reduced systemic toxicity | [319] |
| Gold nanoparticles | Melanoma | Active targeting using specific ligands; photothermal therapy | Increased tumor regression; minimal side effects | [320] |
| Dendrimers | Prostate cancer | Passive targeting through size and surface modifications; controlled drug release | Higher drug concentration in cancerous lymph nodes; lower adverse effects | [321] |
| Polymeric nanoparticles | Lung cancer | Targeted delivery via surface functionalization; co-delivery of drugs and genes | Enhanced therapeutic efficacy; reduced drug resistance | [322] |
| Magnetic nanoparticles | Head and neck cancer | Magnetic targeting; hyperthermia therapy | Localized treatment; improved survival rates | [323] |
| Quantum dots | Colorectal cancer | Image-guided drug delivery; real-time monitoring of drug distribution | Precise targeting; optimized dosage and treatment monitoring | [324] |
| Carbon nanotubes | Cervical cancer | Active targeting with antibodies; combination therapy (drug and heat) | Synergistic effect of chemotherapy and hyperthermia; improved treatment response | [325] |
| Micelles | Ovarian cancer | Enhanced drug solubility and stability; targeted delivery through surface modifications | Improved targeting of metastatic sites; reduced off-target effects | [326] |
| Silica nanoparticles | Pancreatic cancer | Site-specific drug release; enzyme-responsive drug release in tumor environment | Higher therapeutic index; minimal impact on healthy tissue | [327] |
| Lipid-based nanocarriers | Bladder cancer | Mucoadhesive properties for intravesical therapy; sustained drug release | Increased drug retention in bladder; enhanced local efficacy | [328] |
| Exosome-based delivery | Gastric cancer | Natural biocompatibility; targeted delivery through surface proteins | Reduced immune response; improved drug delivery to metastatic lymph nodes | [329] |
| Polymeric Micelles | Thyroid cancer | Active targeting with thyroid-specific ligands; controlled release kinetics | Selective accumulation in thyroid cancer cells; low systemic toxicity | [330] |
| Metal–organic frameworks | Renal cancer | High drug loading capacity; stimuli-responsive release | Efficient drug delivery to tumor sites; reduced renal clearance | [331] |
| Hybrid nanoparticles | Glioblastoma | Blood–brain barrier penetration; dual drug delivery system | Enhanced delivery to brain tumors; synergistic therapeutic effects | [332] |
| Albumin nanoparticles | Skin cancer (non-melanoma) | Tumor microenvironment targeting; enhanced permeation | Improved localization at tumor sites; reduced toxicity to normal cells | [333] |
| Mesoporous silica nanoparticles | Sarcoma | High drug loading, pH-sensitive release in tumor microenvironment | Efficient targeting of sarcoma cells; decreased systemic side effects | [334] |
| Nanodiamonds | Leukemia | Drug delivery via surface adsorption; biocompatibility | Sustained drug release in target cells; low immunogenicity | [335] |
| Polymeric nanocapsules | Brain cancer | Enhanced blood–brain barrier penetration; targeted delivery to tumor cells | Increased drug concentration in brain tumors; reduced peripheral toxicity | [336] |
| Iron oxide nanoparticles | Lymphoma | Magnetic targeting; diagnostic and therapeutic (theranostic) applications | Targeted therapy with real-time imaging; improved treatment monitoring | [337] |
| Nanogels | Esophageal cancer | Responsive drug release; protection of therapeutic agents | Enhanced delivery to esophageal cancer cells; reduced degradation of drugs | [338] |
| Hollow nanospheres | Osteosarcoma | Targeted delivery and controlled release; high drug encapsulation efficiency | Increased accumulation in tumor tissues; effective treatment of metastasis | [339] |
| Hydrogel nanoparticles | Hepatocellular carcinoma | Enhanced liver targeting; slow and controlled drug release | Improved drug concentration in liver tumors; reduced systemic exposure | [340] |
| Chitosan nanoparticles | Endometrial cancer | Mucoadhesive properties for targeted delivery; bioresponsive degradation | Increased retention and efficacy in endometrial tissue; lower adverse effects | [341] |
| Nanoemulsions | Thyroid cancer | Enhanced solubility of poorly water-soluble drugs; active targeting using thyroid-specific antibodies | Improved bioavailability; specific targeting of thyroid cancer cells | [342] |
| Poly(lactic-co-glycolic acid) (PLGA) Nanoparticles | Kidney cancer | Biodegradable and biocompatible carrier; sustained drug release | Reduced nephrotoxicity; enhanced accumulation in renal cancer cells | [343] |
| magnetic liposomes | Prostate cancer | Magnetic field-directed targeting; combination of drug and hyperthermia therapy | Localized treatment effects; synergistic improvement in tumor reduction | [344] |
| Cerium oxide nanoparticles | Colorectal cancer | Antioxidant properties; protection of normal cells from oxidative stress | Reduced side effects; enhanced targeting of colorectal tumor cells | [345] |
| Silver nanoparticles | Bladder cancer | Anti-microbial properties; prevention of post-surgical infections | Lowered risk of infection in bladder cancer patients undergoing treatment | [346] |
| Zeolite nanoparticles | Ovarian cancer | High surface area for drug adsorption; targeted delivery using ovarian-specific ligands | Efficient delivery to ovarian tumors; reduced off-target effects | [347] |
| Carbon nanocapsules | Pancreatic cancer | High drug loading efficiency; protection of encapsulated drugs from degradation | Improved stability and efficacy of the therapeutic agent in pancreatic tumors | [348] |
| Peptide nanofibers | Breast cancer | Targeted delivery using breast cancer-specific peptides; enhanced cellular uptake | Higher specificity for breast cancer cells; reduced impact on healthy tissue | [349] |
| Nanocrystals | Squamous cell carcinoma | Improved solubility and bioavailability of poorly soluble drugs; passive targeting to tumor sites | Enhanced drug delivery to tumor sites; improved treatment efficacy | [350] |
| Polymer-dendrimer hybrid nanoparticles | Liver cancer | Targeted delivery to liver cells; dual drug loading capacity | Efficient delivery and reduced toxicity in liver cancer treatment | [351] |
| Bimetallic nanoparticles | Oral cancer | Theranostic application; imaging and therapy | Enhanced tumor imaging and targeted therapy; improved treatment monitoring | [352] |
| Polyethylene glycol (PEG) nanoparticles | Glioma | Enhanced brain penetration; targeted delivery to tumor cells | Improved drug delivery across the blood–brain barrier; targeted action at tumor sites | [353] |
| Nanostructured lipid carriers | Cervical cancer | Improved stability and prolonged release of drugs; targeted delivery using cervical cancer-specific ligands | Enhanced efficacy and reduced systemic toxicity in cervical cancer treatment | [354] |
| Inorganic nanocarriers (e.g., silica, gold) | Osteosarcoma | Targeted drug delivery; multimodal therapy options (e.g., thermal ablation) | Improved targeting and treatment outcomes; potential for combination therapies | [355] |
| Biodegradable nanospheres | Melanoma | Controlled release; targeted delivery with melanoma-specific ligands | Enhanced drug accumulation in melanoma cells; minimal side effects | [356] |
| Nanobubbles | Bladder cancer | Ultrasound-mediated drug delivery; enhanced permeability and retention effect | Targeted drug delivery and improved treatment efficacy in bladder cancer | [357] |
| Stimuli-responsive Nanoparticles | Lung cancer | Targeted delivery triggered by pH/tumor microenvironment; controlled release | Enhanced targeting and treatment efficacy in lung cancer cells | [358] |
| Superparamagnetic iron oxide nanoparticles | Brain tumors | Magnetic targeting; imaging contrast agents | Improved imaging of tumor sites; targeted drug delivery with magnetic guidance | [359] |
| Gold nanorods | Oral squamous cell carcinoma | Photothermal therapy; localized heating to release drugs | Efficient tumor ablation; targeted drug release at tumor site | [360] |
| Lipid-polymer hybrid nanoparticles | Colorectal cancer | Enhanced drug stability; targeted delivery with colorectal-specific ligands | Improved drug retention in colorectal tumors; reduced side effects | [361] |
| Carbon quantum dots | Breast cancer | Fluorescence imaging; targeted drug delivery | Effective tumor imaging and targeted therapy; low toxicity | [362] |
| Hollow gold nanospheres | Pancreatic cancer | Photothermal therapy; targeted heat-induced drug release | Selective tumor cell destruction; controlled drug release | [363] |
| Nanostructured lipid carriers | Prostate cancer | Improved solubility of hydrophobic drugs; sustained release | Higher drug bioavailability in prostate tumors; reduced systemic toxicity | [364] |
| Polymeric nanogels | Ovarian cancer | Targeted delivery using ovarian cancer-specific markers; responsive drug release | Increased targeting accuracy; enhanced drug effectiveness with reduced side effects | [365] |
| Silica-based nanoparticles | Renal cell carcinoma | High drug loading capacity; controlled and sustained release | Efficient delivery of therapeutics to renal tumors; minimal renal toxicity | [366] |
| Multifunctional nanoparticles | Head and neck cancer | Combination therapy delivery; targeted imaging and treatment | Enhanced drug delivery efficiency; improved treatment monitoring and outcomes | [367] |
| Quantum dot nanocarriers | Thyroid cancer | Targeted imaging and drug delivery; real-time tumor tracking | Precise drug delivery with imaging; improved treatment efficacy | [368] |
| Nanoscale metal–organic frameworks | Bladder cancer | High drug loading; controlled release in tumor microenvironment | Targeted therapy with reduced systemic toxicity; improved therapeutic outcomes | [369] |
| Polymeric nanobubbles | Skin cancer (melanoma) | Ultrasound-triggered drug release; enhanced tumor penetration | Improved drug delivery to deep-seated tumors; enhanced treatment efficacy | [370] |
| Self-assembling nanofibers | Pancreatic cancer | Targeted drug delivery; enhanced penetration in dense tumor stroma | Improved drug delivery in fibrotic pancreatic tumors; reduced systemic side effects | [371] |
| Magnetic nanoclusters | Osteosarcoma | Magnetic field-directed targeting; enhanced delivery to bone tumors | Improved targeting to osteosarcoma sites; potential for hyperthermia therapy | [372] |
| Nanostructured surfaces for drug delivery | Gastric cancer | Enhanced mucosal adhesion; localized and sustained drug release | Increased drug concentration at the tumor site; reduced systemic absorption | [373] |
| Lipid-based nanovesicles | Cervical cancer | Targeted drug delivery via cervical cancer-specific antigens | Enhanced specificity for cervical cancer cells; minimized impact on non-cancerous cells | [374] |
| Biodegradable nanofibers | Liver cancer | Controlled drug release; specific targeting to liver cells | Improved drug delivery to liver tumors with minimal off-target effects | [375] |
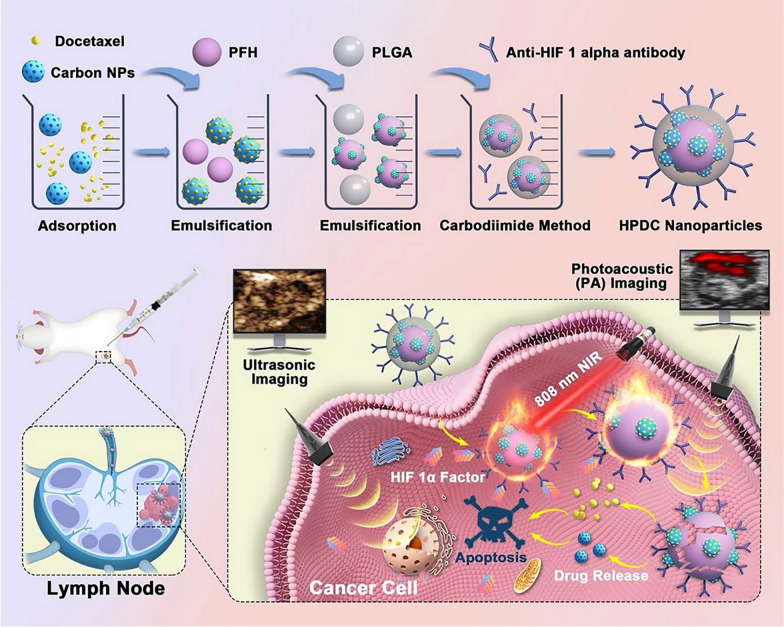
Fig. 5.
illustrates a precision medicine platform designed for visualizing metastatic lymph nodes. This platform employs ultrasonic/photoacoustic dual-modal imaging to guide targeted hyperthermia and combined chemotherapy directly at the site. The image, copyrighted in 2021 by Springer Nature [282], features several components: nanoparticles (NP), perfluorohexane (PFH), poly(lactic-co-glycolic acid) (PLGA), and lymph nodes (LN)
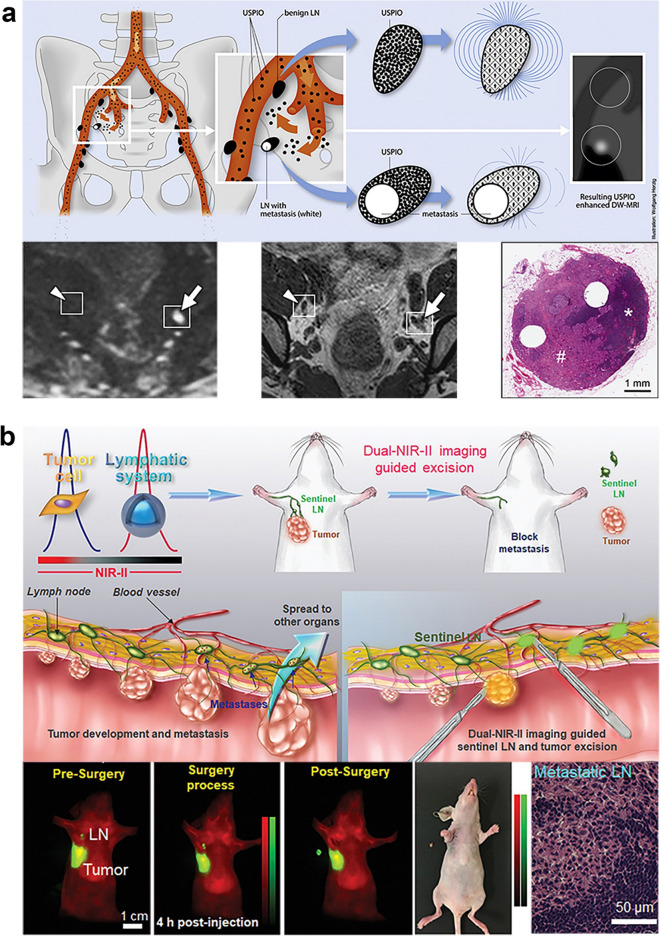
Fig. 6.
Two different applications: a The use of ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles for identifying metastases in normally-sized pelvic LN in individuals with bladder and prostate cancer. These nanoparticles are absorbed by macrophages, resulting in a reduced signal in T2- or T2*-weighted magnetic resonance imaging (MRI). This reduction is not observed in malignant LN (indicated by an arrow) due to their lower macrophage count and minimal absorption of USPIO nanoparticles, unlike benign LN (marked by an arrowhead).
Copyright 2013 by the European Association of Urology [283]. b The implementation of a near-infrared (NIR) probe for detecting lymph node metastasis (LNM) in mice. The images show NIR imaging-assisted sentinel lymph node (SLN) surgery in a mouse model of orthotopic 4T1 breast cancer. Copyright 2020 by Wiley–VCH [284]
Liposomes are a pivotal component in the treatment of lymph node metastasis in cancer, primarily due to their unique structural and compositional characteristics [283]. As spherical vesicles formed from lipid bilayers, they encapsulate chemotherapeutic drugs, protecting them from degradation and ensuring targeted delivery [284]. Once administered, liposomes circulate in the bloodstream and accumulate in the cancer-affected lymph nodes [285]. Their design allows them to release the encapsulated drugs specifically at the metastatic site [286]. This targeted release ensures a higher concentration of the drug at the tumor site, enhancing therapeutic efficacy [287]. Moreover, liposomes can be engineered to be sensitive to the microenvironment of cancer cells, such as pH or temperature, triggering drug release precisely where needed [288]. This not only maximizes the impact on cancer cells but also significantly reduces the systemic toxicity often associated with chemotherapy [289]. The use of liposomes in targeting lymph node metastasis exemplifies the progress in personalized medicine, offering a more effective and safer alternative to conventional cancer treatments [290].
Dendrimers are a novel class of nanoparticles playing a transformative role in targeting lymph node metastasis in cancer treatments [291]. These nanosized, branched polymers have a unique architecture that allows for the attachment of multiple drug molecules [292]. The multivalency of dendrimers enables the simultaneous delivery of different therapeutic agents, potentially enhancing treatment efficacy [293]. Their size and surface functionality can be precisely controlled, allowing for targeted delivery to metastatic sites in the lymph nodes [294]. Dendrimers can be engineered to interact with specific cancer cell markers, ensuring that the drug payload is delivered directly to cancer cells, thereby reducing the impact on healthy cells [295]. Furthermore, the branched structure of dendrimers provides a controlled release mechanism, ensuring that the drugs are released over a sustained period, which can be crucial for effective cancer treatment [296]. This controlled release also reduces the frequency of drug administration, improving patient compliance and comfort [297]. Dendrimers' versatility and efficiency in drug delivery systems represent a significant advancement in nanotechnology-based cancer therapies, providing a promising approach to treating lymph node metastasis [298].
Quantum dots (QDs) are semiconductor nanoparticles that offer groundbreaking applications in the management of lymph node metastasis in cancer [299]. Their primary role lies in imaging and tracking the spread of cancer to the lymph nodes [300]. Quantum dots have unique optical properties, such as size-tunable light emission, which makes them highly effective for biomedical imaging [301]. When used in cancer patients, QDs can be engineered to bind to specific tumor markers, allowing for precise visualization of metastatic cancer cells in the lymph nodes [302]. This targeted imaging provides invaluable information about the extent and progression of cancer, aiding in the formulation of effective treatment strategies [303]. Additionally, the high brightness and stability of QDs enhance the quality of imaging, facilitating early detection of metastasis, which is crucial for successful cancer treatment [304]. The real-time tracking capability of quantum dots also allows for monitoring the efficacy of therapeutic interventions, enabling adjustments in treatment plans as needed [305]. The integration of quantum dots into cancer management underscores the potential of nanotechnology in advancing diagnostic and therapeutic approaches in oncology [306].
Solid lipid nanoparticles (SLNs) and polymeric nanoparticles are critical in cancer treatment, particularly for targeting lymph node metastasis [307]. SLNs are characterized by their solid lipid core, which can effectively encapsulate lipophilic drugs, enhancing their solubility and stability [308]. This feature is particularly beneficial in the treatment of cancer, where many chemotherapeutic agents are hydrophobic [309]. The biodegradable nature of SLNs ensures minimal toxicity, and their ability to bypass biological barriers allows for efficient drug delivery to lymph nodes [310]. On the other hand, polymeric nanoparticles offer versatility in terms of composition, structure, and functionality [311]. They can be engineered to have specific shapes, sizes, and surface characteristics, enabling targeted drug delivery [312]. Polymers can be functionalized with ligands that bind specifically to cancer cells, ensuring that the drug payload is delivered directly to the affected lymph nodes [313]. Both SLNs and polymeric nanoparticles can be designed to release their drug payload in a controlled manner, providing a sustained therapeutic effect [314]. This controlled release reduces the need for frequent dosing, improving patient compliance [315]. The use of SLNs and polymeric nanoparticles in targeting lymph node metastasis represents a significant advancement in nanotechnology-based drug delivery systems, offering a more effective and patient-friendly approach to cancer treatment [316]. Figure 7 depicts recent advancements in gene delivery for melanoma using polymeric and lipid-based nanocarriers.
Fig. 7.
Recent advancements in polymer and lipid-based nanocarriers for gene therapy in melanoma. This includes A, i a diagram of RRPHC ternary complexes, (ii) a comparison of transfection efficiency in B16F10 cells using PF33/pGFP, RRPHC/pGFP, and Lipofectamine 2000/pGFP, and (iii) the administration of HAC/pDNA and RRPHC/pDNA through intravenous injection, followed by in vivo and ex vivo fluorescence imaging in A375 tumor-bearing nude mice (specifically RRPHC). This information, originally from L. Li et al., 2016, is used with Elsevier's permission [317]. Section (B) focuses on human skin samples that were intradermally injected with lipid nanoparticle (LNP) formulations varying in lipid composition and dosage. This part includes (i) ex vivo imaging of the samples after 11 days and (ii) quantification of the luciferase image. This section is reproduced with permission from Blakney et al., 2019, and is under the copyright of the American Chemical Society [318]
Nanobiotechnology in enhancing immunotherapy
Nanoparticles play a crucial role in enhancing the efficacy of immunotherapy for treating lymph node metastases in cancer [376]. Their small size and customizable surface properties allow them to target and accumulate in lymph nodes effectively [377]. This targeted delivery is vital in improving the immunotherapy response [378]. For instance, liposomal nanoparticles can deliver PD-1 inhibitors directly to lymph nodes, significantly enhancing T-cell activation against cancer cells [379]. PD-1, or Programmed Death-1, is a crucial gene in regulating the immune system's response to cancer cells [380]. Inhibiting PD-1 allows T-cells to attack cancer cells more effectively [381]. Similarly, nanoparticles carrying CTLA-4 antibodies can inhibit the CTLA-4 pathway, promoting an immune response against tumor cells [382]. CTLA-4, or Cytotoxic T-Lymphocyte-Associated protein 4, is another immune checkpoint that, when blocked, can enhance the immune system's ability to fight cancer [383]. Additionally, the delivery of siRNA targeting genes like TGF-β, which plays a role in immune suppression in the tumor microenvironment, can further enhance the immune response [384]. TGF-β, or Transforming Growth Factor Beta, is often involved in creating an environment that suppresses the immune system's ability to combat cancer [385]. Moreover, nanoparticles can modulate the tumor microenvironment by altering the expression of factors like VEGF and IL-10 [336]. VEGF, or Vascular Endothelial Growth Factor, promotes tumor growth and angiogenesis, while IL-10, or Interleukin-10, is known to suppress immune responses [337]. By targeting these factors, nanoparticles create a more favorable environment for immunotherapy [338]. Lastly, nanoparticles delivering CRISPR/Cas9 gene-editing tools targeting genes like FOXP3 can reduce immune suppression [339]. FOXP3 is often overexpressed in regulatory T cells in cancer and plays a significant role in suppressing the immune response [340]. By editing this gene, nanoparticles can enhance the effectiveness of immunotherapy in treating lymph node metastases in cancer [341].
In the context of cancer immunotherapy, specific genes and factors like PD-1, CTLA-4, TGF-β, VEGF, and IL-10 play pivotal roles, and nanoparticles are engineered to target these effectively [342]. PD-1, or Programmed Death-1, is a gene that codes for a protein on the surface of T-cells [343]. It negatively regulates the immune response, and cancer cells often exploit this pathway to evade immune detection [344]. Nanoparticles can deliver PD-1 inhibitors to lymph nodes, thereby blocking this pathway and enhancing the T-cell-mediated attack on cancer cells [345]. CTLA-4, or Cytotoxic T-Lymphocyte-Associated protein 4, is another checkpoint protein that inhibits T-cell activation [346]. Nanoparticles carrying CTLA-4 antibodies can effectively block this pathway, promoting a stronger immune response against tumor cells [347]. TGF-β, or Transforming Growth Factor Beta, is a multifunctional cytokine that plays a role in immune suppression within the tumor microenvironment [348]. Nanoparticles delivering siRNA to silence TGF-β can prevent this suppression, thereby enhancing the immune system's ability to fight cancer [349]. VEGF, or Vascular Endothelial Growth Factor, is crucial in tumor growth and angiogenesis [350]. Nanoparticles can be designed to modulate VEGF expression, thereby hindering tumor growth and metastasis [351]. Lastly, IL-10, or Interleukin-10, is an anti-inflammatory cytokine that can suppress immune responses in the tumor microenvironment [352]. By targeting IL-10, nanoparticles can shift the balance towards a pro-inflammatory and anti-tumor environment, facilitating more effective immunotherapy [353]. Nanoparticles significantly contribute to the modulation of the tumor microenvironment by targeting factors like VEGF and IL-10 [354]. VEGF, or Vascular Endothelial Growth Factor, is a key protein that stimulates angiogenesis, the formation of new blood vessels, which is essential for tumor growth and metastasis. Nanoparticles can be engineered to interfere with the VEGF pathway, either by delivering agents that inhibit VEGF expression or by silencing the VEGF gene directly [355]. This intervention can effectively starve the tumor of the necessary blood supply, impeding its growth and spread [356].
IL-10, or Interleukin-10, is an anti-inflammatory cytokine that plays a role in suppressing immune responses in the tumor microenvironment [357]. This suppression is beneficial for the tumor, as it allows cancer cells to evade immune detection and destruction [358]. By targeting IL-10 with nanoparticles, either through the delivery of inhibitory molecules or gene silencing techniques, the tumor microenvironment can be shifted towards a more pro-inflammatory state [359]. This change enhances the effectiveness of immune cells against the tumor, thereby improving the overall response of immunotherapy [360]. The modulation of these factors by nanoparticles creates a more favorable environment for the immune system to attack the tumor [361]. By altering the balance of pro- and anti-tumor factors in the tumor microenvironment, nanoparticles help in orchestrating a more potent and targeted attack against cancer cells, leading to improved outcomes in cancer treatment [362]. The delivery of CRISPR/Cas9 gene-editing tools via nanoparticles is a significant advancement in cancer immunotherapy, particularly in targeting genes like FOXP3 [363]. FOXP3, a gene critical in the regulation of regulatory T cells (Tregs), often gets overexpressed in the cancer setting, leading to an increase in Tregs within the tumor microenvironment [356]. These Tregs play a role in suppressing the immune response against cancer cells [364]. By targeting FOXP3, it’s possible to reduce this suppression, enhancing the immune system's capacity to fight cancer [365]. Nanoparticles offer a precise and efficient means to deliver CRISPR/Cas9 to the tumor site [366]. This gene-editing technology can be used to either knock out or modulate the expression of FOXP3 in Tregs [367]. The ability to directly edit genes within the tumor microenvironment is a groundbreaking approach in cancer treatment, as it allows for a more targeted and effective modification of the immune response [368]. Moreover, the use of nanoparticles ensures that the CRISPR/Cas9 system is delivered specifically to the tumor site, minimizing off-target effects and potential systemic side effects [369]. This localized delivery is crucial in maximizing the therapeutic benefits while reducing the risk of unwanted immune reactions or other complications [370]. The integration of CRISPR/Cas9 gene-editing into nanoparticles represents a novel and promising strategy in the fight against cancer, offering a more precise and potentially powerful tool in cancer immunotherapy [371].
Nanoparticles carrying small interfering RNA (siRNA) targeting genes like TGF-β play a critical role in enhancing the immune system's ability to fight cancer [372]. TGF-β, or Transforming Growth Factor Beta, is a cytokine that is often implicated in promoting immune suppression within the tumor microenvironment [373]. It aids in the progression of cancer by inhibiting the immune system's ability to recognize and destroy cancer cells [374]. By using nanoparticles to deliver siRNA specifically designed to silence the TGF-β gene, it's possible to disrupt this immune suppression [375]. The siRNA works by binding to the mRNA of TGF-β, leading to its degradation and preventing the translation of the TGF-β protein [376]. This reduction in TGF-β levels can alleviate the immune-suppressive conditions in the tumor microenvironment, thereby reactivating the immune system's natural ability to fight cancer [377]. The targeted delivery of siRNA via nanoparticles ensures that the interference with TGF-β occurs directly at the tumor site, maximizing the therapeutic impact while minimizing potential side effects elsewhere in the body [378]. This precision not only increases the efficacy of the treatment but also reduces the risk of systemic immune reactions that could occur with broader immune system activation [379].
Integration of imaging and therapeutics
In the realm of theranostic interventions for lymph node metastasis, several innovative approaches have been employed to enhance both the detection and treatment of cancer [296]. Key theranostic interventions include the use of nanoparticles conjugated with therapeutic agents, which are engineered to target metastatic lymph nodes specifically [306]. These nanoparticles not only deliver drugs directly to the cancer cells but also possess imaging capabilities, allowing for simultaneous tracking and treatment of metastases [296]. Additionally, immunotherapeutic strategies targeting lymph node metastases have been combined with molecular imaging techniques to monitor immune responses in real-time [209].
The imaging methods used in these integrated approaches are varied and advanced [358]. Positron Emission Tomography (PET) and MRI are prominent modalities that provide high-resolution images and functional information about lymph node status. PET imaging, often using radiolabeled tracers, facilitates the detection of metabolic activity associated with cancer cells, while MRI offers detailed anatomical visualization [107]. Optical imaging techniques, such as fluorescence and bioluminescence imaging, are also utilized for their ability to provide real-time visualization of therapeutic agent distribution and tumor response. These imaging methods, when combined with targeted therapeutics, offer a powerful toolkit for the precise and effective management of lymph node metastasis in cancer [304].
HER2, or Human Epidermal Growth Factor Receptor 2, plays a crucial role in the progression of certain breast cancers [380]. This gene, when overexpressed, leads to aggressive cancer growth and spread, including to lymph nodes [381]. In theranostics, the overexpression of HER2 becomes a target for specialized agents that combine diagnostic imaging and therapeutic intervention [382]. By targeting HER2, theranostic agents can accurately localize metastatic sites in lymph nodes and deliver targeted treatment directly to these areas [383]. This approach is particularly effective because it enables personalized therapy, ensuring that patients with HER2-positive breast cancer receive treatments specifically tailored to their genetic profile [384]. Additionally, by focusing on the unique genetic makeup of the cancer cells, theranostic approaches reduce the impact on healthy tissues, minimizing side effects and enhancing treatment efficacy [385].
The CD20 gene, present on the surface of B cells, is instrumental in the theranostic approach to treating lymphomas, particularly those that metastasize to lymph nodes [386]. Targeting CD20 allows for the precise localization and treatment of these lymphomas [387]. Theranostic agents designed to bind to CD20 can be used both for diagnostic imaging and for delivering targeted therapies directly to cancer cells [388]. This targeted approach ensures that therapeutic agents are concentrated at the site of the tumor, maximizing their effectiveness while minimizing damage to healthy cells [388]. In the context of lymph node metastasis, this precise targeting is vital, as it allows for the treatment of cancer cells that have spread beyond the primary tumor site, offering a more comprehensive approach to cancer therapy [389].
Prostate-Specific Membrane Antigen (PSMA) is highly significant in the field of prostate cancer theranostics, particularly for identifying and treating metastatic sites in lymph nodes [390]. PSMA is a protein commonly found on prostate cancer cells, including those that have metastasized to lymph nodes [391]. In theranostic applications, agents targeting PSMA are used both for diagnostic imaging and for delivering targeted therapeutic drugs [392]. This dual functionality allows for the precise visualization of metastatic sites, enabling clinicians to better understand the extent and specific locations of the cancer spread [393]. Subsequently, the same PSMA-targeting agents can deliver treatment directly to these identified sites, ensuring a focused and effective therapeutic response [296]. This approach is particularly valuable in managing metastatic prostate cancer, as it allows for personalized treatment plans based on the specific characteristics of the patient's cancer [394].
KRAS gene mutations are common in various cancers and play a pivotal role in influencing personalized therapy in cancer theranostics, especially concerning lymph node metastasis [395]. These mutations often lead to uncontrolled cell growth and cancer progression [396]. In theranostics, detecting KRAS mutations is essential for developing personalized treatment strategies [397]. Theranostic agents can be tailored to target these specific mutations, allowing for precise treatment of cancers that have spread to lymph nodes [397]. By focusing on the unique genetic alterations of the cancer cells, theranostic approaches enable the delivery of highly specific and effective treatments, thereby improving the overall prognosis [397]. This personalized approach is particularly beneficial in treating cancers with KRAS mutations, as it addresses the genetic basis of the disease, leading to more effective and targeted therapy [398].
The CA125 gene, a marker for ovarian cancer, is crucial in the theranostic approach to treating this disease, especially when it involves lymph node metastasis [399]. In ovarian cancer, the CA125 protein is often overexpressed and can be used as a biomarker for the presence and progression of the disease [399]. In theranostics, agents that target the CA125 gene are employed both for diagnostic imaging and for the targeted delivery of therapeutics [400]. This dual application is particularly beneficial for identifying and treating metastatic sites in lymph nodes [401]. By specifically targeting the CA125 marker, theranostic agents can accurately locate metastatic cancer cells and deliver effective treatment directly to these sites [402]. This targeted approach not only enhances the effectiveness of the treatment but also reduces the likelihood of damaging healthy tissues, thereby improving the safety and outcomes for patients with ovarian cancer [403].
Differentiating sentinel lymph nodes from regional lymph nodes in cancer management
The identification of sentinel lymph nodes versus regional lymph nodes is a pivotal component in the staging and treatment of cancer, particularly in malignancies such as breast cancer and melanoma [376]. Sentinel lymph nodes are the first lymph nodes to which cancer cells are most likely to spread from a primary tumor. This concept is based on the premise that lymphatic drainage from a tumor follows a predictable pathway, initially involving one or a few key nodes before disseminating to a broader regional network [350].
The process of detecting SLNs typically involves injecting a tracer substance, such as a radioactive isotope or a blue dye, near the tumor site. This tracer travels through the lymphatic system and accumulates in the sentinel nodes [317]. Surgeons then use a gamma probe to detect the radioactive signal or visually identify the blue-stained nodes. This targeted approach allows for the removal of only the sentinel nodes for pathological examination, significantly reducing the need for extensive lymph node dissection and its associated morbidities [308].
In contrast, regional lymph nodes encompass a wider array of nodes within the lymphatic drainage basin of the tumor [393]. Assessing regional lymph nodes often involves more comprehensive procedures, such as axillary lymph node dissection (ALND) in breast cancer or inguinal lymph node dissection in melanoma. These procedures aim to remove a larger number of lymph nodes to evaluate the extent of cancer spread. While this provides thorough staging information and helps in planning further treatment, it also carries a higher risk of complications, including lymphedema, infection, and nerve damage [397].
The distinction between SLNs and regional lymph nodes is crucial for personalized cancer treatment. SLN biopsy is less invasive and focuses on the nodes most likely to harbor metastasis, allowing for early detection and timely intervention with minimal impact on the patient's quality of life [381]. If the sentinel nodes are free of cancer, patients can often avoid more extensive lymph node surgery. Conversely, if metastasis is detected in the SLNs, it may necessitate further regional lymph node assessment and more aggressive treatment strategies [384].
Advancements in imaging technologies, such as single-photon emission computed tomography (SPECT) combined with CT (SPECT/CT), have further refined the identification and localization of sentinel lymph nodes [388]. These imaging modalities provide three-dimensional visualization of the tracer uptake, enhancing the accuracy of sentinel lymph node detection and facilitating more precise surgical planning [390].
Clinical trials
The research field of oncology is advancing with multiple clinical trials focusing on the diagnosis and treatment of lymph node metastases across various types of cancer. These trials utilize different models, approaches, and technologies, aiming to improve patient outcomes through more accurate diagnosis and effective treatment plans.
One such trial is the “Prediction Model for Lateral Lymph Node Metastasis” under study ID NCT04635488, which is currently of unknown status. This observational study focuses on rectal cancer, examining the metastasis rate of lateral lymph nodes through a prospective cohort model. The primary procedure involved is lateral lymph node dissection, seeking to observe and potentially predict lymph node involvement.
Similarly, the study "Distribution of Lymph Node Metastases in Esophageal Carcinoma" (NCT03222895) is actively recruiting participants. It aims to map the spread of lymph node metastases in esophageal cancer, testing the accuracy of preoperative diagnostics, and understanding the prognostic value of various lymph node stations. This observational cohort study also adopts a prospective approach, reflecting a growing trend in exploring the spatial distribution of metastatic nodes.
For pancreatic cancer, the "Para-aortic Lymph Node Metastasis in Resectable Pancreatic Cancer" trial (NCT06065891) is recruiting to investigate the prevalence and prognostic significance of para-aortic lymph node involvement. This interventional study involves PALN resection to determine its impact on patient prognosis after curative resection, underpinning the direct intervention approach in surgical oncology research.
Another notable trial, "Selective Lymph Node Dissection Using Fluorescent Dye in Node-positive Breast Cancer" (NCT02781259), though currently of unknown status, employs both a drug (Indocyanine green) and imaging devices to improve the precision of axillary lymph node dissection. This Phase 4, diagnostic-focused trial explores clinicopathological factors associated with lymph node metastasis, illustrating the integration of advanced imaging and pharmacological tools in surgical procedures.
In contrast, the "Detecting Lymph Node Metastasis in Intrahepatic Cholangiocarcinoma" study (NCT06381648), also known as LyMIC, represents an observational, retrospective case–control model aiming to enhance diagnostic accuracy by measuring sensitivity, specificity, and overall accuracy of lymph node metastasis detection in cholangiocarcinoma.
Challenges and future perspectives
NPs and nanoconjugates have shown great promise in the diagnosis and treatment of lymph node metastasis, but they are not without limitations [139]. One key limitation is the difficulty in effectively targeting and delivering NPs/nanoconjugates to lymph nodes. Lymph nodes have a complex microenvironment with various physical and biological barriers that can hinder the accumulation and penetration of NPs [130]. The size, shape, and surface properties of NPs need to be carefully engineered to optimize lymph node targeting, which can be challenging. Additionally, the heterogeneity of lymph node metastases, in terms of their location, size, and degree of metastatic infiltration, makes it difficult to develop a one-size-fits-all NP/nanoconjugate solution [110].
Another limitation is the potential for off-target effects and toxicity. NPs, especially those made of inorganic materials, can potentially accumulate in non-target organs and induce unwanted side effects [45]. This is a concern not only for diagnostic imaging but also for therapeutic applications where NPs/nanoconjugates may be used to deliver cytotoxic drugs or to induce local immune responses. Careful evaluation of the pharmacokinetics, biodistribution, and safety profile of NPs/nanoconjugates is crucial before clinical translation. Despite these limitations, ongoing research continues to address these challenges, with the ultimate goal of developing more effective and safer NP-based strategies for the management of lymph node metastasis [112].
Molecular imaging faces several challenges in accurately detecting lymph node metastasis in cancer [396]. Firstly, sensitivity and specificity remain a primary concern [404]. Traditional imaging techniques may miss micro-metastases, which are crucial for early detection and treatment [405]. Innovations in molecular imaging strive to enhance the detection of these small metastatic sites [406]. For example, the use of novel biomarkers or tracers that target specific cancer cell properties can improve sensitivity [397]. Secondly, there's a challenge in distinguishing between reactive lymph nodes and those with metastatic disease [398]. This differentiation is vital for accurate staging and treatment planning [399]. Thirdly, the integration of molecular imaging data with other diagnostic modalities, like histopathology, enhances the overall diagnostic accuracy but requires sophisticated data analysis techniques [400]. Fourthly, patient safety and comfort are always a priority, necessitating the development of non-invasive and minimally invasive techniques [401]. Finally, the high cost and accessibility of advanced molecular imaging technologies limit their widespread use, especially in resource-limited settings [402]. Addressing these challenges will require continued research and development in molecular imaging technologies, biomarker discovery, and data integration methods [404]. Table 5 highlights the main aspects of the topic, outlines the current challenges faced in each area, and presents the anticipated future developments or perspectives.
Table 5.
Challenges and future perspectives in molecular imaging and targeted therapeutics for lymph node metastasis in cancer
| Aspect | Challenges | Future Perspectives | Refs. |
|---|---|---|---|
| Molecular imaging |
- Limited sensitivity and specificity in detecting early lymph node metastasis - Difficulty in distinguishing between reactive and metastatic lymph nodes - High costs and limited availability of advanced imaging techniques |
- Development of more sensitive and specific imaging agents - Integration of AI and machine learning for better image analysis - Wider accessibility and affordability of advanced imaging modalities |
[420] |
| Targeted therapeutics |
- Resistance to current therapies - Lack of specificity, leading to systemic toxicity - Difficulty in delivering therapeutic agents specifically to lymph nodes |
- Research into more effective and specific therapeutic agents - Development of nanotechnology-based delivery systems for targeted therapy - Personalized medicine approaches based on genetic profiling of tumors |
[421] |
| Diagnostic methods |
- Invasive nature of current diagnostic methods like lymph node biopsy - Risk of false negatives in early-stage metastasis |
- Non-invasive diagnostic tools with higher accuracy - Liquid biopsy techniques for early detection and monitoring |
[422] |
| Clinical trials |
- Ethical and logistical challenges in conducting trials - Difficulty in recruiting a sufficient number of participants |
- More international collaboration for larger, more diverse clinical trials - Use of real-world data to supplement trial findings |
[423] |
| Regulatory approvals |
- Stringent regulatory requirements for new diagnostics and therapeutics - Long approval times delaying access to new treatments |
- Streamlining regulatory processes - Adaptive trial designs to speed up the approval of promising therapies |
[323] |
Targeted therapeutics have significantly advanced the treatment of lymph node metastasis in cancer, offering more personalized and effective approaches (Fig. 8). First, the development of monoclonal antibodies that target specific cancer antigens has led to treatments that are more specific to cancer cells, sparing normal tissues. For instance, trastuzumab targets HER2-positive breast cancer cells, which often metastasize to lymph nodes [406]. Second, small molecule inhibitors disrupt cancer cell signaling pathways critical for tumor growth and metastasis [407]. Drugs like imatinib, targeting the BCR-ABL fusion protein in chronic myeloid leukemia, have shown effectiveness in controlling metastatic spread [408]. Third, immune checkpoint inhibitors, such as pembrolizumab, have revolutionized cancer treatment by enhancing the immune system's ability to recognize and destroy cancer cells, including those in lymph nodes [409]. Fourth, advances in gene therapy, such as the use of CRISPR/Cas9 for gene editing, provide new avenues for targeting the genetic alterations specific to metastatic cells [410]. Lastly, nanoparticle-based drug delivery systems enable targeted delivery and controlled release of therapeutics directly to metastatic lymph nodes, improving treatment efficacy and reducing systemic side effects [411]. The ongoing research in these areas continues to expand the arsenal of targeted therapies for cancer metastasis, potentially transforming the prognosis for patients with advanced disease [412].
Fig. 8.
The sequence and primary challenges involved in gene delivery for melanoma treatment. This includes the creation of the nucleic acid polyplex, its journey through the bloodstream, gathering at the targeted tissue, movement within the cell, and the release of its contents in the nucleus. Re-printed from the Springer Nature [89]
Emerging technologies in molecular imaging for lymph node metastasis are revolutionizing cancer diagnosis and management [413]. Firstly, advanced PET imaging techniques, such as PSMA-PET for prostate cancer, provide superior specificity and sensitivity in detecting metastatic lymph nodes [414]. Secondly, the development of novel contrast agents for MRI, like iron oxide nanoparticles, enhances the visibility of metastatic lymph nodes [415]. Thirdly, optical imaging technologies, including near-infrared fluorescence imaging, offer non-invasive ways to visualize lymph node metastasis during surgery [416]. Fourthly, photoacoustic imaging, which combines ultrasound and optical imaging, provides high-resolution images of lymph node metastases [417]. Lastly, molecular ultrasound, using targeted microbubbles, allows for the real-time visualization of molecular expressions in lymph nodes [418]. These technologies, by providing more accurate and detailed information about the extent and nature of lymph node involvement, aid in better staging, treatment planning, and monitoring of cancer [419].
Conclusion
The review underscored groundbreaking developments in molecular imaging techniques, which have substantially enhanced the detection and characterization of lymph node metastases. This advancement facilitates earlier, more precise diagnoses, and better staging of cancer, crucial for effective treatment planning. Simultaneously, targeted therapeutics have emerged as a pivotal approach, focusing on the specific molecular profiles of cancers. These therapies have shown promise in reducing the adverse side effects typically associated with traditional cancer treatments and improving efficacy. The integration of advanced molecular imaging and targeted therapeutics holds significant implications for cancer care. Enhanced imaging capabilities lead to improved identification of metastatic nodes, thus enabling more accurate prognoses and tailored treatment strategies. Targeted therapeutics, on the other hand, offer a personalized approach to treatment, potentially improving survival rates and quality of life for patients. These advances represent a shift towards more individualized, precise cancer care, moving away from a one-size-fits-all approach. Looking ahead, nanobiotechnology emerges as a promising frontier in oncology. This field has the potential to further refine the accuracy of molecular imaging and the efficacy of targeted therapeutics. Nanoparticles can be engineered to enhance imaging contrast or to deliver therapeutic agents directly to tumor cells, minimizing systemic toxicity. The future of nanobiotechnology in oncology is poised to usher in an era of highly efficient, minimally invasive cancer diagnosis and treatment modalities. This could revolutionize the management of lymph node metastasis in cancer, offering hope for better patient outcomes.
Author contributions
YW, JS, XZ, and NL wrote the main manuscript text All authors reviewed the manuscript.
Funding
The authors declare that no funding was received for the research.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yunhao Wu and Jin Shang are co-first authors and equally contributed to this work.
References
- 1.Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, Foster R, Gardener B, Lerner H. Margolese : relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983;52(9):1551–7. [DOI] [PubMed] [Google Scholar]
- 2.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15(2):290–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci USA. 1998;95(2):548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leong SP, Witte MH. Lymphangiogenesis lymphatic system and lymph nodes cancer lymphangiogenesis and metastasis. In: Leong SP, Nathanson SD, Zager JS, editors. Cancer Metastasis Through the Lymphovascular System. Cham: Springer; 2022. 10.1007/978-3-030-93084-4_21. [Google Scholar]
- 5.Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2(8):573–83. [DOI] [PubMed] [Google Scholar]
- 6.Valtola R, Salven P, Heikkila P, Taipale J, Joensuu H, Rehn M, Pihlajaniemiet T, Weich H, deWaal R, Alitalo K. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol. 1999;154(5):1381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leong SP, Pissas A, Scarato M, Gallon F, Pissas MH, Amore M, Wu M, Faries MB, Lund AW. The lymphatic system and sentinel lymph nodes: conduit for cancer metastasis. Clin Exp Metastasis. 2022;39(1):139–57. 10.1007/s10585-021-10123-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Auwera I, Van Laere SJ, Van den Eynden GG, Benoy I, Van Dam P, Colpaert CG, Fox SB, Turley H, Harris AL, Van Marck EA, Vermeulen PB. Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin Cancer Res. 2004;10(23):7965–71. [DOI] [PubMed] [Google Scholar]
- 9.Schoppmann SF, Fenzl A, Nagy K, Unger S, Bayer G, Geleff S, Gnant M, Horvat R, Jakesz R, Birner P. VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery. 2006;139(6):839–46. [DOI] [PubMed] [Google Scholar]
- 10.Dumont AE. Factor VIII-related antigen. J Natl Cancer Inst. 1993;85(8):674–6. [DOI] [PubMed] [Google Scholar]
- 11.Cunnick GH, Jiang WG, Gomez KF, Mansel RE. Lymphangiogenesis quantification using quantitative PCR and breast cancer as a model. Biochem Biophys Res Commun. 2001;288(4):1043–6. [DOI] [PubMed] [Google Scholar]
- 12.Chehelgerdi M, Doosti A. Effect of the cagW-based gene vaccine on the immunologic properties of BALB/c mouse: an efficient candidate for Helicobacter pylori DNA vaccine. J Nanobiotechnology. 2020;18(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alrushaid N, Khan FA, Al-Suhaimi EA, Elaissari A. Nanotechnology in cancer diagnosis and treatment. Pharmaceutics. 2023;15(3):1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chehelgerdi M, Chehelgerdi M, Allela OQ, Pecho RD, Jayasankar N, Rao DP, Thamaraikani T, Vasanthan M, Viktor P, Lakshmaiya N, Saadh MJ. Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation. Mol Cancer. 2023;22(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Zhu L, Kwok HF. Nanotechnology-based approaches overcome lung cancer drug resistance through diagnosis and treatment. Drug Resist Updates. 2023;1(66): 100904. [DOI] [PubMed] [Google Scholar]
- 16.Dezfuli AA, Abu-Elghait M, Salem SS. Recent insights into nanotechnology in colorectal cancer. Appl Biochem Biotechnol. 2023;26:1–5. [DOI] [PubMed] [Google Scholar]
- 17.Chen YC, Lin YH, Chien HC, Hsu PK, Hung JJ, Huang CS, et al. Preoperative consolidation-to-tumor ratio is effective in the prediction of lymph node metastasis in patients with pulmonary ground-glass component nodules. Thorac Cancer. 2021;12:1203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takenaka T, Yano T, Morodomi Y, Ito K, Miura N, Kawano D, et al. Prediction of true-negative lymph node metastasis in clinical IA non-small cell lung cancer by measuring standardized uptake values on positron emission tomography. Surg Today. 2012;42:934–9. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Wu J, Lv X, Yang Q, Chen J, Zhang D. The value of red blood cell distribution width, neutrophil-to-lymphocyte ratio, and hemoglobin-to-red blood cell distribution width ratio in the progression of non-small cell lung cancer. PLoS ONE. 2020;15: e237947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Zee KJ, Manasseh DE, Bevilacqua JLB, Boolbol SK, Fey JV, Tan LK, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–51. [DOI] [PubMed] [Google Scholar]
- 21.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouvier A, Haas O, Piard F, et al. How many nodes must be examined to accurately stage gastric carcinomas? Results from a population based study. Cancer. 2002;94(11):2862–6. [DOI] [PubMed] [Google Scholar]
- 23.Cao HH, Tang ZQ, Yu Z, et al. Comparison of the 8th union for international cancer control lymph node staging system for gastric cancer with two other lymph node staging systems. Oncol Lett. 2019;17(1):1299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JW, Ali B, Park CH, et al. Different lymph node staging systems in patients with gastric cancer from Korean. Medicine (United States). 2016;95(25): e3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jian-Hui C, Shi-Rong C, Hui W, et al. Prognostic value of three different lymph node staging systems in the survival of patients with gastric cancer following D2 lymphadenectomy. Tumour Biol. 2016;37(8):11105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Bao M, Zhang C. Prognostic value of different lymph node staging methods for node-positive cardia gastric cancer: a register-based retrospective cohort study. BMJ Open. 2021;11(8): e050378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng W, Xu T, Wang Y, et al. Log odds of positive lymph nodes may predict survival benefit in patients with node-positive non-small cell lung cancer. Lung Cancer (Amsterdam, Netherlands). 2018;122:60–6. [DOI] [PubMed] [Google Scholar]
- 28.Hattori A, Hirayama S, Matsunaga T, Hayashi T, Takamochi K, Oh S, et al. Distinct clinicopathologic characteristics and prognosis based on the presence of ground glass opacity component in clinical stage IA lung adenocarcinoma. J Thorac Oncol. 2019;14:265–75. [DOI] [PubMed] [Google Scholar]
- 29.Zhao L, Bai G, Ji Y, Peng Y, Zang R, Gao S. Consolidation tumor ratio combined with pathological features could predict status of lymph nodes of early-stage lung adenocarcinoma. Front Oncol. 2022;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandaliya H, Jones M, Oldmeadow C, Nordman IIC. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. 2019;8:886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Cao L, Li S, Wang F, Huang D, Jiang R. Combination of PD-L1 expression and NLR as prognostic marker in patients with surgically resected non-small cell lung cancer. J Cancer. 2019;10:6703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Zhou N, Zhu R, Li X, Sun Z, Gao Y, et al. Circulating activated immune cells as a potential blood biomarkers of non-small cell lung cancer occurrence and progression. Bmc Pulm Med. 2021;21:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. [DOI] [PubMed] [Google Scholar]
- 34.Lardinois D, Dlelyn P, Vancshil P, Porta R, Waller D, Passlick B, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardio Thorac. 2006;30:787–92. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe K, Sakamaki K, Ito H, Yokose T, Yamada K, Nakayama H, et al. Impact of the micropapillary component on the timing of recurrence in patients with resected lung adenocarcinoma. Eur J Cardio Thorac. 2020;58:1010–8. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y, Wang R, Shen X, Pan Y, Cheng C, Li Y, et al. Minor components of micropapillary and solid subtypes in lung adenocarcinoma are predictors of lymph node metastasis and poor prognosis. Ann Surg Oncol. 2016;23:2099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trejo Bittar HE, Incharoen P, Althouse AD, Dacic S. Accuracy of the IASLC/ATS/ERS histological subtyping of stage I lung adenocarcinoma on intraoperative frozen sections. Modern Pathol. 2015;28:1058–63. [DOI] [PubMed] [Google Scholar]
- 38.Ishiguro F, Matsuo K, Fukui T, Mori S, Hatooka S, Mitsudomi T. Effect of selective lymph node dissection based on patterns of lobe-specific lymph node metastases on patient outcome in patients with resectable non-small cell lung cancer: a large-scale retrospective cohort study applying a propensity score. J Thorac Cardiovasc Surg. 2010;139:1001–6. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y, Mao Y, He J, Gao S, Zhang Z, Ding N, et al. Lobe-specific lymph node dissection in clinical stage IA solid-dominant non–small-cell lung cancer: a propensity score matching study. Clin Lung Cancer. 2021;22:e201–10. [DOI] [PubMed] [Google Scholar]
- 40.Darling GE, Allen MS, Decker PA, Ballman K, Malthaner RA, Inculet RI, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non–small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg. 2011;141:662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383:1561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim E, Harris G, Patel A, Adachi I, Edmonds L, Song F. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer. J Thorac Oncol. 2009;4:1380–8. [DOI] [PubMed] [Google Scholar]
- 43.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Blake SJ, Yong MCR, Harjunpää H, Ngiow SF, Takeda K, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6:1382–99. [DOI] [PubMed] [Google Scholar]
- 45.Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. New Engl J Med. 2018;378:1976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Dómine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1413–22. [DOI] [PubMed] [Google Scholar]
- 47.Liang W, Cai K, Chen C, Chen H, Chen Q, Fu J, et al. Expert consensus on neoadjuvant immunotherapy for non-small cell lung cancer. Transl Lung Cancer Res. 2020;9:2696–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng RS, Sun KX, Zhang SW, et al. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi. 2019;41(1):19–28. [DOI] [PubMed] [Google Scholar]
- 49.Yin L, Liao XH, Zhang JH, et al. Research progress of semi-quantitative parameters assessed on 18F-FDG PET/CT for prognosis of non-small cell lung cancer. Chin J Interv Imaging Ther. 2019;16(8):507–10. [Google Scholar]
- 50.Park SY, Yoon JK, Park KJ, et al. Prediction of occult lymph node metastasis using volume-based PET parameters in small-sized peripheral non-small cell lung cancer. Cancer Imaging. 2015;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouyang ML, Xia HW, Xu MM, et al. Prediction of occult lymph node metastasis using SUV, volumetric parameters and intratumoral heterogeneity of the primary tumor in T1–2N0M0 lung cancer patients staged by PET/CT. Ann Nucl Med. 2019;33(9):671–80. [DOI] [PubMed] [Google Scholar]
- 52.Sano T, Coit D, Kim H, et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20(2):217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coburn N, Swallow C, Kiss A, et al. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer. 2006;107(9):2143–51. [DOI] [PubMed] [Google Scholar]
- 54.Schwarz R, Smith D. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol. 2007;14(2):317–28. [DOI] [PubMed] [Google Scholar]
- 55.Smith D, Schwarz R, Schwarz R. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23(28):7114–24. [DOI] [PubMed] [Google Scholar]
- 56.Deng J, Liu J, Wang W, et al. Validation of clinical significance of examined lymph node count for accurate prognostic evaluation of gastric cancer for the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system. Chin J Cancer Res. 2018;30(5):477–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wd T, Ap B. World Health Organization classification of tumours. WHO classification of tumors. 5th ed. IARC Press: Lyon; 2021. [Google Scholar]
- 58.Ettinger DS, Wood DE, Aisner DL, et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Comp Cancer Netw. 2021;19(3):254–66. [DOI] [PubMed] [Google Scholar]
- 59.Chen S, Zhao Y, Tang Q, et al. Diagnostic performance and prognostic value of preoperative 18F-FDG PET/CT in renal cell carcinoma patients with venous tumor thrombus. Cancer Imaging. 2022;22(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao XH, Cui YG, Chen XQ, et al. Primary metabolic tumor volume from 18F-FDG PET/CT associated with epidermal growth factor receptor mutation in lung adenocarcinoma patients. Nucl Med Commun. 2020;41(11):1210–7. [DOI] [PubMed] [Google Scholar]
- 61.Liao XH, Liu M, Wang RF, et al. Potentials of non-invasive 18F-FDG PET/CT in immunotherapy prediction for non-small cell lung cancer. Front Genet. 2022;12: 810011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmidt-Hansen M, Baldwin DR, Hasler E, et al. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst Rev. 2014;2014(11):D9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bryant AS, Cerfolio RJ, Klemm KM, et al. Maximum standard uptake value of mediastinal lymph nodes on integrated FDG-PET-CT predicts pathology in patients with non-small cell lung cancer. Ann Thorac Surg. 2006;82(2):417–22. [DOI] [PubMed] [Google Scholar]
- 64.Liao XH, Liu M, Wang RF, et al. Relationships of semiquantitative parameters assessed on 18F-FDG PET/CT and EGFR mutation subtypes in lung adenocarcinoma patients. Chin J Interv Imaging Ther. 2020;17(2):98–103. [Google Scholar]
- 65.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–70. [DOI] [PubMed] [Google Scholar]
- 66.Mattes MD, Weber WA, Foster A, Moshchinsky AB, Ahsanuddin S, Zhang Z, et al. A predictive model for lymph node involvement with malignancy on PET/CT in non-small-cell lung cancer. J Thorac Oncol. 2015;10:1207–12. [DOI] [PubMed] [Google Scholar]
- 67.Lv X, Wu Z, Cao J, Hu Y, Liu K, Dai X, et al. A nomogram for predicting the risk of lymph node metastasis in T1–2 non-small-cell lung cancer based on PET/CT and clinical characteristics. Transl Lung Cancer Res. 2021;10:430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fu Y, Xi X, Tang Y, Li X, Ye X, Hu B, et al. Development and validation of tumor-to-blood based nomograms for preoperative prediction of lymph node metastasis in lung cancer. Thorac Cancer. 2021;12:2189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yip R, Li K, Liu L, Xu D, Tam K, Yankelevitz DF, et al. Controversies on lung cancers manifesting as part-solid nodules. Eur Radiol. 2018;28:747–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu L, Saxena S, Singh RK. Neutrophils in the tumor microenvironment. Adv Exp Med Biol. 2020;1224:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sorvillo N, Cherpokova D, Martinod K, Wagner DD. Extracellular DNA NET-works with dire consequences for health. Circ Res. 2019;125:470–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim CY, Yang DH. Adjustment of N stages of gastric cancer by the ratio between the metastatic and examined lymph nodes. Ann Surg Oncol. 2009;16(7):1868–74. [DOI] [PubMed] [Google Scholar]
- 74.Coimbra F, Costa W, Montagnini A, et al. The interaction between N-category and N-ratio as a new tool to improve lymph node metastasis staging in gastric cancer: results of a single cancer center in Brazil. Eur J Surg Oncol. 2011;37(1):47–54. [DOI] [PubMed] [Google Scholar]
- 75.Gu P, Deng J, Sun Z, et al. Superiority of log odds of positive lymph nodes (LODDS) for prognostic prediction after gastric cancer surgery: a multi-institutional analysis of 7620 patients in China. Surg Today. 2021;51(1):101–10. [DOI] [PubMed] [Google Scholar]
- 76.Xu J, Bian YH, Jin X, et al. Prognostic assessment of different metastatic lymph node staging methods for gastric cancer after D2 resection. World J Gastroenterol. 2013;19(12):1975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chung HW, Lee KY, Kim HJ, et al. FDG PET/CT metabolic tumor volume and total lesion glycolysis predict prognosis in patients with advanced lung adenocarcinoma. J Cancer Res Clin Oncol. 2014;140(1):89–98. [DOI] [PubMed] [Google Scholar]
- 78.Moon Y, Choi SY, Park JK, et al. Risk factors for occult lymph node metastasis in peripheral non-small cell lung cancer with invasive component size 3 cm or less. World J Surg. 2020;44(5):1658–65. [DOI] [PubMed] [Google Scholar]
- 79.Pan JB, Hou YH, Zhang GJ. Correlation between efficacy of the EGFR tyrosine kinase inhibitor and serum tumor markers in lung adenocarcinoma patients. Clin Lab. 2014;60(9):1439–47. [DOI] [PubMed] [Google Scholar]
- 80.Isaksson S, Jonsson P, Monsef N, et al. CA 19–9 and CA 125 as potential predictors of disease recurrence in resectable lung adenocarcinoma. PLoS ONE. 2017;12(10): e186284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Noone A, Howlader N, Krapcho M, et al. SEER cancer statistics review, 1975–2015. Bethesda: National Cancer Institute; 2017. [Google Scholar]
- 82.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 83.Siewert J, Böttcher K, Stein H, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German gastric cancer study. Ann Surg. 1998;228(4):449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu C, Hsieh M, Lo S, et al. Relation of number of positive lymph nodes to the prognosis of patients with primary gastric adenocarcinoma. Gut. 1996;38(4):525–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang J, Dang P, Raut C, et al. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Ann Surg. 2012;255(3):478–85. [DOI] [PubMed] [Google Scholar]
- 86.Sun Z, Xu Y, Li DM, et al. Log odds of positive lymph nodes: a novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer. 2010;116(11):2571–80. [DOI] [PubMed] [Google Scholar]
- 87.Yuan M, Yang Y, Li Y, et al. Mucin-like domain of mucosal addressin cell adhesion molecule-1 facilitates integrin α4β7-mediated cell adhesion through electrostatic repulsion. Front Cell Dev Biol. 2020;14(8):603148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vinh-Hung V, Verschraegen C, Promish D, et al. Ratios of involved nodes in early breast cancer. Breast Cancer Res. 2004;6(6):R680-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ji H, Hu C, Yang X, et al. Lymph node metastasis in cancer progression: molecular mechanisms, clinical significance and therapeutic interventions. Sig Transduct Target Ther. 2023;8:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guyatt G, Oxman A, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol. 2011;64(12):1294–302. [DOI] [PubMed] [Google Scholar]
- 91.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. [DOI] [PubMed] [Google Scholar]
- 92.Galbraith R. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. 1988;7(8):889–94. [DOI] [PubMed] [Google Scholar]
- 93.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shi Z, Xiao ZQ, Li LJ, et al. Application of nomogram containing log odds of metastatic lymph node in gallbladder cancer patients. Ann Transl Med. 2020;8(10):655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Modi KB, Sekhon R, Rawal S, et al. Analysis of role of lymph node density, negative lymph node and lodds on survival in cervical cancer. Int J Gynecol Obstet. 2018;143:426–7. [Google Scholar]
- 96.Dziedzic D, Rudzinski P, Orlowski T, et al. Log odds as a novel prognostic indicator superior to the number-based and ratio-based category for non-small cell lung cancer. J Thorac Oncol. 2017;12(1):S731. [Google Scholar]
- 97.Lin P. Prognostic performance of different lymph node staging systems for esophageal squamous cell carcinoma. Dis Esophagus. 2016;29:157A. [Google Scholar]
- 98.Smith DD, Nelson RA, Schwarz RE. A comparison of competing lymph node staging schemes in resectable gastric cancer. Ann Surg Oncol. 2010;17:S64. [DOI] [PubMed] [Google Scholar]
- 99.Zhang QW, Zhang CH, Li XB, et al. Comparison of three lymph node staging schemes for predicting survival in patients with colorectal cancer: a large population database and Chinese multicenter validation. United Eur Gastroenterol J. 2018;6(8):A277. [Google Scholar]
- 100.Pan SW, Wang PL, Xing YN, et al. Retrieved lymph nodes from different anatomic groups in gastric cancer: a proposed optimal number, comparison with other nodal classification strategies and its impact on prognosis. Cancer Commun. 2019;39(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daw NC, Chi YY, Kalapurakal JA, et al. Activity of vincristine and irinotecan in diffuse anaplastic Wilms tumor and therapy outcomes of stage II to IV disease: results of the Children’s Oncology Group AREN0321 Study. J Clin Oncol. 2020;38(14):1558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shen J, Kim S, Cheong J, et al. The impact of total retrieved lymph nodes on staging and survival of patients with pT3 gastric cancer. Cancer. 2007;110(4):745–51. [DOI] [PubMed] [Google Scholar]
- 103.Occhionorelli S, Andreotti D, Vallese P, et al. Evaluation on prognostic efficacy of lymph nodes ratio (LNR) and log odds of positive lymph nodes (LODDS) in complicated colon cancer: the first study in emergency surgery. World J Surg Oncol. 2018;16(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J, Li J, Chen R, et al. Survival effect of different lymph node staging methods on ovarian cancer: an analysis of 10 878 patients. Cancer Med. 2018;7(9):4315–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aurello P, Petrucciani N, Nigri GR, et al. Log odds of positive lymph nodes (LODDS): what are their role in the prognostic assessment of gastric adenocarcinoma? J Gastrointest Surg. 2014;18(7):1254–60. [DOI] [PubMed] [Google Scholar]
- 106.Liu H, Deng J, Zhang R, et al. The RML of lymph node metastasis was superior to the LODDS forevaluating the prognosis of gastric cancer. Int J Surg. 2013;11(5):419–24. [DOI] [PubMed] [Google Scholar]
- 107.Tóth D, Bíró A, Varga Z, et al. Comparison of different lymph node staging systems in prognosis of gastric cancer: a bi-institutional study from Hungary. Chin J Cancer Res. 2017;29(4):323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 110.Qiu MZ, Qiu HJ, Wang ZQ, et al. The tumor-log odds of positive lymph nodes-metastasis staging system, a promising new staging system for gastric cancer after D2 resection in China. PLoS ONE. 2012;7(2): e31736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gong Y, Pan S, Wang X, et al. A novel lymph node staging system for gastric cancer including modified Union for cancer Control/American Joint Committee on cancer and Japanese Gastric Cancer Association criteria. Eur J Surg Oncol. 2020;46:e27-32. [DOI] [PubMed] [Google Scholar]
- 112.Che K, Wang Y, Wu N, et al. Prognostic nomograms based on three lymph node classification systems for resected gastric adenocarcinoma: a large population-based cohort study and external validation. Ann Surg Oncol. 2021;28:8937–49. [DOI] [PubMed] [Google Scholar]
- 113.Lee H, Yang H, Kim W, et al. Influence of the number of lymph nodes examined on staging of gastric cancer. Br J Surg. 2001;88(10):1408–12. [DOI] [PubMed] [Google Scholar]
- 114.Hsieh M, Wang S, Wei C, et al. S1 versus doublet regimens as adjuvant chemotherapy in patients with advanced gastric cancer after radical surgery with D2 dissection—a propensity score matching analysis. Cancers (Basel). 2020;12(9):2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li J, Lin Y, Wang Y, et al. Prognostic nomogram based on the metastatic lymph node ratio for gastric neuroendocrine tumour: SEER database analysis. ESMO Open. 2020;5(2): e000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rawicz-Pruszyński K, Ciseł B, Mlak R, et al. The role of the lymph node ratio in advanced gastric cancer after neoadjuvant chemotherapy. Cancers. 2019;11(12):1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shin J, Kim H, Lee W, et al. The stage migration should be reconsidered in stage IIIA rectal cancer: based on propensity score analysis. Clin Colorectal Cancer. 2021;20(4):e273–8. [DOI] [PubMed] [Google Scholar]
- 118.Li MM, Zhao S, Eskander A, et al. Stage migration and survival trends in laryngeal cancer. Ann Surg Oncol. 2021;28(12):7300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tseng YC, Xu Z, Guley K, Yuan H, Huang L. Lipid-calcium phosphate nanoparticles for delivery to the lymphatic system and SPECT/CT imaging of lymph node metastases. Biomaterials. 2014;35:4688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Urban DA, Rodriguez-Lorenzo L, Balog S, Kinnear C, Rothen-Rutishauser B, Petri-Fink A. Plasmonic nanoparticles and their characterization in physiological fluids. Colloids Surf B Biointerfaces. 2016;137:39–49. [DOI] [PubMed] [Google Scholar]
- 121.Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81–94. [DOI] [PubMed] [Google Scholar]
- 122.Wang G, Li W, Shi G, Tian Y, Kong L, Ding N, et al. Sensitive and specific detection of breast cancer lymph node metastasis through dual-modality magnetic particle imaging and fluorescence molecular imaging: a preclinical evaluation. Eur J Nucl Med Mol Imaging. 2022;49:2723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chaturvedi VK, Singh A, Singh VK, Singh MP. Cancer nanotechnology: a new revolution for cancer diagnosis and therapy. Curr Drug Metab. 2019;20:416–29. [DOI] [PubMed] [Google Scholar]
- 124.Hoogstins CE, Tummers QR, Gaarenstroom KN, de Kroon CD, Trimbos JB, Bosse T, et al. A Novel tumor-specific agent for intraoperative near-infrared fluorescence imaging: a translational study in healthy volunteers and patients with ovarian cancer. Clin Cancer Res. 2016;22:2929–38. [DOI] [PubMed] [Google Scholar]
- 125.Schilham MGM, Zamecnik P, Privé BM, Israël B, Rijpkema M, Scheenen T, et al. Head-to-head comparison of (68)Ga-prostate-specific membrane antigen PET/CT and Ferumoxtran-10-enhanced MRI for the diagnosis of lymph node metastases in prostate cancer patients. J Nucl Med. 2021;62:1258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Unkart JT, Chen SL, Wapnir IL, González JE, Harootunian A, Wallace AM. Intraoperative tumor detection using a ratiometric activatable fluorescent peptide: a first-in-human phase 1 study. Ann Surg Oncol. 2017;24:3167–73. [DOI] [PubMed] [Google Scholar]
- 127.Rossi EC. gynaecology: an evolution in sentinel node mapping for cervical cancer. BJOG. 2020. 10.1111/1471-0528.16568. [DOI] [PubMed] [Google Scholar]
- 128.Zalewski K, Benke M, Mirocha B, Radziszewski J, Chechlinska M, Kowalewska M. Technetium-99m-based radiopharmaceuticals in sentinel lymph node biopsy: gynecologic oncology perspective. Curr Pharm Des. 2018;24:1652–75. [DOI] [PubMed] [Google Scholar]
- 129.Cousins A, Tsopelas C, Balalis G, Thompson SK, Bartholomeusz D, Wedding AB, Thierry B. Hybrid (99m)Tc-magnetite tracer for dual modality sentinel lymph node mapping. J Mater Sci Mater Med. 2018;29:76. [DOI] [PubMed] [Google Scholar]
- 130.Gould EA, Winship T, Philbin PH, Kerr HH. Observations on a “sentinel node” in cancer of the parotid. Cancer. 1960;13:77–8. [DOI] [PubMed] [Google Scholar]
- 131.Amersi F, Hansen NM. The benefits and limitations of sentinel lymph node biopsy. Curr Treat Options Oncol. 2006;7:141–51. [DOI] [PubMed] [Google Scholar]
- 132.Stadelmann WK. The role of lymphatic mapping and sentinel lymph node biopsy in the staging and treatment of melanoma. Clin Plast Surg. 2010;37:79–99. [DOI] [PubMed] [Google Scholar]
- 133.Shahbazi R, Ozpolat B, Ulubayram K. Oligonucleotide-based theranostic nanoparticles in cancer therapy. Nanomedicine. 2016;11(10):1287–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Borroni E, Miola M, Ferraris S, Ricci G, Rožman KŽ, Kostevšek N, Catizone A, Rimondini L, Prat M, Verné E, Follenzi A. Tumor targeting by lentiviral vectors combined with magnetic nanoparticles in mice. Acta Biomater. 2017;1(59):303–16. [DOI] [PubMed] [Google Scholar]
- 135.Lee S, Xie J, Chen X. Peptide-based probes for targeted molecular imaging. Biochemistry. 2010;49:1364–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pelosi E, Arena V, Baudino B, Bellò M, Giusti M, Gargiulo T, Palladin D, Bisi G. Pre-operative lymphatic mapping and intra-operative sentinel lymph node detection in early stage endometrial cancer. Nucl Med Commun. 2003;24:971–5. [DOI] [PubMed] [Google Scholar]
- 137.Mohammad A, Hunter MI. Robot-assisted sentinel lymph node mapping and inguinal lymph node dissection using near-infrared fluorescence in vulvar cancer. J Minim Invasive Gynecol. 2019;26:968–72. [DOI] [PubMed] [Google Scholar]
- 138.He M, Jiang Z, Wang C, Hao Z, An J, Shen J. Diagnostic value of near-infrared or fluorescent indocyanine green guided sentinel lymph node mapping in gastric cancer: a systematic review and meta-analysis. J Surg Oncol. 2018;118:1243–56. [DOI] [PubMed] [Google Scholar]
- 139.Zulfajri M, Abdelhamid HN, Sudewi S, Dayalan S, Rasool A, Habib A, Huang GG. Plant part-derived carbon dots for biosensing. Biosensors (Basel). 2020;10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang W, Hu Z. Targeting peptide-based probes for molecular imaging and diagnosis. Adv Mater. 2019;31: e1804827. [DOI] [PubMed] [Google Scholar]
- 141.Tajima Y, Yamazaki K, Masuda Y, Kato M, Yasuda D, Aoki T, Kato T, Murakami M, Miwa M, Kusano M. Sentinel node mapping guided by indocyanine green fluorescence imaging in gastric cancer. Ann Surg. 2009;249:58–62. [DOI] [PubMed] [Google Scholar]
- 142.Ankersmit M, Bonjer HJ, Hannink G, Schoonmade LJ, van der Pas M, Meijerink W. Near-infrared fluorescence imaging for sentinel lymph node identification in colon cancer: a prospective single-center study and systematic review with meta-analysis. Tech Coloproctol. 2019;23:1113–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shan L. Activatable Alexa Fluor680-conjugated panitumumab and indocyanine green-conjugated trastuzumab cocktail. Molecular Imaging and Contrast Agent Database (MICAD). Bethesda: National Center for Biotechnology Information; 2004. [PubMed] [Google Scholar]
- 144.Olafsen T, Wu AM. Antibody vectors for imaging. Semin Nucl Med. 2010;40:167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu R, Xi L, Luo D, Ma X, Yang W, Xi Y, Wang H, Qian M, Fan L, Xia X, et al. Enhanced targeted anticancer effects and inhibition of tumor metastasis by the TMTP1 compound peptide TMTP1-TAT-NBD. J Control Release. 2012;161:893–902. [DOI] [PubMed] [Google Scholar]
- 146.Wang L, Zhang D, Li J, Li F, Wei R, Jiang G, Xu H, Wang X, Zhou Y, Xi L. A novel ICG-labeled cyclic TMTP1 peptide dimer for sensitive tumor imaging and enhanced photothermal therapy in vivo. Eur J Med Chem. 2022;227: 113935. [DOI] [PubMed] [Google Scholar]
- 147.Zhou Y, Jiang G, Wang W, Wei R, Chen X, Wang X, Wei J, Ma D, Li F, Xi L. A Novel Near-Infrared fluorescent probe TMTP1-PEG4-ICG for in vivo Tumor Imaging. Bioconjug Chem. 2018;29:4119–26. [DOI] [PubMed] [Google Scholar]
- 148.Yeh CY, Hsiao JK, Wang YP, Lan CH, Wu HC. Peptide-conjugated nanoparticles for targeted imaging and therapy of prostate cancer. Biomaterials. 2016;99:1–15. [DOI] [PubMed] [Google Scholar]
- 149.Katsamakas S, Chatzisideri T, Thysiadis S, Sarli V. RGD-mediated delivery of small-molecule drugs. Fut Med Chem. 2017;9:579–604. [DOI] [PubMed] [Google Scholar]
- 150.Dai J, Rabie AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res. 2007;86:937–50. [DOI] [PubMed] [Google Scholar]
- 151.Sallinen H, Anttila M, Grohn O, Koponen J, Hamalainen K, Kholova I, Kosma VM, Heinonen S, Alitalo K, Yla-Herttuala S. Cotargeting of VEGFR-1 and – 3 and angiopoietin receptor Tie2 reduces the growth of solid human ovarian cancer in mice. Cancer Gene Ther. 2011;18:100–9. [DOI] [PubMed] [Google Scholar]
- 152.Kodera Y, Katanasaka Y, Kitamura Y, Tsuda H, Nishio K, Tamura T, Koizumi F. Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3. Breast Cancer Res. 2011;13:R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.García-Caballero M, Paupert J, Blacher S, Van de Velde M, Quesada AR, Medina MA, Noël A. Targeting VEGFR-3/-2 signaling pathways with AD0157: a potential strategy against tumor-associated lymphangiogenesis and lymphatic metastases. J Hematol Oncol. 2017;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gu P, Wusiman A, Wang S, Zhang Y, Liu Z, Hu Y, Liu J, Wang D. Polyethylenimine-coated PLGA nanoparticles-encapsulated Angelica sinensis polysaccharide as an adjuvant to enhance immune responses. Carbohydr Polym. 2019;223: 115128. [DOI] [PubMed] [Google Scholar]
- 155.Proulx ST, Luciani P, Derzsi S, Rinderknecht M, Mumprecht V, Leroux JC, Detmar M. Quantitative imaging of lymphatic function with liposomal indocyanine green. Cancer Res. 2010;70:7053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zheng X, Zhou F, Wu B, Chen WR, Xing D. Enhanced tumor treatment using biofunctional indocyanine green-containing nanostructure by intratumoral or intravenous injection. Mol Pharm. 2012;9:514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zakaria S, Hoskin TL, Degnim AC. Safety and technical success of methylene blue dye for lymphatic mapping in breast cancer. Am J Surg. 2008;196:228–33. [DOI] [PubMed] [Google Scholar]
- 158.Gilmore DM, Khullar OV, Gioux S, Stockdale A, Frangioni JV, Colson YL, Russell SE. Effective low-dose escalation of indocyanine green for near-infrared fluorescent sentinel lymph node mapping in melanoma. Ann Surg Oncol. 2013;20:2357–63. [DOI] [PubMed] [Google Scholar]
- 159.Soltesz EG, Kim S, Laurence RG, DeGrand AM, Parungo CP, Dor DM, Cohn LH, Bawendi MG, Frangioni JV, Mihaljevic T. Intraoperative sentinel lymph node mapping of the lung using near-infrared fluorescent quantum dots. Ann Thorac Surg. 2005;79:269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang W, Luo D, Wang S, Wang R, Chen R, Liu Y, Zhu T, Ma X, Liu R, Xu G, et al. TMTP1, a novel tumor-homing peptide specifically targeting metastasis. Clin Cancer Res. 2008;14:5494–502. [DOI] [PubMed] [Google Scholar]
- 161.Wei R, Jiang G, Lv M, Tan S, Wang X, Zhou Y, Cheng T, Gao X, Chen X, Wang W, et al. TMTP1-modified indocyanine green-loaded polymeric micelles for targeted imaging of cervical cancer and metastasis sentinel lymph node in vivo. Theranostics. 2019;9:7325–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li F, Cheng T, Dong Q, Wei R, Zhang Z, Luo D, Ma X, Wang S, Gao Q, Ma D, et al. Evaluation of (99m)Tc-HYNIC-TMTP1 as a tumor-homing imaging agent targeting metastasis with SPECT. Nucl Med Biol. 2015;42:256–62. [DOI] [PubMed] [Google Scholar]
- 163.Liu R, Ma X, Wang H, Xi Y, Qian M, Yang W, Luo D, Fan L, Xia X, Zhou J, et al. The novel fusion protein sTRAIL-TMTP1 exhibits a targeted inhibition of primary tumors and metastases. J Mol Med (Berl). 2014;92:165–75. [DOI] [PubMed] [Google Scholar]
- 164.Li F, Zhang Z, Cai J, Chen X, Zhou Y, Ma X, Dong Q, Li F, Xi L. Primary preclinical and clinical evaluation of (68)Ga-DOTA-TMVP1 as a novel VEGFR-3 PET imaging radiotracer in gynecological cancer. Clin Cancer Res. 2020;26:1318–26. [DOI] [PubMed] [Google Scholar]
- 165.Iraji M, Salehi M, Malekshah RE, Khaleghian A, Shamsi F. Liposomal formulation of new arsenic schiff base complex as drug delivery agent in the treatment of acute promyelocytic leukemia and quantum chemical and docking calculations. J Drug Deliv Sci Technol. 2022;75: 103600. [Google Scholar]
- 166.Abdelhamid HN. Zeolitic imidazolate frameworks (ZIF-8) for biomedical applications: a review. Curr Med Chem. 2021;28:7023–75. [DOI] [PubMed] [Google Scholar]
- 167.Moharramnejad M, Ehsani A, Shahi M, Gharanli S, Saremi H, Malekshah RE, Basmenj ZS, Salmani S, Mohammadi M. MOF as nanoscale drug delivery devices: synthesis and recent progress in biomedical applications. J Drug Deliv Sci Technol. 2023;81: 104285. [Google Scholar]
- 168.Sun Q, Zhou Z, Qiu N, Shen Y. Rational design of cancer nanomedicine: nanoproperty integration and synchronization. Adv Mater. 2017;29:1606628. [DOI] [PubMed] [Google Scholar]
- 169.Gholivand K, Mohammadpour M, Alavinasab Ardebili SA, Eshaghi Malekshah R, Samadian H. Fabrication and examination of polyorganophosphazene/polycaprolactone-based scaffold with degradation, in vitro and in vivo behaviors suitable for tissue engineering applications. Sci Rep. 2022;12:18407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Noh YW, Kong SH, Choi DY, Park HS, Yang HK, Lee HJ, Kim HC, Kang KW, Sung MH, Lim YT. Near-infrared emitting polymer nanogels for efficient sentinel lymph node mapping. ACS Nano. 2012;6:7820–31. [DOI] [PubMed] [Google Scholar]
- 171.Luo S, Zhang E, Su Y, Cheng T, Shi C. A review of NIR dyes in cancer targeting and imaging. Biomaterials. 2011;32:7127–38. [DOI] [PubMed] [Google Scholar]
- 172.Kraft JC, Ho RJ. Interactions of indocyanine green and lipid in enhancing near-infrared fluorescence properties: the basis for near-infrared imaging in vivo. Biochemistry. 2014;53:1275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.MacDonald RI. Characteristics of self-quenching of the fluorescence of lipid-conjugated rhodamine in membranes. J Biol Chem. 1990;265:13533–9. [PubMed] [Google Scholar]
- 174.Yoon HK, Ray A, Lee YE, Kim G, Wang X, Kopelman R. Polymer-protein hydrogel nanomatrix for stabilization of indocyanine green towards targeted fluorescence and photoacoustic bio-imaging. J Mater Chem B. 2013;1:5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hill TK, Abdulahad A, Kelkar SS, Marini FC, Long TE, Provenzale JM, Mohs AM. Indocyanine green-loaded nanoparticles for image-guided tumor surgery. Bioconjug Chem. 2015;26:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fu X, Fu S, Cai Z, Jin R, Xia C, Lui S, Song B, Gong Q, Ai H. Manganese porphyrin/ICG nanoparticles as magnetic resonance/fluorescent dual-mode probes for imaging of sentinel lymph node metastasis. J Mater Chem B. 2022;10:10065–74. [DOI] [PubMed] [Google Scholar]
- 177.Steeg PS. Targeting metastasis. Nat Rev Cancer. 2016;16:201–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schulze T, Bembenek A, Schlag PM. Sentinel lymph node biopsy progress in surgical treatment of cancer. Langenbecks Arch Surg. 2004;389:532–50. [DOI] [PubMed] [Google Scholar]
- 180.Falk Delgado A, Zommorodi S, Falk DA. Sentinel lymph node biopsy and complete lymph node dissection for melanoma. Curr Oncol Rep. 2019;21:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ulmer A, Kofler L. Sentinel node biopsy and lymph node dissection in the era of new systemic therapies for malignant melanoma. Hautarzt. 2019;70:864–9. [DOI] [PubMed] [Google Scholar]
- 182.Balsat C, Blacher S, Herfs M, Van de Velde M, Signolle N, Sauthier P, Pottier C, Gofflot S, De Cuypere M, Delvenne P, et al. A specific immune and lymphatic profile characterizes the pre-metastatic state of the sentinel lymph node in patients with early cervical cancer. Oncoimmunology. 2017;6: e1265718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Smith NR, Baker D, James NH, Ratcliffe K, Jenkins M, Ashton SE, Sproat G, Swann R, Gray N, Ryan A, et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin Cancer Res. 2010;16:3548–61. [DOI] [PubMed] [Google Scholar]
- 184.Hameed S, Chen H, Irfan M, Bajwa SZ, Khan WS, Baig SM, Dai Z. Fluorescence guided sentinel lymph node mapping: from current molecular probes to future multimodal nanoprobes. Bioconjug Chem. 2019;30:13–28. [DOI] [PubMed] [Google Scholar]
- 185.Chen J, Chen L, Zeng F, Wu S. Aminopeptidase N activatable nanoprobe for tracking lymphatic metastasis and guiding tumor resection surgery via optoacoustic/NIR-II fluorescence dual-mode imaging. Anal Chem. 2022;94:8449–57. [DOI] [PubMed] [Google Scholar]
- 186.Hou X, Tao Y, Pang Y, Li X, Jiang G, Liu Y. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int J Cancer. 2018;143:3050–60. [DOI] [PubMed] [Google Scholar]
- 187.Göppner D, Nekwasil S, Jellestad A, Sachse A, Schönborn KH, Gollnick H. Indocyanine green-assisted sentinel lymph node biopsy in melanoma using the “FOVIS” system. J Dtsch Dermatol Ges. 2017;15:169–78. [DOI] [PubMed] [Google Scholar]
- 188.Verbeek FP, Tummers QR, Rietbergen DD, Peters AA, Schaafsma BE, van de Velde CJ, Frangioni JV, van Leeuwen FW, Gaarenstroom KN, Vahrmeijer AL. Sentinel lymph node biopsy in vulvar cancer using combined radioactive and fluorescence guidance. Int J Gynecol Cancer. 2015;25:1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guo J, Yang H, Wang S, Cao Y, Liu M, Xie F, Liu P, Zhou B, Tong F, Cheng L, et al. Comparison of sentinel lymph node biopsy guided by indocyanine green, blue dye, and their combination in breast cancer patients: a prospective cohort study. World J Surg Oncol. 2017;15:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Helle M, Rampazzo E, Monchanin M, Marchal F, Guillemin F, Bonacchi S, Salis F, Prodi L, Bezdetnaya L. Surface chemistry architecture of silica nanoparticles determine the efficiency of in vivo fluorescence lymph node mapping. ACS Nano. 2013;7:8645–57. [DOI] [PubMed] [Google Scholar]
- 191.Arif U, Haider S, Haider A, Khan N, Alghyamah AA, Jamila N, Khan MI, Almasry WA, Kang IK. Biocompatible polymers and their potential biomedical applications: a review. Curr Pharm Des. 2019;25:3608–19. [DOI] [PubMed] [Google Scholar]
- 192.Mir M, Ahmed N, Rehman AU. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf B Biointerfaces. 2017;159:217–31. [DOI] [PubMed] [Google Scholar]
- 193.Fornaguera C, Feiner-Gracia N, Calderó G, García-Celma MJ, Solans C. PLGA nanoparticles from nano-emulsion templating as imaging agents: versatile technology to obtain nanoparticles loaded with fluorescent dyes. Colloids Surf B Biointerfaces. 2016;147:201–9. [DOI] [PubMed] [Google Scholar]
- 194.Zhang K, Tang X, Zhang J, Lu W, Lin X, Zhang Y, Tian B, Yang H, He H. PEG-PLGA copolymers: their structure and structure-influenced drug delivery applications. J Control Release. 2014;183:77–86. [DOI] [PubMed] [Google Scholar]
- 195.Lin WJ, Lee WC. Polysaccharide-modified nanoparticles with intelligent CD44 receptor targeting ability for gene delivery. Int J Nanomedicine. 2018;13:3989–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhou H, Fan Z, Deng J, Lemons PK, Arhontoulis DC, Bowne WB, Cheng H. Hyaluronidase embedded in nanocarrier PEG Shell for enhanced tumor penetration and highly efficient antitumor efficacy. Nano Lett. 2016;16:3268–77. [DOI] [PubMed] [Google Scholar]
- 197.Mumprecht V, Detmar M. Lymphangiogenesis and cancer metastasis. J Cell Mol Med. 2009;13:1405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cao Y, Song W, Jiang Q, Xu Y, Cai S, Wang S, Yang W. Nanoparticles from ancient ink endowing a green and effective strategy for cancer photothermal therapy in the second near-infrared window. ACS Omega. 2020;5:6177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dai R, Peng X, Lin B, Xu D, Lv R. NIR II luminescence imaging for sentinel lymph node and enhanced chemo-/photothermal therapy for breast cancer. Bioconjug Chem. 2021;32:2117–27. [DOI] [PubMed] [Google Scholar]
- 200.Yang L, Cheng J, Chen Y, Yu S, Liu F, Sun Y, Chen Y, Ran H. Phase-transition nanodroplets for real-time photoacoustic/ultrasound dual-modality imaging and photothermal therapy of sentinel lymph node in breast cancer. Sci Rep. 2017;7:45213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ali MRK, Warner PE, Yu AM, Tong M, Han T, Tang Y. Preventing metastasis using gold nanorod-assisted plasmonic photothermal therapy in xenograft mice. Bioconjug Chem. 2022;33:2320–31. [DOI] [PubMed] [Google Scholar]
- 202.Jiang G, Wang X, Zhou Y, Zou C, Wang L, Wang W, Zhang D, Xu H, Li J, Li F, et al. TMTP1-modified, tumor microenvironment responsive nanoparticles co-deliver cisplatin and paclitaxel prodrugs for effective cervical cancer therapy. Int J Nanomedicine. 2021;16:4087–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ishima Y, Yamazaki N, Chuang VTG, Shimizu T, Ando H, Ishida T. A maleimide-terminally modified PEGylated liposome induced the accelerated blood clearance independent of the production of anti-PEG IgM antibodies. Biol Pharm Bull. 2022;45:1518–24. [DOI] [PubMed] [Google Scholar]
- 204.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92:3566–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Laakkonen P, Waltari M, Holopainen T, Takahashi T, Pytowski B, Steiner P, Hicklin D, Persaud K, Tonra JR, Witte L, Alitalo K. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007;67:593–9. [DOI] [PubMed] [Google Scholar]
- 206.Doroshow JH, Simon RM. On the design of combination cancer therapy. Cell. 2017;171:1476–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li J, Zhuang Z, Jiang B, Zhao P, Lin C. Advances and perspectives in nanoprobes for noninvasive lymph node mapping. Nanomed (Lond). 2015;10:1019–36. [DOI] [PubMed] [Google Scholar]
- 208.Cai X, Liu X, Liao LD, Bandla A, Ling JM, Liu YH, Thakor N, Bazan GC, Liu B. Encapsulated conjugated oligomer nanoparticles for real-time photoacoustic sentinel lymph node imaging and targeted photothermal therapy. Small. 2016;12:4873–80. [DOI] [PubMed] [Google Scholar]
- 209.Feng HY, Yuan Y, Zhang Y, Liu HJ, Dong X, Yang SC, Liu XL, Lai X, Zhu MH, Wang J, et al. Targeted micellar phthalocyanine for lymph node metastasis homing and photothermal therapy in an orthotopic colorectal tumor model. Nanomicro Lett. 2021;13:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shi H, Yan R, Wu L, Sun Y, Liu S, Zhou Z, He J, Ye D. Tumor-targeting CuS nanoparticles for multimodal imaging and guided photothermal therapy of lymph node metastasis. Acta Biomater. 2018;72:256–65. [DOI] [PubMed] [Google Scholar]
- 211.Ji C, Zhao M, Wang C, Liu R, Zhu S, Dong X, Su C, Gu Z. Biocompatible Tantalum Nanoparticles as Radiosensitizers for enhancing therapy efficacy in primary tumor and metastatic sentinel lymph nodes. ACS Nano. 2022;16:9428–41. [DOI] [PubMed] [Google Scholar]
- 212.Ma R, Tang X, Wang M, Du Z, Chen S, Heng Y, Zhu L, Alifu N, Zhang X, Ma C. Clinical indocyanine green-based silk fibroin theranostic nanoprobes for in vivo NIR-I/II fluorescence imaging of cervical diseases. Nanomedicine. 2023;47: 102615. [DOI] [PubMed] [Google Scholar]
- 213.Yang Z, Tian R, Wu J, Fan Q, Yung BC, Niu G, Jacobson O, Wang Z, Liu G, Yu G, et al. Impact of semiconducting perylene diimide nanoparticle size on lymph node mapping and cancer imaging. ACS Nano. 2017;11:4247–55. [DOI] [PubMed] [Google Scholar]
- 214.Kase AM, Menke D, Tan W. Breast cancer metastasis to the bladder: a literature review. BMJ Case Rep. 2018;2018:bcr2017222031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Scott EA, Karabin NB, Augsornworawat P. Overcoming immune dysregulation with immunoengineered nanobiomaterials. Annu Rev Biomed Eng. 2017;19:57–84. [DOI] [PubMed] [Google Scholar]
- 216.Zahin N, Anwar R, Tewari D, Kabir MT, Sajid A, Mathew B, Uddin MS, Aleya L, Abdel-Daim MM. Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environ Sci Pollut Res Int. 2020;27:19151–68. [DOI] [PubMed] [Google Scholar]
- 217.Hoshyar N, Gray S, Han H, Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine. 2016;11:673–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ye B, Fan D, Xiong W, Li M, Yuan J, Jiang Q, Zhao Y, Lin J, Liu J, Lv Y, Wang X, Li Z, Su J, Qiao Y. Oncogenic enhancers drive esophageal squamous cell carcinogenesis and metastasis. Nat Commun. 2021;12:4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li F, Nie W, Zhang F, Lu G, Lv C, Lv Y, Bao W, Zhang L, Wang S, Gao X, Wei W, Xie HY. Engineering magnetosomes for high-performance cancer vaccination. ACS Cent Sci. 2019;5:796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015;91:3–6. [DOI] [PubMed] [Google Scholar]
- 221.Eckhardt BL, Cao Y, Redfern AD, Chi LH, Burrows AD, Roslan S, Sloan EK, Parker BS, Loi S, Ueno NT, Lau PKH, Latham B, Anderson RL. Activation of canonical BMP4-SMAD7 signaling suppresses breast cancer metastasis. Cancer Res. 2020;80:1304–15. [DOI] [PubMed] [Google Scholar]
- 222.Chen X, Wang W, Jiang Y, Qian X. A dual-transformation with contrastive learning framework for lymph node metastasis prediction in pancreatic cancer. Med Image Anal. 2023;5: 102753. [DOI] [PubMed] [Google Scholar]
- 223.Ho AS, Kim S, Tighiouart M, Gudino C, Mita A, Scher KS, Laury A, Prasad R, Shiao SL, Ali N, Patio C, Mallen-St Clair J, Van Eyk JE, Zumsteg ZS. Association of quantitative metastatic lymph node burden with survival in hypopharyngeal and laryngeal cancer. JAMA Oncol. 2018;4:985–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang B, An J, Zhang H, Zhang S, Zhang H, Wang L, Zhang H, Zhang Z. Personalized cancer immunotherapy via transporting endogenous tumor antigens to lymph nodes mediated by nano Fe3 O4. Small. 2018;14: e1801372. [DOI] [PubMed] [Google Scholar]
- 225.Ryan GM, Kaminskas LM, Porter CJ. Nano-chemotherapeutics: maximising lymphatic drug exposure to improve the treatment of lymph-metastatic cancers. J Control Release. 2014;193:241–56. [DOI] [PubMed] [Google Scholar]
- 226.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly (propylene sulfide) nanoparticles. J Control Release. 2006;112:26–34. [DOI] [PubMed] [Google Scholar]
- 227.Schudel A, Francis DM, Thomas SN. Material design for lymph node drug delivery. Nat Rev Mater. 2019;4(6):415–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zwicke GL, Mansoori GA, Jeffery CJ. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012. 10.3402/nano.v3i0.18496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lin N, Qiu J, Song J, Yu C, Fang Y, Wu W, Yang W, Wang Y. Application of nano-carbon and titanium clip combined labeling in robot-assisted laparoscopic transverse colon cancer surgery. BMC Surg. 2021;21:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bottcher JP, Reis e Sousa C. The role of type 1 conventional dendritic cells in cancer immunity. Trends Cancer. 2018;4:784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dammeijer F, et al. The PD-1/PD-L1-checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell. 2020;38:685-700.e688. [DOI] [PubMed] [Google Scholar]
- 232.Clement CC, Wang W, Dzieciatkowska M, Cortese M, Hansen KC, Becerra A, Thangaswamy S, Nizamutdinova I, Moon JY, Stern LJ, Gashev AA, Zawieja D, Santambrogio L. Quantitative profiling of the lymph node clearance capacity. Sci Rep. 2018;8:11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gerner MY, Torabi-Parizi P, Germain RN. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity. 2015;42:172–85. [DOI] [PubMed] [Google Scholar]
- 234.Liu Y, Liu Y, Xu D, Zang J, Zheng X, Zhao Y, Li Y, He R, Ruan S, Dong H, Gu J, Yang Y, Cheng Q, Li Y. Targeting the negative feedback of adenosine-A2AR metabolic pathway by a tailored nanoinhibitor for photothermal immunotherapy. Adv Sci. 2022;9: e2104182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jalkanen S, Salmi M. Lymphatic endothelial cells of the lymph node. Nat Rev Immunol. 2020;20:566–78. [DOI] [PubMed] [Google Scholar]
- 236.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Oh HJ, Yang D, Oh HW, Jeon JG, Kim C, Ahn JY, Han SW, Kim CY. Chronologic trends of cancer-related lymph node research in PubMed: informetrics analysis. Ann Surg Treat Res. 2020;99:305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Scallan JP, Zawieja SD, Castorena-Gonzalez JA, Davis MJ. Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol. 2016;594:5749–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gashev AA. Basic mechanisms controlling lymph transport in the mesenteric lymphatic net. Ann NY Acad Sci. 2010;1207:E16-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Roozendaal R, Mebius RE, Kraal G. The conduit system of the lymph node. Int Immunol. 2008;20:1483–7. [DOI] [PubMed] [Google Scholar]
- 241.Menon I, Bagwe P, Gomes KB, Bajaj L, Gala R, Uddin MN, D’Souza MJ, Zughaier SM. Microneedles: a new generation vaccine delivery system. Micromachines. 2021;12(4):435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Trac N, Chung EJ. Overcoming physiological barriers by nanoparticles for intravenous drug delivery to the lymph nodes. Exp Biol Med (Maywood). 2021;246(22):2358–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Furubayashi T, Inoue D, Kimura S, Tanaka A, Sakane T. Evaluation of the pharmacokinetics of intranasal drug delivery for targeting cervical lymph nodes in rats. Pharmaceutics. 2021;13(9):1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med. 2001;194:1361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Patravale VB, Prabhu RH, Bora CR. Lymphatic delivery: concept, challenges and applications. Indian Drugs. 2017;54:5–22. [Google Scholar]
- 246.Hawley AE, Davis SS, Illum L. Targeting of colloids to lymph nodes: influence of lymphatic physiology and colloidal characteristics. Adv Drug Deliv Rev. 1995;17:129–48. [Google Scholar]
- 247.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ja C, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Schudel A, Chapman AP, Yau MK, Higginson CJ, Francis DM, Manspeaker MP, Avecilla ARC, Rohner NA, Finn MG, Thomas SN. Programmable multistage drug delivery to lymph nodes. Nat Nanotechnol. 2020;15:491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Dukhin SS, Labib ME. Convective diffusion of nanoparticles from the epithelial barrier toward regional lymph nodes. Adv Colloid Interface Sci. 2013;199–200:23–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ke X, Howard GP, Tang H, Cheng B, Saung MT, Santos JL, Mao HQ. Physical and chemical profiles of nanoparticles for lymphatic targeting. Adv Drug Deliv Rev. 2019;151–152:72–93. [DOI] [PubMed] [Google Scholar]
- 252.Montes-Casado M, Sanvicente A, Casarrubios L, Feito MJ, Rojo JM, Vallet-Regí M, Arcos D, Portolés P, Portolés MT. An immunological approach to the biocompatibility of mesoporous SiO2-CaO nanospheres. Int J Mol Sci. 2020;21:8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Patel HM, Boodle KM, Vaughan-Jones R. Assessment of the potential uses of liposomes for lymphoscintigraphy and lymphatic drug delivery. Failure of 99m-technetium marker to represent intact liposomes in lymph nodes. Biochim Biophys Acta. 1984;801:76–86. [DOI] [PubMed] [Google Scholar]
- 254.Punjabi MS, Naha A, Shetty D, Nayak UY. Lymphatic drug transport and associated drug delivery technologies: a comprehensive review. Curr Pharm Des. 2021;27(17):1992–8. [DOI] [PubMed] [Google Scholar]
- 255.Ding Y, Li Z, Jaklenec A, Hu Q. Vaccine delivery systems toward lymph nodes. Adv Drug Deliv Rev. 2021;179: 113914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chen Y, De Koker S, De Geest BG. Engineering strategies for lymph node targeted immune activation. Acc Chem Res. 2020;53(10):2055–67. [DOI] [PubMed] [Google Scholar]
- 257.Gracia G, Cao E, Feeney OM, Johnston APR, Porter CJH, Trevaskis NL. High-density lipoprotein composition influences lymphatic transport after subcutaneous administration. Mol Pharm. 2020;17(8):2938–51. [DOI] [PubMed] [Google Scholar]
- 258.He X, Wang J, Tang Y, Chiang ST, Han T, Chen Q, Qian C, Shen X, Li R, Ai X. Recent advances of emerging spleen-targeting nanovaccines for immunotherapy. Adv Healthc Mater. 2023;8: e2300351. [DOI] [PubMed] [Google Scholar]
- 259.Yoshida T, Kojima H, Sako K, Kondo H. Drug delivery to the intestinal lymph by oral formulations. Pharm Dev Technol. 2022;27(2):175–89. [DOI] [PubMed] [Google Scholar]
- 260.Refaat H, Naguib YW, Elsayed MMA, Sarhan HAA, Alaaeldin E. Modified spraying technique and response surface methodology for the preparation and optimization of propolis liposomes of enhanced anti-proliferative activity against human melanoma cell Line A375. Pharmaceutics. 2019;11:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol. 1964;8:660–8. [DOI] [PubMed] [Google Scholar]
- 262.Sun S, Sun S, Sun Y, Wang P, Zhang J, Du W, Wang S, Liang X. Bubble-manipulated local drug release from a smart thermosensitive cerasome for dual-mode imaging guided tumor chemo-photothermal therapy. Theranostics. 2019;9:8138–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Reichmuth AM, Oberli MA, Jaklenec A, Langer R, Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv. 2016;7:319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jung HS, Neuman KC. Surface modification of fluorescent nanodiamonds for biological applications. Nanomaterials. 2021;11:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Maeki M, Kimura N, Sato Y, Harashima H, Tokeshi M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv Drug Deliv Rev. 2018;128:84–100. [DOI] [PubMed] [Google Scholar]
- 266.Milicic A, Kaur R, Reyes-Sandoval A, Tang CK, Honeycutt J, Perrie Y, Hill AV. Small cationic DDA:TDB liposomes as protein vaccine adjuvants obviate the need for TLR agonists in inducing cellular and humoral responses. PLoS ONE. 2012;7: e34255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Chu Y, Qian L, Ke Y, Feng X, Chen X, Liu F, Yu L, Zhang L, Tao Y, Xu R, Wei J, Liu B, Liu Q. Lymph node-targeted neoantigen nanovaccines potentiate anti-tumor immune responses of post-surgical melanoma. J Nanobiotechnol. 2022;20:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Warashina S, Nakamura T, Sato Y, Fujiwara Y, Hyodo M, Hatakeyama H, Harashima H. A lipid nanoparticle for the efficient delivery of siRNA to dendritic cells. J Control Release. 2016;225:183–91. [DOI] [PubMed] [Google Scholar]
- 269.Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo MB, Mueller S, Irvine DJ. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J Clin Invest. 2015;125:2532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Chen J, Ye Z, Huang C, Qiu M, Song D, Li Y, Xu Q. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc Natl Acad Sci USA. 2022;119: e2207841119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Luozhong S, Yuan Z, Sarmiento T, Chen Y, Gu W, McCurdy C, Gao W, Li R, Wilkens S, Jiang S. Phosphatidylserine lipid nanoparticles promote systemic RNA delivery to secondary lymphoid organs. Nano Lett. 2022;22(20):8304–11. [DOI] [PubMed] [Google Scholar]
- 272.Trimaille T, Verrier B. Micelle-based adjuvants for subunit vaccine delivery. Vaccines. 2015;3:803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Li C, Iqbal M, Jiang B, Wang Z, Kim J, Nanjundan AK, Whitten AE, Wood K, Yamauchi Y. Pore-tuning to boost the electrocatalytic activity of polymeric micelle-templated mesoporous Pd nanoparticles. Chem Sci. 2019;10:4054–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Cui M, Jin M, Han M, Zang Y, Li C, Zhang D, Huang W, Gao Z, Yin X. Improved antitumor outcomes for colon cancer using nanomicelles loaded with the novel antitumor agent LA67. Int J Nanomed. 2020;15:3563–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Li X, Dong Q, Yan Z, Lu W, Feng L, Xie C, Xie Z, Su B, Liu M. MPEG-DSPE polymeric micelle for translymphatic chemotherapy of lymph node metastasis. Int J Pharm. 2015;487:8–16. [DOI] [PubMed] [Google Scholar]
- 276.Thol K, Pawlik P, McGranahan N. Therapy sculpts the complex interplay between cancer and the immune system during tumour evolution. Genome Med. 2022;14:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Cabral H, Makino J, Matsumoto Y, Mi P, Wu H, Nomoto T, Toh K, Yamada N, Higuchi Y, Konishi S, Kano MR, Nishihara H, Miura Y, Nishiyama N, Kataoka K. Systemic targeting of lymph node metastasis through the blood vascular system by using size-controlled nanocarriers. ACS Nano. 2015;9:4957–67. [DOI] [PubMed] [Google Scholar]
- 278.Kumar A, Tan A, Wong J, Spagnoli JC, Lam J, Blevins BD, Thorne GNL, Ashkan K, Xie J, Liu H. Nanotechnology for neuroscience: promising approaches for diagnostics, therapeutics and brain activity mapping. Adv Funct Mater. 2017;27:1700489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Anraku Y, Kuwahara H, Fukusato Y, Mizoguchi A, Ishii T, Nitta K, Matsumoto Y, Toh K, Miyata K, Uchida S, Nishina K, Osada K, Itaka K, Nishiyama N, Mizusawa H, Yamasoba T, Yokota T, Kataoka K. Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat Commun. 2017;8:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ehser S, Chuang JJ, Kleist C, Sandra-Petrescu F, Iancu M, Wang D, Opelz G, Terness P. Suppressive dendritic cells as a tool for controlling allograft rejection in organ transplantation: promises and difficulties. Hum Immunol. 2008;69:165–73. [DOI] [PubMed] [Google Scholar]
- 281.Jewell CM, López SC, Irvine DJ. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc Natl Acad Sci USA. 2011;108:15745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Liu W, et al. A novel targeted multifunctional nanoplatform for visual chemo-hyperthermia synergy therapy on metastatic lymph nodes via lymphatic delivery. J Nanobiotechnol. 2021;19:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Birkhauser FD, et al. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging facilitates detection of metastases in normal-sized pelvic lymph nodes of patients with bladder and prostate cancer. Eur Urol. 2013;64:953–60. [DOI] [PubMed] [Google Scholar]
- 284.Wang J, Lu T, Yang M, Sun D, Xia Y, Wang T. Hydrogel 3D printing with the capacitor edge effect. Sci Adv. 2019;5:eaau8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Chen W, Chen H, Zheng D, Zhang H, Deng L, Cui W, Zhang Y, Santos HA, Shen H. Gene-hydrogel microenvironment regulates extracellular matrix metabolism balance in nucleus pulposus. Adv Sci. 2019;7:1902099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Deng W, Yan Y, Zhuang P, Liu X, Tian K, Huang W, Li C. Synthesis of nanocapsules blended polymeric hydrogel loaded with bupivacaine drug delivery system for local anesthetics and pain management. Drug Deliv. 2022;29:399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhuang X, Wu T, Zhao Y, Hu X, Bao Y, Guo Y, Song Q, Li G, Tan S, Zhang Z. Lipid-enveloped zinc phosphate hybrid nanoparticles for codelivery of H-2K(b) and H-2D(b)-restricted antigenic peptides and monophosphoryl lipid A to induce antitumor immunity against melanoma. J Control Release. 2016;228:26–37. [DOI] [PubMed] [Google Scholar]
- 288.Kjellman P, in ’t Zandt R, Fredriksson S, Strand SE. Optimizing retention of multimodal imaging nanostructures in sentinel lymph nodes by nanoscale size tailoring. Nanomedicine. 2014;2014(10):1089–95. [DOI] [PubMed] [Google Scholar]
- 289.Zaloga J, Janko C, Nowak J, Matuszak J, Knaup S, Eberbeck D, Tietze R, Unterweger H, Friedrich RP, Duerr S, Heimke-Brinck R, Baum E, Cicha I, Dörje F, Odenbach S, Lyer S, Lee G, Alexiou C. Development of a lauric acid/albumin hybrid iron oxide nanoparticle system with improved biocompatibility. Int J Nanomedicine. 2014;9:4847–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zou Y, Liu P, Liu CH, Zhi XT. Doxorubicin-loaded mesoporous magnetic nanoparticles to induce apoptosis in breast cancer cells. Biomed Pharmacother. 2015;69:355–60. [DOI] [PubMed] [Google Scholar]
- 291.Quinto CA, Mohindra P, Tong S, Bao G. Multifunctional superparamagnetic iron oxide nanoparticles for combined chemotherapy and hyperthermia cancer treatment. Nanoscale. 2015;7:12728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Liu Y, Wang Z, Yu F, Li M, Zhu H, Wang K, Meng M, Zhao W. The adjuvant of α-galactosylceramide presented by gold nanoparticles enhances antitumor immune responses of MUC1 antigen-based tumor vaccines. Int J Nanomedicine. 2021;16:403–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Mottas I, Bekdemir A, Cereghetti A, Spagnuolo L, Yang YS, Müller M, Irvine DJ, Stellacci F, Bourquin C. Amphiphilic nanoparticle delivery enhances the anticancer efficacy of a TLR7 ligand via local immune activation. Biomaterials. 2019;190–191:111–20. [DOI] [PubMed] [Google Scholar]
- 294.Oladipo AO, Oluwafemi OS, Songca SP, Sukhbaatar A, Mori S, Okajima J, Komiya A, Maruyama S, Kodama T. A novel treatment for metastatic lymph nodes using lymphatic delivery and photothermal therapy. Sci Rep. 2017;7:45459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Dadfar SM, Roemhild K, Drude NI, von Stillfried S, Knüchel R, Kiessling F, Lammers T. Iron oxide nanoparticles: diagnostic, therapeutic and theranostic applications. Adv Drug Deliv Rev. 2019;138:302–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Pourmadadi M, Rahmani E, Shamsabadipour A, Mahtabian S, Ahmadi M, Rahdar A, Díez-Pascual AM. Role of iron oxide (Fe2O3) nanocomposites in advanced biomedical applications: a state-of-the-art review. Nanomaterials. 2022;12:3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Li AW, Sobral MC, Badrinath S, Choi Y, Graveline A, Stafford AG, Weaver JC, Dellacherie MO, Shih TY, Ali OA, Kim J, Wucherpfennig KW, Mooney DJ. A facile approach to enhance antigen response for personalized cancer vaccination. Nat Mater. 2018;17:528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lu Y, Yang Y, Gu Z, Zhang J, Song H, Xiang G, Yu C. Glutathione-depletion mesoporous organosilica nanoparticles as a self-adjuvant and Co-delivery platform for enhanced cancer immunotherapy. Biomaterials. 2018;175:82–92. [DOI] [PubMed] [Google Scholar]
- 299.Khakpour E, Salehi S, Naghib SM, Ghorbanzadeh S, Zhang W. Graphene-based nanomaterials for stimuli-sensitive controlled delivery of therapeutic molecules. Front Bioeng Biotechnol. 2023;11:1129768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Yang F, Jin C, Yang D, Jiang Y, Li J, Di Y, Hu J, Wang C, Ni Q, Fu D. Magnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. Eur J Cancer. 2011;47:1873–82. [DOI] [PubMed] [Google Scholar]
- 301.Nuhn L, Vanparijs N, De Beuckelaer A, Lybaert L, Verstraete G, Deswarte K, Lienenklaus S, Shukla NM, Salyer AC, Lambrecht BN, Grooten J, David SA, De Koker S, De Geest BG. pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation. Proc Natl Acad Sci USA. 2016;113:8098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.De Koker S, Cui J, Vanparijs N, Albertazzi L, Grooten J, Caruso F, De Geest BG. Engineering polymer hydrogel nanoparticles for lymph node-targeted delivery. Angew Chem Int Ed Engl. 2016;55:1334–9. [DOI] [PubMed] [Google Scholar]
- 303.Urimi D, Hellsing M, Mahmoudi N, Söderberg C, Widenbring R, Gedda L, Edwards K, Loftsson T, Schipper N. Structural characterization study of a lipid nanocapsule formulation intended for drug delivery applications using small-angle scattering techniques. Mol Pharm. 2022;19:1068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Shafiq M, Anjum S, Hano C, Anjum I, Abbasi BH. An overview of the applications of nanomaterials and nanodevices in the food industry. Foods. 2020;9:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Vicente S, Goins BA, Sanchez A, Alonso MJ, Phillips WT. Biodistribution and lymph node retention of polysaccharide-based immunostimulating nanocapsules. Vaccine. 2014;32:1685–92. [DOI] [PubMed] [Google Scholar]
- 306.Li AV, Moon JJ, Abraham W, Suh H, Elkhader J, Seidman MA, Yen M, Im EJ, Foley MH, Barouch DH, Irvine DJ. Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Sci Transl Med. 2013;5: 204ra130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Nawaz M, Yusuf N, Habib S, Shakoor RA, Ubaid F, Ahmad Z, Kahraman R, Mansour S, Gao W. Development and properties of polymeric nanocomposite coatings. Polymers. 2019;11:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Sato Y, Hashiba K, Sasaki K, Maeki M, Tokeshi M, Harashima H. Understanding structure-activity relationships of pH-sensitive cationic lipids facilitates the rational identification of promising lipid nanoparticles for delivering siRNAs in vivo. J Control Release. 2019;295:140–52. [DOI] [PubMed] [Google Scholar]
- 309.Gao W, Fang RH, Thamphiwatana S, Luk BT, Li J, Angsantikul P, Zhang Q, Hu CM, Zhang L. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015;15:1403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hu CM, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, Chien S, Zhang L. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Phillips WT, Klipper R, Goins B. Novel method of greatly enhanced delivery of liposomes to lymph nodes. J Pharmacol Exp Ther. 2000;295:309–13. [PubMed] [Google Scholar]
- 312.Phillips WWT, Klipper R, Goins B. Use of (99m)Tc-labeled liposomes encapsulating blue dye for identification of the sentinel lymph node. J Nucl Med. 2001;42:446–51. [PubMed] [Google Scholar]
- 313.Yuan B, Zhao S, Hu P, Cui J, Niu QJ. Asymmetric polyamide nanofilms with highly ordered nanovoids for water purification. Nat Commun. 2020;11(1):6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Esfand R, Tomalia DA. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6:427–36. [DOI] [PubMed] [Google Scholar]
- 315.Liu C, Liu X, Xiang X, Pang X, Chen S, Zhang Y, Ren E, Zhang L, Liu X, Lv P, Wang X, Luo W, Xia N, Chen X, Liu G. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy. Nat Nanotechnol. 2022;17:531–40. [DOI] [PubMed] [Google Scholar]
- 316.Wang S, Li F, Ye T, Wang J, Lyu C, Qing S, Ding Z, Gao X, Jia R, Yu D, Ren J, Wei W, Ma G. Macrophage-tumor chimeric exosomes accumulate in lymph node and tumor to activate the immune response and the tumor microenvironment. Sci Transl Med. 2021;13:eabb6981. [DOI] [PubMed] [Google Scholar]
- 317.Li L, Song L, Yang X, Li X, Wu Y, He T, Wang N, Yang S, Zeng Y, Yang L, Wu Q. Multifunctional, “core-shell” nanoparticles-based gene delivery for treatment of aggressive melanoma. Biomaterials. 2016;1(111):124–37. [DOI] [PubMed] [Google Scholar]
- 318.Blakney AK, McKay PF, Ibarzo Yus B, Hunter JE, Dex EA, Shattock RJ. The skin you in: are design-of-experiments optimization of lipid nanoparticle self-amplifying RNA formulations in human skin explants. ACS Nano. 2019;13(5):5920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Osborne MP, Richardson VJ, Jeyasingh K, Ryman BE. Radionuclide-labelled liposomes—a new lymph node imaging agent. Int J Nucl Med Biol. 1979;6:75–83. [DOI] [PubMed] [Google Scholar]
- 320.Chiechio RM, Ducarre S, Marets C, Dupont A, Even-Hernandez P, Pinson X, Dutertre S, Artzner F, Musumeci P, Ravel C, Faro MJL, Marchi V. Encapsulation of luminescent gold nanoclusters into synthetic vesicles. Nanomaterials. 2022;12:3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Talanov VS, Regino CA, Kobayashi H, Bernardo M, Choyke PL, Brechbiel MW. Dendrimer-based nanoprobe for dual modality magnetic resonance and fluorescence imaging. Nano Lett. 2006;6:1459–63. [DOI] [PubMed] [Google Scholar]
- 322.Kobayashi H, Kawamoto S, Sakai Y, Choyke PL, Star RA, Brechbiel MW, Sato N, Tagaya Y, Morris JC, Waldmann TA. Lymphatic drainage imaging of breast cancer in mice by micro-magnetic resonance lymphangiography using a nano-size paramagnetic contrast agent. J Natl Cancer Inst. 2004;96:703–8. [DOI] [PubMed] [Google Scholar]
- 323.Niki Y, Ogawa M, Makiura R, Magata Y, Kojima C. Optimization of dendrimer structure for sentinel lymph node imaging: Effects of generation and terminal group. Nanomedicine. 2015;11(8):2119–27. [DOI] [PubMed] [Google Scholar]
- 324.Yakunin S, Chaaban J, Benin BM, Cherniukh I, Bernasconi C, Landuyt A, Shynkarenko Y, Bolat S, Hofer C, Romanyuk YE, Cattaneo S, Pokutnyi SI, Schaller RD, Bodnarchuk MI, Poulikakos D, Kovalenko MV. Radiative lifetime-encoded unicolour security tags using perovskite nanocrystals. Nat Commun. 2021;12:981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Han SJ, Rathinaraj P, Park SY, Kim YK, Lee JH, Kang IK, Moon JS, Winiarz JG. Specific intracellular uptake of herceptin-conjugated CdSe/ZnS quantum dots into breast cancer cells. Biomed Res Int. 2014;2014: 954307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A, Parker JA, Mihaljevic T, Laurence RG, Dor DM, Cohn LH, Bawendi MG, Frangioni JV. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Kim SW, Zimmer JP, Ohnishi S, Tracy JB, Frangioni JV, Bawendi MG. Engineering InAs(x)P(1–x)/InP/ZnSe III-V alloyed core/shell quantum dots for the near-infrared. J Am Chem Soc. 2005;127:10526–32. [DOI] [PubMed] [Google Scholar]
- 328.Dai T, Zhou S, Yin C, Li S, Cao W, Liu W, Sun K, Dou H, Cao Y, Zhou G. Dextran-based fluorescent nanoprobes for sentinel lymph node mapping. Biomaterials. 2014;35:8227–35. [DOI] [PubMed] [Google Scholar]
- 329.Xie F, Zhang D, Cheng L, Yu L, Yang L, Tong F, Liu H, Wang S, Wang S. Intradermal microbubbles and contrast-enhanced ultrasound (CEUS) is a feasible approach for sentinel lymph node identification in early-stage breast cancer. World J Surg Oncol. 2015;13:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Montoya Mira J, Wu L, Sabuncu S, Sapre A, Civitci F, Ibsen S, Esener S, Yildirim A. Gas-stabilizing sub-100 nm mesoporous silica nanoparticles for ultrasound theranostics. ACS Omega. 2020;5:24762–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Nie Z, Luo N, Liu J, Zeng X, Zhang Y, Su D. Multi-mode biodegradable tumour-microenvironment sensitive nanoparticles for targeted breast cancer imaging. Nanoscale Res Lett. 2020;15:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Stride E, Saffari N. The potential for thermal damage posed by microbubble ultrasound contrast agents. Ultrasonics. 2004;42:907–13. [DOI] [PubMed] [Google Scholar]
- 333.Hultborn KA, Larsson LG, Raghult I. The lymph drainage from the breast to the axillary and parasternal lymph nodes, studied with the aid of colloidal Au198. Acta radiol. 1955;43:52–64. [DOI] [PubMed] [Google Scholar]
- 334.Zhou Y, Chakraborty S, Liu S. Radiolabeled cyclic RGD peptides as radiotracers for imaging tumors and thrombosis by SPECT. Theranostics. 2011;1:58–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Wilhelm AJ, Mijnhout GS, Franssen EJ. Radiopharmaceuticals in sentinel lymph-node detection—an overview. Eur J Nucl Med. 1999;26:S36-42. [DOI] [PubMed] [Google Scholar]
- 336.Zhang Y, Cheng H, Chen H, Xu P, Ren E, Jiang Y, Li D, Gao X, Zheng Y, He P, Lin H, Chen B, Lin G, Chen A, Chu C, Mao J, Liu G. A pure nanoICG-based homogeneous lipiodol formulation: toward precise surgical navigation of primary liver cancer after long-term transcatheter arterial embolization. Eur J Nucl Med Mol Imaging. 2022;49:2605–17. [DOI] [PubMed] [Google Scholar]
- 337.Naz S, Shamoon M, Wang R, Zhang L, Zhou J, Chen J. Advances in therapeutic implications of inorganic drug delivery nano-platforms for cancer. Int J Mol Sci. 2019;20:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Eid HM, Ali AA, Ali AMA, Eissa EM, Hassan RM, Abo El-Ela FI, Hassan AH. Potential use of tailored citicoline chitosan-coated liposomes for effective wound healing in diabetic rat model. Int J Nanomedicine. 2022;17:555–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Lee J, Kang S, Park H, Sun JG, Kim EC, Shim G. Nanoparticles for lymph node-directed delivery. Pharmaceutics. 2023;15:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Cheng H, Yang X, Liu G. Superstable homogeneous iodinated formulation technology: revolutionizing transcatheter arterial chemoembolization. Sci Bull. 2020;65:1685–7. [DOI] [PubMed] [Google Scholar]
- 341.Ma JJ, Zhang DB, Zhang WF, Wang X. Application of nanocarbon in breast approach endoscopic thyroidectomy thyroid cancer surgery. J Laparoendosc Adv Surg Tech A. 2020;30:547–52. [DOI] [PubMed] [Google Scholar]
- 342.Wang R, Mo S, Liu Q, Zhang W, Zhang Z, He Y, Cai G, Li X. The safety and effectiveness of carbon nanoparticles suspension in tracking lymph node metastases of colorectal cancer: a prospective randomized controlled trial. Jpn J Clin Oncol. 2020;50:535–42. [DOI] [PubMed] [Google Scholar]
- 343.Schilham MGM, Zamecnik P, Privé BM, Israël B, Rijpkema M, Scheenen T, Barentsz JO, Nagarajah J, Gotthardt M. Head-to-head comparison of 68Ga-prostate-specific membrane antigen PET/CT and ferumoxtran-10-enhanced MRI for the diagnosis of lymph node metastases in prostate cancer patients. J Nucl Med. 2021;62:1258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wallace AM, Hoh CK, Ellner SJ, Darrah DD, Schulteis G, Vera DR. Lymphoseek: a molecular imaging agent for melanoma sentinel lymph node mapping. Ann Surg Oncol. 2007;14:913–21. [DOI] [PubMed] [Google Scholar]
- 345.Bradbury MS, Pauliah M, Zanzonico P, Wiesner U, Patel S. Intraoperative mapping of sentinel lymph node metastases using a clinically translated ultrasmall silica nanoparticle. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:535–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.He P, Ren E, Chen B, Chen H, Cheng H, Gao X, Liang X, Liu H, Li J, Li B, Chen A, Chu C, Chen X, Mao J, Zhang Y, Liu G. A super-stable homogeneous lipiodol-hydrophilic chemodrug formulation for treatment of hepatocellular carcinoma. Theranostics. 2022;12:1769–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.He P, Xiong Y, Ye J, Chen B, Cheng H, Liu H, Zheng Y, Chu C, Mao J, Chen A, Zhang Y, Li J, Tian J, Liu G. A clinical trial of super-stable homogeneous lipiodol-nanoICG formulation-guided precise fluorescent laparoscopic hepatocellular carcinoma resection. J Nanobiotechnology. 2022;20:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Peng X, Wang J, Zhou F, Liu Q, Zhang Z. Nanoparticle-based approaches to target the lymphatic system for antitumor treatment. Cell Mol Life Sci. 2021;78:5139–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Angelousi A, Hayes AR, Chatzellis E, Kaltsas GA, Grossman AB. Metastatic medullary thyroid carcinoma: a new way forward. Endocr Relat Cancer. 2022;29(7):R85–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Jiang T, Xie L, Zhou S, Liu Y, Huang Y, Mei N, et al. Metformin and histone deacetylase inhibitor based anti-inflammatory nanoplatform for epithelial-mesenchymal transition suppression and metastatic tumor treatment. J Nanobiotechnol. 2022;20(1):394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Sugiyama S, Iwai T, Baba J, Oguri S, Izumi T, Sekino M, et al. MR lymphography with superparamagnetic iron oxide for sentinel lymph node mapping of N0 early oral cancer: a pilot study. Dento Maxillo Fac Radiol. 2021;50(4):20200333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Sreedhar S, Maloney J, Hudson S. Introducing SentiMag in a rural setting: a 5-year experience. ANZ J Surg. 2021;91(11):2404–10. [DOI] [PubMed] [Google Scholar]
- 353.Pouw JJ, Grootendorst MR, Bezooijen R, Klazen CA, De Bruin WI, Klaase JM, et al. Pre-operative sentinel lymph node localization in breast cancer with superparamagnetic iron oxide MRI: the SentiMAG Multicentre Trial imaging subprotocol. Br J Radiol. 2015;88(1056):20150634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Nune SK, Gunda P, Majeti BK, Thallapally PK, Forrest ML. Advances in lymphatic imaging and drug delivery. Adv Drug Deliv Rev. 2011;63(10–11):876–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Ganeshalingam S, Koh DM. Nodal staging. Cancer Imaging. 2009;9(1):104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Griffin LR, Frank C, Rao S, Seguin B. Lymphotropic nanoparticle magnetic resonance imaging for diagnosing metastatic lymph nodes in dogs with malignant head and neck tumours. Vet Comp Oncol. 2023. 10.1111/vco.12901. [DOI] [PubMed] [Google Scholar]
- 357.Philips BWJ, Stijns RCH, Rietsch SHG, Brunheim S, Barentsz JO, Fortuin AS, et al. USPIO-enhanced MRI of pelvic lymph nodes at 7-T: preliminary experience. Eur Radiol. 2019;29(12):6529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Froehlich JM, Triantafyllou M, Fleischmann A, Vermathen P, Thalmann GN, Thoeny HC. Does quantification of USPIO uptake-related signal loss allow differentiation of benign and malignant normal-sized pelvic lymph nodes? Contrast Media Mol Imaging. 2012;7(3):346–55. [DOI] [PubMed] [Google Scholar]
- 359.Fortuin AS, Smeenk RJ, Meijer HJ, Witjes AJ, Barentsz JO. Lymphotropic nanoparticle-enhanced MRI in prostate cancer: value and therapeutic potential. Curr Urol Rep. 2014;15(3):389. [DOI] [PubMed] [Google Scholar]
- 360.Fortuin A, van Asten J, Veltien A, Philips B, Hambrock T, Johst S, et al. Small suspicious lymph nodes detected on ultrahigh-field magnetic resonance imaging (MRI) in patients with prostate cancer with high risk of nodal metastases: the First In-patient study on ultrasmall superparamagnetic iron oxide-enhanced 7T MRI. Eur Urol. 2023;83(4):375–7. [DOI] [PubMed] [Google Scholar]
- 361.Pultrum BB, van der Jagt EJ, van Westreenen HL, van Dullemen HM, Kappert P, Groen H, et al. Detection of lymph node metastases with ultrasmall superparamagnetic iron oxide (USPIO)-enhanced magnetic resonance imaging in oesophageal cancer: a feasibility study. Cancer Imaging. 2009;9(1):19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.de Gouw D, Maas MC, Slagt C, Muhling J, Nakamoto A, Klarenbeek BR, et al. Controlled mechanical ventilation to detect regional lymph node metastases in esophageal cancer using USPIO-enhanced MRI; comparison of image quality. Magn Reson Imaging. 2020;74:258–65. [DOI] [PubMed] [Google Scholar]
- 363.Oghabian MA, Gharehaghaji N, Amirmohseni S, Khoei S, Guiti M. Detection sensitivity of lymph nodes of various sizes using USPIO nanoparticles in magnetic resonance imaging. Nanomed Nanatechnol Biology Med. 2010;6(3):496–9. [DOI] [PubMed] [Google Scholar]
- 364.Zamecnik P, Israel B, Feuerstein J, Nagarajah J, Gotthardt M, Barentsz JO, et al. Ferumoxtran-10-enhanced 3-T magnetic resonance angiography of pelvic arteries: initial experience. Eur Urol Focus. 2022;8(6):1802–8. [DOI] [PubMed] [Google Scholar]
- 365.Harisinghani MG, Dixon WT, Saksena MA, Brachtel E, Blezek DJ, Dhawale PJ, et al. MR lymphangiography: imaging strategies to optimize the imaging of lymph nodes with ferumoxtran-10. Radiographics. 2004;24(3):867–78. [DOI] [PubMed] [Google Scholar]
- 366.Griffin L, Frank CB, Seguin B. Pilot study to evaluate the efficacy of lymphotropic nanoparticle enhanced MRI for diagnosis of metastatic disease in canine head and neck tumours. Vet Comp Oncol. 2020;18(2):176–83. [DOI] [PubMed] [Google Scholar]
- 367.Kim SH, Oh SN, Choi HS, Lee HS, Jun J, Nam Y, et al. USPIO enhanced lymph node MRI using 3D multi-echo GRE in a rabbit model. Contrast Media Mol Imaging. 2016;11(6):544–9. [DOI] [PubMed] [Google Scholar]
- 368.Pandharipande PV, Mora JT, Uppot RN, Goehler A, Braschi M, Halpern EF, et al. Lymphotropic nanoparticle-enhanced MRI for independent prediction of lymph node malignancy: a logistic regression model. AJR Am J Roentgenol. 2009;193(3):W230–7. [DOI] [PubMed] [Google Scholar]
- 369.Minamiya Y, Ito M, Hosono Y, Kawai H, Saito H, Katayose Y, et al. Subpleural injection of tracer improves detection of mediastinal sentinel lymph nodes in non-small cell lung cancer. Eur J Cardio Thorac Surg. 2007;32(5):770–5. [DOI] [PubMed] [Google Scholar]
- 370.Zhang F, Zhu L, Huang X, Niu G, Chen X. Differentiation of reactive and tumor metastatic lymph nodes with diffusion-weighted and SPIO-enhanced MRI. Mol Imaging Biol. 2013;15(1):40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Song J, Hu Q, Huang J, Chen T, Ma Z, Shi H. MR targeted imaging for the expression of tenascin-C in cervical cancer. Br J Radiol. 2018;91(1090):20170681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Jain R, Dandekar P, Patravale V. Diagnostic nanocarriers for sentinel lymph node imaging. J Controlled Release. 2009;138(2):90–102. [DOI] [PubMed] [Google Scholar]
- 373.Anzai Y, Piccoli CW, Outwater EK, Stanford W, Bluemke DA, Nurenberg P, et al. Evaluation of neck and body metastases to nodes with ferumoxtran 10-enhanced MR imaging: phase III safety and efficacy study. Radiology. 2003;228(3):777–88. [DOI] [PubMed] [Google Scholar]
- 374.Shen N, Tan J, Wang P, Wang J, Shi Y, Lv W, et al. Indirect magnetic resonance imaging lymphography identifies lymph node metastasis in rabbit pyriform sinus VX2 carcinoma using ultra-small super-paramagnetic iron oxide. PLoS ONE. 2014;9(4): e94876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Triantafyllou M, Studer UE, Birkhauser FD, Fleischmann A, Bains LJ, Petralia G, et al. Ultrasmall superparamagnetic particles of iron oxide allow for the detection of metastases in normal sized pelvic lymph nodes of patients with bladder and/or prostate cancer. Eur J Cancer. 2013;49(3):616–24. [DOI] [PubMed] [Google Scholar]
- 376.Choi SH, Kim KH, Moon WK, Kim HC, Cha JH, Paik JH, et al. Comparison of lymph node metastases assessment with the use of USPIO-enhanced MR imaging at 15 T versus 30 T in a rabbit model. J Magn Reson Imaging. 2010;31(1):134–41. [DOI] [PubMed] [Google Scholar]
- 377.Jayaprakasam VS, Alvarez J, Omer DM, Gollub MJ, Smith JJ, Petkovska I. Watch-and-wait approach to rectal cancer: the role of imaging. Radiology. 2023;307(1): e221529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Davenport MS. Incidental findings and low-value care. AJR Am J Roentgenol. 2023. 10.2214/AJR.22.28926. [DOI] [PubMed] [Google Scholar]
- 379.Bowen SR, Nyflot MJ, Gensheimer M, Hendrickson KR, Kinahan PE, Sandison GA, et al. Challenges and opportunities in patient-specific, motion-managed and PET/CT-guided radiation therapy of lung cancer: review and perspective. Clin Translational Med. 2012;1(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Sadrkhanloo M, Entezari M, Orouei S, Ghollasi M, Fathi N, Rezaei S, et al. STAT3-EMT axis in tumors: modulation of cancer metastasis, stemness and therapy response. Pharmacol Res. 2022;182: 106311. [DOI] [PubMed] [Google Scholar]
- 381.Yang Y, Gu J, Li X, Xue C, Ba L, Gao Y, et al. HIF-1alpha promotes the migration and invasion of cancer-associated fibroblasts by miR-210. Aging Dis. 2021;12(7):1794–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Zheng H, Yuan C, Cai J, Pu W, Wu P, Li C, et al. Early diagnosis of breast cancer lung metastasis by nanoprobe-based luminescence imaging of the pre-metastatic niche. J Nanobiotechnol. 2022;20(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Qiao C, Wang H, Guan Q, Wei M, Li Z. Ferroptosis-based nano delivery systems targeted therapy for colorectal cancer: insights and future perspectives. Asian J Pharm Sci. 2022;17(5):613–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Montagna G, Zhang J, Sevilimedu V, Charyn J, Abbate K, Gomez EA, et al. Risk factors and racial and ethnic disparities in patients with breast cancer-related lymphedema. JAMA Oncol. 2022;8(8):1195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Gallo J, Long NJ, Aboagye EO. Magnetic nanoparticles as contrast agents in the diagnosis and treatment of cancer. Chem Soc Rev. 2013;42(19):7816–33. [DOI] [PubMed] [Google Scholar]
- 386.Han M, Kang R, Zhang C. Lymph node mapping for tumor micrometastasis. ACS Biomater Sci Eng. 2022;8(6):2307–20. [DOI] [PubMed] [Google Scholar]
- 387.Kurochkin MA, German SV, Abalymov A, Vorontsov Dcapital AC, Gorin DA, Novoselova MV. Sentinel lymph node detection by combining nonradioactive techniques with contrast agents: state of the art and prospects. J Biophotonics. 2022;15(1): e202100149. [DOI] [PubMed] [Google Scholar]
- 388.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–72. [DOI] [PubMed] [Google Scholar]
- 389.Thoeny HC, Barbieri S, Froehlich JM, Turkbey B, Choyke PL. Functional and targeted lymph node imaging in prostate cancer: current status and future challenges. Radiology. 2017;285(3):728–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Pouw JJ, Grootendorst MR, Klaase JM, van Baarlen J, Ten Haken B. Ex vivo sentinel lymph node mapping in colorectal cancer using a magnetic nanoparticle tracer to improve staging accuracy: a pilot study. Colorectal Dis. 2016;18(12):1147–53. [DOI] [PubMed] [Google Scholar]
- 391.Donohoe KJ, Carroll BJ, Chung DKV, Dibble EH, Diego E, Giammarile F, et al. Summary: appropriate use criteria for lymphoscintigraphy in sentinel node mapping and lymphedema/lipedema. J Nucl Med. 2023;64(4):525–8. [DOI] [PubMed] [Google Scholar]
- 392.Feldman AS, McDougal WS, Harisinghani MG. The potential of nanoparticle-enhanced imaging. Urol Oncol. 2008;26(1):65–73. [DOI] [PubMed] [Google Scholar]
- 393.Lai G, Rockall AG. Lymph node imaging in gynecologic malignancy. Semin Ultrasound CT MR. 2010;31(5):363–76. [DOI] [PubMed] [Google Scholar]
- 394.Fortuin AS, Bruggemann R, van der Linden J, Panfilov I, Israel B, Scheenen TWJ, et al. Ultra-small superparamagnetic iron oxides for metastatic lymph node detection: back on the block. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018;10(1): e1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Harisinghani M, Ross RW, Guimaraes AR, Weissleder R. Utility of a new bolus-injectable nanoparticle for clinical cancer staging. Neoplasia. 2007;9(12):1160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Cho HR, Choi SH, Lee N, Hyeon T, Kim H, Moon WK. Macrophages homing to metastatic lymph nodes can be monitored with ultrasensitive ferromagnetic iron-oxide nanocubes and a 15T clinical MR scanner. PLoS ONE. 2012;7(1): e29575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Néel L. Théorie du traînage magnétique des ferromagnétiques en grains fins avec application aux terres cuites. Ann de Géophys. 1949;5:99–136. [Google Scholar]
- 398.Vangijzegem T, Lecomte V, Ternad I, Van Leuven L, Muller RN, Stanicki D, et al. Superparamagnetic iron oxide nanoparticles (SPION): from fundamentals to state-of-the-art innovative applications for cancer therapy. Pharmaceutics. 2023;15(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Vu-Quang H, Yoo MK, Jeong HJ, Lee HJ, Muthiah M, Rhee JH, et al. Targeted delivery of mannan-coated superparamagnetic iron oxide nanoparticles to antigen-presenting cells for magnetic resonance-based diagnosis of metastatic lymph nodes in vivo. Acta Biomater. 2011;7(11):3935–45. [DOI] [PubMed] [Google Scholar]
- 400.Motomura K, Ishitobi M, Komoike Y, Koyama H, Noguchi A, Sumino H, et al. SPIO-enhanced magnetic resonance imaging for the detection of metastases in sentinel nodes localized by computed tomography lymphography in patients with breast cancer. Ann Surg Oncol. 2011;18(12):3422–9. [DOI] [PubMed] [Google Scholar]
- 401.Motomura K, Izumi T, Tateishi S, Sumino H, Noguchi A, Horinouchi T, et al. Correlation between the area of high-signal intensity on SPIO-enhanced MR imaging and the pathologic size of sentinel node metastases in breast cancer patients with positive sentinel nodes. BMC Med Imaging. 2013;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Gupta S, Rajesh A. Magnetic resonance imaging of penile cancer. Magn Reson Imaging Clin N Am. 2014;22(2):191–9. [DOI] [PubMed] [Google Scholar]
- 403.Li X, Xu C, Yu Y, Guo Y, Sun H. Prediction of lymphovascular space invasion using a combination of tenascin-C, cox-2, and PET/CT radiomics in patients with early-stage cervical squamous cell carcinoma. BMC Cancer. 2021;21(1):866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Visscher M, Pouw JJ, van Baarlen J, Klaase JM, Ten Haken B. Quantitative analysis of superparamagnetic contrast agent in sentinel lymph nodes using ex vivo vibrating sample magnetometry. IEEE Trans Bio Med Eng. 2013;60(9):2594–602. [DOI] [PubMed] [Google Scholar]
- 405.Grootendorst DJ, Fratila RM, Visscher M, Haken BT, van Wezel RJ, Rottenberg S, et al. Intra-operative ex vivo photoacoustic nodal staging in a rat model using a clinical superparamagnetic iron oxide nanoparticle dispersion. J Biophotonics. 2013;6(6–7):493–504. [DOI] [PubMed] [Google Scholar]
- 406.Johnson L, Pinder SE, Douek M. Deposition of superparamagnetic iron-oxide nanoparticles in axillary sentinel lymph nodes following subcutaneous injection. Histopathology. 2013;62(3):481–6. [DOI] [PubMed] [Google Scholar]
- 407.Winter A, Kowald T, Engels S, Wawroschek F. Magnetic resonance sentinel lymph node imaging and magnetometer-guided intraoperative detection in penile cancer, using superparamagnetic iron oxide nanoparticles: first results. Urol Int. 2020;104(3–4):177–80. [DOI] [PubMed] [Google Scholar]
- 408.Fortuin AS, Deserno WM, Meijer HJ, Jager GJ, Takahashi S, Debats OA, et al. Value of PET/CT and MR lymphography in treatment of prostate cancer patients with lymph node metastases. Int J Radiat Oncol Biol Phys. 2012;84(3):712–8. [DOI] [PubMed] [Google Scholar]
- 409.Tatsumi Y, Tanigawa N, Nishimura H, Nomura E, Mabuchi H, Matsuki M, et al. Preoperative diagnosis of lymph node metastases in gastric cancer by magnetic resonance imaging with ferumoxtran-10. Gastric Cancer. 2006;9(2):120–8. [DOI] [PubMed] [Google Scholar]
- 410.Tokuhara T, Tanigawa N, Matsuki M, Nomura E, Mabuchi H, Lee SW, et al. Evaluation of lymph node metastases in gastric cancer using magnetic resonance imaging with ultrasmall superparamagnetic iron oxide (USPIO): diagnostic performance in post-contrast images using new diagnostic criteria. Gastric Cancer. 2008;11(4):194–200. [DOI] [PubMed] [Google Scholar]
- 411.Motoyama S, Ishiyama K, Maruyama K, Narita K, Minamiya Y, Ogawa J. Estimating the need for neck lymphadenectomy in submucosal esophageal cancer using superparamagnetic iron oxide-enhanced magnetic resonance imaging: clinical validation study. World J Surg. 2012;36(1):83–9. [DOI] [PubMed] [Google Scholar]
- 412.Lai CH, Yen TC, Ng KK. Surgical and radiologic staging of cervical cancer. Curr Opin Obst Gynecol. 2010;22(1):15–20. [DOI] [PubMed] [Google Scholar]
- 413.Lee Y, Lee JS, Kim CM, Jeong JY, Choi JI, Kim MJ. Area of paradoxical signal drop after the administration of superparamagnetic iron oxide on the T2-weighted image of a patient with lymphangitic metastasis of the liver. Magn Reson Imaging. 2008;26(4):577–82. [DOI] [PubMed] [Google Scholar]
- 414.Kitamura N, Kosuda S, Araki K, Tomifuji M, Mizokami D, Shiotani A, et al. Comparison of animal studies between interstitial magnetic resonance lymphography and radiocolloid SPECT/CT lymphoscintigraphy in the head and neck region. Ann Nucl Med. 2012;26(3):281–5. [DOI] [PubMed] [Google Scholar]
- 415.Lei J, Xue HD, Li Z, Li S, Jin ZY. Possible pathological basis for false diagnoses of lymph nodes by USPIO-enhanced MRI in rabbits. J Magn Reson Imaging: JMRI. 2010;31(6):1428–34. [DOI] [PubMed] [Google Scholar]
- 416.Zhou Z, Chen H, Lipowska M, Wang L, Yu Q, Yang X, et al. A dual-modal magnetic nanoparticle probe for preoperative and intraoperative mapping of sentinel lymph nodes by magnetic resonance and near infrared fluorescence imaging. J Biomater Appl. 2013;28(1):100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Ittrich H, Peldschus K, Raabe N, Kaul M, Adam G. Superparamagnetic iron oxide nanoparticles in biomedicine: applications and developments in diagnostics and therapy. RoFo. 2013;185(12):1149–66. [DOI] [PubMed] [Google Scholar]
- 418.Will O, Purkayastha S, Chan C, Athanasiou T, Darzi AW, Gedroyc W, et al. Diagnostic precision of nanoparticle-enhanced MRI for lymph-node metastases: a meta-analysis. Lancet Oncol. 2006;7(1):52–60. [DOI] [PubMed] [Google Scholar]
- 419.Grootendorst DJ, Jose J, Fratila RM, Visscher M, Velders AH, Ten Haken B, et al. Evaluation of superparamagnetic iron oxide nanoparticles (Endorem(R)) as a photoacoustic contrast agent for intra-operative nodal staging. Contrast Media Mol Imaging. 2013;8(1):83–91. [DOI] [PubMed] [Google Scholar]
- 420.Pantiora E, Tasoulis MK, Valachis A, Eriksson S, Kuhn T, Karakatsanis A, et al. Evolution and refinement of magnetically guided sentinel lymph node detection in breast cancer: meta-analysis. Br J Surg. 2023;110(4):410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Wang G, Li W, Shi G, Tian Y, Kong L, Ding N, et al. Sensitive and specific detection of breast cancer lymph node metastasis through dual-modality magnetic particle imaging and fluorescence molecular imaging: a preclinical evaluation. Eur J Nucl Med Mol Imaging. 2022;49(8):2723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Cleaveland P, Lau M, Parnham A, Murby B, Ashworth D, Manohoran P, et al. Testing the feasibility of SentiMag/Sienna + for detecting inguinal sentinel nodes in penile cancer (SentiPen): an eUROGEN and National cancer research institute trial. Eur Urol. 2019;76(6):874–5. [DOI] [PubMed] [Google Scholar]
- 423.Nishio N, van den Berg NS, van Keulen S, et al. Optical molecular imaging can differentiate metastatic from benign lymph nodes in head and neck cancer. Nat Commun. 2019;10:5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.