Abstract
Background
Colorectal cancer (CRC) has increasingly come into worldwide cancer and almost half of patients have liver metastasis (CRLM) during the progression. Therefore, treatment of colorectal cancer liver metastasis (CRLM) is important to improve the prognosis of CRC patients. Histopathological growth patterns (HGPs) of CRLM have emerged as a reliable prognostic marker. In this study, we investigated the role of prognostic impact of pure desmoplastic HGPs (dHGPs), 100% desmoplastic, in CRLM.
Methods
The present study evaluated the HGPs in 71 patients with CRLM who underwent surgery (R0) between 1995 and 2012. HGPs were classified by international consensus guidelines with H&E stained slides. The pure dHGPs was defined as a complete peripheral fibrotic rim around the tumor.
Results
The dHGP was present in 36.6% (n = 26) and the pure-dHGPs was present in 73% (n = 19) among dHGPs patients. Pure-dHGPs were significantly associated with sex (male), metachronous metastatic period, normal CEA level, shallow tumor invasion and less lymph node metastasis.
Patients with dHGPs had longer overall survival (OS) compared to other HGPs (p < 0.05). Furthermore, pure dHGPs patients had longer OS than non-pure dHGPs (90.9% vs. 51.4%, p < 0.05). Multivariate analysis identified pure dHGPs (p = 0.04) and better primary tumor differentiation (p < 0.001) were identified as independent prognostic indicators for OS. Patients with pure dHGPs also had longer disease-free survival (DFS) compared to other HGPs (p < 0.05). Pure-dHGPs patients had longer DFS than non-pure dHGPs (63.4% vs. 28.8%, p < 0.05). Multivariate analysis identified pure dHGPs (p = 0.04) and better primary tumor differentiation (p = 0.03) as independent prognostic indicators.
Conclusions
Pure desmoplastic HGP might be a good prognostic marker in CRLM.
Keywords: CRLM, HGP, Colorectal cancer
Introductions
The prognosis in patients with colorectal cancer (CRC) is related to the development of liver metastasis. CRC initially metastasizes to the liver in approximately 25% of cases [1]. Consequently, half of cases develop liver metastasis in the course of a disease [2]. Therefore, CRC liver metastasis (CRLM) may be a critical factor to determine the prognosis of CRC patients.
Histopathological growth patterns (HGPs) have been extensively studied as independent prognosticators in CRLM patients [3]. Three distinct HGPs have been reported in CRLM: desmoplastic HGPs (dHGPs), pushing HGPs (pHGPs) and replacement HGPs (rHGPs) [4]. HGPs could be assessed according to the distinct biological and invasive patterns of the interface between tumor cells and normal liver parenchyma. The characteristic of dHGPs is broad peripheral rim which comes from the fibrotic reaction (desmoplasia) and angiogenesis [5]. A previous study found that dHGP patients have superior survival compared to rHGPs and pHGP [6]. Moreover, recent studies have suggested that pure HGPs which show 100% of the interface of growth pattern could predict the effect of systemic chemotherapy [6, 7]. One report showed pure dHGPs patients showed better overall survival (OS) compared to any other type of HGPs (i.e. pHGPS, rHGP or mixed HGPs) in chemo-naïve patients with CRLM [6]. However, there was no report about the prognostic significance of pure-dHGP but not limited to the neoadjuvant chemotherapy.
The aim of this study was to evaluate the prognostic significance of pure dHGPs compared to other HGPs.
Methods
Patients
The present study enrolled 71 patients with CRLM who underwent surgery (R0) between 1995 and 2012 at Tokushima University Hospital. The regular follow-up consisted of measurements of the carcinoembryonic antigen (CEA), carbohydrate 19–9 (CA19-9), computed tomography, ultrasonography and colonoscopy was performed up to 5 years after surgery. The median follow-up period was 47.5 months. The classifications of liver metastasis, such as H-stage and grade, were defined in accordance with the Japanese Classification of Colorectal Carcinoma, Second English Edition [8]. Briefly, H-stage was classified by the number and maximum diameter of liver tumors. Grade classifications were determined by H-stage, mesenteric lymph node metastasis, and extrahepatic metastasis. This retrospective study was approved by the institutional review board of the Tokushima University and informed consent was obtained from all participating patients (#2395 authorized in August 31, 2015).
Histological assessment
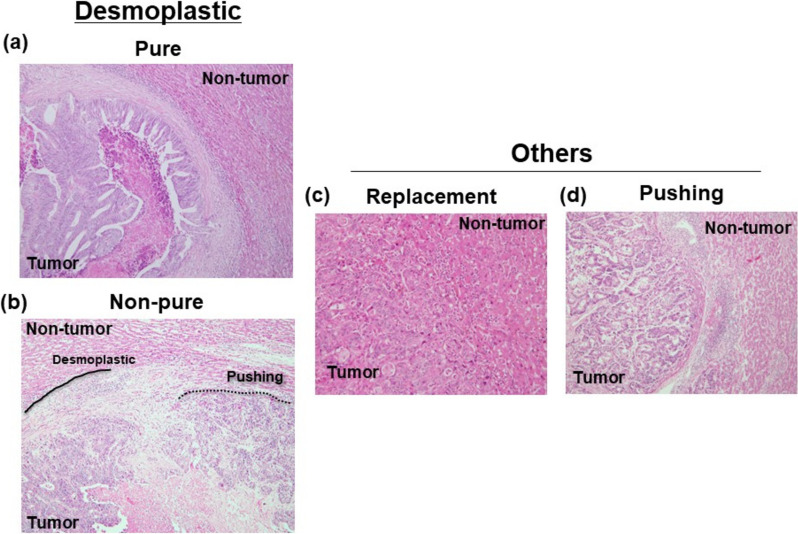
Hematoxylin and eosin-stained tissue sections from formalin-fixed paraffin-embedded blocks were used to evaluate growth patterns. The definition of HGP was followed by the international consensus guidelines [9]. The group of HGPs (replacement, desmoplastic, pushing) was categorized by predominant type of HGP. Pure dHGPs is consisted of complete circumference rim.
Statistical analysis
JMP 8.0.1 software (SAS, Cary, NC, USA) was used for statistical analysis. The statistical method to evaluate the relationship between two groups was the chi-squared test. Continuous variables are presented as the median and were compared by the Mann–Whitney test. Survival was calculated by Kaplan–Meier analysis and compared using the log-rank test. Multivariable Cox regression analysis was performed for variables with p < 0.05 in the univariate analysis. Overall survival (OS) was defined as the time from curative surgery to death [10]. P < 0.05 was considered statistically significant.
Results
Clinical and histopathologic characteristics
Representative images of each HGPs are shown in Fig. 1. Twenty-six tumors showed dHGPs. Moreover, nineteen tumors a pure dHGPs at the tumor-liver interface among them. Of the 71 cases, 19 cases (27%) were classified into rHGPs, and 26 cases (37%) were classified into pHGPs. Clinicopathological characteristics in accordance with pure-HGPs are shown in Table 1. dHGPs was significantly associated with sex (men) compared to others (p = 0.03). Furthermore, dHGPs was significantly associated with shallow tumor depth (p = 0.003) regardless of pure of non-pure. On the other hand, pure-dHGPs was significantly correlated to less lymph node metastasis compared to others (p = 0.05). There was no difference between non-pure dHGPs and others in tumor differentiation of primary site and lymph node metastasis.
Fig. 1.
Representative images of HGPs. a. Pure-dHGPs b. Non-pure dHGPs c. rHGPs. d. pHGPs
Table 1.
Clinicopathological characteristics in accordance with pure-dHGPs
| Variables | Pure dHGPs (n = 19) |
Non-pure dHGPs (n = 7) |
Others (n = 45) |
p-value |
|---|---|---|---|---|
| < Metastatic tumor characteristics > | ||||
| Age (< 70 years / ≥ 70 years) | 6/13 | 3/4 | 20/25 | 0.62 |
| Sex (men / women) | 17/2** | 5/2** | 28/19 | 0.03 |
|
Tumor maximum size (< 5 cm / ≥ 5 cm) |
14/5 | 5/2 | 35/9 | 0.85 |
| Tumor number (solitary/ multiple) | 12/7 | 2/5 | 19/26 | 0.18 |
| H-stage (H1 / H2, 3) | 13/6 | 5/3 | 27/18 | 0.73 |
| Grade (A / B,C) | 14/5 | 5/2 | 35/9 | 0.85 |
| Metastasis period (synch / meta) | 7/12 | 4/3 | 30/15 | 0.08 |
| Pre-operative chemotherapy (- / +) | 18/1 | 7/0 | 44/1 | 0.67 |
| < Primary tumor characteristics > | ||||
| Tumor differentiation (diff./undiff.) | 18/1 | 6/1 | 43/2 | 0.67 |
| T (2,3/4) | 19/0** | 9/0** | 31/14 | 0.003 |
| Lymph node metastasis (-/ +) | 13/6** | 2/5 | 17/28 | 0.05 |
Synch/meta synchronous/metachronous, diff./undiff differentiated histological type/undifferentiated histological type, HGP histopathological growth pattern.*vs. Non-pure dHGPs. ** vs. Others
Influence of pure-dHGPs on survival
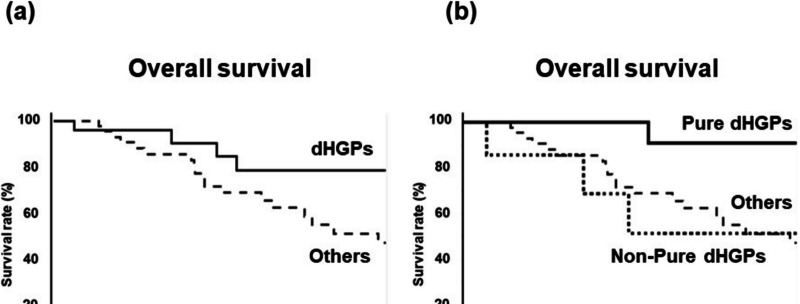
The 5-year OS rate of dHGPs was higher than that of others (78.4% vs. 47.1%, p = 0.05) (Fig. 2a). Furthermore, the 5-year OS rate of pure dHGPs was significantly higher than that of non-pure dHGPs (90.9% vs. 51.4%, p = 0.02) and that of others (90.9% vs. 47.1%, p = 0.01) (Fig. 2b). Univariate analysis of OS is shown in Table 2. H1 stage (p = 0.02), metastatic grade A (p = 0.01), pure HGPs (p = 0.01), better differentiation in the primary site (p < 0.001), and shallow tumor invasion in the primary site (p = 0.04) were significant prognostic factors for longer OS. Multivariate analysis revealed that pure dHGPs [hazard ratio (HR): 8.14, p = 0.04) and differentiation (HR: 46.4, p < 0.001) were independent prognostic indicators for OS.
Fig. 2.
Overall survival according to HGPs. Kaplan–Meier survival analysis of CRLM patients with HGPs: a.OS: dHGPs (pure dHGPs + non-pure dHGPs) vs. others (rHGPs and pHGPs) b. OS: Pure dHGPs) vs non-pure dHGPs vs. others (rHGPs and pHGPs)
Table 2.
Univariate and multivariate analyses of clinicopathological factors associated with overall survival after hepatectomy
| Variable | 5-year OS rate (%) | Uni- variate P-value |
Multivariate analysis | |
|---|---|---|---|---|
|
HR (95% CI) |
P-value | |||
| < Metastatic tumor characteristics > | ||||
| Age (< 70 years / ≥ 70 years) | 64.3 / 59.8 | 0.97 | ||
| Sex (men/women) | 64.1 / 56.3 | 0.70 | ||
|
Tumor maximum size (< 5 cm / ≥ 5 cm) |
65.4 / 50.0 | 0.09 | ||
| Tumor number (< 5 / ≥ 5) | 66.4 / 52.8 | 0.27 | ||
| H-stage (H1/H2, 3) | 72.7 / 44.4 | 0.02 |
1.73 (0.44–6.83) |
0.43 |
| Grade (A/B,C) | 77.6 / 46.2 | 0.01 |
1.58 (0.39–6.38) |
0.52 |
| Metastasis period (synch/meta) | 52.4 / 66.8 | 0.35 | ||
|
Pre-operative chemotherapy (- / +) |
56.2 / 100 | 0.31 | ||
| Pure HGPs (- / +) | 48.8 / 78.8 | 0.01 |
8.14 (1.00–66.14) |
0.04 |
| < Primary tumor characteristics > | ||||
| Tumor differentiation (diff./undiff.) | 64.3 / 25.0 | < 0.001 |
46.4 (5.58–386.00) |
< 0.001 |
| T (2,3/4) | 70.8 / 37.1 | 0.04 |
1.12 (0.42–3.00) |
0.47 |
| Lymph node metastasis (- / +) | 67.4 / 57.0 | 0.91 | ||
Synch/meta synchronous/metachronous, diff./undiff differentiated histological type/undifferentiated histological type
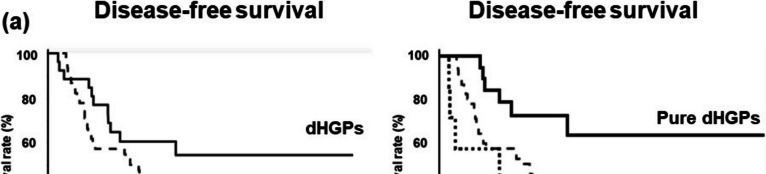
The 5-year DFS rate of dHGPs was higher than that of others (54.4% vs. 19.8%, p = 0.06) (Fig. 3a). Furthermore, the 5-year DFS rate of pure dHGPs was significantly higher than that of non-pure dHGPs (63.5.% vs. 28.6%, p = 0.03) and that of others (63.5% vs. 19.8%, p = 0.02) (Fig. 3b). Univariate analysis of DFS is shown in Table 3. Metastatic grade A (p = 0.04), pure HGPs (p = 0.01), better differentiation in the primary site (p = 0.03), and no lymph node metastasis in the primary site (p = 0.04) were significant prognostic factors for longer DFS. Multivariate analysis revealed that pure dHGPs [hazard ratio (HR): 2.42, p = 0.04) and differentiation (HR: 4.07, p = 0.03) were independent prognostic indicators for OS.
Fig. 3.
Disease-free survival according to HGPs. Kaplan–Meier survival analysis of CRLM patients with HGPs: a. DFS: dHGPs (pure dHGPs + non-pure dHGPs) vs. others (rHGPs and pHGPs) b. DFS: Pure dHGPs) vs non-pure dHGPs vs. others (rHGPs and pHGPs)
Table 3.
Univariate and multivariate analyses of clinicopathological factors associated with disease-free survival after hepatectomy
| Variable | 5-year DFS rate (%) | Uni- variate P-value |
Multivariate analysis | |
|---|---|---|---|---|
|
HR (95% CI) |
P-value | |||
| < Metastatic tumor characteristics > | ||||
| Age (< 70 years / ≥ 70 years) | 21.8 / 37.0 | 0.72 | ||
| Sex (men/women) | 35.3 / 22.0 | 0.77 | ||
|
Tumor maximum size (< 5 cm / ≥ 5 cm) |
30.6 / 30.0 | 0.84 | ||
| Tumor number (< 5 / ≥ 5) | 31.4 / 28.2 | 0.36 | ||
| H-stage (H1/H2, 3) | 35.7 / 22.2 | 0.24 | ||
| Grade (A/B,C) | 39.5 / 20.5 | 0.04 |
1.60 (0.89–2.89) |
0.11 |
| Metastasis period (synch/meta) | 19.1 / 46.9 | 0.06 | ||
|
Pre-operative chemotherapy (- / +) |
29.9 / 50.0 | 0.72 | ||
| Pure HGPs (- / +) | 21.3 / 64.5 | 0.01 |
2.42 (1.01–5.77) |
0.04 |
| < Primary tumor characteristics > | ||||
| Tumor differentiation (diff./undiff.) | 31.6 / 25.0 | 0.03 |
4.07 (1.12–14.72) |
0.03 |
| T (2,3/4) | 33.7 / 21.4 | 0.45 | ||
| Lymph node metastasis (- / +) | 40.9 / 23.0 | 0.04 |
1.32 (0.69–2.52) |
0.40 |
Synch/meta synchronous/metachronous, diff./undiff differentiated histological type/undifferentiated histological type
Discussion
The main histological characteristics of tumor growth in CRC include pushing, replacement, and desmoplastic [4]. The desmoplastic subtype shows the clear tumor border. Tumor cells separate from the surrounding liver parenchyma by desmoplastic stroma formation [6, 7]. Patients with dHGPs have a better prognosis after curative liver resection compared with patients with the rHGPs. The current study revealed that pure-dHGPs has strongest prognostic impact in CRLM patients both for OS and DFS beyond the use of neoadjuvant chemotherapy. Dominant dHGPs which include other HGPs besides on desmoplastic pattern does not have any impact on prognosis.
The different histological patterns of CRLM are associated with different types of tumor vascularization [4]. The replacement subtype shows a non-angiogenic growth pattern in contrast to the desmoplastic subtype [3]. In the dHGPs, the sprouting angiogenesis could be seen. One of the reasons for good prognosis with dHGPs is this angiogenesis and vascularization. The tumor in dHGPs showed angiogenetic features such as a proliferated endothelial cells and increased blood vessels and rich vascular hot spots [5]. The vascular architecture does not resemble the surrounding liver tissue and not use the vascular architecture of the adjacent liver tissue. On the other hand, the co-opted capillary bed from normal liver is observed in rHGPs. Rich vascularity means minimal hypoxia and contributes cancer cell motility and invasion during replacement growth [11]. Predominant dHGPs, not-pure dHGPs, has another characteristic derived from other HGPs. Our result revealed, the prognosis of not-pure dHGPs is almost similar to other HGPs. This may be a reason that tumors with not-pure dHGPs show aggressive characteristics derived from other HGPs resulting in a poor prognosis. Furthermore, CRLM with dHGPs respond better than rHGPs to antiangiogenetic therapy [3]. Furthermore, dHGPs correlated to better overall survival than rHGPs, especially after the use of anti-VEGF monoclonal antibody [12]. Our result showed that there was no correlation between type of HGPs and the tumor differentiation in primary site. The factors which may affect the difference of tumor vascularization should be investigated in the future study.
The other reason for the difference between rHGPs and dHGPs may be the immune response to metastatic tumors. Desmoplastic rim originated from anti-tumor reaction plays a protective role against tumor progression. A previous study revealed that CD8 + TILs in dHGPs are associated with longer survival [13]. Furthermore, a tumor with dHGPs has more infiltration of CD8 + immune cells [14]. Previous report showed that desmoplastic capsule strongly associated to higher peri-tumor infiltration of CD4 + , CD45RO + and CD8 + cells in stroma [14]. Furthermore, cancer-associated fibroblasts (CAFs) play a critical role for desmoplasia through producing cytokines and growth factors and remodeling the extracellular matrix (CM) [15]. The desmoplastic rim may be a complicated reaction by tumor microenvironment such as blood vessels, fibroblasts and immune cells. Since the rim functions as a barrier to protect normal tissues from the malignant cells, complete rim would be necessary to avoid the invasion. Thus, to confirm and clarify this association, further studies are required to delineate the role and influence of stromal cells to the histological characteristics of tumor growth.
This study has certain limitations. Firstly, this was a retrospective single-center study with an inherent risk of selection bias. Secondly, the sample size was relatively small. Moreover, future research should perform to investigate the detailed mechanism of desmoplastic formation such as stromal cells including TILs.
Conclusions
Our present study demonstrates the strong influence of pure dHGPs on the prognostic outcomes of CRLM patients. The dHGPs served as a better independent prognostic factor both for OS and DFS. These results contribute to a better understanding of the biology of CRLM and thereby enable prediction of the prognoses of CRLM patients.
Acknowledgements
Not applicable.
Authors’ contributions
Authors' contributions: Conception and design: CT, MS; Development of methodology: CT, MN, KY, TT, HK; Acquisition of data: CT, YW, TY; Analysis and interpretation of data: CT, YW; Writing, review of the manuscript: CT, SM; Study supervision: MS, YM, TT. All authors have read and approved the manuscript.
Funding
The authors have no financial ties to disclose.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Tokushima University Hospital Institutional Ethics Committee 2395), and informed consent was obtained from all participating patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adam R, de Gramont A, Figueras J, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015;41:729–41. [DOI] [PubMed] [Google Scholar]
- 2.van der Pool AE, Damhuis RA, Ijzermans JN, et al. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: a population-based series. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2012;14:56–61. [DOI] [PubMed] [Google Scholar]
- 3.Frentzas S, Simoneau E, Bridgeman VL, et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med. 2016;22:1294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vermeulen PB, Colpaert C, Salgado R, et al. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol. 2001;195:336–42. [DOI] [PubMed] [Google Scholar]
- 5.Stessels F, Van den Eynden G, Van der Auwera I, et al. Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br J Cancer. 2004;90:1429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galjart B, Nierop PMH, van der Stok EP, et al. Angiogenic desmoplastic histopathological growth pattern as a prognostic marker of good outcome in patients with colorectal liver metastases. Angiogenesis. 2019;22:355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buisman FE, van der Stok EP, Galjart B, et al. Histopathological growth patterns as biomarker for adjuvant systemic chemotherapy in patients with resected colorectal liver metastases. Clin Exp Metastasis. 2020;37:593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rectum. JSfCotCa. Japanese Classification of Colorectal Carcinoma, Second English edition. Tokyo: Kanehara; 2009.
- 9.van Dam PJ, van der Stok EP, Teuwen LA, et al. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer. 2017;117:1427–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oba M, Hasegawa K, Matsuyama Y, et al. Discrepancy between recurrence-free survival and overall survival in patients with resectable colorectal liver metastases: a potential surrogate endpoint for time to surgical failure. Ann Surg Oncol. 2014;21:1817–24. [DOI] [PubMed] [Google Scholar]
- 11.Bentolila LA, Prakash R, Mihic-Probst D, et al. Imaging of Angiotropism/Vascular Co-Option in a Murine Model of Brain Melanoma: Implications for Melanoma Progression along Extravascular Pathways. Sci Rep. 2016;6:23834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazaris A, Amri A, Petrillo SK, et al. Vascularization of colorectal carcinoma liver metastasis: insight into stratification of patients for anti-angiogenic therapies. J Pathol Clin Res. 2018;4:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stremitzer S, Vermeulen P, Graver S, et al. Immune phenotype and histopathological growth pattern in patients with colorectal liver metastases. Br J Cancer. 2020;122:1518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunner SM, Kesselring R, Rubner C, et al. Prognosis according to histochemical analysis of liver metastases removed at liver resection. Br J Surg. 2014;101:1681–91. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Vicien G, Mezheyeuski A, Banuls M, Ruiz-Roig N, Mollevi DG. The Tumor Microenvironment in Liver Metastases from Colorectal Carcinoma in the Context of the Histologic Growth Patterns. Int J Mol Sci. 2021;22:1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.