Abstract
Background
The objective of this study was to evaluate the clinicopathological characteristics and patterns of care among women diagnosed with vulvar malignancy at a tertiary care teaching institute. Additionally, the study aimed to analyse the implications of revised FIGO staging system on stage shift and patient outcomes.
Methods
A retrospective observational study was conducted, wherein hospital records of biopsy-proven cases of vulvar cancers managed over a period of 10 years were comprehensively reviewed. The assignment of FIGO staging was performed utilizing both 2009 and 2021 FIGO staging systems for comparative analysis. Statistical analysis was performed using STATA version 17. Survival curves were constructed using the Kaplan-Meier method, with differences assessed using the log-rank test. Additionally, multivariable analysis was conducted using the Cox proportional hazard model.
Results
A total of 82 cases meeting the inclusion criteria were enrolled in the study. Management patterns varied widely, with the majority undergoing surgery (73.2%), followed by definitive radiotherapy with or without chemotherapy (10.9%), neoadjuvant radiotherapy and subsequent surgery (4.9%), and palliative care (10.9%). Post-operative radiotherapy was administered in 31.7% of cases. The disease-specific recurrence rate was found to be 32.9%, and the mortality rate was 30.5%. The median Disease-Free Survival (DFS) was 17 months (interquartile range [IQR]: 1–36 months), while the Overall Survival (OS) was 27 months (IQR: 9–52 months). Upon application of the 2021 staging system, a stage shift was observed in 18% of cases of advanced vulvar cancer. The 3-year DFS and OS were reduced for stage IIIA and stage IVA, while showing improvement for stage IIIB.
Conclusions
The revised FIGO 2021 staging system offers enhanced simplicity in its application within clinical practice and demonstrates improved correlation with prognosis. Approximately 18% cases experienced restaging under the updated system.
Trial registration number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-024-03612-1.
Keywords: Carcinoma vulva, Disease free survival, FIGO 2021 staging, Patterns of treatment, Overall survival
Background
Vulvar cancer, while uncommon, is recognized for its aggressive nature, with a majority of cases reported in high-income countries [1]. In recent decades, there has been a gradual increase in its incidence worldwide. According to GLOBOCAN 2020 data, there were 45,240 new cases of vulvar cancer and 17,427 associated deaths globally [2].
Vulvar cancer predominantly affects elderly women, with over 50% of cases diagnosed in women aged 70 years or older [1]. Prognosis is strongly dependent on the stage at diagnosis, with advanced stages disease often needing multimodal therapy including surgical interventions as well as adjuvant radiotherapy and chemotherapy. In India, the estimated age-adjusted standardized incidence rate in 2020 was reported as 0.51 per 100,000 women, with a mortality rate of 0.25 per 100,000 women [2]. Patients commonly present with large, advanced-stage tumors, posing significant challenges for effective management [3]. Despite therapeutic interventions, recurrence rates exceed 40%, contributing to an overall poor prognosis [4]. Due to the rarity of disease, there is a dearth of studies investigating patterns of care and survival outcomes in affected women, highlighting the need for further research in this area.
In the field of oncology, staging systems serve as crucial tools, enabling clinicians to accurately prognosticate patient outcomes, identify high-risk individuals, determine the need for intensive treatment and follow-up strategies, and facilitate standardized comparisons across different clinical settings. In October 2021, the International Federation of Gynaecology and Obstetrics (FIGO) revised the staging system for vulvar cancer, prompted by the inconsistent prognostic performance of the previous schema (FIGO 2009). The new staging was based on data from the National Cancer Data Base (NCDB) spanning 2010 to 2017 [1]. The revised 2021 staging schema has reclassified stages IIIA and IIIB based on the size of nodal metastasis, as well as introducing changes in stage IVA. However, it is essential to validate the revised FIGO staging system outside the NCDB database and assess whether it fulfils the staging goals with improved accuracy compared to the previous staging [5]. This is crucial for providing insights into the applicability and effectiveness of the updated staging system in diverse clinical settings beyond the original database.
Materials and methods
The present study aims to evaluate the clinical profile and management patterns of vulvar cancer and to assess the impact of the revised FIGO staging on outcomes.
A retrospective observational study was conducted following the receipt of institutional ethical clearance (IEC-258/04.03.2022). The study involved the review of case records of patients with biopsy-proven vulvar malignancy diagnosed between 2011 and 2020. Inclusion criteria encompassed patients aged over 18 years who completed primary therapy at our centre, while individuals presenting with non-squamous histology and recurrent disease were excluded from the analysis. Comprehensive clinical data, including patient demographics, tumour characteristics, surgical details, adjuvant therapy, recurrence status, and date of death or last follow-up, were collected. Post-operative radiotherapy (PORT) was administered to patients exhibiting specific risk factors, including close margins, nodes with extracapsular extension, or more than two positive nodes. The standard PORT dose comprised 50.4 Gy delivered in 28 fractions over 5.5 weeks, with interstitial brachytherapy reserved for cases involving large tumours in proximity to the urethra and anus. Treatment delivery encompassed nine-field computed tomography (CT)-guided intensity-modulated radiation therapy using 6MV photons, with treatment volumes conforming to standard contouring guidelines.
The duration of follow-up was calculated from the date of primary treatment completion to the date of death or last follow-up. Disease-free survival (DFS) was defined as the duration from diagnosis to the date of confirmed recurrence, while overall survival (OS) was determined from the date of diagnosis to death from any cause or last follow-up, whichever occurred first. Descriptive statistics were applied using the 2009 FIGO staging schema, with a subsequent reassessment of staging according to the FIGO 2021 system (Table 1) based on confirmation from specimen block review. The main outcome measures included stage shift and the prognostic performance of FIGO stage, with cases classified as upstaged if reclassified to a higher substage and down-staged if reclassified to a lower stage.
Table 1.
FIGO 2009 and 2021 staging for vulvar cancer [1]
| FIGO 2009 staging | FIGO 2021 staging | |
|---|---|---|
| Stage 1 | Tumour confined to vulva with no nodal metastasis | |
| IA | Lesion ≤ 2 cm, with stromal invasion ≤ 1 mm | |
| IB | Lesion > 2 cm, with stromal invasion > 1 mm | |
| II | Any size of tumour with/without extension to adjacent perineal structures (1/3 lower urethra, 1/3rd vagina or anus) with negative regional lymph nodes | |
| III | Tumour of any size with/without extension to adjacent perineal structures (1/3 lower urethra, 1/3rd vagina, anus) with positive inguinofemoral nodes | Tumor of any size with extension to upper part of perineal structures, or with any number of non-fixed, non-ulcerated regional lymh node |
| IIIA |
i. With 1 lymph node having metastasis ≥ 5 mm ii. With 1–2 lymph node having metastasis < 5 mm |
Tumor extending to upper 2/3rd of urethra or vagina, bladder mucosa, rectal mucosa, or regional lymph node metastases ≤ 5 mm |
| IIIB |
i. With 2 or more nodal metastasis (≥ 5 mm) ii. ≥ 3 nodal metastasis (< 5 mm) |
Regional lymph node metastases > 5 mm |
| IIIC | Positive nodes with extracapsular extension |
Regional lymph node metastases with extracapsular spread |
| IV | Tumor invades other regional (2/3 upper urethra, 2/3 upper vagina), or distant structures | Tumour of any size fixed to bone, or fixed ulcerated lymph node metastasis, or distant metastasis |
| IVA |
Tumor invades any of the following: (i) upper urethral and/or vaginal mucosa, bladder mucosa, rectal mucosa, or (ii) fixed or ulcerated inguino-femoral lymph nodes (iii) or fixed to pelvic bone |
Disease fixed to pelvic bone, or fixed or ulcerated regional lymph node metastasis |
| IVB | Any distant metastasis including pelvic lymph nodes | Distant metastasis |
NB :
In the 2009 staging, the depth of invasion is defined as the measurement of the tumour from the epithelial- stromal junction of the adjacent most superficial dermal papilla to the deepest point of invasion
In the 2021 staging, the depth of invasion is defined as the measurement of the tumour from the basement membrane of the deepest, adjacent, dysplastic tumour free rete-ridge to the deepest point of invasion
Statistical analysis was performed using STATA version 17. Survival curves were constructed using the Kaplan-Meier method, with differences assessed using the log-rank test. Additionally, multivariable analysis was conducted using the Cox proportional hazard model.
Results
The medical records of 102 patients diagnosed with vulvar malignancy during the study period were reviewed. Following this review, four cases were excluded due to non-squamous histology, specifically one case each of fibrosarcoma, small cell carcinoma, malignant melanoma, and adenoid cystic carcinoma. Additionally, 16 cases with incomplete data were omitted from the analysis. This resulted in 82 cases with squamous histology that had completed primary therapy, which were included in the final analysis. Notably, for three of these cases, the specimen blocks were unavailable for review by pathologists; consequently, revised staging could not be assigned.
The median age of the patients was 61 years (range: 24–92), with 81.7% (67/82) being post-menopausal. The mean duration of symptoms was 17.7 ± 39.3 months. The most commonly reported presenting complaints were vulvar swelling and itching. The median tumour size measured by the largest dimension was 3 cm (range: 1–8). Among the patients, 46 (56.1%) had co-morbidities, with hypertension being the most prevalent affecting 17 patients. Other reported co-morbidities included hypothyroidism (n = 5), diabetes (n = 4), cardiac ailments (n = 4), pulmonary disease (n = 4), and ten patients with multiple co-morbidities. Additionally, 18 (21.9%) patients had received prior therapy for vulvar dystrophy. Notably, three patients had a history of cervical malignancy; of these, two had undergone prior chemoradiation (CTRT), while one patient had received a radical hysterectomy. The most common sites of involvement were labia minora (80%) and majora (72.8%). The clitoris was involved in 43 (53%) cases, while involvement of the vagina, urethra, and anus was observed in 37.5%, 20%, and 2.5% of cases, respectively. Imaging results were available for 88.2% (75/82) of cases, with CT scans performed in 34, magnetic resonance imaging (MRI) in 29, and positron emission tomography (PET) scans in 12 cases. Four cases underwent multiple imaging studies. Surgical intervention was the primary treatment modality in 60 (73.2%) patients, with radical vulvectomy combined with bilateral inguinofemoral lymphadenectomy being the most commonly performed procedure (50/63, 79.4%). None of the patients underwent pelvic lymphadenectomy as part of surgical staging. The distribution of final stage assignment is presented in Table 2. PORT was received by 31.7% (26/82) of patients. After a median follow-up of 34.7 months (range: 27–110 months), recurrence was observed in 26 (32.9%) cases. The most frequent site of recurrence was the vulva (18/26, 69.2%), followed by distant metastasis (4/26, 15.4%), groin lymph nodes (3/26, 11.5%), and pelvic disease (1/26, 3.8%).
Table 2.
Stage distribution of cases according to the FIGO 2009 and 2021 schema
| FIGO 2009 Schema | FIGO 2021 schema | |
|---|---|---|
| IA | 2 (1.2%) | 2 (1.3%) |
| IB | 25 (32.1%) | 25 (33.3%) |
| II | 5 (6.1%) | 5 (6.4%) |
| IIIA | 8 (9.8%) | 7 (9.0%) |
| IIIB | 15 (18.5%) | 18 (23.1%) |
| IIIC | 12 (14.8%) | 12 (15.4%) |
| IV A | 10 (12.3%) | 5 (6.4%) |
| IV B | 4 (4.9%) | 4 (5.1%) |
| Incompletely staged | 1 (1.2%) | 1 (1.2%) |
| Total cases | N = 82 |
N = 79 Revised staging could not be assigned in 3 cases |
The disease-specific mortality rate was 30.5% (25/82). The median DFS for the study cohort was 37 months, while the median overall survival (OS) was 56 months. Overall survival was significantly affected by the presence of lymph node involvement [HR 3.53 (95% CI 1.36–9.13); P = .009] and failure to receive adjuvant therapy [HR 5.04 (95% CI 1.55–16.31); P = .007]. Other variables including patient age, midline tumour involvement, tumour diameter, positive surgical margins, depth of invasion, lymph vascular space invasion (LVSI) and tumour grade did not demonstrate statistically significant impact on OS. The stage-wise distribution according to FIGO 2009 and 2021 schema is depicted in Table 2.
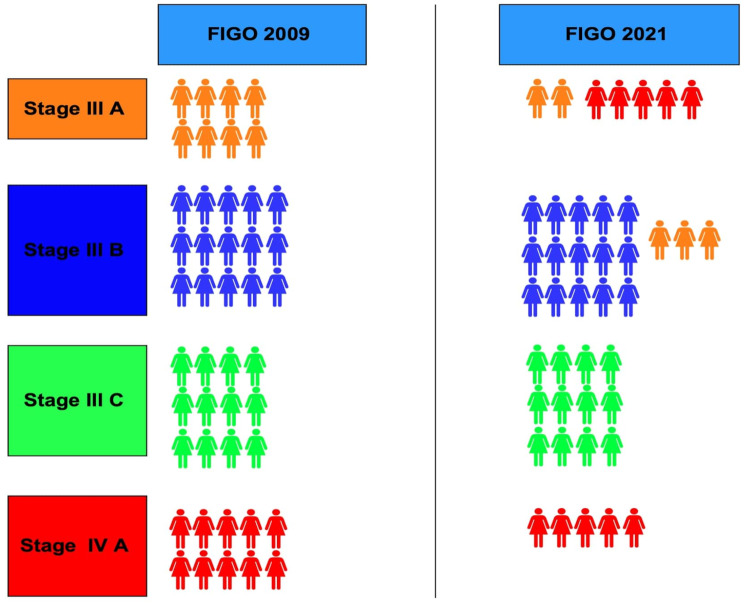
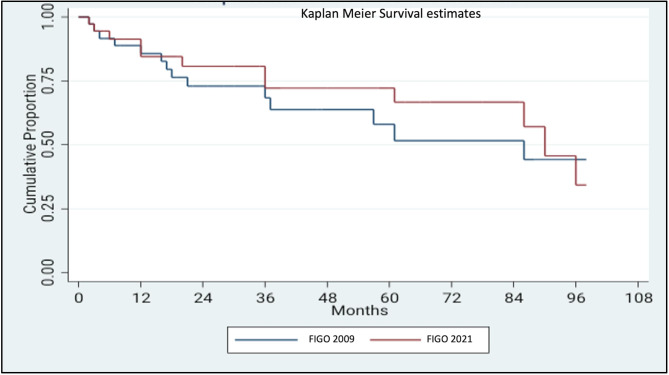
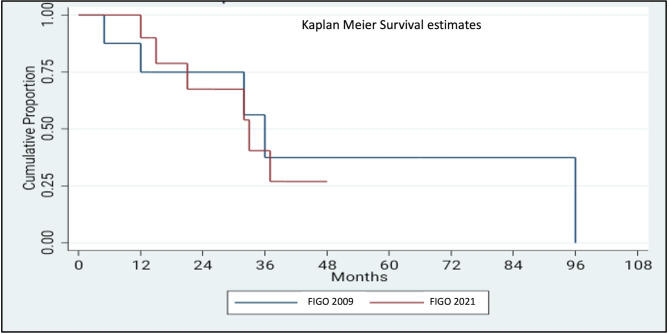
A stage-shift was observed in eight (17.4%) cases of advanced vulvar cancer, encompassing stages IIIA, IIIB, and IVA (Fig. 1). Specifically, 37.5% (3/8) of cases initially classified as stage IIIA, were upstaged to stage IIIB, while 50% (5/10) of cases initially categorized as stage IVA were down-staged to stage IIIA. The proportion of cases assigned to stage IIIB increased after implementation of the FIGO 2021 staging schema, rising from 18.3 to 21.9% (Fig. 1). The impact of the revised staging on survival outcome was further analysed by Kaplan Meier survival curve using log-rank test (Figs. 2 and 3). For stage III disease, the 3-year survival rate was 55% versus 60.7% for FIGO 2021 and 2009 staging schema, respectively (P = .63). Similarly, for stage IV disease the 1-year survival rate was 50% versus 66.5% (P = .69). Comparison of overall survival (OS) revealed that survival was reduced for stages IIIA and IVA, while it improved for stage IIIB (Table 3; Figs. 2 and 3). Disease-free survival (DFS) could be assessed for stage IIIB, while the 3-year and 5-year DFS improving from 60.0 to 67.9% and from 20.0 to 45.2%, respectively. However, DFS data could not be reliably determined for other FIGO substages.
Fig. 1.
Impact of 2021 staging system on the 2009 staging assignments
Fig. 2.
Comparison of survival estimates for stage III according to FIGO 2009 and FIGO 2021 schema
Fig. 3.
Comparison of survival estimates for stage IV according to FIGO 2009 and FIGO 2021 schema
Table 3.
Stage wise overall survival (OS) in accordance with FIGO 2009 and 2021 staging schema for substages in advanced disease
| 3-year OS | 5-year OS | ||
|---|---|---|---|
| IIIA | 2009 | 100% | 83.3% |
| 2021 | 40% | 20% | |
| III B | 2009 | 44.8% | 33.6% |
| 2021 | 63.8% | 56.7% | |
| IIIC | 2009 | 66.3% | 66.3% |
| 2021 | 66.3% | 66.3% | |
| IV A | 2009 | 28.6% | 14.3% |
| 2021 | 33.3% | - | |
Discussion
There is notable heterogeneity in the patterns of care for vulvar malignancy in India and similar low- and middle-income countries, with variations in incidence, diagnostic approaches, and treatment protocols [6–8]. Patients typically present with bulky tumours and often have multiple comorbidities [3]. Over 50% of patients presenting in the locally advanced stage and consequently receiving radical chemoradiotherapy as the primary treatment modality [4]. However, our findings diverge from this trend, as 74% of cases in our study cohort received surgery as the primary therapy. This variance may be attributed to differences in the availability of surgical expertise across different regions.
Vulvar cancer management has evolved significantly over recent decades. The historical approach of radical vulvectomy, associated with substantial morbidity, has shifted towards more conservative, personalized surgical options such as wide radical excision or hemivulvectomy [9]. Inguinofemoral lymphadenectomy remains crucial, given the nodal status’s prognostic significance, regardless of patient age or complication risks [10]. This paradigm shift stems from improved understanding of tumor biology, advanced imaging, and implementation of sentinel lymph node biopsy techniques. These advancements reflect a broader trend in oncological surgery towards more precise, less invasive interventions that optimize both survival outcomes and quality of life [11].
The management of locally advanced vulvar cancer (LAVC) presents a significant challenge, as evidenced by the varied treatment approaches observed in our study cohort. Some patients received radical chemoradiotherapy (CRT), while others underwent neoadjuvant therapy followed by salvage surgery. Notably, none of the patients with LAVC underwent extensive surgical resection or pelvic exenteration. A meta-analysis supports the use of neoadjuvant chemoradiotherapy, reporting operability rates ranging from 63 to 92%, with disease-free rates ranging from 26 to 63% after follow-up periods ranging from 6 months to 10 years [12]. Thus, tailored therapy can potentially avert the need for morbid surgery in these cases. The indications for PORT also vary in the literature, often based on institutional protocols related to definitions of close margins or considerations of the number of positive nodes [13]. These variations underscore the importance of adhering to established recommendations in clinical practice. While MRI is regarded as the most effective imaging modality for pre-operative evaluation of vulvar cancer, our findings suggest that other imaging techniques, or in some cases, no imaging at all, were employed in the initial management of vulvar cancer cases. Several prognostic factors have been identified that impact the outcome of vulvar cancer, including the number and extent of nodal metastasis, margin positivity, lymphovascular space invasion (LVSI) positivity, and tumor size. However, due to the rarity of the disease and smaller sample size in most studies, establishing statistical correlations for these factors has been challenging [14]. Regarding the most appropriate imaging modality, MRI is considered the most useful. However, variations in practice were noted in our study, highlighting the diversity in imaging approaches [15]. A review of available Indian studies has also highlighted similar heterogeneity in the management patterns of women with vulvar cancer in India, underscoring the need for standardizing treatment protocols for this uncommon malignancy [4].
Cancer staging systems are dynamic entities that evolve with advancements in scientific knowledge and technology [1]. The primary aim of a standardized staging system is to enable accurate prognostication, identifying patients at the highest risk of treatment failure who may require more aggressive treatment strategies, and providing a standardized formats for comparing outcomes. Initially, the staging system for vulvar cancer relied on clinical parameters but underwent its first modification in 1988 to incorporate surgico-pathological prognostic variables [1]. This modification enhanced the system’s efficiency in assigning stages to patients and improved prognostic capabilities, particularly given that vulvar cancer is primarily treated surgically and lymph node involvement is a significant prognostic factor. However, further investigations revealed limitations in the modified staging system. It was observed that tumours with larger diameters but negative nodal involvement were categorized as low-risk; nevertheless, there was no significant difference in survival between stages I and II. Furthermore, stage III was found to be heterogeneous, with variable survival outcomes depending on the number and morphological characteristics of nodal involvement. In response to these insights, the staging system underwent another revision in 2009, which incorporated additional prognostic variables such as tumour size, depth of invasion, type, and number of nodal involvements [16, 17]. Although this revision was based on an extensive review of published evidence, the 2009 staging system was found to lack prognostic capability. A retrospective comparison between the 1988 and 2009 staging systems performed by Tabbaa et al. reported that 31% of cases were down-staged, with only one case being upstaged after the application of the 2009 staging schema. Moreover, the 2009 staging system failed to differentiate the 10-year cancer-specific survival (CSS) between stages I and II. Similarly, there was no observed difference in survival among the three sub-stages of stage III [18].
Recently, the staging system for vulvar cancer was revised based on the analysis of prospectively collected data, with the goals of simplification and enhanced prognostic granularity [1]. The current study aimed to investigate the impact of the revised 2021 FIGO vulvar cancer staging system in a retrospective cohort from a single centre. It was observed that under the revised staging, one in five cases were reassigned to a new substage, with changes predominantly observed in stages IIIA, IIIB, and IVA. The survival outcomes for stages IIIA and IVA decreased, while it increased for stage IIIB compared to the 2009 schema. This could be explained by cases with better prognostic features being shifted from stage IIIA to stage IIIB.
In a retrospective cohort of 889 women from the SEER database, Matsuo et al. validated the revised 2021 staging system. They observed a 25.8% stage shift in women with advanced vulvar cancer following the application of the 2021 schema, which is higher than the 18% stage shift observed in our study [5]. The overall survival (OS) for stage IIIA decreased from 48.9 to 45.6% in Matsuo et al.‘s validation cohort. In contrast, our cohort exhibited a 3-year and 5-year OS for stage IIIA that declined from 100.0 to 40.0% and 83.3–20.0%, respectively. Similarly, for stage IVA, the 3-year OS decreased from 33.3 to 28.6% in our cohort and from 25.1 to 13.9% in Matsuo et al.‘s validation cohort. Conversely, for stage IIIB, the survival improved from 44.2 to 47.0% in Matsuo et al.‘s study, whereas in our cohort, the 3-year and 5-year OS improved from 44.8 to 63.8% and from 33.6 to 56.7%, respectively. The 5-year OS for stage IVA, reduced significantly in both cohorts. While, the comparative trends are similar, the wider differences observed in our study may be attributed to its smaller sample size. Matsuo et al. observed no change in 5-year OS rates for stage IVA and IVB disease (13.9% vs. 14.5%; P = .99) and for stage IIIA and IIIB disease (45.6% vs. 47.0%). This highlights the need for further investigation into the future utility of the revised staging system [5].
The main strength of our study is the long follow-up duration of cases, which provides valuable insights into the outcomes of patients with vulvar cancer. However, several limitations should be acknowledged. Firstly, the retrospective nature of the study limits our ability to account for the effects of treatment individualization and the evolving landscape of treatment strategies over extended periods. Additionally, the limited number of cases within each sub-stage increases the risk of type-II error, rendering the study underpowered to accurately compare survival differences across sub-stages.
Conclusion
Women with vulvar cancer frequently present with a diverse array of symptoms, and the wide variation in patterns of care can significantly impact outcomes. There is a pressing need for the implementation of standardized and contextually relevant treatment guidelines to optimize patient care. The revised FIGO 2021 staging system provides a simpler and more user-friendly framework, resulting in stage-shifts in a substantial proportion of cases and demonstrating better correlation with patient outcomes. However, further studies are necessary to thoroughly assess its prognostic implications and to inform future revisions of the staging system.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to Dr. Ashish Datt Upadhyay, Clinical Research Unit, All India Institute of Medical Sciences, New Delhi, India for the statistical analysis. We thank Dr. Haritha Maddirala, Dr Chandrima Ray and Dr Saroj Rajan for supporting data collection and clinical follow-up of patients in the current study.
Abbreviations
- FIGO
The International Federation of Gynecology and Obstetrics
- DFS
Disease Free Survival
- OS
Overall Survival
- CSS
Cancer Specific Survival
- PORT
Post operative radiotherapy
- CRT
Chemoradiotherapy
- LAVC
Locally advanced vulvar cancer
- MRI
Magnetic Resonance Imaging
- LVSI
Lymph vascular space invasion
- CT
Computerized Tomography
- SEER
Surveillance, Epidemiology, and End Results Program
Author contributions
SS: conception, design of the work, the acquisition, analysis, interpretation of data; have drafted the work and revisions DS: design of the work, the acquisition, analysis, interpretation of data; have drafted the work and revisionsSM: the acquisition, analysis, interpretation of data; have contributed substantially to revisionsST: design of the work, the acquisition, analysis, interpretation of data; have drafted the work and revisions JM: data acquisition, have drafted and revised the work AS: data acquisition, have drafted the workNB : conception, design of the work, the acquisition, analysis, interpretation of data; have drafted the work and revisions.
Funding
None.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study was approved by institutional ethical clearance committee (IEC-258/04.03.2022).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Olawaiye AB, Cotler J, Cuello MA, Bhatla N, Okamoto A, Wilailak S, et al. FIGO staging for carcinoma of the vulva: 2021 revision. Int J Gynecol Obstet. 2021;155(1):43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Can. 2021;149(4):778–89. [DOI] [PubMed] [Google Scholar]
- 3.Singhal S, Kumar S, Sharma DN, Bharti J, Meena J, Yadav A. Retrospective analysis of surgically treated cases of squamous cell carcinoma vulva. J Cancer Res Ther. 2021;17:186–90. [DOI] [PubMed] [Google Scholar]
- 4.Kaur S, Garg H, Nandwani M. The Unmet needs in the management of Vulvar Cancer and a review of Indian literature. JCO Global Oncol. 2022;8:e2200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuo K, Klar M, Nishio S, Mikami M, Roman LD, Wright JD. Validation of the 2021 FIGO staging schema for advanced vulvar cancer. Int J Gynecol Cancer. 2022;32(4). [DOI] [PubMed]
- 6.Chhabra S, Bhavani M, Deshpande A. Trends of vulvar cancer. J Obstet Gynaecol. 2014;34:165–8. [DOI] [PubMed] [Google Scholar]
- 7.Nandwani M, Barmon D, Begum D, Liegise H, Kataki AC. An overview of vulvar cancer: a single-center study from Northeast India. J Obstet Gynaecol India. 2019;69:541–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deka P, Barmon D, Shribastava S, Kataki AC, Sharma JD, Bhattacharyya M. Prognosis of vulval cancer with lymph node status and size of primary lesion: a survival study. J Mid-Life Health. 2014;5:10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannini A, D’Oria O, Chiofalo B, Bruno V, Baiocco E, Mancini E, et al. The giant steps in surgical downsizing toward a personalized treatment of vulvar cancer. J Obstet Gynaecol Res. 2022;48(3):533–40. 10.1111/jog.15103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panici PB, Tomao F, Domenici L, Giannini A, Giannarelli D, Palaia I, et al. Prognostic role of inguinal lymphadenectomy in vulvar squamous carcinoma: younger and older patients should be equally treated. A prospective study and literature review. Gynecol Oncol. 2015;137(3):373–9. 10.1016/j.ygyno.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 11.Hacker NF, Barlow EL. Conservative Management of Vulvar Cancer-where should we draw the line? Cancers (Basel). 2024;16(17):2991. 10.3390/cancers16172991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Doorn HC, Ansink A, Verhaar-Langereis M, Stalpers L. Neoadjuvant chemora- diation for advanced primary vulvar cancer. Cochrane Database Syst Rev, 2006(3): CD003752. [DOI] [PubMed]
- 13.National Comprehensive Cancer Network (NCCN): NCCN Clinical Practice Guidelines in Oncology. Vulvar Cancer Version 1.2022. Philadelphia, PA, National Comprehensive Cancer Network. 2021. https://www.nccn.org/professionals/physician_gls/pdf/vulvar.pdf
- 14.Te Grootenhuis NC, Pouwer AF, de Bock GH, Hollema H, Bulten J, van der Zee AG, et al. Prognostic factors for local recurrence of squamous cell carcinoma of the vulva: a systematic review. Gynecol Oncol. 2018;148(3):622–31. 10.1016/j.ygyno.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 15.Nikolić O, Cunha TM, Nikolić MB, Otero-García MM, Gui B, Nougaret S, et al. Vulvar cancer staging: guidelines of the European Society of Urogenital Radiology (ESUR). Insights into Imaging. 2021;12(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–4. [DOI] [PubMed] [Google Scholar]
- 17.Hacker NF. Revised FIGO staging for carcinoma of the vulva. Int J Gynaecol Obstet. 2009;105:105–6. [DOI] [PubMed] [Google Scholar]
- 18.Tabbaa ZM, Gonzalez J, Sznurkowski JJ, Weaver AL, Mariani A, Cliby WA. Impact of the new FIGO 2009 staging classification for vulvar cancer on prognosis and stage distribution. Gynecol Oncol. 2012;127(1):147–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.