Abstract
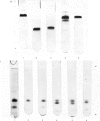
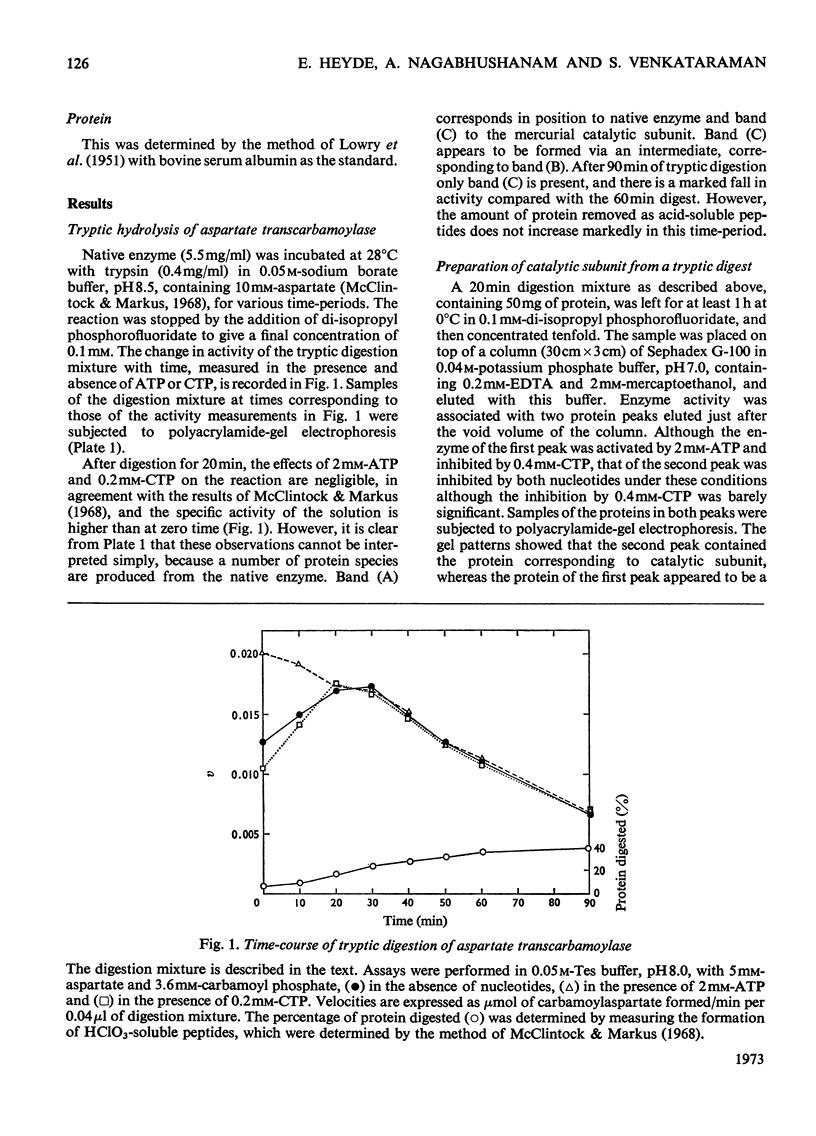
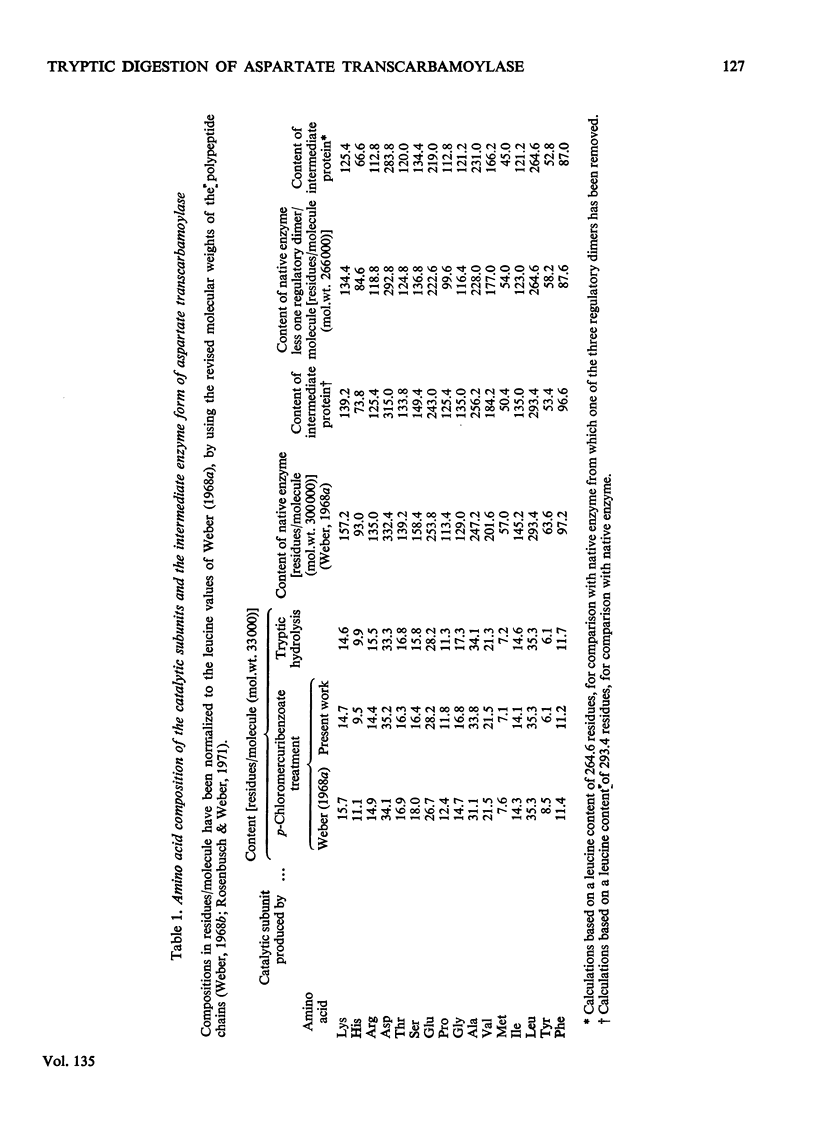
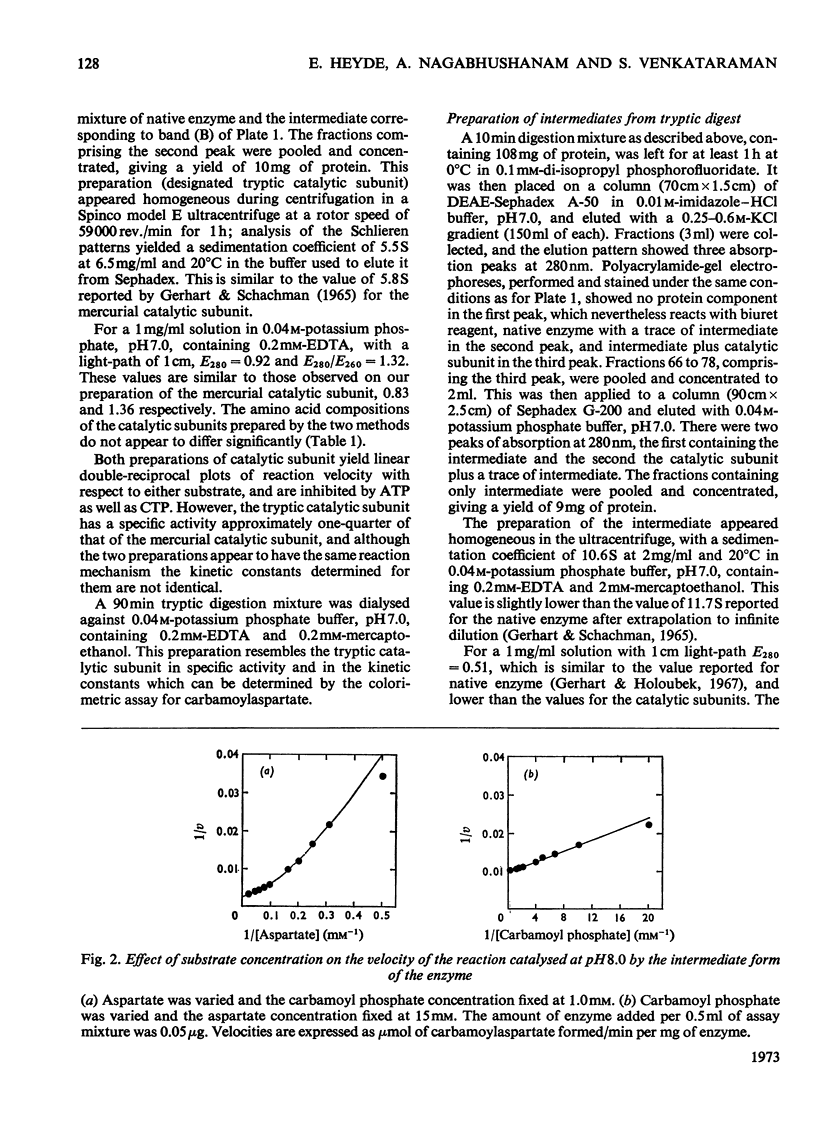
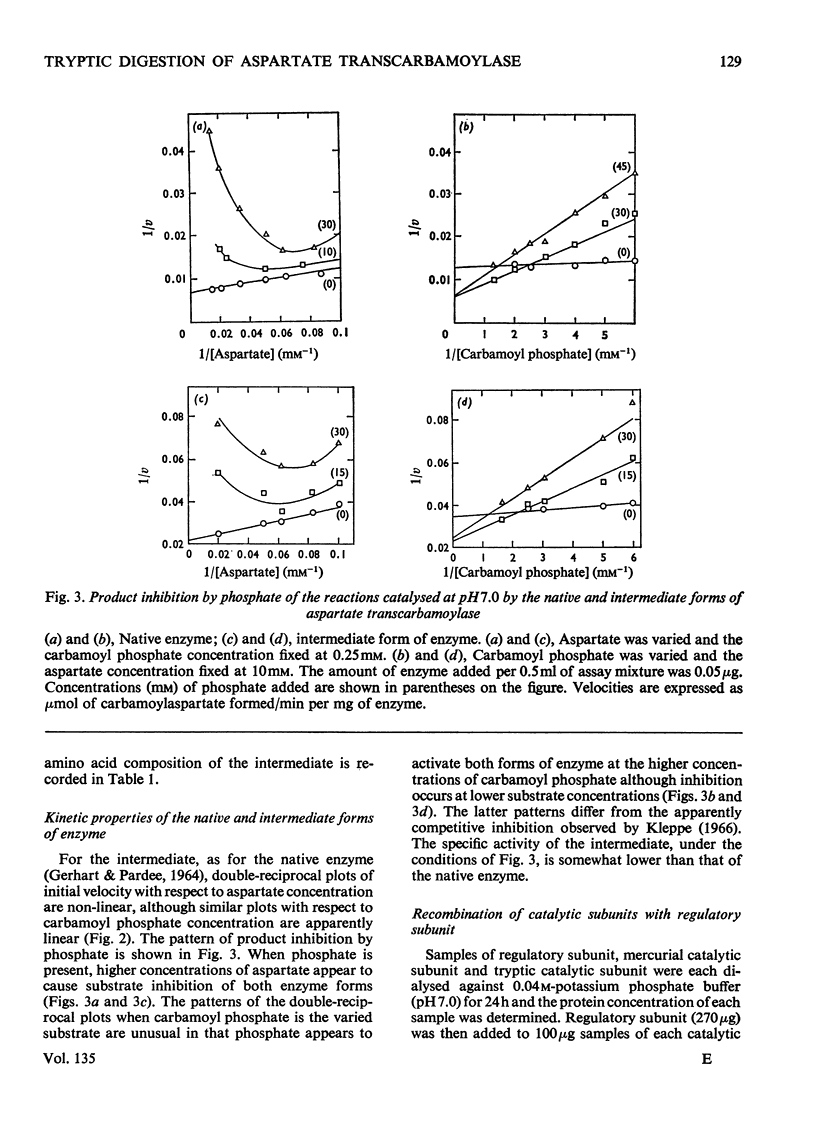
1. The time-course of tryptic hydrolysis of aspartate transcarbamoylase (aspartate carbamoyltransferase, EC 2.1.3.2) was followed by activity measurements in the presence and absence of allosteric effectors, and by polyacrylamide-gel electrophoresis. 2. Two proteins with enzyme activity are formed in this way from native enzyme, and the isolation and some properties of these species are reported. The larger protein (10.6S) resembles native enzyme in that it contains regulatory subunits and is sensitive to allosteric effectors, as well as in a more detailed kinetic investigation. It appears from the time-course of tryptic digestion to be an intermediate in the formation of a catalytic subunit (5.5S) which is similar to, but not identical with, the catalytic subunit produced by mercurial treatment of the native enzyme. 3. Sodium dodecyl sulphate–polyacrylamide-gel electrophoresis of the different enzyme forms demonstrates that trypsin can hydrolyse bonds in the catalytic polypeptide chains as well as completely remove the regulatory polypeptide chains. 4. Both preparations of catalytic subunit can recombine with regulatory subunit to form enzymes which resemble the native enzyme in being activated by ATP, although they do not appear to be inhibited by CTP. 5. This study is consistent with the models of the enzyme that propose that the catalytic subunits are held together in the native enzyme by three pairs of regulatory polypeptide chains.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GERHART J. C., PARDEE A. B. ASPARTATE TRANSCARBAMYLASE, AN ENZYME DESIGNED FOR FEEDBACK INHIBITION. Fed Proc. 1964 May-Jun;23:727–735. [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Gerhart J. C., Holoubek H. The purification of aspartate transcarbamylase of Escherichia coli and separation of its protein subunits. J Biol Chem. 1967 Jun 25;242(12):2886–2892. [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Gros C., Labouesse B. Study of the dansylation reaction of amino acids, peptides and proteins. Eur J Biochem. 1969 Feb;7(4):463–470. doi: 10.1111/j.1432-1033.1969.tb19632.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Markus G., McClintock D. K., Bussel J. B. Conformational changes in aspartate transcarbamylase. 3. A functional model for allosteric behavior. J Biol Chem. 1971 Feb 10;246(3):762–771. [PubMed] [Google Scholar]
- McClintock D. K., Markus G. Conformational changes in aspartate transcarbamylase. I. Proteolysis of the intact enzyme. J Biol Chem. 1968 Jun 10;243(11):2855–2862. [PubMed] [Google Scholar]
- Rosenbusch J. P., Weber K. Subunit structure of aspartate transcarbamylase from Escherichia coli. J Biol Chem. 1971 Mar 25;246(6):1644–1657. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Weber K. Aspartate transcarbamylase from Escherichia coli. Characterization of the polypeptide chains by molecular weight, amino acid composition, and amino-terminal residues. J Biol Chem. 1968 Feb 10;243(3):543–546. [PubMed] [Google Scholar]
- Weber K. New structural model of E. coli aspartate transcarbamylase and the amino-acid sequence of the regulatory polypeptide chain. Nature. 1968 Jun 22;218(5147):1116–1119. doi: 10.1038/2181116a0. [DOI] [PubMed] [Google Scholar]