Abstract
Background
In the past few years, an increasing number of research studies have documented the utilization of durvalumab in the field of immunotherapy for cancerous tumors. However, there remains insufficient documentation regarding its associated adverse event (AEs). In order to enhance our comprehension of its toxicological profile, this investigation retrospectively examined the AEs linked to durvalumab using data from the US Food and Drug Administration adverse event reporting system (FAERS).
Methods
Using data from FAERS for the period 2004 to 2024, the reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN) and mu-item gamma Poisson shrinker (MGPS) four algorithms were used to quantify durvalumab related AEs. SAS 9.4 was used for statistical analysis.
Results
We collected nonduplicated reported 17,629,340 patients from the FAERS database and 19,709 AEs cases in the target population with durvalumab as the primary drug of suspicion. There were 6 significantly disproportionate preferred terms (PTs) that fit all four algorithms simultaneously. The AEs commonly reported include death, radiation pneumonitis, pneumonitis, and lung disorders. Furthermore, durvalumab has been associated with additional AEs, such as metastases to the central nervous system and drug-induced liver injury.
Conclusions
The study revealed that durvalumab immunotherapy is associated with AEs including death, radiation pneumonitis, pneumonitis, metastases to the central nervous system, lung disorder and drug-induced liver injury. In clinical practice, it is crucial to be vigilant and prevent the occurrence of these AEs.
Keywords: Durvalumab, FAERS, PD-L1, Adverse events
Introduction
Malignant neoplasms are presently the second most common cause of mortality worldwide [1, 2]. In recent years, there has been an increasing emphasis on adopting a holistic approach to the treatment of malignant tumors, with immunotherapy gaining recognition as a highly effective therapeutic modality for these malignancies which has significantly advanced the systemic therapy for patients with advanced cancer [3].
The primary immunotherapy agents currently employed in clinical settings encompass antibodies targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death 1 (PD-1)/ programmed death ligand 1 (PD-L1) pathways [4]. PD-L1 has been confirmed to be a member of the B7/CD28 protein family that is highly expressed on many tumor cells and avoids immune surveillance and clearance by inhibiting anti-tumor T cell responses [5]. The inhibitors of PD-L1 present extensive and varied opportunities for enhancing antitumour immunity, with the potential to generate long-lasting clinical responses [6]. Several clinical trials have demonstrated the potential of immunotherapy in enhancing prognosis and extending survival duration among individuals diagnosed with advanced malignancies like non-small cell lung cancer (NSCLC), advanced renal-cell carcinoma, and advanced hepatocellular carcinoma [7–9].
A variety of immunotherapeutic agents are currently available and being utilized in clinical settings, including pembrolizumab, bcemiplimab, toripalimab, camrelizumab, and durvalumab [10]. Durvalumab is a monoclonal antibody of the human IgG1κ with high selectivity and affinity that inhibits the interaction between PD-L1 and both PD-1 and CD80, thereby facilitating T cell recognition and elimination of tumor cells [11, 12]. The efficacy of this treatment has been demonstrated in several clinical studies, such as NSCLC, SCLC and bladder cancer [10, 13–15]. However, the AEs of durvalumab have started to surface gradually. In a meta-analysis by wang et al., anti-PD-1/ PD-L1-related deaths were generally due to pneumonitis (115/333, 35%), hepatitis (74/333, 22%) and neurotoxic effects (50/333, 15%) [16]. Although these were mixed with nivolumab, pembrolizumab, atezolizumab, avelumab and durvalumab. Fatigue was reported in a study of durvalumab plus tremelimumab, 30.1% (95% CI 23.8–36.3); diarrhea, 21.7% (95% CI 17.8–25.6); pruritus 17.9% (95% CI 14.4–21.3); decreased appetite, 17.7% (95% CI 13.7–22.0); nausea, 15.6% (95% CI 12.1–19.6) [17]. The safety concerns associated with the utilization of durvalumab have hindered its implementation in clinical settings. Due to the limited duration of its usage, a comprehensive demonstration of all potential adverse events (AEs) is lacking. Therefore, it is crucial for us to investigate the range of toxicities and AEs exhibited by durvalumab.
The US Food and Drug Administration (FDA) adverse event reporting system (FAERS) database serves the purpose of detecting potential links between medications and negative effects during the post-market monitoring of drug safety. Data are lacking regarding the real-world safety of durvalumab. Given the inherent constraints of clinical trials, including stringent trial protocols, strict participant selection criteria, relatively limited sample size, and restricted duration of monitoring, spontaneous reporting systems have been employed in pharmacovigilance to evaluate the safety of suspected AEs and have significantly contributed to signal detection [18]. We conducted a comprehensive analysis of the AEs associated with durvalumab by conducting a thorough search in FAERS, with the goal of presenting a comprehensive overview of its clinical AEs.
Methods
Data source
The FAERS database comprises AE reports associated with drugs, complaints regarding product quality, and incidents of medication errors. Its purpose is to facilitate the FDA’s post-market surveillance for therapeutic biologic products and medications. The data in the FAERS database has been anonymized in accordance with regulatory guidelines. Due to the spontaneous reporting method used for data collection, there may be instances of duplicate reports or reports that have been withdrawn/deleted from the database. The data cleaning process involves deduplicating reports based on FDA-recommended methods and selecting specific fields (PRIMARYID, CASEID, and FDA_DT) from the DEMO table. Reports are sorted by CASEID, FDA_DT, and PRIMARYID, ensuring that for each unique CASEID, the report with the highest FDA_DT value is retained along with the report having the highest PRIMARYID value. In the determination of the target drug user population, only the drug primarily suspected by the patient is considered. In the background database for analysis, if the drug primarily suspected by the patient is the target drug under study, it will be included in the target drug population, while other patients will be classified into the other drug population.
The FDA releases updated FAERS files on a quarterly basis. For our research, we extracted reports submitted from Q1 2004 to Q1 2024. All individual AEs based on Medical Dictionary for Regulatory Activities (MedDRA), system organ class (SOC) and preferred term (PT) level recorded on durvalumab reports were identified to describe the spectrum of toxicities.
Statistical analysis
Our research follows a case/non-case design, similar to a case-control study. We examined AEs associated with investigational drugs rather than disease conditions. To identify any potential signals indicating an elevated risk of drug-related AEs, we conducted a disproportionality analysis using the reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN) and muti-item gamma Poisson shrinker (MGPS). The ROR, PRR, BCPNN and MGPS values were calculated through the case/non-case approach, enabling us to detect spontaneous signals [19, 20]. Patients treated with durvalumab who reported a specific AE were considered as ‘cases’, while all other potential patients were categorized as ‘non-cases’. The ROR was calculated by analyzing the reported event counts for both the specific drug and other drugs using two-by-two contingency tables. ROR serves as an indicator of the likelihood of a particular outcome occurring in relation to exposure to a drug, reflecting the level of association between drug exposure and the probability of experiencing a specific outcome [21].
A positive signal was generated when (i) number of cases > 3, (ii) lower limit of ROR 95% confidence interval (CI) > 1, (iii) PRR ≥ 2 and chi-square value (χ²) ≥ 4, (iv) the lower limit of IC confidence interval (IC025) > 0, (v) the lower limit of 95% CI of EBGM (EBGM05) > 2. SAS 9.4 was used for all data processing and statistical analysis. SAS software is one of the statistical analysis software recommended by FDA website for FAERS database mining.
Results
Descriptive results
From the Q1 of 2004 to the Q1 of 2024, after eliminating 3,534,477 duplicate reports, a total of 70,993 patients were encompassed in the analysis of the background population. Among them, 9447 patients who were mainly suspected of using durvalumab were incorporated into the target population. There were 19,709 AEs cases in the target population. The clinical characteristics of events with durvalumab were described in Table 1. Among all AEs, male patients accounted for the majority (56.94%), and more than half of the patients were older than 45 years old (65.28%). Among all reports with durvalumab as the primary drug of suspicion, physicians provided more reports (60.41%), and nearly half of these reports were from Asia (47.63%). Severe cases accounted for the vast majority (93.37%), and hospitalization was a common outcome (34.23%). Most patients were reported in 2017 or later (99.94%).
Table 1.
Characteristics of reports associated with durvalumab from Q1 2004 to Q1 2024
| Indicators | Number of patients (%) |
|---|---|
| Overall number of patients | 9447 |
| Gender | |
| Female | 2620 (27.73) |
| Male | 5379 (56.94) |
| Not Specified | 1448 (15.33) |
| Age (years) | |
| < 18 | 4 (0.04) |
| 18–44 | 190 (2.01) |
| 45–64 | 2296 (24.30) |
| 65–74 | 2525 (26.73) |
| ≥ 75 | 1346 (14.25) |
| Not Specified | 3086 (32.67) |
| Mean (SD) | 66.25 (10.38) |
| Median (Q1, Q3) | 67.83 (60.00, 73.07) |
| Min, Max | 0.00, 100.00 |
| Reported person | |
| Consumer | 1550 (16.41) |
| Pharmacist | 1091 (11.55) |
| Physician | 5707 (60.41) |
| Other health-professional | 142 (1.50) |
| Not Specified | 957 (10.13) |
| Reported countries | |
| Japan | 2559 (27.09) |
| United States of America | 2115 (22.39) |
| China | 1113 (11.78) |
| Canada | 650 (6.88) |
| France | 562 (5.95) |
| Serious report | |
| Serious | 8821 (93.37) |
| Non-Serious | 626 (6.63) |
| Outcome | |
| Life-Threatening | 790 (8.36) |
| Hospitalization-Initial or Prolonged | 3234 (34.23) |
| Disability | 193 (2.04) |
| Death | 2870 (30.38) |
| Congenital Anomaly | 5 (0.05) |
| Intervention | 11 (0.12) |
| Other | 4503 (47.67) |
| Onset time (days) | |
| 0–30 | 1552 (16.43) |
| 31–60 | 664 (7.03) |
| 61–90 | 391 (4.14) |
| 91–120 | 266 (2.82) |
| 121–150 | 165 (1.75) |
| 151–180 | 104 (1.10) |
| 181–360 | 324 (3.43) |
| > 360 | 121 (1.28) |
| Not Specified | 5860 (62.03) |
| Mean (SD) | 83.74 (136.60) |
| Median (Q1, Q3) | 41.00 (14.00,99.00) |
| Min, Max | 0.00, 3657.00 |
| Reporting year | |
| 2014 | 2 (0.02) |
| 2015 | 1 (0.01) |
| 2016 | 3 (0.03) |
| 2017 | 178 (1.88) |
| 2018 | 621 (6.57) |
| 2019 | 1037 (10.98) |
| 2020 | 1880 (19.90) |
| 2021 | 1323 (14.00) |
| 2022 | 1290 (13.66) |
| 2023 | 2239 (23.70) |
| 2024 | 873 (9.24) |
For patients included in the analysis and administered the target medication, statistical description was conducted based on individual patient data. In cases where multiple adverse events occurred simultaneously within a patient, only one adverse event was considered for counting purposes
Positive signal values associated with durvalumab
Table 2 described the reported signal intensities for durvalumab at the SOC level. A positive signal was generated when number of cases ≥ 3, ROR 95% CI > 1, PRR ≥ 2, χ²≥4, IC025 > 0, and EBGM05 > 2 were simultaneously met. We found that AEs with durvalumab as the primary suspected (PS) drug were associated with 13 SOCs.Among them, 18 signals were identified in “Neoplasms benign, malignant and unspecified (incl cysts and polyps)” (29.51%), followed by 12 positive signals in “Respiratory, thoracic and mediastinal disorders” (19.67%) and 11 in “Infections and infestations” (18.03%), respectively.
Table 2.
Signal strength of AEs of durvalumab at the SOC level in FAERS database
| SOC | Cases (%) | Positive signal (%) |
|---|---|---|
| General disorders and administration site conditions | 3372 (17.11) | 3 (4.92) |
| Respiratory, thoracic and mediastinal disorders | 2418 (12.27) | 12 (19.67) |
|
Neoplasms benign, malignant and unspecified (incl cysts and polyps) |
1879 (9.53) | 18 (29.51) |
| Investigations | 1422 (7.21) | 4 (6.56) |
| Gastrointestinal disorders | 1374 (6.97) | 1 (1.64) |
| Infections and infestations | 1281 (6.50) | 11 (18.03) |
| Injury, poisoning and procedural complications | 1263 (6.41) | 3 (4.92) |
| Nervous system disorders | 916 (4.65) | 1 (1.64) |
| Blood and lymphatic system disorders | 852 (4.32) | 1 (1.64) |
| Hepatobiliary disorders | 738 (3.74) | 2 (3.28) |
| Skin and subcutaneous tissue disorders | 695 (3.53) | 0 |
| Musculoskeletal and connective tissue disorders | 618 (3.14) | 0 |
| Cardiac disorders | 590 (2.99) | 0 |
| Metabolism and nutrition disorders | 535 (2.71) | 0 |
| Endocrine disorders | 432 (2.19) | 0 |
| Renal and urinary disorders | 331 (1.68) | 0 |
| Vascular disorders | 313 (1.59) | 2 (3.28) |
| Psychiatric disorders | 205 (1.04) | 0 |
| Eye disorders | 174 (0.88) | 1 (1.64) |
| Immune system disorders | 160 (0.81) | 2 (3.28) |
| Ear and labyrinth disorders | 41 (0.21) | 0 |
| Surgical and medical procedures | 27 (0.14) | 0 |
| Reproductive system and breast disorders | 26 (0.13) | 0 |
| Social circumstances | 20 (0.10) | 0 |
| Congenital, familial and genetic disorders | 14 (0.07) | 0 |
| Product issues | 12 (0.06) | 0 |
| Pregnancy, puerperium and perinatal conditions | 1 (0.01) | 0 |
| Total | 19,709 (100.00) | 61 (100.00) |
The positive signal generation conditions were as follows: i) number of cases ≥ 3; ii) ROR 95% CI lower limit > 1; iii) PRR ≥ 2, χ²≥4; iv) IC025 > 0; v) EBGM05 > 2. SOC, system organ class
We further analyzed the PT signals and 6 significantly disproportional PT signals that simultaneously fit the four algorithms (Table 3). Death (PT: 10011906, ROR 3.19, 95% CI 3.01–3.37), radiation pneumonitis (PT: 10037765, ROR 56.06, 95% CI 45.94–68.42), pneumonitis (PT: 10035742, ROR 3.41, 95% CI 3.08–3.77) and lung disorder (PT: 10025082, ROR 2.54, 95% CI 2.06–3.13) were usually reported in patients treated with durvalumab. In this study, durvalumab also demonstrated AEs in terms of metastases to central nervous system (PT: 10059282, ROR 9.07, 95% CI 7.47–11.01) and drug-induced liver injury (PT: 10072268, ROR 3.83, 95% CI 3.07–4.78).
Table 3.
Signal strength of AEs of durvalumab at the PT level in FAERS database
| PT | Case (n) |
ROR (95% CI) |
PRR (χ²) |
IC (IC025) |
EBGM (EBGM05) |
|---|---|---|---|---|---|
| Death* | 1719 |
3.19 (3.01–3.37) |
3.00 (1760.73) |
1.31 (1.23) |
2.48 (2.35) |
| Malignant neoplasm progression | 882 |
1.17 (1.09–1.26) |
1.16 (18.53) |
0.19 (0.09) |
1.14 (1.06) |
| Radiation pneumonitis* | 704 |
56.06 (45.94–68.42) |
54.10 (5096.23) |
3.06 (2.89) |
8.34 (6.84) |
| Pneumonitis* | 540 |
3.41 (3.08–3.77) |
3.34 (646.53) |
1.43 (1.28) |
2.69 (2.43) |
| Pneumonia | 288 |
1.68 (1.48–1.91) |
1.67 (66.28) |
0.65 (0.46) |
1.57 (1.38) |
| Dyspnoea | 263 |
1.53 (1.35–1.75) |
1.53 (41.12) |
0.53 (0.34) |
1.45 (1.27) |
| Pyrexia | 236 |
0.85 (0.75–0.98) |
0.85 (5.37) |
-0.20 (-0.40) |
0.87 (0.76) |
| Interstitial lung disease | 233 |
1.28 (1.11–1.47) |
1.28 (12.28) |
0.31 (0.11) |
1.24 (1.08) |
| Diarrhoea | 232 |
0.82 (0.71–0.93) |
0.82 (8.72) |
-0.26 (-0.46) |
0.83 (0.73) |
| Neutrophil count decreased | 219 |
2.37 (2.04–2.75) |
2.35 (134.46) |
1.04 (0.82) |
2.06 (1.78) |
| Metastases to central nervous system* | 209 |
9.07 (7.47–11.01) |
8.98 (730.39) |
2.30 (2.02) |
4.92 (4.06) |
| Febrile neutropenia | 190 |
1.71 (1.46-2.00) |
1.70 (46.16) |
0.67 (0.43) |
1.59 (1.36) |
| Fatigue | 186 |
0.65 (0.56–0.75) |
0.65 (32.33) |
-0.56 (-0.78) |
0.68 (0.58) |
| Rash | 170 |
0.77 (0.66–0.90) |
0.77 (10.98) |
-0.34 (-0.57) |
0.79 (0.67) |
| Platelet count decreased | 163 |
1.35 (1.14–1.59) |
1.34 (12.43) |
0.38 (0.13) |
1.30 (1.10) |
| Myelosuppression | 152 |
1.83 (1.53–2.18) |
1.82 (46.89) |
0.75 (0.49) |
1.68 (1.41) |
| Hepatic function abnormal | 131 |
1.24 (1.03–1.49) |
1.24 (5.43) |
0.28 (0.01) |
1.21 (1.01) |
| Pleural effusion | 131 |
1.78 (1.47–2.15) |
1.77 (36.92) |
0.72 (0.44) |
1.64 (1.36) |
| Cough | 125 |
1.70 (1.40–2.05) |
1.69 (29.64) |
0.66 (0.37) |
1.58 (1.30) |
| Hypothyroidism | 121 |
0.76 (0.63–0.92) |
0.76 (8.21) |
-0.35 (-0.62) |
0.78 (0.65) |
| Off label use | 121 |
0.45 (0.38–0.54) |
0.46 (75.84) |
-1.05 (-1.31) |
0.48 (0.40) |
| Asthenia | 119 |
0.78 (0.64–0.94) |
0.78 (6.92) |
-0.33 (-0.60) |
0.80 (0.66) |
| Nausea | 116 |
0.63 (0.52–0.76) |
0.63 (23.60) |
-0.61 (-0.88) |
0.66 (0.54) |
| Lung disorder* | 113 |
2.54 (2.06–3.13) |
2.53 (81.10) |
1.13 (0.81) |
2.18 (1.77) |
| Drug-induced liver injury* | 112 |
3.83 (3.07–4.78) |
3.81 (161.86) |
1.56 (1.22) |
2.96 (2.37) |
| Decreased appetite | 111 |
0.60 (0.49–0.72) |
0.60 (28.13) |
-0.68 (-0.96) |
0.62 (0.52) |
| Disease progression | 110 |
0.80 (0.66–0.98) |
0.80 (4.84) |
-0.29 (-0.57) |
0.82 (0.67) |
| Vomiting | 109 |
0.91 (0.75–1.11) |
0.91 (0.85) |
-0.12 (-0.41) |
0.92 (0.75) |
| Colitis | 109 |
0.92 (0.75–1.12) |
0.92 (0.69) |
-0.11 (-0.40) |
0.93 (0.76) |
| Anaemia | 108 |
0.73 (0.60–0.89) |
0.73 (9.94) |
-0.41 (-0.70) |
0.75 (0.62) |
*, positive signal. The positive signal generation conditions were as follows: i) number of cases ≥ 3; ii) ROR 95% CI lower limit > 1; iii) PRR ≥ 2, χ²≥4; iv) IC025 > 0; v) EBGM05 > 2. Only the top 30 most frequent PTs are shown. PT, preferred term
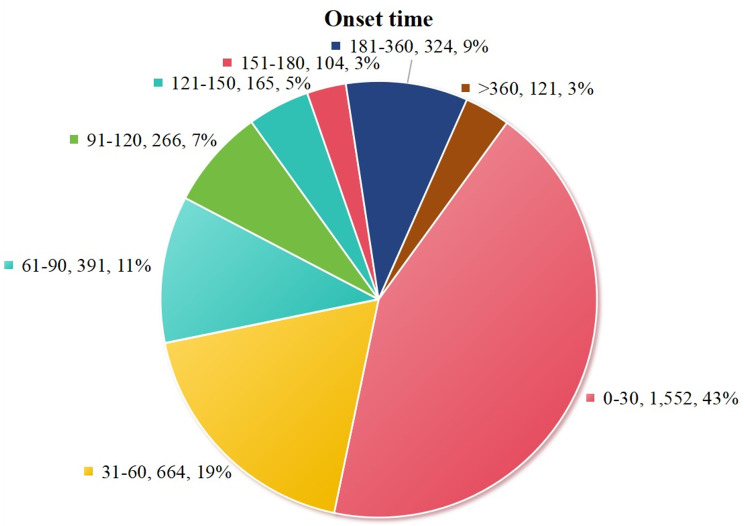
Onset time of events
As shown in Table 1, and Fig. 1, among the durvalumab related AEs collected in the FAERS database, 3,587 (37.97%) had reported time of onset after excluding unreported or unknown time of onset reports, and the median time to onset was 41 days [interquartile range (IQR) 14–99 days]. Among them, a large proportion of the onset time was less than half a year (33.26%). Nevertheless, most instances were observed during the initial month after the commencement of durvalumab (16.43%). The occurrence rates of AEs recorded at 2 month (7.03%), 3 month (4.14%), 4 month (2.82%), 5 month (1.75%) and 6 month (1.10%) were found to be similar throughout the first year of treatment, suggesting that AEs can manifest at any time within the initial half year period.
Fig. 1.
Time to onset of durvalumab-related AEs
Discussion
In the ICIs for the treatment of advanced cancer, durvalumab as an inhibitor of PD-L1 has shown a significant improvement in the prognosis of cancers such as NSCLC [12, 13]. However, the reports of AEs to durvalumab are rare, and its safety is not fully understood, which has always affected its clinical use [16]. We conducted a FAERS database-based analysis of AEs associated with durvalumab use to improve the systematic review of durvalumab-related AEs. To the best of our knowledge, this is the largest comprehensively study to report AEs for durvalumab through the FAERS database.
In our study, durvalumab associated with AEs such as death, radiation pneumonitis and pneumonitis, was consistent as reported by Antonia et al. [13]. They suggested that death, radiation pneumonitis and other pneumonitis were expected outcome after definitive chemoradiotherapy. A meta-analysis of ICI fatal AEs found that 35% of anti-PD-1/ anti-PD-L1-related deaths were due to pneumonitis [16]. This association established a link between the occurrence of AEs resulting in death and the development of pneumonitis. Radiation pneumonitis is a process of cell damage caused by ionizing radiation, in which various cytokines and their related pathways regulate and participate in the damage and post-damage repair [22]. Radiotherapy and ICI are important treatment methods for lung cancer, and studies have shown that radiotherapy combined with ICI can play a better role in the treatment of lung cancer [23]. Compared with treatment alone, combination therapy significantly improves the efficacy, but can also cause pulmonary toxicity. Radiation treatment of lung tissue can lead to oxidative damage of DNA and proteins, induce the release of tumor antigens and inflammatory factors, and lead to the aggregation of inflammatory cells and cytokines in the alveolar cavity and produce inflammatory response [24]. In addition, ICI enhances the anti-tumor immune response by inducing lymphocyte differentiation and up-regulating the levels of cytokines and autoantibodies, so that higher levels of immune cells and cytokines enter the lung tissue treated with radiotherapy, which may cause damage to tumor cells and normal lung tissue at the same time [25–27]. Considering the existing lung conditions such as chronic obstructive pulmonary disease and pulmonary fibrosis, individuals diagnosed with NSCLC might face an increased susceptibility to pneumonitis. We still need large-scale studies to confirm the relationship between the risk and role of the PD-1/ PD-L1 pathway in the development of pneumonitis [28]. Drug-induced liver injury has also been identified in previous studies. It has been reported that immunotherapy for metastatic cancer can be complicated by immune-related AEs in the liver, and acute hepatitis resulting from treatment is rare (3.5%) and in most cases mild. In patients receiving immunotherapy for metastatic cancer, liver biopsy is helpful in the diagnosis and assessment of the severity of liver injury if immune-mediated hepatitis develops [29].
The FAERS database is a spontaneous reporting system and due to its inherent limitations, spontaneous reporting is not restricted to health care professionals and consumers can also provide relevant AEs reports, however the medical expertise of consumers is limited. In addition, some of the associated AEs may be controversial because the reported causality has not been proven.In the present study, there are still certain tasks that remained unaccomplished, such as performing a classification analysis of the AEs associated with durvalumab in a particular disease. Furthermore, on account of the limitations of the database, we are unable to ascertain whether there are any additional complications among the reported patients and whether these complications have a considerable influence on the outcomes. Our findings provide an alert for future clinical use of durvalumab and direction for clinical trials. While vigilant surveillance and identification of these AEs in all populations is recommended, future large-scale prospective trials are needed to confirm our findings and fully elucidate the underlying biologic mechanisms and risk factors of durvalumab in order to enhance risk management strategies.
Conclusion
The study revealed that durvalumab immunotherapy is associated with AEs including death, radiation pneumonitis, pneumonitis, metastases to the central nervous system, lung disorder and drug-induced liver injury. In clinical practice, it is crucial to be vigilant and prevent the occurrence of these AEs.
Acknowledgements
None.
Author contributions
Conceptualization was conducted by Ting Zou and Yusi Hua. Methodology was developed by Ting Zou and Zhuoyang Li. Software was managed by Ting Zou, Zhuoyang Li, and Tianhong Wang. Statistical analysis was performed by Ting Zou and Zhuoyang Li. The original draft was written by Ting Zou, Zhuoyang Li, Shuang Deng, and Siman Wang. Review and editing were carried out by Ting Zou, Zhuoyang Li, Tianhong Wang, Shuang Deng, Siman Wang, and Yusi Hua.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study used the public database which did not need ethics approval and consent to participate (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html).
Consent for publication
All authors consent to publish the article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ting Zou and Zhuoyang Li contributed equally to this work and should be considered as co-first authors.
References
- 1.Siegel RL, Giaquinto AN, Jemal A, Cancer statistics. 2024. CA Cancer J Clin. 2024 Jan-Feb;74(1):12–49. 10.3322/caac.21820. [DOI] [PubMed]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209– 49. 10.3322/caac.21660. [DOI] [PubMed]
- 3.Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22(1):40. 10.1186/s12943-023-01740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38(1):255. 10.1186/s13046-019-1259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart R, Morrow M, Hammond SA, Mulgrew K, Marcus D, Poon E et al. Identification and characterization of MEDI4736, an antagonistic Anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3(9):1052–62. 10.1158/2326-6066.Cir-14-0191. [DOI] [PubMed]
- 6.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. 10.1038/nrc3239. [DOI] [PMC free article] [PubMed]
- 7.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–33. 10.1056/NEJMoa1606774. [DOI] [PubMed]
- 8.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1803–13. 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed]
- 9.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502. 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed]
- 10.Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21(1):28. 10.1186/s12943-021-01489-2. [DOI] [PMC free article] [PubMed]
- 11.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33(17):1974–82. 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed]
- 12.Mezquita L, Planchard D. Durvalumab in non-small-cell lung cancer patients: current developments. Future Oncol. 2018;14(3):205–22. 10.2217/fon-2017-0373. [DOI] [PubMed] [Google Scholar]
- 13.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in Stage III Non-small-cell Lung Cancer. N Engl J Med. 2017;377(20):1919–29. 10.1056/NEJMoa1709937. [DOI] [PubMed]
- 14.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379(24):2342–50. 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 15.Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L et al. Five-Year survival outcomes from the PACIFIC trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol. 2022;40(12):1301–11. 10.1200/jco.21.01308. [DOI] [PMC free article] [PubMed]
- 16.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects Associated with Immune Checkpoint inhibitors: a systematic review and Meta-analysis. JAMA Oncol. 2018;4(12):1721–8. 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto H, Somekawa K, Horita N, Ueda S, Kaneko M, Kaneko A, et al. Adverse events induced by durvalumab and tremelimumab combination regimens: a systematic review and meta-analysis. Ther Adv Med Oncol. 2023;15:17588359231198453. 10.1177/17588359231198453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou C, Peng S, Lin A, Jiang A, Peng Y, Gu T et al. Psychiatric disorders associated with immune checkpoint inhibitors: a pharmacovigilance analysis of the FDA Adverse Event Reporting System (FAERS) database. EClinicalMedicine. 2023;59:101967. 10.1016/j.eclinm.2023.101967. [DOI] [PMC free article] [PubMed]
- 19.Zink RC, Huang Q, Zhang LY, Bao WJ. Statistical and graphical approaches for disproportionality analysis of spontaneously-reported adverse events in pharmacovigilance. Chin J Nat Med. 2013;11(3):314–20. 10.1016/S1875-5364(13)60035-7. [DOI] [PubMed]
- 20.Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, De Freitas RM. A Bayesian neural network method for adverse drug reaction signal generation. European Journal of Clinical Pharmacology. 1998 1998/07/01;54(4):315–21. 10.1007/s002280050466. [DOI] [PubMed]
- 21.Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13(8):519–23. 10.1002/pds.1001. [DOI] [PubMed]
- 22.Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation-Induced Lung Injury: Assessment and Management. Chest. 2019;156(1):150–62. 10.1016/j.chest.2019.03.033. [DOI] [PMC free article] [PubMed]
- 23.Faivre-Finn C, Vicente D, Kurata T, Planchard D, Paz-Ares L, Vansteenkiste JF, et al. Four-year survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-an Update from the PACIFIC Trial. J Thorac Oncol. 2021;16(5):860–7. 10.1016/j.jtho.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Hwang WL, Pike LRG, Royce TJ, Mahal BA, Loeffler JS. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol. 2018;15(8):477–94. 10.1038/s41571-018-0046-7. [DOI] [PubMed]
- 25.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–95. 10.1172/JCI67313. [DOI] [PMC free article] [PubMed]
- 26.Li M, Gan L, Song A, Xue J, Lu Y. Rethinking pulmonary toxicity in advanced non-small cell lung cancer in the era of combining anti-PD-1/PD-L1 therapy with thoracic radiotherapy. Biochim Biophys Acta Rev Cancer. 2019;1871(2):323–30. 10.1016/j.bbcan.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378(2):158–68. 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86–104. 10.3322/caac.21596. [DOI] [PubMed]
- 29.De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68(6):1181–90. 10.1016/j.jhep.2018.01.033. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.