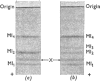
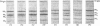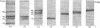Abstract
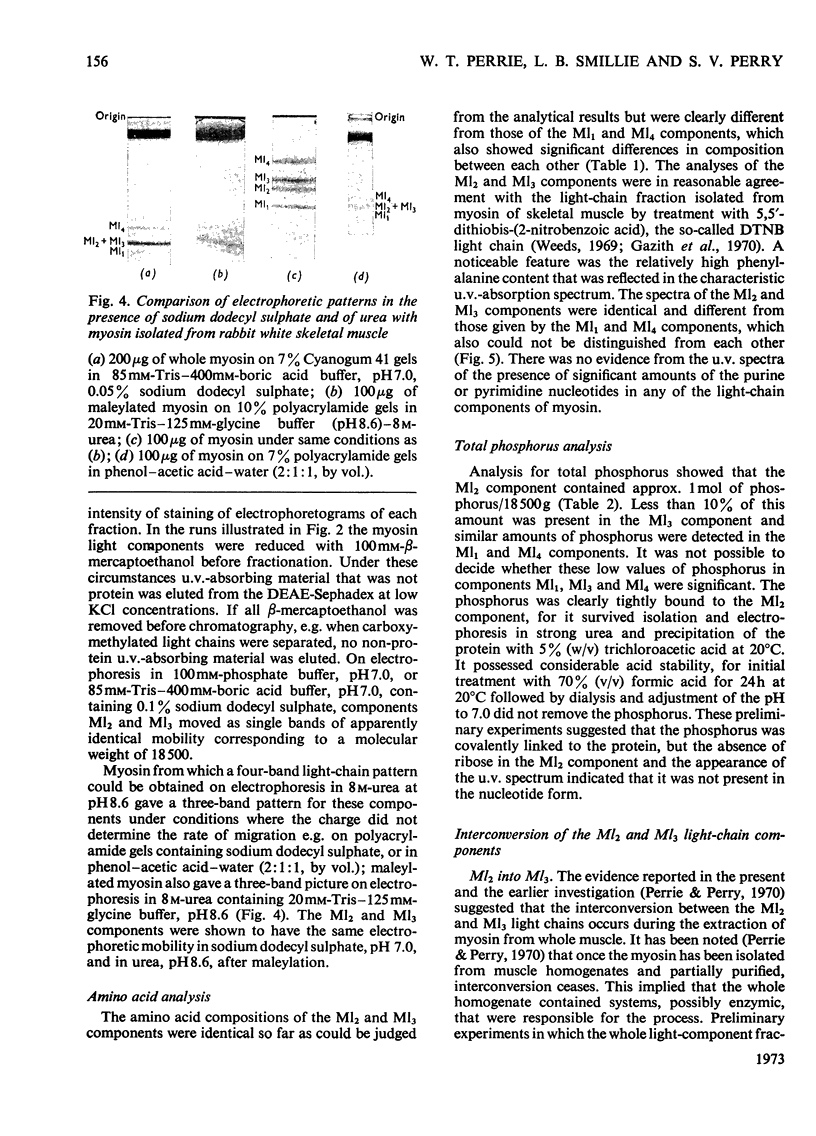
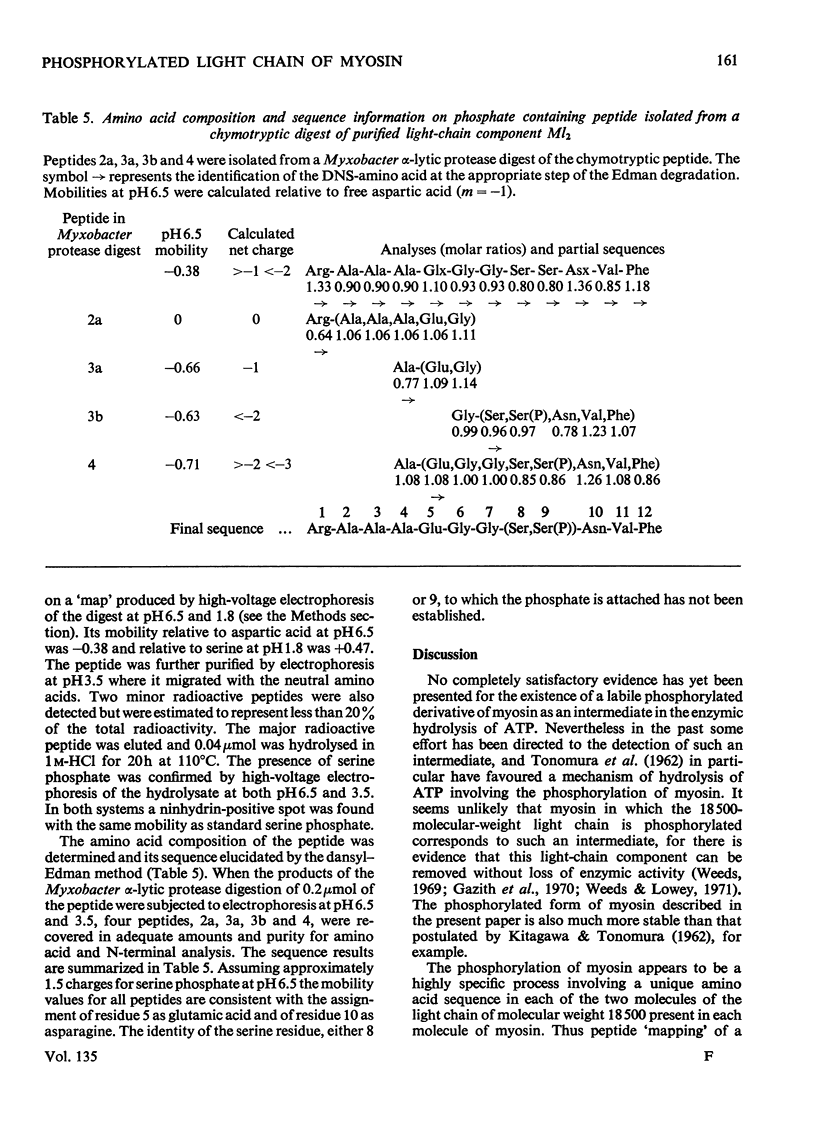
1. The low-molecular-weight components of myosin from rabbit skeletal muscle migrated as four bands on polyacrylamide-gel electrophoresis in 8m-urea but only as three in systems containing sodium dodecyl sulphate. The two bands of intermediate mobility in 8m-urea (Ml2 and Ml3) had identical mobilities in sodium dodecyl sulphate. 2. The isolation of pure samples of all four low-molecular-weight components by DEAE-Sephadex chromatography is described. 3. The amino acid compositions of components Ml2 and Ml3 were identical. Further analyses showed the presence of 1 mol of phosphate/18500g of component Ml2 and less than 10% of this amount in component Ml3. Neither light component contained ribose. 4. Alkaline phosphatase from Escherichia coli converted component Ml2 into Ml3. Incubation with crude preparations of phosphorylase b kinase or protein kinase in the presence of ATP converted component Ml3 into Ml2. 5. Phosphorylation of component Ml3 with the kinases isolated from skeletal muscle and [γ-32P]ATP gave incorporation of 32P only into component Ml2 whether whole myosin or separated low-molecular-weight components were used. 6. High-voltage electrophoresis at pH6.5 and pH1.8 of a chymotryptic digest of 32P-labelled component Ml2 yielded one major radioactive peptide containing serine phosphate. 7. The amino acid sequence of this peptide was shown to be: Arg-Ala-Ala-Ala-Glu-Gly-Gly-(Ser,Ser(P))-Asn-Val-Phe. This sequence shows no obvious similarity to the site phosphorylated in the conversion of phosphorylase b into phosphorylase a by phosphorylase b kinase. 8. Evidence suggests that in vivo all the 18500-molecular-weight light chain is in the phosphorylated form. The extent of dephosphorylation that occurred during myosin extraction depended on the conditions employed.
Full text
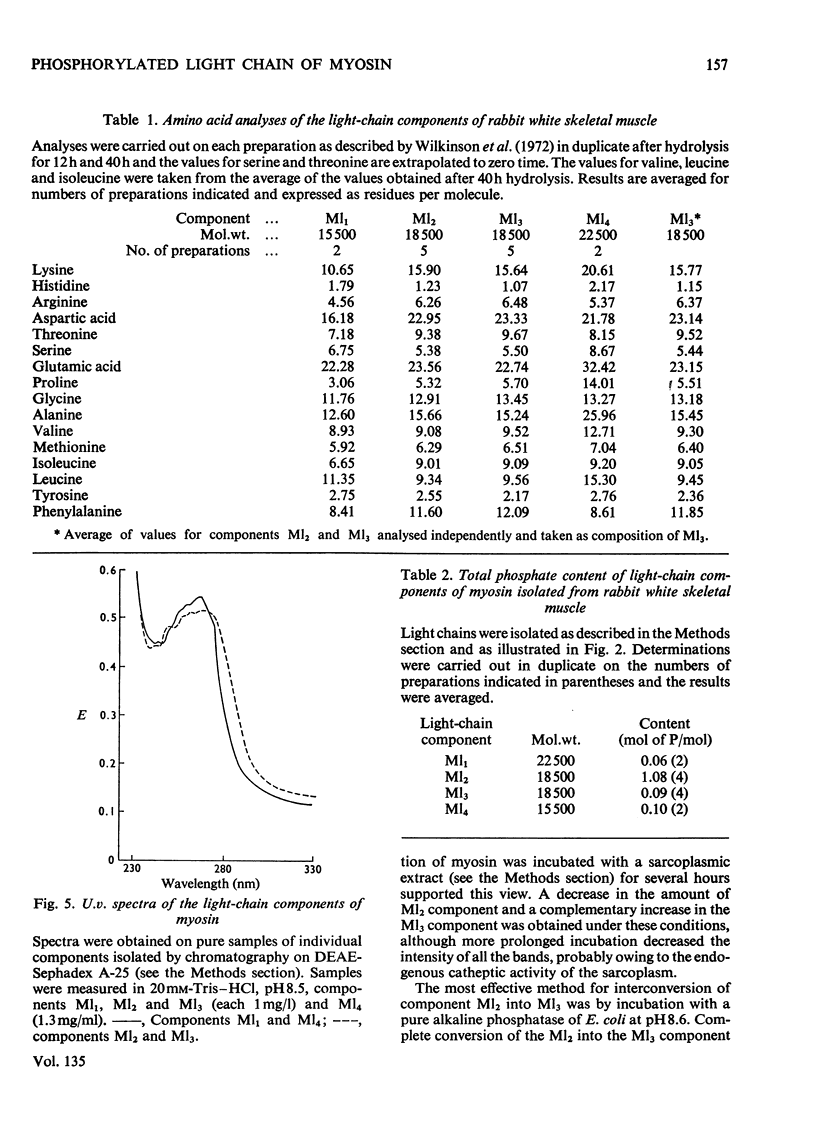
PDF



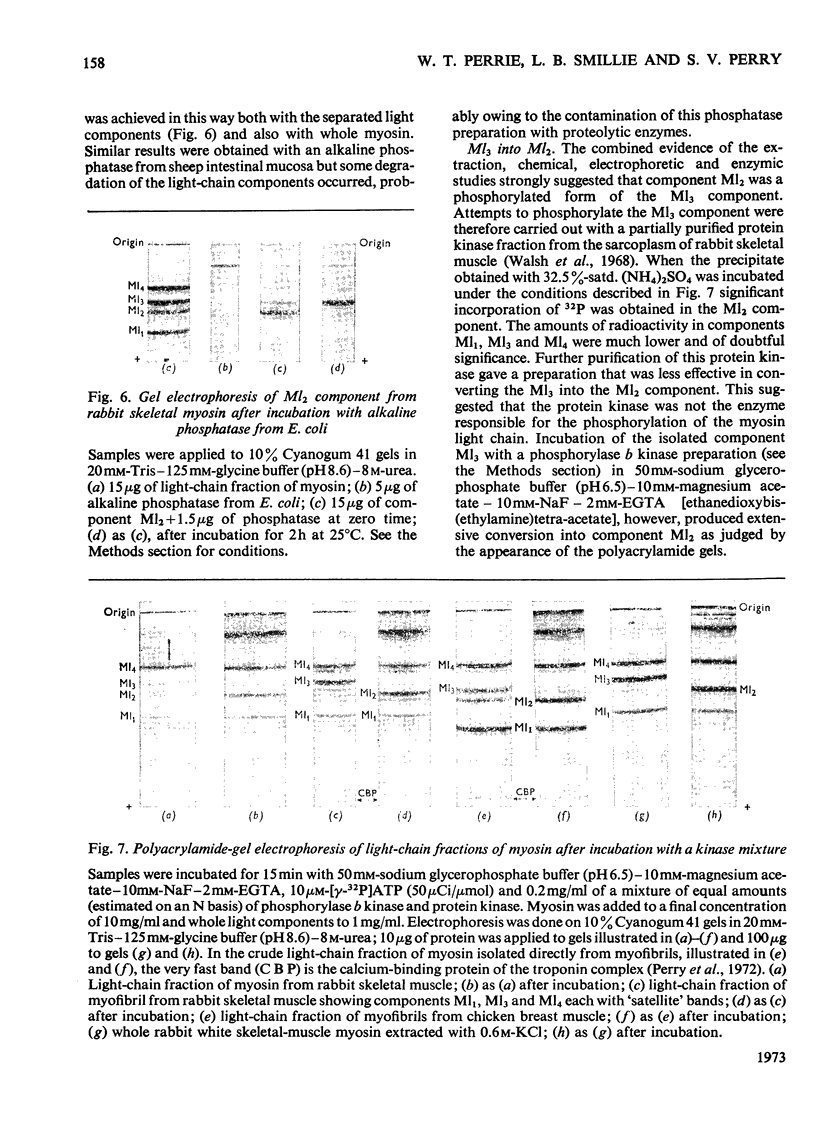




Images in this article
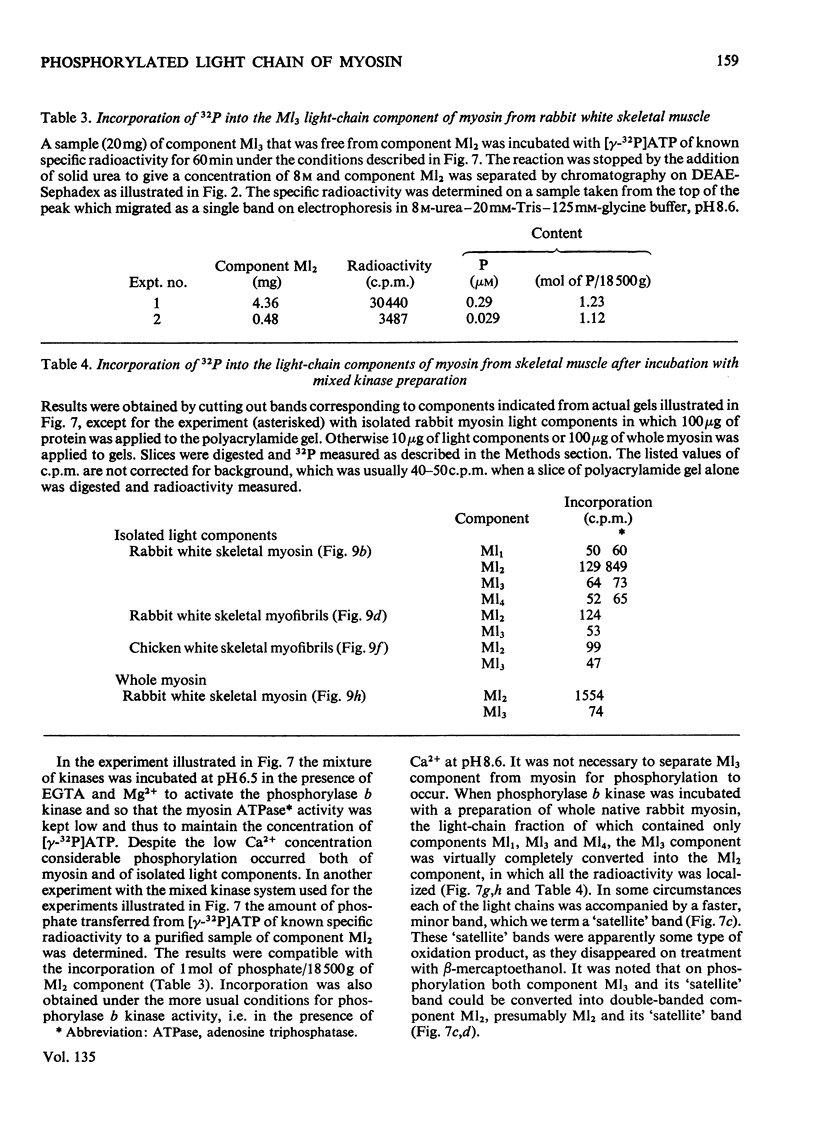
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akroyd P. Acrylamide gel slab electrophoresis in a simple glass cell for improved resolution and comparison of serum proteins. Anal Biochem. 1967 Jun;19(3):399–410. doi: 10.1016/0003-2697(67)90229-1. [DOI] [PubMed] [Google Scholar]
- England P. J., Stull J. T., Krebs E. G. Dephosphorylation of the inhibitor component of troponin by phosphorylase phosphatase. J Biol Chem. 1972 Aug 25;247(16):5275–5277. [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- Gazith J., Himmelfarb S., Harrington W. F. Studies on the subunit structure of myosin. J Biol Chem. 1970 Jan 10;245(1):15–22. [PubMed] [Google Scholar]
- Gould J. M., Cather R., Winget G. D. Advantages of the use of Cerenkov vounting for determination of P 32 in photophosphorylation research. Anal Biochem. 1972 Dec;50(2):540–548. doi: 10.1016/0003-2697(72)90064-4. [DOI] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- KITAGAWA S., TONOMURA Y. Possibility of phosphorylation of myosin as the initial phase of the myosin-adenosinetri-phosphatase reaction. Biochim Biophys Acta. 1962 Feb 26;57:416–418. doi: 10.1016/0006-3002(62)91149-6. [DOI] [PubMed] [Google Scholar]
- KREBS E. G., GRAVES D. J., FISCHER E. H. Factors affecting the activity of muscle phosphorylase b kinase. J Biol Chem. 1959 Nov;234:2867–2873. [PubMed] [Google Scholar]
- KREBS E. G., LOVE D. S., BRATVOLD G. E., TRAYSER K. A., MEYER W. L., FISCHER E. H. PURIFICATION AND PROPERTIES OF RABBIT SKELETAL MUSCLE PHOSPHORYLASE B KINASE. Biochemistry. 1964 Aug;3:1022–1033. doi: 10.1021/bi00896a003. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., DeLange R. J., Kemp R. G., Riley W. D. Activation of skeletal muscle phosphorylase. Pharmacol Rev. 1966 Mar;18(1):163–171. [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowey S., Risby D. Light chains from fast and slow muscle myosins. Nature. 1971 Nov 12;234(5324):81–85. doi: 10.1038/234081a0. [DOI] [PubMed] [Google Scholar]
- NOLAN C., NOVOA W. B., KREBS E. G., FISCHER E. H. FURTHER STUDIES ON THE SITE PHOSPHORYLATED IN THE PHOSPHORYLASE B TO A REACTION. Biochemistry. 1964 Apr;3:542–551. doi: 10.1021/bi00892a013. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Paterson B., Strohman R. C. Myosin structure as revealed by simultaneous electrophoresis of heavy and light subunits. Biochemistry. 1970 Oct 13;9(21):4094–4105. doi: 10.1021/bi00823a010. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of the "37000 component" of the troponin complex (troponin-t). Biochem J. 1973 Feb;131(2):425–428. doi: 10.1042/bj1310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratje E., Heilmeyer L. M.G. Phosphorylation of rabbit muscle troponin and actin by a 3', 5'-c-AMP-dependent protein kinase. FEBS Lett. 1972 Oct 15;27(1):89–93. doi: 10.1016/0014-5793(72)80416-2. [DOI] [PubMed] [Google Scholar]
- RYLE A. P., SANGER F., SMITH L. F., KITAI R. The disulphide bonds of insulin. Biochem J. 1955 Aug;60(4):541–556. doi: 10.1042/bj0600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V., Häcker W. The regulatory proteins of the myofibril. Characterization and biological activity of the calcium-sensitizing factor (troponin A). Biochem J. 1972 Jan;126(1):237–249. doi: 10.1042/bj1260237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V. The regulatory proteins of the myofibril. Characterization and properties of the inhibitory factor (troponin B). Biochem J. 1971 Jul;123(3):367–377. doi: 10.1042/bj1230367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia C. L., Horecker B. L. Dissociation of protein subunits by maleylation. Biochem Biophys Res Commun. 1968 Jun 10;31(5):731–737. doi: 10.1016/0006-291x(68)90622-0. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Purification and characterization of pyruvate-dehydrogenase phosphatase from pig-heart muscle. Eur J Biochem. 1972 Mar 15;26(1):96–105. doi: 10.1111/j.1432-1033.1972.tb01744.x. [DOI] [PubMed] [Google Scholar]
- Sreter F. A., Sarkar S., Gergely J. Myosin light chains of slow twitch (red) muscle. Nat New Biol. 1972 Sep 27;239(91):124–125. doi: 10.1038/newbio239124a0. [DOI] [PubMed] [Google Scholar]
- Starr R., Offer G. Polypeptide chains of intermediate molecular weight in myosin preparations. FEBS Lett. 1971 Jun 2;15(1):40–44. doi: 10.1016/0014-5793(71)80075-3. [DOI] [PubMed] [Google Scholar]
- Stull J. T., Brostrom C. O., Krebs E. G. Phosphorylation of the inhibitor component of troponin by phosphorylase kinase. J Biol Chem. 1972 Aug 25;247(16):5272–5274. [PubMed] [Google Scholar]
- TONOMURA Y., KITAGAWA S., YOSHIMURA J. The initial phase of myosin A-adenosinetriphosphatase and the possible phosphorylation of myosin A. J Biol Chem. 1962 Dec;237:3660–3666. [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weeds A. G. Light chains of myosin. Nature. 1969 Sep 27;223(5213):1362–1364. doi: 10.1038/2231362a0. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Lowey S. Substructure of the myosin molecule. II. The light chains of myosin. J Mol Biol. 1971 Nov 14;61(3):701–725. doi: 10.1016/0022-2836(71)90074-x. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Chemical studies on light chains from cardiac and skeletal muscle myosins. Nature. 1971 Nov 12;234(5324):85–88. doi: 10.1038/234085a0. [DOI] [PubMed] [Google Scholar]
- Whitaker D. R. Lytic enzymes of Sorangium sp. Isolation and enzymatic properties of the alpha- and beta-lytic proteases. Can J Biochem. 1965 Dec;43(12):1935–1954. doi: 10.1139/o65-217. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M., Perry S. V., Cole H. A., Trayer I. P. The regulatory proteins of the myofibril. Separation and biological activity of the components of inhibitory-factor preparations. Biochem J. 1972 Mar;127(1):215–228. doi: 10.1042/bj1270215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]