Abstract
Background
The clinical manifestations of subacute combined degeneration of spinal cord (SCD) in children are complex and vary greatly. Notably, some SCD patients may be complicated with autoimmune diseases, leading to high early misdiagnosis and missed diagnosis rates.
Case presentation
In this study, a case involving an adolescent female with repetitive severe anemia, multiple joint swelling and pain in the left limbs, and paralysis of the bilateral lower limbs with serum vitamin B12 deficiency, polyglandular involvement, and various positive auto-antibodies (anti‑intrinsic factor antibody, anti‑parietal cell antibody, thyroid peroxidase antibody, thyroid globulin antibody and perinuclear anti‑neutrophil cytoplasmic antibody) is reported. The patient was diagnosed with SCD co-existing with autoimmune polyglandular syndrome type 3b (APS 3b) and undifferentiated connective tissue disease (UCTD) based on the symptoms and laboratory tests. However, treatment with high-dose intravenous methylprednisolone pulses, intravenous immunoglobulin, oral naproxen (changed to hydroxychloroquine after 2 weeks), vitamin B12, levothyroxine sodium tablets supplementation, blood transfusion, and rehabilitation significantly improved the patient’s condition.
Conclusion
Co-existence of APS 3b, UCTD, and SCD is rare in children with significantly different clinical manifestations. Nonetheless, early diagnosis and timely treatment of SCD are crucial for improving patient outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-024-05262-4.
Keywords: Subacute combined degeneration, Vitamin B12, Anemia, ANCA antibody, Undifferentiated connective tissue disease, Autoimmune polyglandular syndrome
Background
Subacute combined degeneration of spinal cord (SCD) is a neurodegenerative disease associated with myelopathy and affects the posterior and lateral columns of the spinal cord. SCD is primarily associated with vitamin B12 (Vit B12) deficiency, possibly due to reduced VitB12 intake or metabolic disorders [1–3]. SCD has diverse clinical presentations, which can vary greatly. Besides, SCD may be complicated by autoimmune diseases [4–9], leading to a high rate of misdiagnosis and missed diagnosis. Autoimmune polyglandular syndromes (APS) is a rare group of conditions characterized by the immune-mediated destruction of both endocrine and non-endocrine organs. APS Type 3b is the most prevalent and is characterized by autoimmune thyroid disease, often accompanied by autoimmune gastritis (AIG), and commonly affects middle-aged women [6, 8]. SCD co-existence with APS 3b is rare in children, and only sporadic cases have been reported in China and abroad. In this study, a girl with SCD who was admitted to the Affiliated Hospital of Zunyi Medical University was retrospectively analyzed. The clinical features of SCD with APS 3b in children were also summarized based on the literature.
Case presentation
Clinical data
A 13-year-old female was admitted to Affiliated Hospital of Zunyi Medical University with persistent pain in her left ankle, elbow, and knee joints for over six months. The patient had been experiencing progressive difficulty in walking, multiple episodes of electric shock-like pain in both lower limbs, as well as urinary and fecal incontinence for the past two weeks. The patient had no history of fever, trauma, vomiting, diarrhea, or gastric surgery. There were no identifiable illnesses, vaccinations, or physiological stressors before the onset of symptoms. The patient denied any use of recreational drugs, including nitrous oxide, and there was no family history of consanguinity or neurological disorders. Weakness and numbness in both lower limbs had been reported for about a year. The patient had experienced repetitive megaloblastic anemia in the past two years and was treated with oral VitB12, which was stopped after the symptoms improved. Additionally, the patient had a history of intermittent abdominal pain, and gastroscopy revealed non-atrophic gastritis. The patient appeared anemic after a physical examination. Notably, the patient was a vegan. Neurological assessment indicated normal muscle tension and strength in the upper limbs, while strength in the lower limbs was decreased (power grade 2/5). Furthermore, kinesthesia, tactile sense, position senses were absent in the distal ends of the lower limbs. Swelling and pain were noted in the left ankle, knee, and elbow joints. The patient had lower extremity tremors and increased pain sensitivity. Bilateral knee reflexes were active, with positive findings for bilateral ankle clonus and Babinski’s sign. The patient could not complete the heel-knee-tibia test. Besides, the examination of cranial nerves yielded normal results.
Laboratory tests
Blood routine tests: The patient had reduced red blood cells (2.53 × 1012/L, reference range; 4–12 × 1012/L) and hemoglobin (7.4 g/dL, reference range 11.0–15.0 g/dL) with normal Mean Corpuscular Volume and Mean Corpuscular Hemoglobin Concentration, elevated lactic dehydrogenase (LDH) (1450 U/L, reference range 140–271 U/L) and indirect bilirubin (22.4 µmol/L), decreased serum iron (5.2 µmol/L, reference value 10–30 µmol/L). Further results revealed VitB12 deficiency (13 pg/mL, reference range 189–914 pg/mL), normal folate, and homocysteine. Bone marrow examination suggested that the patient had megaloblastic anemia.
Gastroscopy showed atrophy of gastric corpus mucosa (Fig. 1), while biopsy showed intestinal metaplasia of gastric corpus mucosa. C13 urea breath test showed negative results for Helicobacter pylori infection. Moreover, anti-intrinsic factor antibody (IFA) and anti-parietal cell antibody (PCA) were positive. Thyroid tests showed that the patients had elevated thyroid stimulating hormone levels (TSH) (9.738 pg/mL, reference value 0.5–4.9 µIU/mL) with normal free T4, free T3, T3 and T4, elevated anti-thyroid peroxidase antibody antibodies (TPOAb) (294.4 IU/ml, reference value 0–34 IU/mL) and anti-thyroid globulin antibody (TGAb) (1184 IU/mL, reference value 0-115 IU/mL). Thyroid ultrasound showed thyroid enlargement. Positive anti-nuclear antibody (1: 320) and perinuclear anti-neutrophil cytoplasmic antibody (pANCA) with negative rheumatoid factor and lupus cells were also detected in the patient.
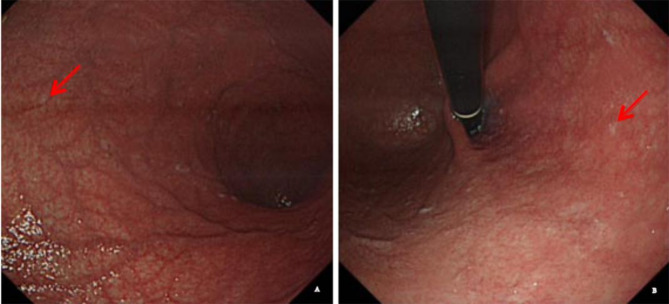
Fig. 1.
The results of gastroscopy of our patients with SCD with autoimmune disease A-B Gastroscopy showed the gastric mucosa at the atrophic site was red and white (arrow)
The cerebrospinal fluid routine examination showed normal results. Antibodies targeting aquaporin 4 (AQP4) and myelin oligodendrocyte glycoprotein (MOG) in the cerebrospinal fluid and serum were negative, ruling out neuromyelitis optica spectrum disease (NMOSD) and AQP4-negative NMOSD. The cranial magnetic resonance image (MRI) showed mild cerebral atrophy. Spinal cord MRI revealed irregular hyperintensity signals in the posterior column of the cervical, thoracolumbar, and lumbar cord on T2 weighted images, as well as an inverted ‘V’ sign in the axial segment of the cervical cord (Fig. 2). Somatosensory-evoked potentials indicated prolonged latency of N20 in both upper extremities and absence of the P40 wave in both lower limbs. Bilateral sural nerve sensory conduction was absent, while electromyography and visual-evoked potentials showed normal results. Echocardiography and abdominal computed tomography scans did not show any abnormalities. Blood tests showed negative results for amino acids, acylcarnitine, and metabolic disease. Urine tests for organic acids also showed negative results. Whole exon sequencing did not reveal any pathogenic variations.
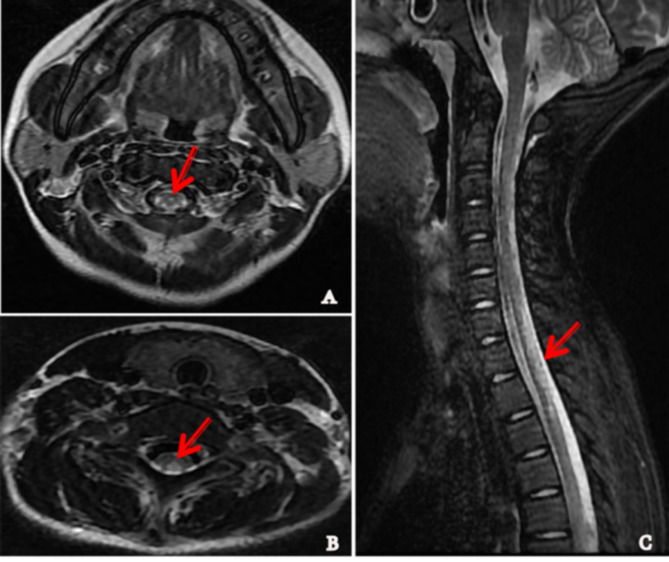
Fig. 2.
The results of MRI of spinal cord in the patient with SCD with autoimmune disease. A-B Axial T2-weighted images of cervical cord showed ‘three point’ sign(A arrow) and inverted ‘V’ sign (B arrow) with symmetrical bilateral high-signal intensity lesions in posterior columns. C Sagittal view showed the diffuse high-signal intensity lesions of posterior column in cervical and thoracic vertebral cord (arrow)
Diagnosis and treatment after admission
The patient was diagnosed with SCD based on clinical symptoms, VitB12 deficiency, inverted ‘V’ signs of spinal cord MRI, and abnormal somatosensory-evoked potentials [2, 3, 10–12]. Additionally, atrophy of the gastric corpus mucosa, positive gastric autoimmune antibodies (IFA and PCA), serum VitB12 deficiency, macrocytic and iron-deficiency anemia, elevated LDH, and indirect bilirubin levels indicated AIG [8]. Hashimoto’s thyroiditis was diagnosed based on diffuse thyroid enlargement and elevated levels of TSH, TPOAb, and TGAb. As a result, the patient met the criteria for autoimmune polyglandular syndrome type 3b (Hashimoto’s thyroiditis with AIG).
The patient received blood transfusions, daily intramuscular injections of VitB12 (500 µg) and VitB1 (100 µg) as well as oral Vit C and iron, levothyroxine sodium tablets, and intravenous immunoglobulin (400 mg/kg/d for 5 days). Notably, symptoms of SCD did not improve after treatment for 9 days. Further MRI examinations of the left ankle and left knee showed slight thickening of the synovium in the left knee joint and small amount of effusion in the joint capsule. Since the patient had a history of joint swelling and pain in multiple joints (elbow, knee, ankle) tested positive for pANCA and antinuclear antibodies, these symptoms were inconsistent with rheumatoid arthritis, juvenile idiopathic arthritis, or systemic lupus erythematosus. Therefore, the patient was diagnosed with undifferentiated connective tissue disease (UCTD) [13]. The patient was treated with high-dose intravenous methylprednisolone pulses and initially received oral naproxen for inflammation. Due to suboptimal results, the treatment was switched to hydroxychloroquine after 2 weeks. Anemia improved after 2 weeks, hemoglobin levels returned to normal (12.6 g/dL), and serum VitB12 levels increased. Furthermore, joint swelling and pain in the left limb disappeared, while numbness and weakness in the lower limbs slightly improved. The patient experienced a sensation of walking on cotton with assistance and was discharged. Rehabilitation continued for 2 months after discharge. Neurological symptoms significantly improved after a 9-month follow-up, and the patient could walk 3 km unassisted. Besides, elevated LDH, indirect bilirubin, and thyroid tests were normal after the 9-month follow-up. IFA and PCA tests were also negative, and T2-weighted hyperintensity in spinal cord MRI nearly disappeared (Fig. 3). The patient regained normal ambulatory function after a 2-year follow-up, with occasional joint pain.
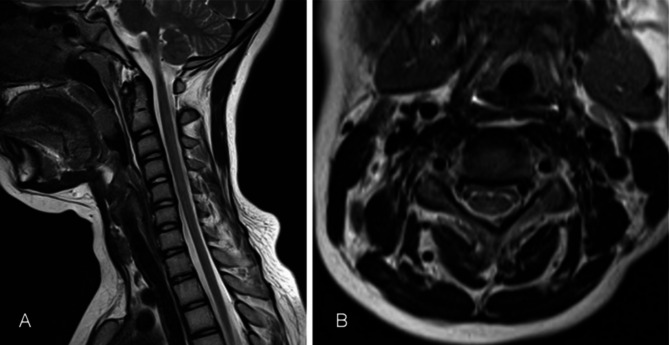
Fig. 3.
Recovered MRI image on cervical spinal after treatment. A. The diffuse high-signal intensity lesions of posterior column in cervical and thoracic vertebral cord in sagittal view were disappeared. B. Inverted ‘V’ sign were almost disappeared in posterior columns in cervical cord after 6 months
Literature review
A systematic search for literature published between January 1, 2001 and July 31, 2024 was conducted in five databases, including Pubmed, ScienceDirect and Ovid, using the keywords ‘subacute combined degeneration of spinal cord’ and ‘children’ in English. A total of 55 articles were found, of which only 6 met the criteria. Articles lacking detailed information or over 14 years old and review articles were excluded. The six articles had 7 SCD cases in children [1–3, 14–16] with symptoms, such as limb weakness, walking difficulty, gait instability, reduced limb activity, deep sensory disorders, and positive Babinski sign. Abnormal spinal cord MRI findings were observed in five of the seven cases, including the inverted ‘V’ sign in 3/7 cases. Additionally, five of seven patients had anemia, and three of seven had reduced Vit B12. Furthermore, the etiology of SCD varied, including factors such as reduced VitB12 intake [16], impaired Vit B12 digestion or absorption [1, 14, 15], and inherited diseases affecting transport and metabolism [2, 3]. Treatment for SCD mainly involves Vit B12 supplementation, with the addition of methylprednisolone in cases where autoimmune diseases co-exist (Table 1).
Table 1.
The clinical features of children with SCD in the literature and in our study
| present case | Mohanty [1] | Arunath V [2] | Cui J [3] | Cui J [3] | Suliman OSM [14] | Manjunatha Yc [15] | Licht [16] | |
|---|---|---|---|---|---|---|---|---|
| gender | female | female | male | female | female | male | female | male |
| Age onset | 13 years old | 12 years old | 4 years old | 13 years old | 6 years old | 12-year-old | three and half years old | 14 years old |
| Past history | megloblastic anemia | Glossitis and loose | silent α-thalassaemia carrier | gastroenteritis | persistent of diarrhea | Vegan diet | ||
| Clinical features | Weakness and walking difficulty | a stamping gait, swaying side-to-side while walking | worsening difficulty in walking for two weeks | unsteady walking | unsteady gait, fell easily | pain and heaviness in both lower limbs and tingling sensations on both feet, developing into completely bedridden | decreased activity and paucity of movements | Stumbling frequently and requiring assistance walking |
| Physical examination | Pallor, power grade 2/5 in lower limbs, motor, touch and position sense were disappeared in the distal end of lower limbs. Active bilateral knee reflexes, positive Babinski Signs, welling and pain of left ankle, knee and elbow joint were observed | pallor, knuckle hyperpigmentation and glossitis. wide-based gait with positive Babinski Sign and Romberg’s sign | generalised hypotonia, grade 4 muscle power in lower limbs, exaggerated knee jerk, extensor plantar response bilaterally | hypoesthesia, inability to perform fine movements with the hands, unwillingness to interact with other human beings, | Bilateral knee tendon reflex was decreased. the heel-to-shin and Romberg tests were abnormal. The joint position sense of the lower limbs was impaired. He did not cooperate for the vibration sense investigation | Pale, dehydration with severe wasting, Lower limbs showed generalised weakness with power grade 3/5, hypotonia with absent deep tendon reflexes at knees and ankles but with a positive Babinski sign. Impaired position sense | pallor, bilateral lower limbs were areflexia and flaccid paraparesis with upgoing plantar reflexes dysmetria and truncal ataxia. | Deep tendon reflexes were 2 + in the upper extremities and absent in the lower extremities. Impaired joint position sense in the lower extremities, a positiveRomberg sign. |
| Blood routine | HB 7.4gd/l | HB 7.7 g/dl, macrocytosis | HB 6.8 g/dL | normal | HB 9.9 g/dL | HB 9.5 g/dl | HB 7 mg/dl | normal |
| Vit B12 | 13pg/ml | < 60pg/ml | 116 pg/ml | normal | normal | Normal(may be associated with dehydration) | < 150pg/ml | 281pg/ml(normal) |
| Bone marrow examination | megloblastic anemia | Not mentioned | megaloblastic erythropoiesis giant metamyelocytes | Not mentioned | Not mentioned | microcytic hypochromic anaemia | Arrest of maturation. | Not mentioned |
| Spinal cord MRI | typical inverted ‘V’ sign of the cervical spinal cord in transverse view | Hyperintense signals in the posterior aspect of the cord in the midline, | symmetrical T2-weighted high signal intensity in the posterior column and characteristic ‘inverted V sign” | Symmetrical long T2 signals in the posterior portion of the spinal cord in the 2 to 6 vertebral bodies. | an abnormally long T2 weighted image signal in the posterior columns from T8 to T11. (“inverted ‘V’ signs | Not mentioned |
abnormal diffuse hyperintensity involving the posterior aspect of the spinal cord in T2 weighted images(inverted ‘V’ sign) |
Normal |
| Nerves conduction velocity | Bilateral sural nerve sensory conduction was absence | normal | normal | Not mentioned | Not mentioned | Not mentioned | delayed motor conduction | mild slowing of the conduction velocities in the sural nerve |
| Other features |
LDH 1450 U/L Positive IFA and PCA and pANCA, elevated TPOAb and TgAb |
LDH 1804 U/L | analysis revealed urine albumin 2 +, non-nephrotic range proteinuria | Two MMACHC mutation. Elevated serum propionyl-carnitine C3/C2 levels, homocysteine level, methylmalonic acid, C3, and homocysteine suggested endogenous vitamin B12 deficiency | Two MMACHC mutation, raised TPOAb and Urinary methylmalonic acid with propionylcarnitine C3 and C3/C2, High methylmalonic acid and serum homocysteine were observed | high anti-tissue transglutaminase (tTG) IgA levels, positive anti-endomyseal antibodies | reduced visual evoked response | Increased methylmalonic acid, 2- methylcitric acid, total homocysteine and cystathionine |
| etiology | Autoimmune Polyglandular Syndrome Type 3b | Not mentioned | Imerslund-Gräsbeck syndrome | late-onset CblC disorder | late-onset CblC disorder | coeliac disease | Cobalamin deficiency | |
| treatments | intravenous methylprednisolone impulse therapy, intravenous immunoglobulin, naproxen followed by hydroxychloroquine, vitamin B12 and levothyroxine sodium tablets supplementation, blood transfusion and rehabilitation. | injection Cyanocobolamine 1000 mg intramuscularly daily for 2 weeks, followed by 1000 mg weekly for a month followed by 1000 mg monthly | Intramuscular hydroxocobalamin 1 mg daily for two weeks followed by 1 mg weekly for eight weeks and then 1 mg monthly | 500ug methycobalamin was injected every day. L-carnitine 1 g twice a day, intramuscular injection of vitamin B12 twice a week, and betaine orally wereadded to the treatment | 500ug methycobal was injected every day. methylcobalamin 500 mg intravenous injection | fluid resuscitation, intravenous methylprednisolone 2 mg/kg/day over 2 days then with oral prednisolone over 2 weeks. Intramuscular (IM) vitamin B12 daily for 1 week, then once per week for 1 month | vitamin B12 injections. | Cobalamin (1 mg IM per day) |
HB: hemoglobin; LDH: lactate dehydrogenase; IFA: Anti-intrinsic factor antibody; PCA: anti-parietal cell antibody; IM:.intramuscular; pANCA: Perinuclear anti-neutrophil cytoplasmic antibody; MRI: magnetic resonance image; TPOAb: anti-thyroid peroxidase antibody antibodies; TgAb: anti-thyroid globulin antibody; TSH: thyroid stimulating hormone
Discussion and conclusion
SCD is a neurodegenerative disease primarily affecting the posterior and lateral columns of the spinal cord. SCD is mainly associated with Vit B12 deficiency or metabolic disorders [1–3, 10]. SCD symptoms include sensory disorders, paresthesia, limb weakness, ataxia, and gait disturbances, often accompanied by positive pathological reflexes and active tendon reflexes. MRI shows high-intensity signals in T2-weighted images and inverted ‘V’ or ‘three point’ sign in the cervical cord on axial sections of SCD patients [2, 3, 10–12, 15, 17]. SCD is common among the elderly and very rare in children.
Vit B12 is a crucial cofactor in various biological processes, such as erythrocyte DNA synthesis, myelination, and fatty acid synthesis, playing a vital role in maintaining the integrity of the neuronal myelin sheath. Vit B12 deficiency can lead to anemia or neuropathy, reduced methionine synthase activity, myelin synthesis disorders (SCD) [15]. Notably, Vit B12 deficiency can be caused by dietary factors (long-term vegetarian diet), non-dietary factors (gastric or ileal surgery, long-term diarrhea, immune (AIG)), and metabolic disorders (methylmalonic acidemia and Imerslund-Gräsbeck syndrome). In this study, Vit B12 deficiency was associated with dietary factors and immune factors, specifically positive IFA and PCA.
AIG-induced Vit B12 deficiency is often undiagnosed due to its asymptomatic nature. Besides, anemia treatment usually overlooks the underlying etiology of Vit B12 deficiency [5, 17–19]. Vit B12 is absorbed in the distal ileum along with intrinsic factors secreted by gastric parietal cells. PCA primarily targets H+/K + ATPase, leading to parietal cell destruction, reduced gastric acid secretion, intrinsic factor deficiency, and subsequent malabsorption of iron and Vit B12. This can result in iron deficiency anemia, megaloblastic anemia, and other related complications, such as hemolytic anemia, consistent with the study results. Hemolytic anemia may be associated with megaloblastic anemia in patients with unbalanced nuclear and cytoplasmic development (cytoplasmic is more mature than the nucleus, “nucleoplasmic aging “), which is prone to in situ hemolysis. The red blood cell membrane becomes more rigid during Vit B12 deficiency, increasing red blood cell splenic lysis [20]. Vit B12 Deficiency leads to ineffective erythropoiesis, resulting in intramedullary hemolysis and the release of LDH, thus causing extramedullary fragmentation and hemolysis [21].
AIG is associated with other autoimmune diseases, particularly Hashimoto’s thyroiditis. Notably, AIG and Hashimoto’s thyroiditis can co-exist in APS [6, 8]. Studies have shown that SCD is a comorbidity of APS, affecting the pancreas and thyroid gland, leading to clinical symptoms of AIG and Hashimoto’s thyroiditis [6–8, 18]. In this study, the patient was diagnosed with APS type 3b. The relationship between APS and SCD pathogenesis is illustrated in Fig. 4.
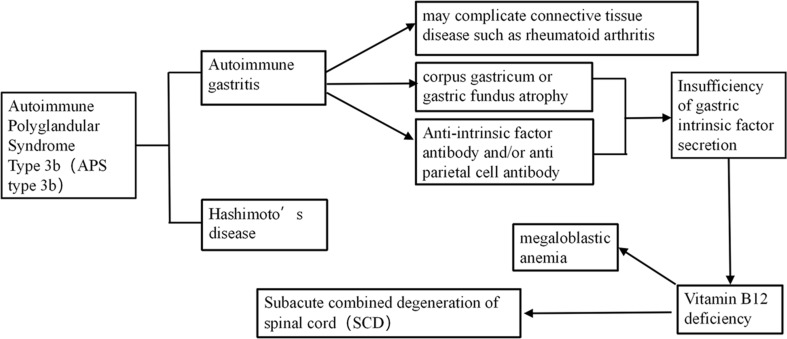
Fig. 4.
Underlying mechanisms between the subacute combined degeneration of the spinal cord and autoimmune polyglandular syndrome 3b including autoimmune gastritis and Hashimoto’s thyroiditis, former led to the absence of intrinic factors or antiparietal cell antibody, followed by deficiency of Vit B12, resulting in subacute combined degeneration of the spinal cord. Furthermore, autoimmune gastritis may be accompanied by connective tissue disease
Studies have also shown that Vit B12 plays an important role in the proper functioning of the immune system. Vit B12 level is negatively associated with autoimmune thyroid diseases (AITD), including autoimmune hypothyroidism. Furthermore, Vit B12 level is significantly lower in patients with AITD than in controls [22]. Furthermore, AITD is often accompanied by autoimmune-related thyroid antibodies, such as anti-thyroid peroxidase antibody (TPOAB), anti-thyroglobulin antibody (TGAB). TPOAb and TgAb may co-exist in some autoimmune connective tissue disease, including UTCD, RA or SLE [23]. Some studies have reported that the frequencies of Vit B12 deficiency among patients with hypothyroidism and AITD are 27%, and 18%, respectively.[23–25]. Rheumatic manifestations are more common in AITD patients (HT) than in controls [26]. Recent studies have suggested that AITD may worsen the cumulative damage of RA through higher disease activity and also worsen secondary osteoarthritis changes [27]. Nonetheless, further studies should assess whether severe Vit B12 deficiency aggravates joint symptoms of connective tissue disease. The limitation of this study is only a case report, and the concrete mechanism has not been further investigated. In this case, the patient had APS, co-existing with SCD and UTCD, making it unclear whether the aggravation of the joint symptoms was related to AITD or Vit B12 deficiency.
Although early treatment with Vit B12 can significantly alleviate symptoms in most SCD patients, only a few can achieve full recovery [10, 28]. Herein, the patient’s condition remained unchanged after treatment with Vit B12 alone. However, the condition gradually improved after treatment with corticosteroid pulses and Vit B12 due to concomitant autoimmune disease. To our knowledge, there are as yet no guidelines regarding a therapeutic regimen for patients with SCD. The recommended course of Vit B12 treatment included intramuscular injections of 0.5-1 mg daily for 2 weeks, followed by 1 mg once a week for 1 month, then 0.5-1 mg intramuscular injection once a month. Notably, lifelong treatment is recommended for AIG patients [1, 2, 4, 6, 17, 28]. Furthermore, anemia, serum Vit B12 levels, and abnormal spinal cord MRI imaging are not significantly correlated with prognosis. Age of onset and disease course are crucial for evaluating SCD outcomes [10]. Also, an increased FT4-to-FT3 ratio at admission is independently associated with a higher risk of poor outcomes [29]. Herein, the patient had normal free T4 and free T3 levels, indicating a good prognosis. Furthermore, the patient had almost fully recovered at the last follow-up after 2 years.
In conclusion, Vit B12 deficiency can lead to severe hematologic and neurological diseases. Megaloblastic anemia, progressive limb weakness, sensory disorders, and characteristic spinal cord MRI findings may indicate SCD. Furthermore, future studies should investigate the etiology of Vit B12 deficiency in megaloblastic anemia. SCD is also related to autoimmune thyroid disease and autoimmune gastritis, indicating that analyses of thyroid function, thyroid antibodies, internal factor antibodies, parietal cell antibodies, and gastroscope are crucial in SCD patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thanks for the Doctor Huang kexin in imaging department and Chen xiaoyan in gastroenterology department for providing the imaging data in the Affiliated Hospital of Zunyi Medical University.
Abbreviations
- AIG
Autoimmune gastritis
- SCD
Subacute combined degeneration of spinal cord
- APS
Autoimmune polyglandular syndromes
- LDH
Lactic dehydrogenase
- VitB12
Vitamin B12
- UCTD
Undifferentiated connective tissue disease
- TSH
Thyroid stimulating hormone
- TPOAb
Thyroid peroxidase antibody antibodies
- TGAb
Thyroid globulin antibody
- pANCA
Perinuclear anti-neutrophil cytoplasmic antibody
- IFA
Intrinsic factor antibody
- PCA
Anti-parietal cell antibody
- AQP4
Aquaporin 4
- MOG
Myelin oligodendrocyte glycoprotein
- MRI
Magnetic resonance image
Author contributions
Lang changhui and Huang pei collected data and draft the main manuscript. Gao jianmei revised the manuscript and prepared figures. He zhixu and Chen yan analyzes and interprets the data, and revised the manuscript. All authors reviewed the final manuscript.
Funding
This work was supported by the National Natural Science foundation of China (32270848), Guizhou Provincial Science and Technology Project (QKHCG[2024]ZD012), Guizhou Provincial High-Level (“Thousand” Level) Innovative Talents Project (gzwjrs2023-034); and The Zunyi Municipal Science and Technology Project (ZSKRPT-2023-6, ZSKHZ-2024-218).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
The studies involving humans were approved by the ethics committee of affiliated hospital of zunyi medical university (ID: KLL-2022-469).
Consent for publication
This study was approved by the patient’s parents. Written informed consent has been obtained from the patient’s parents to publish this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yan Chen, Email: cyz600@163.com.
Zhixu He, Email: hzx@gmc.edu.cn.
References
- 1.Mohanty S, Shobhavat L, Joshi R. Subacute Combined Degeneration of the spinal cord in an adolescent girl. J Trop Pediatr. 2010;56(4):272–4. 10.1093/tropej/fmp111. [DOI] [PubMed] [Google Scholar]
- 2.Arunath V, Hoole TJ, Rathnasri A, Muthukumarana O, Kumarasiri IM, Liyanage ND, et al. A child with Imerslund-Grasbeck syndrome concealed by co-existing alpha-thalassaemia presenting with subacute combined degeneration of the spinal cord: a case report. BMC Pediatr. 2021;21(1):41. 10.1186/s12887-021-02499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui J, Wang Y, Zhang H, Cui X, Wang L, Zheng H. Isolated subacute combined degeneration in late-onset cobalamin C deficiency in children: two case reports and literature review. Med (Baltim). 2019;98(39):e17334. 10.1097/MD.0000000000017334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang TF, Zheng J, Chen X. Subacute combined degeneration of the spinal cord with concomitant autoimmune disease: report of 2 cases. Braz J Med Biol Res. 2021;54(10):e11355. 10.1590/1414-431X2021e11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang N, Li RH, Ma L, Li N, Shan PY, Wang XB et al. Subacute combined degeneration, pernicious anemia and gastric neuroendocrine tumor occurred simultaneously caused by autoimmune gastritis. Front Neurosci, 2019, 13; 1–4. 10.3389/fnins.2019.00001. [DOI] [PMC free article] [PubMed]
- 6.Apolinario M, Brussels A, Cook CB, Yang S, Autoimmune polyglandular syndrome type 3:. A case report of an unusual presentation and literature review. Clin Case Rep. 2022;10(2):e05391. 10.1002/ccr3.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhan HS, Yao X, Hu HY, Han YF, Yue B, Sun LY, et al. Coexistence of primary Sjögren’s syndrome and autoimmune gastritis with pernicious Anemia and Subacute Combined Degeneration of the spinal cord: Case Report and Literature Review. Front Immunol. 2022;13:908528. 10.3389/fimmu.2022.908528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ota K, Yamaguchi R, Tsukahara A, Nishida S, Shigekiyo T, Harada S, et al. Subacute Combined Degeneration of the spinal cord caused by Autoimmune Gastritis. Intern Med. 2020;59(17):2113–6. 10.2169/internalmedicine.4684-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng Y, Bai R, Zhou Y, Ren L. Neuromyelitis Optica spectrum disease coexisting with subacute combined degeneration: a case report. BMC Neurol. 2022;4(1):377. 10.1186/s12883-022-02870-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao J, Su ZY, Xu SB, Liu CC. Subacute Combined Degeneration: a retrospective study of 68 cases with short-term Follow-Up. Eur Neurol. 2018;79(5–6):247–55. 10.1159/000488913. [DOI] [PubMed] [Google Scholar]
- 11.Xiao CP, Ren CP, Cheng JL, Zhang Y, Li Y, Li BB, et al. Conventional MRI for diagnosis of subacute combined degeneration (SCD) of the spinal cord due to vitamin B12 deficiency. Asia Pac J Clin Nutr. 2016;25:34–8. 10.6133/apjcn.2016.25.1.04. [DOI] [PubMed] [Google Scholar]
- 12.Linazi G, Abudureyimu S, Zhang J, Wulamu A, Maimaitiaili M, Wang B, et al. Clinical features of different stage subacute combined degeneration of the spinal cord. Med (Baltim). 2022;101(37):e30420. 10.1097/MD.0000000000030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antunes M, Scirè CA, Talarico R, Alexander T, Avcin T, Belocchi C, et al. Undifferentiated connective tissue disease: state of the art on clinical practice guidelines. RMD Open. 2019;4(Suppl 1):e000786. 10.1136/rmdopen-2018-000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suliman OSM. Sub-acute combined degeneration of the spinal cord as first presentation of coeliac disease in a Sudanese child. Sudan J Paediatr. 2023;23(1):98–103. 10.24911/SJP.106-1639730602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manjunatha YC, Gupta AK, Gupta SK. Subacute Combined Degeneration of the spinal cord in a Child. Indian J Pediatr. 2011;78(2):240–1. 10.1007/s12098-010-0205-z. [DOI] [PubMed] [Google Scholar]
- 16.Licht DJ, Berry GT, Brooks DG, Younkin DP. Reversible subacute combined degeneration of the spinal cord in a 14-year-old due to a strict vegan diet. Clin Pediatr (Phila). 2001;40(7):413–5. 10.1177/000992280104000710. [DOI] [PubMed] [Google Scholar]
- 17.Sun Z, Yu X. A case report: subacute combined degeneration of the spinal cord and pernicious anemia caused by autoimmune gastritis. Med (Baltim). 2022;101(26):e29226. 10.1097/MD.0000000000029226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamada T, Watanabe H, Furuta T, Terao S, Maruyama Y, Kawachi H, et al. Diagnostic criteria and endoscopic and histological findings of autoimmune gastritis in Japan. J Gastroenterol. 2023;58(3):185–95. 10.1007/s00535-022-01954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Fu XX, Luo XX, Hao YX. Misdiagnosed Analysis of 40 Patients with Autoimmune Gastritis. Clincal Misdiagonsis & Mistherapy. 2022; 35(2): 5–8. https://doi.org/10. 3969/j.issn.1002–3429. 2022. 02. 002(Chinese).
- 20.Trivedi M, Areti A, Venishetty N, Parikh A, Didia C. Pernicious Anemia unveiled: unusual hemolytic complications and clinical implications. Cureus. 2024;16(4):e57901. 10.7759/cureus.57901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodford AM, Chaudhry R, Conte GA, Gupta V, Anne M. Chronic Atrophic Gastritis Presenting as Hemolytic Anemia due to Severe Vitamin B12 Deficiency. Case Rep Hematol. 2021; 2021:9571072. 10.1155/2021/9571072 [DOI] [PMC free article] [PubMed]
- 22.Kacharava T, Giorgadze E, Janjgava S, Lomtadze N, Taboridze I. Correlation between vitamin B12 Deficiency and Autoimmune thyroid diseases. Endocr Metab Immune Disord Drug Targets. 2023;23(1):86–94. 10.2174/1871530322666220627145635. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Shangguan J, Zhang Y, Pan Y, Yuan Y, Que W. The prevalence of thyroid autoantibodies in autoimmune connective tissue disease, a systematic review and meta-analysis. Expert Rev Clin Immunol. 2020;16(9):923–30. 10.1080/1744666X.2020.1811089. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Castro KI, Franceschi M, Miraglia C, Russo M, Nouvenne A, Leandro G, et al. Autoimmune diseases in autoimmune atrophic gastritis. Acta Biomed. 2018;89(8–S):100–3. 10.23750/abm.v89i8-S.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benites-Zapata VA, Ignacio-Cconchoy FL, Ulloque-Badaracco JR, Hernandez-Bustamante EA, Alarcón-Braga EA, Al-Kassab-Córdova A, et al. Vitamin B12 levels in thyroid disorders: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14:1070592. 10.3389/fendo.2023.1070592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valderrama-Hinds LM, García-Carrión E, Hernández E, Agostini MI, Reyes-Morales OR, Fung L, et al. Prevalence of undifferentiated inflammatory arthropathy in patients with Hashimoto’s thyroiditis in an endocrinology clinic. Int J Rheum Dis. 2019;22(11):1985–9. 10.1111/1756-185X.13722. [DOI] [PubMed] [Google Scholar]
- 27.Lichtiger A, Fadaei G, Tagoe CE. Autoimmune thyroid disease and rheumatoid arthritis: where the twain meet. Clin Rheumatol. 2024;43(3):895–905. 10.1007/s10067-024-06888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jhunjhnuwala D, Tanglay O, Briggs NE, Yuen MTY, Huynh W. Prognostic indicators of subacute combined degeneration from B12 deficiency: a systematic review. PM R. 2022;14(4):504–14. 10.1002/pmrj.12600. [DOI] [PubMed] [Google Scholar]
- 29.Luo S, Wang XR, Yang LJ, Zou LY. FT4-to-FT3 ratio is a novel prognostic marker in subacute combined spinal cord degeneration patients. Transl Neurosci. 2024;15(1):20220340. 10.1515/tnsci-2022-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.