Abstract
Objective
We aimed to investigate the interrelationships among polygenic risk scores (PRS), healthy lifestyle factors (HLFs), and colorectal cancer (CRC) risk in individuals with prediabetes. To investigate whether adherence to HLFs influence CRC risk in those with elevated PRS within this specific population.
Methods
Data from 22,408 prediabetes participants without CRC at baseline were analyzed from the UK Biobank. HLFs were graded using healthy lifestyle scores (HLSs) and classified as favorable, intermediate, or unfavorable, while the PRS for CRC was categorized as high, medium, or low. Cox proportional hazards models were used to calculate hazard ratios (HR) and 95% confidence intervals (CI) for CRC risk.
Results
High PRS (HR: 2.36; 95% CI: 1.86–3.00) and medium PRS (HR: 1.42; 95% CI: 1.09–1.83) prediabetes were associated with increased CRC risk compared to those with low PRS. HLFs were linked to lower CRC risk in a dose–response manner, with never smoking (HR: 0.69; 95% CI: 0.57–0.84) and maintaining a healthy BMI (HR: 0.64; 95% CI: 0.49–0.82) associated with reduced CRC risk. Adherence to favorable HLFs may reduce the CRC risk in those with medium (HR: 0.51; 95% CI: 0.27–0.95) and high PRS (HR: 0.62; 95% CI: 0.39–0.99) over 15 years of follow-up. In participants with high PRS and unfavorable HLFs, the excess risk due to the additive interaction between PRS and HLFs was 1.41% (p < 0.01), especially for women (1.07%).
Conclusions
There is an additive interaction of PRS and HLFs on CRC risk in individuals with prediabetes. Adopting favorable HLFs should be integrated into the management of prediabetes individuals to reduce the risk of CRC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-024-03552-w.
Keywords: Prediabetes, Colorectal cancer, Genetic susceptibility, Healthy lifestyle factors
Significance Statement
This large population-based prospective cohort study delves into the relationship between PRS, HLFs, and CRC risk in prediabetes individuals. This study suggests that adopting more HLFs can mitigate the CRC risk in prediabetes individuals with moderate to high PRS over 15 years of follow-up. It reveals a nuanced link: Higher PRS and less HLFs correlate with increased CRC risk in prediabetes individuals. Notably, PRS and HLFs exhibit an additive effect on CRC incidence. Participants with high PRS and unfavorable HLFs, the excess risk due to the additive interaction was 1.41% (p<0.01). These findings have significant implications for understanding and managing CRC in prediabetes individuals, prompting a rethink of CRC prevention strategies in this vulnerable population.
Introduction
CRC ranks among the foremost causes of cancer-related morbidity and mortality worldwide [1, 2]. The development, progression, and prognosis of CRC are closely linked to both genetic and lifestyles factors [3]. Traditional genetic risk factors, such as genes associated with chromosomal instability, DNA mismatch repair deficiency, and the CpG island methylator phenotype, play significant roles in CRC development, accounting for about 5–25% of cases [4, 5]. A large multi-center study identified significant correlation between higher PRS and the increased likelihood of CRC [6]. Lifestyle choices are critical modifiable environmental determinants in the prevention and development of various cancers. Unhealthy dietary patterns, such as high consumption of red and processed meats, low fiber intake, and diets rich in saturated fats, are associated with a heightened risk of CRC [3, 7, 8]. Moreover, obesity, physical inactivity, long-term smoking, and excessive alcohol consumption are well-established lifestyle risk factors [8–12]. Alarmingly, a meta-analysis of 18 observational studies revealed that these risk factors not only increase the CRC susceptibility but also result in poorer prognoses for individuals with genetic alterations [13]. These findings emphasize the importance of understanding the multifactorial nature of CRC to develop effective prevention strategies and personalized management plans.
Abnormal glucose metabolism markedly increases tumorigenesis risk. Recent evidence suggested that the incidence of CRC is markedly elevated in diabetes individuals compared to the general population [14, 15]. Of particular concern is the prevalence of prediabetes, a condition characterized by higher-than-normal blood glucose levels that have not yet reached diabetic thresholds. Prediabetes affects 1 in 3 adults in the US and approximately 720 million people worldwide [16]. Prediabetes not only raises the risk of developing type 2 diabetes [17–19] but is also associated with various complications. Recent studies showed that prediabetes is significantly associated with increased risks of cancers in the biliary tract [20], pancreas [21], kidney [22], stomach, colorectum, liver, breast and endometrium [23]. Therefore, could adopting a healthy lifestyle to manage blood glucose levels be a crucial step in preventing tumor development? Numerous studies indicated that the elevated risk of CRC in diabetic individuals can potentially be mitigated through effective management of their blood glucose conditions [24, 25]. Some clinical practices have attempted to reduce the risk of progression from prediabetes via lifestyle interventions like dietary improvements, increased physical activity, and weight loss [16]. For instance, several randomized controlled trials (RCTs) demonstrated that dietary modifications and enhanced physical activity can significantly reduce fasting blood glucose and glycosylated hemoglobin (HbA1c) levels in prediabetes individuals [18, 19, 26, 27]. However, it remains unclear whether these lifestyle changes can decrease CRC risk in prediabetes individuals, highlighting the need for more large-scale prospective studies to validate the long-term impacts of these interventions.
To our knowledge, no comprehensive study has investigated the interaction and joint association between genetics factors, lifestyle choices, and CRC risk in individuals with prediabetes. Furthermore, we aimed to examine whether lifestyle modifications could mitigate CRC risk among individuals with high genetic predisposition. The findings may inform the development of targeted prevention strategies and contribute to reducing CRC risk in individuals with prediabetes, particularly among those with elevated genetic susceptibility.
Methods
Study participants
This study utilized data from the UK Biobank (UKB), an ongoing prospective cohort study that has enrolled over 500,000 eligible participants from 22 centers across England, Wales, and Scotland between 2006 and 2010. At baseline (March 2006 to August 2010), participants underwent extensive assessments, including a touchscreen questionnaire to gather sociodemographic and lifestyle information, physical measurements (e.g., height and weight), and biological sample collection for genetic data. When it comes to whether they have ever had CRC screening, participants are asked a question that "Have you ever had a screening test for bowel (colorectal) cancer? (Please include tests for blood in the stool/faeces or a colonoscopy or a sigmoidoscopy)." The frequency of consumption of main foods and the duration of physical activity were gathered at recruitment through touchscreen questionnaires. The detailed study design and methodologies have been described in prior publications [28].
Diagnosis of CRC and definitions of Prediabetes
The diagnosis of CRC is linked to the International Classification of Diseases, 10th Revision (ICD-10) codes C18, C19, and C20. Follow-up was censored at death or the end of follow-up (2022–10–31), whichever came first. Prediabetes was defined according to the 2021 American Diabetes Association diagnostic criteria as HbA1c levels between 5.7% and 6.5% [29]. HbA1c was measured by HPLC analysis on a Bio-Rad VARIANT II Turbo at recruitment.
Healthy lifestyle components
The healthy lifestyle scores (HLSs) were constructed using five healthy lifestyle factors (HLFs) [30]: physical activity [31], smoking status, body mass index (BMI) [9], alcohol consumption [32] and diet quality (including intakes of fruits, vegetables, fish, red and processed meats, whole grains, refined grains, dairy, and sugar-sweetened beverages) [33]. Each component was scored as 0 or 1, with 1 indicating a more favorable lifestyle behavior (Table S1). Participants were then categorized into three adherence levels based on their cumulative lifestyle score: "favorable HLFs " (scores 4 to 5), "intermediate HLFs " (scores 2 to 3), and "unfavorable HLFs " (scores 0 to 1).
Polygenic risk score
We employed the standard PRS for CRC available in the UKB dataset, as described by Thompson et al [34]. This PRS was generated by Genomics PLC under the UKB project 9659 through a meta-analysis of multiple external genome-wide association study (GWAS) sources. The standardized PRS for CRC was calculated for all individuals in the UKB database. The UKB has defined phenotypes for 28 diseases and 25 quantitative traits, which were used as input data for the standardized PRS assessments. GWAS datasets were identified and meta-analyzed to generate the input data for the PRS releases. Where sufficient external GWAS data were available for a trait, a "standardized" PRS was generated for each participant. Participants were classified into low, medium, and high PRS groups based on the distribution of PRS values.
Statistical analysis
Continuous variables were presented as median [interquartile range], while categorical variables were reported as counts and percentage. Kruskal–Wallis tests for continuous variables and chi-square tests for categorical variables were used to calculate p-value. Separate multivariable Cox proportional hazards models were employed to investigate the associations between genetic risk level and lifestyle score with the risk of CRC, adjusting for covariates such as age, sex, education level, Townsend Deprivation Index [35], and ever had bowel cancer screening. For the multivariable analysis of genetic risk level, additional adjustments were made for genotyping array type (UKB Axiom array, UK BiLEVE array) and the first five principal components of genetic ancestry to account for potential population stratification [36].
To explore the individual contributions of each lifestyle component or dietary pattern to CRC outcomes, we conducted univariable (Model 1) and multivariable (Model 2) Cox proportional hazards regression analyses for each lifestyle factor and dietary structure. We also performed subgroup analysis based on certain dietary factors to investigate their potential interaction with specific genetic variants. Furthermore, we stratified the population by genetic risk level and applied Cox respectively Cox models based on healthy lifestyle scores and three adherence levels to estimate the association between them and CRC incidence within each genetic risk stratum.
We also examined the combined effects of lifestyle adherence (classified as unfavorable, intermediate, and favorable) and genetic risk (classified as low, medium, and high) by creating nine combined categories. The models included the PRS, lifestyle factors, and their interaction terms to assess their multiplicative interaction on hazard ratios (HR). The p-value of the interaction term was used to evaluate the multiplicative interaction. Furthermore, we also used competing risks models (death as the competing risk) as sensitivity analyses to correct for presence of competing events. Multivariable Cox regression models, adjusted for other covariates, were utilized to estimate the 15-year cumulative risks of CRC events for each combination of genetic risk and lifestyle adherence level. For categorical covariates, adjustments were based on the mode of the current combination, and for continuous covariates, the mean value was used. The cumulative risk was calculated as 1 minus the cumulative survival rate. To assess the additive interaction between PRS and lifestyle factors, we regressed the previously calculated cumulative risks on PRS, lifestyle factors, and their interaction term, with the interaction term's p-value considered as the p-value for the additive interaction [37]. The proportional hazards assumptions were assessed using Schoenfeld residuals. Results indicated that genetic risk level and lifestyle score satisfied the proportional hazards assumption (p > 0.05). All analyses were performed using R software (v. 4.2.2), with a two-sided p-value of less than 0.05 considered statistically significant.
Results
Participants included in the study
From the initial cohort of 502,370 individuals, we excluded those with missing lifestyle data (N = 248,102), those who subsequently developed conditions such as pregnancy, organ failure (renal/ heart/ hepatic failure), or systemic inflammatory response syndrome (SIRS) (N = 1,442), those with difference between genetic sex and reported sex (N = 132), those diagnosed with cancer at baseline (N = 20,979), participants not in a prediabetic state at baseline (N = 208,542), those who withdrew from the study (N = 35), and those with missing covariate data (N = 730). With these exclusions, there were 22,408 participants with prediabetes available for the study (Fig. 1).
Fig. 1.
Flowchart of the study population.The difference between genetic sex and reported sex refers to the difference between the sex information obtained from the central registry at the time of recruitment and the sex determined by genotyping analysis
Baseline characteristics
We analyzed the baseline characteristics of 22,408 participants with prediabetes, which included 4,207 individuals with unfavorable HLFs, 13,894 with intermediate HLFs, and 4,307 with favorable HLFs. As shown in Table 1, significant differences were observed among individuals with varying levels of lifestyle adherence in terms of sex, age, blood glucose, HbA1c, smoking status, PRS classification, and BMI category. Compared to the unfavorable lifestyles, individuals with more favorable lifestyle behaviors tended to have blood glucose and HbA1c levels closer to the normal range.
Table 1.
Baseline characteristics of prediabetes participants in the UK Biobank
| Lifestyle categorya | ||||||
|---|---|---|---|---|---|---|
| level | Overall | Unfavorable | Intermediate | Favorable | p-value | |
| N (%) | 22408(100%) | 4207(18.8%) | 13894(62.0%) | 4307(19.2%) | ||
| Sex (%) | Male | 12723 (56.8) | 2883 (68.5) | 8062 (58.0) | 1778 (41.3) | <0.01 |
| Female | 9685 (43.2) | 1324 (31.5) | 5832 (42.0) | 2529 (58.7) | ||
| Age, years (median [IQR]) | 61.00[55.00,65.00] | 60.00[54.00,64.00] | 61.00[55.00,65.00] | 61.00[56.00,65.00] | <0.01 | |
| Blood glucose, mmol/L (median [IQR]) | 5.14[4.78,5.59] | 5.18[4.81,5.67] | 5.14[4.78,5.59] | 5.11[4.76,5.52] | <0.01 | |
| HbA1c, % (median [IQR]) | 5.84[5.76,5.97] | 5.86[5.76,6.00] | 5.84[5.76,5.98] | 5.82[5.75,5.94] | <0.01 | |
| Smoking (%) | Never | 10125 (45.2) | 275 (6.5) | 6116 (44.0) | 3734 (86.7) | <0.01 |
| Previous | 9220 (41.1) | 2822 (67.1) | 5930 (42.7) | 468 (10.9) | ||
| Current | 3063 (13.7) | 1110 (26.4) | 1848 (13.3) | 105 (2.4) | ||
| Ever had CRC screening (%) | No | 14028 (62.6) | 2678 (63.7) | 8689 (62.5) | 2661 (61.8) | 0.20 |
| Yes | 8380 (37.4) | 1529 (36.3) | 5205 (37.5) | 1646 (38.2) | ||
| PRSb (%) | Low | 7469 (33.3) | 1353 (32.2) | 4566 (32.9) | 1550 (36.0) | <0.01 |
| Medium | 7469 (33.3) | 1448 (34.4) | 4661 (33.5) | 1360 (31.6) | ||
| High | 7470 (33.3) | 1406 (33.4) | 4667 (33.6) | 1397 (32.4) | ||
| BMI categoryc (%) | Normal | 5302 (23.7) | 162 (3.9) | 2503 (18.0) | 2637 (61.2) | <0.01 |
| Overweight | 10031 (44.8) | 2176 (51.7) | 6762 (48.7) | 1093 (25.4) | ||
| Obesity | 6998 (31.2) | 1850 (44.0) | 4579 (33.0) | 569 (13.2) | ||
| Others | 77 (0.3) | 19 (0.5) | 50 (0.4) | 8 (0.2) | ||
aThree levels of lifestyle adherence were defined based on the overall lifestyle score variable: “favorable” (scores from 4 to 5), “intermediate” (scores from 2 to 3), and “unfavorable” (scores from 0 to 1).
bBased on the distribution of the polygenic risk score (PRS), the population was categorized into low PRS, medium PRS, and PRS groups.
cBMI was classified as follows: Normal (18.5-25.0), Overweight (25-30), Obesity (≥30), and Others (<18.5).
Increased incidence of CRC in prediabetes individuals with medium or high PRS
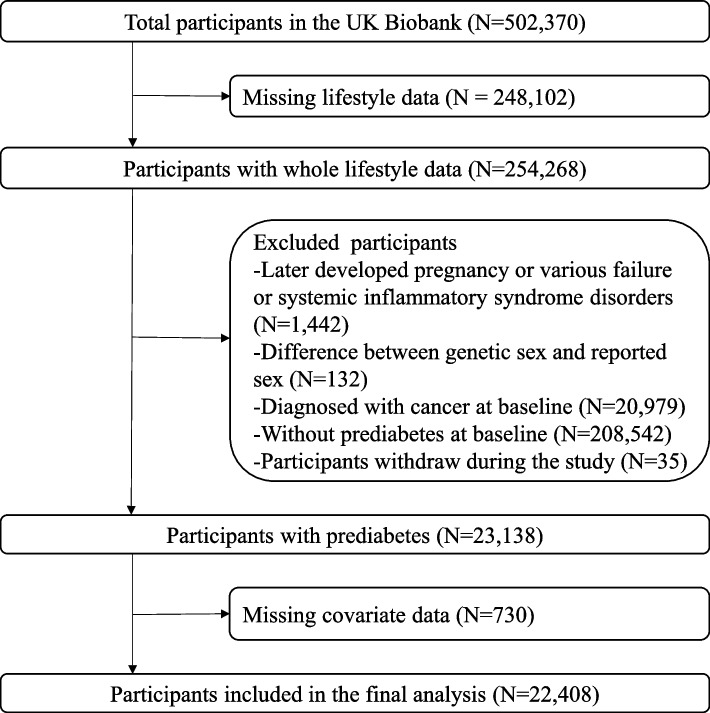
Figure 2A illustrates the relationship between genetic risk levels and CRC incidence among the prediabetes population. Prediabetes individuals with medium PRS had a 42% higher risk (HR: 1.42; 95% CI: 1.09–1.83) and those with high PRS had a 136% higher risk (HR: 2.36; 95% CI: 1.86–3.00) of developing CRC compared to those with low PRS.
Fig. 2.
Associations of genetic risk and health lifestyle score for CRC development in people with prediabetes. A. Associations of genetic risk for CRC in people with prediabetes. B. Associations of health lifestyle score with CRC in people with prediabetes. C. Overall analyses for joint effects of healthy lifestyle score and polygenic risk scores on CRC risk. Cox regression models were adjusted for age at enrollment, sex, Townsend Deprivation Index, education (college or university degree, A levels/AS levels or equivalent, O level/GCSEs or equivalent, CSEs or equivalent, NVQ or HND or HNC or equivalent, other professional qualifications and none of above), ever had bowel cancer screening, the genotype array type and the first 5 principal components of genetic ancestry
Decreased incidence of CRC in prediabetes individuals with healthier lifestyles
As depicted in Fig. 2B, there was a significant inverse trend between the number of HLFs adhered to and the incidence of CRC among the prediabetes population, after adjusting for confounding factors (p for trend < 0.01). Compared to individuals with no HLF, healthier lifestyle factors were associated with a reduced risk of CRC in a dose–response manner. Those adhering to one HLF resulted in a 46% reduction (HR: 0.54; 95% CI: 0.35–0.84), two HLFs had a 42% reduction (HR: 0.58; 95% CI: 0.39–0.87), three HLFs had a 56% reduction (HR: 0.44; 95% CI: 0.29–0.67), four HLFs had a 64% reduction (HR: 0.36; 95% CI: 0.23–0.58), and five HLFs had a 71% reduction (HR: 0.29; 95% CI: 0.14–0.61) of CRC risk.
Associations between healthy lifestyle factors and CRC incidence in prediabetes individuals
Table 2 displays the associations between individual healthy lifestyle components and CRC incidence among the prediabetes population. After adjusting for confounding factors, never smoking, maintaining a healthy BMI, and moderate alcohol consumption were associated with a 31% (HR: 0.69; 95% CI: 0.57–0.84), 36% (HR: 0.64; 95% CI: 0.49–0.82), and 21% (HR: 0.79; 95% CI: 0.64–0.98) lower risk of CRC, respectively, compared to not adhering to these lifestyle factors. In sex-stratified analyses, adherence to the aforementioned lifestyle factors produced similar effects in men (Table S2). For women, never smoking (HR: 0.70; 95% CI: 0.52–0.96) and maintaining a healthy BMI (HR: 0.65; 95% CI: 0.45–0.93) were also with a significant reduction of CRC risk except for moderate alcohol consumption (Table S3). Regarding specific dietary components, consuming three or more servings of whole grains per day and less than two servings of unprocessed red meat per week were associated with a 22% (HR: 0.78; 95% CI: 0.64–0.95) and 59% (HR: 0.41; 95% CI: 0.18–0.92) lower risk of CRC, respectively. In the subgroup analysis based on certain dietary factors, a significant interaction between refined grain intake and PRS was observed. Among individuals consuming refined grains ≤ 2 servings/day, PRS was no longer associated with an increased risk of CRC (Table S4).
Table 2.
Associations between components of the healthy lifestyle and risk of CRC
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Variable | Count | Case | HR (95%CI) | p-value | HR (95%CI) | p-value |
| Physical activity* | ||||||
| Unfavorable | 8387 | 183 | 1.00(Ref.) | 1.00(Reference) | ||
| Favorable | 14021 | 284 | 0.93 (0.77-1.12) | 0.43 | 0.89 (0.74-1.07) | 0.22 |
| Smoking* | ||||||
| Unfavorable | 12283 | 304 | 1.00(Reference) | 1.00(Reference) | ||
| Favorable | 10125 | 163 | 0.63 (0.52-0.76) | <0.01 | 0.69 (0.57-0.84) | <0.01 |
| BMI* | ||||||
| Unfavorable | 17103 | 393 | 1.00(Reference) | 1.00(Reference) | ||
| Favorable | 5305 | 74 | 0.60 (0.47-0.77) | <0.01 | 0.64 (0.49-0.82) | <0.01 |
| Alcohol Consumption* | ||||||
| Unfavorable | 4761 | 112 | 1.00(Reference) | 1.00(Reference) | ||
| Favorable | 17647 | 355 | 0.85 (0.68-1.05) | 0.12 | 0.79 (0.64-0.98) | 0.03 |
| Diet** | ||||||
| Unfavorable | 13270 | 278 | 1.00(Reference) | 1.00(Reference) | ||
| Favorable | 9138 | 189 | 0.98 (0.82-1.18) | 0.84 | 0.98 (0.81-1.18) | 0.82 |
| Fruits (≥ 3 servings/day) | ||||||
| No | 14647 | 305 | 1.00(Reference) | 1.00(Reference) | ||
| Yes | 7761 | 162 | 0.99 (0.82-1.20) | 0.92 | 1.02 (0.84-1.23) | 0.88 |
| Vegetables (≥ 3 servings/day) | ||||||
| No | 21929 | 458 | 1.00(Reference) | 1.00(Reference) | ||
| Yes | 479 | 9 | 0.92 (0.47-1.77) | 0.79 | 0.94 (0.48-1.81) | 0.85 |
| Whole grains (≥ 3 servings/day) | ||||||
| No | 6754 | 162 | 1.00(Reference) | 1.00(Reference) | ||
| Yes | 15654 | 305 | 0.80 (0.66-0.97) | 0.02 | 0.78 (0.64-0.95) | 0.01 |
| Refined grain (≤ 2 servings/day) | ||||||
| No | 20821 | 436 | 1.00(Reference) | 1.00(Reference) | ||
| Yes | 1587 | 31 | 0.94 (0.65-1.35) | 0.72 | 1.00 (0.69-1.44) | 0.99 |
| Fish (≥ 2 servings/day) | ||||||
| No | 15489 | 336 | 1.00(Reference) | 1.00(Reference) | ||
| Yes | 6919 | 131 | 0.87 (0.71-1.07) | 0.19 | 0.83 (0.68-1.02) | 0.08 |
| Unprocessed meats (≤ 2 servings/week) | ||||||
| No | 120 | 6 | 1.00(Reference) | 1.00(Reference) | ||
| Yes | 22288 | 461 | 0.40 (0.18-0.89) | 0.03 | 0.41 (0.18-0.92) | 0.03 |
| Processed meats (≤ 1 servings/week) | ||||||
| No | 8113 | 186 | 1.00(Reference) | 1.00(Reference) | ||
| Yes | 14295 | 281 | 0.85 (0.71-1.02) | 0.08 | 0.90 (0.74-1.09) | 0.29 |
| Dairy (≥ 2 servings/day) | ||||||
| No | 300 | 3 | 1.00(Reference) | 1.00(Reference) | ||
| Yes | 22108 | 464 | 2.07 (0.66-6.44) | 0.21 | 1.96 (0.63-6.11) | 0.25 |
| Sugar-sweetened beverages (Don't drink) | ||||||
| No | 18253 | 362 | 1.00(Reference) | 1.00(Reference) | ||
| Yes | 4155 | 105 | 1.29 (1.04-1.60) | 0.02 | 1.18 (0.95-1.47) | 0.14 |
Model 1 unadjusted for any covariates. Model 2 adjusted for age, sex, Townsend Deprivation Index, education (college or university degree, A levels/AS levels or equivalent, O level/GCSEs or equivalent, CSEs or equivalent, NVQ or HND or HNC or equivalent, other professional qualifications and none of above), and ever had bowel cancer screening.
*Favorable refers to conditions that meet the corresponding criteria in Table S1. Conversely, it is termed unfavorable.
**Favorable refers to meeting five or more conditions corresponding to those in Table S1. Conversely, it is termed unfavorable.
Interaction of genetic risk and healthy lifestyle on CRC incidence in prediabetes individuals
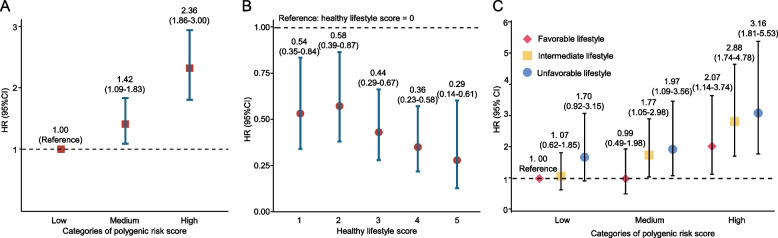
As shown in Fig. 2C, the joint impact of genetic risk and lifestyle adherence on CRC incidence was examined among the prediabetes population. Compared to individuals with low PRS and a favorable lifestyle, those with medium PRS and an intermediate or unfavorable lifestyle had a 77% (HR: 1.77; 95% CI: 1.05–2.98) and 97% (HR: 1.97; 95% CI: 1.09–3.56) higher risk of CRC, respectively. High PRS individuals with favorable lifestyle exhibited a 107% (HR: 2.07; 95% CI: 1.14–3.74), those with medium PRS exhibited a 188% (HR: 2.88; 95% CI: 1.74–4.78), and those with high PRS exhibited a 216% (HR: 3.16; 95% CI: 1.81–5.53) increased risk. It is evident that individuals with high PRS exhibited a comprehensive increase in the risk of developing CRC, but the adoption of unfavorable lifestyle factors exacerbated this outcome. However, we did not observe a statistically significant multiplicative interaction between them (p > 0.05). Additionally, our results from a competing risk model that accounts for mortality risk (Table S5) were similar with those presented in Fig. 2C.
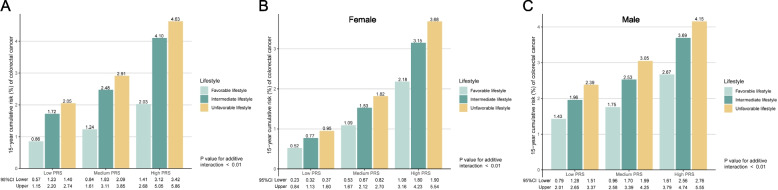
Figure 3A demonstrated that the 15-year cumulative risks of CRC ranged from 2.03% to 4.63% for the high PRS group, 1.24% to 2.91% for the medium PRS group, and 0.86%−2.05% for the low PRS group, depending on their adherence to different healthy lifestyles. Notably, individuals with a high PRS but a healthy lifestyle (2.03%) had a similar 15-year cumulative risk of CRC compared to those with a low PRS but unfavorable lifestyle (2.05%). Among individuals with both high PRS and unfavorable lifestyle, the excess risk attributable to the additive interaction between PRS and lifestyle reached 1.41% (p < 0.01). Our sex-stratified analysis further revealed that, among those with high PRS and unfavorable lifestyle, the excess risk due to the additive interaction between PRS and lifestyle was higher in women (1.07%) (Fig. 3B) than in men (0.52%) (Fig. 3C). However, the 15-year cumulative incidence of CRC was consistently higher in men than in women across all combinations of genetic risk and lifestyle factors (Fig. 3B-C).
Fig. 3.
Estimates of 15-year absolute risk for CRC according to categories of lifestyle and genetic risk for CRC. A. Estimates of 15-year absolute risk for CRC according to categories of lifestyle and genetic risk for CRC in all prediabetes. B. Estimates of 15-year absolute risk for CRC according to categories of lifestyle and genetic risk for CRC in female prediabetes. C. Estimates of 15-year absolute risk for CRC according to categories of lifestyle and genetic risk for CRC in male prediabetes. Cox regression models were adjusted for age at enrollment, sex, Townsend Deprivation Index, education (college or university degree, A levels/AS levels or equivalent, O level/GCSEs or equivalent, CSEs or equivalent, NVQ or HND or HNC or equivalent, other professional qualifications and none of above), ever had bowel cancer screening, the genotype array type and the first 5 principal components of genetic ancestry
The favorable lifestyle adherence linked to lower CRC risk in prediabetes individuals with higher genetic risk
As shown in Table 3, the stratified associations between genetic risk, lifestyle adherence, and CRC incidence were examined among the prediabetes population. In the low PRS group, individuals with an intermediate lifestyle had a 38% (HR: 0.62; 95% CI: 0.39–0.98) lower risk of CRC compared to those with unfavorable lifestyle, but this association was no longer significant after adjusting for covariates. However, among those with medium or high PRS, adherence to a favorable lifestyle was associated with a 49% (HR: 0.51; 95% CI: 0.27–0.95) and 38% (HR: 0.62; 95% CI: 0.39–0.99) decreased risk of CRC, respectively, compared to those adhering unfavorable lifestyle, after adjusting for confounding factors. In addition, we also found that healthy lifestyle scores were associated with a reduced risk of colorectal cancer across different levels of genetic risk. However, the lifestyle scores obtained by adding sleep duration to the five previously mentioned healthy lifestyle factors were no longer associated with a reduced risk of colorectal cancer in individuals with low to medium genetic risk (Table S6).
Table 3.
Stratified analysis of healthy lifestyle adherence and polygenic risk scores on CRC risk
| Count | Case | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | |||
| Low PRS | ||||||
| Health lifestyle scores | 7469 | 98 | 0.79(0.66-0.94) | <0.01 | 0.82(0.69-0.98) | 0.03 |
| Unfavorable lifestyle | 1550 | 17 | 1.00(Reference) | 1.00(Reference) | ||
| Intermediate lifestyle | 4566 | 55 | 0.62(0.39-0.98) | 0.04 | 0.66(0.41-1.05) | 0.08 |
| Favorable lifestyle | 1353 | 26 | 0.55(0.30-1.02) | 0.06 | 0.66(0.35-1.23) | 0.19 |
| Medium PRS | ||||||
| Health lifestyle scores | 7469 | 140 | 0.81(0.70-0.94) | <0.01 | 0.82(0.70-0.96) | 0.01 |
| Unfavorable lifestyle | 1360 | 15 | 1.00(Reference) | 1.00(Reference) | ||
| Intermediate lifestyle | 4661 | 93 | 0.89(0.59-1.32) | 0.55 | 0.91(0.60-1.36) | 0.63 |
| Favorable lifestyle | 1448 | 32 | 0.48(0.26-0.89) | 0.02 | 0.51(0.27-0.95) | 0.03 |
| High PRS | ||||||
| Health lifestyle scores | 7470 | 229 | 0.86(0.77-0.97) | 0.01 | 0.85(0.75-0.96) | <0.01 |
| Unfavorable lifestyle | 1397 | 31 | 1.00(Reference) | 1.00(Reference) | ||
| Intermediate lifestyle | 4667 | 150 | 0.93(0.67-1.28) | 0.65 | 0.90(0.64-1.24) | 0.51 |
| Favorable lifestyle | 1406 | 48 | 0.63(0.40-0.99) | 0.04 | 0.62(0.39-0.99) | 0.04 |
Participants were then categorized into three adherence levels based on their cumulative lifestyle score: "favorable lifestyle " (scores 4 to 5), "intermediate lifestyle " (scores 2 to 3), and "unfavorable lifestyle " (scores 0 to 1). Model 1 unadjusted for any covariates. Model 2 adjusted for age at enrollment, sex, Townsend Deprivation Index, education (college or university degree, A levels/AS levels or equivalent, O level/GCSEs or equivalent, CSEs or equivalent, NVQ or HND or HNC or equivalent, other professional qualifications and none of above), ever had bowel cancer screening, the genotype array type and the first 5 principal components of genetic ancestry.
Discussion
This study provides a comprehensive evaluation of the association between genetic risk factors, lifestyle choices, and the incidence of CRC among individuals with prediabetes. Our findings indicate that individuals with medium to high PRS exhibit an elevated risk of CRC events. Conversely, adherence to a favorable lifestyle can reduce risk of CRC. Specifically, factors, such as non-smoking status, maintaining a healthy BMI, moderate alcohol consumption, and dietary habits including the consumption of three or more servings of whole grains per day and fewer than two servings of unprocessed red meat per week, are significantly linked to lower CRC risk. Unfavorable lifestyle practices markedly increase CRC risk in individuals with medium or high PRS. In contrast, adherence to a healthy lifestyle within high PRS group is associated with a substantial reduction in CRC incidence. These findings provide compelling evidence supporting the importance of lifestyle modifications for the primary prevention of CRC among individuals with prediabetes.
The influence of genetic risk factors and adverse lifestyle behaviors on CRC development has been extensively documented in numerous basic, epidemiological, and clinical studies about general population over recent decades. Individuals with high PRS demonstrated a 2-fold increased risk of CRC compared to those with average genetic risk [38], a finding that aligns with our results. In light of these observations, the National Cancer Institute (NCI) has previously suggested that multigene testing may be advisable for patients with early-onset CRC (aged < 55 years) due to the high prevalence and diversity of hereditary cancer syndromes identified [4]. This impact may arise from genetic mutations or chronic diseases induced by unhealthy lifestyle behaviors, which contribute to genomic instability, alteration of antitumor responses, and persistent inflammatory stimuli, among other factors [39–41].
Numerous studies have reported a close association between unhealthy lifestyle factors and the development of metabolic disorders such as obesity, diabetes, and metabolic syndrome, which are in turn strongly linked to an increased risk of CRC [9, 14, 42]. Our findings support the notion that an increase in the number of healthy lifestyle practices adopted by individuals correlates with a decrease in CRC incidence. Notably, the population in our study consisted of individuals with prediabetes, characterized by elevated blood glucose levels that have not yet reached the diagnostic threshold for prediabetes [16]. Various RCTs have demonstrated that dietary modifications and increased physical activity can significantly lower fasting blood glucose and HbA1c levels in individuals with prediabetes [18, 19, 26, 27]. Our study indicates that individuals with prediabetes who engage in more favorable lifestyle behaviors tend to have blood glucose and HbA1c levels closer to the normal range. These results underscore the crucial role of accumulating favorable lifestyle factors in stabilizing blood glucose levels and delaying the onset of diabetes.
A study utilizing UKB data identified a significant additive interaction between genetic and lifestyle factors affecting the incidence of CRC [43]. Our research observed a similar additive effect among the prediabetes population. However, in contrast to studies focusing on the general population, our investigation specifically examined individuals with prediabetes and revealed that the excess risk associated with high PRS and unfavorable lifestyle was as high as 1.41%. Among prediabetes individuals with high PRS and unfavorable lifestyle, the excess risk due to the additive interaction between PRS and lifestyle was higher in women (1.07%) than in men (0.52%). This suggests that, within the prediabetes cohort, maintaining a healthy lifestyle is particularly critical for women with high PRS to mitigate CRC risk. Additionally, individuals with high PRS who maintained a favorable lifestyle demonstrated a lower 15-year cumulative risk of CRC compared to those with low PRS but unfavorable lifestyle. These findings emphasize the importance of primary prevention strategies tailored to individuals with high PRS within the prediabetes population.
Our findings indicate that the adoption of a healthy lifestyle in individuals with prediabetes can facilitate the normalization of blood glucose levels. Some studies have reported that hyperglycemia is often associated with elevated levels of insulin and insulin-like growth factor (IGF-1), which can activate signaling pathways such as PI3K/AKT and MAPK [44]. These pathways are closely related to cell cycle regulation, metabolism, and anti-apoptosis [45]. Genetic mutations enriched in these pathways may further increase the risk of tumor development, such as common genes associated with CRC, such as PIK3CA and PTEN [46, 47]. On the other hand, the binding of advanced glycation end products (AGEs) to oncogenic KRAS facilitates hypoxia-inducible factor 1 (HIF1) α activation and promotes tumor growth under hypoxic conditions [48]. Meanwhile, the interaction between AGEs and mutant KRAS increases under hypoxia, which in turn sustains KRAS signaling pathways (RAF-MEK-ERK and PI3K-AKT), facilitating stabilization and transcriptional activity of HIF1α [48]. Moreover, previous studies have demonstrated an elevated incidence of DNA oxidative damage among individuals with diabetes mellitus. The accumulation of unrepaired DNA lesions may precipitate various pathological processes, including carcinogenesis. This risk is particularly pronounced in patients exhibiting dMMR or MSI-H [49, 50]. Furthermore, prior research has indicated that increased fruit consumption is associated with a trend towards reduced risk of BRAF-mutated CRC, but not BRAF-wildtype CRC [51]. This differential effect is attributed to the accumulation of cellular reactive oxygen species and subsequent inactivation of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). In BRAF-mutated CRC cells, GAPDH inhibition precipitates an energetic crisis and cellular death through the dual mechanisms of glycolysis inhibition and ATP depletion [52]. These molecular pathways may provide a preliminary explanation for the additive effects observed between HLFs and PRS on CRC incidence in the prediabetes population.
A substantial body of clinical research has demonstrated that individuals with prediabetes can delay or even reverse the onset of diabetes by modifying their lifestyle habits, which also results in short-term improvements in cardiovascular disease (CVD) risk factors, such as systolic blood pressure, low-density lipoprotein cholesterol, and triglyceride levels [16, 53]. However, the impact of these preventive measures on the long-term complications of prediabetes remains unclear. For instance, in the Diabetes Prevention Program (DPP) trial, neither metformin nor lifestyle interventions significantly reduced major cardiovascular events over a median follow-up of 21 years, despite the long-term prevention of diabetes [54]. To our knowledge, research on whether individuals with prediabetes can prevent CRC through lifestyle modifications is currently lacking. Our study found that prediabetes individuals who do not smoke, maintain a healthy weight, and consume alcohol in moderation can reduce their CRC incidence by 31%, 36%, and 21%, respectively, compared to those who do not adhere to these lifestyle practices. Additionally, dietary habits such as consuming three or more servings of whole grains daily or less than two servings of unprocessed red meat per week were associated with a 22% and 59% reduction in CRC incidence, respectively. Global dietary guidelines recommend the inclusion of whole grains in the daily diet due to their association with improved health outcomes and reduced risk of chronic diseases, including obesity, CVD, and various cancers [55]. Interestingly, we also observed that among individuals consuming ≤ 2 servings of refined grains per day, PRS was no longer associated with an increased risk of CRC. Notably, whole grains have been shown to improve gut health and reduce the risk of cancers in the upper gastrointestinal tract and colorectum. A meta-analysis indicated a 17% decrease in CRC risk for each 90 g/day increase in whole grain consumption [56]. These findings are of significant relevance in reducing the incidence of CRC among individuals with prediabetes and provide evidence-based recommendations for maintaining a healthy diet and adopting beneficial lifestyle habits in this population.
This study has several limitations. First, the components of HLSs were based on self-reported questionnaires, which are susceptible to biases such as recall bias, social desirability bias, and comprehension bias. These biases may attenuate the observed associations, potentially leading to an underestimation of risk levels. Second, although our study's HLSs included various variables, it did not encompass all aspects of the American Cancer Society (ACS) Guidelines on Nutrition and Physical Activity for Cancer Prevention or guidelines on tobacco avoidance [57, 58]. This limitation arose because the UKB did not collect comprehensive data on sugary foods, sugary beverages, sedentary behavior, and other relevant activities. Jin D, et al. has reported that individuals who frequently consume calcium, magnesium and phosphorus-rich foods have a significantly lower incidence of CRC compared to those who do not [59]. However, our study focused more on dietary patterns rather than the intake of trace elements in prediabetes. Consequently, the impact of a healthy lifestyle on CRC risk in the prediabetes population may be underestimated. Future research should integrate additional components of the ACS guidelines to more accurately assess the effect of lifestyle on CRC risk among prediabetes individuals. Third, since the SNPs associated with prediabetes and CRC were selected from populations of European descent, it is essential to evaluate the generalizability of these findings to other demographic groups.
Conclusions
In conclusion, our study among the prediabetes population suggests that both genetic factors and unfavorable lifestyle are associated with an increased incidence of CRC. Adherence to a favorable lifestyle may significantly reduce CRC risk in prediabetes individuals, particularly those with a genetic risk. The benefits of maintaining a lifestyle in lowering CRC risk are likely to be most pronounced in individuals with the medium PRS. Our study provides specific lifestyle recommendations that could potentially mitigate CRC risk, supporting the development of personalized cancer prevention strategies for the prediabetes population.
Electronic supplementary material
Acknowledgements
This research has been conducted with the UK Biobank Resource under project 89483.
Abbreviations
- ACS
American Cancer Society
- BMI
Body mass index
- CI
Confidence intervals
- CRC
Colorectal cancer
- CVD
Cardiovascular disease
- DPP
Diabetes Prevention Program
- GWAS
Genome-wide association study
- HbA1c
Glycosylated hemoglobin
- HLF
Healthy lifestyle factor
- HLS
Healthy lifestyle score
- HR
Hazard ratios
- ICD-10
International Classification of Diseases, 10th Revision
- MREC
Multi-centre Research Ethics Committee
- NCI
National Cancer Institute
- PRS
Polygenic risk score
- RCT
Randomized controlled trial
- SIRS
Systemic inflammatory response syndrome
- UKB
UK Biobank
Authors’ contributions
Dr Yiping Cheng had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Wenchen Wang and Yikang Cheng contributed equally to the article. Concept and design: Yiping Cheng, Baoqing Jia. Acquisition, analysis, or interpretation of data: Yiping Cheng. Drafting of the manuscript: Wenchen Wang, Yikang Cheng. Critical revision of the manuscript for important intellectual content: Baoqing Jia, Dawei Yao, Yiping Cheng. Statistical analysis: Yiping Cheng. Administrative, technical, or material support: Wenchen Wang, Qingyu Meng. Supervision: Yiping Cheng, Dawei Yao, Baoqing Jia.
Funding
This study was funded by grants from the National Natural Science Foundation of China (82372784, 82400927), the China Postdoctoral Science Foundation (2023TQ0202 and 2023M742158), and the Shandong Postdoctoral Science Foundation (SDBX2023035).
Data availability
The data can be accessed on the UK Biobank database (https://www.ukbiobank.ac.uk/). The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All participants gave written informed consent. This study complied with the ethical standards of the Declaration of Helsinki and the protocol using secondary data received approval from the UKB data has approval from the North West Multi-centre Research Ethics Committee (MREC) (REC reference: 21/NW/0157).
Consent for publication
Written informed consent for publication of the details in this manuscript, including any personal or clinical information, was obtained from all participants by UKB (https://www.ukbiobank.ac.uk/).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenchen Wang and Yikang Cheng contributed equally to this work.
Contributor Information
Baoqing Jia, Email: baoqingjia@126.com.
Dawei Yao, Email: 18678800909@163.com.
Yiping Cheng, Email: chengyiping93@163.com.
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin Jan-Feb. 2024;74(1):12–49. [DOI] [PubMed] [Google Scholar]
- 2.Morgan E, Arnold M, Gini A, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):338–44. [DOI] [PubMed] [Google Scholar]
- 3.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394(10207):1467–80. [DOI] [PubMed] [Google Scholar]
- 4.Board PDQCGE. Genetics of Colorectal Cancer (PDQ®): Health Professional Version. PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US); 2002.
- 5.Graff RE, Möller S, Passarelli MN, et al. Familial Risk and Heritability of Colorectal Cancer in the Nordic Twin Study of Cancer. Clin Gastroenterol Hepatol. 2017;15(8):1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timofeeva MN, Kinnersley B, Farrington SM, et al. Recurrent Coding Sequence Variation Explains Only A Small Fraction of the Genetic Architecture of Colorectal Cancer. Sci Rep. 2015;5:16286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinicrope FA. Increasing Incidence of Early-Onset Colorectal Cancer. N Engl J Med. 2022;386(16):1547–58. [DOI] [PubMed] [Google Scholar]
- 8.Islami F, Marlow EC, Thomson B, McCullough ML, Rumgay H, Gapstur SM, Patel AV, Soerjomataram I, Jemal A. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States, 2019. CA Cancer J Clin. 2024;74(5):405–32. 10.3322/caac.21858. Epub 2024 Jul 11. [DOI] [PubMed]
- 9.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62(6):933–47. [DOI] [PubMed] [Google Scholar]
- 10.Tsoi KK, Pau CY, Wu WK, Chan FK, Griffiths S, Sung JJ. Cigarette smoking and the risk of colorectal cancer: a meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol. 2009;7(6):682–8 e681–685. [DOI] [PubMed] [Google Scholar]
- 11.Bardou M, Montembault S, Giraud V, et al. Excessive alcohol consumption favours high risk polyp or colorectal cancer occurrence among patients with adenomas: a case control study. Gut. 2002;50(1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Sullivan DE, Sutherland RL, Town S, et al. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20(6):1229-1240.e1225. [DOI] [PubMed] [Google Scholar]
- 13.Thanikachalam K, Khan G. Colorectal Cancer and Nutrition. Nutrients. 2019;11(1):164. 10.3390/nu11010164. [DOI] [PMC free article] [PubMed]
- 14.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(22):1679–87. [DOI] [PubMed] [Google Scholar]
- 15.Wu L, Yu C, Jiang H, et al. Diabetes mellitus and the occurrence of colorectal cancer: an updated meta-analysis of cohort studies. Diabetes Technol Ther. 2013;15(5):419–27. [DOI] [PubMed] [Google Scholar]
- 16.Echouffo-Tcheugui JB, Perreault L, Ji L, Dagogo-Jack S. Diagnosis and Management of Prediabetes: A Review. JAMA. 2023;329(14):1206–16. [DOI] [PubMed] [Google Scholar]
- 17.Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52(6):1475–84. [DOI] [PubMed] [Google Scholar]
- 18.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–44. [DOI] [PubMed] [Google Scholar]
- 19.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–50. [DOI] [PubMed] [Google Scholar]
- 20.Park JH, Hong JY, Han K, Park YS, Park JO. Light-to-Moderate Alcohol Consumption Increases the Risk of Biliary Tract Cancer in Prediabetes and Diabetes, but Not in Normoglycemic Status: A Nationwide Cohort Study. J Clin Oncol. 2022;40(31):3623–32. [DOI] [PubMed] [Google Scholar]
- 21.Liao WC, Tu YK, Wu MS, Lin JT, Wang HP, Chien KL. Blood glucose concentration and risk of pancreatic cancer: systematic review and dose-response meta-analysis. Bmj. 2015;350:g7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JH, Hong JY, Han K, Shen JJ. Association Between Glycemic Status and the Risk of Kidney Cancer in Men and Women: A Nationwide Cohort Study. Diabetes Care. 2023;46(1):38–45. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Cai X, Qiu M, et al. Prediabetes and the risk of cancer: a meta-analysis. Diabetologia. 2014;57(11):2261–9. [DOI] [PubMed] [Google Scholar]
- 24.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut. Feb2006;55(2):285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev. 2010;11(1):19–30. [DOI] [PubMed] [Google Scholar]
- 26.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49(2):289–97. [DOI] [PubMed] [Google Scholar]
- 28.Harris K, Boland C, Meade L, Battise D. Adjunctive therapy for glucose control in patients with type 1 diabetes. Diabetes Metab Syndr Obes. 2018;11:159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Classification and Diagnosis of Diabetes. Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15-s33.33298413 [Google Scholar]
- 30.Sikavi DR, Wang K, Ma W, et al. Aspirin Use and Incidence of Colorectal Cancer According to Lifestyle Risk. JAMA Oncol. 2024;10(10):1354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction. Circulation. 2010;121(4):586–613. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. 8th Edition. 2015. Available at https://odphp.health.gov/our-work/food-nutrition/previous-dietary-guidelines/2015.
- 33.Mozaffarian D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation. 2016;133(2):187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson DJ, Wells D, Selzam S, Peneva I, Moore R, Sharp K, Tarran WA, Beard EJ, Riveros-Mckay F, Duncan, Palmer, Seth P, Harrison J, Futema M, McVean G, Plagnol V, Donnelly P, Weale ME. UK Biobank release and systematic evaluation of optimised polygenic risk scores for 53 diseases and quantitative traits. medRxiv. 2022. Website: https://www.semanticscholar.org/paper/UK-Biobank-release-and-systematic-evaluation-of-for-Thompson-Wells/17728f5acdfdfc9c75d2e4e67f9bbdc1825aee4e.
- 35.Wang X, Ma H, Li X, Heianza Y, Fonseca V, Qi L. Joint association of loneliness and traditional risk factor control and incident cardiovascular disease in diabetes patients. Eur Heart J. 2023;44(28):2583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–9. [DOI] [PubMed] [Google Scholar]
- 37.Wang K, Ma W, Hu Y, et al. Endoscopic Screening and Risk of Colorectal Cancer according to Type 2 Diabetes Status. Cancer Prev Res (Phila). 2022;15(12):847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Archambault AN, Su YR, Jeon J, et al. Cumulative Burden of Colorectal Cancer-Associated Genetic Variants Is More Strongly Associated With Early-Onset vs Late-Onset Cancer. Gastroenterology. 2020;158(5):1274-1286.e1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoffel EM, Mangu PB, Gruber SB, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol. 2015;33(2):209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–91. [DOI] [PubMed] [Google Scholar]
- 42.Jin EH, Han K, Lee DH, et al. Association Between Metabolic Syndrome and the Risk of Colorectal Cancer Diagnosed Before Age 50 Years According to Tumor Location. Gastroenterology. 2022;163(3):637-648.e632. [DOI] [PubMed] [Google Scholar]
- 43.Choi J, Jia G, Wen W, Shu XO, Zheng W. Healthy lifestyles, genetic modifiers, and colorectal cancer risk: a prospective cohort study in the UK Biobank. Am J Clin Nutr. 2021;113(4):810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hua H, Kong Q, Yin J, Zhang J, Jiang Y. Insulin-like growth factor receptor signaling in tumorigenesis and drug resistance: a challenge for cancer therapy. J Hematol Oncol. 2020;13(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stefani C, Miricescu D, Stanescu-Spinu II, Nica RI, Greabu M, Totan AR, Jinga M. Growth Factors, PI3K/AKT/mTOR and MAPK Signaling Pathways in Colorectal Cancer Pathogenesis: Where Are We Now? Int J Mol Sci. 2021;22(19):10260. 10.3390/ijms221910260. [DOI] [PMC free article] [PubMed]
- 46.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. [DOI] [PubMed] [Google Scholar]
- 47.Goel A, Arnold CN, Niedzwiecki D, et al. Frequent inactivation of PTEN by promoter hypermethylation in microsatellite instability-high sporadic colorectal cancers. Cancer Res. 2004;64(9):3014–21. [DOI] [PubMed] [Google Scholar]
- 48.Kang R, Hou W, Zhang Q, et al. RAGE is essential for oncogenic KRAS-mediated hypoxic signaling in pancreatic cancer. Cell Death Dis. 2014;5(10):e1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dandona P, Thusu K, Cook S, et al. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347(8999):444–5. [DOI] [PubMed] [Google Scholar]
- 50.Dizdaroglu M. Oxidatively induced DNA damage: mechanisms, repair and disease. Cancer Lett. 2012;327(1–2):26–47. [DOI] [PubMed] [Google Scholar]
- 51.Hidaka A, Harrison TA, Cao Y, et al. Intake of Dietary Fruit, Vegetables, and Fiber and Risk of Colorectal Cancer According to Molecular Subtypes: A Pooled Analysis of 9 Studies. Cancer Res. 2020;80(20):4578–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yun J, Mullarky E, Lu C, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350(6266):1391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orchard TJ, Temprosa M, Barrett-Connor E, et al. Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet Med. 2013;30(1):46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldberg RB, Orchard TJ, Crandall JP, et al. Effects of Long-term Metformin and Lifestyle Interventions on Cardiovascular Events in the Diabetes Prevention Program and Its Outcome Study. Circulation. 2022;145(22):1632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones JM, Engleson J. Whole grains: benefits and challenges. Annu Rev Food Sci Technol. 2010;1:19–40. [DOI] [PubMed] [Google Scholar]
- 56.Vieira AR, Abar L, Chan DSM, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann Oncol. 2017;28(8):1788–802. [DOI] [PubMed] [Google Scholar]
- 57.Rock CL, Thomson C, Gansler T, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. Jul2020;70(4):245–71. [DOI] [PubMed] [Google Scholar]
- 58.Dietz WH, Douglas CE, Brownson RC. Chronic Disease Prevention: Tobacco Avoidance, Physical Activity, and Nutrition for a Healthy Start. JAMA. 2016;316(16):1645–6. [DOI] [PubMed] [Google Scholar]
- 59.Jin D, Lu Y, Wu W, Jiang F, Li Z, Xu L, Zhang R, Li X, Chen D. Diet-Wide Association, Genetic Susceptibility and Colorectal Cancer Risk: A Prospective Cohort Study. Nutrients. 2023;15(22):4801. 10.3390/nu15224801. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data can be accessed on the UK Biobank database (https://www.ukbiobank.ac.uk/). The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.