Abstract
Objective
Red blood cell distribution width (RDW) is a marker of inflammation and oxidative stress, and its elevation has been associated with poor outcomes in critically ill patients. This meta-analysis aimed to evaluate the association between RDW at admission and short-term mortality in patients with severe burn injury.
Methods
We systematically searched PubMed, Embase, and Web of Science up to August 2024, following PRISMA 2020 and Cochrane guidelines. Cohort studies of adult severe burn patients who reported RDW at admission and short-term mortality were included. Meta-analyses were performed using random-effects models, with mean difference (MD) and relative risk (RR) calculated for RDW and mortality outcomes, respectively. Subgroup and sensitivity analyses were conducted to assess heterogeneity.
Results
Nine cohort studies involving 5268 patients were included. Overall, RDW at admission was higher in nonsurvivors compared to survivors [MD: 0.95%, 95% confidence interval (CI): 0.52–1.38, p < 0.001, I2 = 57%]. In addition, a high RDW was associated with an increased risk of short-term mortality (RR: 1.76, 95% CI: 1.41–2.19, p < 0.001, I2 = 77%). Sensitivity analyses by excluding one dataset at a time confirmed the robustness of these findings. Subgroup analyses revealed no significant differences based on clinical settings, patient age, sex, follow-up duration, or study quality.
Conclusions
RDW at admission is a significant predictor of short-term mortality in patients with severe burn injury. This finding highlights the potential role of RDW as a simple and effective marker for risk stratification in these patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-024-02165-z.
Keywords: Severe burn, Red blood cell distribution width, Mortality, Risk factor, Meta-analysis
Introduction
Severe burn injuries are a significant public health issue, leading to high rates of morbidity and mortality worldwide [1, 2]. Despite advancements in burn care, the prognosis for patients with extensive burns remains poor, especially in those with total body surface area (TBSA) involvement greater than 15% or those requiring intensive care [3–5]. These patients often experience complications such as systemic infections, organ failure, and other critical conditions that can complicate recovery and lead to death [6, 7]. Accurate prediction of short-term mortality is essential for guiding clinical decisions, optimizing resource allocation, and improving patient outcomes [8, 9]. However, identifying a reliable, easy-to-use biomarker for mortality risk stratification in severe burn patients remains a challenge.
Red blood cell distribution width (RDW), a standard component of a complete blood count, reflects the variability in red blood cell size [10]. Elevated RDW has emerged as a marker of inflammation and oxidative stress and has been linked to adverse outcomes in various acute and chronic conditions, including sepsis [11], acute coronary syndrome [12], heart failure [13], chronic kidney disease [14], and cancers [15]. Its value in predicting mortality and morbidity in these settings makes it a convenient and cost-effective tool for risk stratification in critically ill patients [16, 17]. Given the inflammatory response and oxidative stress that accompany severe burn injuries [18, 19], it is plausible that RDW could serve as a useful biomarker in this patient population as well.
In the context of severe burns, increased RDW may be a result of multiple pathophysiological processes. The systemic inflammatory response triggered by severe burn injuries can lead to oxidative stress, disruption of erythropoiesis, and alterations in red blood cell turnover [20]. Moreover, critical illness in burn patients often leads to metabolic dysregulation, which further contributes to red cell abnormalities [21]. Despite these theoretical mechanisms, studies investigating the association between RDW at admission and mortality in patients with severe burn injury have yielded inconsistent results [22–30]. Some research suggests that higher RDW is associated with increased mortality [23, 24, 27–30], while others have found no significant relationship [22, 25, 26], possibly due to differences in study populations, burn severity, and follow-up duration. Given these inconsistencies and the potential role of RDW as a predictive marker for mortality in severe burn patients, this meta-analysis aimed to systematically review and quantify the association between RDW at admission and short-term mortality.
Methods
The study adhered to PRISMA 2020 [31, 32] and the Cochrane Handbook for Systematic Reviews and Meta-analyses [33] guidelines for conducting this meta-analysis, including for the study design, data collection, statistical analysis, and results interpretation. The study protocol has been registered at PROSPERO (https://www.crd.york.ac.uk/prospero) with the identifier CRD42024589800. The PRISMA Checklist of the manuscript is provided in Supplemental Material 1.
Literature search
To identify studies pertinent to this meta-analysis, we searched the PubMed, Embase, and Web of Science databases using an extensive array of search terms, which included: ("red blood cell distribution width" OR "red cell distribution width" OR "RDW") AND ("burn" OR "burns" OR "burned") AND ("mortality" OR "death" OR "deaths" OR "survival" OR "prognosis"). The search was limited to research involving human subjects and we only included studies published in English. The detailed search syntax for each database is shown in Supplemental Material 2. Additionally, we manually reviewed the references of relevant original and review articles to identify further pertinent studies. The literature was assessed from the inception of the searched databases up to August 08, 2024.
Inclusion and exclusion criteria
The PICOS principle was used in developing the inclusion criteria of the meta-analysis.
Population (P): Adult patients (18 years or older) with severe burn injury, which was defined as those with TBSA ≥ 15% or admitted to intensive care unit (ICU).
Exposure (I): RDW was analyzed within 24 h after admission, and patients with a high RDW were considered as exposure. The cutoff value of RDW was determined according to the cutoffs used in the original studies.
Comparison (C): Patients with a high RDW was considered as exposure.
Outcome (O): Short-term mortality following severe burn injury, defined as all-cause mortality during hospitalization or within 90 days of burn injury.
Study design (S): Cohort studies published as full-length articles in English peer-reviewed journals.
The exclusion criteria included reviews, editorials, meta-analyses, preclinical studies, cross-sectional studies, studies including pediatric patients or patients without burn injuries, studies not evaluating RDW as exposure or not measuring at admission, or studies that did not report short-term mortality of the included patients. If two or more studies with overlapping populations were found, the study with the largest sample size was enrolled for the meta-analysis.
Study quality evaluation and data extraction
The literature search, study identification, quality assessment, and data extraction were conducted independently by two authors, with any disagreements resolved through discussion with the corresponding author. Study quality was evaluated using the Newcastle–Ottawa Scale (NOS) [34], which assesses the selection, control of confounders, and outcome measurement and analysis, with scores ranging from 1 to 9, where 9 signifies the highest quality. The data collected for analysis included the study details (author, year, country, and design), patient characteristics (diagnosis, sample size, age, and sex, timing for measuring RDW, follow-up duration, and the number of patients died during follow-up, outcomes reported, and the variables adjusted when analyzing the association between RDW and the risk of all-cause mortality.
Statistics
The primary outcome of the meta-analysis was to compare the RDW at admission between nonsurvivors and survivors of severe burn injury, which was summarized as mean difference (MD) and corresponding 95% confidence interval (CI). The secondary outcome of the meta-analysis was to evaluate the association between RDW at admission and the risk of short-term death of patients with severe burn injury, which was summarized as risk ratio (RR) and 95% CI comparing between patients with a high versus a low RDW at admission. The RR values and their standard errors were computed from 95% CIs or p-values and logarithmically transformed for variance stabilization. To assess heterogeneity, we used the Cochrane Q test and I2 statistics [35], with I2 > 50% indicating significant statistical heterogeneity. A random-effects model was applied to integrate the results, accounting for study variability [33]. Via excluding individual studies sequentially, a sensitivity analysis was performed to evaluate the robustness of the findings. Predefined subgroup analyses were performed to explore the effects of various factors, such as clinical settings (ICU or burn departments), age and sex of the patients, follow-up duration, and NOS scores of the studies. Medians of the continuous variables were selected as the cutoffs for defining the subgroups. Publication bias was evaluated using funnel plots and visual inspection for asymmetry, supplemented by Egger’s regression test [36]. The certainty of evidence was evaluated with the five Grading of Recommendations Assessment, Development, and Evaluation (GRADE) considerations of within- and across-study risk of bias (limitations in the study design and execution or methodological quality), inconsistency (or heterogeneity), indirectness of evidence, and imprecision of the effect estimates and risk of publication bias [37]. Analyses were performed using RevMan (Version 5.1; Cochrane Collaboration, Oxford, UK) and Stata software (version 12.0; Stata Corporation, College Station, TX, USA).
Results
Study inclusion
The study inclusion process is illustrated in Fig. 1. Initially, 99 potentially relevant records were identified from the three searched databases, with 28 initially excluded due to duplication. A subsequent screening of the titles and abstracts led to the further exclusion of 50 studies, primarily because they did not align with the objectives of the meta-analysis. The full texts of the remaining 21 records were reviewed by two independent authors, resulting in the exclusion of 12 more studies for various reasons, as detailed in Fig. 1. Finally, nine cohort studies remained and were deemed appropriate for inclusion in the quantitative analysis [22–30].
Fig. 1.
Flowchart of database search and study inclusion
Overview of the study characteristics
Table 1 shows the summarized characteristics of the available studies included in the meta-analysis. Overall, one prospective cohort study [26] and eight retrospective studies [22–25, 27–30] were included in the meta-analysis. Because one study included two cohorts of patients with severe burn injury [25], these datasets were included in the meta-analysis independently. These studies were published from 2016 to 2024 and were conducted in China, the United States, Uruguay, India, and Korea, respectively. Overall 5268 patients with severe burns were included, with the mean ages of 31.4 to 53.6 years, and the proportion of men ranging from 51 to 88%. All of them excluded patients with comorbidities that could influence RDW levels, such as hematologic diseases, thalassemia, or use of iron, folic acid, or vitamin B12 supplements. Five of the cohorts included patients who were admitted to ICUs [22, 25, 27, 30], while the other five cohorts included patients in burn departments [23, 24, 26, 28, 29]. For each patient, RDW was measured within 24 h after admission and analyzed as exposure. The follow-up durations were within hospitalization in six cohorts [22, 24–26, 29], 60 days in one cohort [30], and 90 days in another three cohorts [23, 27, 28]. A total of 1203 (22.8%) patients died during follow-up. The difference of RDW at admission between nonsurvivors and survivors was reported in nine cohorts [22–27, 29, 30], and the RR for the risk of all-cause death between patients with a high versus a low RDW was reported in six cohorts [22, 23, 27–30]. The cutoff for defining a high RDW at admission was based on the receiver operating characteristic curve analysis in five studies [22, 27–30], and via the third tertile of RDW in another study [23]. Confounding factors such as age, sex, TBSA, inhalation injury, and comorbidities, etc. were adjusted to a varying degree when the association between RDW and the risk of all-cause death was estimated. The NOS scores of the included studies were six to nine, suggesting an overall moderate to good study quality (Table 2).
Table 1.
Characteristics of the included studies
| Study | Country | Design | Diangnosis | No. of patients | Mean age (years) | Men (%) | Timing of RDW measuring | Follow-up duration | Patients died | Outcomes reported | Variables matched or adjusted |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Guo et al. [22] | China | RC | Patients with severe burn admitted to ICU | 149 | 37.2 | 66.1 | At admission | Inhospital | 8 | Difference of RDW, and RR for mortality (cutoff: T3:T1–2, 14.5%) | Age, sex, PLT, HGB, and PCT at admission |
| Qiu et al. [23] | China | RC | Patients with severe burn (TBSA burned of ≥30%) | 610 | 45 | 73.4 | Within 1 days after admission | 90-day | 88 | Difference of RDW, and RR for mortality (cutoff: ROC curve analysis derived, 13.5%) | Age, sex, a high burn index, inhalation injury, MV, and surgery in the first week |
| Sen et al. [24] | USA | RC | Patients with severe burn (TBSA burned of ≥15%) | 191 | 45 | 88 | Within 1 day after admission | Inhospital | 23 | Difference of RDW | None |
| Angulo et al. [25] C1 | Uruguay | RC | Patients with severe burn admitted to ICU | 88 | 47 | 70.5 | Within 1 day after admission | Inhospital | 13 | Difference of RDW | None |
| Angulo et al. [25] C2 | Uruguay | RC | Patients with severe burn admitted to ICU | 95 | 46 | 66.3 | Within 1 day after admission | Inhospital | 27 | Difference of RDW | None |
| Karki et al. [26] | India | PC | Patients with severe burn (TBSA burned of ≥20%) | 157 | 31.4 | 51 | Within 1 day after admission | Inhospital | 94 | Difference of RDW | None |
| Park et al. [27] | Korea | RC | Patients with severe burn admitted to ICU | 731 | 53.6 | 83 | At admission | 90-day | 198 | Difference of RDW, and RR for mortality (cutoff: ROC curve analysis derived, 12.9%) | Age, sex, HTN, DM, TBSA, inhalation injury, HGB, PLT, ALB, and SCr |
| Lai et al. [28] | China | RC | Patients with severe burn (TBSA burned of ≥ 30%) | 342 | 44 | 73.7 | Within 1 day after admission | 90-day | 57 | RR for mortality (cutoff: ROC curve analysis derived, 13.1%) | Age, sex, BMI, TBSA, inhalation injury, transfer time, and etiology |
| Tang et al. [29] | China | RC | Patients with severe burn (TBSA burned of ≥ 30%) | 148 | 48.2 | 73 | Within 1 day after admission | Inhospital | 26 | Difference of RDW, and RR for mortality (cutoff: ROC curve analysis derived, 14.6%) | Age, sex, TBSA, and inhalation injury |
| Kim et al. [30] | Korea | RC | Patients with severe burn admitted to ICU | 2757 | 51.5 | 79.1 | At admission | 60-day | 669 | Difference of RDW, and RR for mortality (cutoff: ROC curve analysis derived, NR) | Age, sex, TBSA, and inhalation injury |
RDW red blood cell distribution width, RC retrospective cohort, PC prospective cohort, ICU intensive care unit, TBSA total body surface area, RR risk ratio, ROC receiver operating characteristic, T tertile, PLT platelet count, HGB hemoglobin, PCT procalcitonin, MV mechanical ventilation, HTN hypertension, DM diabetes mellitus, ALB albumin, SCr serum creatinine, BMI body mass index
Table 2.
Study quality evaluation via the Newcastle–Ottawa Scale
| Study | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome not present at baseline | Control for age and sex | Control for other confounding factors | Assessment of outcome | Enough long follow-up duration | Adequacy of follow-up of cohorts | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Guo et al. [22] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Qiu et al. [23] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Sen et al. [24] | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| Angulo et al. [25] C1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| Angulo et al. [25] C2 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| Karki et al. [26] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 |
| Park et al. [27] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Lai et al. [28] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Tang et al. [29] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Kim et al. [30] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
Difference of RDW at admission between nonsurvivors and survivors
Overall, the pooled results of the nine cohorts [22–27, 29, 30] showed a higher RDW at admission in nonsurvivors as compared to survivors of patients with severe burn injury (MD: 0.95%, 95% CI: 0.52–1.38, p < 0.001; I2 = 57%; Fig. 2A) with a moderate certainty of evidence (Table 3). The sensitivity analyses were performed by excluding one dataset at a time but did not significantly change the results (MD: 0.75–1.03%, p all < 0.05). In particular, omitting the study by Kim 2024 [30] showed similar results but significantly reduced the heterogeneity (MD: 0.75%, 95% CI: 0.44–1.05, p < 0.001; I2 = 0%). Further subgroup analyses showed similar results in patients admitted to ICU or burn department (p for subgroup difference = 0.42; Fig. 2B), in patients with mean ages ≤ or >45 years (p for subgroup difference = 0.21; Fig. 2C), in studies of men ≤ or >73% (p for subgroup difference = 0.36; Fig. 3A), in studies reporting inhospital mortality or 60-day/90-day mortality after burn injury (p for subgroup difference = 0.56; Fig. 3B), and in studies with NOS scores of 6–7 or 8–9 (p for subgroup difference = 0.55; Fig. 3C).
Fig. 2.
Forest plots for the meta-analysis comparing RDW at baseline between nonsurvivors and survivors of patients with severe burn injury; A forest plots for the overall meta-analysis; B forest plots for the subgroup analysis according to clinical settings; and C forest plots for the subgroup analysis according to the mean age of the patients
Table 3.
Summary of findings
| Results | Effect (95% CI) | Patient number (studies) | Certainty of the evidence (GRADE) | Comments |
|---|---|---|---|---|
| Red blood cell distribution width at admission and the short-term mortality of patients with severe burn injury | ||||
| Patients: adult patients (18 years or older) with severe burn injury; Exposure: RDW was analyzed within 24 h after admission; Outcome: short-term mortality | ||||
| Difference of RDW at baseline between nonsurvivors and survivors of patients with severe burn injury | MD 0.95 (0.52–1.38) | 4926 (8 studies) | Moderatea | RDW at admission is likely to be higher in nonsurvivors compared to survivors of patients with severe burn injuries |
| Association between RDW at baseline and the risk of short-term mortality in patients with severe burn injury | RR 1.76 (1.41–2.19) | 4737 (6 studies) | Moderatea | A high RDW at admission is likely to be associated with an increased risk of short-term mortality in patients with severe burn injuries |
GRADE Working Group grades of evidence; High certainty: We are very confident that the true effect lies close to that of the estimated effect. Moderate certainty: We are moderately confident in the estimated effect. The true effect is likely to be close to the estimated effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the estimated effect is limited: The true effect may be substantially different from the estimated effect. Very low certainty: We have very little confidence in the estimated effect. The true effect is likely to be substantially different from the estimated effect
CI confidence interval, MD mean difference, RR risk ratio, RDW Red blood cell distribution
aDowngraded one point as inconsistency due to substantial heterogeneity
Fig. 3.
Forest plots for the subgroup analyses comparing RDW at baseline between nonsurvivors and survivors of patients with severe burn injury; A forest plots for the subgroup analysis according to the proportion of men; B forest plots for the subgroup analysis according to follow-up durations; and C forest plots for the subgroup analysis according to the NOS scores
Association between RDW at admission and the risk of short-term death
The meta-analysis of the six cohorts [22, 24–26, 29] suggested a high RDW at admission was associated with an increased risk of short-term death in patients with severe burn injury (RR: 1.76, 95% CI: 1.41–2.19, p < 0.001; I2 = 77%; Fig. 4A) with a moderate certainty of evidence (Table 3). The sensitivity analyses by excluding one cohort of a time showed similar results (1.66–1.90, p all < 0.05). Further subgroup analyses according to study setting (p for subgroup difference = 0.54; Fig. 4B), mean age of the patients (p for subgroup difference = 0.05; Fig. 4C), proportion of men (p for subgroup difference = 0.53; Fig. 5A), follow-up duration (p for subgroup difference = 0.10; Fig. 5B), or study quality scores (p for subgroup difference = 0.17; Fig. 5C) did not significantly change the results.
Fig. 4.
Forest plots for the meta-analysis of the association between RDW at baseline and the risk of short-term mortality in patients with severe burn injury; A forest plots for the overall meta-analysis; B forest plots for the subgroup analysis according to clinical settings; and C forest plots for the subgroup analysis according to the mean age of the patients
Fig. 5.
Forest plots for the meta-analysis of the association between RDW at baseline and the risk of short-term mortality in patients with severe burn injury; A forest plots for the subgroup analysis according to the proportion of men; B forest plots for the subgroup analysis according to follow-up durations; and C forest plots for the subgroup analysis according to the NOS scores
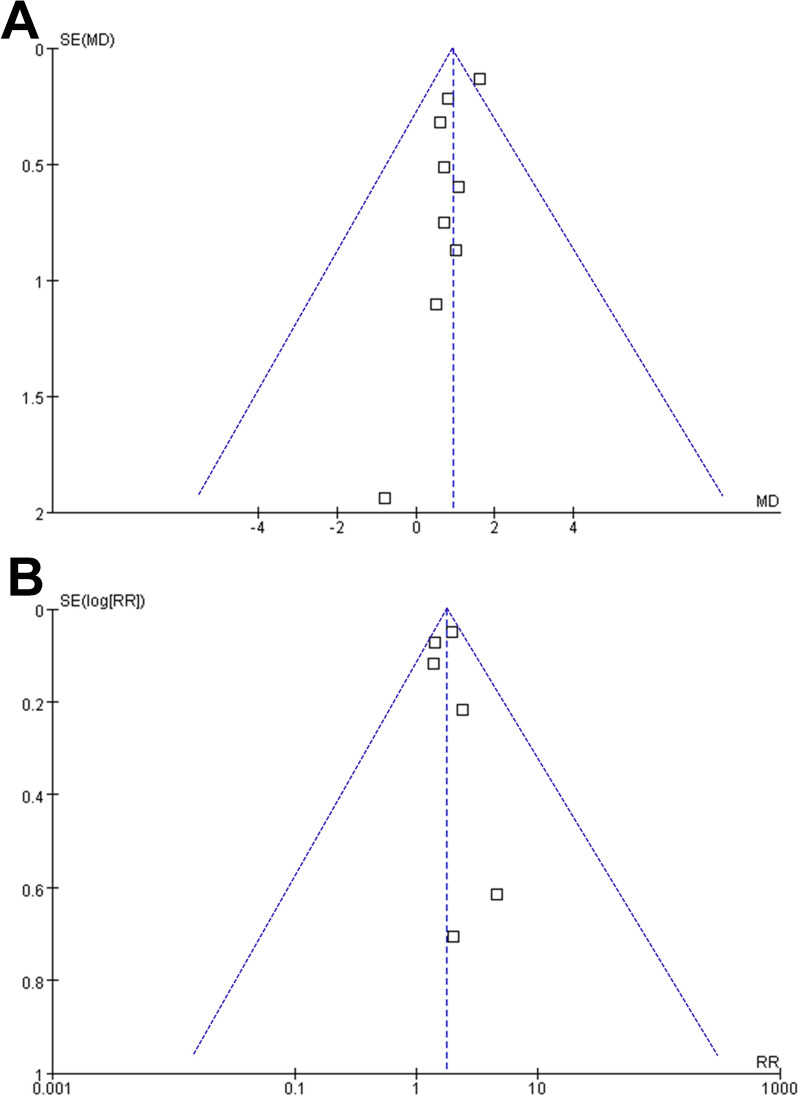
Publication bias
Upon visual inspection, the funnel plots for meta-analyses of the difference of RDW between non-survivors and survivors and RR for short-term death were symmetrical, indicating a low likelihood of publication bias (Fig. 6A, B). Additionally, Egger’s regression test results for the meta-analyses of the difference between RDW and RR for short-term death in patients with severe burn injury (p = 0.42 and 0.66, respectively) also supported this conclusion by suggesting a low risk of publication bias.
Fig. 6.
Funnel plots for the meta-analyses; A funnel plots for the meta-analysis comparing RDW at baseline between nonsurvivors and survivors of patients with severe burn injury; and B funnel plots for the meta-analysis of the association between RDW at baseline and the risk of short-term mortality in patients with severe burn injury
Discussion
This meta-analysis of nine cohort studies, encompassing over 5200 patients with severe burn injuries, revealed a significant association between red RDW at admission and short-term mortality. Our results demonstrated that patients who did not survive had higher RDW values at admission compared to survivors, with an MD of 0.95%. Furthermore, a higher RDW was associated with a 76% increased risk of short-term death in patients with severe burn injury. These findings suggest that RDW may serve as a useful and easily accessible biomarker for early risk stratification in this critically ill population.
The exact mechanisms underlying the association between elevated RDW and poor outcomes in patients with severe burns are not entirely understood, but several possibilities can be proposed. Severe burns trigger a systemic inflammatory response, characterized by the release of pro-inflammatory cytokines, oxidative stress, and alterations in erythropoiesis. These factors disrupt red blood cell homeostasis, leading to increased RDW [38]. Additionally, burn injuries can induce a hypermetabolic state, which exacerbates inflammation, accelerates red cell turnover, and contributes to an imbalance in red blood cell maturation [39]. The oxidative damage caused by these processes may impair erythrocyte membrane stability, further increasing RDW [40]. Elevated RDW could thus be a marker of the severity of the systemic inflammatory response, tissue hypoxia, and metabolic derangements, all of which contribute to poor prognosis in severe burn patients. Consistently, an increased RDW at admission has been related to multiple severe complications in burn patients, such as acute respiratory distress syndrome [41], acute kidney injury [42], and sepsis [30], all of which may at least partly explain its association with a high short-term mortality in these patients.
Subgroup analyses showed that the association between RDW and short-term mortality remained consistent across different clinical settings (ICU vs. burn departments), age groups, and patient populations with varying proportions of men. The absence of significant differences in RDW’s predictive value across these subgroups supports its robustness as a prognostic marker. Similarly, the lack of heterogeneity in outcomes based on follow-up duration (in-hospital vs. 60-day or 90-day mortality) suggests that RDW is a reliable indicator of early mortality, irrespective of the exact timing of death. However, the subgroup analysis based on study quality (NOS scores) did not indicate a significant impact on the overall findings, implying that the predictive value of RDW was not markedly affected by study design or quality. The high heterogeneity (I2 = 77%) observed in the analysis of RDW and the risk of short-term mortality may reflect differences in burn severity, patient management, and RDW cutoff values among the included studies, necessitating caution in interpreting these results.
The strengths of this meta-analysis lie in its comprehensive approach to synthesizing available evidence on the prognostic value of RDW in patients with severe burns. By including a large sample size from multiple geographic regions and utilizing predefined subgroup and sensitivity analyses, we have provided a detailed and robust evaluation of the association between RDW at admission with mortality in this population. Moreover, the consistency of our findings across subgroups adds further weight to the clinical relevance of RDW as a simple, accessible biomarker that could be integrated into routine clinical practice to enhance early risk assessment in patients with severe burn injuries. Additionally, the use of standardized methods for quality assessment and adherence to PRISMA guidelines ensures the reliability of our conclusions.
However, several limitations should be considered when interpreting the findings of this meta-analysis. First, we observed substantial heterogeneity among the included studies. Although all studies excluded patients with comorbidities that could influence RDW, this consistency does help mitigate confounding from these sources. However, only one study reported the cause of burn [29], which restricted our ability to perform subgroup analyses based on burn etiology. This limitation likely contributed to the observed heterogeneity and affected the precision of our findings. Future studies that include detailed data on burn cause and additional medical history would enable more refined analyses and improve the consistency of results. Addressing these factors would be essential for better understanding the relationship between RDW and short-term mortality in severe burn patients. Second, the majority of the included studies were retrospective in nature, which may introduce potential selection bias and confounding factors that were not fully accounted for, despite efforts to adjust for key variables such as age, sex, and burn severity [43]. Moreover, there was variability in the cutoff values used to define high RDW levels across studies, which could have contributed to the heterogeneity observed in the results. Additionally, while we attempted to investigate the effects of various confounders through subgroup analysis, other unmeasured factors may also influence RDW and its association with mortality. Finally, it is important to acknowledge that the statistical robustness of our publication bias assessment is limited due to the small number of studies included in our meta-analysis. While we performed a publication bias assessment, the results should be interpreted with caution, as the limited number of studies may not adequately represent the potential for publication bias. Future research with a larger number of studies may provide more reliable insights into the publication bias and its impact on the findings of RDW as a prognostic marker in burn patients.
In addition to the limitations mentioned earlier, it is crucial to consider potential confounding factors that could affect the association between RDW and mortality in burn patients. While our analysis focused on studies that excluded patients with known hematologic disorders and those receiving supplements such as iron, folic acid, or vitamin B12, other confounding variables may still exist. For instance, factors such as the severity of the burn injury [44], the presence of concurrent infections [45], pre-existing comorbidities [46], nutritional status [47], or interventions received during hospitalization [48] could influence both RDW levels and mortality outcomes. Moreover, inflammatory responses following burn injuries can significantly impact RDW, complicating the interpretation of its prognostic value [23]. A more comprehensive understanding of these confounding factors is essential for accurately assessing the relationship between RDW and mortality in burn patients. Future studies should aim to control for these variables to clarify RDW’s role as a reliable prognostic marker.
From a clinical perspective, our findings suggest that RDW, an inexpensive and widely available laboratory parameter, could be incorporated into the early management of patients with severe burn injuries [49]. Given its association with increased mortality risk, RDW may help clinicians identify high-risk patients who may benefit from closer monitoring or more aggressive interventions. However, RDW should not be used in isolation for prognostication. Future research should aim to explore the combined use of RDW with other markers of inflammation, metabolic derangement, and tissue damage to develop more comprehensive risk models for burn patients. Additionally, further studies are needed to investigate the dynamic changes in RDW over time and how they relate to long-term outcomes, as well as to determine the optimal cutoff values for RDW in this context.
Conclusions
To sum up, this meta-analysis provides evidence that elevated RDW at admission is significantly associated with short-term mortality in patients with severe burn injuries. These findings highlight the potential role of RDW as a convenient and accessible biomarker for early risk stratification. Future studies should focus on refining the prognostic utility of RDW by integrating it with other clinical and laboratory variables, as well as validating its use in prospective, multicenter cohorts.
Supplementary Information
Author contributions
Qing Cao and Lutao Yang designed the study. Qing Cao and Xiafei He performed database search, study identification, and study quality evaluation. Qing Cao, Xiuhuang Chen, Xing Han, and Lutao Yang performed statistical analysis and interpreted the results. Qing Cao drafted the manuscript. All authors revised the manuscript and approved the submission.
Funding
No funding was received for this study.
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smolle C, Cambiaso-Daniel J, Forbes AA, Wurzer P, Hundeshagen G, Branski LK, et al. Recent trends in burn epidemiology worldwide: a systematic review. Burns. 2017;43(2):249–57. 10.1016/j.burns.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S. Burn injury. Nat Rev Dis Primers. 2020;6(1):11. 10.1038/s41572-020-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuo M, Muramatsu K, Matsuda S, Fushimi K, Kaizuka Y, Kamochi M. Age-dependent influence of premorbid underweight status on mortality in severe burn patients: an administrative database study. Burns. 2021;47(6):1314–21. 10.1016/j.burns.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Nitzschke S, Offodile AC 2nd, Cauley RP, Frankel JE, Beam A, Elias KM, et al. Long term mortality in critically ill burn survivors. Burns. 2017;43(6):1155–62. 10.1016/j.burns.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Foppiani JA, Weidman A, Hernandez Alvarez A, Valentine L, Bustos VP, Galinaud C, et al. A meta-analysis of the mortality and the prevalence of burn complications in western populations. J Burn Care Res. 2024;45(4):932–44. 10.1093/jbcr/irae064. [DOI] [PubMed] [Google Scholar]
- 6.Tejiram S, Romanowski KS, Palmieri TL. Initial management of severe burn injury. Curr Opin Crit Care. 2019;25(6):647–52. 10.1097/MCC.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 7.Dobson GP, Morris JL, Letson HL. Pathophysiology of severe burn injuries: new therapeutic opportunities from a systems perspective. J Burn Care Res. 2024;45(4):1041–50. 10.1093/jbcr/irae049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zielinski M, Wroblewski P, Kozielski J. Prognostic factors in patients with burns. Anaesthesiol Intensive Ther. 2020;52(4):330–5. 10.5114/ait.2020.97497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuznetsova TA, Andryukov BG, Besednova NN. Modern aspects of burn injury immunopathogenesis and prognostic immunobiochemical markers (mini-review). BioTech (Basel). 2022;11(2):18. 10.3390/biotech11020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52(2):86–105. 10.3109/10408363.2014.992064. [DOI] [PubMed] [Google Scholar]
- 11.Hu ZD, Lippi G, Montagnana M. Diagnostic and prognostic value of red blood cell distribution width in sepsis: a narrative review. Clin Biochem. 2020;77:1–6. 10.1016/j.clinbiochem.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Parizadeh SM, Jafarzadeh-Esfehani R, Bahreyni A, Ghandehari M, Shafiee M, Rahmani F, et al. The diagnostic and prognostic value of red cell distribution width in cardiovascular disease; current status and prospective. BioFactors. 2019;45(4):507–16. 10.1002/biof.1518. [DOI] [PubMed] [Google Scholar]
- 13.Xanthopoulos A, Giamouzis G, Dimos A, Skoularigki E, Starling RC, Skoularigis J, et al. Red blood cell distribution width in heart failure: pathophysiology, prognostic role, controversies and dilemmas. J Clin Med. 2022;11(7):1951. 10.3390/jcm11071951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang T, Li J, Lin Y, Yang H, Cao S. Association between red blood cell distribution width and all-cause mortality in chronic kidney disease patients: a systematic review and meta-analysis. Arch Med Res. 2017;48(4):378–85. 10.1016/j.arcmed.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Montagnana M, Danese E. Red cell distribution width and cancer. Ann Transl Med. 2016;4(20):399. 10.21037/atm.2016.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo R, Hu J, Jiang L, Zhang M. Prognostic value of red blood cell distribution width in non-cardiovascular critically or acutely patients: a systematic review. PLoS ONE. 2016;11(12): e0167000. 10.1371/journal.pone.0167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichtman MA. Red cell distribution width as a bellwether of prognosis. Blood Cells Mol Dis. 2024;109: 102884. 10.1016/j.bcmd.2024.102884. [DOI] [PubMed] [Google Scholar]
- 18.Burgess M, Valdera F, Varon D, Kankuri E, Nuutila K. The immune and regenerative response to burn injury. Cells. 2022;11(19):3073. 10.3390/cells11193073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parihar A, Parihar MS, Milner S, Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns. 2008;34(1):6–17. 10.1016/j.burns.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Nielson CB, Duethman NC, Howard JM, Moncure M, Wood JG. Burns: pathophysiology of systemic complications and current management. J Burn Care Res. 2017;38(1):e469–81. 10.1097/BCR.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark A, Imran J, Madni T, Wolf SE. Nutrition and metabolism in burn patients. Burns Trauma. 2017;5:11. 10.1186/s41038-017-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo J, Qin Q, Hu H, Zhou D, Sun Y, Deng A. Red cell distribution width (RDW) as a prognostic tool in burn patients. Clin Lab. 2016;62(10):1973–8. 10.7754/Clin.Lab.2016.160222. [DOI] [PubMed] [Google Scholar]
- 23.Qiu L, Chen C, Li SJ, Wang C, Guo F, Peszel A, et al. Prognostic values of red blood cell distribution width, platelet count, and red cell distribution width-to-platelet ratio for severe burn injury. Sci Rep. 2017;7(1):13720. 10.1038/s41598-017-13151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sen S, Hsei L, Tran N, Romanowski K, Palmieri T, Greenhalgh D, et al. Early clinical complete blood count changes in severe burn injuries. Burns. 2019;45(1):97–102. 10.1016/j.burns.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Angulo M, Moreno L, Aramendi I, Dos Santos G, Cabrera J, Burghi G. Complete blood count and derived indices: evolution pattern and prognostic value in adult burned patients. J Burn Care Res. 2020;41(6):1260–6. 10.1093/jbcr/iraa091. [DOI] [PubMed] [Google Scholar]
- 26.Karki D, Dawson L, Muthukumar V, Aggarwal N. Blood indices in adult acute burn: a window into milieu interieur - the future biomarkers? Ann Burns Fire Disasters. 2022;35(1):46–54. [PMC free article] [PubMed] [Google Scholar]
- 27.Park JH, Cho Y, Shin D, Choi SS. Prediction of mortality after burn surgery in critically Ill burn patients using machine learning models. J Pers Med. 2022;12(8):1293. 10.3390/jpm12081293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai H, Cai Z, Wu S, Zhang W, Chen J, Wu G. An Increase in admission RDW value is associated with excess short-term mortality rates in patients with severe burns. Appl Biochem Biotechnol. 2023;195(5):3217–28. 10.1007/s12010-022-04302-y. [DOI] [PubMed] [Google Scholar]
- 29.Tang XD, Qiu L, Wang F, Liu S, Lü XW, Chen XL. Diagnostic value of procalcitonin and red blood cell distribution width at admission on the prognosis of patients with severe burns: a retrospective analysis. Int Wound J. 2023;20(9):3708–16. 10.1111/iwj.14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim M, Kym D, Park J, Yoon J, Cho YS, Hur J, et al. Big data insights into the diagnostic values of CBC parameters for sepsis and septic shock in burn patients: a retrospective study. Sci Rep. 2024;14(1):800. 10.1038/s41598-023-50695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372: n160. 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. The Cochrane Collaboration. 2021; www.training.cochrane.org/handbook.
- 34.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2010; http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 35.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 36.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 38.Korkmaz HI, Flokstra G, Waasdorp M, Pijpe A, Papendorp SG, de Jong E, et al. The complexity of the post-burn immune response: an overview of the associated local and systemic complications. Cells. 2023;12(3):345. 10.3390/cells12030345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knuth CM, Auger C, Jeschke MG. Burn-induced hypermetabolism and skeletal muscle dysfunction. Am J Physiol Cell Physiol. 2021;321(1):C58–71. 10.1152/ajpcell.00106.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orrico F, Laurance S, Lopez AC, Lefevre SD, Thomson L, Moller MN, et al. Oxidative stress in healthy and pathological red blood cells. Biomolecules. 2023;13(8):1262. 10.3390/biom13081262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao CH, Wan J, Liu H, Qiu L, Wang F, Liu S, et al. Red blood cell distribution width is an independent risk factor in the prediction of acute respiratory distress syndrome after severe burns. Burns. 2019;45(5):1158–63. 10.1016/j.burns.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Park J, Kym D, Kim M, Cho YS, Hur J, Chun W, et al. Pioneering predictions of AKI and AKIN severity in burn patients: a comprehensive CBC approach. Sci Rep. 2024;14(1):675. 10.1038/s41598-024-51253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zealley I. Retrospective studies - utility and caveats. J R Coll Physicians Edinb. 2021;51(1):106–10. 10.4997/JRCPE.2021.133. [DOI] [PubMed] [Google Scholar]
- 44.Cassidy JT, Phillips M, Fatovich D, Duke J, Edgar D, Wood F. Developing a burn injury severity score (BISS): adding age and total body surface area burned to the injury severity score (ISS) improves mortality concordance. Burns. 2014;40(5):805–13. 10.1016/j.burns.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Kiley JL, Greenhalgh DG. Infections in burn patients. Surg Clin North Am. 2023;103(3):427–37. 10.1016/j.suc.2023.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Thombs BD, Singh VA, Halonen J, Diallo A, Milner SM. The effects of preexisting medical comorbidities on mortality and length of hospital stay in acute burn injury: evidence from a national sample of 31,338 adult patients. Ann Surg. 2007;245(4):629–34. 10.1097/01.sla.0000250422.36168.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grudziak J, Snock C, Zalinga T, Banda W, Gallaher J, Purcell L, et al. Pre-burn malnutrition increases operative mortality in burn patients who undergo early excision and grafting in a sub-Saharan African burn unit. Burns. 2018;44(3):692–9. 10.1016/j.burns.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Britton GW, Wiggins AR, Halgas BJ, Cancio LC, Chung KK. Critical care of the burn patient. Surg Clin North Am. 2023;103(3):415–26. 10.1016/j.suc.2023.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. 2009;169(5):515–23. 10.1001/archinternmed.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.