Abstract
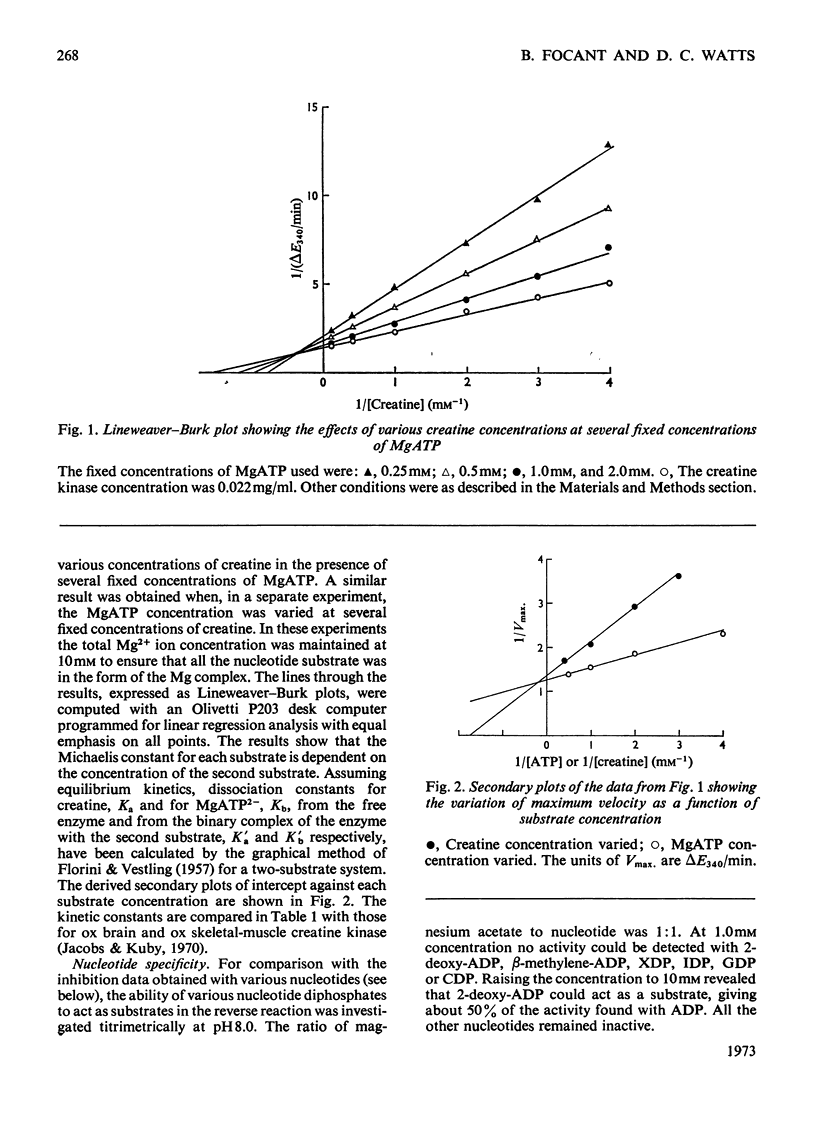
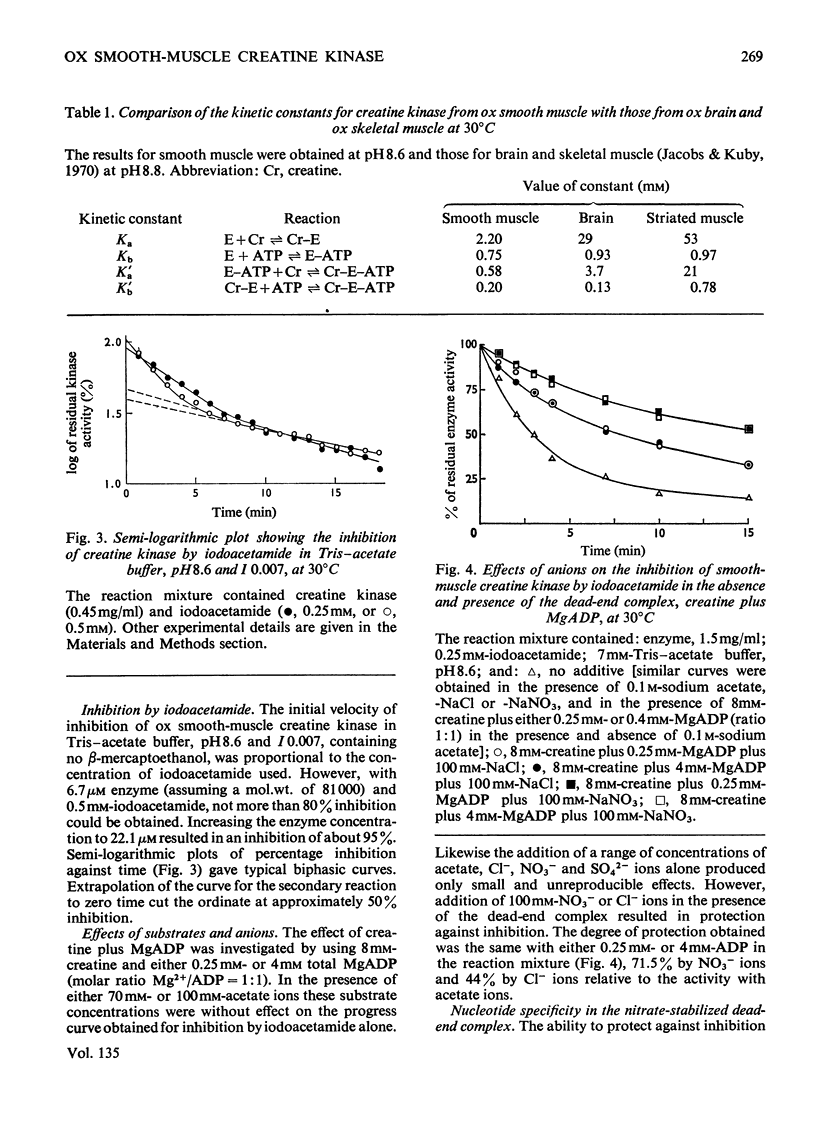
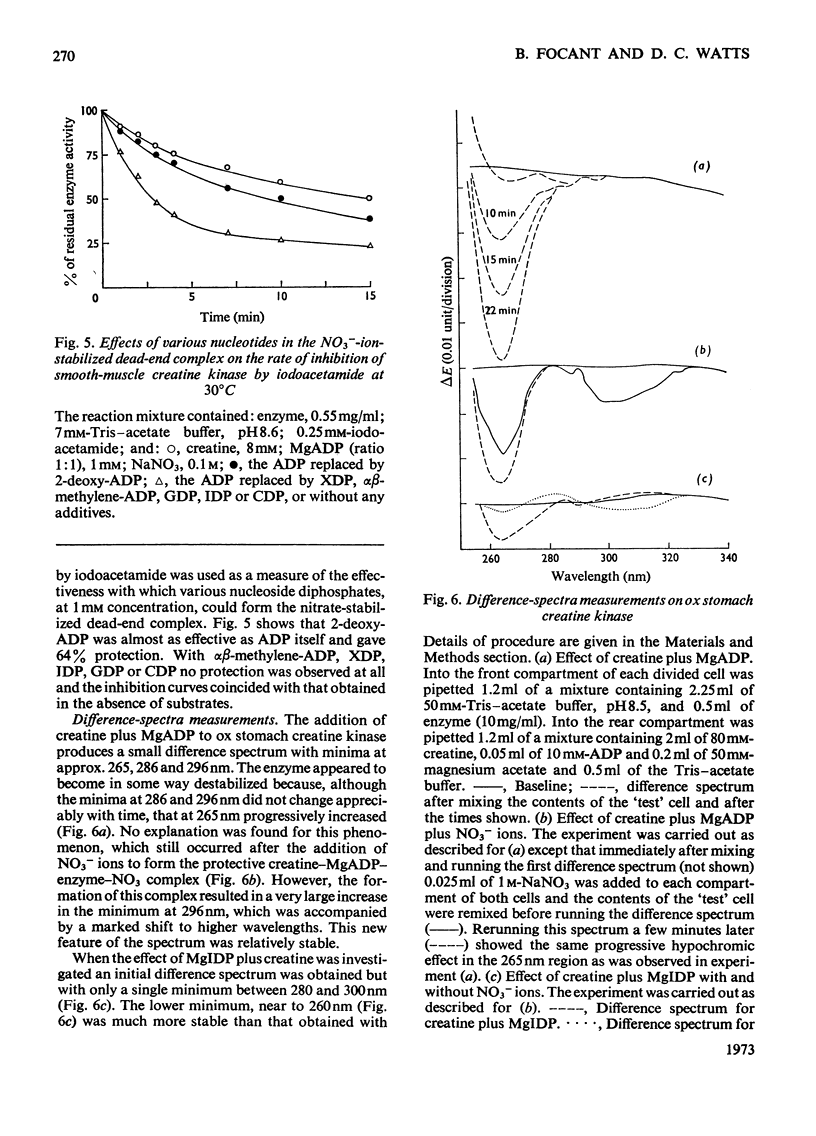
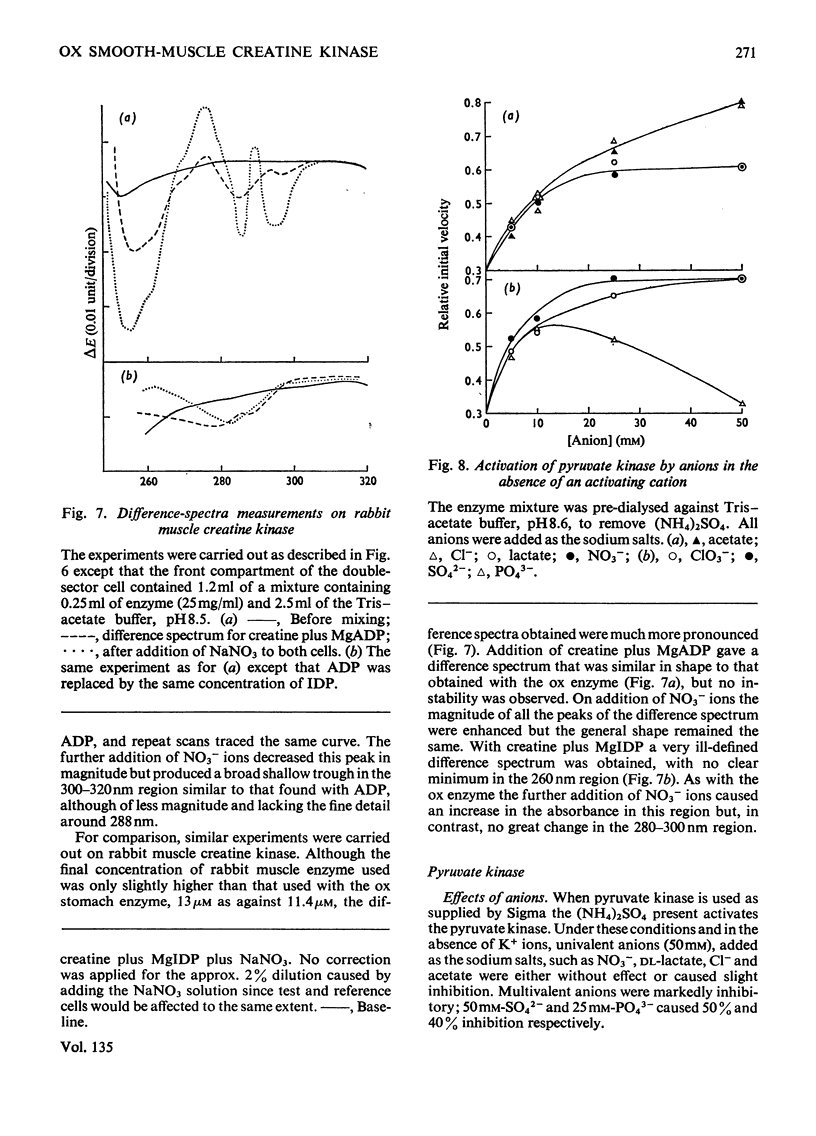
1. An improved purification procedure for the brain-type creatine kinase from ox smooth muscle is described. 2. Michaelis constants show the characteristic dependence on the concentration of the second substrate: the derived constants are compared with those for the enzyme from ox brain. 3. Inhibition by iodoacetamide gives a biphasic curve and the total extent of the reaction depends on the enzyme concentration. The rate of inhibition at pH8.6 is not affected by creatine plus MgADP or by a range of simple anions. Addition of creatine plus MgADP plus either NO3− or Cl− ions affords 71.5 and 44% protection respectively. ADP could be replaced by 2-deoxy-ADP but not by αβ-methylene ADP, XDP, IDP, GDP or CDP. Nucleotides that did not protect would not act as substrates. 4. Difference-spectra measurements support the interpretation that addition of NO3− ions to the enzyme–creatine–MgADP complex causes further conformational changes in the enzyme accompanying the formation of a stable quaternary enzyme–creatine–NO3−–MgADP complex that simulates an intermediate stage in the transphosphorylation reaction. However, the enzyme structure is partially destabilized by quaternary-complex formation. IDP apparently fails to act as a substrate because it cannot induce the necessary conformational change. This behaviour is compared with that of rabbit skeletal muscle creatine kinase. 5. With pyruvate kinase from rabbit muscle, anions activate in the absence of an activating cation and either inhibit or have no effect in its presence. 6. Both activation and inhibition were competitive with respect to the substrate, phosphoenolpyruvate, and curved double-reciprocal plots were obtained. The results may be interpreted in terms of co-operatively induced conformational changes, and this is supported by difference-spectra measurements. However, the Hill coefficient of 1 was not significantly altered. 7. Inhibition by lactate plus pyruvate is less than additive, indicating that both bind to the same site on the enzyme, whereas that by lactate plus NO3− is additive, indicating binding at separate sites. It is inferred that a quaternary enzyme–pyruvate–NO3−–MgADP complex could form, but no evidence was obtained to suggest that it possessed special properties comparable with those found with creatine kinase. The implications of these findings for the unidirectional nature of the mechanism of pyruvate kinase is discussed. 8. Lactate or α-hydroxybutyrate could not act instead of pyruvate to form a stable quaternary complex, although both activate the K+-free enzyme. Only the former inhibits the K+-activated enzyme. The activating cation both lowers the Michaelis constant for phosphoenolpyruvate and tightens up the specificity of its binding site.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton R. S., Laws J. F., Thomson A. R. Alkylation of bovine brain creatine kinase. Biochem J. 1970 Aug;118(5):903–904. doi: 10.1042/bj1180903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGER A., RICHTERICH R., AEBI H. DIE HETEROGENITAET DER KREATIN-KINASE. Biochem Z. 1964 Jan 28;339:305–314. [PubMed] [Google Scholar]
- Dawson D. M., Eppenberger H. M., Kaplan N. O. Creatine kinase: evidence for a dimeric structure. Biochem Biophys Res Commun. 1965 Nov 22;21(4):346–353. doi: 10.1016/0006-291x(65)90200-7. [DOI] [PubMed] [Google Scholar]
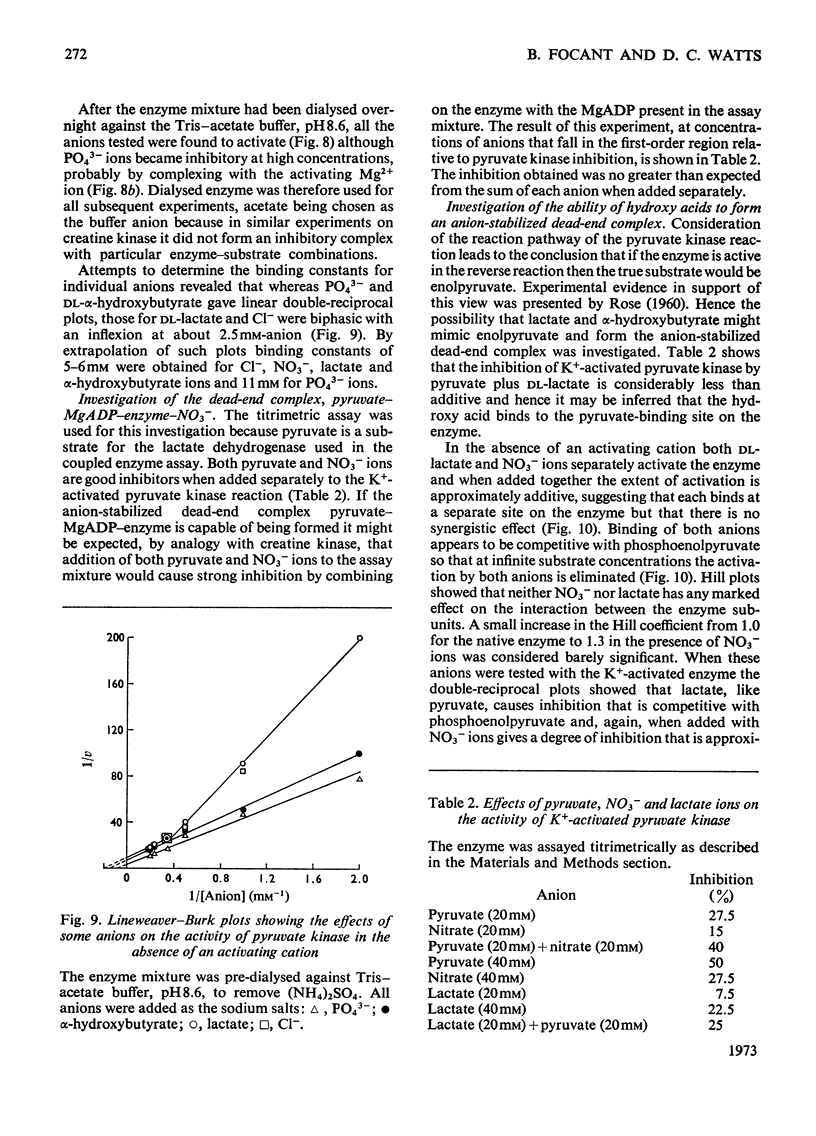
- EPPENBERGER H. M., EPPENBERGER M., RICHTERICH R., AEBI H. THE ONTOGENY OF CREATINE KINASE ISOZYMES. Dev Biol. 1964 Aug;10:1–16. doi: 10.1016/0012-1606(64)90002-8. [DOI] [PubMed] [Google Scholar]
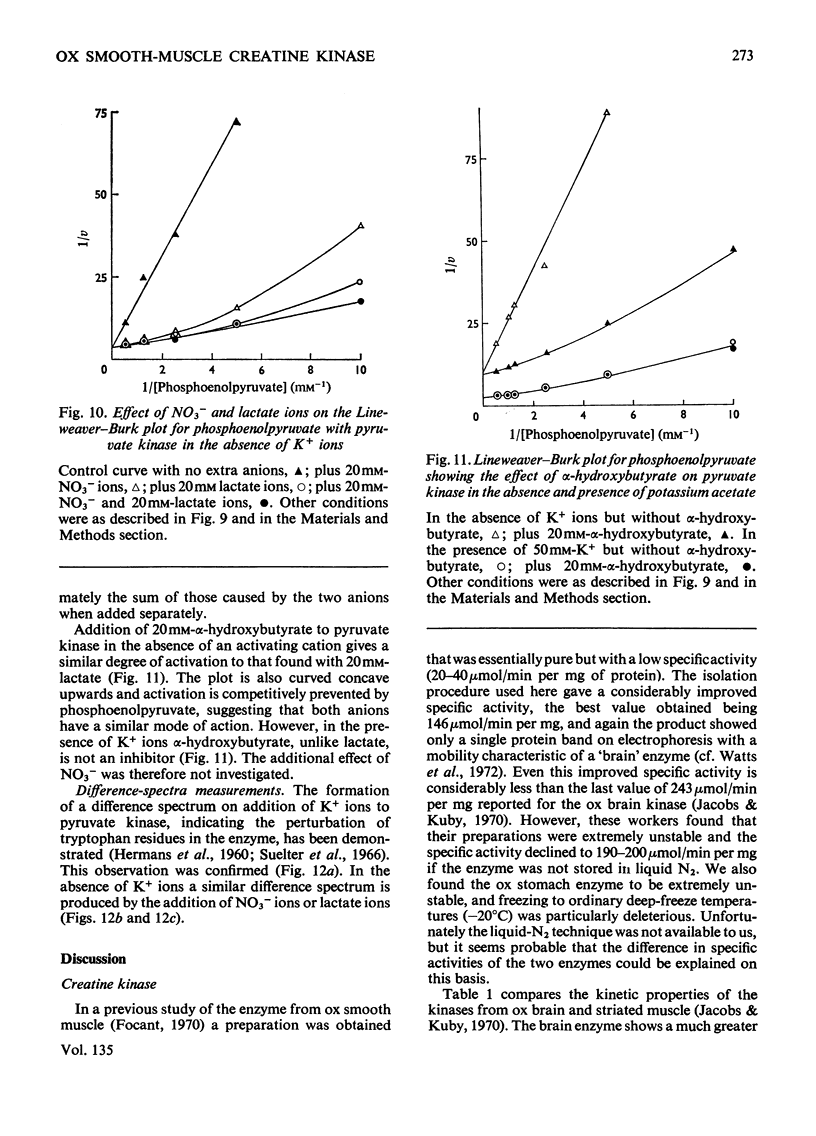
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Eppenberger H. M., Dawson D. M., Kaplan N. O. The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. J Biol Chem. 1967 Jan 25;242(2):204–209. [PubMed] [Google Scholar]
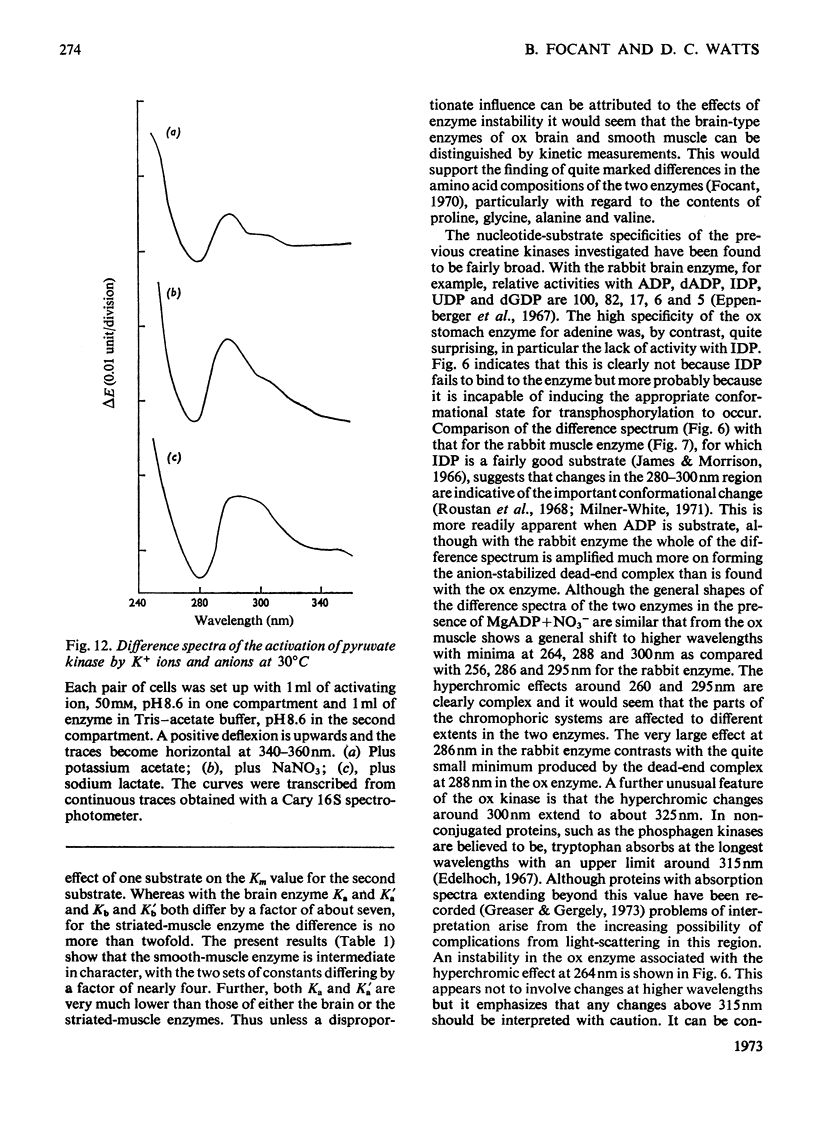
- FLORINI J. R., VESTLING C. S. Graphical determination of the dissociation constants for two-substrate enzyme systems. Biochim Biophys Acta. 1957 Sep;25(3):575–578. doi: 10.1016/0006-3002(57)90529-2. [DOI] [PubMed] [Google Scholar]
- Focant Bruno. Isolement et proprietes de la creatine-kinase de muscle lisse de boeuf. FEBS Lett. 1970 Sep 18;10(1):57–61. doi: 10.1016/0014-5793(70)80415-x. [DOI] [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Purification and properties of the components from troponin. J Biol Chem. 1973 Mar 25;248(6):2125–2133. [PubMed] [Google Scholar]
- HERMANS J., Jr, DONOVAN J. W., SCHERAGA H. A. Thermodynamic data from difference spectra. J Biol Chem. 1960 Jan;235:91–93. [PubMed] [Google Scholar]
- Hooton B. T. Creatine kinase isoenzymes and the role of thiol groups in the enzymic mechanism. Biochemistry. 1968 Jun;7(6):2063–2071. doi: 10.1021/bi00846a007. [DOI] [PubMed] [Google Scholar]
- Jacobs H. K., Kuby S. A. Studies on adenosine triphosphate transphosphorylases. IX. Kinetic properties of the crystalline adenosine triphosphate-creatine transphosphorylase from calf brain. J Biol Chem. 1970 Jul 10;245(13):3305–3314. [PubMed] [Google Scholar]
- James E., Morrison J. F. The reaction of nucleotide substrate analogues with denosine triphosphate-creatine phosphotransferase. J Biol Chem. 1966 Oct 25;241(20):4758–4770. [PubMed] [Google Scholar]
- Kumudavalli I., Moreland B. H., Watts D. C. Properties and reaction with iodoacetamide of adenosine 5'-triphosphate-creatine phosphotransferase from human skeletal muscle. Further evidence about the role of the essential thiol group in relation to the mechanism of action. Biochem J. 1970 Apr;117(3):513–523. doi: 10.1042/bj1170513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-White E. J., Watts D. C. Inhibition of adenosine 5'-triphosphate-creatine phosphotransferase by substrate-anion complexes. Evidence for the transition-state organization of the catalytic site. Biochem J. 1971 May;122(5):727–740. doi: 10.1042/bj1220727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNARD A. M., HASS L. F., JACOBSEN D. D., BOYER P. D. The correlation of reaction kinetics and substrate binding with the mechanism of pyruvate kinase. J Biol Chem. 1961 Aug;236:2277–2283. [PubMed] [Google Scholar]
- ROSE I. A. Studies on the enolization of pyruvate by pyruvate kinase. J Biol Chem. 1960 Apr;235:1170–1177. [PubMed] [Google Scholar]
- Reed G. H., Cohn M. Structural changes induced by substrates and anions at the active site of creatine kinase. Electron paramagnetic resonance and nuclear magnetic relaxation rate studies of the manganous complexes. J Biol Chem. 1972 May 25;247(10):3073–3081. [PubMed] [Google Scholar]
- Roustan C., Kassab R., Pradel L. A., van Thoai N. Interaction des ATP: guanidine phosphotransférases avec leurs substrats, étudiée par spectrophotometrie différentielle. Biochim Biophys Acta. 1968 Oct 8;167(2):326–338. doi: 10.1016/0005-2744(68)90212-x. [DOI] [PubMed] [Google Scholar]
- Simonarson B., Watts D. C. Purification and properties of adenosine triphosphate-creatine phosphotransferase from muscle of the dogfish Scylliorhinus canicula. Biochem J. 1972 Aug;128(5):1241–1253. doi: 10.1042/bj1281241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suelter C. H., Singleton R., Jr, Kayne F. J., Arrington S., Glass J., Mildvan A. S. Stuies on the interaction of substrate and monovalent and divalent cations with pyruvate kinase. Biochemistry. 1966 Jan;5(1):131–139. doi: 10.1021/bi00865a017. [DOI] [PubMed] [Google Scholar]
- TANZER M. L., GILVARG C. Creatine and creatine kinase measurement. J Biol Chem. 1959 Dec;234:3201–3204. [PubMed] [Google Scholar]
- TIETZ A., OCHOA S. Fluorokinase and pyruvic kinase. Arch Biochem Biophys. 1958 Dec;78(2):477–493. doi: 10.1016/0003-9861(58)90372-2. [DOI] [PubMed] [Google Scholar]
- WOOD T. Adenosine triphosphate-creatine phosphotransferase from ox brain: purification and isolation. Biochem J. 1963 Jun;87:453–462. doi: 10.1042/bj0870453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D. C., Focant B., Moreland B. M., Watts R. L. Formation of a hybrid enzyme between echinoderm arginine kinase and mammalian creatine kinase. Nat New Biol. 1972 May 10;237(71):51–53. doi: 10.1038/newbio237051a0. [DOI] [PubMed] [Google Scholar]


