Abstract
Numerous existing studies reported the negative impacts of outdoor nitrogen dioxide (NO2) on respiratory mortality. However, the evidence of related high-risk populations was considerably limited, especially associated with ages, causes of death, and district-level characteristics. In addition, most earlier studies were based on monitored areas, thus previous risk estimates of NO2 could be biased to provide nationwide risk estimates and high-risk populations. Therefore, this study performed a nationwide time-stratified case-crossover study to evaluate the association between short-term ambient NO2 and respiratory mortality in South Korea (2015–2019). A machine learning-ensemble daily NO2 prediction model was used to cover unmonitored areas. To examine high-risk populations, we assessed NO2 risk estimates by age group, sex, cause of mortality, and district-level characteristics. In the total population, NO2 was weakly associated with increased mortality risk due to respiratory disease (OR [odds ratio]: 1.011, 95% CI [confidence interval]: 0.995–1.027), and the association became evident only in individuals aged 80 y or older (1.022, 1.000–1.044), especially related to pneumonia. Further, in people aged 60–69 years, NO2 was marginally associated with mortality for chronic lower respiratory diseases. Lower district-level socioeconomic status and medical services were marginally related to higher respiratory mortality risks related to NO2. The excess respiratory mortality fractions and YLL (year of life lost) attributable to NO2 were 4.13% and 93,851.63 years, and around 70% of the excess deaths were due to noncompliance with the World Health Organization air quality guidelines (daily average NO2 > 25 µg/m3). This study provides evidence for high-risk populations and the appropriateness of target-specific action plans against NO2. In addition, based on the excess death estimates, we suggest stricter NO2 standards are required.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-21048-w.
Keywords: Nitrogen dioxide, Respiratory mortality, High-risk populations
Introduction
NO2 has been known as a common outdoor air pollutant that has hazardous impacts on the respiratory system [22]. NO2 is involved in the secondary formation of fine particulate matter [31], and it is one of the highly reactive nitrogen oxides (NOx) from high-temperature combustion processes. Traffic, power plants, and off-road equipment are the major contributors to ambient NO2 concentrations [10, 17, 37], thus NO2 concentrations have been considered a proxy of traffic-related air pollutant levels [1].
Historically, multiple human or animal toxicology studies [20] identified that NO2 is a major risk factor for dysfunctions in the respiratory system including respiratory infections, [11], asthma [1], and decreasing pulmonary function [40]. Existing in vitro and experimental studies revealed that exposure to NO2 increases oxidative stress, inflammatory response to inhaled allergen in bronchi, and the reactive oxygen and nitrogen species [3, 4]. NO2 inhalation also can lead to lung malfunctions, accelerate pulmonary infections, and degenerate lung diseases through a pro-inflammatory response (which is an automatic immune response) [20].
Furthermore, the association between outdoor NO2 and respiratory mortality is less understood, compared to particulate matter and ozone [37]. First, because many previous studies have investigated the association between NO2 and all-cause or non-accidental mortality [31, 32], epidemiological evidence for the NO2 risk of respiratory mortality with more specific causes of death was limited, despite plausible biological mechanisms we mentioned earlier, such as oxidative stress, inflammatory response, and increased pulmonary infections. Second, since monitoring stations were generally operated in metropolitan urban areas or industrial areas, many relevant studies have investigated well-monitored areas [31, 32]. This point suggests that previous NO2 risk estimates might be biased due to limited information on less-monitored [16]. Third, to our knowledge, few studies have examined spatial differences in NO2-related respiratory mortality risks depending on regional characteristics with large data [37], although examining regional differences could be important for effective and righteous public health resource allocation and mobilization. Lastly, studies on the excess mortality burden attributable to NO2 were limited.
To address these gaps in knowledge, we performed a nationwide time-stratified case-crossover study, with the national respiratory mortality records and a machine learning-based NO2 prediction model from 2015 through 2019 in South Korea. To reveal detailed high-risk populations, this study examined the heterogeneous associations between NO2 and respiratory mortality by age group, cause of death, sex, and district-level characteristics. Further, we estimated the excess mortality burdens due to the entire NO2 and due to NO2 concentrations above the 2021 World Health Organization (WHO) air quality guidelines (daily average NO2 > 25 µg/m3).
Methodology
Study populations
This study included all people residing in South Korea who died of respiratory disease and were registered in Statistics Korea. We obtained individual-level respiratory mortality data covering all 247 districts of inland South Korea (except Jeju and Ulleung islands) from 2015 to 2019. The International Classification of Disease 10th Revision (ICD-10) was used to define respiratory death in our study (ICD-10: J00–J99).
However, due to the identification problem, Statistics Korea only provides the causes of respiratory death based on the three-character “category of the diagnosis” of ICD-10. Thus, we could collect three specific causes of respiratory death: pneumonia (J12–J18), chronic lower respiratory diseases [CLR] (J40–J47), and other respiratory diseases (respiratory diseases that were not classified into the above three specific diseases). We were unable to analyze the mortality for influenza separately because of the small sample size (less than 3,000 cases, annually). Thus, we classified influenza into the “other respiratory disease” category.
Further, to examine the different associations between NO2 and mortality by age, we classified ages at death into five categories: total (all ages), individuals aged 0–59 y, 60–69 y, 70–79 y, and those aged 80 or older.
Study design
This study adopted a time-stratified case-crossover design to estimate the association between NO2 and respiratory mortality. This approach has been recognized as a standardized method to estimate the association between short-term exposure to air pollution and health outcomes [21, 25] by controlling for time-invariant (or less variant) confounders and time trends. Detailed explanations of the study design are in the Supplementary Materials (“1. Study design”).
Air pollution and environmental data
We collected nationwide district-level modeled NO2 (ppm) and PM2.5 (μg/m3) from machine-learning ensemble prediction models from 2015 to 2019 (study years). These models were developed by the AiMS-CREATE team and their models were used in previous published studies [24, 33]. More detailed information on the ensemble prediction model is provided in the Supplemental Materials, “2. Air pollution prediction models”. The prediction models for daily NO2 and PM2.5 showed good performances during the study period: cross-validated R2 values of 0.95 and 0.94 (Table S1). Although previous studies used a relatively short lag period of NO2 (e.g., lag 0–1) as the main exposure [6, 12, 15, 31], several studies reported that the impacts of air pollution on respiratory outcome could have longer lag days; some existing studies suggested the plausible mechanisms that exacerbation of respiratory infections would be expected to take more time [38, 39] and complex interactions between air pollutants and antioxidants in the lung lining fluid compartment could contribute to the delayed deterioration of respiratory reactions [34]. Therefore, we tried to find an optimal moving average period of NO2 (up to a week) based on the lowest Akaike Information Criterion (AIC). As a result, the average NO2 from the same day to five lag days (lag 0–5) was selected as the main exposure.
We also collected district-level 2 m air temperature (K), relative humidity (%), and precipitation (m) from the ERA-5 Land global reanalysis dataset [7]. This ERA-5 dataset has a horizontal resolution of 0.1° × 0.1°, thus we assigned it to each district by averaging the values at grid cells with centroid points inside the boundary of each district [35].
District indicators
To examine the effect modifications by district-level characteristics, we obtained data on six indicators covering urbanicity, accessibility to parks, health behaviors, and socioeconomic levels. Detailed information on data sources and explanations of the selected district indicators is in the Supplementary Materials (“3. District-level indicators”). Briefly, we first collected population density (persons per km2) to address different urbanicity [26]. Second, to examine the urban green spaces [8, 19], the accessibility to parks in the living sphere (hereafter, park accessibility; distance to neighboring parks from each grid) was collected. Third, we obtained two variables related to regional health behaviors: the proportions of current smoking and obesity [23]. To examine the effect modification by regional socioeconomic status, we collected the proportion of people who received the National Basic Livelihood Security and the proportion of people who could not visit the medical facilities when they wanted within a year [23]. We classified each indicator into three categories (high, middle, and low) based on their tertiles for the statistical analysis [8].
Statistical analysis
We created a time-stratified case-crossover dataset for each age group, cause of death, and sex (a total of 40 combinations in the main analysis: five age categories, sexes, and four respiratory deaths). For each case day (i.e., date of death), we set matched control days as days with the same day of the week and month within the same year. This time-stratified self-matching controls the confounding from time-invariant variables or variables that do not change substantially in a month, such as body mass index, and diet [45]. Further, the bidirectional time-stratified matching also controlled for potential confounding for the day of the week, seasonality, and long-term trends of NO2 and outcomes [28]. We performed a conditional logistic regression to evaluate the associations between short-term exposure to NO2 and respiratory mortality in each case-crossover dataset.
As the main analysis, we performed NO2 single pollutant models. To control for potential confounding, we adjusted for relative humidity, precipitation (as linear terms with lag 0–1), and temperatures (lag 0–3) with a natural cubic spline with six degrees of freedom based on previous large studies on air pollution and mortality [6, 29, 31]; however, it was also based on our grid search for optimal modeling specifications based on the lowest Akaike Information Criterion. We measured the association between short-term NO2 and respiratory mortality with odd ratios (ORs) for a 0.01 ppm (10 ppb) increase in lag 0–5 NO2. In addition, to check the potential nonlinear relationship between NO2 (0–5) and respiratory mortality, we conducted a conditional logistic regression with a natural cubic spline (including equally spaced three internal knots for NO2), instead of the linear term of NO2 in the main model.
To find high-risk districts, we performed analyses to examine which district-level characteristics modify the association between NO2 and respiratory mortality. For each case-crossover dataset divided by causes of death, we added an interaction term between NO2 and categorized district indicators (tertiles) and repeated the main analysis. The time-stratified self-matching design already controlled the potential confounding from district-level characteristics that did not change within a month, thus the interaction was included in a conditional logistic model individually for each indicator.
We performed sensitivity analyses to examine the consistency of our results in relation to different modeling specifications and two pollutant models (PM2.5 adjusted). Across all statistical analyses, R software (version 4.2.1) with package “survival” was used.
Excess respiratory mortality and Years of life lost from mortality attributable to NO2
To assess the excess respiratory mortality burden due to short-term exposure to NO2, we translated our estimated ORs into excess deaths and YLL (years of life lost from mortality) attributable to NO2. Detailed processes for the excess respiratory mortality burden estimation are in the Supplementary Materials (“4. Estimation of the excess respiratory mortality burden attributable to short-term exposure to ambient NO2”). Briefly, we first allocated the individual YLLs based on the national life expectancy estimation in 2019 (Statistics Korea 2024). Based on the estimated ORs from the main analysis, we calculated daily and total excess deaths and YLL, and the total excess deaths ratio with the total number of respiratory deaths provide the total excess fraction (%) of respiratory deaths attributable to short-term NO2 exposures. Further, to assess the excess mortality burden due to non-compliance with the current 2021 WHO guidelines, we calculated excess respiratory deaths and YLL only for the subset of days with NO2 levels above (i.e. non-compliance) the WHO air quality guidelines (daily average NO2 > 25 µg/m3).
Results
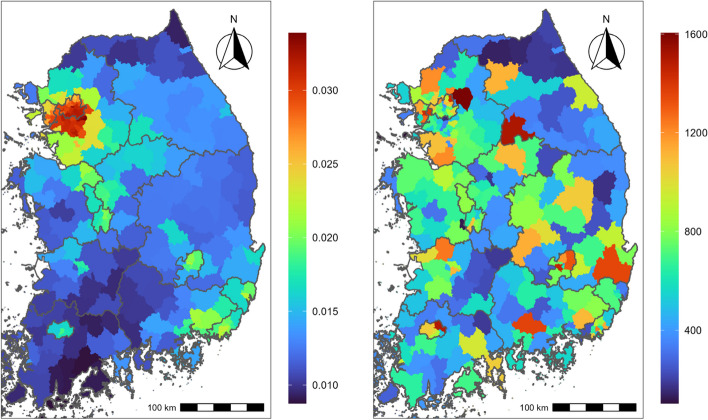
Table 1 shows descriptive statistics of respiratory mortality during the study period. During the study period, a total of 161,059 mortality cases were included in this study. Among causes of respiratory death, pneumonia, CLR, and other respiratory diseases showed 95,201 (59.1%), 33,478 (20.8%), and 32,380 (20.1%) cases, individually. Percentages for the causes of respiratory death were calculated as a proportion of the total number of respiratory disease cases. Individuals aged 80 y or older showed the largest mortality counts for (total) respiratory disease (103,271; 55.7%) and males showed higher counts (89,687) than females (71,371). Figure 1 presents the average geographical distribution of average NO2 (left) during the study period (0.012 ppm on average) and total respiratory death counts (right).
Table 1.
Descriptive statistics on respiratory mortality data the study used (2015–2019)
| Causes of death | Age categories | Case | % |
|---|---|---|---|
| Respiratory disease | Total | 161,059 | 100.0 |
| 0–59 y | 6,396 | 4.0 | |
| 60–69 y | 11,741 | 7.3 | |
| 70–79 y | 39,628 | 24.6 | |
| 80 y and older | 103,271 | 64.1 | |
| Females | 71,372 | 44.3 | |
| Males | 89,687 | 55.7 | |
| Pneumonia | Total | 95,201 | 59.1 |
| 0–59 y | 3,709 | 2.3 | |
| 60–69 y | 5,847 | 3.6 | |
| 70–79 y | 21,100 | 13.1 | |
| 80 y and older | 64,531 | 40.1 | |
| Females | 45,529 | 28.3 | |
| Males | 49,672 | 30.8 | |
| Chronic lower respiratory diseases | Total | 33,478 | 20.8 |
| 0–59 y | 884 | 0.5 | |
| 60–69 y | 2580 | 1.6 | |
| 70–79 y | 8964 | 5.6 | |
| 80 y and older | 21,043 | 13.1 | |
| Females | 12,248 | 7.6 | |
| Males | 21,230 | 13.2 | |
| Other respiratory diseases | Total | 32,380 | 20.1 |
| 0–59 y | 1803 | 1.1 | |
| 60–69 y | 3314 | 2.1 | |
| 70–79 y | 9564 | 5.9 | |
| 80 y and older | 17,697 | 11.0 | |
| Females | 13,595 | 8.4 | |
| Males | 18,785 | 11.7 |
Fig. 1.
Geographical distributions of the district-level averages of daily average NO2 (ppm [left]) and total death counts [right] in South Korea from 2015 through 2019. NO2: nitrogen dioxide
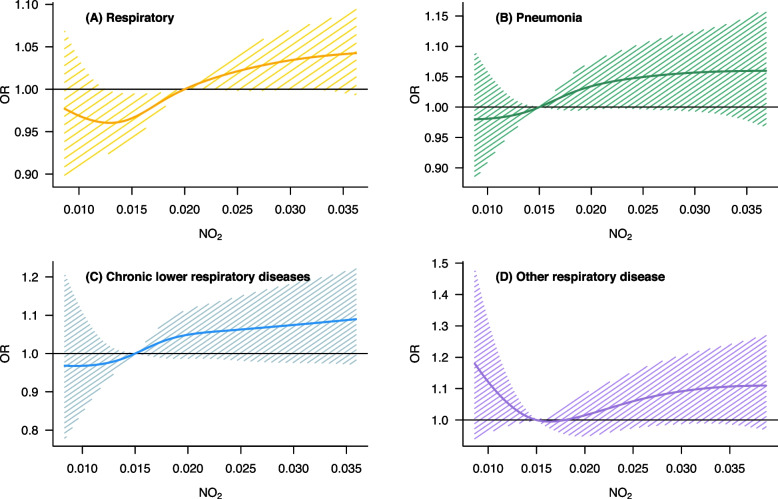
Figure 2 exhibits a nonlinear association between NO2 and respiratory mortalities. Except for mortality due to other respiratory diseases, mortality due to respiratory diseases had an approximately linear association with short-term exposure to NO2. Mortality due to other respiratory diseases also showed a positively linear association with NO2 after around 0.015 ppm.
Fig. 2.
Nonlinear associations between exposure to NO2 (lag 0–5) and respiratory mortalities. A total respiratory deaths, B Deaths due to pneumonia, C Deaths due to chronic lower respiratory diseases, D Deaths due to other respiratory diseases (not classified into pneumonia or chronic lower respiratory-related deaths). NO2: Nitrogen dioxide (ppm)
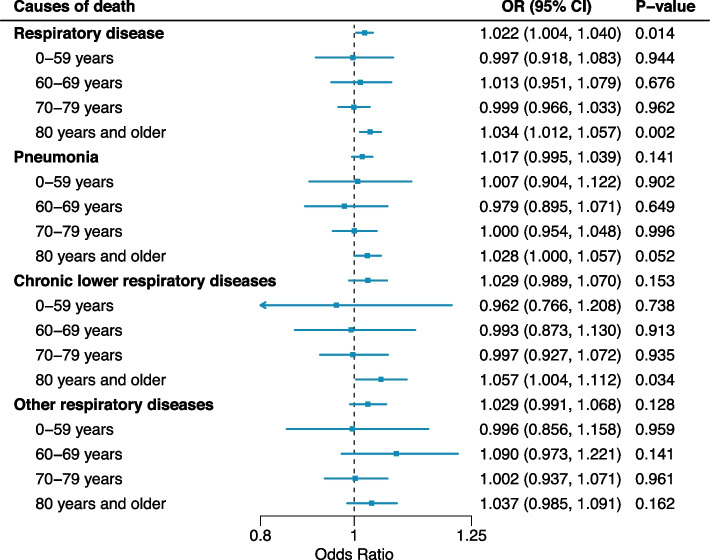
Figure 3 presents the cause-specific associations between NO2 and respiratory mortality in the total age and by age group. In the total age, the associations between NO2 and respiratory mortality were observed, with an OR of 1.022 with 95% CIs: 1.004–1.040. The association with respiratory disease was not evident in people aged less than 80 y, and those aged 80 y or older only showed an association with NO2: OR: 1.034 (95% CI: 1.012–1.057). Other respiratory disease deaths (pneumonia, CLR, and other respiratory diseases) also showed the similar age pattern: individuals aged 80 y or older showed the highest ORs. Figure S1 displays the age-specific association between NO2 and respiratory mortality by sex. Based on the point estimates, we could not observe risk differences between females and males.
Fig. 3.
Associations between short-term NO2 exposure (lag 0–5) and respiratory mortality by age group and cause of respiratory mortality. NO2: nitrogen dioxide. Odd ratios (ORs) for a 0.01 ppm (10 ppb) increase in NO2
Figure 4 shows the associations between NO2 and respiratory death depending on district indicator categories in the total population. P-values in Fig. 3 indicate the statistical test results on the effect modification (i.e. differences in ORs for middle or high levels compared to OR for low levels). We could not find statistically evident effect modifications by district characteristic for the total respiratory deaths. While the association between NO2 and pneumonia death was lower in high density areas than in low density areas (P-value: 0.060). Except for it, we could not find a consistent effect modification.
Fig. 4.
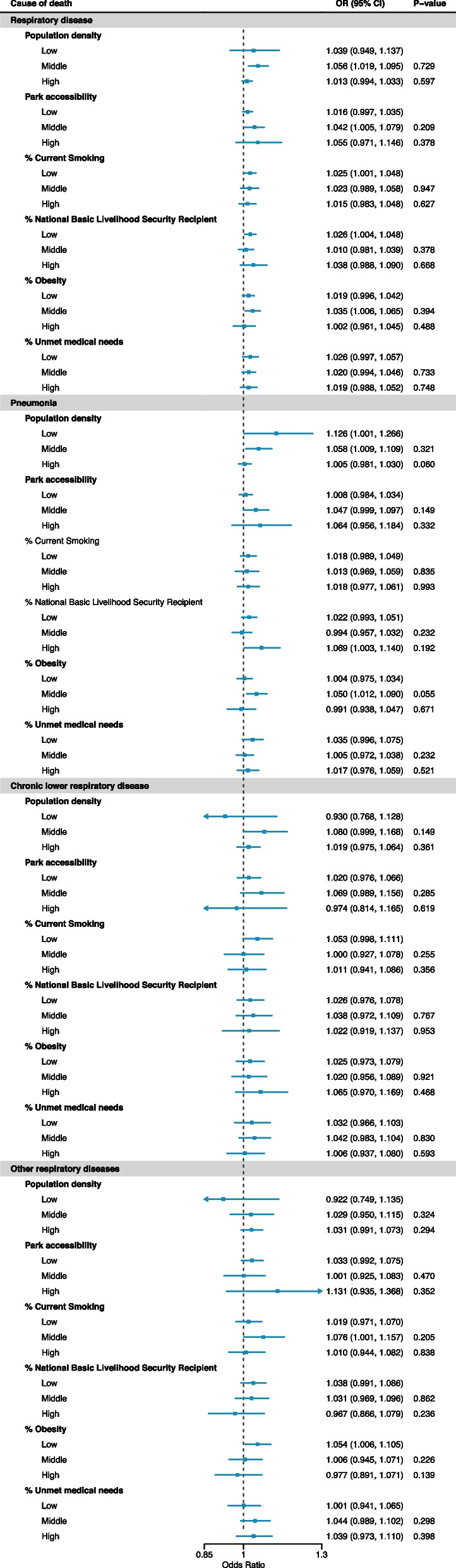
Effect modifications by district-level indicators in the NO2 (lag 0–5)-respiratory mortality risk in the total population. P-values indicate the statistical test results on the effect modification (i.e. magnitude of differences in estimates for middle or high-levels compared to low-level; not for the hypothesis that OR is one). %: proportion. NO2: nitrogen dioxide. Odd ratios (ORs) for a 0.01 ppm (10 ppb) increase in NO2
Figure 5 displays the effect modifications by district-level indications in the associations between NO2 and respiratory death depending on two age groups (individuals aged 50–59 y and aged 80 or older). We could not find a consistent effect modification pattern between the two age groups. The marginal effect modification of the proportion of National Basic Livelihood Security Recipient was observed only in individuals aged 80 or older, especially related to pneumonia and CLR deaths. The corresponding results to Fig. 4 for people aged 60–69 y and 70–79 y are exhibited in Figure S2, and we could not observe evident differences between the two age groups. In addition, although we could not find pronounced differences in effect modification patterns between sexes, the effect modifications by the proportion of Unmet medical needs were marginally more prominent in females than in males based on the point estimates (Figure S3).
Fig. 5.
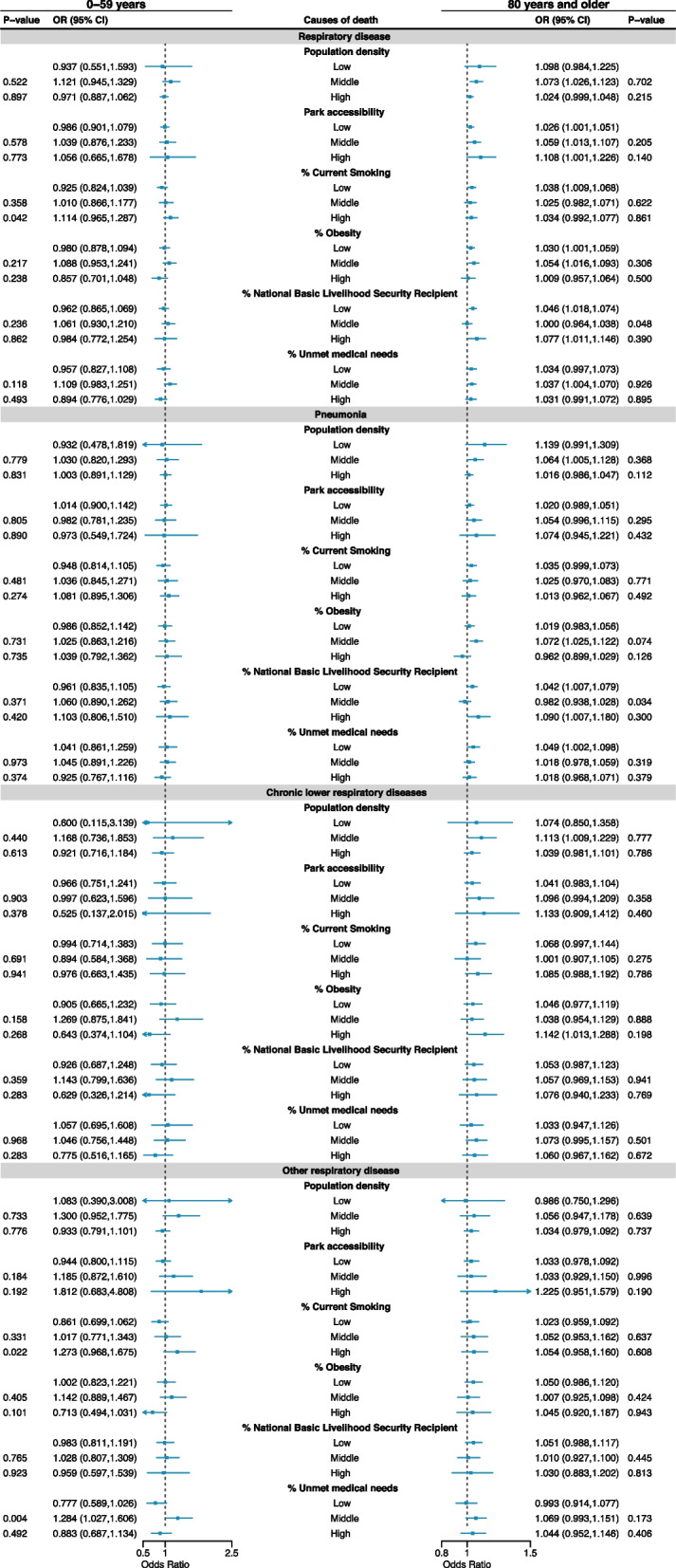
Effect modifications by district-level indicators in the NO2 (lag 0–5)-respiratory mortality risk in the individuals aged 0–59 y and aged 80 y or older. P-values indicate the statistical test results on the effect modification (i.e. magnitude of differences in estimates for middle or high-levels compared to low-level; not for the hypothesis that OR is one). %: proportion. NO2: nitrogen dioxide. Odd ratios (ORs) for a 0.01 ppm (10 ppb) increase in NO2
Table 2 displays the estimated excess mortality and YLL attributable to short-term NO2 exposures by cause of death. For respiratory mortality, the excess mortality fraction attributable to NO2 was 4.13 (95% CI: 0.87–7.40). Based on the point estimates, the excess fraction was higher in CLR mortality (5.29%) than pneumonia mortality in the total age. Further, the excess respiratory mortality fraction attributable to NO2 due to non-compliance with WHO air quality guidelines was 3.06%, and it accounted for over 60% of the total excess respiratory mortality attributable to NO2. Table S2 reports the excess mortality numbers attributable to NO2; the absolute number of excess deaths was the highest in pneumonia deaths due to the large numbers of pneumonia deaths. Table 2 also shows the excess YLL attributable to NO2, and the excess YLL was 93,851.63 years (95% CI: 19,779.11 years–167,983.69 years) for respiratory mortality in the total population, and it was the highest for pneumonia death (42,261.11 years, −13,775.96.24 years–97,815.11 years) compared to other causes of respiratory death.
Table 2.
Excess death fractions and YLL (Year of Life Lost from mortality) attributable to ambient short-term exposure to NO2 (lag 0–5) for respiratory mortalities. NO2: nitrogen dioxide
| Cause of death | All-range of PM2.5 | WHO guideline compliance | |||
|---|---|---|---|---|---|
| Age group | Excess deaths (%) | Excess YLL (years) | Excess deaths (%) | Excess YLL (years) | |
| Respiratory disease | Total |
4.13 (0.87–7.4) |
93,851.63 (19,779.11–167,983.69) |
3.06 (0.65–5.44) |
70,137.1 (14,924.97–124,721.11) |
| 0–59 years |
−0.86 (−18.39–14.72) |
−19,527.33 (−418,028.33–334,285.78) |
−0.77 (−14.43–10.63) |
−17,708.82 (−330,805.7–243,721.42) |
|
| 60–69 years |
2.44 (−10.09–14.01) |
55,505.05 (−229,293.12–317,974.14) |
1.75 (−7.76–10.13) |
40,197.35 (−177,779.19–232,242.61) |
|
| 70–79 years |
−0.18 (−7.12–6.56) |
−4045.92 (−161,835.04–148961.15) |
−0.16 (−5.43–4.84) |
−3555.95 (−124,559.71–110,827.91) |
|
| 80 y and older |
6.38 (2.36–10.37) |
144,936.7 (53,639.82–235,570.93) |
4.7 (1.76–7.57) |
107,715.31 (40,326.26–173,614.0) |
|
| Pneumonia | Total |
3.25 (−1.06–7.51) |
42,261.11 (−13,775.96–97,815.11) |
2.4 (−0.79–5.51) |
31,446.79 (−10,397.91–72,265.19) |
| 0–59 years |
0.8 (−22.4–20.45) |
10,366.55 (−291,683.25–266,162.39) |
0.38 (−17.72–14.53) |
5001.6 (−232,121.31–190,382.81) |
|
| 60–69 years |
−4.6 (−24.91–13.15) |
−59,917.22 (−324,379.29–171,133.55) |
−3.65 (−19.83–9.51) |
−47,859.08 (−259,737.78–124,663.67) |
|
| 70–79 years |
−0.08 (−9.78–9.1) |
−1081.76 (,318.91–118,431.39) |
−0.1 (−7.5–6.65) |
−1355.59 (−98,207.76–87,150.48) |
|
| 80 y and older |
5.16 (0.01–10.22) |
67,144.59 (127.83–133,002.25) |
3.8 (0.01–7.45) |
49,743.69 (96.23–97,599.13) |
|
| Chronic lower respiratory diseases | Total |
5.29 (−1.99–12.31) |
24,599.6 (−9280.38–57,233.47) |
3.74 (−1.45–8.61) |
17,634.43 (−6812.12–40,567.73) |
| 0–59 years |
−10.83 (−71.44–30.64) |
−50,521.6 (−333,313.62–142,297.7) |
−9.22 (−61.22–20.51) |
−43,598.38 (−289,370.71–96,513.75) |
|
| 60–69 years |
−2.17 (−30.78–21.26) |
−10,150.65 (−143,404.53–98,794.91) |
−1.88 (−23.93–14.56) |
−8872.69 (−112,947.0–68525.47) |
|
| 70–79 years |
−0.77 (−15.82–12.87) |
−3606.25 (−73,670.91–59,853.0) |
−0.65 (−11.86–8.99) |
−3066.53 (−55,941.99–42,366.33) |
|
| 80 y and older |
9.89 (0.87–18.41) |
45,973.81 (4030.89–85,569.0) |
6.93 (0.62–12.69) |
32,627.65 (2938.14–59,761.22) |
|
| Other respiratory diseases | Total |
5.59 (−1.63–12.55) |
28,089.73 (−8171.77–63,042.9) |
4.28 (−1.28–9.5) |
21,697.38 (−6459.24–48,134.97) |
| 0–59 years | −2.04(−37.99–25.92) |
−10,262.43 (−191,068.05–130197.1) |
−2.1 (−32.56–18.99) |
−10,590.46 (−164,773.03–96204.4) |
|
| 60–69 years | 15.27(−5.76–32.91) |
76,697.6 (−28,953.94–165,229.39) |
11.31 (−4.56–23.69) |
57,318.75 (−23,117.37–120,027.21) |
|
| 70–79 years | 0.16(−14.33–13.35) |
801.43 (−72,059.09–67063.9) |
0.03 (−11.6–10.09) |
172.12 (−58,739.01–51104.78) |
|
| 80 y and older | 6.92(−2.89–16.15) |
34,780.85 (−14,535.22–81,143.28) |
5.27 (−2.28–12.12) |
26,711.78 (−11,524.58–61,410.34) |
|
Lastly, in the sensitivity analyses (Table S3), we could observe that our risk estimates in the total and by age groups were generally less affected by different modeling specifications.
Discussion
This study assessed the nationwide association between short-term exposure to NO2 and respiratory mortality in South Korea from 2015 through 2019. To our knowledge, this is the first and largest nationwide study addressing the association between NO2 and respiratory mortality as well as the excess respiratory mortality burden due to NO2 in South Korea. We found that the association between NO2 and respiratory disease was the strongest in people aged 80 y or older compared to other age groups, especially for pneumonia death. Although the statistical evidence was weak, we found marginal effect modifications by certain district-level characteristics. Particularly, a higher proportion of National Nasic Liveliuhood Security Recipient was marginally associated with higher NO2-respiratory mortality risk, and the association was slightly more evident in people aged 80 y or older than other age groups. Lastly, we estimated the excess respiratory mortality burden attributable to short-term NO2 and found that over 60% of the excess burden attributable to NO2 was related to non-compliance with the current WHO air quality guidelines.
Our findings are generally consistent with previous studies. A multi-country study with a large dataset including 5.5 million mortality cases showed that short-term NO2 was associated with increased respiratory mortality risk [31]. One European multi-city study also revealed the short-term impact of NO2 on increased respiratory mortality [38]. A systemic review study including 87 research articles from multiple continents reported that short-term NO2 and mortality reported that a 0.01 ppm increase in NO2 was associated with a 2.05% increase in respiratory mortality [43]. Our study estimated around a 1.1% increase in respiratory mortality per 0.01 ppm increase in NO2. However, it should be carefully interpreted because study periods and regions were heterogeneous between our study and the review study.
One of the novel findings of this study was examining the different associations between NO2 and respiratory mortality by age group and the cause of respiratory death. We found that total respiratory mortality risk estimates related to NO2 were the highest in people aged 80 or older, and the association was not generally observed in people less than 80 y. Whereas previous studies reported that the short-term NO2 risks for respiratory disease-related mortalities became more evident in people aged around 65 y [9, 38, 42], which is much lower than our high-risk age. These discrepancies should be interpreted carefully because the traditional concept of “the elderly” might have changed in recent years. Due to the rapid advances in medicine and science, the overall health of people has consistently improved during recent decades, globally [14]. Although the evidence of this study is highly limited, we conjecture that the differences between previous studies and our findings regarding age group might be related to the research period of each study. The three studies we mentioned above have been performed from 1990–1997 in 30 European cities [38], 1997–2007 in Santiago [9], and 1998–2001 in Hong Kong [42]. However, a recent multi-country study involving Jiangsu (China), California (the United States), Central-southern Italy, and Germany from 2015–2019 reported no NO2 risk on mortality or substantially small risk estimates in people aged less than 75 y, and the association between NO2 and mortality became more pronounced after 75 y in all countries [30]. These results are generally consistent with our findings with the same study period: 2015–2019. Of course, although it should be interpreted with extreme caution because there might be a lot of potential confounders, we cautiously suggest that the concepts of high-risk populations regarding “the elderly” might need to be re-discussed in-depth from the perspective of health risks related to air pollution.
In addition, we found that the association between NO2 and mortality for other respiratory disease was more pronounced in people aged 60–69, although the statistical evidence was insufficient. According to the ICD-10 category, the other respiratory disease mortality we used included mortality for influenza, acute respiratory infections, etc. Previous animal and human studies have identified that NO2 inhalation negatively affects these diseases [5, 47]. Although this study is limited in providing exact evidence, previous studies might suggest some hypotheses: existing studies revealed that individuals with early COPD were related to an increased risk of acute respiratory hospitalization and early death [13, 44]. In addition, based on the Korean National Health and Nutrition Examination Survey in 2018 [23], the current smoking rate was much higher in individuals aged 60–69 (14.9%) than in individuals aged 70 y or older (6.6%). Thus, we cautiously surmised that relatively young elderly with risk factors for severe acute respiratory diseases, like influenza, could be more vulnerable to respiratory mortality risks related to NO2 compared to other older age groups because of poorer pulmonary health conditions and health behaviors, although more studies are required.
Our findings related to effect modification by district-level characteristics also should be interpreted carefully. Although the statistical evidence was relatively weak, we found areas with low population density showed a higher NO2-related respiratory mortality risk in total respiratory and pneumonia deaths. Along with a high average age of people living in low-density areas (i.e., rural areas) [23], we conjecture that poorer accessibility to medical facilities in low-density areas could be associated with a higher vulnerability to environmental stressors, which was addressed in a previous study [26]. Further, although the finding was also statistically weak, we observed that areas with the highest proportion of Basic Livelihood Security Recipient needs generally exhibited the highest NO2-respiratory mortality risk, based on the point estimates. However, although the statistical evidence was substantially weak, areas with a low proportion of Unmet medical needs generally showed a higher NO2-respiratory mortality risk compared to areas with a high proportion of Unmet medical needs, especially for pneumonia death. Although this study could provide limited evidence, we carefully conjecture that there might be an ecological bias. In South Korea, the proportion of Unmet medical needs was generally lower in urban cities, although some metropolitan areas (Ulsan and Incheon metropolitan cities) and more urbanized regions (Gyeonggi and Gyeongnam provinces) had a higher proportion of Unmet medical needs [23] due to their large population size. In other words, the reason for the higher NO2-related respiratory death in areas with a low proportion of Unmet medical needs might be confounded by the urbanicity level; however, further studies are required. In addition, we found that the proportion of National Basic Livelihood Security Recipients modified the NO2-respiratory mortality risk in individuals aged 80 or older, especially related to pneumonia death [23]. Several studies have reported that lower socioeconomic status and poorer health behaviors are closely linked to a higher risk of severe respiratory diseases [2, 27, 36]. Thus, although ecological bias from the district-level data could be considered, our findings could suggest that improving community-level socioeconomic status can mitigate respiratory deaths related to NO2, especially in “older seniors” (aged 80 y or older) who likely have lower immunity than other younger groups [46].
In addition, our results on the excess respiratory deaths and YLL attributable to short-term NO2 provide important implications for public health and environmental policies. We found that the excess respiratory mortality fraction attributable to NO2 related to non-compliance with the current WHO air quality guidelines (daily average NO2 > 25 µg/m3) accounted for over 60% of the total excess mortality due to NO2. It indicates the necessity and social benefits of stricter air quality standards regarding NO2 in South Korea, especially, given the current air quality guidelines of South Korea (0.06 ppm [~ 110 µg/m3] for 24-h average NO2) that are much higher than the WHO guidelines.
Several limitations should be acknowledged. First, we adopted a time-stratified case-crossover design to control confounders that did not substantially change within a month. However, unmeasured short-term time-dependent confounders, such as daily high-risk activities and smoking, etc., might affect our risk estimates. Second, because the national mortality data of this study provided only district-level residential addresses at death (the median size of districts in South Korea: 397 km2, which is around 1.7 times larger than the median size of the US zip code areas), thus exposure misclassification could exist. Third, we could not address more specific respiratory diseases, because of the limited data availability. In particular, we were limited in addressing more detailed causes of respiratory death, like asthma, influenza, and COPD, which could be acute, severe, and related to NO2. Lastly, due to the insufficient sample size of each specific category, our risk estimates regarding subgroups were statistically weak in general, except estimates for those aged 80 y or older. Many previous epidemiological journals did not recommend using the P-value of the criteria of the significance [18] because the P-value depends on the sample size and most epidemiological studies were based on observational studies without planned samples to test the primary hypothesis. However, because the P-value and 95% confidence interval could provide evidence for the reliability of the association and possibilities of sampling and information errors, our results should be carefully interpreted when the corresponding statistical evidence is weak. In addition, our results for the total population and people aged 80 years or older (with strong statistical evidence) could provide more reliable evidence on the association between NO2 and respiratory mortality, along with high-risk populations.
Nonetheless, this study has several strengths. First, with high-accuracy machine learning exposure modeling, this study assessed the nationwide association between short-term exposure to outdoor NO2 and respiratory mortality in South Korea. Second, by stratified and interaction analyses, we examined the heterogeneous association between NO2 and respiratory mortality by age group, cause of death, sex, and regional characteristics and suggested specific high-risk populations. Our findings provide important evidence for more targeted action plans to reduce the health impacts of NO2. Third, by estimating the excess mortality burden attributable to non-compliance with the WHO air quality guidelines, this study suggests the social benefits and justification for establishing more stringent NO2 air quality standards.
Conclusion
In summary, we assessed the nationwide association between short-term ambient NO2 and respiratory mortality in South Korea and provided evidence regarding high-risk populations. This study also provides findings that might indicate the latent benefits of stricter NO2 air quality guidelines and potential effect modifications by individual and community-level characteristics that could provide partial evidence for more effective public health resource mobilization.
Supplementary Information
Acknowledgements
This work was supported by Institute of Information & communications Technology Planning & Evaluation (IITP) under the Artificial Intelligence Convergence Innovation Human Resources Development (IITP-2024-RS-2023-00254177) grant funded by the Korea government (MSIT). This work was also supported by the National Institute of Environmental Research (NIER) funded by the Ministry of Environment (MOE) of the Republic of Korea (NIER-2021-03-03-007), the Korea Environment Industry & Technology Institute (KEITI) through "Climate Change R&D Project for New Climate Regime." funded by Korea Ministry of Environment (MOE) [RS-2022-KE002235], and the National Research Foundation of Korea (NRF) grant funded by the Korea government(MSIT) (No. RS-2024-00416848).
Authors’ contributions
Contributions Seoyeong Ahn: Formal analysis, Data curation, Visualization, Writing – Original draft Hyewon Yun: Formal analysis, Data curation, Visualization, Writing – Original draft Sooyoung Kim: Formal analysis, Data curation, Visualization Yejin Kim: Data curation, Writing – Original draft Jieun Oh, Hyemin Jang: Data curation, Writing – review & editing Sojin Ahn: Writing – review & editing Cinoo Kang, Ayoung Kim, Dohoon Kwon, Jinah Park, Insung Song, Jeongmin Moon, Jieun Min, Ejin Kim: Data curation Ho Kim: Writing – review & editing, Funding acquisition. Whanhee Lee: Conceptualization, Methodology, Investigation, Supervision, Writing – Original draft, Funding acquisition.
Funding
None.
Data availability
The data of this study can be provided upon request to the corresponding authors.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board (IRB) of Pusan National University (Review Exemption; IRB number: 2024_196_HR). The IRB approval process also waived the requirement for informed patient participation consent because this study used secondary and publicly available data (https://mdis.kostat.go.kr/index.do). The data of this study did not include any information related to personal identification.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alotaibi R, Bechle M, Marshall JD, Ramani T, Zietsman J, Nieuwenhuijsen MJ, et al. Traffic related air pollution and the burden of childhood asthma in the contiguous united states in 2000 and 2010. Environ Int. 2019;127:858–67. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DO, Ferris BG. Role of tobacco smoking in the causation of chronic respiratory disease. N Engl J Med. 1962;267:787–94. [DOI] [PubMed] [Google Scholar]
- 3.Ayyagari VN, Januszkiewicz A, Nath J. Effects of nitrogen dioxide on the expression of intercellular adhesion molecule-1, neutrophil adhesion, and cytotoxicity: Studies in human bronchial epithelial cells. Inhalation Toxicol. 2007;19:181–94. [DOI] [PubMed] [Google Scholar]
- 4.Barck C, SandstrÖM T, Lundahl J, HalldÉN G, Svartengren M, Strand V, et al. Ambient level of no2 augments the inflammatory response to inhaled allergen in asthmatics. Respir Med. 2002;96:907–17. [DOI] [PubMed] [Google Scholar]
- 5.Becker S, Soukup JM. Effect of nitrogen dioxide on respiratory viral infection in airway epithelial cells. Environ Res. 1999;81:159–66. [DOI] [PubMed] [Google Scholar]
- 6.Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality, and air pollution study. Epidemiology. 2005;16:436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buontempo C, Burgess SN, Dee D, Pinty B, Thépaut J-N, Rixen M, et al. The copernicus climate change service: Climate science in action. 2022;103:E2669–87. [Google Scholar]
- 8.Byun G, Kim S, Choi Y, Kim A, Team A-C, Lee JT, et al. Long-term exposure to pm2.5 and mortality in a national cohort in south korea: Effect modification by community deprivation, medical infrastructure, and greenness. BMC Public Health. 2024;24:1266. [DOI] [PMC free article] [PubMed]
- 9.Cakmak S, Dales RE, Angelica Rubio M, Blanco VC. The risk of dying on days of higher air pollution among the socially disadvantaged elderly. Environ Res. 2011;111:388–93. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri SF, Kerr GH, Anenberg SC, Horton DE. All-cause no2-attributable mortality burden and associated racial and ethnic disparities in the united states. Environ Sci Technol Lett. 2023;10:1159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chauhan AJ, Krishna MT, Frew AJ, Holgate ST. Exposure to nitrogen dioxide (no2) and respiratory disease risk. Rev Environ Health. 1998;13:73–90. [PubMed] [Google Scholar]
- 12.Chen R, Samoli E, Wong C-M, Huang W, Wang Z, Chen B, et al. Associations between short-term exposure to nitrogen dioxide and mortality in 17 chinese cities: The china air pollution and health effects study (capes). Environ Int. 2012;45:32–8. [DOI] [PubMed] [Google Scholar]
- 13.Çolak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Prevalence, characteristics, and prognosis of early chronic obstructive pulmonary disease. The copenhagen general population study. Am J Respir Crit Care Med. 2020;201:671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dattani S, Rodés-Guirao L, Ritchie H, Ortiz-Ospina E, Max R. Life expectancy. OurWorldInData.org:Our World In Data. 2023.
- 15.Di Q, Dai L, Wang Y, Zanobetti A, Choirat C, Schwartz JD, et al. Association of short-term exposure to air pollution with mortality in older adults. JAMA. 2017;318:2446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. Air pollution and mortality in the medicare population. N Engl J Med. 2017;376:2513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, et al. Assessing no2 concentration and model uncertainty with high spatiotemporal resolution across the contiguous united states using ensemble model averaging. Environ Sci Technol. 2020;54:1372–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Editors T. The value of p. Epidemiology. 2001;12:286. [PubMed]
- 19.Fong KC, Hart JE, James P. A review of epidemiologic studies on greenness and health: Updated literature through 2017. Current Environ Health Rep. 2018;5:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hesterberg TW, Bunn WB, McClellan RO, Hamade AK, Long CM, Valberg PA. Critical review of the human data on short-term nitrogen dioxide (no2) exposures: Evidence for no2 no-effect levels. Crit Rev Toxicol. 2009;39:743–81. [DOI] [PubMed] [Google Scholar]
- 21.Janes H, Sheppard L, Lumley T. Overlap bias in the case-crossover design, with application to air pollution exposures. Stat Med. 2005;24:285–300. [DOI] [PubMed] [Google Scholar]
- 22.Kagawa J. Evaluation of biological significance of nitrogen oxides exposure. Tokai J Exp Clin Med. 1985;10(4):348–53. [PubMed] [Google Scholar]
- 23.Kim D, Jeong J, Ko Y, Kwon Y, Kim YT. The construction of database of community health outcomes and health determinants in the republic of korea. Public Health Wkly Rep KCDC. 2018;11(30):979–83.
- 24.Kim Y, Oh J, Kim S, Kim A, Park J, Ahn S, et al. Relationship between short-term ozone exposure, cause-specific mortality, and high-risk populations: A nationwide, time-stratified, case-crossover study. Environ Res. 2024:119712. [DOI] [PubMed]
- 25.Lee JT, Kim H, Schwartz J. Bidirectional case-crossover studies of air pollution: Bias from skewed and incomplete waves. Environ Health Perspect. 2000;108:1107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee W, Choi M, Bell ML, Kang C, Jang J, Song I, et al. Effects of urbanization on vulnerability to heat-related mortality in urban and rural areas in south korea: A nationwide district-level time-series study. Int J Epidemiol. 2021;51:111–21. [DOI] [PubMed] [Google Scholar]
- 27.Lee YS, Oh JY, Min KH, Lee SY, Kang KH, Shim JJ. The association between living below the relative poverty line and the prevalence of chronic obstructive pulmonary disease. J Thorac Dis. 2019;11:427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy D, Lumley T, Sheppard L, Kaufman J, Checkoway H. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology. 2001;12:186–92. [DOI] [PubMed] [Google Scholar]
- 29.Liu C, Chen R, Sera F, Vicedo-Cabrera Ana M, Guo Y, Tong S, et al. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med. 2019;381:705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma Y, Nobile F, Marb A, Dubrow R, Stafoggia M, Breitner S, et al. Short-term exposure to fine particulate matter and nitrogen dioxide and mortality in 4 countries. JAMA Netw Open. 2024;7:e2354607–e2354607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng X, Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Milojevic A, et al. Short term associations of ambient nitrogen dioxide with daily total, cardiovascular, and respiratory mortality: Multilocation analysis in 398 cities. BMJ. 2021;372:n534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mills IC, Atkinson RW, Kang S, Walton H, Anderson HR. Quantitative systematic review of the associations between short-term exposure to nitrogen dioxide and mortality and hospital admissions. BMJ Open. 2015;5:e006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Min J, Lee W, Kang D-H, Ahn S, Kim A, Kang C, et al. Air pollution and acute kidney injury with comorbid disease: A nationwide case-crossover study in south korea. Environ Res. 2024;260:119608. [DOI] [PubMed] [Google Scholar]
- 34.Mudway IS, Kelly FJ. Ozone and the lung: A sensitive issue. Mol Aspects Med. 2000;21:1–48. [DOI] [PubMed] [Google Scholar]
- 35.Park J, Kim A, Kim Y, Choi M, Yoon TH, Kang C, et al. Association between heat and hospital admissions in people with disabilities in south korea: A nationwide, case-crossover study. The Lancet Planetary Health. 2024;8:e217–24. [DOI] [PubMed] [Google Scholar]
- 36.Prescott E, Vestbo J. Socioeconomic status and chronic obstructive pulmonary disease. Thorax. 1999;54:737–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian Y, Li H, Rosenberg A, Li Q, Sarnat J, Papatheodorou S, et al. Long-term exposure to low-level no2 and mortality among the elderly population in the southeastern united states. Environ Health Perspect. 2021;129:127009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samoli E, Aga E, Touloumi G, Nisiotis K, Forsberg B, Lefranc A, et al. Short-term effects of nitrogen dioxide on mortality: An analysis within the aphea project. Eur Respir J. 2006;27:1129–38. [DOI] [PubMed] [Google Scholar]
- 39.Samoli E, Zanobetti A, Schwartz J, Atkinson R, LeTertre A, Schindler C, et al. The temporal pattern of mortality responses to ambient ozone in the aphea project. J Epidemiol Community Health. 2009;63:960–6. [DOI] [PubMed] [Google Scholar]
- 40.Speizer FE, Ferris B, Bishop YMM, Spengler J. Respiratory disease rates and pulmonary function in children associated with no2 exposure. Am Rev Respir Dis. 1980;121:3–10. [DOI] [PubMed] [Google Scholar]
- 41.Statistics Korea. Life expectancy table. 2024. https://kosis.kr/index/index.do. Accessed 1 Dec 2024.
- 42.Sun S, Sarkar C, Kumari S, James P, Cao W, Lee RS-y, et al. Air pollution associated respiratory mortality risk alleviated by residential greenness in the chinese elderly health service cohort. Environ Res. 2020;183:109139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M, Li H, Huang S, Qian Y, Steenland K, Xie Y, et al. Short-term exposure to nitrogen dioxide and mortality: A systematic review and meta-analysis. Environ Res. 2021;202:111766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Li Y, Lin J, Huang J, Zhang Q, Wang F, et al. Prevalence, risk factors, and mortality of copd in young people in the USA: Results from a population-based retrospective cohort. BMJ Open Respir Res. 2023;10:e001550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei Y, Wang Y, Di Q, Choirat C, Wang Y, Koutrakis P, et al. Short term exposure to fine particulate matter and hospital admission risks and costs in the medicare population: Time stratified, case crossover study. BMJ. 2019;367:l6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weyand CM, Goronzy JJ. Aging of the immune system. Mechanisms and therapeutic targets. Ann Am Thorac Soc. 2016;13:S422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng W, Zhao H, Liu R, Yan W, Qiu Y, Yang F, et al. Association between no2 cumulative exposure and influenza prevalence in mountainous regions: A case study from southwest china. Environ Res. 2020;189:109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this study can be provided upon request to the corresponding authors.