Abstract
Background
Pathogenesis of atherosclerosis is largely mediated by inflammatory process. Statins are lipid-lowering drugs which also have anti-inflammatory effects. 18 fluorine radiolabeled fluorodeoxyglucose (18 F-FDG) positron emission tomography-computed tomography (PET-CT) is considered to be a good indicator of arterial wall inflammation. Therefore, in this meta-analysis the role of statins on inflammatory process in the artery wall was evaluated using this method since its actual validity for this purpose is not yet well established.
Methods
PubMed, Scopus, Web of Science, ClinicalTrials.gov, and Google Scholar databases were searched using MESH terms and keywords. Funnel plot, Begg’s rank correlation, and Egger’s weighted regression tests evaluated publication bias in the meta-analysis. In cases where funnel plot asymmetry was observed, the “trim and fill” method was used to check the input of potentially missing studies.
Results
Findings of 10 clinical trials involving 373 subjects showed a remarkable reduction of arterial wall 18 F-FDG uptake according to target-to-background ratio (TBR) index after treatment with statins. Subgroup analysis showed a significant decrease in TBR with high-intensity and non-significant reduction of TBR with low-to-moderate-intensity statin therapy.
Conclusion
Treatment with statins suppressed arterial wall inflammation as shown by using 18 F-FDG PET-CT.
Keywords: Statins, Atherosclerosis, Inflammation, 18F-FDG PET-CT, Cardiovascular disease
Introduction
Statins are among the most widely used medications, which have many beneficial effects on preventing the progression of atherosclerotic cardiovascular disease (ASCVD) [1–3]. The main anti-atherosclerotic mechanism of statins is based on LDL-cholesterol reduction. However, numerous biological and pharmacological effects have also been discovered for these drugs, which are independent of their putative cholesterol-lowering activity [4–14]. Since atherosclerosis is largely driven by inflammatory process, the anti-inflammatory role of statins is also beneficial [15–18]. Atherogenesis is a complex process in which due to binding to adhesion molecules, monocytes migrate into intima where they mature into macrophages which became loaded with cholesterol rich LDL particles and are transformed into foam cells. All these events stimulate inflammatory and immune reactions [19]. Subsequent migration and proliferation of smooth muscle cells lead to deposition of extracellular matrix which contributes to the formation of a fibrous plaque. The clinical manifestations of atherosclerosis occur when vulnerable plaques are ruptured which causes thrombus formation on them which could also be triggered by inflammation [20, 21]. Radionuclide imaging with 18fluorine radiolabeled fluorodeoxyglucose (18 F-FDG) positron emission tomography-computed tomography (PET-CT) is for more than a decade considered to be a possible indicator of arterial wall inflammation. Compared to other cell types, activated inflammatory cells show higher 18 F-FDG uptake because of their increased metabolic activity [22, 23]. It is also a validated method for quantifying atherosclerotic plaque inflammation which might help to quantify plaque vulnerability and thrombus formation on them [24, 25]. There have been a number of studies reporting the impact of statins on arterial wall inflammation using 18 F-FDG PET-CT. Besides, according to some studies these drugs could not reduce arterial wall inflammation in the aorta or carotid arteries of patients with chronic kidney disease [26]. Meta-analysis, as the highest level of evidence, will enable more conclusive evidence from the performed studies and a more accurate estimation of the effect size of statins on arterial wall inflammation. Therefore, a meta-analysis was conducted to find by using 18 F-FDG PET-CT whether statins do have a beneficial effect on reducing vessel wall inflammation.
Materials and methods
Search Strategy.
The study was performed using the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines [27]. PubMed, Google Scholar, Scopus, Web of Science, and ClinicalTrials.gov databases were searched based on the following terms in titles and abstracts: (“statin” OR “lipid lowering agents” OR “HMG-CoA reductase inhibitor” OR “Atorvastatin” OR “Pravastatin” OR “Fluvastatin” OR “Simvastatin” OR “Rosuvastatin” OR “Lovastatin” OR “Pitavastatin”) AND (18 F-fluorodeoxyglucose OR FDG OR “18 F-fluorodeoxyglucose” OR “18 F-FDG” OR “18F-FDG” OR “FDG-18 F” OR “FDG-18F” OR fluorodeoxyglucose OR “18F FDG” OR “18 F FDG” OR “18 FDG” OR 18FDG). The wild-card term ‘‘*’’ was used to improve the sensitivity of the search strategy. The search was performed from inception to September 5, 2023.
Study selection
Among the original studies selected, the inclusion criteria were: studies focused on the effect of statins on arterial wall inflammation by FDG PET-CT, studies that presented arterial wall FDG uptake as target-to-background ratio (TBR) values prior to and after statin administration or presented net change values. Exclusion criteria were: non-clinical and non-interventional studies such as observational studies with case-control, cohort designs, cross-sectional as well as studies that did not provide sufficient data on baseline or post treatment TBR values or arterial wall FDG uptake.
Data extraction
Following data from eligible studies were taken into consideration: (1) first author’s name, (2) the year of publication, (3) the number of included subjects, (4) study design (5) the type of statin therapy used, (6) the dose of statin, (7) the duration of treatment.
Quality Assessment
Cochrane criteria were used to assess the quality of the studies included in this meta-analysis [28].
Quantitative data synthesis
The meta-analysis was conducted using Comprehensive Meta-Analysis (CMA) V4 software [29]. To estimate the heterogeneity of the included publications, a random-effects model and the generic inverse variance weighting method were used [30]. Standard deviations (SDs) were calculated based on the reported data or estimated using formulas. Cochrane Q and I2 statistic were used to assess heterogeneity. The study presented effect sizes as weighted mean difference (WMD) and 95% confidence interval (CI). Sensitivity analysis assessed the influence of each single study on the overall effect size [31].
Meta-regression
A meta-regression model was used to investigate the relationship between the estimated effect size on arterial wall inflammation and potential confounders such as baseline TBR, treatment duration, mean changes in circulating levels of LDL-cholesterol and CRP. The model used random-effects and aimed to assess the impact of these factors on treatment response.
Publication Bias
Funnel plot and Egger’s weighted regression tests as well as Begg’s rank correlation evaluated publication bias in the meta-analysis. In cases where funnel plot asymmetry was observed, the “trim and fill” method was used to check the input of potentially missing studies [32]. The number of potentially missing studies needed to render the p-value non-significant was estimated using the “fail-safe N” method, which serves as another indicator of publication bias.
Results
Study selection process
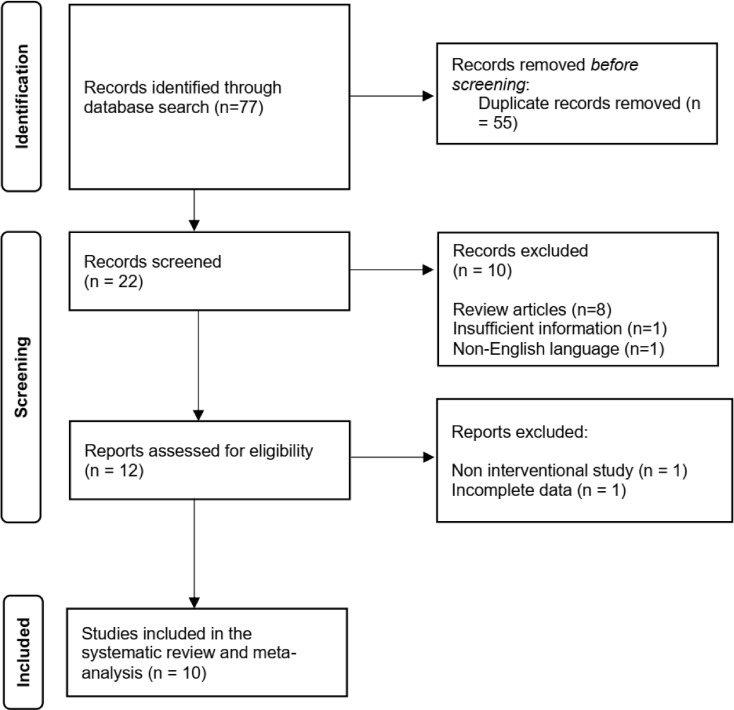
Seventy-seven articles were selected following the search in databases, and 65 were excluded after the review of titles and abstracts. Then, 12 full-text articles were checked and two were excluded because one was a non-interventional study and the other had incomplete data. Thus, 10 clinical trials were included in this meta-analysis (presented as Fig. 1).
Fig. 1.
Flow chart of the number of studies selected for meta-analysis
Study characteristics
Ten clinical trials were included in this analysis involving 373 individuals and 14 different treatment arms. All these publications analyzed the whole vessel TBR of the index vessel. Furthermore, four trials provided data on the TBR of the MDS of the index vessel. Besides the whole vessel TBR four trials also detected TBR of the MDS of the index vessel. The included studies [33–42] used different types and dosages of statins. They were published between 2010 [42] and 2021 [33]. Study designs were open-label [34, 39–42], a prospective randomized trial [33], a prospective interventional study [35] and parallel group studies [36–38]. Selected studies enrolled subjects who had ASCVD risk factors or who had proven atherosclerosis [34, 37–40], stable angina pectoris [42], HIV-infection [33, 36], acute coronary syndrome [35], and ankylosing spondylitis [41]. Table 1 presents the clinical and biochemical characteristics of the included clinical trials.
Table 1.
Clinical and biochemical characteristics of the included clinical trials
| Author | Study design | Target Population | Treatment duration | n | Study groups |
|---|---|---|---|---|---|
| Boczar et al. 2022 [33] | Prospective randomized trial | HIV infection | 6 months |
17 18 |
Rosuvastatin 10 mg/day Control |
| Emami et al. 2015 [34] | Open-label trial | History of atherosclerosis | 3 months |
24 24 |
Atorvastatin 80 mg/day Placebo |
| Ishii et al. 2010 [42] | Randomized, open-label trial | Adults with stable angina pectoris | 6 months |
15 15 |
Atorvastatin 5 mg/day Atorvastatin 20 mg/day |
| Kim et al. 2020 [35] |
Prospective interventional study |
Acute coronary syndrome | 1 month | 13 | Atorvastatin 20 mg/day |
| Lo et al. 2015 [36] | Randomized, double-blind, placebo-controlled | HIV-infected patients | 1 year |
17 20 |
Atorvastatin 40 mg/day Placebo |
| Subramanian et al. 2013 [37] | Randomized, double-blind, active-controlled | Adults with risk factors or with established atherosclerosis | 3 months |
29 29 |
Atorvastatin 10 mg/day Atorvastatin 80 mg/day |
| Tawakol et al. 2013 [38] | Randomized, double-blind trial | Individuals with arterial inflammation | 3 months |
34 34 |
Atorvastatin 10 mg/day Atorvastatin 80 mg/day |
| van der Valk et al. 2016 [41] | Open-label trial | Patients with ankylosing spondylitis | 3 months | 18 | Atorvastatin 40 mg/day |
| Watanabe et al. 2015 [39] | Randomized, open-label trial | Patients with hyperlipidemia | 6 months |
10 10 |
Pitavastatin 2 mg/day Pravastatin 10 mg/day |
| Wu et al. 2012 [40] | Open-label trial | Patients with atherosclerosis | 3 months |
43 34 |
Atorvastatin 40 mg/day Control |
18 F-FDG PET CT procedure
Different studies used 18 F-FDG PET with contrast-enhanced CT imaging to evaluate arterial wall inflammation in different vessels. Ishii et al. [42] measured 18 F-FDG uptake in the ascending aorta and right and left femoral arteries, while Lo et al. [36] assessed 18 F-FDG uptake in the aorta. Emami et al. [34] evaluated the arterial FDG in the left and right carotid and aorta while Boczar et al. assessed 18 F-FDG uptake in the aorta, bone marrow as well as spleen [33]. Two studies [38, 39] performed FDG PET-CT imaging of the thoracic aorta and carotid arteries, while two studies [35, 41] assessed arterial wall inflammation in carotid arteries. Subramanian et al. assessed 18 F-FDG uptake in the periodontium [37]. Wu et al. [40] evaluated FDG uptake in various arterial segments, including the aortic arch, ascending aorta, abdominal aorta, thoracic descending aorta, and both iliofemoral arteries.
Risk of Bias Assessment
In terms of random sequence generation and allocation concealment, three trials which were included had a high risk of bias [35, 40, 41]. Additionally, three studies were found to have a risk of bias in terms of blinding of participants, outcome assessors and personnel [33, 34, 42]. However, all of the selected studies demonstrated a low risk of bias for incomplete outcome data and selective outcome reporting. More information regarding the assessment of bias can be found in Table 2.
Table 2.
Quality of bias assessment of the included studies according to the Cochrane guidelines
| Study | Sequence generation |
Allocation concealment |
Blinding of participants, personnel and outcome assessors | Incomplete outcome data |
Selective outcome reporting | Other sources of bias |
|---|---|---|---|---|---|---|
| Boczar et al. 2022 [33] | U | U | H | L | L | U |
| Emami et al. 2015 [34] | U | U | H | L | L | U |
| Ishii et al. 2010 [42] | U | L | H | L | L | U |
| Kim et al. 2020 [35] | H | H | H | L | L | U |
| Lo et al. 2015 [36] | L | L | L | L | L | L |
| Subramanian et al. 2013 [37] | U | U | L | L | L | L |
| Tawakol et al. 2013 [38] | U | U | U | L | L | U |
| van der Valk et al. 2016 [41] | H | H | H | L | L | U |
| Watanabe et al. 2015 [39] | U | U | H | L | L | U |
| Wu et al. 2012 [40] | H | H | H | L | L | U |
L, low risk of bias; H, high risk of bias; U, unclear risk of bias
Quantitative data synthesis
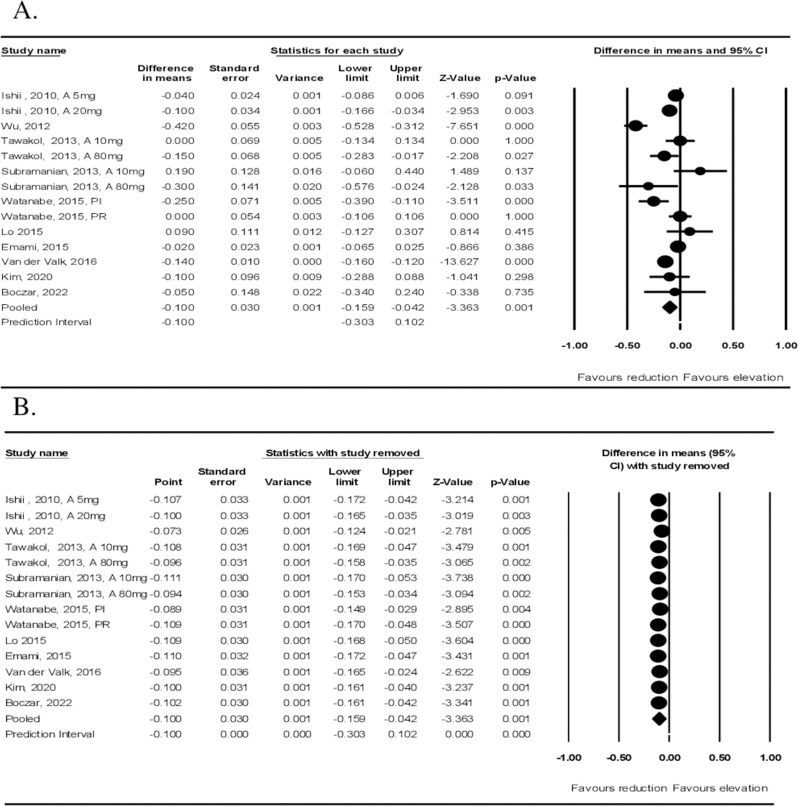
Meta-analysis of data from ten studies including 373 participants and 14 treatment arms showed a significant decrease in 18 F-FDG uptake according to TBR index after treatment with statins (WMD: −0.100, 95% CI: −0.159, − 0.042, p = 0.001; I2: 84.90%) (Fig. 2A). Sensitivity analysis was also performed (Fig. 2B).
Fig. 2.
Quantitative Data Synthesis (arterial wall uptake). A Effect of statin therapy on arterial wall FDG uptake. Forest plot shows weighted mean difference (WMD) and 95% confidence intervals for the effect of statin administration on arterial wall FDG uptake based on whole vessel TBR index. 2 B leave-one-out sensitivity analysis
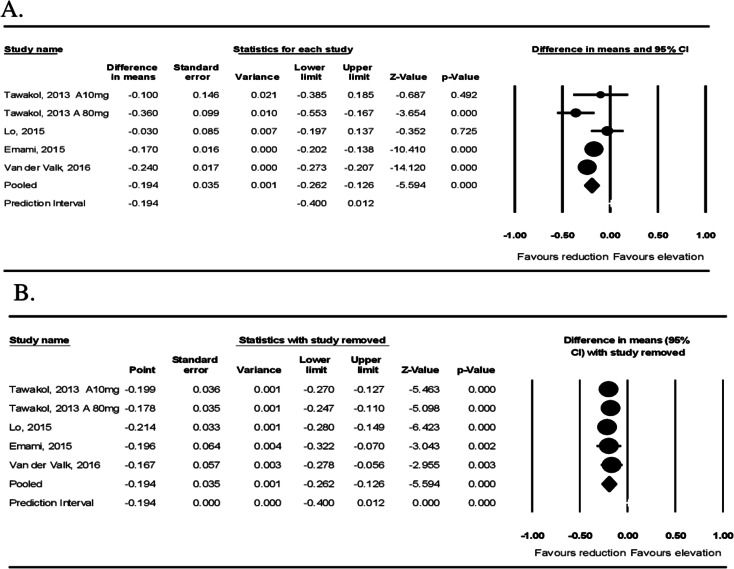
Four studies with five treatment arms showed a significant reduction in arterial MDS TBR after statin administration (WMD: −0.194, 95% CI: −0.272, − 0.126, p < 0.001; I2: 61.71%) (Fig. 3A). Sensitivity analysis was also performed (Fig. 3B).
Fig. 3.
Effect of statin therapy on FDG uptake of the most diseased arterial segment (MDS). Forest plot displays weighted mean difference (WMD) and 95% confidence intervals for the effect of statin administration on arterial wall FDG uptake based on the MDS of vessel TBR
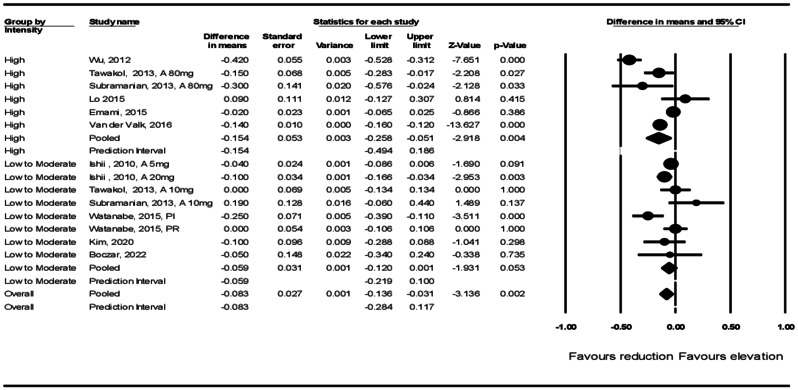
Subgroup analysis showed that arterial wall TBR was significantly reduced in those patients who were treated with high-intensity statins (WMD: −0.154, 95% CI: −0.258, − 0.051, p = 0.004) and there was a non-significant reduction of TBR in those treated with low-to-moderate-intensity (WMD: −0.059, 95% CI: −0.120, 0.001, p = 0.053) statin therapy (Fig. 4).
Fig. 4.
Forest plot stratified according to the intensity of statin therapy
Meta-regression
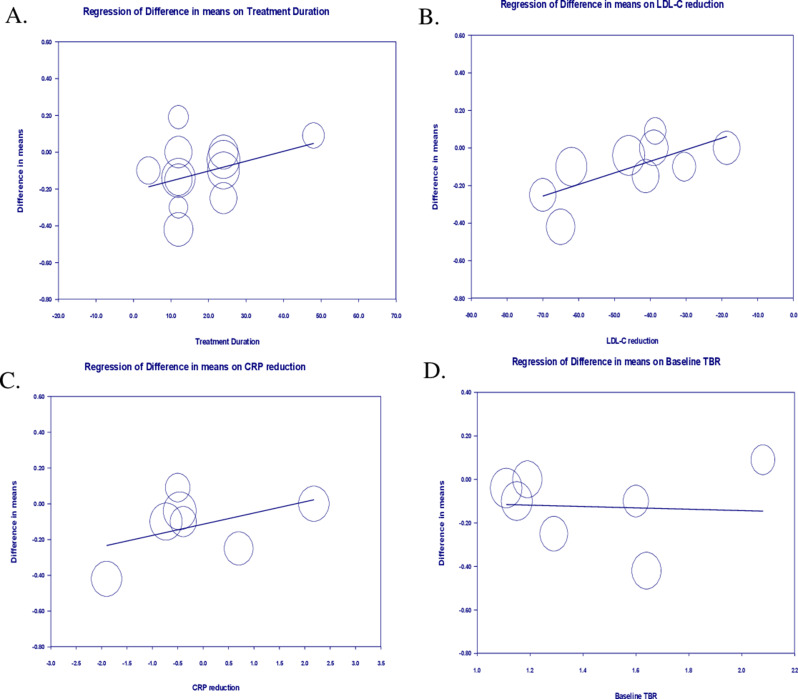
To evaluate the impact of potential confounding factors on the effects of statin treatment on arterial wall inflammation, a random-effects meta-regression was performed. The results suggested a significant association between the effect of statins on TBR and LDL-cholesterol change (slope: 0.006; 95% CI: 0.001, 0.010; p = 0.011), but there were no significant association with treatment duration (slope: 0.006; 95% CI: −0.001, 0.014; p = 0.119), CRP change (slope: 0.062; 95% CI: -0.021, 0.146; p = 0.145), and baseline TBR (slope: -0.031; 95% CI: −0.368, 0.306; p = 0.856) (Fig. 5).
Fig. 5.
The results of meta-regression analyses examining the associations between different potential confounders and changes in arterial wall TBR. The analysis investigated the relationship between (A) treatment duration, (B) alteration in circulating LDL-cholesterol, (C) plasma levels of C-reactive protein, (D) baseline TBR, and mean changes in arterial wall TBR index
Publication Bias
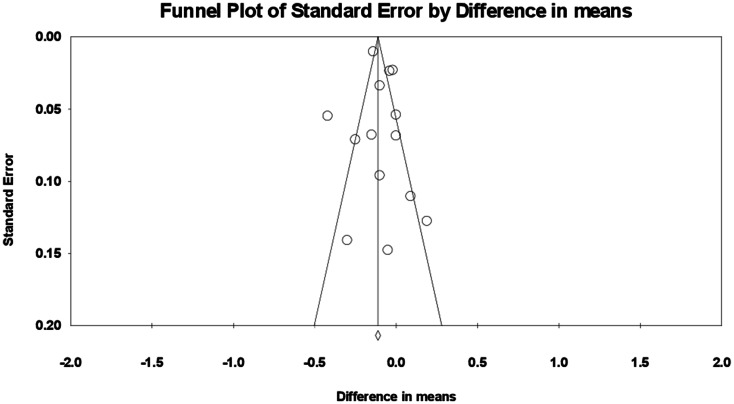
After applying the “trim and fill” method to account for potentially missing studies the corrected effect size was found to be -0.121 (95% CI: -0.18, -0.06) (Fig. 6). Begg’s rank correlation (tau = -0.04, z = 0.21, p = 0.826) and Egger’s regression test (t = 0.52, df = 12, p = 0.609) did not indicate any publication bias. The “fail-safe N” test results suggested that 282 additional publications would be needed to reduce to zero the observed significant result. Since we were able to identify only ten eligible publications (with 14 treatment arms) for this meta-analysis, it is highly unlikely that 282 studies were missed, indicating the absence of any significant publication bias.
Fig. 6.
Funnel plot showing publication bias in the studies
Discussion
This is the second meta-analysis ever made on this topic. It was performed to find whether statins have a beneficial effect on arterial wall inflammation using a 18 F-FDG PET-CT method. Findings from ten studies including 373 participants and 14 treatment arms demonstrated a notable decrease in arterial wall 18 F-FDG uptake according to TBR index following administration of statins. A large effect size was reported in the leave-one-out sensitivity analysis which was not mainly driven by any single study. A significant association between the effect of statins on TBR and LDL-cholesterol change was found, but there were no significant associations with treatment duration, CRP change and baseline TBR.
The first and only similar meta-analysis was published by our group several years ago and it was based on only seven studies that had 287 participants and 10 treatment arms. Following treatment with low to moderate doses of statins, TBR was significantly reduced. TBR values after treatment with statins were not influenced by duration and dosage of drugs, variation in plasma cholesterol and CRP level neither by initial TBR values [31].
Arterial wall inflammation in patients with coronary heart disease who were treated with statins was found to be lower than in those who were not treated [43]. However, most of these studies were estimating the inflammation of the coronary arteries and other arteries wall based upon inflammatory markers in blood such as IL-1β, IL-6 and particularly hsCRP concentration [44, 45]. The 18 F-FDG PET-CT technique was only used in some studies to evaluate the severity of inflammation in atheroma plaques [46]. 18 F-FDG PET-CT has been mostly used for identifying increased tracer uptake in symptomatic carotid plaques, and the tracer uptake has been shown to correlate with plaque inflammation and vulnerability [47]. Therefore, 18 F-FDG PET CT is considered to be a promising method for noninvasive characterization of high-risk atherosclerotic plaques. However, it has been shown already several years ago that 18 F-FDG uptake also predicts major atherosclerotic events (MACE) in a stable population after adjusting for cardiovascular risk factors [48] but as well that 18 F-FDG uptake is higher in plaque-free arterial segments suggesting an arterial inflammatory state even at early stages of aterosclerosis [49]. Neverheless, it has to be stressed again that there are not many studies analyzing the effects of statins on arterial wall inflammation in humans using 18 F-FDG PET CT. Apart from those included in this meta-analysis, it is worth while to mention the results of another study which also used 18 F-FDG PET CT. This study has shown that anti-inflammatory effect of a statin continues throughout its use up to one year, even though yielding stable below-target plasma LDL-cholesterol levels at 3 months [50]. Important are the results of the first study which evaluated the effects of statins on the inflammation of the coronary arteries by using 18 F-FDG PET CT which showed that the anti-inflammatory effect of statins was substantially greater within unstable coronary plaques improving their internal composition and architecture [51].
Since the results of all these studies were to a certain degree controversial, this meta-analysis was performed hoping that it can give some clear answers to the still open questions concerning the effect of statins on inflammation of the artery wall by using the 18 F-FDG PET CT tenique.
This study has some limitations. One of them is that the clinical outcomes were not assessed since none of the included studies had this data. However, our findings suggest that statins had a beneficial effect decreasing inflammation in the arteries’ wall and most probably are even beneficial for the plaque composition because they suppress the intraplaque inflammation. Therefore, there is a possibility that this might be a part of the explanation why is the treatment with statins beneficial preventing ASCVD and decreases clinical outcomes. Another limitation is that we could not explain why the duration of statin therapy, variations in circulating levels of LDL-cholesterol and CRP or initial values of TBR had no impact on TBR values following treatment with statins. Finally, considering the introduction of new cholesterol-lowering agent that can be used on top of statin therapy [52–56], it remains unclear if combination of statins with newer medications in high-risk patients could affect the attenuating impact of statins on arterial wall inflammation.
Conclusion
This meta-analysis clearly showed a decrease of arterial wall inflammation after treatment with statins based on a significant reduction of arterial wall 18 F-FDG uptake according to TBR index using 18 F-FDG PET-CT. It has been also shown that 18 F-FDG PET-CT might be a useful noninvasive method to evaluate the degree of arterial wall inflammation.
Author contributions
Conceptualization: AS Writing-original draft: TJ Writing-review and editing: ZR, LES, WA, SK, AHE, FG, AS Approval of the final version: All authors.
Funding
No funding was received for this study.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tannaz Jamialahmadi, Email: Jamiat931@gmail.com.
Amirhossein Sahebkar, Email: amir_saheb2000@yahoo.com.
References
- 1.Reiner Ž. Statins in the primary prevention of cardiovascular disease. Nat Reviews Cardiol. 2013;10(8):453–64. [DOI] [PubMed] [Google Scholar]
- 2.Salami JA, et al. National trends in statin use and expenditures in the US adult population from 2002 to 2013: insights from the medical expenditure panel survey. JAMA Cardiol. 2017;2(1):56–65. [DOI] [PubMed] [Google Scholar]
- 3.Cheung BM, et al. Meta-analysis of large randomized controlled trials to evaluate the impact of statins on cardiovascular outcomes. Br J Clin Pharmacol. 2004;57(5):640–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin F, et al. The role of statins in lung cancer. Archives Med Sci. 2022;18(1):141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamani S, et al. The effects of statins on the function and differentiation of blood cells. Archives Med Sci. 2023;19(5):1314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamani S et al. The role of statins in the differentiation and function of bone cells. Eur J Clin Invest, 2021. 51(7). [DOI] [PubMed]
- 7.Chruściel P, et al. Impact of statin therapy on plasma adiponectin concentrations: a systematic review and meta-analysis of 43 randomized controlled trial arms. Atherosclerosis. 2016;253:194–208. [DOI] [PubMed] [Google Scholar]
- 8.Kouhpeikar H, et al. The effect of statins through mast cells in the pathophysiology of atherosclerosis: a review. Volume 22. Current Atherosclerosis Reports; 2020. 5. [DOI] [PubMed]
- 9.Mollazadeh H, et al. Effects of statins on mitochondrial pathways. J Cachexia Sarcopenia Muscle. 2021;12(2):237–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahebkar A et al. A comprehensive review on the lipid and pleiotropic effects of pitavastatin. Prog Lipid Res, 2021. 84. [DOI] [PubMed]
- 11.Sahebkar A, et al. Association between statin use and plasma d-dimer levels: a systematic review and meta-analysis of randomised controlled trials. Thromb Haemost. 2015;114(3):546–57. [DOI] [PubMed] [Google Scholar]
- 12.Sahebkar A, et al. The impact of statin therapy on plasma levels of Von Willebrand factor antigen: systematic review and meta-analysis of Randomised placebo-controlled trials. Thromb Haemost. 2016;115(3):520–32. [DOI] [PubMed] [Google Scholar]
- 13.Parizadeh SM, et al. Simvastatin therapy reduces prooxidant-antioxidant balance: results of a placebo-controlled cross-over trial. Lipids. 2011;46:333–40. [DOI] [PubMed]
- 14.Serban C, Sahebkar A, Ursoniu S, Mikhailidis DP, Rizzo M, Lip GY, Kees Hovingh G, Kastelein JJ, Kalinowski L, Rysz J, Banach M. A systematic review and meta-analysis of the effect of statins on plasma asymmetric dimethylarginine concentrations. Sci Rep. 2015;5:9902. [DOI] [PMC free article] [PubMed]
- 15.Bahrami A, et al. Effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors on ageing: molecular mechanisms. Ageing Res Rev. 2020;58:101024. [DOI] [PubMed] [Google Scholar]
- 16.Shakour N, et al. Statins and C-reactive protein: in silico evidence on direct interaction. Archives Med Sci. 2020;16(6):1432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koushki K, et al. Anti-inflammatory action of statins in cardiovascular disease: the role of inflammasome and toll-like receptor pathways. Clin Rev Allergy Immunol. 2021;60:175–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahrami A, et al. Effect of statins on toll-like receptors: a new insight to pleiotropic effects. Pharmacol Res. 2018;135:230–8. [DOI] [PubMed] [Google Scholar]
- 19.Libby P, et al. Atherosclerosis Nat Reviews Disease Primers. 2019;5(1):56–56. [DOI] [PubMed] [Google Scholar]
- 20.Tabas I, García-Cardeña G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Libby P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovascular Res. 2021;117(13):2525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudd JH, Hyafil F, Fayad ZA. Inflammation imaging in atherosclerosis. Thromb Vascular Biology. 2009;29(7):1009–16. Arteriosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tawakol A, et al. Vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48(9):1818–24. [DOI] [PubMed] [Google Scholar]
- 24.Sorci O, et al. 18 F-sodium fluoride PET/CT provides prognostic clarity compared to calcium and Framingham risk scoring when addressing whole-heart arterial calcification. Eur J Nucl Med Mol Imaging. 2020;47:1678–87. [DOI] [PubMed] [Google Scholar]
- 25.McCabe JJ et al. Imaging carotid plaque inflammation using positron emission tomography: emerging role in clinical stroke care, research applications, and future directions. Cells, 2023. 12(16): p. 2073. [DOI] [PMC free article] [PubMed]
- 26.Hoogeveen RM, et al. Atorvastatin treatment does not abolish inflammatory mediated cardiovascular risk in subjects with chronic kidney disease. Sci Rep. 2021;11(1):4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 28.Jpt H. Cochrane handbook for systematic reviews of interventions. cochrane-handbook. org; 2008.
- 29.Borenstein M. Comprehensive meta-analysis software. Systematic reviews in health research: meta‐analysis in context, 2022: pp. 535–548.
- 30.Sutton AJ, et al. Methods for meta-analysis in medical research. Volume 348. Wiley Chichester; 2000.
- 31.Pirro M, et al. Effect of statin therapy on arterial wall inflammation based on 18F-FDG PET/CT: a systematic review and meta-analysis of interventional studies. J Clin Med. 2019;8(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. [DOI] [PubMed] [Google Scholar]
- 33.Boczar KE, et al. Anti-inflammatory effect of rosuvastatin in patients with HIV infection: an FDG-PET pilot study. J Nuclear Cardiol. 2022;29(6):3057–68. [DOI] [PubMed] [Google Scholar]
- 34.Emami H, et al. The effect of BMS-582949, a P38 mitogen-activated protein kinase (P38 MAPK) inhibitor on arterial inflammation: a multicenter FDG-PET trial. Atherosclerosis. 2015;240(2):490–6. [DOI] [PubMed] [Google Scholar]
- 35.Kim CJ, et al. Effect of moderate-intensity statin therapy on plaque inflammation in patients with acute coronary syndrome: a prospective interventional study evaluated by 18F-FDG PET/CT of the carotid artery. Cardiol J. 2020;27(6):762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo J, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2015;2(2):e52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subramanian S, et al. High-dose atorvastatin reduces periodontal inflammation: a novel pleiotropic effect of statins. J Am Coll Cardiol. 2013;62(25):2382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tawakol A, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62(10):909–17. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe T, et al. Anti-inflammatory and morphologic effects of pitavastatin on carotid arteries and thoracic aorta evaluated by integrated backscatter trans-esophageal ultrasound and PET/CT: a prospective randomized comparative study with pravastatin (EPICENTRE study). Cardiovasc Ultrasound. 2015;13:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y-W, et al. The effects of 3-month atorvastatin therapy on arterial inflammation, calcification, abdominal adipose tissue and circulating biomarkers. Eur J Nucl Med Mol Imaging. 2012;39:399–407. [DOI] [PubMed] [Google Scholar]
- 41.van der Valk FM, et al. Increased arterial wall inflammation in patients with ankylosing spondylitis is reduced by statin therapy. Ann Rheum Dis. 2016;75(10):1848–51. [DOI] [PubMed] [Google Scholar]
- 42.Ishii H, et al. Comparison of atorvastatin 5 and 20 mg/d for reducing F-18 fluorodeoxyglucose uptake in atherosclerotic plaques on positron emission tomography/computed tomography: a randomized, investigator-blinded, open-label, 6-month study in Japanese adults scheduled for percutaneous coronary intervention. Clin Ther. 2010;32(14):2337–47. [DOI] [PubMed] [Google Scholar]
- 43.Premnath SM et al. Effect of statins on the inflammatory markers in patients with coronary artery disease. J Lab Physicians, 2023. 15. [DOI] [PMC free article] [PubMed]
- 44.Devaraj S, Siegel D, Jialal I. Statin therapy in metabolic syndrome and hypertension post-JUPITER: what is the value of CRP? Curr Atheroscler Rep. 2011;13:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He WB, et al. The effects of statins on cardiovascular and inflammatory biomarkers in primary prevention: a systematic review and meta-analysis. Heart Lung Circulation. 2023;32(8):938–48. [DOI] [PubMed] [Google Scholar]
- 46.Kelly PJ, et al. Carotid plaque inflammation imaged by 18F-fluorodeoxyglucose positron emission tomography and risk of early recurrent stroke. Stroke. 2019;50(7):1766–73. [DOI] [PubMed] [Google Scholar]
- 47.Ravikanth R. Role of 18F-FDG positron emission tomography in carotid atherosclerotic plaque imaging: a systematic review. World J Nuclear Med. 2020;19(04):327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emami H, et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC: Cardiovasc Imaging. 2015;8(2):121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandez-Friera L, et al. Vascular inflammation in subclinical atherosclerosis detected by hybrid PET/MRI. J Am Coll Cardiol. 2019;73(12):1371–82. [DOI] [PubMed] [Google Scholar]
- 50.Kang M-K, et al. Anti-inflammatory effect of statin is continuously working throughout use: a prospective three time point 18 F-FDG PET/CT imaging study. Int J Cardiovasc Imaging. 2019;35:1745–53. [DOI] [PubMed] [Google Scholar]
- 51.Singh P, et al. Coronary plaque morphology and the anti-inflammatory impact of atorvastatin: a multicenter 18F-fluorodeoxyglucose positron emission tomographic/computed tomographic study. Circ Cardiovasc Imaging. 2016;9(12):e004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahebkar A, Watts GF. New LDL-cholesterol lowering therapies: Pharmacology, clinical trials, and relevance to acute coronary syndromes. Clin Ther. 2013;35(8):1082–98. [DOI] [PubMed] [Google Scholar]
- 53.Sahebkar A, Watts GF. New therapies targeting apoB metabolism for high-risk patients with inherited dyslipidaemias: what can the clinician expect? Cardiovasc Drugs Ther. 2013;27(6):559–67. [DOI] [PubMed] [Google Scholar]
- 54.Raschi E, et al. Beyond statins: New pharmacological targets to decrease LDL-cholesterol and cardiovascular events. Pharmacol Ther. 2023;250:108507. [DOI] [PubMed] [Google Scholar]
- 55.Michaeli DT, et al. Established and emerging lipid-lowering drugs for primary and secondary Cardiovascular Prevention. Am J Cardiovasc Drugs. 2023;23(5):477–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang WZ, et al. A novel small-molecule PCSK9 inhibitor E28362 ameliorates hyperlipidemia and atherosclerosis. Acta Pharmacol Sin. 2024;45(10):2119–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.