Abstract
Background
The Norwood operation (NO) for infants with univentricular physiology has high interstage mortality. This study evaluated outcomes and risk factors for mortality following NO.
Methods
Retrospective single-center study of patients undergoing NO from 2010 to 2020. Analysis used appropriate statistics.
Results
Of 269 patients undergoing NO, 213 (79.2 %) survived to discharge. Non-survivors had longer bypass times, delayed sternal closure, required nitric oxide, higher vasoactive scores, required post-operative catheterization, Extracorporeal Life Support (ECLS), and longer ventilation (p < 0.05). Logistic regression showed moderate-severe atrioventricular valve regurgitation on intraoperative TEE (OR 2.6), requiring nitric oxide (OR 2.63), delayed sternal closure (OR 2.94), post-operative catheterization (OR 10.48), and ECLS (OR 14.54) increased mortality odds (p < 0.05). Multivariable analysis confirmed catheterization (aOR 10.48) and ECLS (aOR 14.54) as significant predictors. Of survivors, 26 (12.3 %) developed new morbidity, 9 (4.2 %) had unfavorable outcomes. Functional status improved from 6.0 to 8.04, mainly in feeding and respiratory domains (p < 0.0001).
Conclusions
Norwood survival was 79.2 %. Requiring post-operative catheterization and ECLS significantly increased mortality risk. Multicenter evaluation of these modifiable risk factors is needed to improve outcomes in this high-risk population.
Keywords: Univentricular physiology patients, Norwood operation, Functional status scale (FSS), New-morbidity, Unfavorable outcome
1. Introduction
Univentricular physiology, particularly hypoplastic left heart syndrome (HLHS), stands as the most prevalent cardiac anomaly leading to congenital heart disease-related mortality within the first year of life [1,2]. These patients necessitate multiple palliative procedures, among which the initial stage is most commonly the Norwood Operation (NO). A substantial portion of mortality within the first year of life occurs subsequent to stage 1 palliation. This once-deemed fatal cardiac ailment now boasts a hospital survival rate ranging from 77 % to 93 %, attributed to ongoing innovation, early diagnosis, and referral to tertiary centers, and improved perioperative care [[3], [4], [5], [6], [7], [8]].
Several single and multicenter studies have been conducted to explore outcomes following NO and identify patient characteristics associated with adverse results. Low weight, prematurity, age, genetic syndromes, and extracardiac anomalies, alongside anatomical factors like HLHS subtype, ascending aorta diameter, restrictive atrial septum, associated cardiac anomalies, right ventricle dysfunction, and tricuspid regurgitation, have all been linked to unfavorable outcomes [[9], [10], [11], [12], [13], [14], [15]].
Despite advancements in surgical techniques, perioperative care, and monitoring, mortality rates remain high in the current era, with no significant change over the past decade [16,17]. In light of this, we conducted a retrospective single-center cohort study at a high-volume cardiac center with three primary objectives: 1) to investigate short-term outcomes in univentricular patients who have undergone a NO, 2) to examine risk factors and their associations with operative mortality, and 3) to assess the functional status of survivors using the Functional Status Scale (FSS) and identify patients who have developed new morbidity and experienced unfavorable outcomes.
2. Materials and methods
This is a single-center retrospective cohort study including all neonates with univentricular physiology who underwent stage-1-palliation with a NO between January 1st, 2010, and December 31st, 2020, at Children's Healthcare of Atlanta (CHOA), a free-standing, university-affiliated quaternary children's hospital. An internal surgical database was queried, and eligible patient encounters were identified. The study was approved by the CHOA Institutional Review Board (IRB# 00001119, approval date: 07/19/2021). Informed consent was waived.
Data and Definitions: All consecutive patients who underwent Norwood Operation (NO) were included without exclusions. We collected comprehensive data including demographic features (age, weight at surgery, sex, race/ethnicity) and clinical characteristics such as chromosomal abnormalities, genetic syndromes, primary cardiac diagnosis, and type of systemic ventricle. The source of pulmonary blood flow was noted, differentiating between modified Blalock-Taussig-Thomas (m-BTT) shunt (a surgical connection from the subclavian or innominate artery to the pulmonary artery), and Sano shunt/right ventricle to pulmonary artery (RV-PA) conduit (a direct connection between the right ventricle and pulmonary artery). Preoperative respiratory support requirements were documented. Echocardiographic data from both preoperative transthoracic echocardiogram (TTE) and intraoperative transesophageal echocardiogram (TEE) were collected, assessing atrioventricular valve regurgitation (AVVR), systemic ventricular function, and native ascending aorta diameter. Hemodynamic data included preoperative and postoperative vasoactive inotropic scores (VIS-score) calculated based on the dosage of medications supporting cardiovascular function at designated time points [18]. Operative variables encompassed cardiopulmonary bypass (CPB) time, representing the duration of extracorporeal support required during surgery to support cardiopulmonary functions; aortic cross-clamp (XC) time, indicating the period of aortic clamping during surgery; and circulatory arrest time, denoting complete circulatory cessation during surgery. Postoperatively, we recorded the requirement for Extracorporeal Life Support (ECLS), an advanced form of life support providing both cardiac and respiratory assistance.
Clinical Outcomes: The aims of this study are three; first, we sought to characterize the short-term outcomes and operative mortality of patients who underwent NO. Secondarily we sought to evaluate risk-factors and their association with mortality post-Norwood operation. Finally, characterize the functional status of survivors using FSS, and determine the change in FSS from admission to discharge in each of the categories.
Functional Status Scale (FSS): The FSS consists of 6 main domains: mental status, sensory, communications, motor function, feeding, and respiratory. Functional status for each domain was categorized from a normal score of 1 to very severe dysfunction with a score of 5, giving total FSS scores ranging from 6 to 30 as previously described by Pollack et al. [19] Functional status scoring for this study involved retrospectively scoring baseline status (i.e., on admission) and again at hospital discharge by examining the appropriate documentation. Newborns who had never achieved a stable baseline function were assigned an FSS score of 6. This was operationalized by assigning a baseline FSS score of 6 to all admissions for infants 0–2 days old and to transfers from another facility for infants 3–6 days old as previously reported [[20], [21], [22], [23]]. New morbidity was defined as an increase in the total FSS score of 3 points or more (³ 3) from admission, while unfavorable outcome was defined by an increase in total FSS score of five points or more (³ 5) as described by Pollack et al. [24].
2.1. Statistical analysis
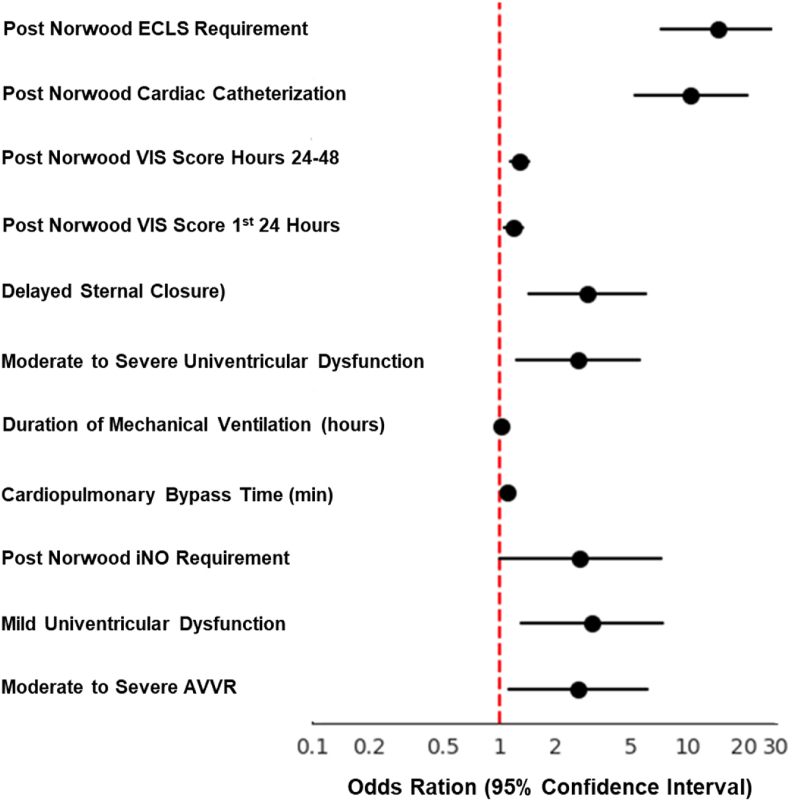
Descriptive statistics were calculated for all variables of interest and include medians and interquartile ranges (IQR) or counts and percentages, as appropriate. Patient characteristics were compared between ECLS cohort vs. non-ECLS cohort and ECLS survivors vs. ECLS non-survivors. Comparisons were made using chi‐square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. When expected cell counts were <5, Fisher's exact test was used in place of the chi‐square test. To identify risk factors for mortality following the Norwood operation, we employed a comprehensive statistical approach. Initially, all variables were subjected to binary logistic regression analyses for univariable assessment. Variables demonstrating statistical significance (p < 0.05) in the univariable analysis were subsequently included in a multivariable logistic regression model. This two-step process allowed us to identify independent risk factors for mortality while accounting for potential confounding effects. The variables that showed significance in the univariable analysis included moderate to severe atrioventricular valve regurgitation (AVVR), mild ventricular dysfunction, CPB duration, duration of mechanical ventilation, post-Norwood inhaled nitric oxide (iNO) use, delayed sternal closure, post-operative vasoactive VIS in the first 24 h and 24–48 h, post-Norwood cardiac catheterization intervention, and ECLS requirement. These variables were then entered into the multivariable model. Odds ratios with 95 % confidence intervals were computed for all variables in both univariable and multivariable analyses, providing a measure of the strength and precision of the associations. This rigorous statistical approach enabled us to identify the most significant predictors of mortality in our patient cohort. Overall FSS and subdomain FSS at admission and at discharge were reported as mean and standard deviation (SD). Paired Student's t-test was employed to compare FSS at admission and FSS at discharge. p-values of <0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R statistical software (version 4.0.2; R Core Team, 2020).
3. Results
Patient Demographics and Characteristics: During the study period, 269 patients with univentricular physiology underwent a NO. The median age was 5 days (IQR 4.0, 7.0), and median weight 3.2 kg (IQR 2.8, 3.5). The majority of patients were male (62.1 %), with a median gestational age of 38 weeks (IQR 37, 39), and 10 % were preterm (<36 weeks gestation). The most common diagnosis was hypoplastic left heart syndrome (HLHS) (75 %), with mitral atresia/aortic atresia (MA/AA) being the most common variant (43.8 %).
Preoperative TTE and intraoperative TEE univentricular Function and Atrioventricular Valve Regurgitation: On preoperative TTE and intraoperative TEE, 83 % and 89 % had no – trivial – mild atrioventricular valve regurgitation (AVVR), and 80 % and 81.5 % had normal univentricular function, respectively. A systemic right ventricle was the most common type at 83.6 %.
Operative Variables: The median CPB time was 163 min (IQR 142, 188), median cross-clamp time was 73 min (IQR 61, 86), and median circulatory arrest time was 3 min (IQR 2, 11) (Table 1).
Table 1.
Patient characteristics of the overall cohort.
| Variables | Overall Cohort (n = 269) |
|---|---|
| Age (days) | 5.0 [4.0, 7.0] |
| Weight (Kg) | 3.2 [2.8, 3.5] |
| Sex | |
| Male Female |
167 (62.1 %) 102 (37.9 %) |
| Race | |
| Caucasian African American Asian Hispanic Other |
146 (54.3 %) 101 (37.5 %) 1 (0.4 %) 15 (5.6 %) 6 (2.2 %) |
| Preterm Birth (<36 weeks gestation) | 27 (10.0 %) |
| Gestational Age (weeks) | 38.0 [37.0, 39.0] |
| Genetic Syndrome | 38 (14.1 %) |
| Chromosomal Abnormality | 23 (8.6 %) |
| Primary Cardiac Diagnosis | |
| HLHS Aortic Atresia & critical aortic Stenosis DILV DORV Single Ventricle Other Single Ventricle, unbalanced AV canal Tricuspid Atresia Single Ventricle, heterotaxia |
202 (75.1 %) 11 (4.1 %) 11 (4.1 %) 10 (3.7 %) 12 (4.5 %) 11 (4.1 %) 10 (3.7 %) 2 (0.7 %) |
| HLHS Variant | |
| MA/AA MA/AS MS/AA MS/AS |
91 (43.8 %) 15 (7.2 %) 55 (26.4 %) 47 (22.6 %) |
| Pre-Norwood Ascending Aorta | |
| Diameter (mm) | 2.80 [2.00, 4.90] |
| z-score | −3.93 [-4.48, −2.30] |
| Pre-Norwood Ascending Aorta Groups Based on Diameter | |
| ≤1.5 mm 1.6–1.9 mm 2.0–3.9 mm ≥4.0 mm |
13 (4.8 %) 54 (20.1 %) 106 (39.4 %) 96 (35.7 %) |
| Pre-Norwood Respiratory Support | |
| RA NC HFNC NIPPV Intubated |
102 (37.9 %) 52 (19.3 %) 41 (15.2 %) 7 (2.6 %) 67 (24.9 %) |
| Pre-Norwood Prostaglandin Dose (mcg/kg/min) | 0.02 [0.01, 0.02] |
| Preoperative VIS score | 0.0 [0.0, 5.0] |
| Pre-Norwood Transthoracic Echocardiogram | |
|
Atrioventricular Valve Regurgitation (AVVR) No – Trivial – Mild AVVR Moderate – Severe Systemic Ventricular Function Normal Mild Dysfunction Moderate – Severe Dysfunction |
222 (83.1 %) 45 (16.9 %) 214 (79.9 %) 19 (7.1 %) 35 (13.1 %) |
| Source of Pulmonary Blood Flow | |
|
m-BTT shunt Sano Shunt |
84 (31.2 %) 185 (68.8 %) |
| Type of Systemic Ventricle | |
| RV LV undetermined |
225 (83.6 %) 25 (9.3 %) 19 (7.1 %) |
| Intraoperative Transesophageal Echocardiogram | |
|
Atrioventricular Valve Regurgitation (AVVR) No – Trivial – Mild AVVR Moderate – Severe Systemic Ventricular Function Normal Mild Dysfunction Moderate – Severe Dysfunction |
222 (89.2 %) 27 (10.8 %) 203 (81.5 %) 26 (10.4 %) 20 (8.0 %) |
| Cardiopulmonary Bypass Time (min) | 163.0 (142.0, 188.0) |
| Cross Clamp Time (min) | 73.0 (61.0, 86.0) |
| Circulatory Arrest Time (min) | 3.0 (2.0, 11.0) |
| Post-Norwood FiO2 on Arrival to CICU | 60.0 (40.0, 100.0) |
| Post-Norwood iNO on Arrival to CICU | 35 (13.0 %) |
| Delayed Sternal Closure | 169 (62.8 %) |
| Post-Norwood VIS Score | |
| First 24 h Hours 24–48 |
18.0 (11.0, 23.0) 17.0 (11.0, 23.0) |
| Post-Norwood Cath Intervention | 83 (31.1 %) |
| Post-Norwood ECLS Required | 65 (24.2 %) |
| Duration of mechanical Ventilation | 168.82 (98.66, 368.77) |
| Operative Mortality | 56 (20.8 %) |
| Length of Stay (LOS) (days) | |
| CICU LOS Postoperative LOS Hospital LOS |
16.0 (10.0, 33.0) 22.0 (14.0, 45.0) 28.0 (19.0, 50.0) |
Results depicted in n (percent), median (interquartile range).
Abbreviations: HLHS: Hypoplastic Left Heart Syndrome; DILV: Double Inlet Left Ventricle; DORV: Double Outlet Right Ventricle; AV Canal: Atrioventricular Canal; MA: Mitral Atresia; MS: Mitral Stenosis; AA, Aortic Atresia; AS: Aortic Stenosis; CICU: Cardiac Intensive Care Unit; RA: Room Air; NC: Nasal Canula; HFNC: High Flow Nasal Canula; NIPPV: Non-Invasive Positive Pressure Ventilation; VIS: Vasoactive Inotropic Score; AVVR: Atrioventricular Valve Regurgitation; m-BTT shunt: modified Blalock-Tausig-Thomas shunt; FiO2: Fraction of Inspired Oxygen; iNO: Inhaled Nitric Oxide; VIS: Vasoactive Inotropic Score; ECLS: Extracorporeal Life Support; E-CPR: Extracorporeal Cardiopulmonary Resuscitation.
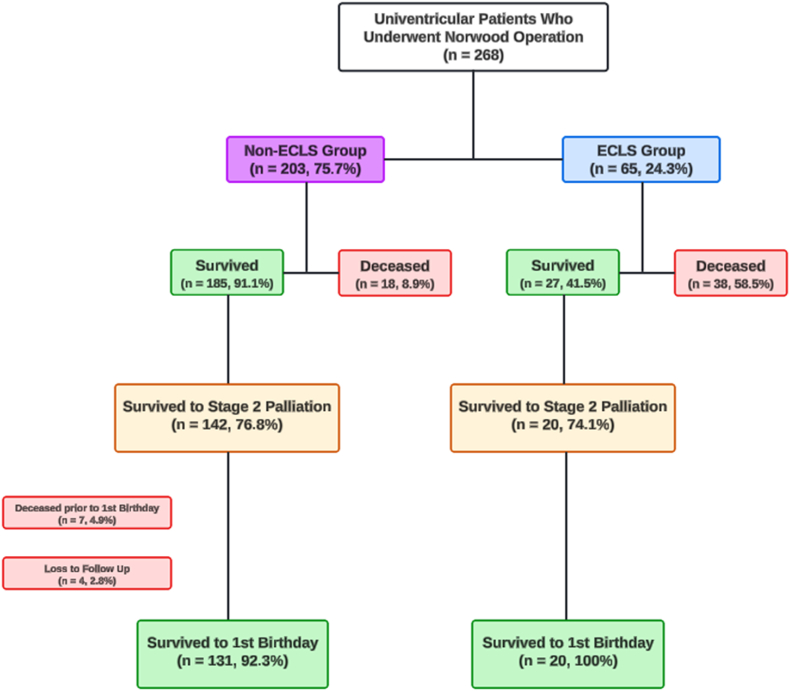
Postoperative Outcomes: The median duration of mechanical ventilation was 168.8 h (IQR 98.66, 368.77), and 31 % of patients underwent cardiac catheterization. The overall operative mortality rate was 20.8 %. Of the 269 patients who underwent NO, 65 patients (24.3 %) required ECLS in the immediate postoperative period. Of these, 27 patients (41.5 %) survived hospital discharge, and 20 of the 27 survivors (74.1 %) successfully completed stage 2 palliation, surviving to one year of age. In contrast, of the 203 patients not requiring ECLS, 185 patients (91.1 %) survived to hospital discharge, with 142 (76.8 %) successfully completing stage 2 palliation, and 131 (92.3 %) surviving to one year of age (Fig. 1). Comparison of Survivors and Non-survivors Survivors had shorter CPB times (162 vs 172.5 min, p = 0.005), were less likely to require iNO on CICU arrival (10.3 vs 23.2 %, p = 0.02), less likely to have delayed sternal closure (58.2 vs 80.4 %, p = 0.004), and had lower vasoactive-inotropic scores (VIS) in the first 24 h and at hours 24–48 (Table 2).
Fig. 1.
Flow Chart of Univentricular Patients who Underwent Norwood Operation Stratified by Post-Norwood Extracorporeal Life Support (ECLS) Requirement.
This flow chart illustrates outcomes for 269 Norwood Operation patients (2010–2021), divided by post-operative ECLS need. It shows survival rates at hospital discharge and one year for ECLS (n = 65) and non-ECLS (n = 204) groups, highlighting the significant impact of ECLS on short-term and long-term survival.
Table 2.
Patient characteristics of overall cohort stratified by operative mortality.
| Variables | Survivors (n = 213) | Non-Survivors (n = 56) | p-value |
|---|---|---|---|
| Age (days) | 5.0 (4.0, 7.0) | 5.0 (4.0, 7.0) | 0.561 |
| Weight (Kg) | 3.2 (2.8, 3.5) | 3.14 (2.8, 3.4) | 0.439 |
| Sex | 0.591 | ||
| Male Female |
130 (61.0 %) 83 (39.0 %) |
37 (66.1 %) 19 (33.9 %) |
|
| Race | 0.701 | ||
| Caucasian African American Asian Hispanic Other |
113 (53.1 %) 81 (38.0 %) 1 (0.5 %) 12 (5.6 %) 6 (2.8 %) |
33 (58.9 %) 20 (35.7 %) 0 (0.0 %) 3 (5.4 %) 0 (0.0 %) |
|
| Gestational Age (weeks) | 38.0 (37.0, 39.0) | 38.0 (37.0, 39.0) | 0.313 |
| Preterm Birth (<36 weeks gestation) | 20 (9.4 %) | 7 (20.5 %) | 0.660 |
| Chromosomal Abnormality | 18 (8.5 %) | 5 (8.9 %) | 1 |
| Genetic Syndrome | 32 (15.0 %) | 6 (10.7 %) | 0.543 |
| Primary Cardiac Diagnosis | 0.871 | ||
| Aortic Atresia & critical aortic Stenosis DILV DORV HLHS Single Ventricle Other Single Ventricle, heterotaxia Single Ventricle, unbalanced AV canal Tricuspid Atresia |
9 (4.2 %) 10 (4.7 %) 8 (3.8 %) 156 (73.2 %) 11 (5.2 %) 2 (0.9 %) 9 (4.2 %) 8 (3.8 %) |
2 (3.6 %) 1 (1.8 %) 2 (3.6 %) 46 (82.1 %) 1 (1.8 %) 0 (0.0 %) 2 (3.6 %) 20 (42.6 %) |
|
| HLHS Variant | 0.177 | ||
| MA/AA MA/AS MS/AA MS/AS |
71 (44.1 %) 15 (7.2 %) 39 (34.2 %) 41 (25.5 %) |
20 (42.6 %) 5 (10.6 %) 16 (34.0 %) 6 (12.8 %) |
|
| Pre-Norwood Ascending Aorta | |||
| Diameter (mm) | 2.90 (2.00, 5.00) | 2.40 (1.90, 4.10) | 0.080 |
| z-score | −3.90 (−4.43, −2.20) | −4.18 (−4.58, −2.77) | 0.078 |
| Pre-Norwood Ascending Aorta Groups Based on Diameter | 0.329 | ||
| ≤1.5 mm 1.6–1.9 mm 2.0–3.9 mm ≥4.0 mm |
10 (4.7 %) 39 (18.3 %) 83 (39.0 %) 81 (38.0 %) |
3 (5.4 %) 15 (26.8 %) 23 (41.1 %) 15 (26.8 %) |
|
| Pre-Norwood Respiratory Support | 0.334 | ||
| RA NC HFNC NIPPV Intubated |
83 (39.0 %) 42 (19.7 %) 33 (15.5 %) 7 (3.3 %) 48 (22.5 %) |
19 (33.9 %) 10 (17.9 %) 8 (14.3 %) 0 (0.0 %) 19 (33.9 %) |
|
| Pre-Norwood Prostaglandin Dose (mcg/kg/min) | 0.02 (0.01, 0.02) | 0.02 (0.01, 0.02) | 0.368 |
| Preoperative VIS score | 0.0 (0.0, 5.0) | 0.0 (0.0, 5.0) | 0.208 |
| Pre-Norwood Transthoracic Echocardiogram | |||
|
Atrioventricular Valve Regurgitation Moderate – Severe No – Trivial – Mild AVVR Systemic Ventricular Function Normal Mild Dysfunction Moderate – Severe Dysfunction |
31 (14.7 %) 180 (85.3 %) 175 (82.5 %) 13 (6.1 %) 24 (11.3 %) |
14 (25.0 %) 42 (75.0 %) 39 (69.6 %) 6 (10.7 %) 11 (19.6 %) |
0.103 0.101 |
| Source of Pulmonary Blood Flow | 1 | ||
|
m-BTT shunt Sano Shunt |
67 (31.5 %) 146 (68.5 %) |
17 (30.4 %) 39 (69.6 %) |
|
| Type of Systemic Ventricle | 0.065 | ||
| RV LV undetermined |
174 (81.7 %) 20 (9.4 %) 19 (8.9 %) |
51 (91.1 %) 5 (8.9 %) 0 (0.0 %) |
|
| Intraoperative Transesophageal Echocardiogram | |||
|
Atrioventricular Valve Regurgitation Moderate – Severe No – Trivial – Mild AVVR Systemic Ventricular Function Normal Mild Dysfunction Moderate – Severe Dysfunction |
17 (8.6 %) 181 (91.4 %) 169 (85.4 %) 16 (8.1 %) 13 (6.6 %) |
10 (19.6 %) 41 (80.4 %) 34 (66.7 %) 10 (19.6 %) 7 (13.7 %) |
0.045 0.009 |
| Cardiopulmonary Bypass Time (min) | 162.0 (140.0, 185.0) | 172.5 (158.0, 219.5) | 0.005 |
| Cross Clamp Time (min) | 74.0 (62.0, 86.0) | 69.0 (60.0, 84.5) | 0.410 |
| Circulatory Arrest Time (min) | 3.0 (2.0, 10.0) | 3.0 (2.0, 16.5) | 0.218 |
| Post-Norwood FiO2 on Arrival to CICU | 60.0 (40.0, 100.0) | 80.0 (40.0, 100.0) | 0.127 |
| Post-Norwood iNO on Arrival to CICU | 22 (10.3 %) | 13 (23.2 %) | 0.02 |
| Delayed Sternal Closure | 124 (58.2 %) | 45 (80.4 %) | 0.004 |
| Post-Norwood VIS Score | |||
| First 24 h Hours 24–48 |
17.0 (10.0, 23.0) 15.0 (10.0, 21.0) |
20.0 (13.7, 28.5) 22.5 (15.0, 25.0) |
0.009 <0.001 |
| Post-Norwood Cath Intervention | 43 (20.3 %) | 40 (72.7 %) | <0.001 |
| Post-Norwood ECLS support | 27 (12.7 %) | 38 (67.9 %) | <0.001 |
| Duration of Mechanical Ventilation (hr) | 145.34 (92.78, 291.35) | 401.12 (193.18, 701.73) | <0.001 |
| Length of Stay (LOS) (days) | |||
| CICU LOS Postoperative LOS Hospital LOS |
16.00 (9.00, 32.00) 23.00 (15.00, 46.25) 29.00 (20.00, 50.50) |
19.00 (10.00, 37.25) 19.50 (11.75, 40.00) 24.50 (16.75, 46.25) |
0.494 0.099 0.186 |
Results depicted in n (percent), median (interquartile range).
Abbreviations: HLHS: Hypoplastic Left Heart Syndrome; DILV: Double Inlet Left Ventricle; DORV: Double Outlet Right Ventricle; AV Canal: Atrioventricular Canal; MA: Mitral Atresia; MS: Mitral Stenosis; AA, Aortic Atresia; AS: Aortic Stenosis; CICU: Cardiac Intensive Care Unit; RA: Room Air; NC: Nasal Canula; HFNC: High Flow Nasal Canula; NIPPV: Non-Invasive Positive Pressure Ventilation; VIS: Vasoactive Inotropic Score; AVVR: Atrioventricular Valve Regurgitation; m-BTT shunt: modified Blalock-Tausig-Thomas shunt; FiO2: Fraction of Inspired Oxygen; iNO: Inhaled Nitric Oxide; ECLS: Extracorporeal Life Support.
Univariable and Multivariable Logistic Regression Assessing Risk Factors for Post-NO Survival: On univariable analysis several factors were associated with increased mortality risk, including moderate to severe AVVR (OR 2.6, 95 % CI 1.11–6.09, p = 0.028), and mild ventricular dysfunction on intraoperative TEE (OR 3.11, 95 % CI 1.3–7.43, p = 0.011), longer CPB time (OR 1.1 per minute, 95 % CI 1.04–1.16, p = 0.001), extended duration of mechanical ventilation (OR 1.02 per hour, 95 % CI 1.01–1.03, p < 0.0001), post-Norwood iNO use on arrival to CICU (OR 2.63, 95 % CI 1.23–5.62, p = 0.013), delayed sternal closure (OR 2.94, 95 % CI 1.44–5.99, p = 0.003), and higher post-operative VIS in the first 24 h (OR 1.19, 95 % CI 1.06–1.33, p = 0.003) and 24–48 h (OR 1.27, 95 % CI 1.14–1.42, p < 0.0001) (Table 3, Fig. 2). On multivariable analysis, both post-Norwood catheter intervention (OR 4.28, 95 % CI 1.76–10.41, p = 0.001) and ECLS requirement (OR 5.35, 95 % CI 2.07–13.83, p = 0.001) emerged as independent predictors of mortality (Table 3, Fig. 3).
Table 3.
Logistic regression examining the association of risk factors with odds of death.
| Variables | Non-Survivor (n = 56) | Survivor (n = 213) | Univariable Analysis |
Multivariable Analysis |
||
|---|---|---|---|---|---|---|
| Odds Ratio | p-value | aOdds Ratio | p-value | |||
|
Intraoperative TEE AVVR No – Trivial – Mild AVVR Moderate – Severe AVVR Systemic Ventricular Function Normal Mild Dysfunction Moderate – Severe Dysfunction |
41 (80.4 %) 10 (19.6 %) 34 (66.7 %) 10 (19.6 %) 7 (13.7 %) |
181 (91.4 %) 17 (8.6 %) 169 (85.4 %) 16 (8.1 %) 13 (6.6 %) |
Reference 2.6 (1.11, 6.09) Reference 3.11 (1.3, 7.43) 2.68 (1, 7.2) |
0.028 0.011 0.051 |
Refence 1.47 (0.46, 4.68) Refence 1.97 (0.62, 6.26) 1.25 (0.31, 4.98) |
0.52 0.248 0.755 |
| Cardiopulmonary Bypass Time (min) | 172.5 [158.0, 219.5] | 162.0 [140.0, 185.0] | 1.1 (1.04, 1.16) | 0.001 | 1.04 (0.96, 1.12) | 0.388 |
| Duration of Mechanical Ventilation (hr) | 401.12 [193.18, 701.73] | 145.34 [92.78, 291.35] | 1.02 (1.01, 1.03) | <0.0001 | 1.01 (1.0,1.02) | 0.197 |
|
Post-Norwood iNO on Arrival to CICU No Yes |
43 (76.8 %) 13 (23.2 %) |
191 (89.7 %) 22 (10.3 %) |
Reference 2.63 (1.23, 5.62) |
0.013 | Reference 1.02 (0.36, 2.88) |
0.971 |
|
Delayed Sternal Closure No Yes |
11 (19.6 %) 45 (80.4 %) |
89 (41.8 %) 124 (58.2 %) |
Reference 2.94 (1.44, 5.99) |
0.003 | Reference 1.51 (0.58, 3.94) |
0.398 |
|
Post-Norwood VIS Score First 24 h Hours 24–48 |
20.0 [13.8, 28.5] 22.5 [15.0, 35.0] |
17.0 [10.0, 23.0] 15.0 [10.0, 21.0] |
1.19 (1.06, 1.33) 1.27 (1.14, 1.42) |
0.003 <0.0001 |
1 (0.78, 1.28) 1.05 (0.84, 1.3) |
0.988 0.666 |
|
Post-Norwood Cath Intervention No Yes |
15 (27.3 %) 40 (72.7 %) |
169 (79.7 %) 43 (20.3 %) |
Reference 10.48 (5.3, 20.72) |
<0.0001 | Reference 4.28 (1.76, 10.41) |
0.001 |
|
Post-Norwood ECLS Requirement No-ECLS ECLS |
18 (32.1 %) 38 (67.9 %) |
186 (87.3 %) 27 (12.7 %) |
Reference 14.54 (7.29, 29.02) |
<0.0001 | Reference 5.35 (2.07, 13.83) |
0.001 |
Abbreviations: TEE: Transesophageal Echocardiogram; AVVR: Atrioventricular Valve Regurgitation; iNO: Inhaled Nitric Oxide; CICU: Cardiac Intensive Care Unit; VIS: Vasoactive Inotropic Score; ECLS: Extracorporeal Life Support.
Note: The odds ratios for continuous factors were calculated for every 5 units change in VIS Score, circulatory arrest time, and every 10 units change in cardiopulmonary bypass time and duration of mechanical ventilation.
Fig. 2.
Forest plot showing of all variables in univariable analysis.
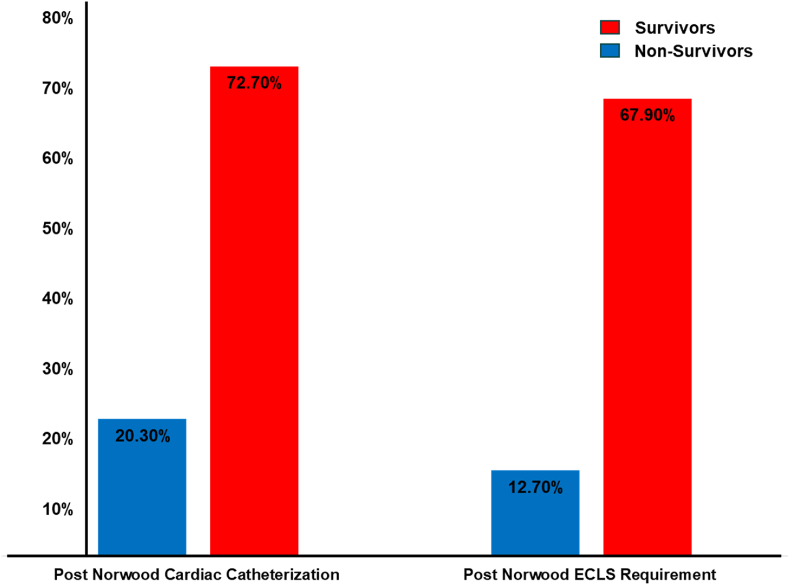
Fig. 3.
Comparison of post-Norwood intervention between survivors and non-survivors.
Predictors of ECLS and Cardiac Catheterization: To understand whether ECLS and post-Norwood cardiac catheterization interventions themselves or pre-existing patient characteristics are linked to reduced survival, we examined the prevalence of these interventions and associated risk factors. Among non-survivors, 72.7 % required post-Norwood catheter intervention compared to 20.3 % of survivors (OR 10.48, 95 % CI 5.3–20.72, p < 0.0001). Similarly, 67.9 % of non-survivors required ECLS, compared to only 12.7 % of survivors (OR 14.54, 95 % CI 7.29–29.02, p < 0.0001) (Fig. 3). Patients requiring these interventions were more likely to have pre-existing risk factors such as: (1) Moderate to severe AVVR: 19.6 % in non-survivors vs 8.6 % in survivors (OR 2.6, 95 % CI 1.11–6.09, p = 0.028); (2) Mild ventricular dysfunction: 19.6 % in non-survivors vs 8.1 % in survivors (OR 3.11, 95 % CI 1.3–7.43, p = 0.011); and (3) Longer cardiopulmonary bypass times: median 172.5 min in non-survivors vs 162.0 min in survivors (OR 1.1 per minute, 95 % CI 1.04–1.16, p = 0.001). They also experienced more post-operative complications, including: (1) Longer median duration of mechanical ventilation 401.12 h in non-survivors vs 145.34 h in survivors (OR 1.02 per hour, 95 % CI 1.01–1.03, p < 0.0001); and (2) Higher VIS score in the first 24 h (OR 1.19, 95 % CI 1.06–1.33, p = 0.003) and hours 24–48 (OR 1.27, 95 % CI 1.14–1.42, p < 0.0001) (Table 3).
Functional Status Scale (FSS) of Survivors: The mean total FSS score for survivors increased from 6.00 (SD 0) on admission/baseline to 8.04 (SD 1.15) at discharge (p < 0.001). When comparing the different domains of FSS on admission and discharge, all reached statistical significance except for sensory function (Table 4). Of the 213 survivors, 26 patients (12.3 %) developed new morbidity and 9 patients (4.2 %) developed an unfavorable functional outcome (Table 5)
Table 4.
Functional status scale (FSS) score for survivors following Norwood operation.
| FSS Domain | Admission Mean (SD) |
Discharge Mean (SD) |
Delta Score (Ref = Admission) Mean Difference (95 % CI) |
p-value |
|---|---|---|---|---|
| Mental Status | 1.00 (0.00) | 1.05 (0.29) | 0.05 (0.01, 0.09) | 0.018 |
| Sensory Function | 1.00 (0.00) | 1.00 (0.07) | 0.001 (−0.005, 0.01) | 0.319 |
| Communication | 1.00 (0.00) | 1.04 (0.19) | 0.04 (0.01, 0.06) | 0.004 |
| Motor Functioning | 1.00 (0.00) | 1.09 (0.49) | 0.09 (0.04, 0.16) | 0.005 |
| Feeding | 1.00 (0.00) | 2.77 (0.64) | 1.77 (1.69, 1.86) | <0.001 |
| Respiratory Status | 1.00 (0.00) | 1.08 (0.29) | 0.08 (0.04, 0.12) | <0.001 |
| Total Score | 6.00 (0.00) | 8.04 (1.15) | 2.21 (2.06, 2.36) | <0.001 |
Results depicted as mean, and standard deviation (S.D.).
Subscale scores range from 1 to 5 Total scores are the sum of subscale scores ranging from 6 to 30.
Table 5.
New morbidity and unfavorable functional outcome for survivors who underwent Norwood operation based on functional status scale change from admission to discharge.
| Survivors | New Morbidity (Change in FSS score ≥3 points) | Unfavorable Outcome (Change in FSS score ≥5 points) |
|---|---|---|
| Overall Cohort of ECLS Survivors (n = 213) | 26 (12.3 %) | 9 (4.2 %) |
FSS: Functional Status Scale.
4. Discussion
In our 11-year experience at a high-volume heart center, 79.2 % of patients who underwent a NO successfully survived to hospital discharge. The non-survivors exhibited prolonged CPB time, delayed sternal closure, required iNO on arrival to the CICU, higher VIS scores post-Norwood, a need for postoperative cardiac catheterization, required post-Norwood ECLS, and an extended duration of mechanical ventilation. In a logistic regression analysis, moderate to severe atrioventricular valve regurgitation (AVVR) detected during intraoperative TEE, the need for iNO upon arrival in the CICU postoperatively, delayed sternal closure, the necessity of post-Norwood cardiac catheterization, and the requirement for ECLS had higher odds of operative mortality. After adjusting for confounding factors, significance remained only for the requirement of postoperative cardiac catheterization and ECLS. Using FSS among survivors, we noted 12.3 % (26/213) developed new morbidity, and 4.2 % (9/213) experienced unfavorable outcomes.
Prior studies have reported the one-year survival rate of patients who underwent NO is 61%–89 %, which is attributed to the preoperative risk stratification [[25], [26], [27]]. The overall one-year survival rate in our cohort was 56.3 %, lower than the reported range. Of note, there was a stark difference in the survival proportional to the requirement of ECLS after NO. The non-ECLS group had a one-year survival rate of 64.5 %, which was more congruent with previously published data. In comparison, the post NO ECLS group had a markedly worse one-year survival rate of 30 %. Thus, postoperative requirement of ECLS significantly impacted the long-term survival in NO patients, emphasizing the necessity for risk stratification and targeted interventions to optimize outcomes in this vulnerable population.
Short-term outcomes for patients post-Norwood operation in the current era appear to be consistent with prior periods. Stasik et al. conducted a study on 111 patients who underwent the Norwood operation between 2001 and 2003, revealing a hospital mortality rate of 21 %. They identified non-cardiac abnormalities, gestational age, and low birth weight as factors related to this mortality [9]. In a retrospective single-center study, Rai et al. observed 85 patients who underwent the Norwood operation between 2007 and 2011, with an early mortality rate of 8.2 % and an overall mortality rate of 28.2 %. Risk factors in this study were found to include an intact atrial septum and coarctation of the aorta [28]. Additionally, Alsoufi et al. analyzed 65 single ventricle variant patients, excluding HLHS, who underwent the Norwood procedure between 2002 and 2012, reporting a 24 % mortality or transplant rate in the cohort at 1 year postoperatively. Risk factors for mortality included a dominant right ventricle, unplanned cardiac reoperation, and the need for postoperative extracorporeal membrane oxygenation [16]. In our study, we report a mortality rate of 21.8 following the NO. Through our analysis, we identified several factors associated with postoperative mortality as shown in Table 2. These factors include longer CPB time, delayed sternal closure, the need for iNO upon arrival at the CICU, higher VIS-score postoperatively, and requirement of postoperative cardiac catheterization. In our logistic regression analysis, we found higher odds of mortality in patients with moderate to severe AVVR observed on intraoperative transesophageal echocardiography (TEE), postoperative moderate to severe ventricular dysfunction, requirement of iNO upon arrival to CICU postoperatively, delayed sternal closure, and the need for post-Norwood cardiac catheterization and ECLS. This is generally consistent with earlier studies discussing risk factors for postoperative mortality attributed to AVVR and ventricular dysfunction [[3], [4], [5],14]. Recent studies have also demonstrated the risk of delayed sternal closure, as observed in a study by Asfari et al., where significantly higher CICU morbidity and mortality were reported among patients with delayed sternal closure [29]. Additionally, reports by both Gaies et al. and Kumar et al. have indicated a correlation between higher VIS-scores and worse outcomes in postoperative pediatric cardiac patients [30,31].
While we did not find a correlation between low birth weight, gestational age, or chromosomal anomalies and postoperative mortality (Table 2), this observation could be attributed to changes in postoperative care for this higher-risk category. This is in line with the findings of Tanem et al., who, in a single-center study, categorized NO patients into standard risk and high-risk categories. The high-risk category included individuals with low birth weight, ventricular dysfunction, AVVR, intact or restrictive atrial septum, or obstructed anomalous pulmonary venous return. One-year survival was lower in the high-risk category, with the lowest survival observed in the intact septum/obstructed veins group, at 54 % [25]. Similarly, Backes et al. and their group analyzed the National Pediatric Cardiology Quality Improvement Collaborative (NPCQIC) Phase II registry data and categorized patients into high-risk and standard-risk groups. Patients were considered high risk if they had a gestational age of less than 37 weeks, a birth weight of less than 2.5 kg, a secondary cardiac lesion, extracardiac anomaly, or a genetic syndrome. Secondary cardiac lesions encompassed conditions such as an intact atrial septum, restrictive atrial septum, moderate or more AVVR, moderate or more ventricular dysfunction, and anomalous pulmonary venous return. The high-risk group exhibited lower survival rates to the first birthday compared to the standard-risk group (76.2 % vs. 88.1 %). Interestingly, having one high-risk diagnosis did not appear to be associated with reduced survival to the first birthday [32].
Of particular interest among the risk factors associated with postoperative Norwood mortality in our study is the revelation that, during the multivariable analysis, post-Norwood cardiac catheterization and post-Norwood ECLS requirement increased the odds of mortality separately. These findings suggest that while both interventions are strongly associated with higher mortality, they likely reflect the severity of the underlying condition rather than being direct causes of mortality themselves. The need for these interventions appears to be influenced by pre-existing patient characteristics and early post-operative complications. Recent studies have focused on this aspect, exemplified by the work of Handler et al. [33]. They reported data from the NPCQIC registry spanning the years 2016–2019, analyzing unplanned reinterventions in the form of cardiac catheterizations or surgical reoperations and their impact on outcomes following the NO. Out of 1367 participants, 24.8 % required reintervention and were found to have a lower likelihood of being discharged before stage two palliation, while their in-hospital mortality rate increased to 17 % [33]. Our findings contribute significantly to the existing literature on risk factors associated with mortality.
Functional outcomes following the Norwood operation using the FSS score have not been previously reported, although such scores have been used in the context of Extracorporeal Life Support (ECLS) post-Norwood procedure. In a study by Berger et al. assessing functional outcomes in patients after cardiac surgery, new morbidity was observed in 4.8 % of survivors. New morbidity rates increased with the complexity of cardiac surgery, as categorized by the Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery (STAT), ranging from 1.7 % in STAT 1 category to 12.9 % in STAT 5 patients [20]. Similar to Berger et al., Han et al. demonstrated that FSS scores increased from admission to discharge, with more complex cardiac surgery associated with higher rates, ranging from 2.0 % in STAT 1–13.3 % in STAT 5 patients. In our study, out of the 269 patients who underwent the Norwood operation, 79.2 % survived to hospital discharge. Among the survivors, 12.3 % developed new morbidity, and 4.2 % experienced unfavorable outcomes. Notably, our data predominantly reflects the STAT 5 category, which encompasses the NO patient population [23]. While our study was not powered to identify specific risk factors for developing new morbidities and unfavorable outcomes, it is essential for the scientific community to conduct further, large-scale investigations to better understand and mitigate these issues, ultimately improving the quality of life for these patients and their families.
5. Limitations
Our findings are subject to several inherent limitations, given the retrospective nature of the study. The ability to generalize our findings is inherently limited due to the study's retrospective design. We acknowledge that certain unmeasured variables, such as objective markers of cardiac output (e.g., NIRS), Qp:Qs calculations, duration of ECLS cannulation, duration of cardiopulmonary resuscitation (CPR), and CPR pauses in patients cannulated onto ECLS, could have played essential roles in patient outcomes. However, some of these data points were not available in our cohort. Moreover, factors such as the expertise of the intensivist and surgeon, as well as the experience levels of other healthcare staff, are challenging to control for and can significantly impact the overall outcomes of patients undergoing the NO. Despite our study being conducted at a large center with a substantial number of patients, it's important to note that the absence of significant findings in our study does not necessarily indicate their absence in reality. Rather, it suggests that our study may have been underpowered to detect such differences. This limitation is particularly relevant for certain key variables in our multivariable analysis. For example, the lack of statistically significant differences in survival between patients with systemic right ventricle (RV) versus left ventricle (LV), or the absence of significant survival differences based on the degree of atrioventricular valve regurgitation in the multivariable analysis, should be interpreted with caution.
6. Conclusions
In our 11-year experience at a high-volume heart center, 79.2 % of patients who underwent a Norwood operation survived to hospital discharge. Our study identified several key factors associated with increased mortality risk, particularly the need for postoperative cardiac catheterization and ECLS requirement. In the multivariable logistic regression, patients who underwent post-Norwood cardiac catheterization had 4.3 times higher odds of mortality and patients who required ECLS had 5.4 times higher odds of mortality. Additionally, we observed that among the survivors, 12.3 % developed new morbidity, and 4.2 % experienced unfavorable outcomes. Based on these findings, we propose several clinical recommendations to enhance the care and outcomes of this high-risk patient cohort.
-
1)
There is a need to develop and implement more comprehensive preoperative risk assessment tools to identify high-risk patients before surgery.
-
2)
Given the association between longer CPB times and mortality, strategies to minimize bypass duration without compromising surgical quality should be a focus of operative management.
-
3)
Early identification of postoperative complications is crucial. Implementing protocols for close monitoring of postoperative patients, with particular attention to signs that may indicate the need for cardiac catheterization or ECLS, could potentially improve outcomes.
-
4)
For patients identified as high-risk, developing specialized care pathways, including more aggressive preoperative optimization and tailored postoperative management strategies, may be beneficial.
-
5)
Furthermore, implementing comprehensive follow-up programs for survivors, focusing on early detection and management of new morbidities and unfavorable outcomes, is essential for improving long-term quality of life.
-
6)
Lastly, fostering collaboration between cardiac surgeons, intensivists, cardiologists, and other specialists to provide integrated care throughout the patient's journey is crucial for optimizing outcomes.
Future research should focus on multicenter prospective evaluations to validate these risk factors, develop and test targeted interventions, and assess their impact on both short-, and long-term survival and functional outcomes.
Funding
No external funding for this manuscript.
Financial disclosure
Authors have no financial relationships relevant to this article to disclose.
CRediT authorship contribution statement
Alaa Aljiffry: Writing – review & editing, Writing – original draft, Methodology, Data curation, Conceptualization. Ashley Harriott: Writing – review & editing, Methodology, Data curation. Shayli Patel: Writing – review & editing, Methodology, Data curation. Amy Scheel: Writing – review & editing, Methodology, Data curation. Alan Amedi: Writing – review & editing, Methodology, Data curation. Sean Evans: Writing – review & editing, Methodology, Data curation. Yijin Xiang: Writing – review & editing, Methodology, Formal analysis. Amanda Harding: Writing – review & editing, Data curation. Subhadra Shashidharan: Writing – review & editing, Methodology. Asaad G. Beshish: Writing – review & editing, Validation, Supervision, Methodology, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Gilboa S.M., et al. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122(22):2254–2263. doi: 10.1161/CIRCULATIONAHA.110.947002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feinstein J.A., et al. Hypoplastic left heart syndrome: current considerations and expectations. J Am Coll Cardiol. 2012;59(1 Suppl):S1–S42. doi: 10.1016/j.jacc.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azakie T., et al. Evolving strategies and improving outcomes of the modified norwood procedure: a 10-year single-institution experience. Ann Thorac Surg. 2001;72(4):1349–1353. doi: 10.1016/s0003-4975(01)02795-3. [DOI] [PubMed] [Google Scholar]
- 4.Gaynor J.W., et al. Risk factors for mortality after the Norwood procedure. Eur J Cardio Thorac Surg. 2002;22(1):82–89. doi: 10.1016/s1010-7940(02)00198-7. [DOI] [PubMed] [Google Scholar]
- 5.Kern J.H., et al. Survival and risk factor analysis for the Norwood procedure for hypoplastic left heart syndrome. Am J Cardiol. 1997;80(2):170–174. doi: 10.1016/s0002-9149(97)00313-5. [DOI] [PubMed] [Google Scholar]
- 6.Tabbutt S., et al. Outcomes after the stage I reconstruction comparing the right ventricular to pulmonary artery conduit with the modified Blalock Taussig shunt. Ann Thorac Surg. 2005;80(5):1582–1590. doi: 10.1016/j.athoracsur.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 7.Tweddell J.S., et al. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation. 2002;106(12 Suppl 1):I82–I89. [PubMed] [Google Scholar]
- 8.Mahle W.T., et al. Survival after reconstructive surgery for hypoplastic left heart syndrome: a 15-year experience from a single institution. Circulation. 2000;102(19 Suppl 3):Iii136–I141. doi: 10.1161/01.cir.102.suppl_3.iii-136. [DOI] [PubMed] [Google Scholar]
- 9.Stasik C.N., et al. Current outcomes and risk factors for the Norwood procedure. J Thorac Cardiovasc Surg. 2006;131(2):412–417. doi: 10.1016/j.jtcvs.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 10.Daebritz S.H., et al. Results of Norwood stage I operation: comparison of hypoplastic left heart syndrome with other malformations. J Thorac Cardiovasc Surg. 2000;119(2):358–367. doi: 10.1016/S0022-5223(00)70192-9. [DOI] [PubMed] [Google Scholar]
- 11.McGuirk S.P., et al. Risk assessment and early outcome following the Norwood procedure for hypoplastic left heart syndrome. Eur J Cardio Thorac Surg. 2006;29(5):675–681. doi: 10.1016/j.ejcts.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 12.Poirier N.C., et al. Modified Norwood procedure with a high-flow cardiopulmonary bypass strategy results in low mortality without late arch obstruction. J Thorac Cardiovasc Surg. 2000;120(5):875–884. doi: 10.1067/mtc.2000.109540. [DOI] [PubMed] [Google Scholar]
- 13.Tabbutt S., et al. Risk factors for hospital morbidity and mortality after the norwood procedure: a report from the pediatric heart network single ventricle reconstruction trial. J Thorac Cardiovasc Surg. 2012;144(4):882–895. doi: 10.1016/j.jtcvs.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghanayem N.S., et al. Interstage mortality after the norwood procedure: results of the multicenter single ventricle reconstruction trial. J Thorac Cardiovasc Surg. 2012;144(4):896–906. doi: 10.1016/j.jtcvs.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alsoufi B., et al. Impact of patient characteristics and anatomy on results of norwood operation for hypoplastic left heart syndrome. Ann Thorac Surg. 2015;100(2):591–598. doi: 10.1016/j.athoracsur.2015.03.106. [DOI] [PubMed] [Google Scholar]
- 16.Alsoufi B., et al. Current outcomes of the Norwood operation in patients with single-ventricle malformations other than hypoplastic left heart syndrome. World J Pediatr Congenit Heart Surg. 2015;6(1):46–52. doi: 10.1177/2150135114558069. [DOI] [PubMed] [Google Scholar]
- 17.Alsoufi B., et al. Factors associated with interstage mortality following neonatal single ventricle palliation. World J Pediatr Congenit Heart Surg. 2018;9(6):616–623. doi: 10.1177/2150135118787723. [DOI] [PubMed] [Google Scholar]
- 18.Gaies M.G., et al. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the pediatric cardiac critical care consortium and virtual PICU system registries. Pediatr Crit Care Med. 2014;15(6):529–537. doi: 10.1097/PCC.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollack M.M., et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009;124(1):e18–e28. doi: 10.1542/peds.2008-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg R.A., et al. Incidence and outcomes of cardiopulmonary resuscitation in PICUs. Crit Care Med. 2016;44(4):798–808. doi: 10.1097/CCM.0000000000001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beshish A.G., et al. Functional status change among children with extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in a pediatric cardiac ICU: a single institution report. Pediatr Crit Care Med. 2018;19(7):665–671. doi: 10.1097/PCC.0000000000001555. [DOI] [PubMed] [Google Scholar]
- 22.Beshish A.G., et al. Functional status change among infants, children, and adolescents following extracorporeal life support: a multicenter report. Asaio j. 2023;69(1):114–121. doi: 10.1097/MAT.0000000000001711. [DOI] [PubMed] [Google Scholar]
- 23.Han B., et al. Early functional status after surgery for congenital heart disease: a single-center retrospective study. Pediatr Crit Care Med. 2022;23(2):109–117. doi: 10.1097/PCC.0000000000002838. [DOI] [PubMed] [Google Scholar]
- 24.Pollack M.M., et al. Relationship between the functional status scale and the pediatric overall performance category and pediatric cerebral performance category scales. JAMA Pediatr. 2014;168(7):671–676. doi: 10.1001/jamapediatrics.2013.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanem J., et al. Survival after norwood procedure in high-risk patients. Ann Thorac Surg. 2020;109(3):828–833. doi: 10.1016/j.athoracsur.2019.07.070. [DOI] [PubMed] [Google Scholar]
- 26.Selenius S. 2023. Risk factors for mortality in patients with hypoplastic left heart syndrome after the Norwood procedure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnaout A.Y. 2023. Mortality and survival after Norwood procedure comparison between shunt type in patients with hypoplastic left heart syndrome or its variants: a systematic review and meta-analysis study. [Google Scholar]
- 28.Rai V., et al. Outcome of Norwood operation for hypoplastic left heart syndrome. Indian J Thorac Cardiovasc Surg. 2018;34(3):337–344. doi: 10.1007/s12055-017-0603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asfari A., et al. Norwood operation: immediate vs delayed sternal closure. Ann Thorac Surg. 2023;115(3):649–654. doi: 10.1016/j.athoracsur.2022.06.046. [DOI] [PubMed] [Google Scholar]
- 30.Gaies M.G., et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11(2):234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 31.Kumar M., et al. Vasoactive Inotrope Score as a tool for clinical care in children post cardiac surgery. Indian J Crit Care Med. 2014;18(10):653–658. doi: 10.4103/0972-5229.142174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Backes E.R., et al. Cumulative comorbid conditions influence mortality risk after staged palliation for hypoplastic left heart syndrome and variants. J Thorac Cardiovasc Surg. 2023;165(1):287–298.e4. doi: 10.1016/j.jtcvs.2022.01.056. [DOI] [PubMed] [Google Scholar]
- 33.Handler S.S., et al. Impact of reintervention during stage 1 palliation hospitalization: a national, multicenter study. Ann Thorac Surg. 2023;115(4):975–981. doi: 10.1016/j.athoracsur.2022.10.014. [DOI] [PubMed] [Google Scholar]