Abstract
Background
Aging is associated with acquired comorbidities that potentially influence the natural history and outcomes of adults with congenital heart disease (CHD). The purpose of this study was to compare the clinical characteristics, as well as the incidence and correlates of all-cause mortality between different age groups.
Method
Adults with CHD were categorized into 3 age groups based on age at baseline encounter: Group 1 (age 18–40 years); Group 2 (age 41–65 years), and Group 3 (age >65 years).
Results
Of 5930 patients (age 37 ± 15 years), 3009 (51%), 2422 (41%), and 499 (8%) were in Groups 1, 2 and 3, respectively. Compared to Group 1, patients in Groups 2 and 3 were less likely to have complex CHD, but more likely to have acquired comorbidities, end-organ dysfunction, ventricular systolic dysfunction, and valvular heart disease. Compared to Group 1, Groups 2 and 3 had higher incidence of all-cause mortality (7.2 versus 15.3 versus 47.8 per 1000 patient-years, respectively, p < 0.001), and lower proportion of deaths from cardiovascular causes (87% versus 77% versus 71%, respectively, p < 0.001). Furthermore, the correlates of all-cause mortality were different between the age groups, with acquired comorbidities such as hypertension, coronary artery disease, and hepatorenal dysfunction being associated with mortality in Group 3, while indices of CHD severity such as number of prior cardiac surgery, and presence of complex CHD being associated with all-cause mortality in Group 1.
Conclusions
These results suggest the need for management strategies tailored to address the correlates of outcomes in each age group.
Keywords: Congenital heart disease, Age, Comorbidities, Prognostication
Highlights
-
•
About half of adults with CHD were young adults at the time of initial presentation.
-
•
Young adults with had different clinical characteristics and risk profile as compared to the middle-aged and older patients.
-
•
These results suggest a need for management strategies tailored to address the correlates of outcomes in each age group.
Abbreviations
- ACC/AHA
American College of Cardiology/American Heart Association
- CHD
Congenital heart disease
- CI
Confidence interval
- CIED
Cardiac implantable electronic devices
- HR
Hazard ratio
- MELD-XI
Model for end-stage liver disease excluding international normalized ratio
1. Introduction
More than 95% of babies born with congenital heart disease (CHD) now survive to adulthood, and more than 90% live beyond the age of 40 years because of significant improvements in medical, transcatheter, and surgical therapies for CHD over time [[1], [2], [3]]. As a result, there has been a significant expansion of the adult CHD population in the past 2 decades, and CHD is now the most common cause of cardiovascular death in young adults [[2], [3], [4]]. Similar to the general population, adults with CHD develop comorbidities in the course of ageing, and these comorbidities influence the natural history of the underlying CHD lesion, and overall longevity of the patients [[5], [6], [7], [8], [9]]. In spite of these expected age-related changes in health profile, there is a general tendency to approach adults with CHD as a homogenous population without taking into account the impact of age, and comorbidities that come with ageing. The purpose of this study was to compare the clinical characteristics, as well as, the incidence and correlates of all-cause mortality between different age groups using a well-characterized cohort of adults with CHD.
2. Methods
2.1. Study population
This is a retrospective cohort study of adults (>18 years old) with CHD that received care at Mayo Clinic, Rochester, MN from January 1, 2003, to December 31, 2021. The patients were identified through the MACHD (Mayo Adult Congenital Heart Disease) Registry. The first clinical encounter in the adult CHD clinic was considered the baseline encounter, and the patients were then divided into 3 age groups based on their age at baseline encounter: (1) Young adults (Group 1) defined as age 18–40 years at baseline encounter; (1) Middle age (Group 2) defined as age 41–65 years at baseline encounter; (3) Older adults (Group 3) defined as age >65 years at baseline encounter. This study was approved by the Mayo Clinic institutional Review Board.
2.2. Data collection
The following medical records were reviewed: clinic notes, hospital dismissal notes, procedure notes, laboratory data, and echocardiograms. Clinical data obtained within 6 months from the baseline encounter were used to define the baseline characteristics of the cohorts. The patients were classified into 3 anatomic groups (simple, moderate, and complex CHD) based on CHD severity according to the 2018 American College of Cardiology/American Heart Association (ACC/AHA) guidelines [10].
Comprehensive echocardiogram was performed according to contemporary guidelines [[11], [12], [13]], and offline image analyses and measurements were performed in all patients. Systemic ventricular dysfunction was defined as systemic left ventricular ejection fraction <50% or systemic right ventricular fractional area change <35% [[11], [12], [13]]. Hemodynamically significant atrioventricular valve regurgitation was defined as ≥ moderate regurgitation assessed using qualitative Doppler echocardiography [[11], [12], [13]]. Hepatorenal function was assessed using the model for end-stage liver disease excluding international normalized ratio (MELD-XI), and hepatorenal dysfunction was defined as MELD-XI score >11 [14].
All-cause mortality was ascertained by review of medical records, from the baseline encounter to the last follow-up. The patients that did not meet the study outcome (all-cause mortality) were censored at the time of last follow-up. Cardiovascular death was defined as death due to heart failure, arrhythmia/sudden cardiac death, postprocedural death after cardiac procedure, and cardiovascular hemorrhage/stroke-related death [15].
2.3. Statistical analysis
Data were presented as mean ± standard deviation, median (interquartile range), and count (%). Normality was assessed using Shapiro-Wilk test. Between-group comparison was performed using chi-squared test and analysis of variance test as appropriate. The incidence of all-cause mortality was calculated as the quotient of the total number of events and the total duration of at-risk period and expressed as events (95% confidence interval [CI]) per 1000 patient years. The correlates of all-cause mortality were assessed in each age group using multivariable Cox regression analysis. The models were adjusted for the following covariates: (1) Demographic indices (age, sex); (2) Indices of CHD severity as defined by the ACC/AHA CHD severity class, number of prior cardiac surgeries, New York Heart Association functional class, prosthetic heart valves, and cardiac implantable electronic devices (CIED); (3) Echocardiographic indices of ventricular and valvular dysfunction; (4) Comorbidities (hypertension, coronary artery disease, type 2 diabetes, atrial fibrillation, chronic kidney disease, and hepatorenal dysfunction). The final covariate selection for the multivariable Cox regression models were determined using stepwise backwards selection with p < 0.05 required for a covariate to remain in the model. All statistical analyses were performed with BlueSky Statistics software (version. 7.10; BlueSky Statistics LLC, Chicago, IL, USA). A p value < 0.05 was considered statistically significant for all analyses.
3. Results
3.1. Baseline characteristics
There were 5930 patients that met study inclusion criteria (mean age 37 ± 15, and 3039 [51%] were males). Of the 5930 patients, 3009 (51%) were in Group 1 (age 18–40 years), 2422 (41%) were in Group 2 (age 41–65 years), and 499 (8%) were in Group 3 (age >65 years). Table 1 shows a comparison of the baseline characteristics of the cohort. Compared to Group 1, patients in Groups 2 and 3 were less likely to have complex CHD, but more likely to have comorbidities, end-organ dysfunction, ventricular systolic dysfunction, and valvular heart disease. Table 2 shows the prevalence of each CHD diagnosis in the different age groups.
Table 1.
Baseline characteristics.
| All (N = 5930) | Age 18–40 y (N = 3,009, 51%) | Age 41–65 y (N = 2,422, 41%) | Age >65 y (N = 499, 8%) | P | |
|---|---|---|---|---|---|
| Age, years | 37 ± 15 | 25 ± 6 | 48 ± 7* | 69 ± 3* | <0.001 |
| Male sex | 3039 (51%) | 1535 (51%) | 1249 (52%) | 255 (51%) | 0.7 |
| Body mass index, kg/m2 | 26 (23–30) | 24 (22–28) | 27 (24–31) * | 27 (24–31) * | <0.001 |
| Prosthetic valves | 1475 (25%) | 723 (24%) | 682 (28%) * | 70 (14%) * | <0.001 |
| CIED | 658 (11%) | 286 (10%) | 308 (13%) * | 64 (13%) * | 0.008 |
| CHD anatomic severity | |||||
| Simple | 832 (14%) | 286 (10%) | 369 (15%) | 177 (36%) | <0.001 |
| Moderate | 3801 (64%) | 1845 (61%) | 1670 (69%) | 286 (57%) | |
| Complex | 1297 (22%) | 878 (29%) | 383 (16%) | 36 (7%) | |
| Comorbidities | |||||
| Hypertension | 1458 (25%) | 397 (13%) | 780 (32%) * | 281 (56%) * | <0.001 |
| Type 2 Diabetes | 406 (7%) | 92 (3%) | 242 (10%) * | 72 (14%) * | <0.001 |
| Coronary artery disease | 313 (5%) | 29 (1%) | 182 (8%) * | 102 (20%) * | <0.001 |
| Hyperlipidemia | 1280 (22%) | 276 (9%) | 758 (31%) * | 246 (49%) * | <0.001 |
| Obesity | 1187 (20%) | 481 (16%) | 579 (24%) * | 127 (26%) * | <0.001 |
| Atrial fibrillation | 917 (16%) | 256 (9%) | 482 (20%) * | 179 (36%) * | <0.001 |
| Atrial flutter/tachycardia | 749 (13%) | 339 (11%) | 331 (14%) | 79 (16%) * | 0.002 |
| Chronic kidney disease ≥ III | 221 (4%) | 53 (2%) | 119 (5%) | 49 (10%) * | <0.001 |
| Hepatorenal renal dysfunction | 1541 (26%) | 499 (17%) | 841 (35%) * | 201 (40%) * | <0.001 |
| Medications | |||||
| Diuretics | 1385 (23%) | 531 (18%) | 683 (28%) * | 171 (34%) * | <0.001 |
| Beta blockers | 2010 (34%) | 822 (27%) | 968 (40%) * | 220 (44%) * | <0.001 |
| Calcium channel blockers | 531 (9%) | 152 (5%) | 272 (11%) * | 107 (21%) * | <0.001 |
| ACEI/ARB | 1780 (30%) | 783 (26%) | 784 (32%) | 213 (43%) * | <0.001 |
| MRA | 419 (7%) | 194 (6%) | 187 (8%) | 38 (8%) | 0.2 |
| Vitamin K antagonist | 1786 (30%) | 747 (25%) | 854 (35%) * | 185 (37%) * | <0.001 |
| DOAC | 268 (5%) | 64 (2%) | 126 (5%) | 78 (16%) | <0.001 |
| Laboratory data | |||||
| MELD-XI score | 9.6 (9.4–12.9) | 9.5 (9.4–10.9) | 10.4 (9.8–11.6)* | 10.9 (10.1–12.5)* | <0.001 |
| eGFR, ml/min/1.73 m2 | 98 ± 39 | 118 ± 26 | 91 ± 21 * | 85 ± 23 * | <0.001 |
| Echocardiogram | |||||
| S-ventricular systolic dysfn | 711 (12%) | 307 (10%) | 311 (13%)* | 93 (19%)* | <0.001 |
| ≥Moderate S-AVVR | 299 (5%) | 121 (4%) | 139 (6%)* | 39 (8%)* | 0.2 |
| NS-ventricular systolic dysfn | 1998 (34%) | 881 (29%) | 903 (37%)* | 214 (43%)* | <0.001 |
| ≥Moderate NS-AVVR | 1183 (20%) | 405 (13%) | 615 (25%)* | 163 (33%)* | <0.001 |
Abbreviations: ACEI: Angiotensin converting enzyme inhibitor; ARB: Angiotensin-II receptor blockers; CHD: congenital heart disease; AVVR: atrioventricular valve regurgitation; CIED: cardiac implantable electronic devices; DOAC: Direct oral anticoagulant; eGFR: estimated glomerular filtration rate; MRA: Mineralocorticoid receptor antagonist; MELD-XI: Model for end-stage liver disease excluding international normalized ratio; NS: non-systemic; S: systemic; TGA: transposition of great arteries.
Footnote: p values were derived from comparisons across all 3 groups using analysis of variance test and chi squared test for continuous and categorical variables respectively. “*” denotes statistically significant difference from pairwise comparisons using the age group 18–40 years as the reference. Ventricular systolic dysfunction was defined as calculated or estimated left ventricular ejection fraction <50% or right ventricular fractional area change <35%. Atrioventricular valve regurgitation was based on qualitative Doppler assessment.
Table 2.
Congenital heart lesions.
| All (N = 5930) | Age 18–40 y (N = 3,009, 51%) | Age 41–65 y (N = 2,422, 41%) | Age >65 y (N = 499, 8%) | |
|---|---|---|---|---|
| Fontan palliation | 442 (8%) | 363 (12%) | 79 (3%) | 0 |
| Tetralogy of Fallot | 893 (15%) | 492 (16%) | 360 (15%) | 41 (8%) |
| Coarctation of aorta | 893 (15%) | 485 (16%) | 318 (13%) | 90 (18%) |
| Ebstein anomaly | 753 (13%) | 351 (12%) | 319 (13%) | 83 (17%) |
| cc-TGA | 234 (4%) | 99 (3%) | 108 (5%) | 27 (5%) |
| d-TGA s/p atrial switch op | 189 (3%) | 127 (4%) | 62 (3%) | 0 |
| Valvular pulmonic stenosis | 291 (5%) | 131 (4%) | 124 (5%) | 36 (7%) |
| Pulmonary atresia with IVS | 53 (0.9%) | 51 (2%) | 2 (0.1%) | 0 |
| Truncus arteriosus | 46 (0.8%) | 37 (1%) | 9 (0.4%) | 0 |
| d-TGA s/p arterial switch/Rastelli op | 164 (3%) | 144 (5%) | 19 (0.8%) | 1 (0.2%) |
| Aortic stenosis | 757 (13%) | 273 (9%) | 453 (19%) | 31 (6%) |
| Atrioventricular canal defect | 335 (6%) | 174 (6%) | 135 (6%) | 26 (5%) |
| ASD/PAPVR | 430 (7%) | 116 (4%) | 193 (8%) | 121 (24%) |
| Double chambered right ventricle | 37 (0.6%) | 15 (0.5%) | 14 (0.6%) | 8 (2%) |
| Subaortic stenosis | 170 (3%) | 70 (2%) | 85 (4%) | 15 (3%) |
| Ventricular septal defect | 74 (1%) | 24 (0.8%) | 38 (2%) | 12 (2%) |
| Others | 169 (3%) | 57 (2%) | 104 (4%) | 8 (2%) |
Abbreviation: ASD: Atrial septal defect; cc-TGA: Congenitally corrected transposition of great arteries; IVS: Intact ventricular septum; PAPVR: Partial anomalous pulmonary venous return.
3.2. Outcomes
3.2.1. All-cause mortality
Of the 5930 patients, 774 (13%) died during 10.9 ± 3.6 years of follow-up, and the average age at the time of death of 53 ± 8 years. Of the 611 patients with documented cause of death, 483 (79%) died from a cardiovascular cause. The unadjusted incidence of all-cause mortality in the overall cohort was 12.1 (95% CI 11.7–12.5) per 1000 patient-years.
Of the 3009 patients in Group 1, 250 (8%) died during a mean follow-up of 11.6 ± 3.2 years, and the average age at the time of death was 34 ± 5 years. Of the 204 patients with documented cause of death, 178 (87%) died from a cardiovascular cause. The unadjusted incidence of all-cause mortality of 7.2 (95% CI 6.8–7.6) per 1000 patient-years. The correlates of all-cause mortality in Group 1 were number of prior cardiac surgeries (hazard ratio [HR] 1.07, 95% CI 1.03–1.11, p = 0.02) for every additional prior cardiac surgery, having complex CHD versus simple/moderately complex CHD (HR 2.63, 95% CI 1.84–3.43, p < 0.001), history of atrial fibrillation (HR 1.89, 95% CI 1.52–2.31, p < 0.001), prior CIED implantation (HR 1.53, 95% CI 1.27–1.81, p = 0.008), and having ≥moderate systemic ventricular systolic dysfunction (HR 1.12, 95% CI 1.06–1.18, p = 0.02), Table 3.
Table 3.
Cox regression model showing correlates of all-cause mortality in group 1 (age 18–40 years at baseline encounter).
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age, years | 1.04 (1.02–1.06) | 0.01 | ||
| Male sex | 1.22 (0.86–1.65) | 0.3 | ||
| Number cardiac surgeries | 1.09 (1.04–1.14) | <0.001 | 1.07 (1.03–1.11) | 0.02 |
| NYHA III/IV (vs I/II) | 2.18 (1.46–2.75) | <0.001 | ||
| Complex CHD (vs simple/moderate) | 3.17 (1.94–4.43) | <0.001 | 2.63 (1.84–3.42) | <0.001 |
| Prosthetic valve | 1.67 (1.33–2.08) | 0.004 | ||
| CIED | 1.82 (1.39–2.46) | 0.002 | 1.53 (1.27–1.81) | 0.008 |
| Comorbidities | ||||
| Hypertension | 1.08 (0.97–1.17) | 0.4 | ||
| Coronary artery disease | 1.44 (1.12–1.78) | 0.01 | ||
| Type 2 diabetes | 1.04 (0.67–1.48) | 0.6 | ||
| Atrial fibrillation | 2.16 (1.44–2.86) | <0.001 | 1.89 (1.52–2.31) | <0.001 |
| Chronic kidney disease ≥ III | 0.98 (0.83–1.14) | 0.3 | ||
| Hepatorenal dysfunction (MELD-XI >11) | 1.08 (1.02–1.14) | 0.03 | ||
| Echocardiographic indices | ||||
| Systemic ventricular systolic dysfunction | 1.14 (1.05–1.26) | 0.02 | 1.12 (1.06–1.18) | 0.02 |
| Non-systemic ventricular systolic dysfunction | 1.10 (1.03–1.17) | 0.01 | ||
| ≥Moderate systemic AVVR | 0.94 (0.74–1.16) | 0.4 | ||
| ≥Moderate non-systemic AVVR | 1.27 (1.08–1.49) | 0.03 | ||
Abbreviation: AVVR: Atrioventricular valve regurgitation; CIED: cardiac implantable electronic device; CI: confidence interval; CHD: congenital heart disease; HR: hazard ratio; MELD-XI: Model for end-stage liver disease excluding international normalized ratio; NYHA: New York Heart Association.
Footnote: The multivariable model was created using stepwise backwards variable selection, and only the variables with p < 0.05 remained in the model.
Of the 2422 patients in Group 2, 374 (15%) died during a mean follow-up of 10.1 ± 3.9 years, and the average age at the time of death was 56 ± 8 years. Of the 286 patients with documented cause of death, 218 (77%) died from a cardiovascular cause. The unadjusted incidence of all-cause mortality of 15.3 (95%CI 14.6–16.1) per 1000 patient-years. The correlates of all-cause mortality in Group 2 were older age (HR 1.04, 95% CI 1.01–1.07, p = 0.01) per 1 year increase in age at baseline encounter, having complex CHD versus simple/moderately complex CHD (HR 1.95, 95% CI 1.42–2.47, p = 0.008), prior CIED implantation (HR 1.37, 95% CI 1.09–1.71, p = 0.002), hepatorenal dysfunction (HR 1.12, 95% CI 1.06–1.18, p = 0.02), history of atrial fibrillation (HR 1.64, 95% CI 1.34–1.96, p = 0.07), presence of ≥moderate systemic ventricular systolic dysfunction (HR 1.15, 95% CI 1.07–1.22, p = 0.01), and presence of ≥moderate non-systemic atrioventricular valve regurgitation (HR 1.19, 95% CI 1.07–1.26, p = 0.01), Table 4.
Table 4.
Multivariable cox regression model showing correlates of all-cause mortality in group 2 (age 41–65 years at baseline encounter).
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age, years | 1.06 (1.03–1.09) | 0.006 | 1.04 (1.01–1.07) | 0.01 |
| Male sex | 1.18 (0.92–1.55) | 0.2 | ||
| Number cardiac surgeries | 1.06 (1.02–1.10) | 0.007 | ||
| NYHA III/IV (vs I/II) | 1.74 (1.33–2.06) | <0.001 | ||
| Complex CHD (vs simple/moderate) | 2.43 (1.84–3.57) | <0.001 | 1.95 (1.42–2.47) | 0.008 |
| Prosthetic valve | 1.27 (1.03–1.53) | 0.006 | ||
| CIED | 1.53 (1.22–1.92) | 0.004 | 1.37 (1.09–1.71) | 0.002 |
| Comorbidities | ||||
| Hypertension | 1.04 (0.91–1.13) | 0.5 | ||
| Coronary artery disease | 1.29 (1.14–1.63) | 0.008 | ||
| Type 2 diabetes | 1.08 (0.84–1.57) | 0.4 | ||
| Atrial fibrillation | 1.97 (1.42–2.46) | <0.001 | 1.64 (1.34–1.96) | 0.007 |
| Chronic kidney disease ≥ III | 1.03 (0.81–1.19) | 0.2 | ||
| Hepatorenal dysfunction (MELD-XI >11) | 1.15 (1.04–1.26) | 0.03 | 1.12 (1.06–1.18) | 0.02 |
| Echocardiographic indices | ||||
| Systemic ventricular systolic dysfunction | 1.19 (1.08–1.31) | 0.01 | 1.15 (1.07–1.22) | 0.01 |
| Non-systemic ventricular systolic dysfunction | 1.06 (1.01–1.12) | 0.03 | ||
| ≥Moderate systemic AVVR | 1.26 (0.81–1.64) | 0.5 | ||
| ≥Moderate non-systemic AVVR | 1.22 (1.08–1.49) | 0.03 | 1.19 (1.12–1.26) | 0.01 |
Abbreviation: AVVR: Atrioventricular valve regurgitation; CIED: cardiac implantable electronic device; CI: confidence interval; CHD: congenital heart disease; HR: hazard ratio; MELD-XI: Model for end-stage liver disease excluding international normalized ratio; NYHA: New York Heart Association.
Footnote: The multivariable model was created using stepwise backwards variable selection, and only the variables with p < 0.05 remained in the model.
Of the 499 patients in Group 3, 150 (30%) died during a mean follow-up of 6.3 ± 2.94 years, and the average age at the time of death was 74 ± 5 years. Of the 121 patients with documented cause of death, 88 (71%) died from a cardiovascular cause. The unadjusted incidence of all-cause mortality of 47.8 (95%CI 43.6–51.2) per 1000 patient-years. The correlates of all-cause mortality in Group 3 were older age (HR 1.26, 95% CI 1.14–1.38, p < 0.001) per 1 year increase in age at baseline encounter, hypertension (HR 1.21, 95% CI 1.04–1.42, p = 0.008), coronary artery disease (HR 1.68, 95% CI 1.30–1.97, p = 0.009), history of atrial fibrillation (HR 1.46, 1.21–1.72, p = 0.02), hepatorenal dysfunction (HR 1.10, 95% CI 1.03–1.17, p = 0.03), presence of ≥moderate systemic ventricular systolic dysfunction (HR 1.17, 95% CI 1.04–1.28, p = 0.02), and presence of ≥moderate non-systemic ventricular systolic dysfunction (HR 1.14, 95% CI 1.09–1.20, p = 0.009), Table 5.
Table 5.
Multivariable cox regression models showing correlates of all-cause mortality in group 3 (age >65 years at baseline encounter).
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age, years | 1.08 (1.05–1.11) | <0.001 | 1.26 (1.14–1.38) | <0.001 |
| Male sex | 1.12 (0.96–1.44) | 0.3 | ||
| Number cardiac surgeries | 1.02 (0.94–1.06) | 0.4 | ||
| NYHA III/IV (vs I/II) | 1.61 (1.39–1.84) | 0.008 | ||
| Complex CHD (vs simple/moderate) | 1.22 (1.07–1.41) | 0.04 | ||
| Prosthetic valve | 1.16 (1.07–1.26) | 0.03 | ||
| CIED | 1.43 (1.17–1.75) | 0.04 | ||
| Comorbidities | ||||
| Hypertension | 1.37 (1.14–1.63) | 0.004 | 1.21 (1.04–1.42) | 0.01 |
| Coronary artery disease | 2.01 (1.42–2.72) | 0.003 | 1.68 (1.30–1.97) | 0.009 |
| Type 2 diabetes | 1.09 (0.87–1.35) | 0.2 | ||
| Atrial fibrillation | 1.51 (1.26–2.03) | 0.005 | 1.46 (1.21–1.72) | 0.02 |
| Chronic kidney disease ≥ III | 1.06 (0.84–1.22) | 0.4 | ||
| Hepatorenal dysfunction (MELD-XI >11) | 1.12 (1.02–1.23) | 0.01 | 1.10 (1.03–1.17) | 0.03 |
| Echocardiographic indices | ||||
| Systemic ventricular systolic dysfunction | 1.38 (1.14–1.74) | 0.009 | 1.17 (1.04–1.28) | 0.02 |
| Non-systemic ventricular systolic dysfunction | 1.09 (1.03–1.15) | 0.01 | 1.14 (1.09–1.20) | 0.009 |
| ≥Moderate systemic AVVR | 1.18 (0.86–1.45) | 0.4 | ||
| ≥Moderate non-systemic AVVR | 1.26 (1.12–1.51) | 0.02 | ||
Abbreviation: AVVR: Atrioventricular valve regurgitation; CIED: cardiac implantable electronic device; CI: confidence interval; CHD: congenital heart disease; HR: hazard ratio; MELD-XI: Model for end-stage liver disease excluding international normalized ratio; NYHA: New York Heart Association.
Footnote: The multivariable model was created using stepwise backwards variable selection, and only the variables with p < 0.05 remained in the model.
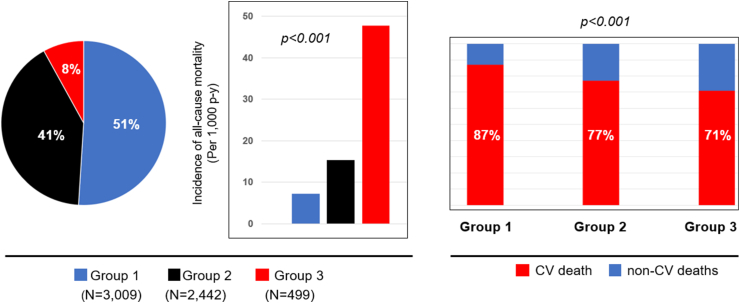
Compared to Group 1, Group 2 had a higher unadjusted incidence of all-cause mortality (15.3 [95%CI 14.6–16.1] versus 7.2 [95% CI 6.8–7.6] per 1000 patient-years, p < 0.001), and lower proportion of deaths from cardiovascular causes (77% versus 87%, p = 0.003) (Fig. 1). Similarly, Group 3 had a higher unadjusted incidence of all-cause mortality (47.8 [95% CI 43.6–51.2] versus 7.2 [95% CI 6.8–7.6] per 1000 patient-years, p < 0.001), and lower proportion of deaths from cardiovascular causes (71% versus 87%, p < 0.001), compared to Group 1 (Fig. 1). Furthermore, the correlates of all-cause mortality were different between the age groups, with acquired comorbidities such as hypertension, coronary artery disease, and hepatorenal dysfunction having significant association with mortality in Group 3, while indices of CHD severity such as number of prior cardiac surgeries, presence of complex CHD, and prior CIED implantation having significant association with mortality in Group 1 (Table 3, Table 4, Table 5).
Fig. 1.
(Left) Pie chart showing the proportion of patients in each age group. Group 1 (age 18–40 y), Group (age 41–65 y), Group (age >65 y). (Middle) Bar graphs comparing the incidence of all-cause mortality between Groups 1, 2 and 3. P value represents comparison across the 3 groups. (Right) Bar graphs comparing the proportion of deaths due to cardiovascular (CV) deaths between Groups 1, 2 and 3. P value represents comparison across the 3 groups.
4. Discussion
In this study, we assessed the clinical characteristics, as well as the incidence and correlates of all-cause mortality across different age groups of adults with CHD. The main findings were: (1) About half of adults with CHD were less than 40 years of age, and only 8% were over the age of 65 years at the time of baseline encounter in a tertiary adult CHD center. (2) Patients in the older age group were less likely to have complex CHD, but more likely to have acquired comorbidities, end-organ dysfunction, ventricular systolic dysfunction, and valvular heart disease as compared to the younger age groups. (3) The older age groups had significantly higher incidence of all-cause mortality (as expected), but lower proportion of death due to cardiovascular causes as compared to the younger age groups. (4) The correlates of all-cause mortality in the older age group were more likely be acquired comorbidities in contrast to the younger age groups where the indices of CHD severity were the more significant correlates of mortality. (5) Systemic ventricular dysfunction was associated with all-cause mortality in the overall cohort, and across all age groups.
Prior to the 2000s, CHD was predominantly a pediatric disease because a greater proportion of CHD patients were less than 18 years of age [2,3,16,17]. In the current era, on the other hand, CHD is now a predominantly an adult disease because a great proportion of CHD patients are now older than 18 years of age [2,3,[16], [17], [18]]. This demographic change is due to improved long-term survival of CHD patients. While longevity is a desired outcome, aging is associated with acquired comorbidities and end-organ dysfunction. These changes are expected to modify the natural history ofthe underlying CHD. The current study showS that young adults (Group 1) had a higher prevalence of complex CHD but lower prevalence of acquired comorbidities, and the indices of CHD severity were important correlates of all-cause mortality as compared to the other age groups. In contrast to the younger adults (Group 1), the middle-aged (Group 2) and older patients (Group 3), had a relatively higher prevalence of acquired comorbidities and end-organ dysfunction as compared to the young adult group, and these comorbidities (instead of CHD severity) were associated with all-cause mortality.
Several studies have reported the incidence, etiology, and risk factors for mortality in adults with CHD [2,3,16,17]. In a retrospective cohort study based on the Dutch CONCOR (Congenital Corvita) registry, Verheugt et al. reported that, among 6933 patients, the median age at the time of death was 49 years, and that 77% of deaths were due to cardiovascular causes [19]. The correlates of mortality in that cohort were older age and CHD severity [19]. Similar findings were reported in another retrospective cohort study based on the German National Registry for Congenital Heart Defects containing data of 2596 adults with CHD [20]. In that study, the annual incidence of all-cause mortality was 1.7% per year, the median age at the time of death was 40 years, and more than 80% of the patients died from cardiovascular causes [20]. While the estimate from the previous studies were comparable to the results of the current study, a novel finding from the current study was that the correlates of all-cause mortality differed across the age groups, which in turn, suggests that different sets of interventions would be required to address the risk factors in each age group. These findings would enable the providers to prioritize care around factors associated with mortality for the different age groups. For instance, the primary focus should be on the underlying congenital heart lesion in the younger age group, while there should be a greater emphasis on screening and treatment of hypertension and coronary artery disease, as well as prevention of end-organ dysfunction in the middle age and older age groups.
Another important observation from the current study was the consistent association between systemic ventricular dysfunction and all-cause mortality in the overall cohort, and across all 3 age groups. This provides an important target for intervention since standard guideline directed medical therapy for heart failure has been shown to be effective in reversing systemic ventricular systolic dysfunction, and reducing the risk of cardiovascular events in CHD patients with systemic left ventricle [21,22]. This underscores the need for early initiation and titration of heart failure therapy in order to reduce the incidence of mortality in this population.
4.1. Limitations
This is a retrospective single center study and is therefore prone to selection and ascertainment bias. The proportion of patients with moderate and complex CHD in this study is significantly higher than reported in other studies, and this may limit the generalizability of the results. We relied on mortality data from the medical records, and hence we could have underestimated the risk of mortality in this cohort.
5. Conclusions
About half of adults with CHD were young adults at the time of baseline encounter in our adult CHD clinic, and these patients had different clinical characteristics and risk profile as compared to the middle-aged and older patients. These results suggest a need for management strategies tailored to address the correlates of outcomes in each age group, and to modify these management strategies as the patients progress from one age group to the next. Furthermore, the association between systemic ventricular dysfunction and mortality was consistent across all age groups, thereby providing a viable target for intervention considering the known benefits of medical therapy for this lesion.
Funding
Dr. Egbe is supported by National Heart, Lung, and Blood Institute (NHLBI) grants (R01 HL158517 and R01 HL160761). The MACHD Registry is supported by the Al-Bahar Research grant.
CRediT authorship contribution statement
Alexander C. Egbe: Writing – original draft, Writing – review & editing. William R. Miranda: Writing – original draft, Writing – review & editing. Marwan Ahmed: Writing – original draft, Writing – review & editing. Snigdha Karnakoti: Writing – original draft, Writing – review & editing. Sriharsha Kandlakunta: Writing – original draft, Writing – review & editing. Muhammad Eltony: Writing – original draft, Writing – review & editing. Marianne Meshreky: Writing – original draft, Writing – review & editing. Luke J. Burchill: Writing – original draft, Writing – review & editing. Heidi M. Connolly: Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Moons P., Bovijn L., Budts W., Belmans A., Gewillig M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation. 2010;122:2264–2272. doi: 10.1161/CIRCULATIONAHA.110.946343. [DOI] [PubMed] [Google Scholar]
- 2.Gilboa S.M., Devine O.J., Kucik J.E., Oster M.E., Riehle-Colarusso T., Nembhard W.N., Xu P., Correa A., Jenkins K., Marelli A.J. Congenital heart defects in the United States estimating the magnitude of the affected population in 2010. Circulation. 2016;134:101. doi: 10.1161/CIRCULATIONAHA.115.019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marelli A.J., Ionescu-Ittu R., Mackie A.S., Guo L., Dendukuri N., Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130:749–756. doi: 10.1161/CIRCULATIONAHA.113.008396. [DOI] [PubMed] [Google Scholar]
- 4.Lopez K.N., Morris S.A., Sexson Tejtel S.K., Espaillat A., Salemi J.L. US mortality attributable to congenital heart disease across the lifespan from 1999 through 2017 exposes persistent racial/ethnic disparities. Circulation. 2020;142:1132–1147. doi: 10.1161/CIRCULATIONAHA.120.046822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth G.A., Johnson C., Abajobir A., Abd-Allah F., Abera S.F., Abyu G., Ahmed M., Aksut B., Alam T., Alam K., Alla F., Alvis-Guzman N., Amrock S., Ansari H., Arnlov J., Asayesh H., Atey T.M., Avila-Burgos L., Awasthi A., Banerjee A., Barac A., Barnighausen T., Barregard L., Bedi N., Belay Ketema E., Bennett D., Berhe G., Bhutta Z., Bitew S., Carapetis J., Carrero J.J., Malta D.C., Castaneda-Orjuela C.A., Castillo-Rivas J., Catala-Lopez F., Choi J.Y., Christensen H., Cirillo M., Cooper L., Jr., Criqui M., Cundiff D., Damasceno A., Dandona L., Dandona R., Davletov K., Dharmaratne S., Dorairaj P., Dubey M., Ehrenkranz R., El Sayed Zaki M., Faraon E.J.A., Esteghamati A., Farid T., Farvid M., Feigin V., Ding E.L., Fowkes G., Gebrehiwot T., Gillum R., Gold A., Gona P., Gupta R., Habtewold T.D., Hafezi-Nejad N., Hailu T., Hailu G.B., Hankey G., Hassen H.Y., Abate K.H., Havmoeller R., Hay S.I., Horino M., Hotez P.J., Jacobsen K., James S., Javanbakht M., Jeemon P., John D., Jonas J., Kalkonde Y., Karimkhani C., Kasaeian A., Khader Y., Khan A., Khang Y.H., Khera S., Khoja A.T., Khubchandani J., Kim D., Kolte D., Kosen S., Krohn K.J., Kumar G.A., Kwan G.F., Lal D.K., Larsson A., Linn S., Lopez A., Lotufo P.A., El Razek H.M.A., Malekzadeh R., Mazidi M., Meier T., Meles K.G., Mensah G., Meretoja A., Mezgebe H., Miller T., Mirrakhimov E., Mohammed S., Moran A.E., Musa K.I., Narula J., Neal B., Ngalesoni F., Nguyen G., Obermeyer C.M., Owolabi M., Patton G., Pedro J., Qato D., Qorbani M., Rahimi K., Rai R.K., Rawaf S., Ribeiro A., Safiri S., Salomon J.A., Santos I., Santric Milicevic M., Sartorius B., Schutte A., Sepanlou S., Shaikh M.A., Shin M.J., Shishehbor M., Shore H., Silva D.A.S., Sobngwi E., Stranges S., Swaminathan S., Tabares-Seisdedos R., Tadele Atnafu N., Tesfay F., Thakur J.S., Thrift A., Topor-Madry R., Truelsen T., Tyrovolas S., Ukwaja K.N., Uthman O., Vasankari T., Vlassov V., Vollset S.E., Wakayo T., Watkins D., Weintraub R., Werdecker A., Westerman R., Wiysonge C.S., Wolfe C., Workicho A., Xu G., Yano Y., Yip P., Yonemoto N., Younis M., Yu C., Vos T., Naghavi M., Global Murray C. Regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth G.A., Nguyen G., Forouzanfar M.H., Mokdad A.H., Naghavi M., Murray C.J. Estimates of global and regional premature cardiovascular mortality in 2025. Circulation. 2015;132:1270–1282. doi: 10.1161/CIRCULATIONAHA.115.016021. [DOI] [PubMed] [Google Scholar]
- 7.Berry J.D., Dyer A., Cai X., Garside D.B., Ning H., Thomas A., Greenland P., Van Horn L., Tracy R.P., Lloyd-Jones D.M. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkins J.T., Ning H., Berry J., Zhao L., Dyer A.R., Lloyd-Jones D.M. Lifetime risk and years lived free of total cardiovascular disease. JAMA, J Am Med Assoc. 2012;308:1795–1801. doi: 10.1001/jama.2012.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenland P., Knoll M.D., Stamler J., Neaton J.D., Dyer A.R., Garside D.B., Wilson P.W. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA, J Am Med Assoc. 2003;290:891–897. doi: 10.1001/jama.290.7.891. [DOI] [PubMed] [Google Scholar]
- 10.Stout K.K., Daniels C.J., Aboulhosn J.A., Bozkurt B., Broberg C.S., Colman J.M., Crumb S.R., Dearani J.A., Fuller S., Gurvitz M., Khairy P., Landzberg M.J., Saidi A., Valente A.M., Van Hare G.F. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e81–e192. doi: 10.1016/j.jacc.2018.08.1029. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell C., Rahko P.S., Blauwet L.A., Canaday B., Finstuen J.A., Foster M.C., Horton K., Ogunyankin K.O., Palma R.A., Velazquez E.J. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American society of echocardiography. J Am Soc Echocardiogr: Off Pub Am Soc Echocardiograp. 2019;32:1–64. doi: 10.1016/j.echo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., Lancellotti P., Muraru D., Picard M.H., Rietzschel E.R., Rudski L., Spencer K.T., Tsang W., Voigt J.U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr: Off Pub Am Soc Echocardiograp. 2015;28 doi: 10.1016/j.echo.2014.10.003. 1–39 e14. [DOI] [PubMed] [Google Scholar]
- 13.Rudski L.G., Lai W.W., Afilalo J., Hua L., Handschumacher M.D., Chandrasekaran K., Solomon S.D., Louie E.K., Schiller N.B. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr: Off Pub Am Soc Echocardiograp. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 14.Egbe A.C., Miranda W.R., Dearani J., Kamath P.S., Connolly H.M. Prognostic role of hepatorenal function indexes in patients with ebstein anomaly. J Am Coll Cardiol. 2020;76:2968–2976. doi: 10.1016/j.jacc.2020.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hicks K.A., Mahaffey K.W., Mehran R., Nissen S.E., Wiviott S.D., Dunn B., Solomon S.D., Marler J.R., Teerlink J.R., Farb A., Morrow D.A., Targum S.L., Sila C.A., Thanh Hai M.T., Jaff M.R., Joffe H.V., Cutlip D.E., Desai A.S., Lewis E.F., Gibson C.M., Landray M.J., Lincoff A.M., White C.J., Brooks S.S., Rosenfield K., Domanski M.J., Lansky A.J., McMurray J.J.V., Tcheng J.E., Steinhubl S.R., Burton P., Mauri L., O'Connor C.M., Pfeffer M.A., Hung H.M.J., Stockbridge N.L. Chaitman BR, temple RJ and standardized data collection for cardiovascular trials I. 2017 cardiovascular and stroke endpoint definitions for clinical trials. J Am Coll Cardiol. 2018;71:1021–1034. doi: 10.1016/j.jacc.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 16.Gilboa S.M., Salemi J.L., Nembhard W.N., Fixler D.E., Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122:2254–2263. doi: 10.1161/CIRCULATIONAHA.110.947002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Leary J.M., Siddiqi O.K., de Ferranti S., Landzberg M.J., Opotowsky A.R. The changing demographics of congenital heart disease hospitalizations in the United States, 1998 through 2010. JAMA, J Am Med Assoc. 2013;309:984–986. doi: 10.1001/jama.2013.564. [DOI] [PubMed] [Google Scholar]
- 18.Marelli A.J., Mackie A.S., Ionescu-Ittu R., Rahme E., Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 19.Verheugt C.L., Uiterwaal C.S., van der Velde E.T., Meijboom F.J., Pieper P.G., van Dijk A.P., Vliegen H.W., Grobbee D.E., Mulder B.J. Mortality in adult congenital heart disease. Eur Heart J. 2010;31:1220–1229. doi: 10.1093/eurheartj/ehq032. [DOI] [PubMed] [Google Scholar]
- 20.Engelings C.C., Helm P.C., Abdul-Khaliq H., Asfour B., Bauer U.M., Baumgartner H., Kececioglu D., Korten M.A., Diller G.P., Tutarel O. Cause of death in adults with congenital heart disease - an analysis of the German National Register for Congenital Heart Defects. Int J Cardiol. 2016;211:31–36. doi: 10.1016/j.ijcard.2016.02.133. [DOI] [PubMed] [Google Scholar]
- 21.Egbe A.C., Miranda W.R., Pellikka P.A., DeSimone C.V., Connolly H.M. Prevalence and prognostic implications of left ventricular systolic dysfunction in adults with congenital heart disease. J Am Coll Cardiol. 2022;79:1356–1365. doi: 10.1016/j.jacc.2022.01.040. [DOI] [PubMed] [Google Scholar]
- 22.Egbe A.C., Miranda W.R., Anderson J.H., Pellikka P.A., Connolly H.M. Prognostic value of left ventricular global longitudinal strain in patients with congenital heart disease. Circul Cardiovasc Imag. 2022 doi: 10.1161/CIRCIMAGING.122.014865. [DOI] [PMC free article] [PubMed] [Google Scholar]