Abstract
Background
Limited epidemiological evidence exists regarding the role of healthy eating patterns in reducing the risk of Crohn’s disease (CD) and ulcerative colitis (UC). This study aimed to investigate the association between adherence to four established healthy eating patterns and subsequent CD or UC risk, and further examined whether these associations are linked to anti-inflammatory mechanisms.
Methods
We conducted a prospective cohort study of 197,391 participants from the UK Biobank who completed at least one dietary questionnaire and were free from inflammatory bowel disease or cancer at baseline. Four dietary patterns were assessed, including Alternate Mediterranean Diet (AMED), Healthy Eating Index 2015 (HEI-2015), Healthful Plant-based Diet Index (HPDI), and EAT-Lancet. Cox proportional models with restricted cubic splines were applied to explore the associations. The potential role of low-grade inflammation in these associations was examined through mediation analysis.
Results
During 2,193,436 person-years follow-up, 260 CD and 601 UC cases were identified. Higher AMED and HEI-2015 scores were associated with a reduced risk of CD but no UC, with no evidence against nonlinearity. These associations remained consistent across multiple sensitive and subgroup analyses. For dietary components, the fruits and monounsaturated fatty acids: saturated fatty acids ratio in AMED, and total fruits, total protein foods and fatty acid in HEI-2015 were linked to a decreased CD risk. Both diets were also associated with lower plasma inflammation biomarkers. Mediation analysis indicated that 7.66% and 13.40% of the reductions in CD risk attributed to AMED and HEI-2015 diets, respectively, were mediated by low-grade inflammation scores.
Conclusions
Higher adherence to AMED and HEI-2015 might significantly reduce CD risk, partly due to their anti-inflammatory properties.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03809-x.
Keyword: Healthy dietary patterns, Crohn’s disease, Ulcerative colitis, Low-grade inflammation score
Background
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic and relapsing inflammatory disorder affecting the gastrointestinal tract globally. The reported prevalence of IBD in 2019 has reached 4.9 million cases, with a rapid increase across various age and gender groups in both developed and developing countries, notably in Europe and North America [1, 2]. The etiology of IBD is complex and multifactorial, with genetic and environmental factors playing significant roles. Diet, among these factors, has been highlighted as one of the most influential environmental contributors, affecting the gut microbiota and influencing systemic inflammation and immune responses [3–5], which are associated with IBD development. However, these associations are observed after diagnosis, and no study has established direct causality.
Recent studies have suggested the potential benefits of specific nutrients, such as omega-3 fatty acids [6], dietary fiber [7, 8], and food items like fruits and vegetables [8] in mitigating the onset and symptoms of IBD. Meanwhile, several studies have indicated that the consumption of ultra-processed foods [9], high red meat consumption [10], and a pro-inflammation dietary pattern [11] were associated with an increased risk of CD or UC. However, due to the complex interplay of nutrients and foods, precise dietary recommendations for IBD prevention remain challenging. Notably, dietary guidelines, including the European Food-Based Dietary Guidelines (FBDGs) [12] and the Dietary Guidelines for Americans (DGAs) [13, 14], have shifted their focus from individual nutrients to healthy eating patterns for disease prevention, with various recommendations being made up to 2020 and updated in subsequent editions up to 2020–2025 [15, 16]. This dietary pattern approach better reflects real-world dietary practices and considers the cumulative effects of different dietary components and has been extensively studied in relation to chronic inflammatory diseases, such as rheumatoid arthritis [17], chronic obstructive pulmonary disease [18], cardiovascular diseases [19], and diabetes [20]. However, comprehensive research on the long-term effects of specific healthy eating patterns on IBD risk is still lacking.
To address this gap, we conducted a prospective analysis using data from the UK Biobank database. We selected four established healthy eating patterns, quantified by various dietary scores including the Alternate Mediterranean Diet (AMED) score, Healthy Eating Index 2015 (HEI-2015), Healthful Plant-based Diet Index (HPDI), and EAT-Lancet score, to analyze their associations with the risk of developing CD and UC. These dietary scores were selected because they represent comprehensive measures of adherence to healthy eating patterns, encompassing a wide range of dietary components known to impact inflammation and immune responses [21–23], both of which are associated with IBD development. These indices have also been extensively validated and widely used in epidemiological researches [19, 24–29], to assess the overall quality of diet but have distinct characteristics that capture different aspects of dietary patterns. For example, the AMED score is based on the Mediterranean diet, emphasizing high consumption of fruits, vegetables, whole grains, nuts, legumes, and olive oil, along with moderate intake of fish and low intake of red and processed meats, with light alcohol consumption [30]. The HEI-2015 assesses adherence to the Dietary Guidelines for Americans, promoting a diet rich in fruits, vegetables, whole grains, and lean proteins while limiting saturated fats, added sugars, and sodium [31] The HPDI emphasizes the health benefits associated with plant-based foods while minimizing consumption of animal-based products [8]. The EAT-Lancet score reflects a holistic approach to diet, considering both human health and environmental impact [32].
Low-grade inflammatory response refers to a subtle increase in peripheral pro-inflammatory markers, such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), even in the absence of noticeable clinical symptoms. These markers have been implicated in the pathogenesis and disease activity of IBD [33–35]. Moreover, elevated levels of these markers have been associated with poorer treatment outcomes and prognosis in IBD patients. For example, a high baseline level of high-sensitivity CRP has been linked to a reduced remission rate in ulcerative colitis (UC) patients [36]. However, while inflammatory markers and scores are known to be associated with various diseases, their specific relationship with the risk of developing IBD remains unclear and warrants further investigation. It is also important to explore whether these markers mediate the association between diet and IBD risk, particularly considering the potential anti-inflammatory effects of certain dietary patterns, such as the Mediterranean diet [5]. Previous studies have suggested significant relationships between dietary patterns and changes in inflammatory biomarkers. However, these associations have primarily been identified in retrospective studies, with a notable lack of prospective investigations [4].
In the present study, we aimed to assess which dietary pattern demonstrates the most significant association with lower IBD risk and investigate potential relationships with anti-inflammatory mechanisms. By examining these associations, our study may provide valuable insights into optimal dietary recommendations for individuals at risk of IBD, contribute to the development of targeted dietary interventions for IBD prevention, and provide initial clues for further research on related mechanisms.
Methods
Study population
This study utilized data from the UK Biobank (https://www.ukbiobank.ac.uk) with application number 51671 [37], a comprehensive resource comprising detailed genetic and health information obtained from 500,000 individuals aged 40–69 years since 2006. The UK Biobank, established with the support of the UK Biobank Board and approved by the National Research Ethics Committee (REC ID: 16/NW/0274), serves as a valuable platform for investigating the interplay between genetic, environmental, and lifestyle factors in disease development. Participants in the UK Biobank provide extensive data, including demographic information, physical measurements, and biological samples (such as blood, urine, and saliva), along with detailed health and lifestyle questionnaires [38]. This rich dataset enables researchers to explore the intricate relationships between genetic and environmental factors in disease development [39] and to discover new biomarkers for early disease detection [40]. Similar studies utilizing UK Biobank data have been published, contributing to a growing body of literature examining various aspects of health and disease. Access to the UK Biobank data is granted to approved researchers upon payment and submission of study proposals for review and approval through the UK Biobank Access Management System. This ensures that research conducted using UK Biobank data is transparent, rigorous, and aligned with the principles of data protection and privacy. Detailed information about the UK Biobank can be found at https://www.ukbiobank.ac.uk/. Participants provided informed consent by signing consent forms. Our study analyzed a sample of 197,391 participants who completed at least one dietary questionnaire, excluding those with lost follow-up or self-reported/diagnosed IBD or cancer at baseline (Additional file 1: Fig. S1). We also excluded participants with implausible energy intake (< 500 kcal or > 5000 kcal), as previously suggested [21].
Assessment of dietary scores
Dietary information was collected through the Oxford WebQ (www.ceu.ox.ac.uk/research/oxford-webq), a web-based 24-h recall questionnaire designed for large population studies. The validation of the Oxford WebQ has been previously confirmed [41]. Participants within the UK Biobank completed the Oxford WebQ on five separate occasions over 5 years, and mean values were computed from available data based on previous studies [42]. From April 2009 to September 2010, participants were invited to complete an online 24-h recall dietary questionnaire. A total of 70,655 individuals completed the first questionnaire. Subsequent follow-ups were conducted, with the first follow-up from February 2011 to April 2011 collecting 100,517 questionnaires, the second follow-up collecting 83,200 questionnaires (June 2011 to September 2011), the third follow-up collecting 103,698 questionnaires (October 2011 to December 2011), and the fourth follow-up collecting 100,168 questionnaires (April 2012 to June 2012). In this study, participants who completed at least one questionnaire, totaling 291,579 individuals, were included in the analysis. Participant responses for each dietary item were based on assigned portion sizes, with options like “None,” “1/2,” “1,” “2,” “3,” “4,” and “5 + ,” varying across items. Certain components like sodium, saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) were indirectly estimated from food consumption [43]. During the calculation of dietary scores, original food portion sizes were also converted to grams using the same portion size definition as specified in the Oxford WebQ [44].
Detailed components and criteria for scoring each dietary pattern are available in Additional file 1: Tables S1–4, based on official definitions or prior studies [29, 30, 45, 46]. Specifically, the AMED score encompasses 9 components with a range of 0 to 9 [30]. HEI-2015 calculations involved converting food units to the appropriate units utilizing the Food Patterns Equivalents Database from US Department of Agriculture [47]. HEI-2015 is composed of 13 components, ranging from 0 to 100 [31]. The HPDI consists of 18 components within a range of 18 to 90 [48], and the EAT-Lancet score incorporates 14 components ranging from 0 to 14 [29]. Higher scores mean greater adherence to healthy eating patterns.
Assessment of outcome
Participants were monitored by linking their data to the Health and Social Care Information Centre (in England and Wales) and the National Health Service Central Register (in Scotland). The study outcomes were the incidences of CD and UC, classified based on the International Classification of Diseases (ICD)−10 codes (K50 for CD, K51 for UC), identified through linkage to hospital inpatient database, primary care database, and cancer and death registries. This method of case ascertainment, using national health databases, provides a robust and validated approach, as confirmed by previous related study [49].
Assessment of low-grade inflammation level
To evaluate the degree of low-grade inflammation, we introduced an aggregated index known as the low-grade inflammation score (INFLA-score), which has been validated in various studies [50, 51]. The INFLA-score is a novel scoring system that takes into account various biomarkers of systemic inflammation to quantify the concept of low-grade inflammation. This score integrates four distinct plasma inflammation biomarkers: white blood cell (WBC) count, platelet (PLT) count, C-reactive protein (CRP) level, and neutrophil-to-lymphocyte ratio (NLR) [52]. All biomarkers used to calculate the low-grade inflammation score were assessed at the time of participant recruitment, during the period from 2006 to 2010. These biomarkers were chosen based on their established roles in reflecting systemic inflammatory status and their relevance in previous research on inflammation and chronic diseases [53, 54]. Within this framework, each inflammation biomarker was allocated a score based on its position within the distribution. Specifically, scores ranging from 1 to 4 were assigned to biomarkers within the 7th to 10th deciles, while scores ranging from − 4 to 1 were assigned to those in the 1st to 4th deciles. The calculation of the INFLA-score involved assigning each of the four components a value from − 4 to 4 based on their respective deciles, with the summation of these values yielding an overall INFLA-score that could range from − 16 to 16. A higher INFLA-score represented an increased intensity of low-grade inflammation.
Assessment of covariates
Participants provided self-reported information on sociodemographic factors (e.g., age, sex, ethnicity), lifestyle factors (e.g., smoking status, alcohol consumption, and daily sleeping time), medication intake (multivitamins, mineral supplements, aspirin, non-aspirin non-steroidal anti-inflammatory drug (NSAIDs), statins), and comorbidities (e.g., hypertension, hypercholesterolemia, and diabetes). Information on socioeconomic status, measured by index of multiple deprivation (IMD), and self-reported longstanding illness were also collected through the baseline questionnaire. Trained research staff measured participants’ height and weight to calculate their body mass index (BMI). Physical activity levels were assessed using the International Physical Activity Questionnaire-Short Form (IPAQ-SF) [55]. These covariates were selected as confounders in our analysis based on existing literature reporting their association with both the exposure and the risk of CD and UC [56, 57]. This method ensured that critical variables were considered in our analyses to mitigate potential confounding effects.
Statistical analysis
In descriptive analyses, values were presented as either a mean (standard deviation) or number (percentage). Some variables, including the IMD, BMI, and physical activity, had missing values. To address this, we employed multiple imputation techniques to fill in the missing data. Our origin time is defined as the participant’s enrollment date in the UK Biobank, which occurred between 2006 and 2010. The start time in our survival analysis is the date when each participant first completed their dietary assessment. The end time corresponds to the first occurrence of events, including the diagnosis of CD or UC, death, or the end of follow-up (December 31, 2021). Person-years were computed from the date of the first dietary information assessment to CD/UC diagnosis, death, or the end of follow-up (December 31, 2021), whichever came first. We employed multivariable Cox regression models, taking age as the underlying timescale, to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association of healthy eating patterns with subsequent risk of incidence of CD and UC. The assumption of proportional hazards was tested by Schoenfeld tests in the UK Biobank and no violation of this assumption was found.
We stratified the analyses jointly by age, sex, and UK Biobank assessment centers in the basic model (model 1). To control for potential confounding by sociodemographic factors, lifestyle variables, medications, and health status, we additionally adjusted for ethnicity (white or other), index of multiple deprivation (continuous), BMI (continuous), smoking status (never, previous, current), alcohol consumption (special occasions only/never, 1–3 times a month, 1–4 times a week, daily or almost daily), physical activity (expressed in MET hours/week as a continuous variable), and daily sleep duration (continuous), as well as binary variables including medication use (multivitamins, mineral supplements, aspirin, non-aspirin NSAIDs, statins), comorbidities (e.g., hypertension, hypercholesterolemia, diabetes), and longstanding illness in model 2. To mitigate the possibility of reverse causality, participants who developed IBD in the initial years of follow-up were excluded, as we previously reported [49]. We utilized restricted cubic splines with four knots at the 5th, 35th, 65th, and 95th centiles to model the potential non-linear associations between dietary scores and the risk of CD and UC. This choice aligns with recommendations from Harrell’s “Regression Modeling Strategies” [58], which suggests that four knots provide a balance between model fit and smoothness while avoiding overfitting. Cubic splines are a flexible and powerful tool for capturing complex, nonlinear associations in data by fitting a series of cubic polynomials between predefined knots [59]. By utilizing cubic splines, we were able to accurately assess the shape of the relationship between each estimated dietary score and the outcome variables. This method allows us to explore potential non-linear relationships between dietary patterns and the risk of IBD, providing a more nuanced understanding of how different levels of dietary adherence may impact IBD risk.
We conducted several sensitivity analyses to test the robustness of our findings. Firstly, we excluded IBD cases that occurred within the first 2 years of follow-up to mitigate any potential reverse causation effects. Secondly, given that Oxford WebQ relies on diet recall for a single day and may not fully represent participants’ typical dietary habits [42], we performed a sensitivity analysis that excluded participants reporting atypical diets during any of the five follow-up assessments. Thirdly, considering the association between dietary intake and BMI, which can influence health outcomes [60], we examined the potential over-adjustment bias by comparing associations estimated from models both with and without BMI categories. Fourthly, digestive system disorders may affect the dietary habits of participants. Therefore, we are excluding participants with pre-existing digestive system disorders at baseline and those who developed digestive system disorders during the period between baseline and completion of the dietary questionnaire. Lastly, as covariates were collected prior to dietary assessments, there is a potential for temporal mismatch that might introduce variability in the results. To minimize this risk, we conducted sensitivity analyses excluding participants with a time gap of more than 2 years between covariate and dietary data collection. Furthermore, to explore potential effect modification, we analyzed whether the association between healthy diet patterns and IBD risk varied across subgroups defined by sex, age, obesity, smoking, drinking, physical activity, and regular NSAIDs use. Interaction tests were conducted by introducing dietary scores and these covariates as multiplicative interaction terms in our models. We did not perform multiple testing correction in the present study.
Mediation analysis allows for the decomposition of the total effect of an exposure on an outcome into direct and indirect effects, with the indirect effect operating through one or more mediator variables [61]. This approach enables the estimation of the natural direct and indirect effects, as well as the proportion mediated, while accounting for potential confounding factors. By conducting mediation analyses, it provides insight into how much of the association between diet and IBD risk can be explained by changes in inflammatory biomarkers, thus highlighting the importance of inflammation as a potential mediator. To investigate whether inflammation mediated the association of healthy eating patterns with the risk of CD and UC, we first conducted the linear regression analysis to confirm the association of healthy eating patterns with INFLA-score and four inflammation biomarkers. Subsequently, we conducted multivariable Cox regression analysis to verify the correlation between the INFLA-score, the four inflammation biomarkers, and the onset of CD and UC. Next, we conducted multivariable Cox regression analysis to confirm the association of INFLA-score and four inflammation biomarkers with the incidences of CD and UC. Finally, we estimated the proportion of the total association mediated through inflammation using the “mediator” package in R. Statistical analysis was conducted under the guidance and supervision of experienced biostatisticians with expertise in epidemiological research methods and statistical techniques. We performed all analyses using the R software (version 3.5.0, R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
A total of 197,391 participants from the UK Biobank were included in this study, from 2006 to 2010 (Additional file 1: Fig. S1). Table 1 presents the characteristics of the study participants based on the distribution of dietary scores. Participants with higher dietary scores tended to be female, engaged in regular exercise, had a lower BMI and IMD, and tended not to have hypercholesterolemia, diabetes, or longstanding illness. Additionally, those with higher scores were more inclined to use vitamin or mineral supplements and less likely to use medications such as aspirin, NSAIDs, or statins.
Table 1.
Baseline characteristics across healthy eating patterns quartiles among UK Biobank participants
| Characteristics | AMED score | HEI-2015 tertile | HPDI tertile | EAT-Lancet tertile | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–3 | 4–5 | 6–9 | Q1 | Q2 | Q3 | Q1 | Q2 | Q3 | Q1 | Q2 | Q3 | |
| No. of participants | 92,598 | 70,255 | 34,538 | 65,797 | 65,797 | 65,797 | 59,051 | 66,239 | 72,101 | 55,905 | 55,675 | 85,811 |
| Mean (SD)a age, years | 56.1 (8.07) | 56.6 (7.87) | 56.8 (7.76) | 55.5 (8.16) | 56.5 (7.95) | 57.2 (7.64) | 55.6 (8.09) | 56.5 (7.95) | 56.9 (7.78) | 56.7 (7.90) | 56.5 (7.97) | 56.2 (7.96) |
| Female, N (%) | 48,632 (52.5) | 38,767 (55.2) | 20,525 (59.4) | 31,666 (48.1) | 35,426 (53.8) | 40,832 (62.1) | 27,772 (47.0) | 35,666 (53.8) | 44,486 (61.7) | 26,783 (47.9) | 29,899 (53.7) | 51,242 (59.7) |
| White, N (%) | 87,962 (95.0) | 67,398 (95.9) | 33,443 (96.8) | 62,448 (94.9) | 62,968 (95.7) | 63,387 (96.3) | 56,680 (96.0) | 63,503 (95.9) | 68,620 (95.2) | 54,213 (97.0) | 53,464 (96.0) | 81,126 (94.5) |
| Mean (SD) index of multiple deprivation | 16.2 (12.9) | 15.0 (12.0) | 14.3 (11.4) | 16.3 (13.0) | 15.2 (12.2) | 14.7 (11.7) | 15.7 (12.6) | 15.4 (12.4) | 15.2 (12.1) | 15.5 (12.5) | 15.4 (12.3) | 15.4 (12.2) |
| Mean (SD) BMI, kg/m2 | 27.4 (4.74) | 26.8 (4.58) | 26.2 (4.44) | 27.2 (4.70) | 27.0 (4.67) | 26.6 (4.57) | 27.6 (4.90) | 27.0 (4.59) | 26.4 (4.43) | 27.4 (4.75) | 27.1 (4.65) | 26.6 (4.56) |
| Never smoker, N (%) | 50,815 (54.9) | 40,597 (57.8) | 20,975 (60.7) | 36,354 (55.3) | 37,621 (57.2) | 38,412 (58.4) | 33,994 (57.6) | 37,580 (56.7) | 40,813 (56.6) | 30,882 (55.2) | 31,603 (56.8) | 49,902 (58.2) |
| Never drinker, N (%) | 16,052 (17.3) | 11,231 (16.0) | 4576 (13.2) | 10,839 (16.5) | 10,463 (15.9) | 10,557 (16.0) | 9510 (16.1) | 10,305 (15.6) | 12,044 (16.7) | 8147 (14.6) | 8749 (15.7) | 14,963 (17.4) |
| Median (IQR)b physical activity, MET hours/week | 41.6 (42.5) | 41.3 (40.2) | 42.5 (39.6) | 41.5 (42.8) | 41.5 (40.7) | 42.0 (39.9) | 39.6 (40.5) | 41.4 (41.1) | 43.7 (41.7) | 40.2 (40.6) | 41.4 (41.3) | 42.8 (41.4) |
| Above 8 h of daily sleeping time, N (%) | 69,960 (75.6) | 54,589 (77.7) | 27,296 (79.0) | 49,643 (75.4) | 50,692 (77.0) | 51,510 (78.3) | 45,279 (76.7) | 51,026 (77.0) | 55,540 (77.0) | 43,112 (77.1) | 43,001 (77.2) | 65,732 (76.6) |
| Hypertension, N (%) | 52,082 (56.2) | 38,839 (55.3) | 18,330 (53.1) | 36,133 (54.9) | 36,702 (55.8) | 36,416 (55.3) | 33,838 (57.3) | 36,816 (55.6) | 38,597 (53.5) | 32,845 (58.8) | 31,003 (55.7) | 45,403 (52.9) |
| Hypercholesterolemia, N (%) | 15,961 (17.2) | 11,493 (16.4) | 4997 (14.5) | 10,903 (16.6) | 10,923 (16.6) | 10,625 (16.1) | 10,075 (17.1) | 10,887 (16.4) | 11,489 (15.9) | 10,142 (18.1) | 9295 (16.7) | 13,014 (15.2) |
| Diabetes, N (%) | 4986 (5.4) | 3103 (4.4) | 1217 (3.5) | 3334 (5.1) | 3096 (4.7) | 2876 (4.4) | 2937 (5.0) | 3107 (4.7) | 3262 (4.5) | 2883 (5.2) | 2695 (4.8) | 3728 (4.3) |
| Longstanding illness, N (%) | 26,668 (28.8) | 19,478 (27.7) | 9053 (26.2) | 18,919 (28.8) | 18,324 (27.8) | 17,956 (27.3) | 17,438 (29.5) | 18,359 (27.7) | 19,402 (26.9) | 16,405 (29.3) | 15,529 (27.9) | 23,265 (27.1) |
| Multivitamin use, N (%) | 13,154 (14.2) | 10,964 (15.6) | 5794 (16.8) | 8897 (13.5) | 9864 (15.0) | 11,151 (16.9) | 8150 (13.8) | 9814 (14.8) | 11,948 (16.6) | 7739 (13.8) | 8336 (15.0) | 13,837 (16.1) |
| Intake of mineral supplements, N (%) | 19,968 (21.6) | 16,267 (23.2) | 8316 (24.1) | 13,868 (21.1) | 14,692 (22.3) | 15,991 (24.3) | 12,517 (21.2) | 14,639 (22.1) | 17,395 (24.1) | 11,608 (20.8) | 12,160 (21.8) | 20,783 (24.2) |
| Aspirin use, N (%) | 11,725 (12.7) | 8735 (12.4) | 3994 (11.6) | 8198 (12.5) | 8210 (12.5) | 8046 (12.2) | 7605 (12.9) | 8171 (12.3) | 8678 (12.0) | 7649 (13.7) | 6931 (12.4) | 9874 (11.5) |
| Non-aspirin NSAIDs use, N (%) | 16,256 (17.6) | 11,370 (16.2) | 5258 (15.2) | 11,638 (17.7) | 10,996 (16.7) | 10,250 (15.6) | 10,237 (17.3) | 11,107 (16.8) | 11,540 (16.0) | 9109 (16.3) | 9298 (16.7) | 14,477 (16.9) |
| Statin use, N (%) | 13,858 (15.0) | 9941 (14.1) | 4303 (12.5) | 9492 (14.4) | 9466 (14.4) | 9144 (13.9) | 8810 (14.9) | 9477 (14.3) | 9815 (13.6) | 8929 (16.0) | 8047 (14.5) | 11,126 (13.0) |
Abbreviations: SD Standard deviation, IQR Interquartile range, Q Quantile, BMI Body mass index, MET hours/week Hours of physical activity per week, NSAIDs Non-steroidal anti-inflammatory drugs, AMED Alternate Mediterranean Diet, HEI-2015 Healthy Eating Index 2015, HPDI Healthful Plant-based Diet Index
aWe use the means (SD) for normally distributed variables and the medians (IQR) for non-normally distributed variables
bInterquartile range (IQR): the range from values of the 25th to the 75th deciles
Healthy dietary patterns and risk of IBD
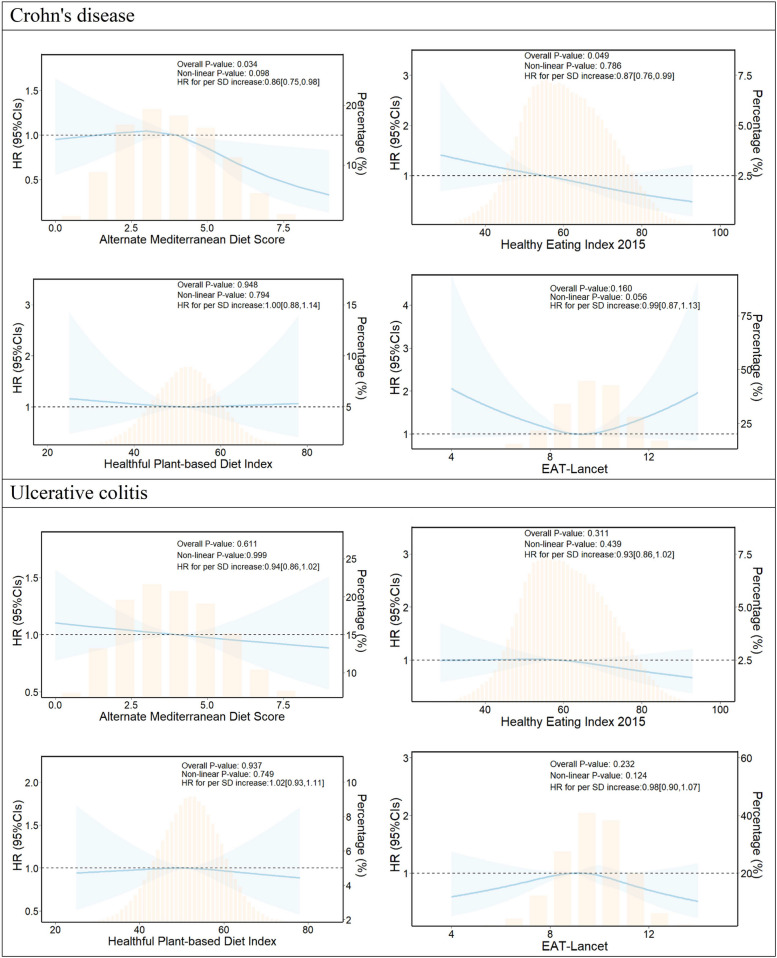
During up to the 2,193,436 person-years of follow-up, 260 CD cases and 601 UC cases were documented (Table 2). The results revealed that higher scores on the AMED and the HEI-2015 were associated with a reduced risk of CD (P trend < 0.05). Specifically, compared to participants in the lowest category of AMED score, those with AMED scores of 4–5 and 6–9 had age and gender-adjusted HRs of 0.87 (95% CI, 0.66–1.14) and 0.51 (95% CI, 0.33–0.78), respectively, for CD. These associations remained consistent even after adjusting for potential confounders, including sociodemographic factors, lifestyle factors, medication intake, and comorbidities. Similar trends were observed for the association of HEI-2015 category with CD risk. For each SD increase in AMED and HEI-2015 scores, the risk of CD decreased by 0.86 (95% CI, 0.75–0.98) and 0.87 (95% CI, 0.76–0.99), respectively, with no evidence against nonlinearity (Fig. 1). There was insufficient evidence to support an association between the HPDI score and the EAT-Lancet score with CD risk. Furthermore, no sufficient evidence of association was found between any of the four healthy eating patterns and the risk of UC.
Table 2.
Association between healthy eating patterns and risk of Crohn’s disease and ulcerative colitis
| Variables | Cases | Person-years | Incidence ratea | Age and gender-stratified HR [95% CI]b | Multivariable-adjusted HR [95% CI]c |
|---|---|---|---|---|---|
| Crohn’s disease | |||||
| AMED score | |||||
| 0–3 | 141 | 1,025,234 | 13.8 | 1.00 [reference] | 1.00 [reference] |
| 4–5 | 89 | 782,806 | 11.4 | 0.87 [0.66, 1.14] | 0.92 [0.70, 1.21] |
| 6–9 | 30 | 385,396 | 7.8 | 0.51 [0.33, 0.78] | 0.49 [0.31, 0.77] |
| P trend | 0.014 | 0.024 | |||
| Per SD increase | 0.85 [0.75, 0.97] | 0.86 [0.75, 0.98] | |||
| HEI-2015 | |||||
| Q1 | 108 | 727,931 | 14.8 | 1.00 [reference] | 1.00 [reference] |
| Q2 | 83 | 731,752 | 11.3 | 0.81 [0.60, 1.09] | 0.82 [0.61, 1.10] |
| Q3 | 69 | 733,753 | 9.4 | 0.65 [0.47, 0.89] | 0.65 [0.47, 0.90] |
| P trend | 0.029 | 0.032 | |||
| Per SD increase | 0.87 [0.76, 0.99] | 0.87 [0.76, 0.99] | |||
| HPDI | |||||
| Q1 | 73 | 656,081 | 11.1 | 1.00 [reference] | 1.00 [reference] |
| Q2 | 83 | 735,836 | 11.3 | 1.04 [0.75, 1.44] | 1.06 [0.76, 1.48] |
| Q3 | 104 | 801,519 | 13 | 1.20 [0.88, 1.65] | 1.24 [0.90, 1.72] |
| P trend | 0.877 | 0.958 | |||
| Per SD increase | 0.99 [0.87, 1.12] | 1.00 [0.88, 1.14] | |||
| EAT-Lancet score | |||||
| Q1 | 73 | 621,822 | 11.7 | 1.00 [reference] | 1.00 [reference] |
| Q2 | 66 | 618,605 | 10.7 | 0.88 [0.62, 1.25] | 0.88 [0.62, 1.26] |
| Q3 | 121 | 953,008 | 12.7 | 1.08 [0.80, 1.45] | 1.09 [0.80, 1.48] |
| P trend | 0.779 | 0.909 | |||
| Per SD increase | 0.98 [0.86, 1.12] | 0.99 [0.87, 1.13] | |||
| Ulcerative colitis | |||||
| AMED score | |||||
| 0–3 | 307 | 1,024,461 | 30 | 1.00 [reference] | 1.00 [reference] |
| 4–5 | 200 | 782,244 | 25.6 | 0.85 [0.70, 1.02] | 0.90 [0.75, 1.09] |
| 6–9 | 94 | 385,115 | 24.4 | 0.76 [0.60, 0.98] | 0.82 [0.64, 1.07] |
| P trend | 0.020 | 0.155 | |||
| Per SD increase | 0.90 [0.83, 0.98] | 0.94 [0.86, 1.02] | |||
| HEI-2015 | |||||
| Q1 | 213 | 727,460 | 29.3 | 1.00 [reference] | 1.00 [reference] |
| Q2 | 211 | 731,111 | 28.9 | 0.96 [0.79, 1.18] | 1.01 [0.82, 1.24] |
| Q3 | 177 | 733,248 | 24.1 | 0.80 [0.65, 0.99] | 0.86 [0.69, 1.07] |
| P trend | 0.020 | 0.124 | |||
| Per SD increase | 0.90 [0.83, 0.98] | 0.93 [0.86, 1.02] | |||
| HPDI | |||||
| Q1 | 181 | 655,559 | 27.6 | 1.00 [reference] | 1.00 [reference] |
| Q2 | 208 | 735,268 | 28.3 | 1.11 [0.90, 1.37] | 1.13 [0.91, 1.40] |
| Q3 | 212 | 800,993 | 26.5 | 1.03 [0.83, 1.28] | 1.08 [0.87, 1.34] |
| P trend | 0.993 | 0.714 | |||
| Per SD increase | 1.00 [0.92, 1.09] | 1.02 [0.93, 1.11] | |||
| EAT-Lancet score | |||||
| Q1 | 164 | 621,365 | 26.4 | 1.00 [reference] | 1.00 [reference] |
| Q2 | 191 | 618,087 | 30.9 | 1.20 [0.96, 1.49] | 1.21 [0.97, 1.51] |
| Q3 | 246 | 952,367 | 25.8 | 0.98 [0.79, 1.21] | 1.02 [0.83, 1.27] |
| P trend | 0.395 | 0.721 | |||
| Per SD increase | 0.96 [0.89, 1.05] | 0.98 [0.90, 1.07] | |||
Abbreviations: HR Hazard ratio, CI Confidence interval, SD Standard deviation, Q Quantile, AMED Alternate Mediterranean Diet, HEI-2015 Healthy Eating Index 2015, HPDI Healthful Plant-based Diet Index
aPer 100,000 person-years
bHazard ratio and 95% CI were estimated from crude Cox regression model stratified by sex, age, and assessment center
cFully adjusted model additionally adjusted for sociodemographic characteristics (ethnicity, index of multiple deprivations, and BMI), lifestyle factor (smoking status, alcohol consumption, physical activity, and daily sleeping time), medications (multivitamins, mineral, aspirin, non-aspirin NSAIDs, and statins use), and comorbidities (hypercholesterolemia, hypertension, diabetes, and longstanding illness)
Fig. 1.
Estimated nonlinear association between healthy eating patterns and risk of Crohn’s disease and ulcerative colitis. Abbreviation: HR, hazard ratio; CI, confidence interval; SD, standard deviation. Separate models were fitted for Crohn’s disease and ulcerative colitis with a restricted cubic spline for each healthy eating pattern. All models were adjusted for sociodemographic characteristics, lifestyle factors, medications, and comorbidities (see footnote in Table 2). Shaded areas represent 95% confidence intervals
These primary results remained robust in sensitivity analyses (Additional file 1: Table S5). Lagging the exposure by 2 years or considering only participants who reported their typical diet did not substantially alter the associations between healthy eating pattern scores and the risk of CD and UC. The associations remained stable when not adjusting for BMI categories, and after excluding participants with pre-existing digestive system disorders at baseline and those who developed them between baseline and the completion of the dietary questionnaire. The sensitivity analyses, excluding participants with covariate-dietary assessment time gaps exceeding 2 years, yielded consistent results. We further explored potential effect modification across various subgroups defined by sex, age, obesity, smoking, drinking, physical activity, and regular NSAIDs use, and no significant evidence for effect modification was observed (all P-interaction > 0.05, Additional file 1: Tables S6–7).
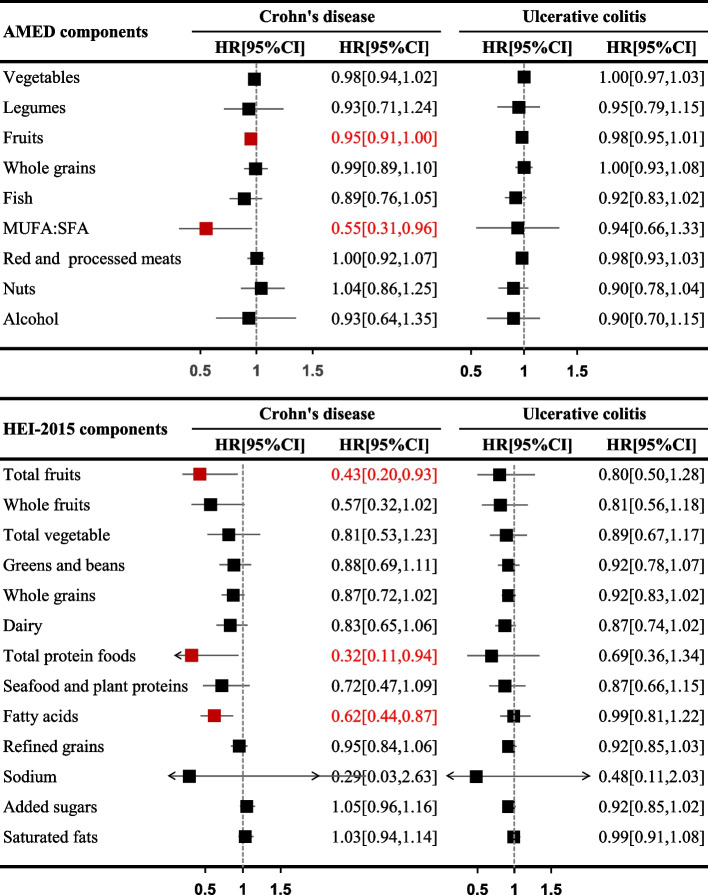
Given the significant associations of AMED and HEI-2015 scores with the reduced risk of CD, we conducted further investigations into the individual components of these scores (Fig. 2). Per SD increase in the fruits and MUFA:SFA ratio within the AMED component were associated with 5% (HR = 0.95, 95% CI 0.91–1.00) and 45% (HR = 0.55, 95% CI 0.31–0.96) lower risk of CD, respectively. Meanwhile, in the HEI-2015 component, higher intake of total fruits (HR = 0.43, 95% CI 0.20–0.93), total protein foods (HR = 0.32, 95% CI 0.11–0.94), and fatty acids (defined as (MUFA + PUFA):SFA, HR = 0.62, 95% CI 0.44–0.87) were associated with a decreased risk of CD.
Fig. 2.
Association between each food group of AMED and HEI-2015 with risk of CD and UC. Abbreviation: CD, Crohn’s disease; UC, ulcerative colitis; HR, hazard ratio; CI, confidence interval; AMED, Alternate Mediterranean Diet; HEI-2015, Healthy Eating Index 2015; MUFA, monounsaturated fatty acid, SFA, saturated fatty acid. Estimated effects were based on the fully adjusted model (see footnote in Table 2)
Mediation analyses of inflammation in the association of healthy diet patterns with IBD risk
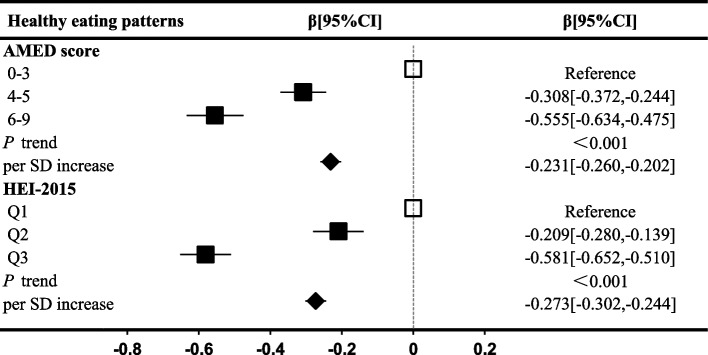
To explore potential mechanisms underlying the association between healthy dietary patterns and IBD risk, we estimated the association of AMED and HEI-2015 scores with markers of inflammation. In the fully adjusted model, higher healthy dietary scores were inversely associated with all plasma inflammatory factors and cumulative INFLA-score (Fig. 3 and Additional file 1: Table S8). For instance, participants with AMED scores of 4–5 and 6–9 had a decrease of − 0.308 (95% CI, − 0.372 to − 0.244) and − 0.555 (95% CI, − 0.634 to − 0.475), respectively, in the INFLA-score compared to those in the lowest category of AMED score. Continuous trends were also significant, with each SD increase in AMED and HEI-2015 associated with a decrease of − 0.231 (95% CI, − 0.260 to − 0.202) and − 0.273 (95% CI, − 0.302 to − 0.244) in the INFLA-score, respectively (Fig. 3).
Fig. 3.
Association of healthy eating patterns with low-grade inflammation scores. Abbreviation: CI, confidence interval; SD, standard deviation; Q, quantile; AMED, Alternate Mediterranean Diet; HEI-2015, Healthy Eating Index 2015; HPDI, Healthful Plant-based Diet Index. Regression coefficient beta and 95% CI were estimated from multivariate-linear regression models stratified by sex, age, and assessment center and adjusted for sociodemographic characteristics (ethnicity, index of multiple deprivations, and BMI), lifestyle factor (smoking status, alcohol consumption, physical activity, and sleep quality), medication (multivitamins, mineral, aspirin, non-aspirin NSAIDs, and statins use), and baseline disease (hypercholesterolemia, hypertension, diabetes, and longstanding illness)
Next, we examined the relationships between inflammatory factors and the long-term risk of CD and UC (Additional file 1: Table S9). A higher INFLA-score, along with elevated levels of WBC, CRP, and NLR, was found to be statistically significantly associated with an increased risk of CD and UC. No significant association was found between PLT and CD or UC risk.
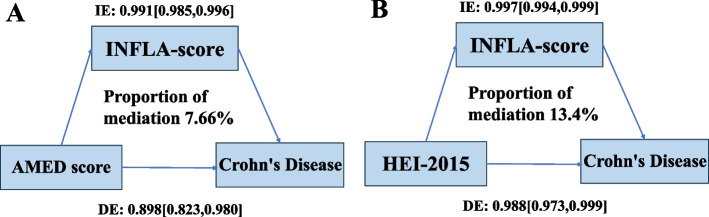
Finally, mediation analyses were performed to explore the potential role of inflammation in mediating the associations between AMED and HEI-2015 diets and reduced CD risk. The results showed that low-grade inflammation partially mediated the associations. Specifically, 7.66% of the reduced CD risk for AMED diet and 13.40% for HEI-2015 diet were mediated through the INFLA-score (Fig. 4).
Fig. 4.
Mediation proportion of the association between healthy eating patterns and CD risk mediated by INFLA-score. Abbreviations: CD, Crohn’s disease; INFLA-score, low-grade inflammation score; AMED, Alternate Mediterranean Diet; HEI-2015, Healthy Eating Index 2015. All models were adjusted for sociodemographic characteristics, lifestyle factors, medications, and comorbidities (see footnote in Table 2)
Discussion
In this large prospective cohort of middle-aged men and women in UK, we comprehensively investigated the link between healthy dietary patterns and the risk of IBD. Our findings demonstrated that higher adherence to the AMED and HEI-2015 was associated with a reduced risk of CD but not UC. These findings were consistent across multiple sensitivity and subgroup analyses. Furthermore, we identified specific components of these dietary patterns, such as the fruits and MUFA:SFA ratio in AMED, and total fruits, total protein foods and fatty acids in HEI-2015, that were significantly associated with a decreased risk of CD. Notably, our analysis indicated a potential association between the anti-inflammatory properties of the AMED and HEI-2015 diets and reduced CD risk. These findings suggest that adopting healthy dietary patterns may hold promise in preventing CD, warranting further investigation and potential dietary interventions for IBD prevention.
Prior investigations into the association between healthy eating patterns and the risk of IBD have yielded inconsistent findings. For instance, a recent investigation based on two Swedish cohorts found no substantial association between HEI-2015 and the risk of older-onset CD. However, this study also reported that, compared to the lowest quartile, the modified Mediterranean Diet Score (mMED) with the highest quartile has an age-adjusted HR of 0.54 (95% CI, 0.30–0.96), while the HPDI with the highest quartile has a multivariable-adjusted HR of 0.52 (95% CI, 0.32–0.85) [62], which partially aligns with our findings. Similarly, within the Lifelines cohort, AMED scores and adherence to the HEI-2015 were negatively associated with CD risk, although it was not statistically significant [63]. Conversely, a case–control study reported a lower odds ratio of 0.34 for UC (95% CI, 0.12–0.96), but not CD, among individuals with higher HEI-2015 scores [64]. These inconsistencies among studies may be, at least in part, attributed to the diversity in study populations, study designs, sample sizes, and methods used to assess dietary patterns. It is worth noting that conclusive health effect associations for the EAT-Lancet diet have yet to be established based on prior research. Further research is needed to verify the relationship of the EAT-Lancet diet on IBD risk.
Among several healthy dietary patterns, AMED and HEI-2015 were associated with a reduced risk of CD, while other dietary patterns did not show this link. This difference may be attributed to the unique characteristics of AMED and HEI-2015, compared to other dietary patterns. Both the Mediterranean diet and HEI-2015 emphasize the intake of unsaturated fatty acids. Omega-3 PUFAs, a sort of unsaturated fatty acids, as an inhibitor of arachidonic acid metabolism in pharmacological mechanisms, the anti-inflammatory effect of n-3 PUFA has been confirmed in animal models to have certain therapeutic effects on inflammatory diseases such as rheumatoid arthritis [65] and IBD [66]. Our study also found that this component was related to a decreased risk of CD (Fig. 2). Additionally, the Mediterranean diet encourages moderate alcohol consumption and the use of olive oil, which contains a variety of polyphenolic substances and has potential anti-inflammatory properties [67, 68]. The exact mechanisms underlying the association between AMED and HEI-2015 with reduced CD risk require further research to elucidate.
Consistent with prior studies demonstrating a protective effect of dietary fiber and unsaturated fatty acid intake against IBD risk, our study has suggested a significant reduction in CD risk associated with specific components (i.e., fruits and MUFA:SFA ratio) of the AMED. These findings align with previous research [5, 8, 69, 70] and are further supported by our observation of a negative association between total fruit intake and the ratio of (PUFA + MUFA) to SFA within the HEI-2015. Fruits are known to be rich sources of dietary fiber, antioxidants, vitamins, and minerals, all of which possess potential anti-inflammatory properties [71]. A previous meta-analysis suggested an association between fruit consumption and a reduced risk of CD [8]. MUFAs prominently found in sources like olive oil, canola oil, and peanut oil [72] have been associated with a lowered CD risk in a previous cohort study [69]. The potential therapeutic benefits of olive oil for IBD patients have been documented in animal and in vitro studies [70]. The anti-inflammatory potential of PUFAs, particularly the well-recognized omega-3 PUFAs, has garnered significant attention due to their ability to inhibit arachidonic acid metabolism, an important pathway of inflammation [73]. Abundantly present in oily fish such as salmon, trout, and herring [72], these omega-3 PUFAs have been linked to a reduced CD risk [74]. Interestingly, our study also identified a protective component against CD in the total protein foods category, encompassing meat, poultry, eggs, seafood, nuts, and legumes as defined in HEI-2015. However, there is inconsistent with existing research on the relationship between this aggregated component and IBD [75–77]. Additionally, it does not differentiate between plant protein and animal protein, limiting our ability to determine which type of protein is associated with the observed outcomes. This disparity highlights the importance of prospective investigations in further confirming and enhancing the validity of our findings.
The justification for conducting the mediation analysis in our study is based on the causal hypothesis that dietary patterns may influence the risk of IBD through their effects on low-grade inflammation. Our finding of an association between the AMED score and low inflammation scores aligns with Bonaccio et al.’s study [5], confirming the external validity of our results and highlighting the anti-inflammatory benefits of the Mediterranean diet. Both AMED and HEI-2015 diets are rich sources of anti-inflammatory components, such as omega-3 fatty acids, fruits, vegetables, and whole grains, which have consistently been linked to a reduction in the occurrence of IBD and the alleviation of its symptoms [78]. Moreover, these health-promoting dietary regimens also restrict the consumption of pro-inflammatory items, including red and processed meat, sugar-sweetened beverages, and high-fat dairy products [79], resulting in a strong negative correlation with plasma inflammatory biomarkers as observed in our study (WBC, PLT, CRP, NLR, and the aggregated INFLA-score). This alignment with previous research reinforces the well-established association between plant-based dietary patterns and reduced inflammation [80]. To quantify the extent to which inflammation mediates the relationship between diet and IBD risk, we performed a mediation analysis. Our results indicated that the proportion of mediation by low-grade inflammation was 7.66% for the association between the AMED score and CD risk and 13.40% for the HEI-2015 score and CD risk. These values suggest that while low-grade inflammation plays a role in mediating the negative association of these dietary patterns, other mechanisms are also likely involved. For instance, AMED and HEI-2015 may exert influence on other mechanisms critical to the development of IBD, including the modulation of the gut microbiota and the maintenance of intestinal barrier integrity [81]. Thus, to attain a comprehensive understanding of these intricate mechanisms, further in-depth investigation is warranted.
The strengths of this study lie in its prospective design with a large sample size, high-quality data source, and robust adjustment for confounding factors, facilitating a direct comparison of multiple dietary patterns for IBD. We also explored associations with constituent components, and further provided insights into the potential mechanistic pathways through which these dietary patterns were linked to the protection against Crohn’s disease. However, certain limitations must be acknowledged. Firstly, our study population primarily consisted of middle-aged participants, which may raise concerns about the generalizability of our findings to younger individuals at risk of IBD. Nonetheless, our investigation remains valuable in identifying modifiable risk factors for elderly-onset disease, considering that environmental factors may play a more significant role in IBD [82]. Moreover, it is important to note that many of the beneficial effects of health diet patterns, such as the Mediterranean diet, have specifically been demonstrated in older adults [83]. Secondly, the use of a 24-h recall for dietary measurements may not fully capture participants’ typical intake and could be subject to recall bias [84]. The timing of when participants started to consume a particular diet is also unknown. However, prior validation studies have demonstrated that dietary data collected using the Oxford WebQ provide reasonably valid estimates of dietary intake [41]. Furthermore, we conducted sensitivity analyses considering only participants who reported their typical diet in the questionnaire, and the results remained consistent with the original findings. Thirdly, the lack of updated dietary data during the follow-up period is a limitation. However, evidence from other studies suggests that individuals’ dietary intake tends to remain relatively stable over time [85], reducing the likelihood of significant changes in their diet categorization. Fourthly, the “healthy volunteer” effect in the UK Biobank may limit the generalizability of our findings, but the large size and heterogeneity of exposure measures in the UK Biobank allow for valid scientific inferences of exposure-outcome relationships that are applicable to other populations [86]. Fifthly, despite our comprehensive adjustment for confounders, residual confounding effects cannot be entirely ruled out. Lastly, as with any observational study, causation cannot be established. Further research, including interventional studies, is required to confirm the causal relationship between these dietary patterns and IBD risk.
Conclusions
In conclusion, our study suggests a possible link between adherence to healthy dietary patterns, such as the AMED and the HEI-2015, and a reduced risk of CD. It is important to note that our study is observational in nature, and causation cannot be inferred. Our study also highlights the potential role of inflammation as a mediator in the relationship between healthy dietary patterns and CD risk. This potentially underscores the feasibility and mechanisms of dietary interventions in IBD prevention. Our findings may have implications for public health initiatives aimed at promoting healthy eating habits, but further research, including interventional studies, is needed to establish causal relationships and elucidate the underlying mechanisms.
Supplementary Information
Acknowledgements
The authors would like to appreciate the participants from the UK Biobank studies for their contributions. This research has been conducted using the UK Biobank resource (https://www.ukbiobank.ac.uk) under application number 51671.
Abbreviations
- AMED
Alternate Mediterranean Diet
- BMI
Body mass index
- CD
Crohn’s disease
- CI
Confidence interval
- CRP
C-reactive protein
- DGAs
Dietary Guidelines for Americans
- FBDGs
European Food-Based Dietary Guidelines
- HEI-2015
Healthy Eating Index 2015
- HPDI
Healthful Plant-based Diet Index
- HR
Hazard ratio
- IBD
Inflammatory bowel disease
- ICD
International Classification of Diseases
- IL-6
Interleukin-6
- IMD
Index of multiple deprivation
- INFLA-score
Low-grade inflammation score
- IPAQ-SF
International Physical Activity Questionnaire-Short Form
- MD
Mediterranean diet
- mMED
Modified Mediterranean Diet Score
- MUFAs
Monounsaturated fatty acids
- NLR
Neutrophil-to-lymphocyte ratio
- NSAIDs
Non-steroidal anti-inflammatory drugs
- PLT
Platelet
- PUFAs
Polyunsaturated fatty acids
- SD
Standard deviation
- SFAs
Saturated fatty acids
- TNF-α
Tumor necrosis factor-alpha
- UC
Ulcerative colitis
- WBC
White blood cell
Authors’ contributions
B.X., P.W., J.Q.: conceived and designed the research question; B.X., J.Q., Y.L., L.H., P.X.: analyzed data. B.X., Y.L., L.H.: prepared the figures and tables, and wrote the first draft of the manuscript; B.X., Y.L., L.H., P.X.: substantively revised the manuscript. All Authors: critically reviewed the manuscript, and read and approved the final manuscript.
Funding
This work was supported by the Shenzhen Medical Research Fund (No. A2403069, C2401002), the Natural Science Foundation of China (grant number 82103913, 82473707), the Research Supporting Start-up Fund for Associate researcher, of SAHSYSU (Grant No. ZSQYRSSFAR0004), the Startup Fund for the 100 Top Talents Program, SYSU (392012), and the Funding of Shenzhen Clinical Research Center for Gastroenterology (Gastrointestinal Surgery, No. LCYSSQ20220823091203008).
Data availability
This research got access to the UK Biobank (https://www.ukbiobank.ac.uk) under application number 51671. Further information is available from the corresponding author upon request.
Availability of data and materials
The data that support the findings of this study are available from UK Biobank project site, subject to registration and application process. Further details can be found at https://www.ukbiobank.ac.uk. The code used in this study is available upon reasonable request to the corresponding author.
Declarations
Ethics approval and consent to participate
The UK Biobank was approved by the National Research Ethics Committee (REC ID: 16/NW/0274). Electronic written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declared that no competing interests exist.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bin Xia, Yan Li, Linmin Hu and Peng Xie these authors contributed equally to this work and shared the first authorship.
Contributor Information
Jian Qi, Email: qijian@sysush.com.
Pengpeng Wang, Email: wp7221@zzu.edu.cn.
Jinqiu Yuan, Email: yuanjq5@mail.sysu.edu.cn.
References
- 1.Park J, Jeong GH, Song M, Yon DK, Lee SW, Koyanagi A, et al. The global, regional, and national burden of inflammatory bowel diseases, 1990–2019: a systematic analysis for the global burden of disease study 2019. Dig Liver Dis. 2023;55:1352–9. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal M, Jess T. Implications of the changing epidemiology of inflammatory bowel disease in a changing world. United Eur Gastroenterol J. 2022;10(10):1113–20. 10.1002/ueg2.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolodziejczyk AA, Zheng DP, Elinav E. Diet-microbiota interactions and personalized nutrition. Nat Rev Microbiol. 2019;17(12):742–53. 10.1038/s41579-019-0256-8. [DOI] [PubMed] [Google Scholar]
- 4.Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71(8):511–27. 10.1111/nure.12035. [DOI] [PubMed] [Google Scholar]
- 5.Bonaccio M, Pounis G, Cerletti C, Donati MB, Iacoviello L, de Gaetano G. Mediterranean diet, dietary polyphenols and low grade inflammation: results from the MOLI-SANI study. Br J Clin Pharmacol. 2017;83(1):107–13. 10.1111/bcp.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Fuchs CS, et al. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut. 2014;63(5):776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fritsch J, Garces L, Quintero MA, Pignac-Kobinger J, Santander AM, Fernandez I, et al. Low-fat, high-fiber diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021;19(6):1189-+. 10.1016/j.cgh.2020.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Milajerdi A, Ebrahimi-Daryani N, Dieleman LA, Larijani B, Esmaillzadeh A. Association of dietary fiber, fruit, and vegetable consumption with risk of inflammatory bowel disease: a systematic review and meta-analysis. Adv Nutr. 2021;12(3):735–43. 10.1093/advances/nmaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babaei A, Pourmotabbed A, Talebi S, Mehrabani S, Bagheri R, Ghoreishy SM, et al. The association of ultra-processed food consumption with adult inflammatory bowel disease risk: a systematic review and dose-response meta-analysis of 4 035 694 participants. Nutr Rev. 2024;82(7):861–71. 10.1093/nutrit/nuad101. [DOI] [PubMed] [Google Scholar]
- 10.Dong C, Chan SSM, Jantchou P, Racine A, Oldenburg B, Weiderpass E, et al. Meat intake is associated with a higher risk of ulcerative colitis in a large European prospective cohort study. J Crohns Colitis. 2022;16(8):1187–96. 10.1093/ecco-jcc/jjac054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo CH, Lochhead P, Khalili H, Song M, Tabung FK, Burke KE, et al. Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2020;159(3):873-83.e1. 10.1053/j.gastro.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwingshackl L, Watzl B, Meerpohl JJ. The healthiness and sustainability of food based dietary guidelines. BMJ. 2020;370:m2417. 10.1136/bmj.m2417. [DOI] [PubMed] [Google Scholar]
- 13.Nelson ME, Hamm MW, Hu FB, Abrams SA, Griffin TS. Alignment of healthy dietary patterns and environmental sustainability: a systematic review. Adv Nutr. 2016;7(6):1005–25. 10.3945/an.116.012567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millen BE, Abrams S, Adams-Campbell L, Anderson CA, Brenna JT, Campbell WW, et al. The 2015 dietary guidelines advisory committee scientific report: development and major conclusions. Adv Nutr. 2016;7(3):438–44. 10.3945/an.116.012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuire S. U.S. Department of Agriculture and U.S. Department of Health and Human Services, dietary guidelines for Americans, 2010. 7th edition, Washington, DC: U.S. Government Printing Office, January 2011. Adv Nutr. 2011;2(3):293–4. 10.3945/an.111.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willett WC, Ludwig DS. The 2010 dietary guidelines–the best recipe for health? N Engl J Med. 2011;365(17):1563–5. 10.1056/NEJMp1107075. [DOI] [PubMed] [Google Scholar]
- 17.Nezamoleslami S, Ghiasvand R, Feizi A, Salesi M, Pourmasoumi M. The relationship between dietary patterns and rheumatoid arthritis: a case-control study. Nutr Metab (Lond). 2020;1775. 10.1186/s12986-020-00502-7. [DOI] [PMC free article] [PubMed]
- 18.Yu W, Pan L, Cao W, Lv J, Guo Y, Pei P, et al. Dietary patterns and risk of chronic obstructive pulmonary disease among Chinese adults: an 11-year prospective study. Nutrients. 2022;14(5): 996. 10.3390/nu14050996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shan Z, Li Y, Baden MY, Bhupathiraju SN, Wang DD, Sun Q, et al. Association between healthy eating patterns and risk of cardiovascular disease. JAMA Intern Med. 2020;180(8):1090–100. 10.1001/jamainternmed.2020.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martín-Peláez S, Fito M, Castaner O. Mediterranean diet effects on type 2 diabetes prevention, disease progression, and related mechanisms. A review. Nutrients. 2020;12(8):2236. 10.3390/nu12082236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu F, Du B, Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: a review. Crit Rev Food Sci Nutr. 2018;58(8):1260–70. 10.1080/10408398.2016.1251390. [DOI] [PubMed] [Google Scholar]
- 22.Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10(11): 1618. 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutiérrez S, Svahn SL, Johansson ME. Effects of omega-3 fatty acids on immune cells. Int J Mol Sci. 2019;20(20):5028. 10.3390/ijms20205028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ. 2008;337:a1344. 10.1136/bmj.a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. 2017;9(10): 1063. 10.3390/nu9101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shan Z, Wang F, Li Y, Baden MY, Bhupathiraju SN, Wang DD, et al. Healthy eating patterns and risk of total and cause-specific mortality. JAMA Intern Med. 2023;183(2):142–53. 10.1001/jamainternmed.2022.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, et al. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13(6): e1002039. 10.1371/journal.pmed.1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satija A, Hu FB. Plant-based diets and cardiovascular health. Trends Cardiovasc Med. 2018;28(7):437–41. 10.1016/j.tcm.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knuppel A, Papier K, Key TJ, Travis RC. EAT-Lancet score and major health outcomes: the EPIC-Oxford study. Lancet. 2019;394(10194):213–4. 10.1016/s0140-6736(19)31236-x. [DOI] [PubMed] [Google Scholar]
- 30.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119(8):1093–100. 10.1161/circulationaha.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–602. 10.1016/j.jand.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, et al. Food in the anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet (London, England). 2019;393(10170):447–92. 10.1016/s0140-6736(18)31788-4. [DOI] [PubMed] [Google Scholar]
- 33.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14(5):329–42. 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 34.Reinisch W, Gasché C, Tillinger W, Wyatt J, Lichtenberger C, Willheim M, et al. Clinical relevance of serum interleukin-6 in Crohn’s disease: single point measurements, therapy monitoring, and prediction of clinical relapse. Am J Gastroenterol. 1999;94(8):2156–64. 10.1111/j.1572-0241.1999.01288.x. [DOI] [PubMed] [Google Scholar]
- 35.Solem CA, Loftus EV Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(8):707–12. 10.1097/01.mib.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
- 36.Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer S, Schreiber S, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60(6):780–7. 10.1136/gut.2010.221127. [DOI] [PubMed] [Google Scholar]
- 37.UK Biobank data. 2021. https://www.ukbiobank.ac.uk/.
- 38.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026–34. 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elliott LT, Sharp K, Alfaro-Almagro F, Shi S, Miller KL, Douaud G, et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature. 2018;562(7726):210–6. 10.1038/s41586-018-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.You J, Guo Y, Wang YJ, Zhang Y, Wang HF, Wang LB, et al. Clinical trajectories preceding incident dementia up to 15 years before diagnosis: a large prospective cohort study. Mol Psychiatry. 2024. 10.1038/s41380-024-02570-0. [DOI] [PubMed]
- 41.Greenwood DC, Hardie LJ, Frost GS, Alwan NA, Bradbury KE, Carter M, et al. Validation of the Oxford WebQ online 24-hour dietary questionnaire using biomarkers. Am J Epidemiol. 2019;188(10):1858–67. 10.1093/aje/kwz165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho FK, Gray SR, Welsh P, Petermann-Rocha F, Foster H, Waddell H, et al. Associations of fat and carbohydrate intake with cardiovascular disease and mortality: prospective cohort study of UK Biobank participants. BMJ. 2020;368:m688. 10.1136/bmj.m688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez-Cornago A, Pollard Z, Young H, van Uden M, Andrews C, Piernas C, et al. Description of the updated nutrition calculation of the Oxford WebQ questionnaire and comparison with the previous version among 207,144 participants in UK Biobank. Eur J Nutr. 2021;60(7):4019–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mills A, Patel S, Crawley H, Great Britain. Ministry of Agriculture, Fisheries and Food. Food portion sizes. 2nd ed. London: H.M. Stationery Office; 1993. ISBN: 0112429610.
- 45.Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–602. 10.1016/j.jand.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W, et al. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in US adults. J Am Coll Cardiol. 2017;70(4):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kohl J, Hohberg V, Hauff P, Lang C, Faude O, Gollhofer A, et al. Development of a metric Healthy Eating Index-2015 and comparison with the healthy eating index-2015 for the evaluation of dietary quality. Front Nutr. 2022;9:9952223. 10.3389/fnut.2022.952223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W, et al. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J Am College Cardiol. 2017;70(4):411–22. 10.1016/j.jacc.2017.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia B, Yang M, Nguyen LH, He Q, Zhen J, Yu Y, et al. Regular use of proton pump inhibitor and the risk of inflammatory bowel disease: pooled analysis of 3 prospective cohorts. Gastroenterology. 2021;161(6):1842-52 e10. [DOI] [PubMed] [Google Scholar]
- 50.Bonaccio M, Di Castelnuovo A, Pounis G, De Curtis A, Costanzo S, Persichillo M, et al. A score of low-grade inflammation and risk of mortality: prospective findings from the Moli-sani study. Haematologica. 2016;101(11):1434–41. 10.3324/haematol.2016.144055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi H, Schweren LJ, Ter Horst R, Bloemendaal M, van Rooij D, Vasquez AA, et al. Low-grade inflammation as mediator between diet and behavioral disinhibition: a UK Biobank study. 2022;106100–10. 10.1016/j.bbi.2022.07.165. [DOI] [PubMed]
- 52.Pounis G, Bonaccio M, Di Castelnuovo A, Costanzo S, de Curtis A, Persichillo M, et al. Polyphenol intake is associated with low-grade inflammation, using a novel data analysis from the Moli-sani study. Thromb Haemost. 2016;115(2):344–52. 10.1160/th15-06-0487. [DOI] [PubMed] [Google Scholar]
- 53.Wu M, Zhang X, Chen J, Zha M, Yuan K, Huang K, et al. A score of low-grade inflammation for predicting stroke recurrence in patients with ischemic stroke. J Inflamm Res. 2021;14:4605–14. 10.2147/jir.S328383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andreis A, Solano A, Balducci M, Picollo C, Ghigliotti M, Giordano M, et al. INFLA-score: a new diagnostic paradigm to identify pericarditis. Hellenic J Cardiol. 2024. 10.1016/j.hjc.2024.03.010. [DOI] [PubMed]
- 55.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. 10.1249/01.Mss.0000078924.61453.Fb. [DOI] [PubMed] [Google Scholar]
- 56.Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology. 2019;157(3):647-+. 10.1053/j.gastro.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 57.Christensen C, Knudsen A, Arnesen EK, Hatlebakk JG, Sletten IS, Fadnes L. Diet, food, and nutritional exposures and inflammatory bowel disease or progression of disease: an umbrella review. Adv Nutr. 2024;15(5). 10.1016/j.advnut.2024.100219. [DOI] [PMC free article] [PubMed]
- 58.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Springer; 2001. 10.1007/978-3-319-19425-7.
- 59.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–61. 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 60.Schisterman EF, Cole SR, Platt RWJE. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–82. 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 62.Khalili H, Hakansson N, Casey K, Lopes E, Ludvigsson JF, Chan AT, et al. Diet quality and risk of older-onset Crohn’s disease and ulcerative colitis. J Crohns Colitis. 2023;17(5):746–53. 10.1093/ecco-jcc/jjac184. [DOI] [PubMed] [Google Scholar]
- 63.Peters V, Bolte L, Schuttert E, Andreu-Sanchez S, Dijkstra G, Weersma R, et al. Western and carnivorous dietary patterns are associated with greater likelihood of IBD development in a large prospective population-based cohort. J Crohns Colitis. 2022;16(6):931–9. 10.1093/ecco-jcc/jjab219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rahmani J, Varkaneh HK, Ryan PM, Zarezadeh M, Rashvand S, Clark C, et al. Healthy eating index-2015 as a predictor of ulcerative colitis risk in a case-control cohort. J Dig Dis. 2019;20(12):649–55. 10.1111/1751-2980.12826. [DOI] [PubMed] [Google Scholar]
- 65.Calder PC. Session 3: Joint Nutrition Society and Irish Nutrition and Dietetic Institute Symposium on ‘nutrition and autoimmune disease’ PUFA, inflammatory processes and rheumatoid arthritis. Proc Nutr Soc. 2008;67(4):409–18. 10.1017/s0029665108008690. [DOI] [PubMed] [Google Scholar]
- 66.Calder PC. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res. 2008;52(8):885–97. 10.1002/mnfr.200700289. [DOI] [PubMed] [Google Scholar]
- 67.Cicerale S, Lucas L, Keast R. Biological activities of phenolic compounds present in virgin olive oil. Int J Mol Sci. 2010;11(2):458–79. 10.3390/ijms11020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scoditti E, Calabriso N, Massaro M, Pellegrino M, Storelli C, Martines G, et al. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch Biochem Biophys. 2012;527(2):81–9. 10.1016/j.abb.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Khalili H, Håkansson N, Chan SS, Chen Y, Lochhead P, Ludvigsson JF, et al. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: results from two large prospective cohort studies. Gut. 2020;69(9):1637–44. 10.1136/gutjnl-2019-319505. [DOI] [PubMed] [Google Scholar]
- 70.Vrdoljak J, Kumric M, Vilovic M, Martinovic D, Tomic IJ, Krnic M, et al. Effects of olive oil and its components on intestinal inflammation and inflammatory bowel disease. Nutrients. 2022;14(4). 10.3390/nu14040757. [DOI] [PMC free article] [PubMed]
- 71.Barrea L, Muscogiuri G, Frias-Toral E, Laudisio D, Pugliese G, Castellucci B, et al. Nutrition and immune system: from the Mediterranean diet to dietary supplementary through the microbiota. Crit Rev Food Sci Nutr. 2021;61(18):3066–90. 10.1080/10408398.2020.1792826. [DOI] [PubMed] [Google Scholar]
- 72.Dietary guidelines for Americans, 2015–2020. U.S. Government Printing Office; 2016. https://www.dietaryguidelines.gov/about-dietary-guidelines.
- 73.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6):1505S-19S. [DOI] [PubMed] [Google Scholar]
- 74.Chan S, Luben R, Olsen A, Tjonneland A, Kaaks R, Lindgren S, et al. Association between high dietary intake of the n-3 polyunsaturated fatty acid docosahexaenoic acid and reduced risk of Crohn’s disease. Aliment Pharmacol Ther. 2014;39(8):834–42. 10.1111/apt.12670. [DOI] [PMC free article] [PubMed]
- 75.Talebi S, Zeraattalab-Motlagh S, Rahimlou M, Naeini F, Ranjbar M, Talebi A, et al. The association between total protein, animal protein, and animal protein sources with risk of inflammatory bowel diseases: a systematic review and meta-analysis of cohort studies. Adv Nutr. 2023;14(4):752–61. 10.1016/j.advnut.2023.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feng R, Tian ZY, Mao R, Ma RQ, Luo WR, Zhao M, et al. Gut microbiome-generated phenylacetylglutamine from dietary protein is associated with Crohn’s disease and exacerbates colitis in mouse model possibly via platelet activation. J Crohns Colitis. 2023. 10.1093/ecco-jcc/jjad098. [DOI] [PubMed]
- 77.Taylor L, Almutairdi A, Shommu N, Fedorak R, Ghosh S, Reimer RA, et al. Cross-sectional analysis of overall dietary intake and Mediterranean dietary pattern in patients with Crohn’s disease. Nutrients. 2018;10(11):1761. 10.3390/nu10111761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Campmans-Kuijpers MJ, Dijkstra G. Food and food groups in inflammatory bowel disease (IBD): the design of the Groningen anti-inflammatory diet (GrAID). Nutrients. 2021;13(4):1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, et al. High-fat, western-style diet, systemic inflammation, and gut microbiota: a narrative review. Cells. 2021;10(11):3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aleksandrova K, Koelman L, Rodrigues CE. Dietary patterns and biomarkers of oxidative stress and inflammation: a systematic review of observational and intervention studies. Redox Biol. 2021;42:101869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wark G, Samocha-Bonet D, Ghaly S, Danta M. The role of diet in the pathogenesis and management of inflammatory bowel disease: a review. Nutrients. 2021;13(1). 10.3390/nu13010135. [DOI] [PMC free article] [PubMed]
- 82.Ruel J, Ruane D, Mehandru S, Gower-Rousseau C, Colombel JF. IBD across the age spectrum—is it the same disease? Nat Rev Gastroenterol Hepatol. 2014;11(2):88–98. [DOI] [PubMed] [Google Scholar]
- 83.Maijo M, Ivory K, Clements SJ, Dainty JR, Jennings A, Gillings R, et al. One-year consumption of a Mediterranean-like dietary pattern with vitamin D3 supplements induced small scale but extensive changes of immune cell phenotype, co-receptor expression and innate immune responses in healthy elderly subjects: results from the United Kingdom arm of the NU-AGE trial. Front Physiol. 2018;9:997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Poslusna K, Ruprich J, de Vries JH, Jakubikova M, van’t Veer P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr. 2009;101(S2):S73–85. [DOI] [PubMed] [Google Scholar]
- 85.Wang DD, Leung CW, Li Y, Ding EL, Chiuve SE, Hu FB, et al. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174(10):1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This research got access to the UK Biobank (https://www.ukbiobank.ac.uk) under application number 51671. Further information is available from the corresponding author upon request.
The data that support the findings of this study are available from UK Biobank project site, subject to registration and application process. Further details can be found at https://www.ukbiobank.ac.uk. The code used in this study is available upon reasonable request to the corresponding author.