Abstract
Background
Previous studies have revealed the impact of heavy metals (HMs) on gut microbiota and intestinal structure. However, the relationship between HMs and fecal incontinence (FI) remains unclear. Therefore, this study aims to evaluate the association between blood HMs exposure and FI.
Methods
Data for this study were obtained from the 2005–2010 cycles of National Health and Nutrition Examination Survey (NHANES). Information regarding FI was retrieved from the Bowel Health Questionnaire, while data on HMs were collected through laboratory examinations. Weighted logistic regression, two-indices weighted quantile sum (2iWQS), quantile g-computation (qgcomp), and restricted cubic splines (RCS) were employed to explore the relationships between blood levels of cadmium (Cd), lead (Pb), and mercury (Hg) and FI. Additionally, Subgroup analyses were conducted to discern specific associations within distinct populations.
Results
A total of 12,142 participants aged 20 years and above were included in this study. Weighted logistic regression indicated a positive association between Cd (Crude model: OR = 1.21, 95% CI: 1.09–1.35, p < 0.001) and Pb (Crude model: OR = 1.01, 95% CI: 1.01–1.02, p < 0.001) with FI. After adjusting for all covariates, the positive associations remained significant for Cd (Model 2: Q1 vs. Q3, OR = 1.38, 95% CI: 1.04–1.83, p = 0.026) and Pb (Model 2: OR = 1.01, 95% CI: 1.00–1.01, p = 0.004). The 2iWQS regression analysis demonstrated a positive correlation between the mixture of three blood HMs and FI (OR = 1.18, 95% CI: 1.05–1.32, p = 0.005), with Cd having the highest weight among the metals (0.76). The qgcomp analysis confirmed this finding (OR = 1.12, 95% CI: 1.01–1.26, p = 0.036; weight = 0.72). Subgroup analysis revealed that the positive association between Cd and FI was more pronounced among males; Mexican Americans; those with a poverty income ratio (PIR) > 2; individuals with college or above education; overweight participants; never-smokers; heavy drinkers; those with hypertension; and non-diabetes individuals. Conversely, the association between Pb and FI was stronger among participants aged 40–60, overweight participants, and never-smokers.
Conclusion
Exposure to blood HMs, particularly Cd, is associated with FI in American adults. Future research should focus on elucidating the causal relationships and underlying mechanisms.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-20958-z.
Keywords: Heavy metals, Blood, Fecal incontinence, NHANES, Cadmium, Lead
Introduction
In recent years, the health issues caused by heavy metals (HMs) exposure have garnered widespread attention. Ubiquitous in daily life, HMs are found in soil, air, dust, drinking water, food, and industrial products [1, 2]. However, existing studies have demonstrated that long-term bioaccumulation of HMs can inflict damage on various tissues and organs and may even lead to increased mortality rates [3]. For instance, exposure to cadmium (Cd) is primarily linked to the increased risk of cardiovascular diseases, renal damage, and osteoporosis [4–6]. Lead (Pb) and mercury (Hg), due to their neurotoxicity, are associated with central nervous system disorders, such as cognitive decline [7–10]. Furthermore, emerging research underscores the significant health impacts of co-exposure to multiple HMs, with associations to conditions such as hypertension, diabetes, metabolic syndrome, dyslipidemia, as well as liver damage, pulmonary harm, and psoriasis [11–16]. Therefore, it is crucial to identify which organs or systems are adversely affected by HMs.
Diet is a significant source of HMs [17]. Research indicates that over 90% of non-occupational Cd exposure in the general population (excluding smokers) comes from the consumption of grains, vegetables, and other plant-based foods [18]. According to a study by the Centers for Disease Control and Prevention (CDC), Hg exposure in young children and childbearing-aged women primarily arises from the consumption of seafood, including freshwater fish, marine fish, and shellfish [19]. Given that the digestive tract is the primary site for food digestion and absorption, exposure to HMs can cause substantial damage to the gastrointestinal system, including a reduced abundance and diversity of gut microbiota and alterations in intestinal structure [20]. Despite these concerns, there is limited research investigating the relationship between HMs and gastrointestinal diseases.
Fecal incontinence (FI) is a prevalent gastrointestinal disorder characterized by the involuntary leakage of solid or liquid feces [21]. The global prevalence of FI is approximately 8%, with higher rates observed among the elderly and females [22]. FI not only significantly impacts quality of life but is also associated with various conditions, including gastrointestinal cancers, lymphomas, depression, and sarcopenia [23–26]. The etiologies of FI encompass neurological degenerative diseases, muscle damage, and gastrointestinal disorders [27]. Given the neurotoxic effects of HMs and their impact on the gastrointestinal tract, a potential link between HMs and FI may exist; however, supporting evidence remains limited.
Therefore, this study aims to investigate the associations between blood Cd, Pb, Hg, as well as their mixture and FI using data from 12,142 participants in NHANES 2005–2010. To evaluate these relationships, we employed weighted logistic regression, two-indices weighted quantile sum (2iWQS), quantile g-computation (qgcomp), and restricted cubic spline (RCS) analyses.
Methods
Study design and population
The National Health and Nutrition Examination Survey (NHANES), conducted by the National Center for Health Statistics (NCHS) under the CDC, is a large-scale survey designed to assess the health and nutritional status of U.S. adults and children. The survey employs a multistage probability sampling method, drawing representative samples from various counties across the United States every two years for health and nutrition assessments. All NHANES projects receive approval from the NCHS Research Ethics Review Board, and participants provide written consent upon enrollment (NHANES—NCHS Research Ethics Review Board Approval (cdc.gov)).
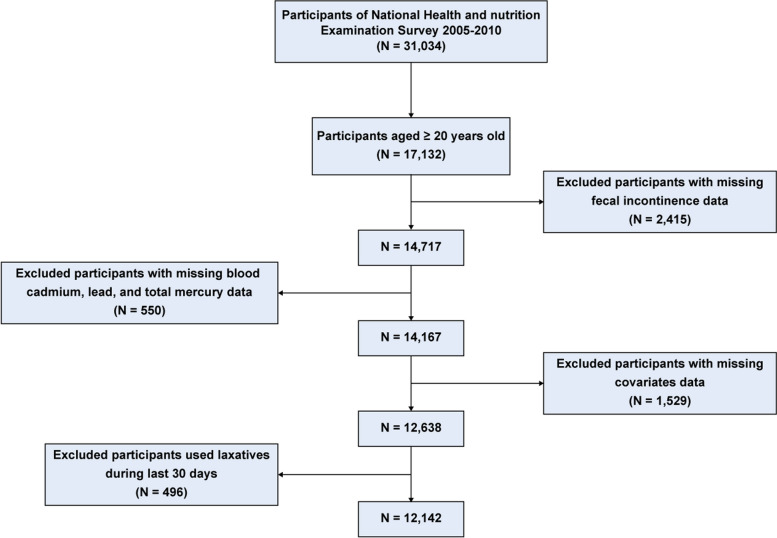
In this study, data were retrieved from the NHANES cycles of 2005–2006, 2007–2008, and 2009–2010. We excluded participants who were under the age of 20 (N = 13,902), had missing FI data (N = 2,415), missing blood HMs data (N = 550), missing covariate data (N = 1,529), or had used laxatives in the past 30 days (N = 496). A total of 12,142 participants were ultimately included in the final analysis (Fig. 1).
Fig. 1.
Flowchart of participant selection
Definition of FI
In the NHANES 2005–2010 questionnaire section, the Bowel Health Questionnaire (BHQ) offered detailed information on FI and bowel function for adults aged 20 or older. In the Mobile Examination Center (MEC), participants were asked about the involuntary leakage of feces, including solids, liquids, mucus, or gas, and the frequency of these occurrences over the past 30 days. Additionally, the Bristol Stool Form Scale (BSFS) cards were used to assess stool morphology. Consistent with previous studies [28, 29], FI in this study was defined as the involuntary discharge of solid, mucus, or liquid stools at least once in the past month, excluding gas.
Measurement of blood HMs
Considering that the NHANES 2005–2010 dataset includes data only on three blood HMs (Cd, Pb, and Hg), this study focuses exclusively on their relationship with FI, excluding manganese (Mn) and selenium (Se), which were introduced in later survey cycles. Within the MECs, trained personnel collected blood samples directly from participants, which were then stored at −30℃ and sent to the National Center for Environmental Health and the CDC for subsequent analysis. Blood concentrations of Cd, Pb, and total Hg were measured using inductively coupled plasma mass spectrometry (ICP-MS). Detailed experimental procedures and methods are outlined in the NHANES laboratory protocol. According to the NHANES analysis guidelines, measurements below the limit of detection (LOD) were substituted with LOD divided by the square root of 2 () [30]. Additionally, the concentrations of the three blood HMs in this study are expressed in μg/L. The specific LOD values for the three metals, as well as the proportions of values below the detection limit, can be found in Table S1 and Table S2.
Covariates
Based on previous research [31–33], we included several potentially significant variables that could affect FI as confounders, such as demographic characteristics (age, sex, race, education level, family poverty income ratio [PIR]); body mass index (BMI); lifestyle behaviors (smoking status, alcohol consumption); and chronic conditions (hypertension, diabetes). A directed acyclic graph was created, as shown in Figure S1.
Race was categorized as non-Hispanic Black, non-Hispanic White, Mexican American, and other races (including other Hispanics and multiple races). Education level was classified as less than high school, high school, and college or above. BMI was categorized as: normal (< 25 kg/m2), overweight (25–29.9 kg/m2), and obese (≥ 30 kg/m2). The continuous variable PIR was dichotomized into a binary variable (PIR < 2, PIR ≥ 2). Smoking status was defined as: never (having smoked fewer than 100 cigarettes in a lifetime), former (having smoked more than 100 cigarettes in a lifetime but not currently smoking), and now (having smoked more than 100 cigarettes in a lifetime and currently smoking). Alcohol consumption was categorized as: never (fewer than 12 alcoholic drinks in a lifetime); former (12 or more alcoholic drinks in a lifetime but none in the past 2 years); mild (women consuming more than 1 drink per day, men more than 2 drinks per day); moderate (women 2 drinks per day, men 3 drinks per day); and heavy (women 3 or more drinks per day, men 4 or more drinks per day). Hypertension was defined as: [1] a doctor’s diagnosis of hypertension; [2] use of antihypertensive medication; or [3] systolic blood pressure greater than 140 mmHg or diastolic blood pressure greater than 90 mmHg. Diabetes was defined as: [1] a doctor’s diagnosis of diabetes; [2] glycated hemoglobin ≥ 6.5%; [3] fasting blood glucose ≥ 7.0 mmol/L, random blood glucose ≥ 11.1 mmol/L, or 2-h OGTT blood glucose ≥ 11.1 mmol/L; or [4] use of antidiabetic medication or insulin.
Statistical analysis
Continuous variables were presented as weighted means (± standard errors), while categorical variables were shown as unweighted counts (weighted percentages). Comparisons between continuous variables were performed using t-tests, whereas comparisons between categorical variables were conducted using chi-square tests. The associations among the three blood HMs were assessed using Pearson correlation analysis.
First, weighted logistic regression analysis was used to assess the relationship between each HM and FI. Three models were constructed to adjust for confounding variables: the crude model, unadjusted; Model 1, adjusted for age, sex, race, education level, and PIR; and Model 2, further adjusted for BMI, smoking status, alcohol consumption, hypertension, and diabetes. Subsequently, we performed a test for linear trends by inputting the median value of each metal as a continuous variable into the model.
Second, given that traditional WQS regression models may be limited by the directional effects of mixtures on outcomes, and that mixtures may contain both “good” and “bad” components [34], we employed the two-indices WQS (2iWQS) regression model to separately assess the positive and negative effects of HMs [35]. Specifically, data were randomly divided into training and validation sets with a 4:6 ratio. We performed 200 iterations in the training set to obtain weights for each HM and tested their significance in the validation set. The 2iWQS index comprises “PWQS” and “NWQS,” which represent the positive and negative effects, respectively. Additionally, to further validate the robustness of the results, quantile g-computation (qgcomp) analysis was conducted to explore the association between HMs and FI.
Restricted Cubic Splines (RCS) were used to assess the nonlinear relationship between HMs and FI. To avoid underfitting or overfitting and ensure model accuracy, the optimal number of knots for RCS was selected based on the minimum Akaike Information Criterion (AIC). Ultimately, the RCS curve was fitted with 4 knots. Finally, subgroup analyses were conducted to explore the relationship between HMs and FI based on age, sex, race, education level, PIR, BMI, smoking status, alcohol consumption, hypertension, and diabetes.
All analyses in this study were conducted using R software (version 4.3.2). Weighted logistic regression, 2iWQS, qgcomp, and RCS analyses were performed using the “survey,” “gWQS,” “qgcomp,” and “rms” packages, respectively. A p-value of less than 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 12,142 participants were included in this study, with a mean age of 46.6 years (± 0.3). Among them, 40.4% were aged between 40 and 59 years, 50.2% were female, and the majority were non-Hispanic white (72.6%). FI was observed in 1,074 participants, accounting for 8.8% of the sample. The blood levels of HMs were 0.52 (± 0.01) μg/L for Cd, 16.53 (± 0.27) μg/L for Pb, and 1.65 (± 0.05) μg/L for Hg, with no significant correlations among them (r < 0.2) (Figure S2). Compared to the non-FI individuals, FI was more common among older individuals, females, non-Hispanic Whites, those with lower PIR, obesity, smokers, heavy drinkers, and those with hypertension or diabetes. Additionally, FI participants had higher blood levels of Cd (0.60 vs 0.51, p < 0.001) and Pb (19.27 vs 16.28, p < 0.001) compared to those without FI. However, FI participants had lower blood levels of Hg, though this difference was not statistically significant (Table 1).
Table 1.
Baseline characteristics of normal and FI participants in NHANES 2005–2010
| Characteristic | Overall(N = 12,142) | Normal(N = 11,068) | FI(N = 1,074) | P value |
|---|---|---|---|---|
| Age, years | < 0.001 | |||
| 20–39 | 4,034 (36.8%) | 3,869 (38.6%) | 165 (16.8%) | |
| 40–59 | 4,112 (40.4%) | 3,747 (40.1%) | 365 (43.0%) | |
| ≥ 60 | 3,996 (22.9%) | 3,452 (21.3%) | 544 (40.2%) | |
| Gender | < 0.001 | |||
| female | 5,947 (50.2%) | 5,357 (49.6%) | 590 (56.7%) | |
| male | 6,195 (49.8%) | 5,711 (50.4%) | 484 (43.3%) | |
| Race | < 0.001 | |||
| non-Hispanic black | 2,351 (10.3%) | 2,164 (10.4%) | 187 (9.2%) | |
| non-Hispanic white | 6,201 (72.6%) | 5,549 (72.1%) | 652 (78.6%) | |
| Mexican American | 2,155 (7.8%) | 2,025 (8.1%) | 130 (4.8%) | |
| other races | 1,435 (9.3%) | 1,330 (9.4%) | 105 (7.4%) | |
| PIR | 0.044 | |||
| < 2 | 5,552 (31.9%) | 5,024 (31.6%) | 528 (35.8%) | |
| ≥ 2 | 6,590 (68.1%) | 6,044 (68.4%) | 546 (64.2%) | |
| Education level | 0.068 | |||
| less than high school | 3,326 (17.8%) | 2,994 (17.5%) | 332 (20.8%) | |
| high school | 2,901 (24.2%) | 2,656 (24.2%) | 245 (23.4%) | |
| college or above | 5,915 (58.0%) | 5,418 (58.2%) | 497 (55.8%) | |
| BMI, kg/m2 | 0.002 | |||
| < 25 | 3,503 (31.4%) | 3,228 (31.8%) | 275 (27.3%) | |
| 25–29.9 | 4,150 (33.5%) | 3,812 (33.7%) | 338 (30.7%) | |
| ≥ 30 | 4,489 (35.1%) | 4,028 (34.5%) | 461 (42.0%) | |
| Smoking status | 0.032 | |||
| never | 6,277 (52.2%) | 5,793 (52.7%) | 484 (47.4%) | |
| former | 3,096 (25.0%) | 2,756 (24.6%) | 340 (29.1%) | |
| now | 2,769 (22.8%) | 2,519 (22.7%) | 250 (23.5%) | |
| Alcohol consumption | < 0.001 | |||
| never | 1,549 (10.3%) | 1,403 (10.2%) | 146 (11.6%) | |
| former | 2,402 (16.5%) | 2,128 (16.0%) | 274 (21.6%) | |
| mild | 3,809 (34.7%) | 3,458 (34.4%) | 351 (37.2%) | |
| moderate | 1,787 (16.4%) | 1,658 (16.7%) | 129 (13.8%) | |
| heavy | 2,595 (22.1%) | 2,421 (22.7%) | 174 (15.8%) | |
| Hypertension | < 0.001 | |||
| no | 7,102 (63.8%) | 6,632 (65.1%) | 470 (49.2%) | |
| yes | 5,040 (36.2%) | 4,436 (34.9%) | 604 (50.8%) | |
| Diabetes | < 0.001 | |||
| no | 10,039 (87.4%) | 9,240 (88.0%) | 799 (80.6%) | |
| yes | 2,103 (12.6%) | 1,828 (12.0%) | 275 (19.4%) | |
| Blood heavy metals, μg/L | ||||
| Cd | 0.52 (± 0.01) | 0.51 (± 0.01) | 0.60 (± 0.03) | < 0.001 |
| Pb | 1.65 (± 0.03) | 1.63 (± 0.03) | 1.93 (± 0.06) | < 0.001 |
| Hg | 1.65 (± 0.05) | 1.66 (± 0.05) | 1.51 (± 0.09) | 0.381 |
FI fecal incontinence, NHANES National Health and Nutrition Examination Survey, PIR family poverty income ratio, BMI body mass index, Cd cadmium, Pb lead, Hg mercury
Mean (± SE) for continuous and n (%) for categorical variables
Bold: p < 0.05
Multiple logistic analysis
Logistic regression analysis showed a significant positive association between blood Cd levels and FI when Cd was analyzed as a continuous variable (Crude model: OR = 1.21, 95% CI: 1.09–1.35, p < 0.001; Model 1: OR = 1.14, 95% CI: 1.01–1.28, p < 0.001). However, after adjusting for all covariates, this association was no longer significant (Model 2: OR = 1.10, 95% CI = 0.96–1.27, p = 0.156). When Cd was categorized into tertiles, the highest tertile (Q3) showed a significant positive association with FI compared to the lowest tertile (Q1) (Crude model: Q3 vs. Q1, OR = 1.83, 95% CI: 1.47–2.28, p < 0.001, p for trend < 0.001; Model 1: OR = 1.38, 95% CI: 1.09–1.73, p = 0.008, p for trend = 0.008). This relationship remained robust after adjusting for all confounders (Model 2: Q3 vs. Q1, OR = 1.38, 95% CI = 1.04–1.83, p = 0.026, p for trend = 0.026) (Table 2).
Table 2.
Multiple logistic analysis between blood heavy metals and FI
| Blood heavy metals(μg/L) | Crude model | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|---|
| OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | ||
| Cd | Continuous | 1.21(1.09,1.35) | <0.001 | 1.14(1.01,1.28) | 0.029 | 1.10(0.96,1.27) | 0.156 |
| Q1 | ref | ref | ref | ||||
| Q2 | 1.59(1.28,2.00) | <0.001 | 1.18(0.94,1.49) | 0.157 | 1.20(0.94,1.53) | 0.146 | |
| Q3 | 1.83(1.47,2.28) | <0.001 | 1.38(1.09,1.73) | 0.008 | 1.38(1.04,1.83) | 0.026 | |
| p for trend | <0.001 | 0.008 | 0.026 | ||||
| Pb | Continuous | 1.12(1.07,1.16) | <0.001 | 1.10(1.02,1.12) | 0.007 | 1.07(1.03,1.12) | 0.004 |
| Q1 | ref | ref | ref | ||||
| Q2 | 1.56(1.26,1.94) | <0.001 | 1.18(0.93,1.51) | 0.171 | 1.20(0.94,1.52) | 0.150 | |
| Q3 | 1.86(1.52,2.30) | <0.001 | 1.23(0.95,1.60) | 0.109 | 1.27(0.98,1.63) | 0.075 | |
| p for trend | <0.001 | 0.112 | 0.075 | ||||
| Hg | Continuous | 0.96(0.92,1.01) | 0.128 | 0.95(0.91,1.00) | 0.061 | 0.96(0.92,1.01) | 0.124 |
| Q1 | ref | ref | ref | ||||
| Q2 | 0.90(0.77,1.05) | 0.189 | 0.86(0.73,1.02) | 0.091 | 0.88(0.74,1.05) | 0.149 | |
| Q3 | 0.89(0.73,1.08) | 0.229 | 0.83(0.67,1.02) | 0.078 | 0.87(0.70,1.08) | 0.186 | |
| p for trend | 0.239 | 0.084 | 0.196 | ||||
Crude model was not adjusted, Model 1 was adjusted for age, gender, race, PIR, and education level, Model 2 was adjusted for age, gender, race, PIR, education level, BMI, smoking status, alcohol consumption, hypertension and diabetes
OR odds ratio, CI confidence interval, Q quantile, Cd: Q1=[0.01, 0.26], Q2=(0.26,0.49]; Q3=(0.49,10.8]; Pb: Q1=[0.13, 1.07]; Q2=(1.07, 1.89]; Q3=(1.89, 33.1]; Hg: Q1=[0.10, 0.62], Q2=(0.62, 1.37], Q3=(1.37, 85.7]
Similarly, when blood Pb was analyzed as a continuous variable, logistic regression analysis revealed a significant positive association between blood Pb levels and FI, with this relationship maintaining statistical significance across all three models (Crude model: OR = 1.01, 95% CI: 1.01–1.02, p < 0.001; Model 1: OR = 1.01, 95% CI: 1.00–1.01, p = 0.007; Model 2: OR = 1.01, 95% CI: 1.01–1.01, p = 0.004). However, when Pb was considered as a categorical variable, a significant positive association between Pb and FI was observed only in the unadjusted model (Crude model: Q2 vs Q1, OR = 1.58, 95% CI: 1.28–1.94, p < 0.001; Q3 vs Q1, OR = 1.88, 95% CI: 1.53–2.30, p < 0.001). This statistical significance not observed after adjusting for confounders.
Regarding blood Hg, logistic regression analysis showed no significant association between blood Hg levels and FI, whether Hg was considered as a continuous or categorical variable (all p-values > 0.05).
2iWQS and qgcomp model
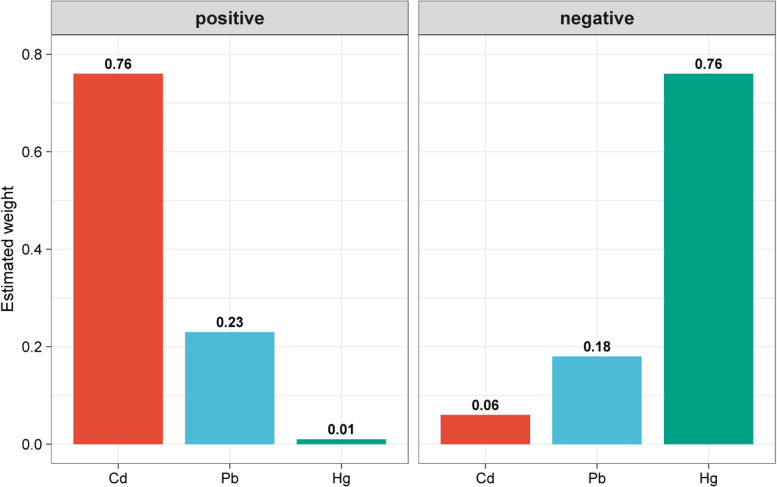
As shown in Table 3, the 2iWQS regression analysis indicated a positive association between combined exposure to the three blood HMs and FI (OR = 1.18, 95% CI: 1.05–1.32, p = 0.005), with no negative association observed. The qgcomp analysis corroborated this finding (OR = 1.12, 95% CI: 1.01–1.26, p = 0.036). The estimated weights for individual HMs in the WQS analysis are shown in Fig. 2. Among the three HMs, Cd had the highest positive weight (0.76), while Hg had the highest negative weight (0.76); however, no significant negative association was observed. Similarly, the qgcomp analysis confirmed these findings, demonstrating the robustness of the findings (Figure S3).
Table 3.
Result of the 2iWQS and qgcomp models
| Model | OR | 95%CI | P value |
|---|---|---|---|
| 2iWQS | |||
| positive | 1.18 | 1.05–1.32 | 0.005 |
| negative | 0.97 | 0.86–1.09 | 0.521 |
| qgcomp | 1.12 | 1.01–1.26 | 0.036 |
Both the WQS and qgcomp model were adjusted for age, gender, race, PIR, education level, BMI, smoking status, alcohol consumption, hypertension and diabetes
Fig. 2.
WQS model regression index weights for blood heavy metals exposure on fecal incontinence
RCS
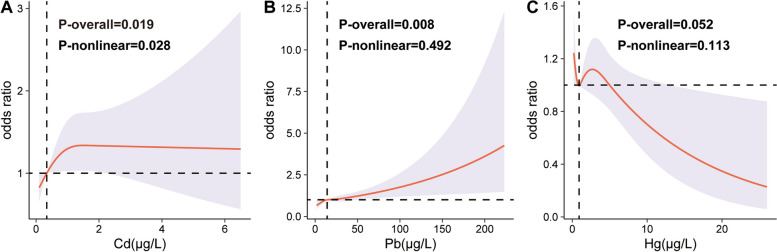
The RCS curves demonstrated a significant nonlinear association between Cd and FI after adjusting for all covariates (p-overall = 0.019, p-nonlinear = 0.028) (Fig. 3A). In contrast, a linear positive correlation was observed between Pb and FI (p-overall = 0.008, p-nonlinear = 0.492) (Fig. 3B). No significant relationship was found between Hg and FI (Fig. 3C).
Fig. 3.
RCS curves between blood Cd(A), Pb(B), Hg(C) and fecal incontinence. ALL were adjusted for age, gender, race, PIR, education level, BMI, smoking status, alcohol consumption, hypertension and diabetes
Subgroup analyses
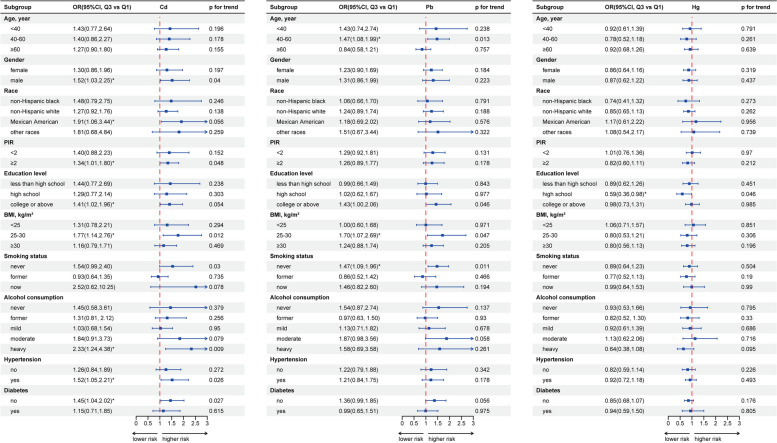
As shown in Fig. 4, the subgroup analysis revealed that the positive correlation between Cd and FI was more pronounced among males, Mexican Americans, those with PIR > 2, individuals with college or above education, overweight participants, never-smokers, heavy drinkers, those with hypertension, and non-diabetic individuals. The positive association between Pb and FI was stronger among participants aged 40–60, overweight participants, and never-smokers. No specific subgroup exhibited a significant correlation between Hg and FI.
Fig. 4.
Forest plot of subgroup analysis based on age, gender, race, PIR, education level, BMI, smoking status, alcohol consumption, hypertension and diabetes. * represents P < 0.05
Discussion
Utilizing a large-scale, nationally representative study, we identified a significant positive correlation between blood HMs and FI, which remains robust even after adjusting for various confounding factors. Multivariate logistic regression analysis revealed that exposure to Cd and Pb is associated with FI. Both the 2iWQS and qgcomp analyses confirmed that the positive association between three blood HMs and FI persists, with Cd emerging as the most substantial contributor. The RCS curves suggested a nonlinear positive correlation between Cd and FI and a linear positive correlation between Pb and FI. Subgroup analysis highlighted that the positive association between Cd and FI was more pronounced among males, Mexican Americans, those with a PIR > 2, individuals with college or above education, overweight individuals, heavy drinkers, those with hypertension, and non-diabetics. The positive association between Pb and FI is more evident in the 40–60 age group, overweight individuals, and non-smokers. To our knowledge, this is the first study to explore the relationship between blood HMs and FI.
However, the specific mechanisms underlying the relationship between HMs and FI remain unclear. We propose that this relationship may be influenced by several factors. Firstly, exposure to HMs can alter the diversity and structure of gut microbiota. Trevors et al. demonstrated that Cd inhibited the growth of intestinal microbiota by interfering with protein synthesis and the function of various enzyme systems [36]. Zhang et al. showed that Cd disrupted the composition of gut microbiota in mice, notably reducing the abundance of Firmicutes and Proteobacteria while increasing the population of Bacteroidetes [37]. Even at low concentrations, Cd exposure has been observed to decrease both the abundance and diversity of gut microbiota [38]. Moreover, Cd exposure leads to a significant reduction in bacteria that produce short-chain fatty acids (SCFAs), impairing the function of intestinal epithelial cells, increasing intestinal permeability, and causing intestinal inflammation [39]. Similarly, Pb exposure has been found to affect the composition of gut microbiota and the integrity of the gut barrier. For instance, a recent study administering low concentrations of Pb to mice over 15 weeks found a significant increase in the abundance of Bacteroidetes and a decrease in fungi within the gut [40]. Human studies have also indicated that the α- and β-diversity of gut microbiota are influenced by urinary Pb levels [41]. Increasing evidence supports a “bidirectional relationship” between HMs exposure and the gut microbiota [42], suggesting that HMs exposure can alter the metabolism of normal gut microbiota, which in turn can influence the biotransformation and toxicological effects of HMs. In summary, the impact of HMs on the gut microbiota and gut structure likely represents a significant mechanism contributing to the occurrence of FI.
Secondly, the neurotoxicity of Cd and Pb directly impacts on the central nervous system, contributing to cognitive decline [43]. A recent NHANES study found a negative correlation between blood levels of Pb and Cd and cognitive function, while blood selenium demonstrated a positive correlation with cognitive function in older adults, particularly among men [44]. Additionally, HMs have been found to positively correlate with systemic immune-inflammation index (SII) and systemic inflammation response index (SIRI). Given that inflammation is a crucial factor in the development of FI, this relationship may further elucidate the pathway by which HMs contribute to FI [45, 46].
In summary, this cross-sectional study identified a positive correlation between blood HMs and FI. This finding not only offers new insights into the detrimental effects of HMs on human health but also provides valuable reference for the prevention of FI. Reducing the intake of foods with high levels of HMs could significantly aid in preventing the onset of FI. Moreover, reducing the release of pollutants, especially waste gases and wastewater containing HMs, is essential for maintaining safe environmental levels of these metals. This is particularly important considering that a significant portion of inhaled Cd eventually enters the gastrointestinal tract [47].
Our study possesses several strengths. Firstly, it is based on a comprehensive national population dataset, providing a large sample size. Secondly, we employed multivariate logistic regression, 2iWQS, qgcomp, and RCS to adjust for multiple potential confounders, enhancing the reliability of our findings. However, there are also some limitations. Firstly, as a cross-sectional study, it cannot establish causal relationships between HMs and FI. Secondly, due to limitations in the NHANES dataset, we were unable to adjust for other potentially relevant variables associated with FI, such as congenital diseases, surgeries, and radiotherapy. Thirdly, using the NHANES-recommended method of dividing metal concentrations below the detection limit by the square root of 2 may affect the accuracy of the study results. Lastly, our study focused exclusively on the association between blood HMs and FI, leaving the relationship between dietary or urinary HMs and FI unexplored. Future research is needed to further investigate these associations.
Conclusion
Given the various health risks posed by HMs in blood and the significant prevalence of FI, understanding the connection between these factors is essential. Our study identifies an association between exposure to blood HMs, particularly cadmium (Cd), and FI. These findings provide valuable insights into the potential implications of heavy metal exposure and could inform preventive strategies for FI. However, the cross-sectional nature of this study presents limitations, emphasizing the need for future investigations to establish causal relationships and explore the underlying biological mechanisms.
Supplementary Information
Supplementary Material 1: Table S1.LODs of blood Cd, Pb and Hg in NHANES 2005-2010. Table S2. Proportion of blood Cd, Pb, and Hg below the limit of detection in NHANES 2005-2010. Figure S1. Directed acyclic graph. PIR, poverty income ratio; BMI, body mass index. Figure S2. Correlation between heavy metals in blood. Figure S3. Quantile g-computation scaled weights of blood Cd, Pb, and Hg.
Acknowledgements
Sincere thanks to the NHANES staff and participants for their invaluable contributions.
Abbreviations
- HMs
Heavy metals
- FI
Fecal incontinence
- NHANES
National Health and Nutrition Examination Survey
- Cd
Cadmium
- Pb
Lead
- Hg
Mercury
- LOD
Low limit of detection
- 2iWQS
Two-indices weighted quantile sum
- Qgcomp
Quantile g-computation
- RCS
Restrict cubic splines
- PIR
Poverty income ratio
- BMI
Body mass index
- OR
Odds ratio
- CI
Confidence interval
Authors’ contributions
Z.G.L performed the described studies, analyzed the data, and prepared the manuscript. S.Q.P and D.C.Z conducted the statistical analysis and revised the manuscript. L.L.L checked the data, review & editing and approved the final manuscript.
Funding
No Funding.
Data availability
Publicly available datasets were analyzed in this study. This data can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.
Declarations
Ethics approval and consent to participate
All NHANES projects receive approval from the NCHS Research Ethics Review Board, and participants provide written consent upon enrollment (NHANES—NCHS Research Ethics Review Board Approval (cdc.gov)).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kavamura VN, Esposito E. Biotechnological strategies applied to the decontamination of soils polluted with heavy metals. Biotechnol Adv. 2010;28(1):61–9. [DOI] [PubMed] [Google Scholar]
- 2.Clemens S, Ma JF. Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. Annu Rev Plant Biol. 2016;67:489–512. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Wang Y, Li R, Ni B, Chen R, Huang Y, et al. Low-grade systemic inflammation links heavy metal exposures to mortality: A multi-metal inflammatory index approach. Sci Total Environ. 2024;947: 174537. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Morata I, Schilling K, Glabonjat RA, Domingo-Relloso A, Mayer M, McGraw KE, et al. Association of Urinary Metals With Cardiovascular Disease Incidence and All-Cause Mortality in the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2024;150(10):758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu H, Tang X, Gu C, Chen R, Liu Y, Chu H, et al. Assessment of human exposure to cadmium and its nephrotoxicity in the Chinese population. Sci Total Environ. 2024;918: 170488. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, Ran D, Shi X, Zhao H, Liu Z. Cadmium toxicity: A role in bone cell function and teeth development. Sci Total Environ. 2021;769: 144646. [DOI] [PubMed] [Google Scholar]
- 7.Shao K, Yu Y, Ritz B, Paul KC. DNA methylation biomarkers for cumulative lead exposures and cognitive impairment. Environ Res. 2024;264(Pt 2):120304. [DOI] [PubMed]
- 8.Reuben A. Childhood Lead Exposure and Adult Neurodegenerative Disease. J Alzheimers Dis. 2018;64(1):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saavedra S, Fernández-Recamales Á, Sayago A, Cervera-Barajas A, González-Domínguez R, Gonzalez-Sanz JD. Impact of dietary mercury intake during pregnancy on the health of neonates and children: a systematic review. Nutr Rev. 2022;80(2):317–28. [DOI] [PubMed] [Google Scholar]
- 10.Shah S, Jeong KS, Park H, Hong YC, Kim Y, Kim B, et al. Environmental pollutants affecting children’s growth and development: Collective results from the MOCEH study, a multi-centric prospective birth cohort in Korea. Environ Int. 2020;137: 105547. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Han X, Guo S, Ma Y, Zhang Y. Associations between patterns of blood heavy metal exposure and health outcomes: insights from NHANES 2011–2016. BMC Public Health. 2024;24(1):558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zha B, Liu Y, Xu H. Associations of mixed urinary metals exposure with metabolic syndrome in the US adult population. Chemosphere. 2023;344: 140330. [DOI] [PubMed] [Google Scholar]
- 13.Wang G, Fang L, Chen Y, Ma Y, Zhao H, Wu Y, et al. Association between exposure to mixture of heavy metals and hyperlipidemia risk among U.S. adults: A cross-sectional study. Chemosphere. 2023;344:140334. [DOI] [PubMed]
- 14.Li W, Li X, Su J, Chen H, Zhao P, Qian H, et al. Associations of blood metals with liver function: Analysis of NHANES from 2011 to 2018. Chemosphere. 2023;317: 137854. [DOI] [PubMed] [Google Scholar]
- 15.Wu L, Cui F, Ma J, Huang Z, Zhang S, Xiao Z, et al. Associations of multiple metals with lung function in welders by four statistical models. Chemosphere. 2022;298: 134202. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Pan Z, Shen J, Wu Y, Fang L, Xu S, et al. Associations of exposure to blood and urinary heavy metal mixtures with psoriasis risk among U.S. adults: A cross-sectional study. The Science of the total environment. 2023;887:164133. [DOI] [PubMed]
- 17.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy Metal Toxicity and the Environment. In: Luch A, editor. Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology. Basel: Springer Basel; 2012. p. 133–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clemens S, Aarts MG, Thomine S, Verbruggen N. Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci. 2013;18(2):92–9. [DOI] [PubMed] [Google Scholar]
- 19.Blood mercury levels in young children and childbearing-aged women--United States, 1999–2002. MMWR Morbidity and mortality weekly report. 2004;53(43):1018–20. [PubMed]
- 20.Liu X, Zhang J, Si J, Li P, Gao H, Li W, et al. What happens to gut microorganisms and potential repair mechanisms when meet heavy metal(loid)s. Environmental pollution (Barking, Essex : 1987). 2023;317:120780. [DOI] [PubMed]
- 21.Bharucha AE, Dunivan G, Goode PS, Lukacz ES, Markland AD, Matthews CA, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110(1):127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mack I, Hahn H, Gödel C, Enck P, Bharucha AE. Global Prevalence of Fecal Incontinence in Community-Dwelling Adults: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2024;22(4):712-31.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ten Hoor MBC, Trzpis M, Broens PMA. The Severity of Fecal Problems Is Negatively Associated With Quality of Life in a Dutch Population Without Bowel Function Comorbidities. Dis Colon Rectum. 2024;67(3):448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adelborg K, Veres K, Sundbøll J, Gregersen H, Sørensen HT. Risk of cancer in patients with fecal incontinence. Cancer Med. 2019;8(14):6449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Li N, Zhou Q, Wang P. Fecal incontinence was associated with depression of any severity: insights from a large cross-sectional study. Int J Colorectal Dis. 2023;38(1):271. [DOI] [PubMed] [Google Scholar]
- 26.Mizuno S, Wakabayashi H, Yamakawa M, Wada F, Kato R, Furiya Y, et al. Sarcopenia Is Associated with Fecal Incontinence in Patients with Dysphagia: Implication for Anal Sarcopenia. J Nutr Health Aging. 2022;26(1):84–8. [DOI] [PubMed] [Google Scholar]
- 27.Menees S, Chey WD. Fecal Incontinence: Pathogenesis, Diagnosis, and Updated Treatment Strategies. Gastroenterol Clin North Am. 2022;51(1):71–91. [DOI] [PubMed] [Google Scholar]
- 28.Ditah I, Devaki P, Luma HN, Ditah C, Njei B, Jaiyeoba C, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005–2010. Clin Gastroenterol Hepatol. 2014;12(4):636–43.e1–2. [DOI] [PubMed]
- 29.Whitehead WE, Borrud L, Goode PS, Meikle S, Mueller ER, Tuteja A, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137(2):512–7, 7.e1–2. [DOI] [PMC free article] [PubMed]
- 30.Duan W, Xu C, Liu Q, Xu J, Weng Z, Zhang X, et al. Levels of a mixture of heavy metals in blood and urine and all-cause, cardiovascular disease and cancer mortality: A population-based cohort study. Environmental pollution (Barking, Essex : 1987). 2020;263(Pt A):114630. [DOI] [PubMed]
- 31.Mahjoob DM, Knol-de Vries GE, de Boer M, van Koeveringe GA, Blanker MH. The association of fecal incontinence, constipation, and pelvic pain with the course of lower urinary tract symptoms in community-dwelling men and women. Neurourology and urodynamics. Neurourol Urodyn. 2024;43(7):1566–73. [DOI] [PubMed]
- 32.Seitz V, Calata J, Mei L, Davidson ERW. Racial Disparities in Sacral Neuromodulation for Idiopathic Fecal Incontinence. Urogynecology (Phila). 2024;30(11):873–9. [DOI] [PubMed]
- 33.Hiramoto B, Flanagan R, Muftah M, Shah ED, Chan WW. Centrally Distributed Adiposity as a Modifiable Risk Factor for Fecal Incontinence: United States Population-based Analysis. Clin Gastroenterol Hepatol. 2024;22(9):1908–16.e1. [DOI] [PMC free article] [PubMed]
- 34.Yu L, Liu W, Wang X, Ye Z, Tan Q, Qiu W, et al. A review of practical statistical methods used in epidemiological studies to estimate the health effects of multi-pollutant mixture. Environmental pollution (Barking, Essex : 1987). 2022;306:119356. [DOI] [PubMed]
- 35.Renzetti S, Gennings C, Calza S. A weighted quantile sum regression with penalized weights and two indices. Front Public Health. 2023;11:1151821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trevors JT, Stratton GW, Gadd GM. Cadmium transport, resistance, and toxicity in bacteria, algae, and fungi. Can J Microbiol. 1986;32(6):447–64. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Jin Y, Zeng Z, Liu Z, Fu Z. Subchronic Exposure of Mice to Cadmium Perturbs Their Hepatic Energy Metabolism and Gut Microbiome. Chem Res Toxicol. 2015;28(10):2000–9. [DOI] [PubMed] [Google Scholar]
- 38.Ba Q, Li M, Chen P, Huang C, Duan X, Lu L, et al. Sex-Dependent Effects of Cadmium Exposure in Early Life on Gut Microbiota and Fat Accumulation in Mice. Environ Health Perspect. 2017;125(3):437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He X, Qi Z, Hou H, Qian L, Gao J, Zhang XX. Structural and functional alterations of gut microbiome in mice induced by chronic cadmium exposure. Chemosphere. 2020;246: 125747. [DOI] [PubMed] [Google Scholar]
- 40.Xia J, Jin C, Pan Z, Sun L, Fu Z, Jin Y. Chronic exposure to low concentrations of lead induces metabolic disorder and dysbiosis of the gut microbiota in mice. The Science of the total environment. 2018;631–632:439–48. [DOI] [PubMed] [Google Scholar]
- 41.Eggers S, Safdar N, Sethi AK, Suen G, Peppard PE, Kates AE, et al. Urinary lead concentration and composition of the adult gut microbiota in a cross-sectional population-based sample. Environ Int. 2019;133(Pt A): 105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duan H, Yu L, Tian F, Zhai Q, Fan L, Chen W. Gut microbiota: A target for heavy metal toxicity and a probiotic protective strategy. The Science of the total environment. 2020;742: 140429. [DOI] [PubMed] [Google Scholar]
- 43.Deng Y, Lin X, Zhou J, Li M, Fu Z, Song D. Concurrent serum lead levels and cognitive function in older adults. Front Neurosci. 2023;17:1180782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song S, Liu N, Wang G, Wang Y, Zhang X, Zhao X, Chang H, Yu Z, Liu X. Sex Specificity in the Mixed Effects of Blood Heavy Metals and Cognitive Function on Elderly: Evidence from NHANES. Nutrients. 2023;15(13):2874. [DOI] [PMC free article] [PubMed]
- 45.He YS, Cao F, Musonye HA, Xu YQ, Gao ZX, Ge M, et al. Serum albumin mediates the associations between heavy metals and two novel systemic inflammation indexes among U.S. adults. Ecotoxicol Environ Saf. 2024;270:115863. [DOI] [PubMed]
- 46.Li Z, Chen X, Huang J, Cheng F, Wu Z, Yuan L, et al. Association between dietary inflammatory index and fecal incontinence in American adults: a cross-sectional study from NHANES. 2024;2005–2010:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zalups RK, Ahmad S. Molecular handling of cadmium in transporting epithelia. Toxicol Appl Pharmacol. 2003;186(3):163–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Table S1.LODs of blood Cd, Pb and Hg in NHANES 2005-2010. Table S2. Proportion of blood Cd, Pb, and Hg below the limit of detection in NHANES 2005-2010. Figure S1. Directed acyclic graph. PIR, poverty income ratio; BMI, body mass index. Figure S2. Correlation between heavy metals in blood. Figure S3. Quantile g-computation scaled weights of blood Cd, Pb, and Hg.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.