Abstract
Background
Currently there are no data regarding medical therapy of aortic dilatation in pediatric patients with normally functioning bicuspid aortic valve (BAV). Aim of the study was to describe the rates of change of aortic root diameters in untreated pediatric patients with normally functioning BAV and in patients with documented progressive dilatation treated with medical therapy.
Methods
Retrospective analysis performed on 191 pediatric patients with normally functioning BAV followed from 2005 to 2021 with serial examinations.
Results
Aortic root dilatation was observed in 46.3% of patients, was mainly localized at the proximal ascending aorta and judged mild. After a mean follow-up of 3.7 ± 2.7 years among 175 untreated patients 52.6% presented a new onset or progressive aortic root dilatation (“progressive”) while 47.4% presented normal and stable aortic diameters. Eight percent of untreated patients with a mild aortic dilatation at baseline presented a normalization of aortic diameters. “Progressive” patients presented more frequently a BAV with a raphe (73.9% vs 57.8%, p = .037) and a mild aortic regurgitation (76% vs 45.8%, p = .00007). Thirty “progressive” patients were treated with medical therapy. After a mean follow-up of 3.3 ± 2.3 years no significant differences were observed between aortic root z score progression in “stable”, “progressive” and “treated” patients.
Conclusions
In a small cohort of patients with normally functioning BAV a raphe and a mild regurgitation are common in progressive aortic dilatation. Medical therapy didn't affect aortic dilatation in patients with progressive and mild dilatation. A randomized controlled trial is needed.
Keywords: Bicuspid aortic valve, Aortic root dilatation, Ascending aorta, Aneurysm, Losartan
1. Introduction
Bicuspid aortic valve (BAV) is the most common form of congenital heart disease, occurring in about 0.5%–1.4% of all live births [1]. BAV is known to be associated with aortic valve dysfunction and ascending aorta dilatation [2]. Aortic root dilatation is common in BAV patients, even with normal valve function, and is progressive [3,4]. Aortic dilatation is frequent in pediatric patients, though most have mild dilatation and present minimal progression during childhood and adolescence [5], and patients with significant valve dysfunction might have a more relevant progressive ascending aorta dilatation [6]. Recently Flyer JN et al. [7] reported that medical therapy in young patients with at least moderate and progressive bicuspid aortopathy resulted associated with reduced proximal aortic growth rates and in 2006 Warren AE et al. [8] observed a slower increase of aortic z score in pediatric patients treated with beta-blockers.
Nowadays there are no data regarding medical therapy for prophylaxis and treatment of BAV related aortic dilatation in pediatric patients with normally functioning BAV. From a longitudinal data set, we assessed the prevalence and progression of aortic root dilatation in pediatric patients with normally functioning BAV and we evaluated the effects of medical therapy in patients with a documented progressive aortic dilatation.
2. Methods
We retrospectively reviewed the records of all pediatric patients with BAV followed in two Italian Pediatric Cardiology centers, the Pediatric Cardiology and Adult Congenital Heart Disease Program, IRCCS Azienda Ospedaliero-Universitaria di Bologna and the Cardiologia Pediatrica Azienda Ospedaliero-Universitaria di Parma, from May 2005 through June 2021. Any patient with BAV who underwent at least two echocardiograms and was younger than 18 years of age at the last echocardiographic evaluation was included. Exclusion criteria were associated congenital heart disease, any significant valvular disease (classified as moderate or severe), moderate or severe aortic valve stenosis (peak velocity ≥3 m/s and mean gradient ≥20 mmHg), moderate or severe aortic valve regurgitation, prior aortic balloon valvuloplasty or valve repair or replacement or aortic root replacement, known genetic syndromes with potential aortic dilatation (Marfan syndrome, Loeys-Dietz syndrome, Ehlers-Danlos syndrome, Turner syndrome, 22q11.2 deletion syndrome, and other heritable thoracic aortic diseases) or family history of aortic aneurism or dissection, documented hypertension or atrioventricular block.
Informed consent from all parents or legal guardians were obtained and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee. Two-dimensional transthoracic echocardiograms were obtained every 12 months as part of routine clinical care. Echocardiographic studies were performed using a Philips iE33 Ultrasound system (Philips Medical Systems, Bothell, WA, USA) with phased-array transducers (4, 8 and 12 MHz). Offline measurements with automatic calibration were performed using a computer workstation (Xcelera R3.2L1 SP2; Philips Medical Systems Nederland BV, Best, The Netherlands). Measurements of aortic root diameters were taken at the aortic anulus, sinuses of Valsalva (SOV), sinotubular junction (STJ) and proximal ascending aorta (PAA) with the use of a parasternal long-axis view. Measurements were performed with the leading edge-to leading edge technique in diastole following recommendations by Tierney [9]. A mean of three replications by the same investigator (M.G.), unaware of the patient's treatment status, was used in analysis. A z score was calculated for each aortic diameter with the use of Gautier's method [10], as nomograms were based on diastolic measurements and on a large sample size. Body surface area was computed using the DuBois formula [11]. Aortic dilatation was defined as a z score > + 2, mild aortic dilatation as a z-score > + 2 but ≤ + 4, moderate dilatation as a z-score > + 4 but ≤ + 6, and severe dilatation as a z-score > + 6. The pattern of dilatation was described according to involvement of aortic sinuses alone (“root type”), ascending aorta alone (“ascending aorta type”), or both the aortic sinuses and ascending aorta (“both”) [2]. The first follow-up was at one year and yearly thereafter, coinciding with routine outpatient clinical visits. We identified 30 pediatric patients who had begun medical therapy between May 2005 and June 2020 and had received therapy for at least 1 year of follow-up. The decision to initiate therapy in these patients was made on clinical grounds during routine visits. Although no formal inclusion criteria were applied, the factor that influenced the decision to prescribe therapy was evidence of progressive aortic root dilatation: aortic z score > +2 and increased as compared to prior echocardiographic evaluation. The choice of agent was at the discretion of the treating cardiologist with no specific recommendations. We collected patient data in an electronic case report form (eCRF) assigning all patients with a unique study identifier so that personal identifiable data could be removed at the hospital source, ensuring anonymity and protecting confidentiality.
2.1. Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD) or median and interquartile range (IQR), depending on their distribution, and were compared using two sample t tests, Mann Whitney U tests, Wilcoxon test and Kruscal Wallis test, as appropriate. Nominal and ordinal variables are summarized as frequencies and percentages and were compared using χ2 tests or Fisher's exact test, as appropriate. The strength of the association between clinical or imaging characteristics and aortic dilatation and progression was tested using Spearman's rho test. Statistical significance was based on a two-sided type I error rate of 0.05. In all analyses, a P ≤ .05 was accepted for statistical significance. The statistical analyses were conducted using SPSS Statistics version 21.
3. Results
One hundred and ninety-one consecutive pediatric patients with BAV were recruited. Among them 175 received two or more echocardiographic evaluation and remained untreated during follow-up, while 30 patients with documented progressive aortic dilatation received a medical therapy. Losartan was the agent most frequently prescribed.
3.1. Prevalence of aortic dilatation in untreated patients
One hundred and seventy-five patients were included, mean age at first echocardiographic evaluation was 8.7 years (SD 4.6) and 73% were male. In 66% a raphe was identified, 61.7% presented fusion of the right-left coronary leaflets (R-L), 37.7% fusion of right-non coronary leaflets (R–N) and 0.6% fusion of left-non coronary leaflets (L-N). Mild aortic stenosis was observed in 3.4% of patients and mild aortic regurgitation in 54%. Aortic root dilatation was observed in 46.3% of patients at first echocardiographic evaluation and was judged mainly mild and rarely moderate.
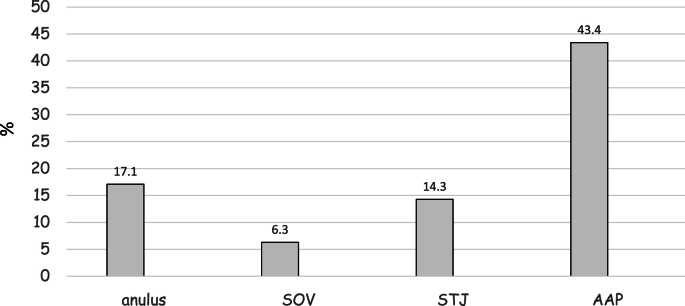
Aortic annulus was dilated in 12% of patients, SOV in 8%, STJ in 9.7% and PAA in 38.3%. The most common type of aortic dilatation was “ascending aorta type” in 32%, “both” were observed in 6.3% and “root type” in 1.7%. Considering all untreated patients aortic root z score, prevalence of aortic dilatation (46.3% vs 52.6%, p = .28) and severity of aortic dilatation remained stable after a mean follow-up of 3.7 years (SD 2.7), while prevalence of aortic regurgitation increased from 54% to 65.7% (p = .04). Supplemental table 1 contains clinical and echocardiographic features of untreated patients at first and last echocardiographic evaluation. Fig. 1 shows the site of aortic dilatation at the last echocardiographic evaluation in untreated patients.
Fig. 1.
Shows the site of aortic dilatation at the last echocardiographic evaluation in untreated patients. Most of the patients presented proximal ascending aorta dilatation. PAA, proximal ascending aorta; SOV, sinuses of Valsalva; STJ, sinotubular junction.
At the last echocardiographic evaluation we identified two subgroups of patients: the first group, made of 83 untreated patients, presented normal aortic root diameters after two or more echocardiographic evaluations (47.4%, “stable”), the second group, made of 92 untreated patients, presented a new onset or progressive aortic root dilatation (52.6%, “progressive”). Among patients classified as “stable” we observed 15 patients (8.6% of untreated patients) who presented a mild aortic dilatation at first echocardiographic evaluation and a normalization of aortic diameters at the last evaluation after a mean of 3.8 years (SD 1.9). The median aortic z score at the first and last echocardiogram were +0.7 (IQR -0.2 - +1.6) and +0.3 (IQR -0.2 - +1.3), respectively, at SOV level (p = .77), and +2.3 (IQR +1.9 - +2.7) and +1.2 (IQR +0.8 - +1.5), respectively, at PAA (p = .59). Proximal ascending aorta z score of patients who presented aortic normalization was ≤ +3 and their mean age at first and last echocardiographic evaluation was 10.2 years (SD 4.9) and 14.1 years (SD 4.9), respectively.
Ninety-two patients (52.6% of untreated patients) presented a new onset or progressive aortic root dilatation after a mean follow-up of 3.7 years (SD 2.7). The comparison of clinical and echocardiographic characteristics of untreated patients who presented a new onset or progressive aortic dilatation (“progressive”) and untreated patients who presented normal aortic diameters at the last echocardiographic evaluation is shown in Table 1. Progressive patients presented more frequently a BAV with a raphe (73.9% vs 57.8%, p = .037) and a mild aortic regurgitation (76% vs 45.8%, p = .00007). All progressive patients presented a slightly and not significant increment of aortic z score in comparison of stable patients.
Table 1.
Comparison of clinical and echocardiographic characteristics of untreated patients who presented a new onset or progressive aortic dilatation (“progressive”) and untreated patients who presented normal aortic diameters at the last echocardiographic evaluation (“stable”).
| Progressive Mean ± SD or median (IQR) or number (%) | Stable Mean ± SD or median (IQR) or number (%) | P | |
|---|---|---|---|
| Number of patients | 92 | 83 | |
| Follow-up (years) | 3.7 ± 2.7 | 3.7 ± 2.4 | 1 |
| Age at last echocardiogram (years) | 12.9 ± 4.8 | 11.9 ± 4.6 | .16 |
| Male sex | 69 (75%) | 58 (69.9%) | .56 |
| Weight (kg) | 48 ± 21.2 | 44.1 ± 18.3 | .20 |
| Height (cm) | 151.6 ± 25.2 | 146.9 ± 23.9 | .21 |
| BSA (m2) | 1.4 ± 0.4 | 1.3 ± 0.4 | .10 |
| Aortic valve fusion type: | |||
| R-L | 52 (56.5%) | 56 (67.5%) | .18 |
| R–NC | 40 (43.4%) | 26 (31.3%) | .13 |
| L-NC | 0 | 1 (1.2%) | .47 |
| Raphe | 68 (73.9%) | 48 (57.8%) | .037 |
| Mild AS (27 ≤ dp ≤ 35 mmHg) | 5 (5.4%) | 5 (6%) | 1 |
| Mild AR | 70 (76%) | 38 (45.8%) | .00007 |
| Z anulus | +1.4 (+0.6 - +2.2) | +0.2 (−0.3 - +0.9) | .03 |
| Z SOV | +0.7 (+0.03 - +1.5) | −0.4 (−1.1 - +0.3) | .04 |
| Z STJ | +1.4 (+0.4 - +2.2) | −0.1 (−1 - +0.5) | .04 |
| Z AAP | +2.8 (+2.2 - +3.1) | +0.8 (−0.01 - +1.4) | .003 |
| Anulus z score change | +0.31 (−0.37 - +1.18) | −0.21 (−0.88 - +0.44) | .31 |
| SOV z score change | +0.12 (−0.49 - +0.79) | −0.25 (−0.83 - +0.32) | .62 |
| STJ z score change | +0.30 (−0.47 - +1.11) | −0.07 (−0.92 - +0.62) | .32 |
| PAA z score change | +0.32 (−0.33 - +0.65) | −0.009 (−0.66 - +0.55) | .33 |
| Annual change of anulus z score | +0.1 (−0.10 - +0.31) | −0.07 (−0.29 - +0.16) | .71 |
| Annual change of SOV z score | +0.03 (−0.11 - +0.27) | −0.08 (−0.27 - +0.12) | .92 |
| Annual change of STJ z score | +0.13 (−0.19 - +0.27) | −0.03 (−0.33 - +0.12) | .46 |
| Annual change of PAA z score | +0.07 (−0.11 - +0.20) | −0.003 (−0.25 - +0.18) | .89 |
AR, aortic regurgitation; AS, aortic stenosis; BSA, body surface area; ECO 1, first echocardiographic evaluation; ECO 2, last echocardiographic evaluation; IQR, interquartile range; L-N, left and noncoronary cusp fusion; R-L, right and left coronary cusp fusion; R–N, right and noncoronary cusp fusion; PAA, proximal ascending aorta; SD, standard deviation; SOV, sinuses of Valsalva; STJ, sinotubular junction; z, z score.
3.2. Aortic dilatation rate in progressive and treated patients
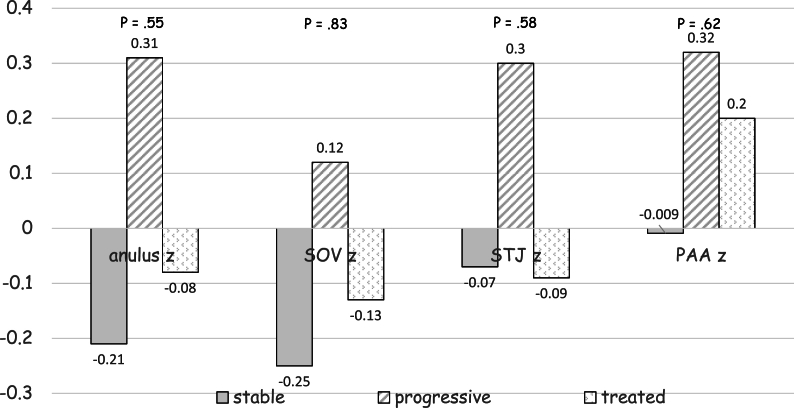
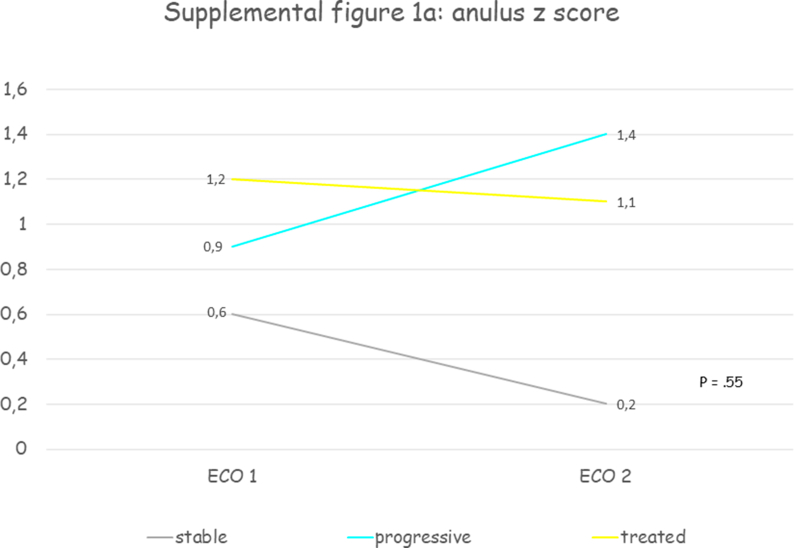
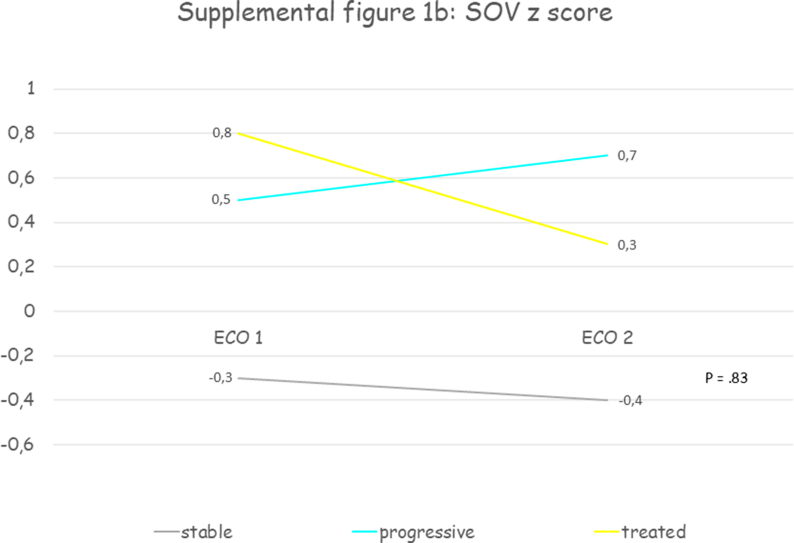
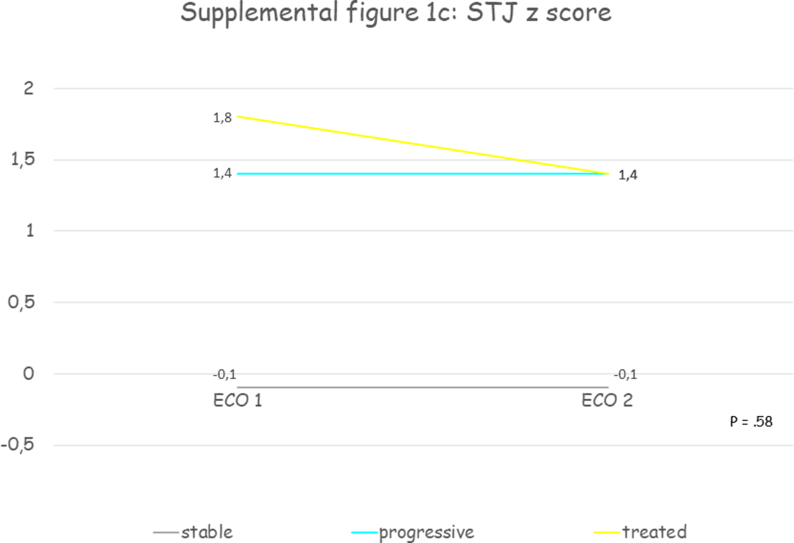
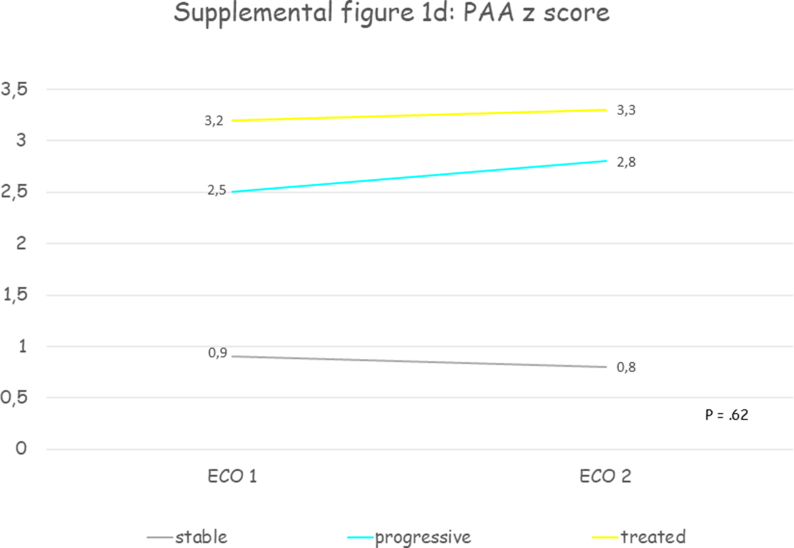
Thirty patients were included, mean age at first echocardiographic evaluation was 11.7 years (SD 3.7) and 70% were male. All patients presented a progressive aortic root dilatation that was localized at the PAA level in 73.3% of patients. Aortic dilatation was judged mild in 70% of cases, moderate in 26.7% and only one patient presented a severe dilatation. Median PAA z score was +3.2 (IQR +2.2 - +4.3) at the time medical therapy was initiated. Losartan was the drug most prescribed (93.4%), one patient received enalapril and another atenolol. The initial mean oral dose of Losartan was 0.5 mg (SD 0.2) per kilogram of body weight per day, of Atenolol was 0.5 mg per kilogram of body weight per day, of Enalapril was 0.1 mg per kilogram of body weight per day and at the last echocardiographic evaluation the dose was unchanged. After a mean follow-up of 3.3 years (SD 2.3) aortic diameters remained stable. Supplemental table 2 contains clinical and echocardiographic features of progressive and treated patients at first and last echocardiographic evaluation. Table 2 reports the comparison between clinical and echocardiographic features of untreated patients who presented progressive aortic dilatation (“progressive”) and patients with progressive aortic dilatation who were treated (“treated”): patients treated were older and with a greater BSA, presented more frequently mild aortic stenosis (33.3% vs 5.4%, p = .0003) and higher median PAA z scores (+3.3 vs 2.8, p = .0002) and slightly lower annulus, SOV and STJ z scores, but aortic root z scores progression weren't significantly different. Fig. 2 and supplemental figure 1 show the comparison between aortic root z scores progression in “stable”, “progressive” and “treated” patients: despite a trend towards aortic root z scores stabilization in treated patients no significant differences between groups were observed.
Table 2.
Comparison between aortic root Z score progression in untreated patients who presented a new onset or progressive aortic dilatation (“progressive”) and patients with progressive aortic dilatation who were treated (“treated”).
| Progressive Mean ± SD or median (IQR) or number (%) | Treated Mean ± SD or median (IQR) or number (%) | P | |
|---|---|---|---|
| Number of patients | 92 | 30 | |
| Follow-up (years) | 3.7 ± 2.7 | 3.3 ± 2.3 | .47 |
| Age at last echocardiogram (years) | 12.9 ± 4.8 | 15 ± 3 | .03 |
| Male sex | 69 (75%) | 21 (70%) | .59 |
| Weight (kg) | 48 ± 21.2 | 57.1 ± 13.3 | .03 |
| Height (cm) | 151.6 ± 25.2 | 164 ± 14.2 | .01 |
| BSA (m2) | 1.4 ± 0.4 | 1.6 ± 0.2 | .01 |
| Aortic valve fusion type: | |||
| R-L | 52 (56.5%) | 20 (66.7%) | .33 |
| R–NC | 40 (43.4%) | 10 (33.3%) | .44 |
| L-NC | 0 | 0 | 1 |
| Raphe | 68 (73.9%) | 23 (76.7%) | .53 |
| Mild AS (27 ≤ dp ≤ 35 mmHg) | 5 (5.4%) | 10 (33.3%) | .0003 |
| Mild AR | 70 (76%) | 27 (90%) | .12 |
| Z anulus | +1.4 (+0.6 - +2.2) | +1.1 (+0.1 - +1.9) | .0001 |
| Z SOV | +0.7 (+0.03 - +1.5) | +0.3 (−0.4 - +1.3) | .00006 |
| Z STJ | +1.4 (+0.4 - +2.2) | +1.4 (+0.5 - +1.9) | .0004 |
| Z AAP | +2.8 (+2.2 - +3.1) | +3.3 (+2.3 - +4.2) | .0002 |
| Anulus z score change | +0.31 (−0.37 - +1.18) | −0.08 (−1.13 - +1.21) | .98 |
| SOV z score change | +0.12 (−0.49 - +0.79) | −0.13 (−0.63 - +0.63) | .92 |
| STJ z score change | +0.30 (−0.47 - +1.11) | −0.09 (−0.47 - +1.01) | .97 |
| PAA z score change | +0.32 (−0.33 - +0.65) | +0.20 (−0.32 - +0.58) | .86 |
| Annual change of anulus z score | +0.1 (−0.10 - +0.31) | −0.05 (−0.39 - +0.32) | .90 |
| Annual change of SOV z score | +0.03 (−0.11 - +0.27) | −0.05 (−0.34 - +0.12) | 1 |
| Annual change of STJ z score | +0.13 (−0.19 - +0.27) | −0.04 (−0.22 - +0.23) | .90 |
| Annual change of PAA z score | +0.07 (−0.11 - +0.20) | +0.08 (−0.10 - +0.26) | .91 |
AR, aortic regurgitation; AS, aortic stenosis; BSA, body surface area; ECO 1, first echocardiographic evaluation; ECO 2, last echocardiographic evaluation; IQR, interquartile range; L-N, left and noncoronary cusp fusion; R-L, right and left coronary cusp fusion; R–N, right and noncoronary cusp fusion; PAA, proximal ascending aorta; SD, standard deviation; SOV, sinuses of Valsalva; STJ, sinotubular junction; z, z score.
Fig. 2.
Shows the comparison between aortic root Z score progression in “stable”, “progressive” and “treated” patients: despite a trend towards aortic root Z score stabilization in treated patients no significant differences between groups were observed. PAA, proximal ascending aorta; SOV, sinuses of Valsalva; STJ, sinotubular junction; z, Z score.
3.3. Aortic dilatation rate in progressive patients before and after treatment
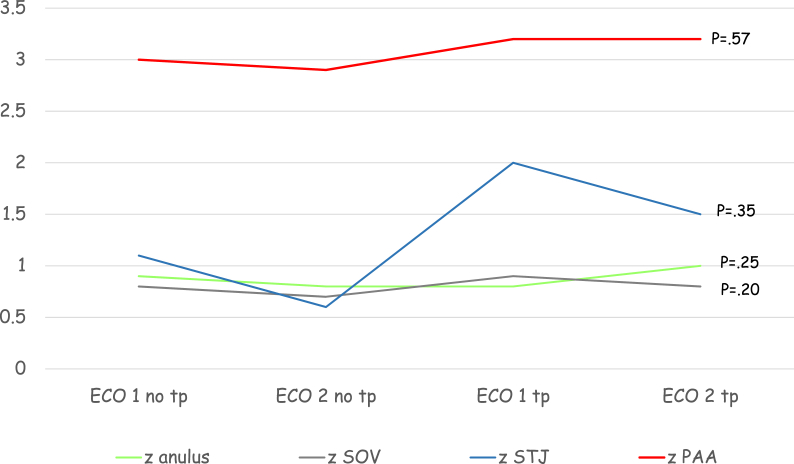
In a small group of patients, 14 subjects, two echocardiographic evaluations both before and after treatment were available. Fig. 3 shows the comparison between aortic root z scores progression before and after treatment: no significant difference was observed despite a trend towards stabilization of aortic root z scores during treatment (p = .42-.94).
Fig. 3.
Shows the comparison between aortic root Z score progression before and after treatment: no significant difference was observed despite a trend towards aortic root Z score stabilization during treatment. Eco, echocardiogram; PAA, proximal ascending aorta; SOV, sinuses of Valsalva; STJ, sinotubular junction; tp, therapy; z, z score.
4. Discussion
Aortic root dilatation is common in BAV patients, even with normal valve function, and is progressive [3,4]. Prevalence increases with age, beginning in childhood and continuing throughout life, and is estimated 56% in those aged <30 years old, up to 88% in those aged >80 years old [3,12].
Aortic dilatation is frequent even in pediatric patients, though most have mild dilatation and present minimal progression during childhood and adolescence [5,6]. A Report from the MIBAVA Consortium [6], a multicenter, retrospective, cross-sectional study of 2122 pediatric BAV patients, range 0–17.9 years, reported that fifty percent of patients had aortic sinus or ascending aorta dilatation, or both with the majority having isolated ascending aorta dilatation. Fernandes e coll [5]. in her cohort of 333 patients with BAV, median age of 13.5 years (range: 0–30 years) at most recent follow-up, reported that the aortic root was dilated in 22% and the ascending aorta in 49%. They also observed that a moderate or severe (Z > +4) aortic root and ascending aortic dilatation was present in 5% and 16% of patients, respectively. These observations have a relevant clinical impact because aortic dilatation can evolve to aneurysm and has a propensity for dissection and rupture if left untreated, making it a potentially lethal disease, and BAV is the most common congenital cardiac abnormality, occurring in 0.5%–1.4% of the population.
Marfan syndrome and BAV aortic disease share common histopathological findings, including medial degeneration, increased matrix metalloproteinase (MMP) activity, and decreased fibrillin-1 in the aortic wall [13,14]. Many studies and randomized controlled trials in patients with Marfan syndrome reported the effect of β-blockers, atenolol or propranolol, or angiotensin II–receptor blockers (ARBs), mainly losartan or irbesartan, on aortic root size and growth rates [14,15]. On the basis of current evidence all patients with known or suspected Marfan syndrome and aortic root dilatation should receive medical therapy with adequate doses of either β-blockers or ARBs. The Pediatric Heart Network trial, a prospective randomized trial comparing losartan with atenolol in a large cohort of children and young adults with Marfan's syndrome and a dilated aortic root, showed that atenolol and losartan each reduced the rate of aortic dilatation. The authors of this trial also reported that both were more effective in younger subjects, which suggests that medical therapy should be prescribed even in the youngest children [15].
The histopathological similarities between Marfan and BAV aortopathy and the results of trials in pediatric Marfan patients led to hypothesize that treatment with β-blockers or ARBs might be effective for the prevention and treatment of aortic root enlargement in patients with BAV. Hussain A et al. [16] reported that medical therapy is often prescribed by the majority of Canadian Pediatric Cardiologists to reduce the rate of aortic dilatation and prevent aortic dissection in patients with BAV. Even in Italy is quite common to observe medical therapy prescription in pediatric patients with BAV aortopathy even for a minimal aortic dilatation.
Nowadays there are no specific recommendations about medical treatment of aortic dilatation in pediatric patients with BAV. According to the 2020 ESC Guidelines for the management of adult congenital heart disease [17] it may be reasonable to consider beta blockers or ARBs as first-line treatment in patients with BAV and arterial hypertension, conversely the 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases [18] stated that beta-blockers may be considered in patients with BAV and dilated aortic root >40 mm (IIb, C). Currently there are few data regarding medical therapy for prophylaxis and treatment of BAV related aortic root dilatation in the pediatric age. The effects of medical therapy on aortic growth rates in moderate to severe bicuspid aortopathy have been recently evaluated in a single-center retrospective study of young patients (1 day–29 years) with bicuspid aortopathy (aortic root z-score ≥ + 4 SD or absolute dimension ≥4 cm), treated with either losartan or atenolol and followed at Boston Children's Hospital [7]. Treatment was associated with decreases in aortic root diameters and z-scores for both losartan and atenolol in young patients with at least moderate and progressive bicuspid aortopathy. Warren AE et al. [8] analyzed 88 pediatric patients with BAV and observed that ascending aortic Z score increased at an average rate of 0.4/year and a faster rate of increase in Z score was predicted by both larger initial aortic valve gradient and non-use of beta-blockers. Both these two studies included patients with BAV and aortic valve dysfunction and with associated congenital heart disease.
To our knowledge, this is the first study which describes the progression of aortic dilatation in treated and untreated pediatric patients with normally functioning BAV without associated congenital heart disease or any significant valvular disease.
In our study among untreated patients only 52.6% presented a new onset or progressive aortic root dilatation after a mean follow-up of 3.7 years (SD 2.7), while others presented normal and stable aortic root diameters. Furthermore 8.6% of untreated patients with a mild aortic dilatation at first echocardiographic evaluation presented a normalization of aortic diameters at the last evaluation. Our observations underline the importance of not labeling as pathologic the first observation of a mild dilatation and of confirming a pathological finding through an evolutive follow-up during the pediatric age and adolescence, when even a minimal variation of the aortic diameter can affect the result of z scores.
In our study after a mean follow-up of 3.3 years (SD 2.3) no significant differences were observed between aortic root Z score progression in “stable”, “progressive” and “treated” patients despite a trend towards aortic root Z score stabilization in treated patients. A raphe and a mild aortic regurgitation are the only differences between “stable” and “progressive” patients. The raphe could represent a cause of increased anomalous flow direction in ascending aorta. Four-dimensional (4D)-flow cardiovascular magnetic resonance imaging (CMR) has been used to visualize abnormal hemodynamic flow patterns such as helical and vortical flow, wall shear stress and flow displacement in adults with congenital heart disease. Lenz A e coll [19]. evaluated the hemodynamic changes of aortic valve repair in patients with bicuspid and unicuspid valve with 4D flow CMR. In patients with bicuspid aortic valve 4D flow CMR reveals an accelerated eccentric asymmetric flow jet and a pronounced helical flow pattern in the ascending aorta, the flow jet impacts and travels along the right aortic wall. After surgery, velocity-coded 4D flow CMR shows reduced helical flow with a more cohesive central flow pattern more parallel to the vessel wall of the ascending aorta.
Finally mild aortic regurgitation can be caused by aortic root dilatation but in our cohort with only mild and prevalent proximal ascending aorta dilatation aortic regurgitation is a consequence of the presence of the raphe which reduces cusp mobility avoiding an effective cusp coaptation.
From the results of our study, we might conclude that the presence of a raphe and a mild aortic regurgitation are most common in progressive aortic dilatation and prescribing low doses of losartan in patients with normally functioning BAV and mild aortic dilatation seems not justified.
The main limitation of our study is its retrospective nature and the short length of follow-up; however, all the patients were regularly followed and we didn't have patients lost at follow-up. There was heterogeneity in the criteria to prescribe medical therapy and dosages used were extremely low compared to the trials which demonstrated a benefit of therapy in Marfan patients and this could have influenced the scarce results of medical therapy observed in our study. Medication dosing and duration of therapy were variable, based on individual prescriber and patient tolerance, and treated patients presented larger ascending aorta diameters as compared to progressive untreated patients. Nevertheless treated group cannot be considered a selected and worse group because aortic z scores of treated patients were mainly mildly dilated as untreated progressive patients. Treated patients were older and presented more frequently a mild aortic stenosis at last echocardiographic evaluation, these factors could affect outcomes. For all these limitations a multicenter randomized controlled trial is needed to establish the role of medical therapy in this patient population.
5. Conclusions
Aortic root dilatation is common in pediatric patients with normally functioning BAV and mainly affects proximal ascending aorta. In our small cohort of patients with normally functioning BAV and mild aortic dilatation we have observed that the presence of a raphe and a mild aortic regurgitation are most common in progressive aortic dilatation and the use of medical therapy didn't affect the rate of aortic dilatation in patients with progressive aortic dilatation. No significant difference was observed during treatment compared with before treatment. These findings require confirmation in a randomized controlled trial with long-term follow-up. Large multicentric studies are warranted to elucidate the most appropriate drugs and dosages and timing for therapy in these patients.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Disclosures
The authors declare that they have no known competing financial interests or personal relations that could have appeared to influence the work reported in this study.
Fundings
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
This author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcchd.2022.100385.
Contributor Information
Elisabetta Mariucci, Email: mariaelisabetta.mariucci@aosp.bo.it.
Marta Guidarini, Email: marta.guidarini@hotmail.it.
Ylenia Bartolacelli, Email: ylenia.bartolacelli@aosp.bo.it.
Bertrand Tchana, Email: btchana@ao.pr.it.
Lucio Careddu, Email: lucio.careddu@aosp.bo.it.
Gaetano Gargiulo, Email: gaetano.gargiulo@aosp.bo.it.
Susanna Maria Roberta Esposito, Email: susannamariaroberta.esposito@unipr.it.
Andrea Donti, Email: andrea.donti@aosp.bo.it.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
Fig. S2.
Fig. S3.
Fig. S4.
References
- 1.Benjamin E.J., Virani S.S., Callaway C.W., et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Verma S., Siu S.C. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370:1920–1929. doi: 10.1056/NEJMra1207059. [DOI] [PubMed] [Google Scholar]
- 3.Hahn R.T., Roman M.J., Mogtader A.H., Devereux R.B. Association of aortic dilatation with regurgitant, stenotic and functionally normal bicuspid aortic valves. J Am Coll Cardiol. 1992;19:283–288. doi: 10.1016/0735-1097(92)90479-7. [DOI] [PubMed] [Google Scholar]
- 4.Ferencik M., Pape L.A. Changes in size of ascending aorta and aortic valve function with time in patients with congenitally bicuspid aortic valves. Am J Cardiol. 2003;92:43–46. doi: 10.1016/s0002-9149(03)00462-4. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes S., Khairy P., Graham D.A., et al. Bicuspid aortic valve and associated aortic dilation in the young. Heart. 2012 Jul;98(13):1014–1019. doi: 10.1136/heartjnl-2012-301773. [DOI] [PubMed] [Google Scholar]
- 6.Grattan M., Prince A., Rumman R.K., et al. Predictors of bicuspid aortic valve-associated aortopathy in childhood: a report from the MIBAVA Consortium. Circ Cardiovasc Imag. 2020 Mar;13(3) doi: 10.1161/CIRCIMAGING.119.009717. [DOI] [PubMed] [Google Scholar]
- 7.Flyer J.N., Sleeper L.A., Colan S.D., Singh M.N., Lacro R.V. Effect of losartan or atenolol on children and young adults with bicuspid aortic valve and dilated aorta. Am J Cardiol. 2021 Apr 1;144:111–117. doi: 10.1016/j.amjcard.2020.12.050. [DOI] [PubMed] [Google Scholar]
- 8.Warren A.E., Boyd M.L., O'Connell C., Dodds L. Dilatation of the ascending aorta in paediatric patients with bicuspid aortic valve: frequency, rate of progression and risk factors. Heart. 2006 Oct;92(10):1496–1500. doi: 10.1136/hrt.2005.081539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tierney E.S.S., Levine J.C., Chen S., et al. Echocardiographic methods, quality review, and measurement accuracy in a randomized multicenter clinical trial of Marfan syndrome. J Am Soc Echocardiogr. 2013;26:657–666. doi: 10.1016/j.echo.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gautier M., Detaint D., Fermanian C., et al. Nomograms for aortic root diameters in children using two-dimensional echocardiography. Am J Cardiol. 2010;105:888–894. doi: 10.1016/j.amjcard.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 11.DuBois D., DuBois E.F. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 12.Tadros T.M., Klein M.D., Shapira O.M. Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation. 2009 Feb 17;119(6):880–890. doi: 10.1161/CIRCULATIONAHA.108.795401. [DOI] [PubMed] [Google Scholar]
- 13.Nataatmadja M., West M., West J., et al. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in Marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108:II329–II334. doi: 10.1161/01.cir.0000087660.82721.15. [DOI] [PubMed] [Google Scholar]
- 14.Singh M.N., Lacro R.V. Recent clinical drug trials evidence in marfan syndrome and clinical implications. Can J Cardiol. 2016 Jan;32(1):66–77. doi: 10.1016/j.cjca.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Lacro R.V., Dietz H.C., Sleeper L.A., et al. Atenolol versus losartan in children and young adults with Marfan's syndrome. N Engl J Med. 2014;371:2061–2071. doi: 10.1056/NEJMoa1404731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain A., Warren A.E., Chen R.P.C., Dhillon S.S. Practice variation among Canadian pediatric cardiologists in medical management of dilated ascending aorta in patients with bicuspid aortic valve. CJC Open. 2019 Apr 12;1(3):119–122. doi: 10.1016/j.cjco.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumgartner H., De Backer J., Babu-Narayan S.V., et al. ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021 Feb 11;42(6):563–645. doi: 10.1093/eurheartj/ehaa554. [DOI] [PubMed] [Google Scholar]
- 18.Erbel R., Aboyans V., Boileau C., et al. ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014 Nov 1;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 19.Lenz A., Petersen J., Riedel C., et al. 4D flow cardiovascular magnetic resonance for monitoring of aortic valve repair in bicuspid aortic valve disease. J Cardiovasc Magn Reson. 2020 Apr 30;22(1):29. doi: 10.1186/s12968-020-00608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.