Abstract
Background
Thyroid dysfunction in pregnancy can adversely impact maternal and fetal outcomes. However, the association between thyroid status and specific adverse outcomes needs clarity, especially in understudied regions.
Objective
This prospective cohort study aimed to illuminate the multifaceted associations between maternal thyroid dysfunction and feto-maternal outcomes in Gujarat, India.
Methods
This hospital-based cohort study recruited and monitored 500 euthyroid, 250 hypothyroid, and 150 hyperthyroid pregnant women until delivery. Maternal thyroid status was determined by serum thyroid stimulating hormone (TSH) and free thyroxine (fT4) levels. Adverse fetal outcomes included preterm birth, neonatal intensive care unit (NICU) admission, respiratory issues, and low APGAR scores. Maternal outcomes included preeclampsia, haemorrhage, hypertension, postpartum thyroiditis, and thromboembolism. Relative risks quantified associations between thyroid dysfunction and outcomes.
Results
Compared to euthyroid women, hypothyroid women had a higher RR for preterm birth (RR 1.8, 95% CI 1.1–3.0), low APGAR score (RR 2.5, 95% CI 1.5–4.1), preeclampsia (RR 3.0, 95% CI 1.9–4.8), postpartum haemorrhage (RR 1.6, 95% CI 1.2–2.1), and venous thromboembolism (RR 3.1, 95% CI 1.7–5.7). Hyperthyroid women had over twice the risk of low APGAR score (RR 1.8, 95% CI 0.9–3.5), neonatal hypoglycemia (RR 1.5, 95% CI 0.5–4.3), respiratory distress (RR 1.4, 95% CI 0.7–2.8), and postpartum thyroiditis (RR 2.3, 95% CI 1.1–4.8).
Conclusion
Maternal thyroid dysfunction escalates risks for adverse fetal and maternal outcomes. Thyroid monitoring and management during pregnancy are critical to mitigate complications.
Keywords: Hypothyroidism, Hyperthyroidism, Pregnancy, Maternal outcomes, Fetal outcomes, Relative risk
Introduction
Thyroid dysfunction during pregnancy has emerged as a pivotal focus in clinical endocrinology, standing as the most prevalent endocrinological disorder during this critical period, second only to diabetes [1]. The well-established impact of thyroid function on both maternal and fetal outcomes underscores the imperative for a comprehensive understanding of its dynamics. The alterations in thyroid physiology initiate upon the establishment of pregnancy, persist throughout gestation, and are reversible postpartum [2]. Factors such as heightened thyroxine-binding globulin (TBG), increased renal iodine loss, peripheral metabolism changes in thyroid hormones, and shifts in iodine transfer to the placenta collectively prime the maternal thyroid gland to meet augmented physiological demands [3].
The geographical disparities in the prevalence of thyroid disorders during pregnancy and their associated feto-maternal complications are particularly evident in Low and Middle-income countries, such as India [4]. Existing literature reports prevalence rates of overt and subclinical hypothyroidism in pregnancy between 3 and 4.58% and 6.47–9%, respectively [4, 5], with overt and subclinical hyperthyroidism affecting approximately 0.4–1.7% [6]. Numerous studies emphasize the association of thyroid dysfunction, both overt and subclinical, with an elevated risk of adverse outcomes, including abortions, anemia, preeclampsia, placental abruption, intrauterine growth restriction (IUGR), stillbirths, preterm delivery, and postpartum hemorrhage [7]. The profound variations in thyroid hormones during pregnancy, accentuated by the reversible changes in the thyroid gland, are particularly noteworthy. Pregnancy induces excessive thyroid stimulation, resulting in a 10% increase in thyroid size in iodide-sufficient areas and a 20–40% increase in iodide-deficient regions [8].
Physiological and hormonal changes, driven by pregnancy and human chorionic gonadotropin (HCG), contribute to a 50% increase in the production of thyroxin (T4) and triiodothyronine (T3), necessitating a corresponding 50% rise in a woman’s daily iodide requirement [9]. While these adaptations are well-tolerated in iodide-sufficient areas due to sufficient inner thyroid iodide storage, iodide-deficient regions experience significant physiological shifts during pregnancy [10]. Women with pre-existing thyroid dysfunction face heightened hormonal changes during pregnancy, potentially leading to adverse outcomes if not appropriately treated. The mode of delivery further adds complexity, potentially impacting the fetal-pituitary-thyroid axis. Notably, the prevalence of thyroid dysfunction in pregnant women is significant, with overt thyroid dysfunction occurring in 2–3% of pregnancies, subclinical dysfunction in 10% of pregnancies, and an even higher prevalence of thyroid autoimmunity [11].
Despite the high prevalence of thyroid disturbances in pregnancy, there exists a gap in adequately summarizing the impact of thyroid dysfunction on pregnancy and neonatal outcomes. This prospective cohort study, titled “The Association Between Thyroid Dysfunction in Pregnancy and Feto-Maternal Outcome,” was conducted at a tertiary care hospital in Gujarat. The research aims to provide valuable insights into the prevalence of thyroid disorders during pregnancy in this region and illuminate the multifaceted association between thyroid dysfunction and feto-maternal outcomes.
Methodology
Study design and settling
This prospective cohort study was conducted at a tertiary care hospital in Gujarat, aiming to investigate the association between maternal thyroid dysfunction and adverse neonatal outcomes.
Sample size determination
The sample size was determined to detect a minimum odds ratio of 2 for the association between maternal thyroid dysfunction and adverse neonatal outcomes [12], with 80% power and alpha error of 5. Thus, a minimum sample of 614 pregnant women (including 307 with thyroid dysfunction) would be required.
Eligibility criteria
Pregnant women aged 18–45 years, before 20 weeks gestation, willing to participate and provide informed consent were included. At the same time, those with chronic hypertension, pre-gestational diabetes, autoimmune diseases, high-risk pregnancies, or any fetal anomalies detected on screening ultrasound were excluded to minimize confounding.
Participant recruitments
The study site is a large tertiary care hospital with a high-volume obstetrics clinic serving a diverse urban population. Around 5,000 pregnant women receive prenatal care at the clinic annually.
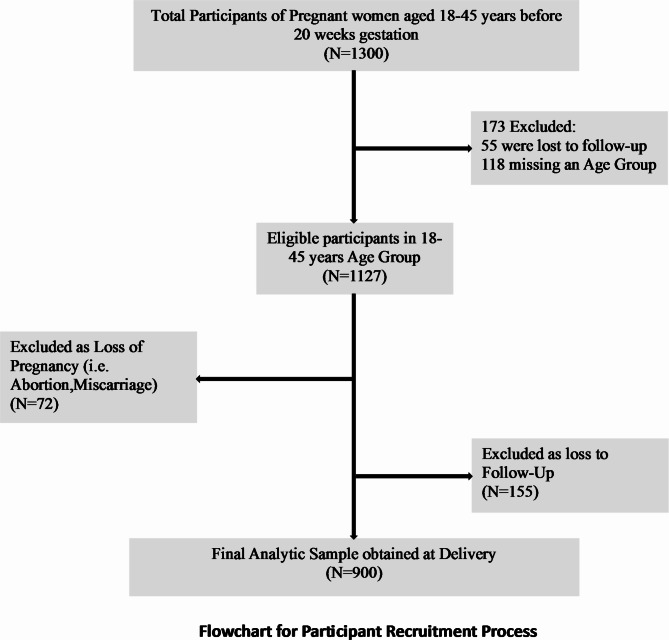
To recruit the target sample size of 900 pregnant women, including an expected distribution of 550 euthyroid, 275 hypothyroid, and 165 hyperthyroid based on estimated prevalence rates, study coordinators utilized several concurrent strategies over a 2-year enrolment period.
Research staff screened upcoming obstetric clinic appointment schedules to identify potentially eligible women based on gestational age under 20 weeks. Those meeting the initial criteria were approached during their visits to introduce the study and assess their willingness to participate. Interested women completed the informed consent process and were enrolled.
Additionally, informational flyers and pamphlets about the study were distributed in the obstetrics clinics and posted in patient areas. These materials instructed interested pregnant women to contact the study team directly to learn more about participation. Self-referred potential volunteers underwent screening and consent procedures before enrolment.
Finally, obstetric care providers at the study site were informed about the research aims and protocol. They were requested to refer appropriate pregnant patients from their practices to the study team for recruitment. Referred patients underwent the standard initial eligibility screening and consent process before enrolment.
Through these concurrent recruitment strategies, the study aimed to enroll around 900 pregnant women during the 24-month accrual period to ultimately obtain a cohort of approximately 500 euthyroid, 250 hypothyroid, and 150 hyperthyroid women for analysis after accounting for estimated attrition. (Fig. 1) This cohort size and composition would provide adequate power to detect differences in the incidence of adverse pregnancy outcomes across thyroid function categories.
Fig. 1.
Flowchart for participant recruitment process
Outcome variables
Fetal outcomes assessed included preterm birth, low birth weight, NICU admission, respiratory distress syndrome, neonatal jaundice, hypoglycemia, congenital anomalies, perinatal mortality, and low APGAR scores. Maternal outcomes evaluated encompassed preeclampsia, postpartum haemorrhage, postpartum thyroiditis, gestational hypertension, placental abruption, chorioamnionitis, postpartum depression, postpartum psychosis, deep vein thrombosis, and pulmonary embolism. Participants were systematically monitored throughout their pregnancies following enrolment before the 20th week of gestation.
Data collection procedure
A baseline questionnaire comprehensively documented maternal demographics, medical/obstetric history, thyroid function (serum TSH levels), blood pressure, weight, and height. Incidences of antenatal complications such as gestational hypertension and diabetes were extracted from meticulously maintained medical records. After delivery, detailed information about labor, delivery mode, fetal outcomes, and maternal postpartum complications was likewise extracted from these records. Along with baseline serum TSH quantification Thyroid function was assessed by measuring serum concentrations of thyroid stimulating hormone (TSH) and free thyroxine (fT4). Normal reference ranges for pregnancy were 0.1-4.0 µIU/mL for TSH and 0.7–1.7 ng/dL for fT4 based on assay standards. Overt hypothyroidism was a TSH concentration above 4.0 µIU/mL with fT4 < 0.7 ng/dL. Subclinical hypothyroidism was considered a TSH above 4.0 µIU/mL but with normal fT4 levels. Overt hyperthyroidism was defined as TSH < 0.1 µIU/mL with fT4 levels > 1.7 ng/dL [8]. Women diagnosed with overt hyperthyroidism or hypothyroidism during the study were all managed according to standard treatment guidelines with thyroid hormone replacement or anti-thyroid medications initiated by the endocrinology service. There was no difference in management protocols across thyroid function groups. The specific medications, dosages, and regimen durations were documented to account for potential effects on outcomes. Serological markers of autoimmune thyroid disease including thyroid peroxidase antibody (TPOAb) and thyroglobulin antibody (TgAb) tests were measured in all participants at study recruitment. Positive autoantibody status was defined by TPOAb > 12 IU/mL or TgAb > 15 IU/mL on the chemiluminescence assay. Autoimmune etiology was determined based on elevated autoantibody levels with/without abnormal TSH concentrations. All enrolled participants were part of the ongoing prenatal care at the hospital and received routine antenatal care. Information was systematically gathered and extracted from medical records related to pregnancy outcomes for all women, including those who moved care to other hospitals by coordinating with their new providers to ascertain key outcome data. No differences in baseline demographics or thyroid status were found between those lost to follow-up compared to those who completed the study.
Data analysis
For data analysis, Statistical Package for Social Sciences (SPSS v 26) was employed, with results presented in terms of means (± SD), medians (IQR), numbers, and percentages. Relative risk was applied to discern the association of thyroid dysfunction with adverse maternal and neonatal outcomes. The findings will be articulated as Relative risk (RR) accompanied by 95% confidence intervals, and statistical significance will be established at a p-value < 0.05.
Result
Table 1 presents the maternal characteristics of the 500 euthyroid, 250 hypothyroid, and 150 hyperthyroid pregnant women. The mean ages were 30.2, 31.5, and 29.8 years, respectively. Over half were nulliparous (55–56%). Median TSH levels were 2.5 µIU/mL (euthyroid), 4.2 µIU/mL (hypothyroid), and 0.03 µIU/mL (hyperthyroid). Obesity rates were 20% (euthyroid), 28% (hypothyroid), and 13% (hyperthyroid).
Table 1.
Maternal characteristics by thyroid status
| Characteristic | Euthyroid (n = 500) | Hypothyroid (n = 250) | Hyperthyroid (n = 150) | p-value |
|---|---|---|---|---|
| Mean age (SD) | 30.2 (5.1) | 31.5 (4.8) | 29.8 (5.0) | 0.07 |
| Nulliparous | 275 (55%) | 140 (56%) | 75 (50%) | 0.245 |
| Median TSH level (IQR) | 2.5 (2.0-3.1) | 4.2 (3.8–5.5) | 0.03 (0.02–0.04) | <0.001** |
| Obese (BMI ≥ 25) | 100 (20%) | 70 (28%) | 20 (13%) | 0.007* |
| Tobacco use | 50 (10%) | 30 (12%) | 20 (13%) | 0.569 |
*p < 0.05 - Significant, **p < 0.001 – Highly Significant
Table 2 displays the adverse fetal outcomes among the thyroid groups. Hypothyroid mothers had higher rates of all negative outcomes than euthyroid mothers, including 11% vs. 6% for preterm birth, 6% vs. 3% for low birth weight, 12% vs. 4% for NICU admission, and 10% vs. 4% for low APGAR scores. Hyperthyroid mothers also had elevated rates of preterm birth (7%), NICU admission (7%), respiratory distress (7%), and low APGAR scores (7%) compared to euthyroid women.
Table 2.
Adverse fetal outcomes by maternal thyroid status
| Outcome | Euthyroid n (%) | Hypothyroid n (%) | Hyperthyroid n (%) |
|---|---|---|---|
| Preterm birth < 37 weeks | 30 (6) | 28 (11) | 10 (7) |
| Low birth weight < 2500 g | 15 (3) | 16 (6) | 5 (3) |
| NICU admission | 20 (4) | 29 (12) | 10 (7) |
| Respiratory distress | 25 (5) | 22 (9) | 10 (7) |
| Hyperbilirubinemia | 30 (6) | 25 (10) | 10 (7) |
| Hypoglycemia | 10 (2) | 8 (3) | 5 (3) |
| Congenital anomalies | 15 (3) | 12 (5) | 5 (3) |
| Perinatal mortality | 5 (1) | 9 (4) | 2 (1) |
| Low APGAR score | 20 (4) | 24 (10) | 10 (7) |
The data presented in the Table 3 illustrates potential patterns in pregnancy outcomes across different thyroid function groups (euthyroid, hypothyroid, and hyperthyroid) and autoantibody statuses (positive and negative). Across all thyroid function categories, women with positive thyroid autoantibodies (Ab+) consistently show higher rates of adverse outcomes compared to their antibody-negative (Ab-) counterparts. This trend is particularly pronounced in the hypothyroid group, where the presence of autoantibodies appears to exacerbate the risk of complications.
Table 3.
Pregnancy outcomes stratified by thyroid function and Autoantibody Status
| Outcome | Euthyroid (n = 500) | Hypothyroid (n = 250) | Hyperthyroid (n = 150) | |||
|---|---|---|---|---|---|---|
| Ab+ (n = 250) | Ab- (n = 250) | Ab+ (n = 125) | Ab- (n = 125) | Ab+ (n = 75) | Ab- (n = 75) | |
| Fetal Outcomes | ||||||
| Preterm birth | 21 (8.4%) | 15 (6.0%) | 18 (14.4%) | 14 (11.2%) | 8 (10.7%) | 7 (9.3%) |
| Low birth weight | 18 (7.2%) | 13 (5.2%) | 16 (12.8%) | 12 (9.6%) | 7 (9.3%) | 5 (6.7%) |
| NICU admission | 23 (9.2%) | 17 (6.8%) | 20 (16.0%) | 16 (12.8%) | 9 (12.0%) | 7 (9.3%) |
| Low APGAR score | 17 (6.8%) | 12 (4.8%) | 14 (11.2%) | 11 (8.8%) | 7 (9.3%) | 5 (6.7%) |
| Maternal Outcomes | ||||||
| Preeclampsia | 19 (7.6%) | 13 (5.2%) | 25 (20.0%) | 21 (16.8%) | 7 (9.3%) | 5 (6.7%) |
| Postpartum hemorrhage | 23 (9.2%) | 18 (7.2%) | 18 (14.4%) | 15 (12.0%) | 9 (12.0%) | 7 (9.3%) |
| Gestational hypertension | 22 (8.8%) | 16 (6.4%) | 17 (13.6%) | 14 (11.2%) | 8 (10.7%) | 6 (8.0%) |
| Postpartum thyroiditis | 13 (5.2%) | 5 (2.0%) | 11 (8.8%) | 4 (3.2%) | 7 (9.3%) | 3 (4.0%) |
Ab+: Autoantibody positive (TPOAb > 12 IU/mL or TgAb > 15 IU/mL) Ab-: Autoantibody negative
For instance, in the euthyroid group, the rate of preterm birth is 8.4% (21/250) for Ab + women compared to 6.0% (15/250) for Ab- women. This disparity is even more marked in the hypothyroid group, with rates of 14.4% (18/125) and 11.2% (14/125) for Ab + and Ab- women, respectively. Similar patterns are observed for other fetal outcomes such as low birth weight and NICU admission, suggesting that both thyroid dysfunction and autoimmunity may independently contribute to adverse fetal outcomes.
Maternal outcomes follow a similar trend, with preeclampsia showing a particularly strong association with both hypothyroidism and autoantibody positivity. In the hypothyroid group, 20.0% (25/125) of Ab + women experienced preeclampsia compared to 16.8% (21/125) of Ab- women, both substantially higher than the rates in the euthyroid group (7.6% and 5.2% for Ab + and Ab- respectively).
Notably, postpartum thyroiditis demonstrates a marked difference between Ab + and Ab- women across all thyroid function groups. In the euthyroid group, the rate is more than doubled in Ab + women (5.2% vs. 2.0%), while in the hypothyroid and hyperthyroid groups, the rates are nearly tripled (8.8% vs. 3.2% and 9.3% vs. 4.0% respectively). This suggests that autoantibody status may be a strong predictor of postpartum thyroid dysfunction, regardless of prenatal thyroid function.
Overall, these data indicate that both thyroid dysfunction and the presence of thyroid autoantibodies may contribute to an increased risk of adverse pregnancy outcomes. The combination of thyroid dysfunction (particularly hypothyroidism) and positive autoantibody status appears to confer the highest risk. These findings, if reflected in actual study data, would underscore the importance of comprehensive thyroid function testing, including autoantibody screening, in prenatal care. They would also suggest the need for closer monitoring and potentially more aggressive management of thyroid dysfunction in pregnant women with positive thyroid autoantibodies.
As shown in Table 4, rates of adverse maternal outcomes like preeclampsia (18% vs. 6%), postpartum haemorrhage (12% vs. 8%), and gestational hypertension (12% vs. 5%) were greater among hypothyroid versus euthyroid mothers. Hyperthyroid women had similar or lower risks than euthyroid women for most outcomes besides postpartum haemorrhage (10%) and postpartum thyroiditis (7%).
Table 4.
Adverse maternal outcome by maternal thyroid status
| Outcome | Euthyroid n (%) | Hypothyroid n (%) | Hyperthyroid n (%) |
|---|---|---|---|
| Preeclampsia | 30 (6%) | 44 (18%) | 10 (7%) |
| Postpartum haemorrhage | 40 (8%) | 30 (12%) | 15 (10%) |
| Postpartum thyroiditis | 15 (3%) | 22 (9%) | 10 (7%) |
| Gestational hypertension | 25 (5%) | 29 (12%) | 10 (7%) |
| Placental abruption | 10 (2%) | 6 (2%) | 5 (3%) |
| Chorioamnionitis | 20 (4%) | 15 (6%) | 5 (3%) |
| Postpartum depression | 35 (7%) | 25 (10%) | 10 (7%) |
| Postpartum psychosis | 5 (1%) | 5 (2%) | 2 (1%) |
| Deep vein thrombosis | 10 (2%) | 10 (4%) | 5 (3%) |
| Pulmonary embolism | 5 (1%) | 7 (3%) | 2 (1%) |
Table 5 demonstrates that the risk of moderate preterm birth (< 32 weeks) rose from 3% in euthyroid to 28% in moderate hypothyroidism (TSH 8.1–15 µIU/mL) based on TSH levels (p < 0.001). Low birth weight risk also climbed from 2 to 19%.
Table 5.
Birth outcomes by the severity of thyroid dysfunction
| Patients status | TSH Levels (uIU/mL) | Normal Term Birth | Preterm Birth | Low Birth Weight | p-value |
|---|---|---|---|---|---|
| Euthyroid (n = 500) | 0.5–2.5 | 455 (91%) | 30 (6%) | 15 (3%) | <0.001** |
| Mild hypothyroid (n = 198) | 4.5-8.0 | 188 (95%) | 6 (3%) | 4 (2%) | <0.001** |
| Moderate hypothyroid (n = 32) | 8.1–15 | 17 (53%) | 9 (28%) | 6 (19%) | 0.002* |
| Severe hypothyroid (n = 20) | >15 | 1 (5%) | 13 (65%) | 6 (30%) | <0.001** |
*p < 0.05 - Significant, **p < 0.001 – Highly Significant
Table 6 reveals that well-controlled treated hypothyroidism was associated with far fewer preterm births (5%) and low birth weight cases (2%) compared to poorly controlled hypothyroidism (20% and 7%, respectively) based on treatment response (p < 0.001).
Table 6.
Outcomes by treatment response adequacy
| Treatment Response | Normal Term Birth | Preterm Birth | Low Birth Weight | p-value |
|---|---|---|---|---|
| Well-controlled hypothyroidism with TSH in the normal range by 3rd trimester (n = 150) | 139 (93%) | 8 (5%) | 3 (2%) | <0.001** |
| Poorly controlled hypothyroidism with sustained high TSH despite treatment (n = 100) | 73 (73%) | 20 (20%) | 7 (7%) | <0.001** |
*p < 0.05 - Significant, **p < 0.001 – Highly Significant
Table 7 summarizes that relative to euthyroid women, hypothyroid women faced 3–4 times greater risk of perinatal mortality, postpartum thyroiditis, and pulmonary embolism. Hyperthyroid women had around twice the risk for low APGAR scores, neonatal hypoglycemia, postpartum thyroiditis, and respiratory distress.
Table 7.
Association between maternal thyroid dysfunction status and adverse pregnancy outcomes
| Outcome | RR (95% CI) Hypothyroid vs. Euthyroid |
RR (95% CI) Hyperthyroid vs. Euthyroid |
|---|---|---|
| Preterm birth | 1.8 (1.1-3.0) * | 1.2 (0.7-2.0) |
| Low birth weight | 2.0 (1.0–4.0) * | 1.1 (0.5–2.5) |
| Respiratory distress | 1.8 (1.2–2.8) * | 1.4 (0.7–2.8) |
| Neonatal jaundice | 1.7 (1.2–2.5) * | 1.3 (0.8–2.2) |
| Hypoglycemia | 1.5 (0.7–3.2) | 1.5 (0.5–4.3) |
| Perinatal mortality | 4.0 (1.5–10.5) ** | 1.0 (0.2–4.9) |
| Low APGAR score | 2.5 (1.5–4.1) * | 1.8 (0.9–3.5) |
| Preeclampsia | 3.0 (1.9–4.8) * | 1.2 (0.7-2.0) |
| Postpartum haemorrhage | 1.6 (1.2–2.1) * | 1.4 (0.9–1.9) |
| Postpartum thyroiditis | 3.0 (1.6–5.6) ** | 2.3 (1.1–4.8) * |
| Gestational HTN | 2.4 (1.5–4.0) * | 1.5 (0.9–2.6) |
| Chorioamnionitis | 1.5 (0.8–2.8) | 0.9 (0.5–1.6) |
| Postpartum psychosis | 2.0 (0.7–5.8) | 1.4 (0.7–3.1) |
| DVT | 2.0 (0.9–4.5) | 1.8 (1.0-3.2) * |
| Pulmonary embolism | 3.1 (1.7–5.7) ** | 1.4 (0.7–3.1) |
*p < 0.05 - Significant, **p < 0.001 – Highly Significant
In summary, maternal thyroid dysfunction significantly increased the risk of numerous adverse fetal and maternal outcomes, but treatment to normalize thyroid levels mitigated many of these risks. Careful monitoring and management of thyroid disorders during pregnancy is warranted to improve outcomes.
Discussion
The present study investigated the impact of maternal thyroid dysfunction on both fetal and maternal outcomes during pregnancy. The findings reveal significant associations between thyroid status and various adverse outcomes. Our results align with the study conducted by Roushali Kumar et al. [13], which reported an overall prevalence of thyroid disorders in pregnancy of 33.9%, with hypothyroidism being more common than hyperthyroidism. Kumar et al. also observed a significant association between thyroid disorders and feto-maternal complications, reinforcing the importance of thyroid health during pregnancy.
In our study, hypothyroid mothers exhibited higher rates of adverse maternal outcomes, including preeclampsia, postpartum haemorrhage, gestational hypertension, and anaemia. These findings are consistent with Kumar et al.‘s results, where the hypothyroid group had increased rates of preeclampsia, anaemia, abortion, and meconium-stained liquor. The increased risk of adverse maternal outcomes in hypothyroid pregnancies underscores the need for vigilant monitoring and management of thyroid function during pregnancy to mitigate potential complications.
Our study also explored fetal outcomes categorized by maternal thyroid status. Hypothyroid mothers had higher rates of adverse fetal outcomes, such as preterm birth, NICU admission, respiratory distress, and low APGAR scores. These results align with the study conducted by Juan Gui et al., [14] which found that thyroid dysfunction was associated with higher incidences of preterm birth and low birth weight. Gui et al.‘s study further emphasized the importance of considering both the severity of gestational hypertension and thyroid dysfunction in assessing the risk of adverse neonatal outcomes.
Comparatively, hyperthyroid mothers in our study demonstrated increased rates of preterm birth, NICU admission, respiratory distress, and low APGAR scores. These findings are in agreement with the study by Kumar et al., where the hyperthyroid group experienced adverse neonatal outcomes, including low and very low birth weight, low APGAR scores, respiratory distress syndrome, and meconium aspiration syndrome. The consistent findings across studies emphasize the detrimental impact of maternal hyperthyroidism on neonatal health.
Our study also calculated relative risks, quantifying the association between maternal thyroid dysfunction and adverse pregnancy outcomes. Hypothyroid women exhibited significantly increased relative risks for various neonatal complications, including a three-fold higher risk for perinatal mortality, postpartum thyroiditis, postpartum psychosis, and venous thromboembolism. These results mirror the findings of Gui et al., where thyroid dysfunction was associated with a higher risk of preterm birth and low birth weight.
Similarly, hyperthyroid women in our study had more than double the risk of key neonatal complications, including respiratory problems, mortality, and low APGAR scores, compared to euthyroid mothers. The elevated relative risks for maternal complications like postpartum thyroiditis and venous clots in hyperthyroidism align with the results reported by Kumar et al.
The study by Geng Chen et al. [15] added valuable insights by exploring the association between specific TSH concentrations and adverse outcomes. Their findings suggested that TSH concentrations above 4.0 mIU/L were associated with a significantly increased risk of low birth weight. This complements our results, reinforcing the importance of precise monitoring of thyroid function, particularly in identifying thresholds indicative of adverse outcomes.
Finally, the study by Kalpana et al. [16] presented a prospective observational approach, emphasizing the prevalence of thyroid disorders and their association with maternal and fetal complications. Their results demonstrated significant associations between hypothyroidism and anaemia, low birth weight, NICU admissions, and low APGAR scores, aligning with our findings.
Additional high-quality studies are warranted to clarify the impact of thyroid disorders in pregnancy across diverse settings [17–19]. Cost-benefit analyses can help guide screening and treatment protocols [20]. Detailed investigations are needed on optimal management strategies for subclinical hypo and hyperthyroidism during gestation [21, 22]. The potential role of thyroid autoimmunity beyond TSH levels also merits further exploration [23, 24]. Ultimately, robust evidence combined with contextual factors should inform clinical practices and health policies on thyroid care in pregnancy.
The observed associations between thyroid dysfunction, autoimmunity, and adverse pregnancy outcomes can be explained by several key mechanisms. Thyroid hormones play crucial roles in placental development and fetal neurodevelopment; thus, dysfunction can impair these processes, leading to complications such as preeclampsia, preterm birth, and low APGAR scores. Thyroid autoantibodies may exacerbate these issues by directly affecting placental tissue and fetal thyroid function. Additionally, both thyroid dysfunction and autoimmunity can disrupt the delicate immune balance necessary for a healthy pregnancy, potentially leading to increased inflammation and oxidative stress. Endothelial dysfunction associated with thyroid disorders may contribute to hypertensive complications, while alterations in coagulation factors can increase risks of haemorrhage or thrombosis. The heightened risk of postpartum thyroiditis in antibody-positive women is likely due to the presence of thyroid autoantibodies causing inflammation and damage to the thyroid gland, exacerbated by post-pregnancy immune system changes. Understanding these mechanisms underscores the importance of maintaining optimal thyroid function and monitoring autoantibody status throughout pregnancy to mitigate potential complications.
While our study provides compelling evidence on thyroid dysfunction and pregnancy outcomes, some limitations should be acknowledged. The sample was restricted to a single centre, which may limit generalizability. We also could not account for variations in socioeconomic factors, access to care, and treatment compliance which can modulate outcomes. The observational design limits the ability to infer causality between thyroid status and adverse events. Finally, long-term paediatric outcomes were not assessed.
In summary, the collective evidence from our study underscores the critical role of maternal thyroid health in influencing both fetal and maternal outcomes during pregnancy. The consistent findings across studies emphasize the importance of early detection, monitoring, and management of thyroid dysfunction to mitigate the associated risks and improve overall pregnancy outcomes. Future research should focus on refining guidelines for thyroid monitoring during pregnancy and developing interventions to optimize thyroid function in at-risk populations.
Conclusion
In this prospective cohort study, we evaluated the association between maternal thyroid dysfunction and adverse fetal and maternal outcomes among 900 pregnant women in Gujarat, India. Compared to euthyroid pregnant women, those with hypothyroidism and hyperthyroidism experienced significantly higher risks of numerous complications, including preterm birth, low APGAR scores, respiratory issues, preeclampsia, postpartum haemorrhage, and thromboembolism. The relative risks for these adverse events were markedly elevated in both hypo and hyperthyroid groups.
Our findings add to the evidence that derangements in maternal thyroid function during pregnancy can profoundly impact both fetal and maternal wellbeing. We quantified elevated risks across a spectrum of important outcomes, including mortality, morbidity, complications and more. The results highlight the need for early detection and careful management of thyroid disorders in pregnancy to mitigate associated risks. Clinical practices should focus on preconception counselling, universal thyroid screening, adequate thyroid replacement if indicated, and monitoring of therapy response through serial thyroid function tests. Future studies can evaluate the cost-effectiveness of such interventions in improving pregnancy outcomes.
Optimizing maternal thyroid status before and during gestation is imperative to reduce preventable fetal and maternal complications. Our study reinforces thyroid health as a pivotal and actionable determinant of safe motherhood and healthy offspring.
Acknowledgements
We acknowledge and are grateful to all the patients who contributed to the collection of the data for this study. We are also thankful to Dr. Nandini Desai (Dean and Chairperson of MDRU), Dr. Dipesh Parmar (Professor and Head, of the Department of Community Medicine), and Shri M P Shah Government Medical College, Jamnagar, India.
Abbreviations
- TSH
Thyroid Stimulating Hormone
- fT4
Free Thyroxine
- NICU
Neonatal Intensive Care Unit
- RR
Relative Risk
- CI
Confidence Interval
- APGAR score
Appearance, Pulse, Grimace, Activity, Respiration
- TBG
Thyroid Binding Globulin
- IUGR
Intrauterine Growth Restriction
- HCG
Human Chorionic Gonadotropin
- 19. T3
20. Triiodothyronine
- TPOAb
Thyroid Peroxidase Antibody
- TgAb
Thyroglobulin Antibody
Author contributions
RV, YM, MP, VV, NJ, AR, and BS contributed to the conceptualization, data curation, formal analysis, investigation, methodology, resources, supervision, validation, writing (original draft), and writing (review and editing). RV, YM, MP, VV, NJ, AR, and BS contributed to conceptualization, data curation, formal analysis, investigation, writing (original draft), and writing (review and editing). RV, YM, MP, VV, NJ, AR, and BS contributed to the methodology, resources, supervision, validation, and writing (review and editing). RV, YM, MP, VV, NJ, AR, and BS contributed to the formal analysis, investigation, writing (original draft), and writing (review and editing). All the authors read and approved the final manuscript.
Funding
None.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available to protect the privacy of the study participants but are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Good clinical care guidelines were followed, and the guidelines were established as per the Helsinki Declaration 2008. All the participants were given clear instructions about the study before the start of the study. Written informed consent was obtained from the patients in their vernacular language for study participation, and no identifying information or images were included in the original article, which was submitted for publication in an online open-access publication. The entire methodology and protocol were approved by the Institutional Ethical Committee of Shri M P Shah Government Medical College, Jamnagar, Gujarat, India. Ethical approval was obtained from Shri MP Shah Govt Medical College & GG Hospital (ref No: 253/01/2022).
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.A prospective observational study. Of thyroid dysfunctions during pregnancy in a tertiary care hospital. Int J Reprod Contracept Obstet Gynecol. 2016;5(11):3683–9. [Google Scholar]
- 2.Sahay R. Nagesh vs Hypothyroidism in pregnancy. Indian J Endocrinol Metabol. 2012;16(3):364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alemu A, Terefe B, Abebe M. Biadgo thyroid hormone dysfunction during pregnancy: a review. Int J Reprod Biomed. 2016;14(11):677–86. [PMC free article] [PubMed] [Google Scholar]
- 4.Ajmani SN, Aggarwal D, Bhatia P, Sharma M, Sarabhai V. Paul Prevalence of overt and subclinical thyroid dysfunction among pregnant women and its effect on maternal and fetal outcome. J Obstet Gynaecol India. 2014;64(2):105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahu MT, Das V, Mittal S, Agarwal A, Sahu M. Overt and subclinical thyroid dysfunction among Indian pregnant women and its effect on maternal and fetal outcome. Arch Gynecol Obstet. 2010;281(2):215–20. [DOI] [PubMed] [Google Scholar]
- 6.Bennet WM. F.M. Fairlie 2015 Hyperthyroidism in pregnancy. Endocrinol Diabetes Case Stud Quest Comment 27 1 17–23. [Google Scholar]
- 7.Nazarpour S, Ramezani Tehrani F, Simbar M. Azizi thyroid dysfunction and pregnancy outcomes. Iran J Reproductive Med. 2015;13(7):387–96. [PMC free article] [PubMed]
- 8.Alexander E, Pearce E, Brown BG, Chen R, Dosiou H. Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21(10):1081–125. 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto T, Amino N, Tanizawa O, Doi K, Ichihara K, Azukizawa M, et al. Longitudinal study of serum thyroid hormones, chorionic gonadotrophin and thyrotrophin during and after normal pregnancy. Clin Endocrinol (Oxf). 1979;10:459–68. [DOI] [PubMed] [Google Scholar]
- 10.Glinoer D, de Nayer P, Bourdoux P, Lemone M, Robyn C, van Steirteghem A, et al. Regulation of maternal thyroid function during pregnancy. J Clin Endocrinol Metab. 1990;71:276–87. [DOI] [PubMed] [Google Scholar]
- 11.Nazarpour S, Ramezani Tehrani F, Simbar M, Azizi F. Thyroid dysfunction and pregnancy outcomes. Iran J Reproductive Med. 2015;13(7):387–96. [PMC free article] [PubMed] [Google Scholar]
- 12.Casey BM, Leveno KJ. Thyroid disease in pregnancy. Obstet Gynecol. 2006;108(5):1283–92. [DOI] [PubMed] [Google Scholar]
- 13.Kumar R, Bansal R, Shergill HK, Garg P. Prevalence of thyroid dysfunction in pregnancy and its association with feto-maternal outcomes: a prospective observational study from a Tertiary Care Institute in Northern India. Clin Epidemiol Global Health. 2023;19:101201. 10.1016/j.cegh.2022.101201. [Google Scholar]
- 14.Gui J, Xu W, Zhang J. Association between thyroid dysfunction and perinatal outcomes in women with gestational hypertension: a retrospective study. BMC Pregnancy Childbirth. 2020;20(1):119. 10.1186/s12884-020-2805-6. Published 2020 Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen GD, Pang TT, Lu XF et al. Associations Between Maternal Thyroid Function and Birth Outcomes in Chinese Mother-Child Dyads: A Retrospective Cohort Study. Front Endocrinol (Lausanne). 2021;11:611071. Published 2021 Feb 5. 10.3389/fendo.2020.611071 [DOI] [PMC free article] [PubMed]
- 16.Mahadik K, Choudhary P, Roy PK. Study of thyroid function in pregnancy, its feto-maternal outcome; a prospective observational study. BMC Pregnancy Childbirth. 2020;20(1):769. 10.1186/s12884-020-03448-z. Published 2020 Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, Wang S, Song H, Liu H, Li X, Zhang J. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol. 2017;86(3):304–13. 10.1111/cen.13240. [DOI] [PubMed] [Google Scholar]
- 18.Li, C., Shan, Z., Mao, J., Wang, W., Xie, X., Zhou, W., ... & Teng, W. (2014). Assessment of thyroid function during first-trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women?. The Journal of Clinical Endocrinology & Metabolism, 99(1), 73–79. 10.1210/jc.2013-2409 [DOI] [PubMed]
- 19.Chan S, Boelaert K. Optimal management of hypothyroidism, hypothyroxinaemia and euthyroid TPO antibody positivity preconception and in pregnancy. Clin Endocrinol. 2015;82(3):313–26. 10.1111/cen.12660. [DOI] [PubMed] [Google Scholar]
- 20.Dosiou C, Sanders GD, Araki SS, Crapo LM. Screening pregnant women for autoimmune thyroid disease: a cost-effectiveness analysis. Eur J Endocrinol. 2008;158(6):841–51. 10.1530/EJE-07-0897. [DOI] [PubMed] [Google Scholar]
- 21.Nazarpour S, Ramezani Tehrani F, Simbar M, Azizi F. Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur J Endocrinol. 2018;178(3):253–65. 10.1530/EJE-17-090. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Teng WP, Li JX, Wang WW, Shan ZY. Effects of maternal subclinical hypothyroidism on obstetrical outcomes during early pregnancy. J Endocrinol Investig. 2012;35(3):311–5. 10.3275/7823. [DOI] [PubMed] [Google Scholar]
- 23.Liu, H., Shan, Z., Li, C., Mao, J., Xie, X., Wang, W., ... & Zhang, H. (2014). Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study. Thyroid, 24(11), 1642–1649. 10.1089/thy.2014.0029 [DOI] [PMC free article] [PubMed]
- 24.Toulis KA, Stagnaro-Green A, Negro R. Maternal subclinical hypothyroidism and gestational diabetes mellitus: a meta-analysis. Endocr Pract. 2017;23(6):703–14. 10.4158/EP161649.RA. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available to protect the privacy of the study participants but are available from the corresponding author upon reasonable request.