Abstract
Background
ICU-acquired weakness (ICU-AW) is a common complication among ICU patients. We used machine learning techniques to construct an ICU-AW inflammatory factor prediction model to predict the risk of disease development and reduce the incidence of ICU-AW.
Methods
The least absolute shrinkage and selection operator (LASSO) technique was used to screen key variables related to ICU-AW. Eleven indicators, such as the presence of sepsis, glucocorticoids (GC), neuromuscular blocking agents (NBAs), length of ICU stay, Acute Physiology and Chronic Health Evaluation (APACHE II) II score, and the levels of albumin (ALB), lactate (LAC), glucose (GLU), interleukin-1β (IL-1β), interleukin-6 (IL-6), and interleukin-10 (IL-10), were used as variables to establish the prediction model. We divided the data into a dataset that included inflammatory factors and a dataset that excluded inflammatory factors. Specifically, 70% of the participants in both datasets were used as the training set, and 30% of the participants were used as the test set. Three machine learning methods, logistic regression (LR), random forest (RF), and extreme gradient boosting (XGB), were used in the 70% participant training set to construct six different models, which were validated and evaluated in the remaining 30% of the participants as the test set. The optimal model was visualized for prediction using nomograms.
Results
The logistic regression model including the inflammatory factors demonstrated excellent performance on the test set, with an area under the curve (AUC) of 82.1% and the best calibration curve fit, outperforming the other five models. The optimal model is represented visually in the nomograms.
Conclusion
This study used easily accessible clinical characteristics and laboratory data that can aid in early clinical recognition of ICU-AW. The inflammatory factors IL-1β, IL-6, and IL-10 have high value for predicting ICU-AW.
Trial registration
The trial was registered at the Chinese Clinical Trial Registry with the registration number ChiCTR2300077968.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12883-024-03981-w.
Keywords: ICU-acquired weakness, Inflammatory factors, Machine learning, Model prediction, Nomogram
Introduction
ICU-acquired weakness (ICU-AW) is a common neuromuscular complication in ICU patients [1]. The incidence of ICU-AW is 45.5%, and it is characterized mainly by decreased muscle strength of the extremities, prolonged mechanical ventilation, and increased patient mortality [2–4]. There is currently no consensus regarding ICU-AW diagnostic methods [5], and the most common diagnostic method still follows the 2009 Medical Research Council (MRC)-based muscle strength scoring process, which requires the patient to be awake and cooperative [3, 6]. Sedated ICU patients are unable to undergo MRC scoring. The inflammatory response in ICU patients may exacerbate the development of ICU-AW by damaging the neuromuscular system and microcirculation. Inflammatory factors such as TNF-α and IL-6 directly promote muscle proteolysis and worsen muscle atrophy [7, 8]. Additionally, inflammation affects the microcirculatory system, leading to poor oxygenation of localized tissues, which further impairs neurological and muscular function. Testing for inflammatory factors in blood is simple and accessible. Therefore, exploring the relationships between these factors and ICU-AW may facilitate early prediction and intervention. To test this hypothesis, two models were developed in this study—one with and one without inflammatory factors—to assess their role in predicting ICU-AW.
Recently, computer-aided diagnosis through machine learning has become a hot topic [9, 10], with machine learning being able to capture high dimensionality in data and interactions between clinical features to support the prediction of data [11]. We extracted clinical data from 527 ICU patients using machine learning techniques and constructed six ICU-AW prediction models using different methods. Finally, we compared these models and created a model with the best predictive performance that could help diagnose and identify patients with ICU-AW. In addition, to improve the usability of the optimized model, the optimized model was transformed into a nomogram.
The goal of this study was to develop a machine learning-based predictive model for building ICU-AW inflammatory factors to improve the in-depth understanding of the impact of inflammatory factors and to provide richer reference information for clinicians.
Materials and methods
Data source and extraction
This study was designed as an observational cohort study. It was conducted in accordance with the Declaration of Helsinki regulations, approved by the Medical Ethics Committee of the Affiliated Hospital of Xuzhou Medical University under No. XYFY2023-KL225-013, and reported to the China Clinical Trial Registry under No. ChiCTR2300077968. Consecutive patients admitted to multiple ICUs in our hospital from April to December 2023 were included in the study.
The inclusion criteria were as follows: patient was aged ≥ 18 years; patient was conscious during bedside muscle strength assessment (awake and responding to at least three of the following simple commands: open or close your eyes, look at me, stick out your tongue, nod your head, and frown); estimated ICU stay > 24 h; and informed consent provided by the patient or his or her agent.
The exclusion criteria were as follows: patients had a history of mental illness or cognitive impairment; patient had neuromuscular diseases such as myasthenia gravis, Guillain Barré syndrome, or sequelae of stroke before admission to the ICU; patients was unable to perform a bedside muscle strength assessment test, such as in patients with severe trauma; patient had central nervous system (CNS) disease (stroke, traumatic brain or spinal cord injury, CNS infection, or CNS tumor); patient was admitted to the hospital for cardiac arrest or spinal injury; patient was expected to die within 48 h.
Diagnosis of ICU-AW
Bedside muscle strength in patients with intensive care unit acquired weakness was assessed using the Medical Research Council (MRC) score recommended by the American Thoracic Society (ATS) in 2014 [12]. The sedative infusion was discontinued at least 30 min before using the MRC scale, which required the patient to be awake and able to respond to at least three of the following simple commands: open or close your eyes, look at me, stick out your tongue, nod your head, or frown. After these commands were performed, muscle strength was assessed via the MRC scale. The MRC scale assessment consisted of six muscle groups (shoulder abductors, elbow flexors, wrist flexors, hip flexors, knee extensors, and ankle dorsiflexors), each scored on a scale ranging from 0 to 5, and was applied to assess both the right and left sides of the body. The total score ranged from 0 to 60, with a total score less than 48 as the basis for diagnosing ICU-AW. The overall Cronbach’s α coefficient of the MRC scale was 0.912. The evaluation criteria for the MRC scale were as follows: (1) generalized weakness developing after the onset of critical illness; (2) diffuse weakness (involving both proximal and distal muscles), symmetric, flaccid, and generally spares cranial nerves; (3) Medical Research Council (MRC) sum scale score of < 48, or average MRC scale score of < 4; (4) dependence on mechanical ventilation; and (5) weakness not related to the underlying critical illness.
For the data collection, all researchers involved in this study were trained in the use of the scale according to the protocol described by Hermans G, available at http://links.lww.com/CCM/A780, to ensure consistency. The researchers were involved and documented throughout the data collection process, including once-daily assessments via the MRC scale by critical care nurses and researchers while awake. If the patient experienced ICU-AW, the evaluation was stopped; if the patient did not experience ICU-AW, the evaluation was continued by another researcher on Day 2 until the patient was transferred out of the ICU. Once the study began, the researchers summarized the collected data weekly and provided timely feedback and adjustments for any problems that arose during the data collection process. The MRC score was used for the diagnosis of ICU-AW patients.
Model input features
Thirty-seven potentially useful variables were identified through a review of the literature and in the context of the hospital.
These variables included basic information such as sex, age, BMI, history of alcoholism, smoking, and marital status; disease-related factors such as the mean arterial pressure; the Acute Physiology and Chronic Health Evaluation II (APACHE II); diabetes mellitus; and sepsis.
Treatment-related factors such as total length of hospital stay, length of ICU stay, and the use of glucocorticoids (GCs) and nerve blocking agents (NBAs) were included.
Laboratory markers, such as albumin (ALB), blood urea nitrogen (BUN), creatinine (CRE), C-reactive protein (CRP), procalcitonin (PCT), lactate (LAC), glucose (GLU), aspartate transaminase (AST), alanine transaminase (ALT), total bilirubin (T-BIL), and white blood cell count (WBC), were measured at the time of admission.
Interleukin-1β (IL-1β), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), interleukin-12p70 (IL-12p70), interleukin-17 (IL-17), interferon-α (IFN-α), interferon-γ (IFN-γ), and tumor necrosis factor α (TNF-α), which were measured for the first time during the patient’s stay in the ICU, were collected.
The least absolute shrinkage and selection operator (LASSO) was employed to identify key variables linked to ICU-AW. Finally, 11 variables, such as the presence of sepsis, GC, NBAs, length of ICU stay, and APACHE II score, as well as ALB, LAC, GLU, IL-1β, IL-6, and IL-10, were incorporated into the model.
The selection of model hyperparameters utilized 10-fold cross-validation on the training datasets. In the 10-fold cross-validation, the datasets were divided into ten partitions, where nine-tenths of the data were used to build the models, and the remaining one tenth was used as the testing datasets. This process was repeated such that each partition was used as a testing dataset only once and training dataset nine times. Cross-validation ensured a better assessment of model performance by averaging the metrics over multiple trials.
In this study, we constructed two different prediction models: one with inflammatory factors and the other without these factors. With this design, we were able to validate the stability and applicability of these models and quantify the contributions of these factors to ICU-AW prediction. On the basis of different modeling approaches, including logistic regression (LR), random forest (RF), and extreme gradient boosting (XGB), we compared the performance of these two models to determine the effects of inflammatory factors on the model.
Sample size
The study included 37 variables in total, with the modeling sample size formula indicating the need for 5 to 10 ICU-AW patients per independent variable [13]. The median incidence of ICU-AW reported in a sample of 1421 patients was 45.5% [3], with a confidence level of 95%, a standard error of estimate of 5%, and an expected loss of 5%. Therefore, the required sample size was approximately 205 to 410 patients. On the basis of the inclusion and exclusion criteria, a total of 527 patients were selected for this study, with 369 in the modeling group and 158 in the validation group, according to a 30% ratio.
Statistical analysis
Data analysis was conducted using R version 4.3.2 by the R Development Core Team. The Shapiro‒Wilk test was used to check for a normal distribution of numeric variables. Continuous variables with a normal distribution were compared via the independent-sample t test, whereas nonnormally distributed variables were analyzed via the Mann–Whitney U test. Categorical data were analyzed via the chi-square test or Fisher’s exact probability test. Leveraging LASSO regression analysis allowed us to determine the importance of each variable in the training datasets.
The synthetic minority oversampling technique (SMOTE) and cross-validation are widely used techniques in machine learning for contending with class imbalance [14].
We optimized model performance by tuning the hyperparameters using grid and random searches to balance accuracy and generalization. A stepwise regression was used in the logistic regression model. In random forest model construction, two parameters will mainly affect its work efficiency: (1) the number of trees (ntree) and (2) the candidate feature subset (mtry). In XGBoost model construction, two parameters will mainly affect its performance: (1) the learning rate; (2) the number of trees (ntree) (3) max depth. Therefore, selecting appropriate parameters can ensure the stability of the model. The predictive performance of the model was validated via discrimination and calibration techniques. The area under the curve (AUC) expresses measurement of discrimination, and the calibration curve expresses the measurement of calibration. Furthermore, the SHAP value was used to visually represent the importance and contribution of each factor to the occurrence of ICU-AW.
The LR and RF models were developed using the “caret” and “randomForest” packages, respectively, and the XGB model was created with the “xgboost” package.
Results
Patient characteristics
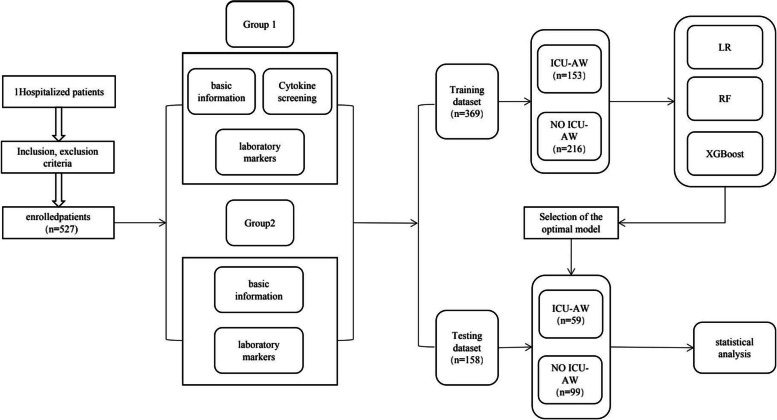
A total of 527 patients were included in this study. The dataset (n = 527) was randomly divided into training and test sets at a 7:3 ratio. The training set contained data from 369 patients, and the test set contained data from 158 patients. The data collected from the training dataset were used to assess important variables related to ICU-AW and to construct predictive models. The patients in the ICU-AW and non-ICU-AW groups were categorized according to the MRC scale. Data from the test dataset were utilized to validate the predictive model. The patient recruitment flowchart is depicted in Fig. 1, and the patient details are presented in Tables 1 and 2.
Fig. 1.
Patient recruitment flowchart
Table 1.
Basic information and clinical characteristics in the train and test datasets
| Property | Train dataset (369) | Test dataset (158) | P |
|---|---|---|---|
| Sex (%) | 232 (63) | 107 (68) | 0.120 |
| Age [y, M (QL, QU) | 62 (52,72) | 64 (54,72) | 0.519 |
| BMI (kg/m2, X ± S) | 24.12 ± 3.5 | 24.06 ± 3.69 | 0.874 |
| History of alcoholism (%) | 41 (11) | 19 (12) | 0.859 |
| History of smoking (%) | 68 (18) | 24 (15) | 0.458 |
| History of marital (%) | 347 (94) | 152 (97) | 0.269 |
| Mean arterial pressure (mmHg, X ± S) | 96.7 ± 16.02 | 94.43 ± 16.94 | 0.153 |
| Reason for admission | |||
| Planned surgery (%) | 113 (31) | 52 (33) | 0.477 |
| Emergency surgery (%) | 91 (24) | 33 (21) | 0.201 |
| Medical (%) | 165 (45) | 73 (46) | 0.196 |
| Sepsis, (%) | 19 (5) | 12 (8) | 0.363 |
| Diabetes (%) | 68 (18) | 38 (24) | 0.164 |
| Length of ICU stay [d, M(QL, QU)] | 6 (3,11) | 6 (3,12) | 0.679 |
| Length of hospital stay [d, M(QL, QU)] | 16 (11,24) | 17 (11,25) | 0.413 |
| GCS [M(QL, QU)] | 15 (12,15) | 15 (13,15) | 0.902 |
| APACHE II [M(QL, QU)] | 9 (6,13) | 9 (7,14) | 0.679 |
| GCa (%) | 130 (35) | 56 (36) | 0.989 |
| NBAsb (%) | 58 (16) | 20 (13) | 0.456 |
| AST [U/L, M(QL, QU)] | 35 (21,64) | 34 (22,71) | 0.675 |
| ALT [U/L, M(QL, QU)] | 25 (17,49) | 27 (18,53) | 0.466 |
| ALB [g/L, M(QL, QU)] | 33.50 (28.6,38.70) | 33.70 (28.30,38.40) | 0.345 |
| T-BIL [umol/L, M(QL, QU)] | 12.50 (7.50,20.60) | 11.20 (7.70,20,00) | 0.523 |
| BUN [mmol/L, M(QL, QU)] | 7.33 (5.21,10.64) | 7.34 (5.74,11.71) | 0.392 |
| CRE [mmol/L, M(QL, QU)] | 65 (47,89) | 63 (53,109) | 0.291 |
| PCT [ng/ml, M(QL, QU)] | 0.143 (0.12,1.87) | 0.42 (0.01,2.26) | 0.760 |
| WBC [10^9/L, M(QL, QU)] | 10.40 (7.30,14.50) | 10.60 (7.80,14.50) | 0.548 |
| CRP [mg/L, M(QL, QU)] | 80.0 (18.1,149.3) | 79.3 (18.2,151.7) | 0.433 |
| GLU [mmol/L, M(QL, QU)] | 8.80 (7.10,11.40) | 8.50 (7.10,11.40) | 0.529 |
| LAC [mmol/L, M(QL, QU)] | 1.50 (1.00,2.60) | 1.40 (0.90,2.50) | 0.099 |
Table 2.
Inflammatory factors in the train and test datasets
| Property | Train dataset (369) | Test dataset (158) | P |
|---|---|---|---|
| IL-1β [pg/ml, M(QL, QU)] | 13.53 (5.62,13.95) | 10.83 (4.99,13.97) | 0.113 |
| IL-2 [pg/ml, M(QL, QU)] | 3.08 (1.77,3.21) | 2.9 (1.45,3.24) | 0.595 |
| IL-4 [pg/ml, M(QL, QU)] | 2.20 (1.27,2.35) | 2.02 (1.18,2.33) | 0.936 |
| IL-5 [pg/ml, M(QL, QU)] | 3.88 (2.42,4.03) | 3.39 (1.81,4.01) | 0.553 |
| IL-6 [pg/ml, M(QL, QU)] | 90.85 (15.88,116.62) | 69.86 (12.4,113.45) | 0.929 |
| IL-8 [pg/ml, M(QL, QU)] | 19.49 (10.10,41.1) | 19.22 (8.68,33.60) | 0.057 |
| IL-10 [pg/ml, M(QL, QU)] | 6.01 (2.64,7.76) | 3.87 (2.23,7.69) | 0.803 |
| IL-12p70 [pg/ml, M(QL, QU)] | 2.96 (1.84,3.06) | 2.91 (1.66,3.04) | 0.358 |
| IL-17 [pg/ml, M(QL, QU)] | 4.37 (1.59,7.47) | 3.32 (1.19,5.66) | 0.472 |
| IFN-α [pg/ml, M(QL, QU)] | 2.93 (2.28,3.21) | 2.88 (1.75,3.02) | 0.931 |
| IFN-γ [pg/ml, M(QL, QU)] | 8.90 (3.30,12.60) | 7.63 (2.76,12.42) | 0.788 |
| TNF-α [pg/ml, M(QL, QU)] | 2.22 (1.53,2.34) | 2.22 (1.36,2.35) | 0.390 |
The data are presented as the number of patients (%) or median (IQR)
aGlucocorticoids (application time > 24 h)
bneuromuscular blockers (application time > 24 h)
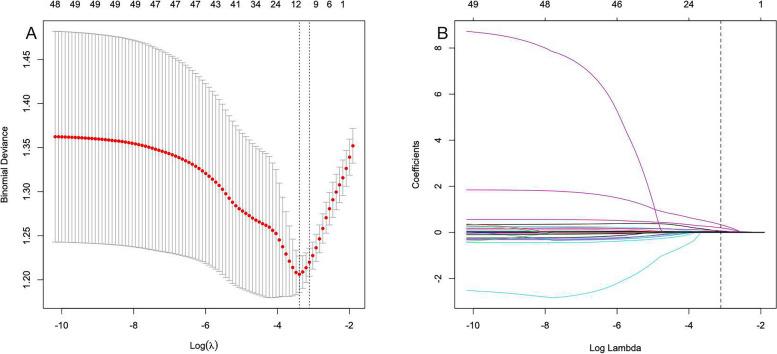
In this study, no statistically significant disparities were observed in patient characteristics between the training and testing sets. To determine the optimal parameters (lambda) in the LASSO model, a rigorous 10-fold cross-validation approach was employed. Dashed vertical lines were plotted at the optimal values determined by both the minimum criterion and one standard error of the minimum criterion (1-SE criterion). Additionally, vertical lines were drawn at values selected through 10-fold cross-validation, where the optimal λ resulted in 11 nonzero coefficients (Fig. 2A, B).
Fig. 2.
Selection of demographic and clinical descriptions via LASSO regression. a In the LASSO model, the coefficient profiles of 37 texture features were drawn from the log (λ) sequence. The vertical dotted lines represent the minimum mean square error and the standard error of the minimum distance. b The use of 10-fold cross-validation to draw vertical lines at selected values, where the optimal lambda produces 11 nonzero coefficients
In the LASSO regression analysis, we screened eleven key characteristics with nonzero coefficients, including the presence of sepsis, GC, NBAs, length of ICU stay, and APACHE II score, as well as the presence of ALB, LAC, GLU, IL-1β, IL-6, and IL-10 levels (Table 3).
Table 3.
LASSO regression results for significant variables related to ICU-AW
| Variables | Coefficient |
|---|---|
| Sepsis | 0.3337085334 |
| Length of ICU stay | 0.0388214232 |
| APACHE II | 0.0190878577 |
| GC | 0.1986876377 |
| NBAs | 0.0261176827 |
| Albumin | −0.0084301482 |
| Glucose | 0.0039358184 |
| Lactate | 0.0722583870 |
| IL-1β | 0.0074240080 |
| IL-6 | 0.0000715233 |
| IL-10 | 0.0071618905 |
Model performance
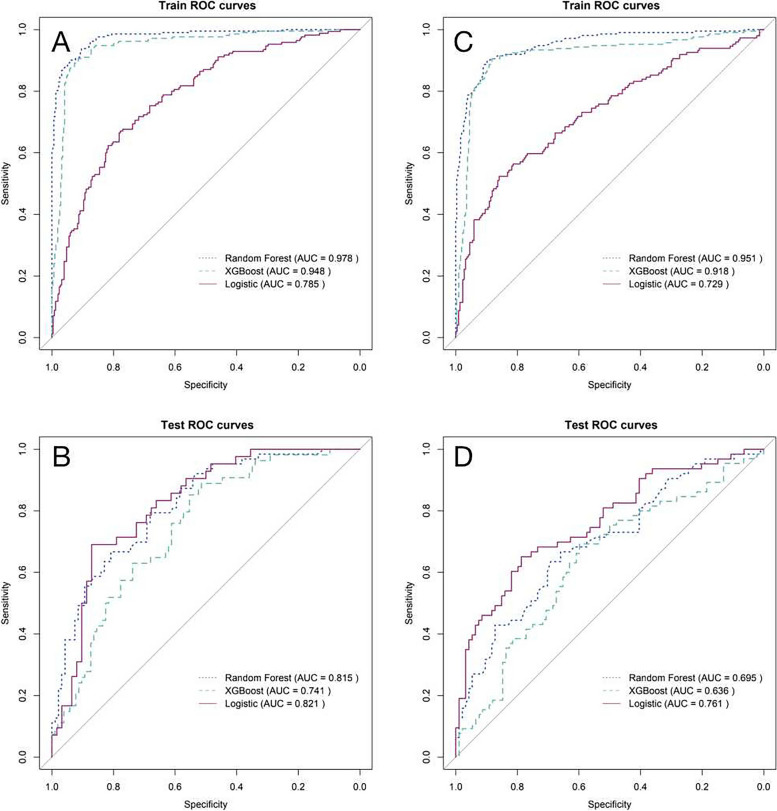
We used 3 machine learning algorithms to build 6 predictive models for ICU-AW. The following values were observed in the training dataset, which included inflammatory factors: 97.8% AUC for the RF model; 94.8% AUC for the XGB model; and 78.5% AUC for the LR model (Supplementary Table 3). For the test dataset, we obtained an AUC of 81.5% for the RF model, 74.1% for the XGB model, and 82.1% for the LR model (Supplementary Table 4). The ROC curves of the models, including inflammatory factors in the training dataset and the test dataset, are shown in Fig. 3A and B. The following values were observed in the training dataset for the model excluding inflammatory factors: 95.1% AUC for the RF model; 91.8% AUC for the XGB model; and 72.9% AUC for the LR model (Supplementary Table 5). For the test dataset, we obtained the following values: an AUC of 69.5% for the RF model, an AUC of 63.6% for the XGB model, and an AUC of 76.1% for the LR model (Supplementary Table 6). The ROC curves of the models excluding inflammatory factors in the training and test datasets are shown in Fig. 3C and D.
Fig. 3.
ROC curves for the models in the training and test datasets A is the training set model including the inflammatory factor; B is the test set model including the inflammatory factor; C is the training set model excluding the excluding factor; D is the test set model excluding the inflammatory factor
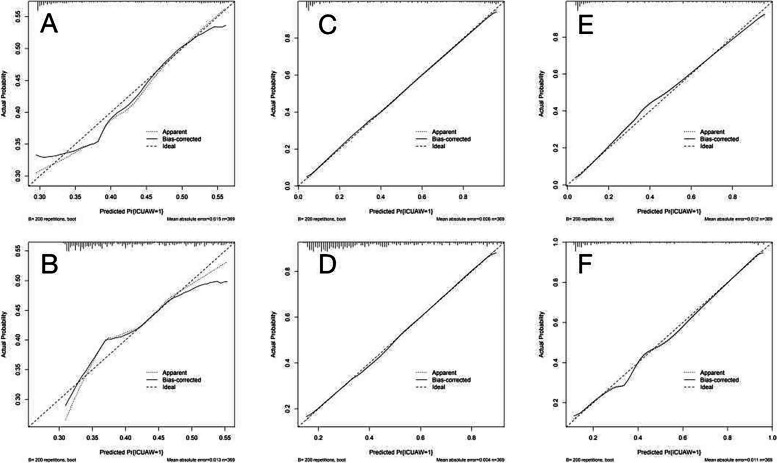
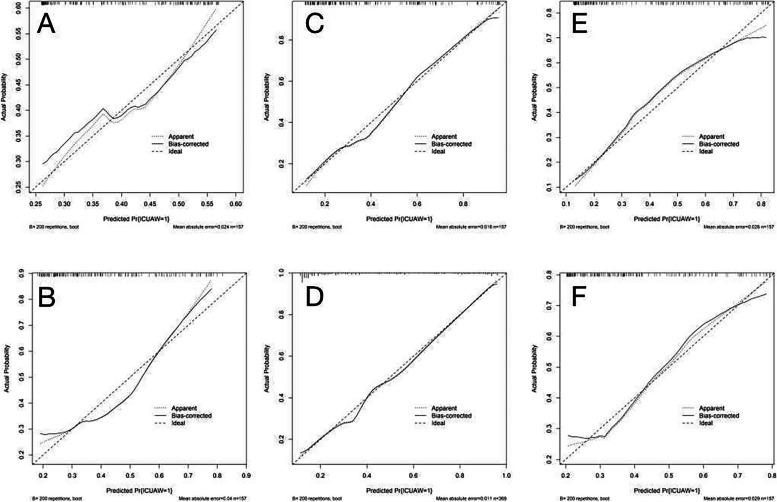
The three models that included inflammatory factors had higher AUC values and performed better than the three models that excluded inflammatory factors. The calibration graphs of the six models including and excluding inflammatory factors in the training set and test set are shown in Figs. 4 and 5. The alignment of the LR models along the X-axis and the Y-axis suggests a high level of agreement.
Fig. 4.
Train set model calibration plots A is the XGB model including inflammatory factors; B is the XGB model excluding inflammatory factors; C is the LR model including inflammatory factors; D is the LR model excluding inflammatory factors; E is the RF model including inflammatory factors; F is the RF model excluding inflammatory factors
Fig. 5.
Test set model calibration plots A XGB model including inflammatory factors; B XGB model excluding inflammatory factors; C LR model including inflammatory factors; D LR model excluding inflammatory factors; E RF model including inflammatory factors; F RF model excluding inflammatory factors
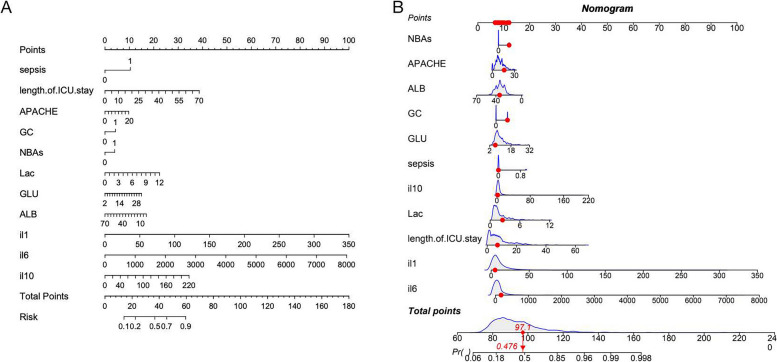
Finally, the LR model was converted to a nomogram to understand and use the model (Fig. 6A, B). On the left side, the names of the factors are listed, and the tick marks on the lines represent the range of possible values for each factor. The length of the line clearly demonstrates the significant influence of each factor on the occurrence of ICU-AW. The sum of individual scores (referred to as “points”) for each factor at different values was calculated to obtain a total score (referred to as the “total score”). The “risk” indicates the likelihood of developing ICU-AW. SHAP values were utilized to summarize the features: sepsis, GC, NBAs, length of ICU stay, and APACHE II score, as well as the presence levels of ALB, LAC, GLU, IL-1β, IL-6, and IL-10 (Fig. 7).
Fig. 6.
Nomogram for estimation of ICU-AW (the ICU-AW risk associated with each patient’s total score can be read from the risk at the bottom of (A). The model-calculated total score for the 24th case in the dataset was 97.1, which correlates to a p value of 0.476 in (B)
Fig. 7.
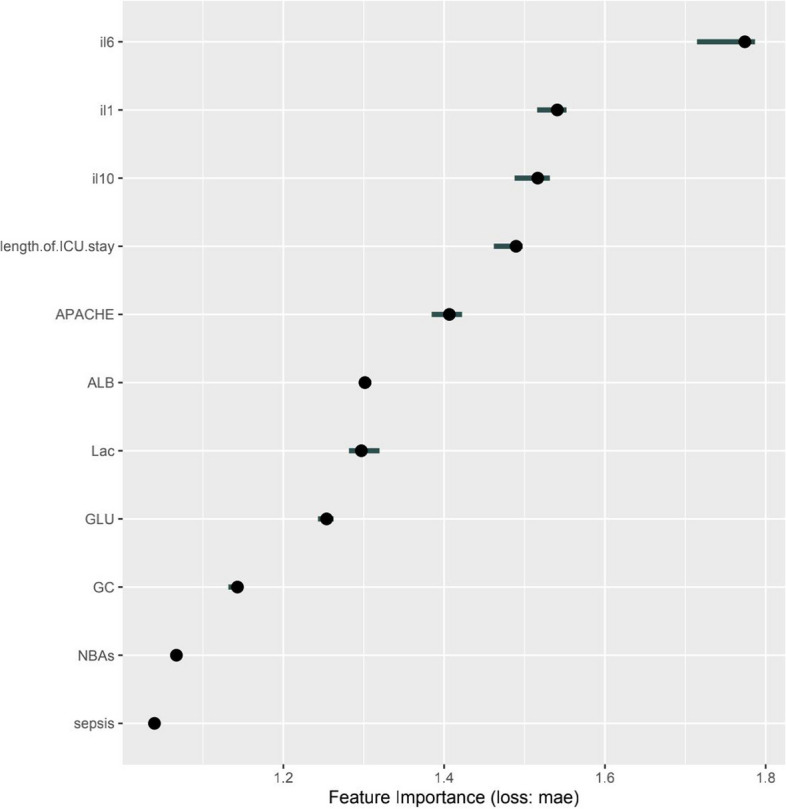
SHAP interprets the model
Discussion
ICU-AW refers to ICU patients with no reasonable explainable cause of weakness other than critical illness [3]. ICU-AW is associated with multiple risk factors, such as sepsis, multiple organ system failure, systemic inflammation, prolonged sedation, prolonged mechanical ventilation, prolonged immobilization, hyperglycemia, glucocorticoids, and neuromuscular blocking agents [15, 16]. Critically ill patients with ICU-AW may experience a prolonged decline in quality of life after discharge from the hospital, including difficulty swallowing, inability to maintain an upright posture, and physical weakness that lasts for months or even years [17, 18]. ICU-AW may result in higher rehab care costs per patient [19]. Multiple diagnostic methods for ICU-AW, including muscle biopsy, MRC scale, electromyography, and muscle ultrasound [20], which are very limited in their application owing to the complexity of the technique, its high cost and the poor state of consciousness of the patient, have been considered, and researchers are working to identify improved methods for diagnosing the occurrence of ICU-AW. Therefore, the establishment of a disease prediction model may provide a completely new approach for the diagnosis of ICU-AW. Through this model, timely diagnosis of ICU-AW can be achieved while increasing the level of public awareness of the disease. To enhance the model’s general applicability, this study included easily accessible laboratory tests for several inflammatory mediators, such as IL-1β, IL-6, and IL-10.
ICU-AW risk prediction models have also been developed in previous studies to predict the probability of ICU-AW in ICU patients using statistical models. However, the model described by Wieske et al. [21] provided a prediction only based on each of the three variables, whereas ICU-AW was associated with multiple factors, and the model had an AUC value of 71%. Our research has several significant advantages. Supplementary Table 2 shows the results of our study in comparison with those of Wieske et al. [21]. First, machine learning provides us with multiple algorithm choices. Second, inflammatory factors, as important biomarkers, help improve the predictive effect of the model and have important clinical applications. Third, our model relies on early and easily accessible clinical data, providing clinicians with early intervention. Finally, using a nomogram, we visualize the prediction results, which enhances clinicians’ understanding and ease of use. We used 11 variables included in the construction of the model. The optimal prediction model was represented by a nomogram. The LR model exhibited favorable performance, with AUC values of 73.99% and 80.44% in the training and testing datasets, respectively, which was higher than the model reported by Wieske et al. [21]. Moreover, the calibration curve of the LR model demonstrated strong concordance between the predicted probabilities and the observed outcomes. Leveraging this predictive model, the nomogram serves as a valuable tool for predicting ICU-AW in patients, enabling targeted interventions for individuals at high risk.
Sepsis leads to protein imbalances that cause muscle atrophy and ICU-AW [22]. Sepsis increases protein degradation and decreases protein synthesis in skeletal muscle [23], resulting in protein imbalances in muscle tissue. Importantly, although sepsis is one of the main factors triggering muscle atrophy and ICU-AW, it is not the only factor contributing to this condition, and other potential factors may contribute to the development of ICU-AW.
A prolonged ICU stay increases the risk of ICU-AW [24]. There is a significant correlation between ICU stay and ICU-AW. ICU patients are hospitalized for longer periods of time, and the risk of muscle atrophy increases accordingly because of prolonged immobilization.
The APACHE II score is considered one of the important susceptibility factors for ICU-AW and reflects the severity of pathophysiologic changes in patients [25]. Patients with higher APACHE II scores are more likely to have ICU-AW.
Corticosteroids have been identified as predictors of ICU-AW [26]. Corticosteroid therapy in ICU patients causes muscle dysfunction and nerve damage, inhibits protein synthesis, and promotes muscle atrophy [27].
Neuromuscular blocking agents are associated with the development of ICU-AW, which is 25% more likely to occur in patients using such drugs compared with those who are not using them [28]. ICU-AW is more common in patients treated with high doses of neuromuscular blocking agents.
Hypoproteinemia occurs in critically ill patients for a number of reasons, and reduced albumin levels in ICU-AW patients may be a macroscopic manifestation of decreased protein synthesis and enhanced protein degradation in patients [29]. In addition, low blood albumin levels can reflect an inflammatory state, leading to more rapid loss of muscle proteins and causing muscle atrophy.
Previous studies have demonstrated that ICU-AW patients have significantly higher lactate levels than patients without ICU-AW and that hyperlactatemia is independently associated with the development of ICU-AW [30]. Lowering the lactate concentration may help reduce the incidence of ICU-AW.
Hyperglycemia is significantly associated with ICU-AW, which can lead to a decrease in diaphragm function, causing dyspnea and adverse effects, promoting the development of ICU-AW and increasing patient mortality [31]. Hyperglycemia can exacerbate the inflammatory response, leading to decreased complement activity, immune system imbalance, and mitochondrial damage [32].
IL-1β and IL-6, as well as IL-10, are important factors in the development of systemic inflammation and anti-inflammatory responses, which affect muscle protein metabolism, leading to muscle atrophy and the induction of ICU-AW [33]. They play an important role in unbalanced inflammatory responses, as observed in sepsis and multiple organ dysfunction syndrome (MODS) [34]. IL-lβ reduces muscle weight and protein content and decreases the rate of protein synthesis in the gastrocnemius muscle of rats, which consists mainly of fast-contracting muscle [35]. IL-6 production is stimulated by IL-1β and may contribute to systemic inflammation in critical illness and sepsis. IL-6 plays an important role in maintaining energy status during exercise, but sustained elevated levels of IL-6 may accelerate the hydrolysis of muscle proteins. In critical illness, the sustained release of IL-6 may lead to a systemic inflammatory response and interfere with the balance of the systemic inflammatory response, increasing the risk of multiorgan failure and muscle damage. IL-6 recruits cytokines to muscle tissue, contributing to the production of leukocytes, which triggers protein hydrolysis, myocyte degeneration, and muscle atrophy [36]. IL-10, an anti-inflammatory cytokine, inhibits the production of the proinflammatory cytokine IL-6, but IL-6 can inhibit its own production via compensatory increases in IL-10 levels to limit the persistent proinflammatory state. However, the compensatory increase in IL-10 does not completely offset the increase in IL-6. Eventually, the proinflammatory state dominates, leading to an imbalance between pro-inflammatory and anti-inflammatory cytokines, which results in muscle damage [37]. In this study, several laboratory test items for inflammatory factors were included in the modeling process, and models that included and excluded inflammatory factors were compared. The results showed that models including inflammatory factors had better predictive results, highlighting the associations of ICU-AW with IL-1β, IL-6, and IL-10.
In our study, the machine learning models random forest and extreme gradient boosting exhibited significant overfitting, manifested by the significantly improved performance on the training set than the test set. Machine models typically require large amounts of data to avoid overfitting, and the size of the current dataset might not be sufficiently large to support the complexity of these models. In subsequent studies, we will increase the sample size, expand the dataset size, and use methods such as L1/L2 regularization to reduce the sensitivity to noisy data. These approaches will facilitate the generalization ability of the model and avoid overfitting. The performance of the LR model on the test set is better than on the training set, which is indeed an unusual phenomenon. Measures such as feature screening and model regularization allow the model to predict the target more consistently across datasets, which may lead to slightly better results on the test set. To ensure the stability of the results, we repeated the experiments with different data splits and multiple cross-validations, and found that the fluctuations in the test set performance were within reasonable limits. The ICU-AW in this study was assessed only by the MRC, which is not applicable to all ICU patients, especially those who are not able to undergo MRC assessment. This limitation may indeed lead to some bias in the training of the model, which was limited to patients who could use the MRC scale, and thus might differ from the actual target group. In future studies, we will introduce a variety of ICU-AW assessment methods and combine them with other assessment tools, such as grip strength measurements, gait assessments or electrophysiological data, to form a more comprehensive ICU-AW assessment criterion to reduce model bias.
The model classification results in this study yielded 72% sensitivity and 70% precision. This result reflects the limitations of the model in practical applications, especially in terms of precision. Although the model performed well in terms of sensitivity, the high false-positive rate may lead to over-treatment or unnecessary further investigations. Therefore, in clinical practice, it is recommended to incorporate other diagnostic information and clinical judgment to improve the accuracy and reliability of predictive results.
There are several limitations to this study. First, because the data for this study were obtained from a single large medical center, the applicability of the results to other health care settings may be limited. When applying this model in a different health care setting, recalibration may be necessary to adjust the weights of the variables to the new setting. Second, the sample size of this study was small; therefore, larger samples sizes are needed in future studies to improve the reliability of the results. Third, the diagnosis of ICU-AW relied only on the MRC scale, which was measured in patients who were awake, and intubated comatose patients may not have been counted in time. Fourth, the number of days of ICU stay utilized by the model was the real-time number of days during the patient’s ICU stay, not the total number of days after discharge. We realize that this criterion limits the model’s ability to predict ICU-AW early during a patient’s admission. Finally, owing to the low disease severity of the patients in the cohort, the results may not be fully applicable to more critically ill ICU patients. Low APACHE scores and CRP levels may lead to an underestimation of the role of inflammatory factors in the development of ICU-AW. Future studies should more fully evaluate patients with more severe disease to reduce the potential bias introduced by patients with less severe disease.
Our study provides clear high-risk factors for clinical practice and suggests that clinical teams should prevent and manage ICU-AW through early identification, individualized management, and multidisciplinary collaboration. In the future, further research can help optimize these interventions and ensure their wider application and validation in clinical practice.
Conclusion
In this study, we used easily accessible clinical characteristics and laboratory data to construct a predictive model of ICU-AW inflammatory factors to aid in the early clinical recognition of ICU-AW. The inflammatory factors IL-1β, IL-6, and IL-10 have high value for predicting ICU-AW.
Supplementary Information
Acknowledgements
We would like to thank all clinical workers who helped to recruit and evaluate participants for the study.
Abbreviations
- ICU-AW
ICU-acquired weakness
- MRC
Medical Research Council
- GBS
Guillain Barré syndrome
- GC
Glucocorticoids
- NBAs
Neuromuscular blocking agents
- APACHE II
Acute Physiology and Chronic Health Evaluation Score II
- ALB
Albumin
- BUN
Blood urea nitrogen
- CRE
Creatinine
- CRP
C-reactive protein
- PCT
Procalcitonin
- LAC
Lactate
- BLU
Glucose
- AST
Aspartate transaminase
- ALT
Alanine transaminase
- T-BIL
Total bilirubin
- WBC
White blood cell count
- IL-1β
Interleukin-1β
- IL-2
Interleukin-2
- IL-4
Interleukin-4
- IL-5
Interleukin-5
- IL-6
Interleukin-6
- IL-8
Interleukin-8
- IL-10
Interleukin-10
- IL-12p70
Interleukin-12p70
- IL-17
Interleukin-17
- IFN-α
Interferon-α
- IFN-γ
Interferon-γ
- TNF-α
Tumor Necrosis Factorα
- LASSO
Least absolute shrinkage and selection operator
- LR
Logistic regression
- RF
Random forest
- XGB
Extreme gradient boosting
- AUC
Area under the curve
- UPS
Ubiquitin-proteasome system
- MODS
Multiple organ dysfunction syndrome
Authors' contributions
YYG: Writing - review & editing, Writing - original draft, Visualization, Validation, Software, Project administration, Methodology, Formal analysis.WPS: Resources, Investigation, Data curation. JX: Writing - review & editing, Supervision, Project administration, Formal analysis, Conceptualization. All authors reviewed the manuscript.
Funding
The study was supported by the National Key Research and Development Program of the Ministry of Science and Technology of the People’s Republic of China (No. 2020YFC2006604).
Data availability
All relevant data are described within the paper. Deidentified data can be requested. Data can be requested by all interested researchers, who can be contacted via the corresponding author.
Declarations
Ethics approval and consent to participate
The Ethics Committee of the Affiliated Hospital of Xuzhou Medical University approved this research (No. XYFY2023-KL225-013). The China Clinical Trial Registry approved this research (No. ChiCTR2300077968). All the participants provided written informed consent before the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wolfe KS, Patel BK, MacKenzie EL, Giovanni SP, Pohlman AS, Churpek MM, et al. Impact of vasoactive medications on ICU-Acquired weakness in mechanically ventilated patients. Chest. 2018;154(4):781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herridge MS, Batt J, Santos CD. ICU-acquired weakness, morbidity, and death. Am J Respir Crit Care Med. 2014;190(4):360–2. [DOI] [PubMed] [Google Scholar]
- 3.Stevens RD, Marshall SA, Cornblath DR, Hoke A, Needham DM, de Jonghe B, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37:S299-308. [DOI] [PubMed] [Google Scholar]
- 4.Bissett BM, Leditschke IA, Neeman T, Boots R, Paratz J. Inspiratory muscle training to enhance recovery from mechanical ventilation: a randomised trial. Thorax. 2016;71(9):812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latronico N, Gosselink R. A guided approach to diagnose severe muscle weakness in the intensive care unit. Rev Bras Ter Intensiva. 2015;27:27(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermans G, Casaer MP, Clerckx B, Güiza F, Vanhullebusch T, Derde S, et al. Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: a subanalysis of the EPaNIC trial. Lancet Respir Med. 2013;1(8):621–9. [DOI] [PubMed] [Google Scholar]
- 7.Witteveen E, Wieske L, van der Poll T, van der Schaaf M, van Schaik IN, Schultz MJ, et al. Increased early systemic inflammation in ICU-Acquired weakness; a prospective Observational Cohort Study. Crit Care Med. 2017;45(6):972–9. [DOI] [PubMed] [Google Scholar]
- 8.Tuttle CSL, Thang LAN, Maier AB. Markers of inflammation and their association with muscle strength and mass: a systematic review and meta-analysis. Ageing Res Rev. 2020;64: 101185. [DOI] [PubMed] [Google Scholar]
- 9.Handelman GS, Kok HK, Chandra RV, Razavi AH, Lee MJ, Asadi H. eDoctor: machine learning and the future of medicine. J Intern Med. 2018;284(6):603–19. [DOI] [PubMed] [Google Scholar]
- 10.Greener JG, Kandathil SM, Moffat L, Jones DT. A guide to machine learning for biologists. Nat Rev Mol Cell Biol. 2022;23(1):40–55. [DOI] [PubMed] [Google Scholar]
- 11.Schwalbe N, Wahl B. Artificial intelligence and the future of global health. Lancet (London England). 2020;395(10236):1579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jolley SE, Bunnell AE, Hough CL. ICU-acquired weakness. Chest. 2016;150(5):1129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Du H, Wei BH, Chang XN, Dong CM. Development and validation of risk-stratification delirium prediction model for critically ill patients: a prospective, observational, single-center study. Medicine. 2017;96(29):e7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LemaÃŽtre G, Nogueira F, Aridas CK. Imbalanced-learn: a python toolbox to tackle the curse of imbalanced datasets in machine learning. J Mach Learn Res. 2017;18(17):1–5. [Google Scholar]
- 15.de Jonghe B, Lacherade J-C, Sharshar T, Outin H. Intensive care unit-acquired weakness: risk factors and prevention. Crit Care Med. 2009;37(10):S309-15. [DOI] [PubMed] [Google Scholar]
- 16.Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med. 2020;46(4):637–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kny M, Fielitz J. Hidden agenda - the involvement of endoplasmic reticulum stress and unfolded protein response in inflammation-Induced muscle wasting. Front Immunol. 2022;13: 878755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wischmeyer PE, San-Millan I. Winning the war against ICU-acquired weakness: new innovations in nutrition and exercise physiology. Crit Care. 2015;19(S3):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermans G, Van Mechelen H, Clerckx B, Vanhullebusch T, Mesotten D, Wilmer A, et al. Acute outcomes and 1-Year mortality of Intensive Care unit–acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190(4):410–20. [DOI] [PubMed] [Google Scholar]
- 20.Xiaomin W, Xiaoping Z. Occurrence, diagnosis, and rehabilitation of intensive care unit-acquired weakness. Chin Crit Care Med. 2020;32(08):1020–4. [DOI] [PubMed] [Google Scholar]
- 21.Wieske L, Witteveen E, Verhamme C, Dettling-Ihnenfeldt DS, van der Schaaf M, Schultz MJ, et al. Early prediction of intensive care unit-acquired weakness using easily available parameters: a prospective observational study. PLoS ONE. 2014;9(10): e111259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schefold JC, Bierbrauer J, Weber-Carstens S. Intensive care unit—acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle. 2010;1(2):147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callahan LA, Supinski GS. Sepsis-induced myopathy. Crit Care Med. 2009;37:S354-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paolo F, Valentina DG, Silvia C, Tommaso P, Elena C, Martin D, et al. The possible predictive value of muscle ultrasound in the diagnosis of ICUAW in long-term critically ill patients. J Crit Care. 2022;71: 154104. [DOI] [PubMed] [Google Scholar]
- 25.KISA NG, Cevik KISAE. BE. Prediction of Mortality in Patients After Oncologic Gastrointestinal Surgery: Comparison of the ASA, APACHE II, and POSSUM Scoring Systems. Cureus. 2021. [DOI] [PMC free article] [PubMed]
- 26.De Jonghe B. Paresis Acquired in the Intensive Care Unit: A Prospective Multicenter Study. JAMA. 2002;288(22):2859. [DOI] [PubMed] [Google Scholar]
- 27.Aare S, Radell P, Eriksson LI, Akkad H, Chen Y-W, Hoffman EP, et al. Effects of corticosteroids in the development of limb muscle weakness in a porcine intensive care unit model. Physiol Genom. 2013;45(8):312–20. [DOI] [PubMed] [Google Scholar]
- 28.Price DR, Mikkelsen ME, Umscheid CA, Armstrong EJ. Neuromuscular blocking agents and neuromuscular dysfunction acquired in critical illness: a systematic review and Meta-analysis. Crit Care Med. 2016;44(11):2070–8. [DOI] [PubMed] [Google Scholar]
- 29.Reid MB, Judge AR, Bodine SC. CrossTalk opposing view: the dominant mechanism causing disuse muscle atrophy is proteolysis. J Physiol. 2014;592(24):5345–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang T, Li Z, Jiang L, Xi X. Hyperlactacidemia as a risk factor for intensive care unit-acquired weakness in critically ill adult patients. Muscle Nerve. 2021;64(1):77–82. [DOI] [PubMed] [Google Scholar]
- 31.Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64(8):1348–53. [DOI] [PubMed] [Google Scholar]
- 32.Amour J, Brzezinska Anna K, Jager Z, Sullivan C, Weihrauch D, Du J, et al. Hyperglycemia adversely modulates endothelial nitric oxide synthase during Anesthetic Preconditioning through tetrahydrobiopterin– and heat shock protein 90–mediated mechanisms. Anesthesiology. 2010;112(3):576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meduri GU, Schwingshackl A, Hermans G. Prolonged glucocorticoid treatment in ARDS: impact on intensive care unit-acquired weakness. Front Pead. 2016;4:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aziz M, Jacob A, Yang W-L, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol. 2012;93(3):329–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedrich O, Yi B, Edwards JN, Reischl B, Wirth-Hücking A, Buttgereit A, et al. IL-1α Reversibly inhibits skeletal muscle ryanodine receptor. A novel mechanism for critical illness myopathy? Am J Respir Cell Mol Biol. 2014;50(6):1096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkelman C. Inactivity and inflammation: selected cytokines as biologic mediators in muscle dysfunction during critical illness. AACN Adv Crit Care. 2004;15(1):74–82. [DOI] [PubMed] [Google Scholar]
- 37.Liang Z, Zhang T, Liu H, Li Z, Peng L, Wang C, et al. Inflammaging: the ground for Sarcopenia? Exp Gerontol. 2022;168: 111931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are described within the paper. Deidentified data can be requested. Data can be requested by all interested researchers, who can be contacted via the corresponding author.