Abstract
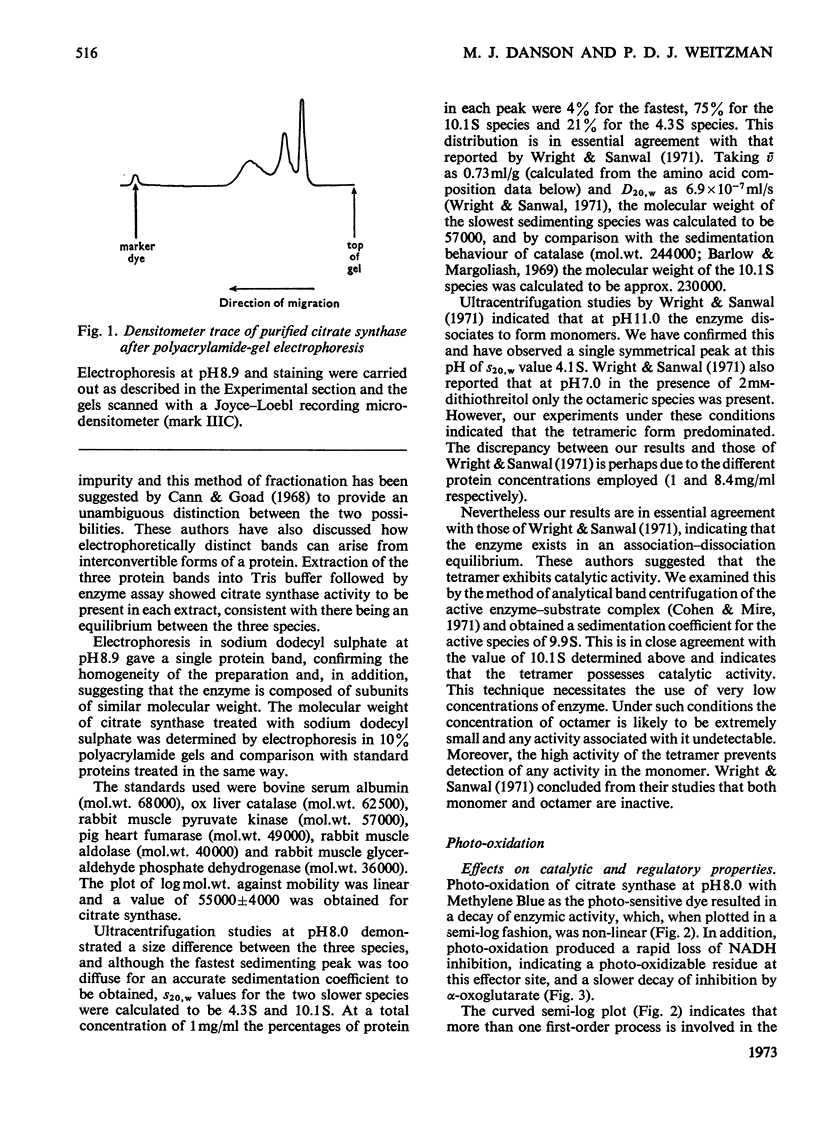
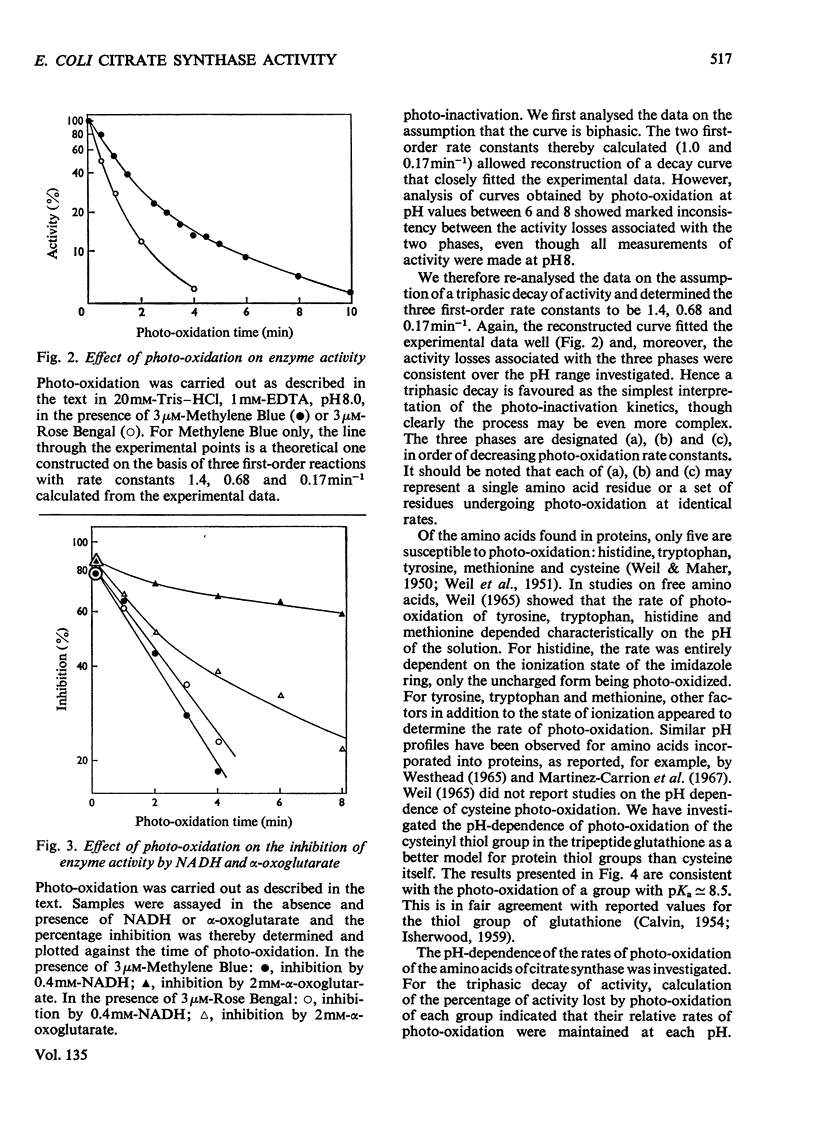
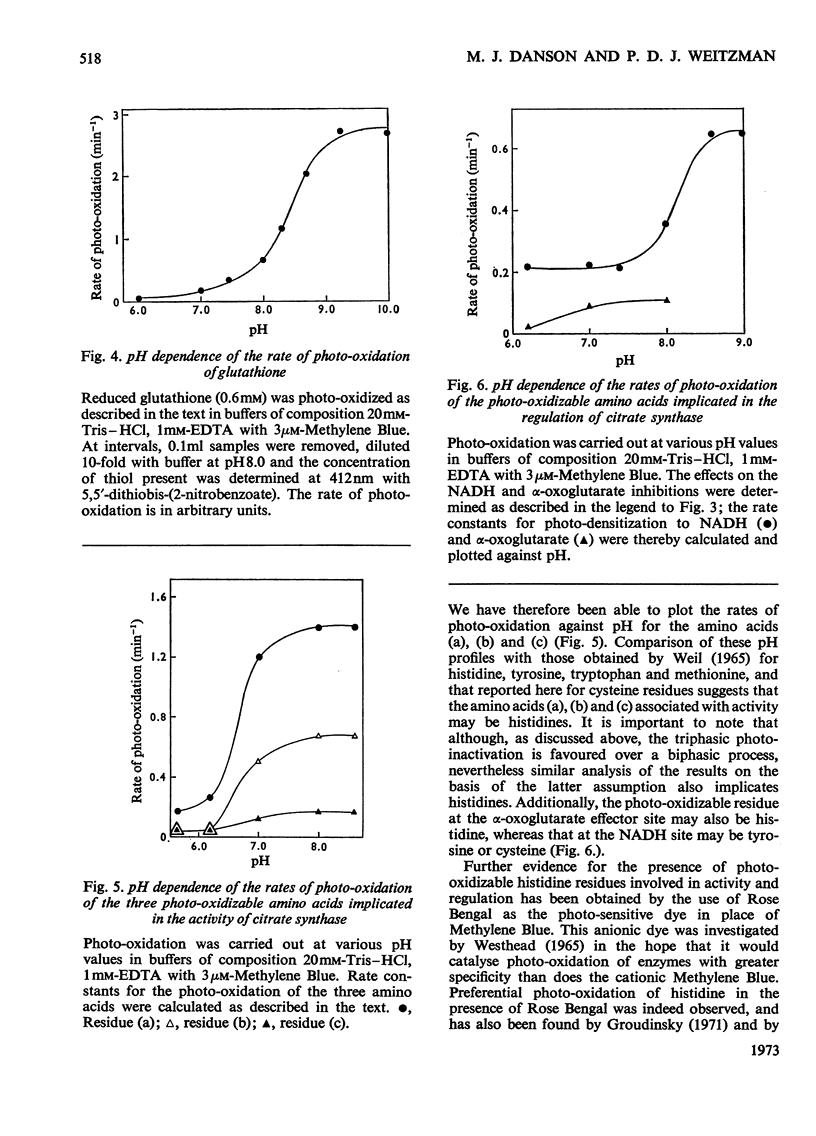
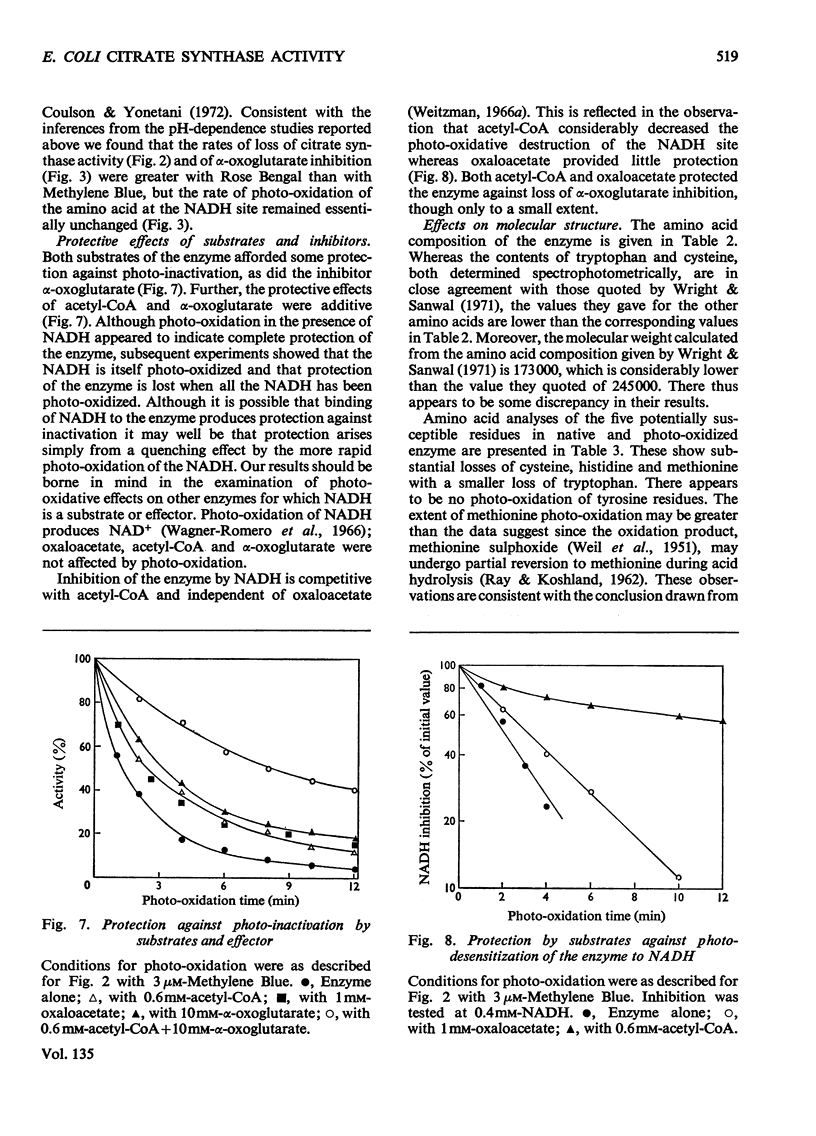
1. Citrate synthase has been purified from Escherichia coli and shown to exist at an equilibrium between three forms: monomer (mol.wt. 57000), tetramer (mol.wt. 230000) and, possibly, octamer. Modification of the enzyme by photo-oxidation and by treatment with specific chemical reagents has been carried out to gain information on the amino acid residues involved in enzymic activity and in the inhibition of activity by NADH and α-oxoglutarate. 2. Several photo-oxidizable amino acids appear to be involved in activity. The nature of the pH-dependence of their rates of photo-oxidation with Methylene Blue suggests that these are histidines, a conclusion supported by the greater rate of photo-inactivation with Rose Bengal and the destruction of activity by diethyl pyrocarbonate. 3. The participation of histidine at the α-oxoglutarate effector site is indicated by photo-oxidation and the participation of cysteine at the NADH effector site suggested by photo-oxidation is confirmed by the desensitization to NADH produced by treatment with 5,5′-dithiobis-(2-nitrobenzoate). Inactivation of the enzyme after modification with this reagent suggests the additional involvement of cysteine in catalytic activity. 4. Amino acid analyses of native and photo-oxidized enzyme are consistent with these conclusions. 5. Modification with 2-hydroxy-5-nitrobenzyl bromide indicates the participation of tryptophan in the activity of the enzyme.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin G. E., Ferdinand W. Accelerated amino acid analysis with lithium citrate buffers. The effect of resin cross-linkage on elution patterns and the avoidance of microbial contamination of columns. J Chromatogr. 1971 Nov 26;62(3):373–381. doi: 10.1016/s0021-9673(00)91388-8. [DOI] [PubMed] [Google Scholar]
- Atkin G. E., Ferdinand W. Accelerated amino acid analysis: studies on the use of lithium citrate buffers and the effect of n-propanol, in the analysis of physiological fluids and protein hydrolyzates. Anal Biochem. 1970 Dec;38(2):313–329. doi: 10.1016/0003-2697(70)90456-2. [DOI] [PubMed] [Google Scholar]
- Barlow G. H., Margoliash E. Sedimentation studies on catalase Complex II using photoelectric scanning optics. Biochim Biophys Acta. 1969 Aug 12;188(1):159–161. doi: 10.1016/0005-2795(69)90057-9. [DOI] [PubMed] [Google Scholar]
- Barman T. E., Koshland D. E., Jr A colorimetric procedure for the quantitative determination of tryptophan residues in proteins. J Biol Chem. 1967 Dec 25;242(23):5771–5776. [PubMed] [Google Scholar]
- Barman T. E. The modification of the tryptophan residues of bovine alpha-lactalbumin with 2-hydroxy-5-nitrobenzyl bromide and with dimethyl(2-hydroxy-5-nitrobenzyl)sulphonium bromide. Biochim Biophys Acta. 1972 Feb 29;257(2):297–313. doi: 10.1016/0005-2795(72)90282-6. [DOI] [PubMed] [Google Scholar]
- Cann J. R., Goad W. B. Two or more electrophoretic zones from a single macromolecule. Ann N Y Acad Sci. 1968 Jun 14;151(1):638–649. doi: 10.1111/j.1749-6632.1968.tb11924.x. [DOI] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Cohen R., Mire M. Analytical-band centrifugation of an active enzyme-substrate complex. 1. Principle and practice of the centrifugation. Eur J Biochem. 1971 Nov 11;23(2):267–275. doi: 10.1111/j.1432-1033.1971.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Coulson A. F., Yonetani T. Interaction of rose bengal with apo-hemoproteins. An essential histidine residue in cytochrome c peroxidase. Eur J Biochem. 1972 Mar 15;26(1):125–131. doi: 10.1111/j.1432-1033.1972.tb01748.x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- FOWLKS W. L. The mechanism of the photodynamic effect. J Invest Dermatol. 1959 Feb;32(2 Pt 2):233–247. [PubMed] [Google Scholar]
- Faloona G. R., Srere P. A. Escherichia coli citrate synthase. Purification and the effect of potassium on some properties. Biochemistry. 1969 Nov;8(11):4497–4503. doi: 10.1021/bi00839a041. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Perkins D. J. Chemical modifications of the tryptophan groups of transferrin. Eur J Biochem. 1972 Feb;25(3):415–419. doi: 10.1111/j.1432-1033.1972.tb01710.x. [DOI] [PubMed] [Google Scholar]
- Groudinsky O. Study of heme-protein linkage in cytochrome b2. Destruction of a crucial histidine residue by photooxidation of "apo" cytochrome b2 core in the presence of rose bengal. Eur J Biochem. 1971 Feb;18(4):480–484. doi: 10.1111/j.1432-1033.1971.tb01267.x. [DOI] [PubMed] [Google Scholar]
- HORTON H. R., KOSHLAND D. E., Jr A HIGHLY REACTIVE COLORED REAGENT WITH SELECTIVITY FOR THE TRYPTOPHAN RESIDUE IN PROTEINS. 2-HYDROXY-5-NITROBENZYL BROMIDE. J Am Chem Soc. 1965 Mar 5;87:1126–1132. doi: 10.1021/ja01083a033. [DOI] [PubMed] [Google Scholar]
- ISHERWOOD F. A. Chemistry and biochemistry of glutathione. Biochem Soc Symp. 1959;17:3–16. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLAR D. B., SCHWERT G. W. LACTIC DEHYDROGENASE. IX. EFFECT OF PHOTO-OXIDATION UPON ACTIVITY AND COMPLEX FORMATION. J Biol Chem. 1963 Oct;238:3249–3255. [PubMed] [Google Scholar]
- Martinez-Carrion M., Kuczenski R., Tiemeier D. C., Peterson D. L. The structure and enzyme-coenzyme relationship of supernatant aspartate transaminase after dye sensitized photooxidation. J Biol Chem. 1970 Feb 25;245(4):799–805. [PubMed] [Google Scholar]
- Martinez-Carrion M., Turano C., Riva F., Fasella P. Evidence of a critical histidine residue in soluble aspartic aminotransferase. J Biol Chem. 1967 Apr 10;242(7):1426–1430. [PubMed] [Google Scholar]
- Melchior W. B., Jr, Fahrney D. Ethoxyformylation of proteins. Reaction of ethoxyformic anhydride with alpha-chymotrypsin, pepsin, and pancreatic ribonuclease at pH 4. Biochemistry. 1970 Jan 20;9(2):251–258. doi: 10.1021/bi00804a010. [DOI] [PubMed] [Google Scholar]
- Mühlrad A., Hegyi G., Horányi M. Studies on the properties of chemically modified actin. 3. Carbethoxylation. Biochim Biophys Acta. 1969 May;181(1):184–190. doi: 10.1016/0005-2795(69)90240-2. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Price P. A. The identification of a tryptophan residue essential to the catalytic activity of bovine pancreatic deoxyribonuclease. J Biol Chem. 1971 Jun 25;246(12):4041–4045. [PubMed] [Google Scholar]
- RAY W. J., Jr, KOSHLAND D. E., Jr Identification of amino acids involved in phosphoglucomutase action. J Biol Chem. 1962 Aug;237:2493–2505. [PubMed] [Google Scholar]
- Sémériva M., Dufour C., Desnuelle P. On the probable involvement of a histidine residue in the active site of pancreatic lipase. Biochemistry. 1971 May 25;10(11):2143–2149. doi: 10.1021/bi00787a029. [DOI] [PubMed] [Google Scholar]
- VODRAZKA Z., CEJKA J., SALAK J. A titration and electrophoretic study on blood proteins photooxidized in the presence of sensitizers. Biochim Biophys Acta. 1961 Sep 16;52:342–348. doi: 10.1016/0006-3002(61)90683-7. [DOI] [PubMed] [Google Scholar]
- WEIL L., BUCHERT A. R. Photoöxidation of crystalline beta-lactoglobulin in the presence of methylene blue. Arch Biochem Biophys. 1951 Nov;34(1):1–15. doi: 10.1016/s0003-9861(51)80003-1. [DOI] [PubMed] [Google Scholar]
- WEIL L., GORDON W. G., BUCHERT A. R. Photooxidation of amino acids in the presence of methylene blue. Arch Biochem Biophys. 1951 Aug;33(1):90–109. doi: 10.1016/0003-9861(51)90084-7. [DOI] [PubMed] [Google Scholar]
- WEIL L. ON THE MECHANISM OF THE PHOTO-OXIDATION OF AMINO ACIDS SENSITIZED BY METHYLENE BLUE. Arch Biochem Biophys. 1965 Apr;110:57–68. doi: 10.1016/0003-9861(65)90154-2. [DOI] [PubMed] [Google Scholar]
- WEIL L., SEIBLES T. S. Photooxidation of crystalline ribonuclease in the presence of methylene blue. Arch Biochem Biophys. 1955 Feb;54(2):368–377. doi: 10.1016/0003-9861(55)90049-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weil L., Seibles T. S., Herskovits T. T. Photooxidation of bovine insulin sensitized by methylene blue. Arch Biochem Biophys. 1965 Aug;111(2):308–320. doi: 10.1016/0003-9861(65)90191-8. [DOI] [PubMed] [Google Scholar]
- Weitzman P. D.J., Dunmore P. Regulation of citrate synthase activity by alpha-ketoglutarate. Metabolic and taxonomic significance. FEBS Lett. 1969 Jun;3(4):265–267. doi: 10.1016/0014-5793(69)80154-7. [DOI] [PubMed] [Google Scholar]
- Weitzman P. D., Jones D. Regulation of citrate synthase and microbial taxonomy. Nature. 1968 Jul 20;219(5151):270–272. doi: 10.1038/219270a0. [DOI] [PubMed] [Google Scholar]
- Weitzman P. D. Regulation of citrate synthase activity in escherichia coli. Biochim Biophys Acta. 1966 Oct 17;128(1):213–215. doi: 10.1016/0926-6593(66)90166-4. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Maeba P., Sanwal B. D. Allosteric regulation of the activity of citrate snythetase of Escherichia coli by alpha-ketoglutarate. Biochem Biophys Res Commun. 1967 Oct 11;29(1):34–38. doi: 10.1016/0006-291x(67)90536-0. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Sanwal B. D. Regulatory mechanisms involving nicotinamide adenine nucleotides as allosteric effectors. IV. Physicochemical study and binding of ligands to citrate synthase. J Biol Chem. 1971 Mar 25;246(6):1689–1699. [PubMed] [Google Scholar]


