Abstract
1. Kinetic experiments suggested the possible existence of at least two different NAD+-dependent aldehyde dehydrogenases in rat liver. Distribution studies showed that one enzyme, designated enzyme I, was exclusively localized in the mitochondria and that another enzyme, designated enzyme II, was localized in both the mitochondria and the microsomal fraction. 2. A NADP+-dependent enzyme was also found in the mitochondria and the microsomal fraction and it is suggested that this enzyme is identical with enzyme II. 3. The Km for acetaldehyde was apparently less than 10μm for enzyme I and 0.9–1.7mm for enzyme II. The Km for NAD+ was similar for both enzymes (20–30μm). The Km for NADP+ was 2–3mm and for acetaldehyde 0.5–0.7mm for the NADP+-dependent activity. 4. The NAD+-dependent enzymes show pH optima between 9 and 10. The highest activity was found in pyrophosphate buffer for both enzymes. In phosphate buffer there was a striking difference in activity between the two enzymes. Compared with the activity in pyrophosphate buffer, the activity of enzyme II was uninfluenced, whereas the activity of enzyme I was very low. 5. The results are compared with those of earlier investigations on the distribution of aldehyde dehydrogenase and with the results from purified enzymes from different sources.
Full text
PDF


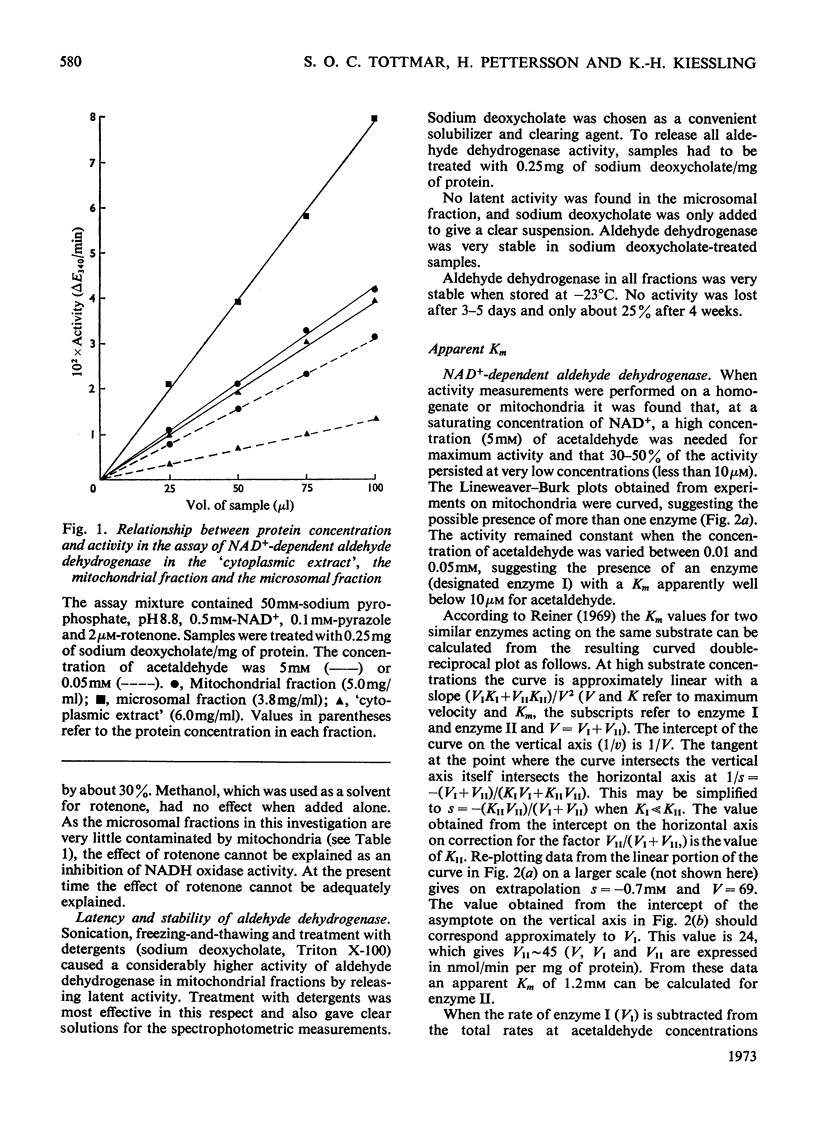
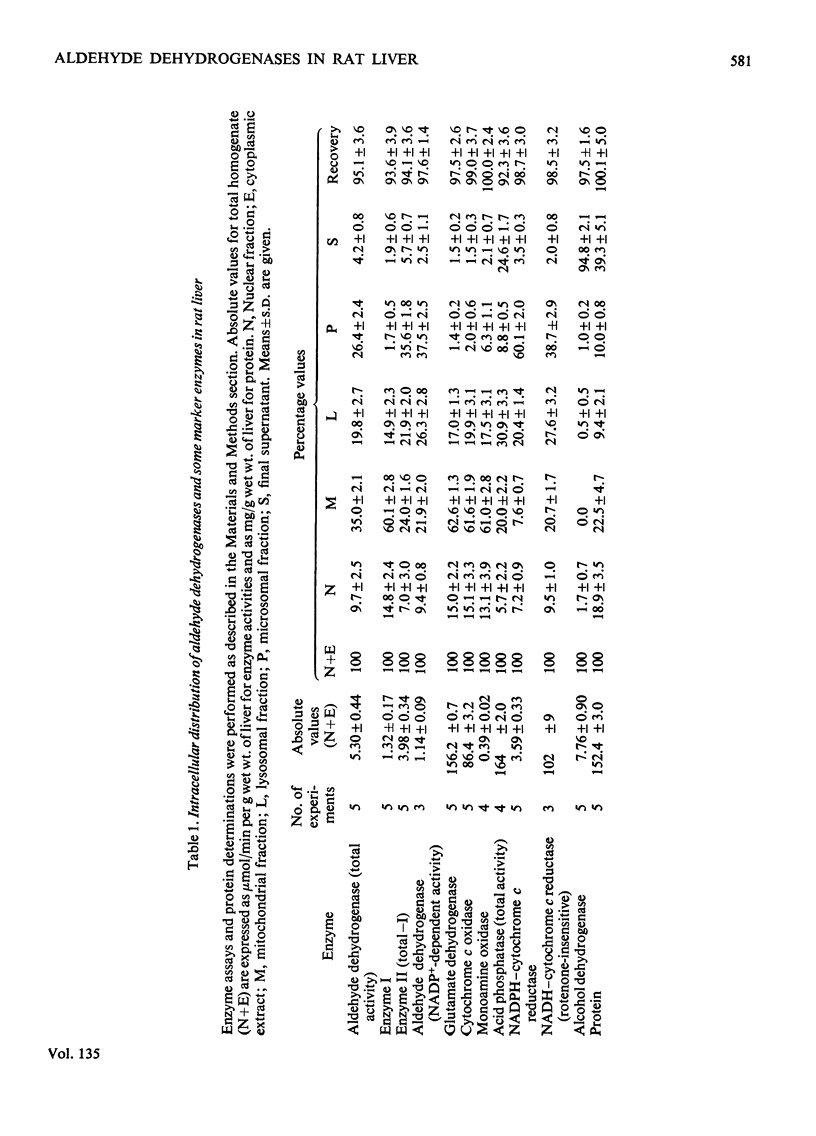
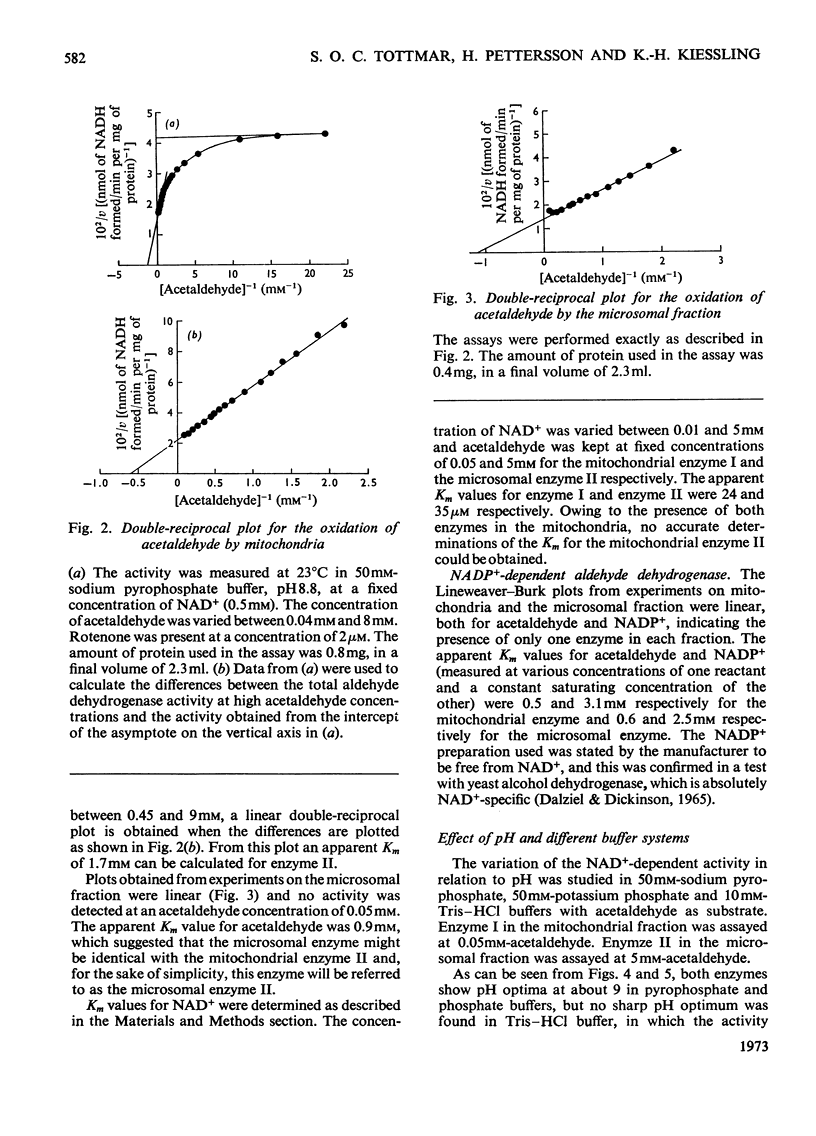
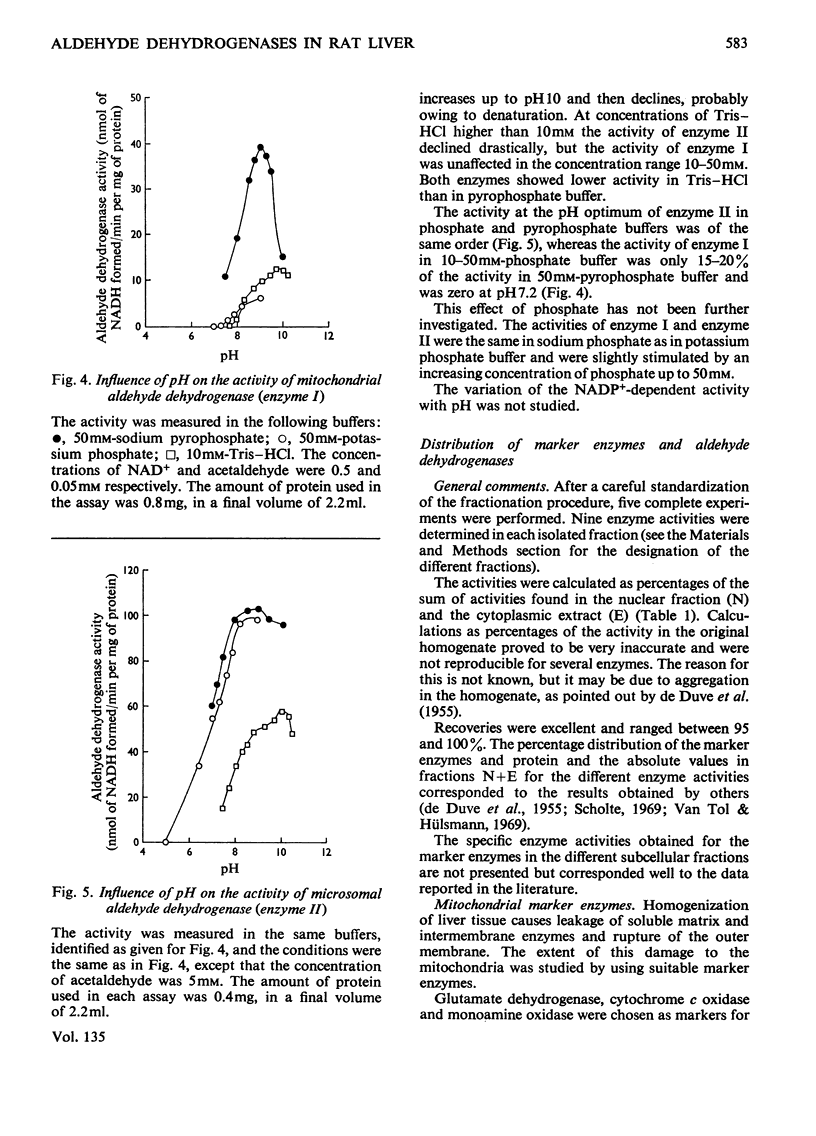



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair A. H., Bodley F. H. Human liver aldehyde dehydrogenase: partial purification and properties. Can J Biochem. 1969 Mar;47(3):265–272. doi: 10.1139/o69-041. [DOI] [PubMed] [Google Scholar]
- DALZIEL K., DICKINSON F. M. THE ACTIVITY OF LIVER ALCOHOL DEHYDROGENASE WITH NICOTINAMIDE-ADENINE DINUCLEOTIDE PHOSPHATE AS COENZYME. Biochem J. 1965 May;95:311–320. doi: 10.1042/bj0950311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitrich R. A. Tissue and subcellular distribution of mammalian aldehyde-oxydizing capacity. Biochem Pharmacol. 1966 Dec;15(12):1911–1922. doi: 10.1016/0006-2952(66)90220-6. [DOI] [PubMed] [Google Scholar]
- Duncan R. J., Tipton K. F. The kinetics of pig brain aldehyde dehydrogenase. Eur J Biochem. 1971 Oct 26;22(4):538–543. doi: 10.1111/j.1432-1033.1971.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Duncan R. J., Tipton K. F. The purification and properties of the NAD-linked aldehyde dehydrogenase from pig brain. Eur J Biochem. 1971 Sep 24;22(2):257–262. doi: 10.1111/j.1432-1033.1971.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Engel P. C., Ferdinand W. The significance of abrupt transitions in Lineweaver-Burk plots with particular reference to glutamate dehydrogenase. Negative and positive co-operativity in catalytic rate constants. Biochem J. 1973 Jan;131(1):97–105. doi: 10.1042/bj1310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin V. G., Deitrich R. A. Brain aldehyde dehydrogenase. Localization, purification, and properties. J Biol Chem. 1966 Aug 10;241(15):3533–3539. [PubMed] [Google Scholar]
- Feldman R. I., Weiner H. Horse liver aldehyde dehydrogenase. I. Purification and characterization. J Biol Chem. 1972 Jan 10;247(1):260–266. [PubMed] [Google Scholar]
- GLENN J. L., VANKO M. Choline and aldehyde oxidation by rat liver. Arch Biochem Biophys. 1959 May;82(1):145–152. doi: 10.1016/0003-9861(59)90099-2. [DOI] [PubMed] [Google Scholar]
- Hassinen I. E., Ylikahri R. H., Kähönen M. T. Effect of ethanol, thyrxine and fructose on the intracellular redox state of a perfused liver as studied by surface fluorometry. Ann Med Exp Biol Fenn. 1970;48(3):176–183. [PubMed] [Google Scholar]
- Hedlung S. G., Kiessling K. H. The physiological mechanism involved in hangover. 1. The oxidation of some lower aliphatic fusel alcohols and aldehydes in rat liver and their effect on the mitochondrial oxidation of various substrates. Acta Pharmacol Toxicol (Copenh) 1969;27(5):381–396. doi: 10.1111/j.1600-0773.1969.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Kraemer R. J., Deitrich R. A. Isolation and characterization of human liver aldehyde dehydrogenase. J Biol Chem. 1968 Dec 25;243(24):6402–6408. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUNDQUIST F., FUGMANN U., KLANING E., RASMUSSEN H. The metabolism of acetaldehyde in mammalian tissues: reactions in rat-liver suspensions under anaerobic conditions. Biochem J. 1959 Jul;72:409–419. doi: 10.1042/bj0720409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDQUIST F., FUGMANN U., RASMUSSEN H., SVENDSEN I. The metabolism of acetaldehyde in mammalian tissues. Reactions in rat-liver suspensions under aerobic conditions. Biochem J. 1962 Aug;84:281–286. doi: 10.1042/bj0840281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindros K. O., Vihma R., Forsander O. A. Utilization and metabolic effects of acetaldehyde and ethanol in the perfused rat liver. Biochem J. 1972 Feb;126(4):945–952. doi: 10.1042/bj1260945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjanen L. Intracellular localization of aldehyde dehydrogenase in rat liver. Biochem J. 1972 May;127(4):633–639. doi: 10.1042/bj1270633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte H. R. The intracellular and intramitochondrial distribution of malonyl-CoA decarboxylase and propionyl-CoA carboxylase in rat liver. Biochim Biophys Acta. 1969 Mar 18;178(1):137–144. doi: 10.1016/0005-2744(69)90140-5. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R., Albersheim P., Mcclearn G. Aldehyde dehydrogenase and ethanol preference in mice. J Biol Chem. 1970 Jun 10;245(11):2876–2882. [PubMed] [Google Scholar]
- Shum G. T., Blair A. H. Aldehyde dehydrogenases in rat liver. Can J Biochem. 1972 Jul;50(7):741–748. doi: 10.1139/o72-103. [DOI] [PubMed] [Google Scholar]
- Smith L., Packer L. Aldehyde oxidation in rat liver mitochondria. Arch Biochem Biophys. 1972 Jan;148(1):270–276. doi: 10.1016/0003-9861(72)90141-5. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- TIETZ A., LINDBERG M., KENNEDY E. P. A NEW PTERIDINE-REQUIRING ENZYME SYSTEM FOR THE OXIDATION OF GLYCERYL ETHERS. J Biol Chem. 1964 Dec;239:4081–4090. [PubMed] [Google Scholar]
- WALKENSTEIN S. S., WEINHOUSE S. Oxidation of aldehydes by mitochondria of rat tissues. J Biol Chem. 1953 Feb;200(2):515–523. [PubMed] [Google Scholar]
- Werner S., Neupert W. Functional and biogenetical heterogeneity of the inner membrane of rat-liver mitochondria. Eur J Biochem. 1972 Feb 15;25(2):379–396. doi: 10.1111/j.1432-1033.1972.tb01707.x. [DOI] [PubMed] [Google Scholar]
- van Tol A., Hülsmann W. C. The localization of plmitoyl-CoA: carnitine palmitoyl-transferase in rat liver. Biochim Biophys Acta. 1969;189(3):342–353. doi: 10.1016/0005-2728(69)90165-0. [DOI] [PubMed] [Google Scholar]


