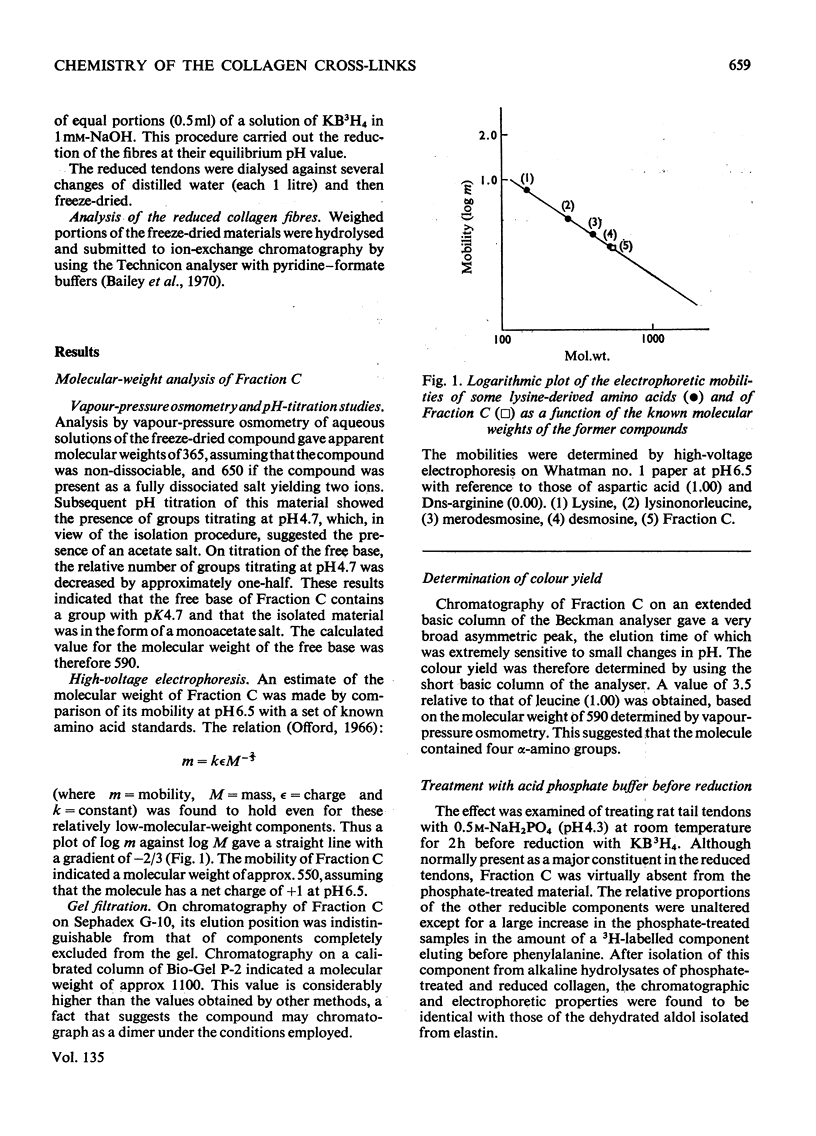
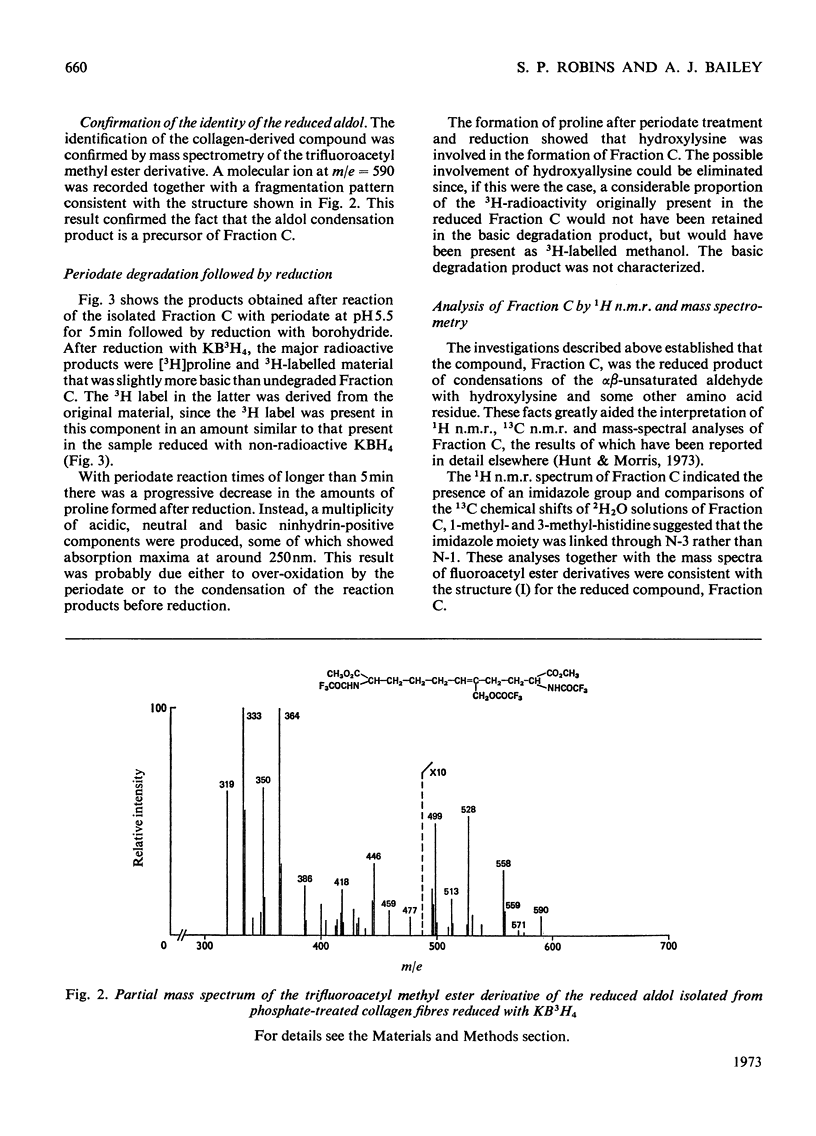
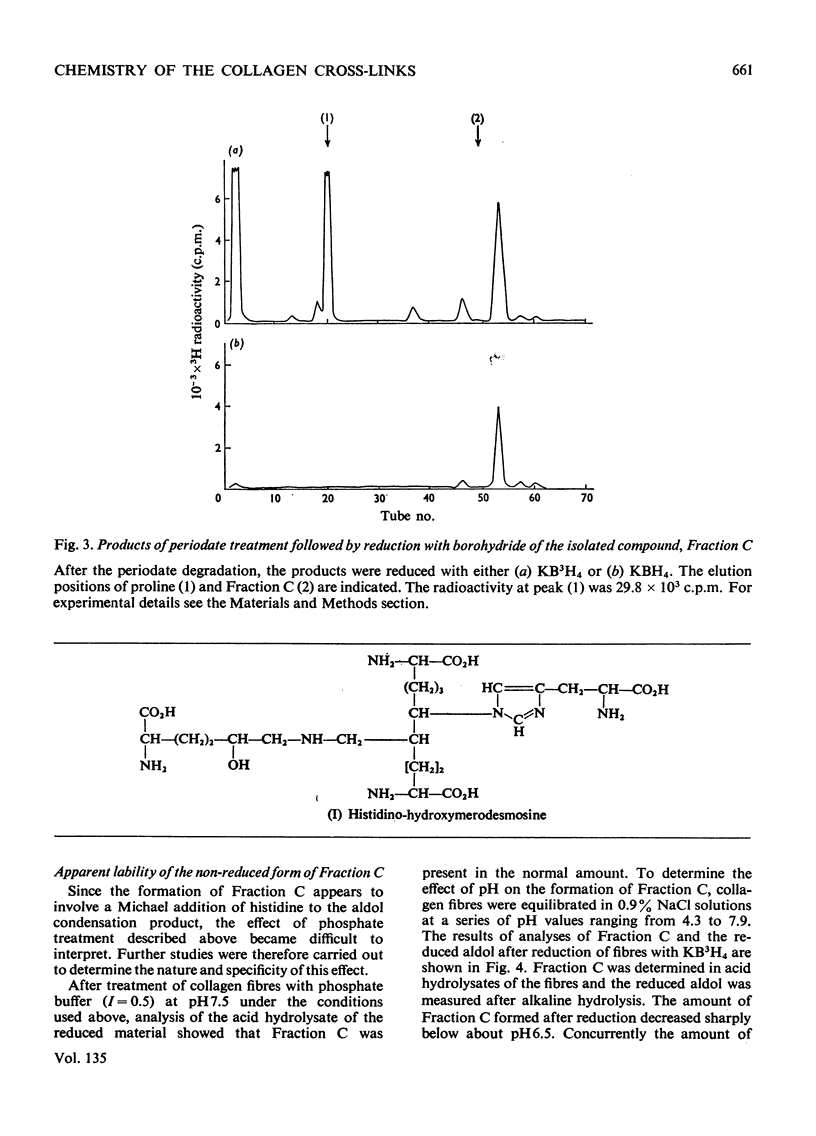
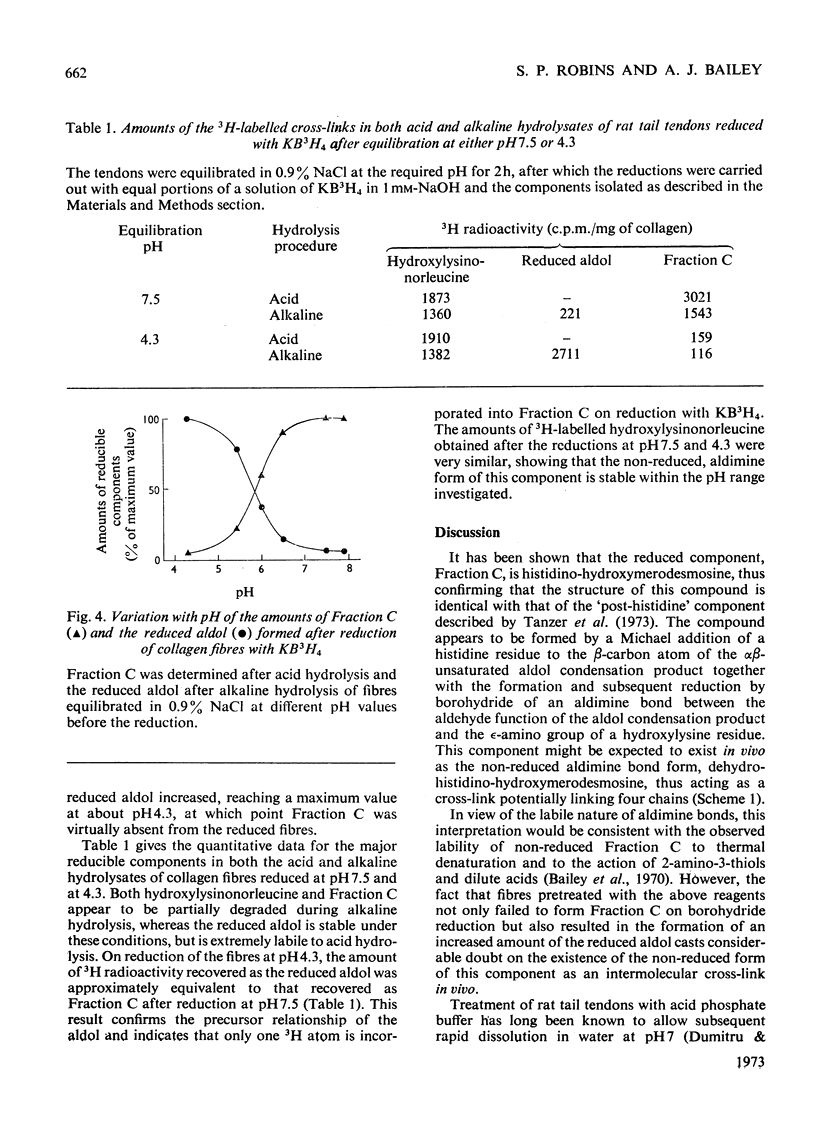
Abstract
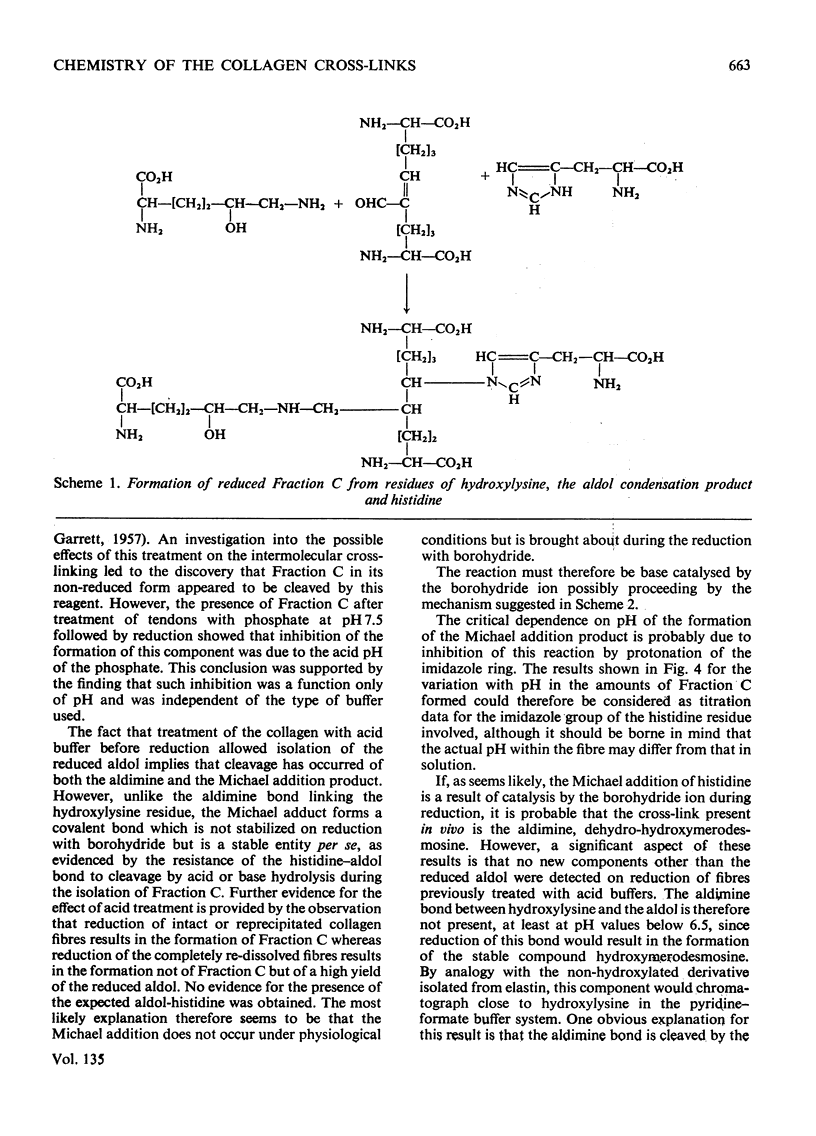
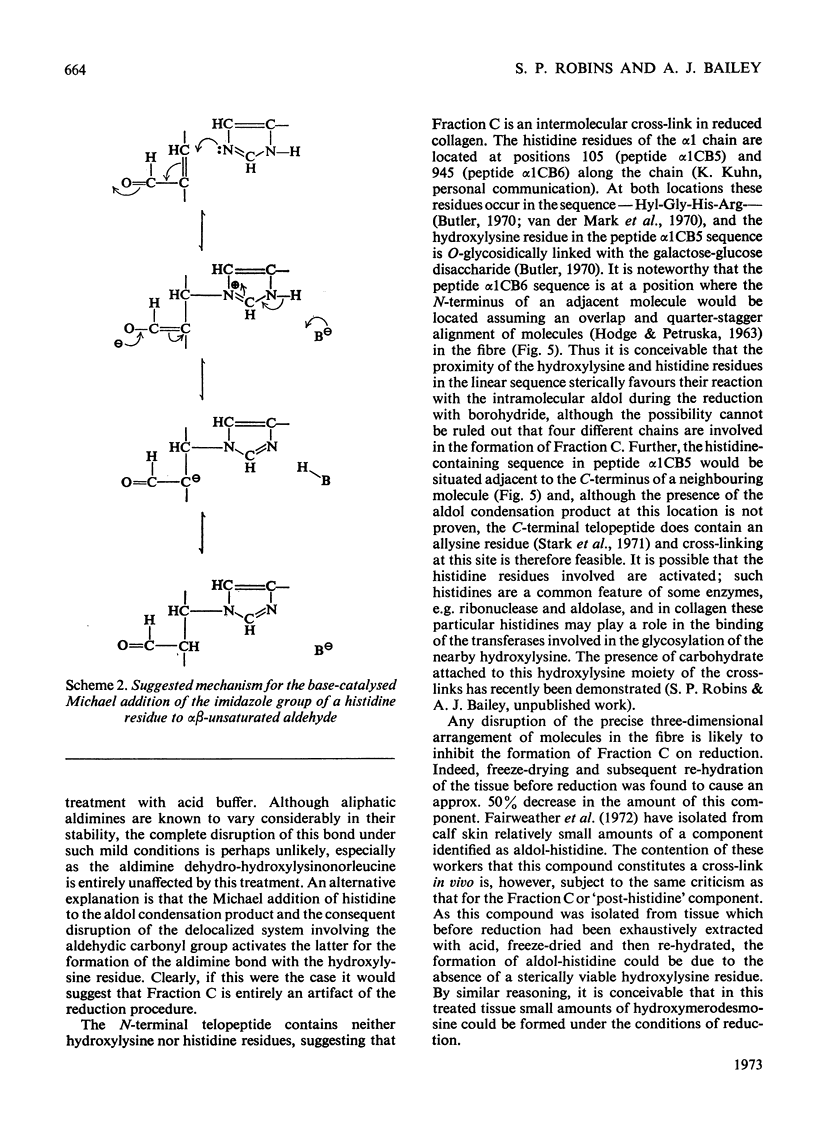
The present paper describes the isolation and identification of a major radioactive component of borotritide-reduced collagen, previously designated Fraction C. The derived structure for the compound confirms that it is identical with the `post-histidine' component described by Tanzer et al. (1973) and given the trivial name histidino-hydroxymerodesmosine. Detailed studies of the effects of acid pH on the formation of Fraction C after borohydride reduction demonstrated the apparent lability of the non-reduced form, thus confirming our previous findings (Bailey & Lister, 1968). Inhibition of the formation of this component by the acid treatment appears to be due to protonation of the histidine imidazole group. Since the only new component formed on reduction of the acid-treated fibres was the reduced aldol condensation product, these results indicate that neither the histidine nor the hydroxylysine residues can be involved in covalent linkage with the aldol condensation product in the native fibre. It is suggested therefore that the proposed non-reduced aldimine form of Fraction C does not exist as an intermolecular cross-link in vivo. Thus the presence of histidino-hydroxymerodesmosine as a tetrafunctional cross-link in reduced collagen fibres is a result of a base-catalysed reaction promoted by the borohydride-reduction procedure and this component must therefore be considered as an artifact.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J. Intermediate labile intermolecular crosslinks in collagen fibres. Biochim Biophys Acta. 1968 Aug 13;160(3):447–453. doi: 10.1016/0005-2795(68)90216-x. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Lister D. Thermally labile cross-links in native collagen. Nature. 1968 Oct 19;220(5164):280–281. doi: 10.1038/220280a0. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Peach C. M., Fowler L. J. Chemistry of the collagen cross-links. Isolation and characterization of two intermediate intermolecular cross-links in collagen. Biochem J. 1970 May;117(5):819–831. doi: 10.1042/bj1170819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A. J., Peach C. M. Isolation and structural identification of a labile intermolecular crosslink in collagen. Biochem Biophys Res Commun. 1968 Dec 9;33(5):812–819. doi: 10.1016/0006-291x(68)90233-7. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Shimokomaki M. S. Age related changes in the reducible cross-links of collagen. FEBS Lett. 1971 Aug 1;16(2):86–88. doi: 10.1016/0014-5793(71)80338-1. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Piez K. A. The nature of the intramolecular cross-links in collagen. The separation and characterization of peptides from the cross-link region of rat skin collagen. Biochemistry. 1966 Nov;5(11):3460–3473. doi: 10.1021/bi00875a012. [DOI] [PubMed] [Google Scholar]
- Butler W. T. Chemical studies on the cyanogen bromide peptides of rat skin collagen. The covalent structure of alpha 1-CB5, the major hexose-containing cyanogen bromide peptide of alpha 1. Biochemistry. 1970 Jan 6;9(1):44–50. doi: 10.1021/bi00803a006. [DOI] [PubMed] [Google Scholar]
- DUMITRU E. T., GARRETT R. R. Solubilization of rat tail tendon collagen. Arch Biochem Biophys. 1957 Jan;66(1):245–247. doi: 10.1016/0003-9861(57)90557-x. [DOI] [PubMed] [Google Scholar]
- Deshmukh A., Deshmukh K., Nimni M. E. Synthesis of aldehydes and their interactions during the in vitro aging of collagen. Biochemistry. 1971 Jun 8;10(12):2337–2342. doi: 10.1021/bi00788a025. [DOI] [PubMed] [Google Scholar]
- Fairweather R. B., Tanzer M. L., Gallop P. M. Aldol-histidine, a new trifunctional collagen crosslink. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1311–1315. doi: 10.1016/0006-291x(72)90854-6. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Faris B., Franzblau C. The in vitro formation of intermolecular cross-links in chick skin collagen. Biochem Biophys Res Commun. 1970 Apr 8;39(1):175–182. doi: 10.1016/0006-291x(70)90774-6. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Shimokomaki M., Bailey A. J. The chemistry of the collagen cross-links. Age-related changes in the reducible components of intact bovine collagen fibres. Biochem J. 1973 Apr;131(4):771–780. doi: 10.1042/bj1310771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M., Rauterberg J., Kühn K. Evidence for a non-helical region at the carboxyl terminus of the collagen molecule. FEBS Lett. 1971 Feb 19;13(2):101–104. doi: 10.1016/0014-5793(71)80209-0. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L., Housley T., Berube L., Fairweather R., Franzblau C., Gallop P. M. Structure of two histidine-containing crosslinks from collagen. J Biol Chem. 1973 Jan 25;248(2):393–402. [PubMed] [Google Scholar]
- von der Mark Klaus, Wendt Peter, Rexrodt Friedrich, Kühn Klaus. Direct evidence for a correlation between amino acid sequence and cross striation pattern of collagen. FEBS Lett. 1970 Nov 18;11(2):105–108. doi: 10.1016/0014-5793(70)80503-8. [DOI] [PubMed] [Google Scholar]


