Abstract
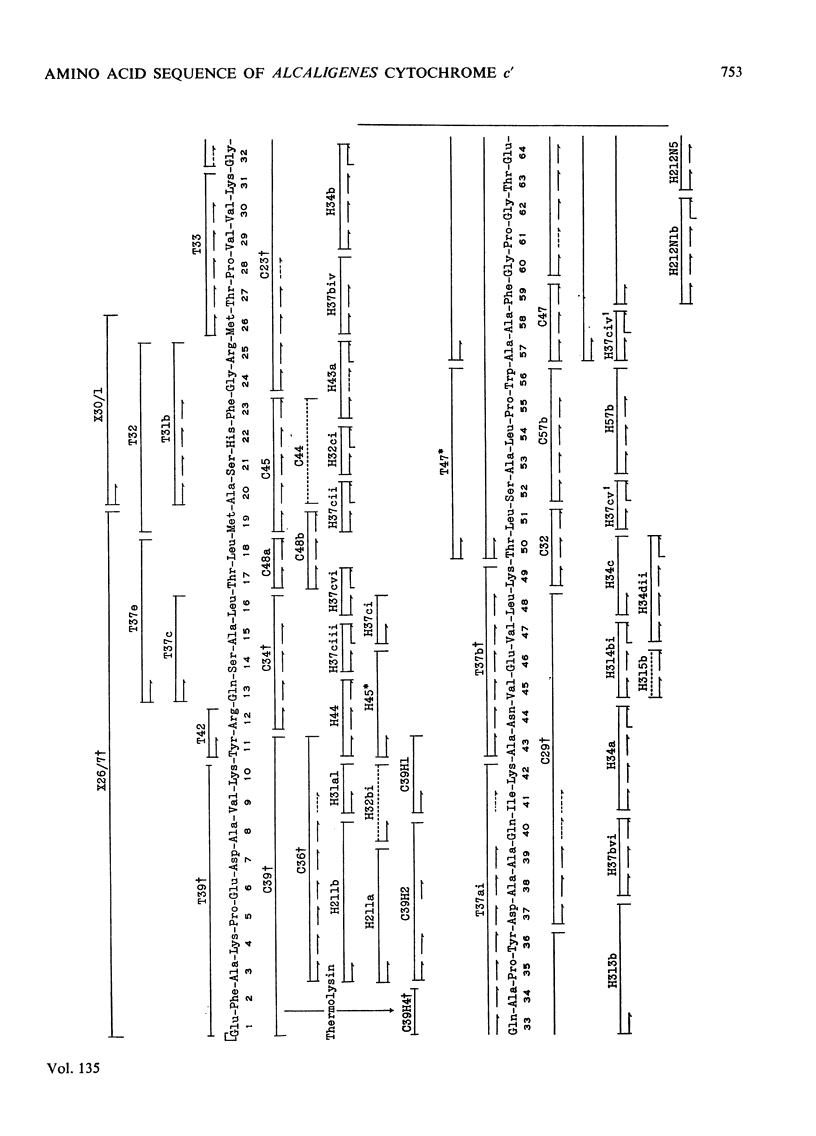
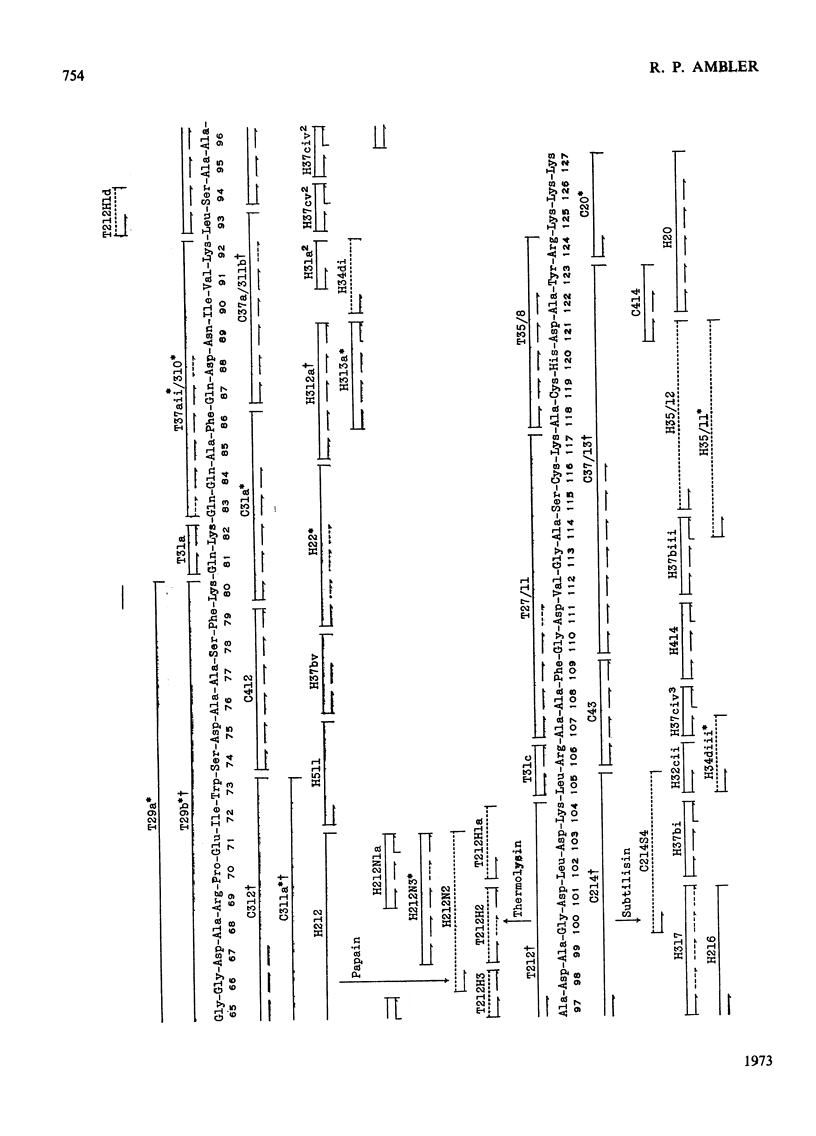
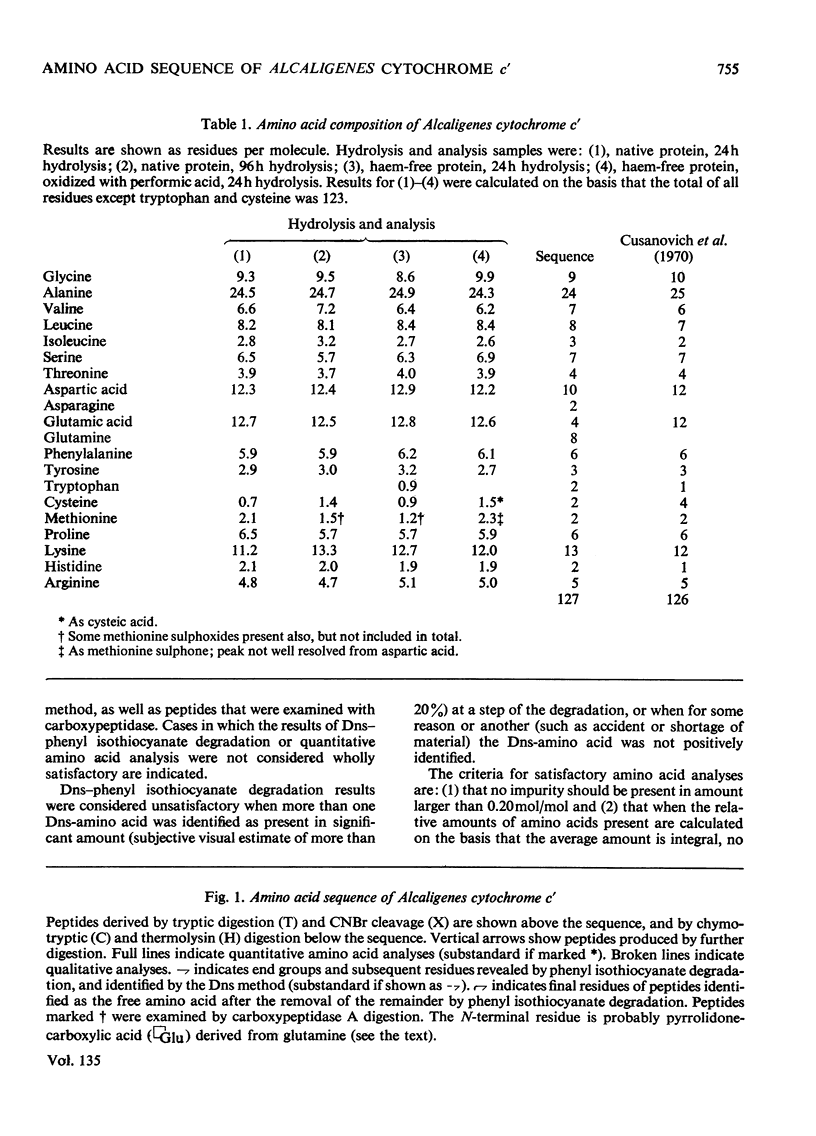
The amino acid sequence of the cytochrome c′ from Alcaligenes sp. N.C.I.B. 11015 (Iwasaki's `Pseudomonas denitrificans') has been determined. This organism is the only non-photosynthetic bacterium in which the protein has been found. The protein consists of a single polypeptide chain of 127 residues, with a single haem covalently attached to two cysteines. Unlike normal cytochromes c, the haem attachment site is very close to the C-terminus. The amino acid sequence around the haem attachment site is very similar to that of Chromatium vinosum D cytochrome c′. Detailed evidence for the amino acid sequence of the protein has been deposited as Supplementary Publication SUP 50022 at the British Library (Lending Division), (formerly the National Lending Library for Science and Technology), Boston Spa, Yorks. LS23 7BQ, U.K., from whom copies may be obtained on the terms given in Biochem. J. (1973) 131, 5.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMBLER R. P. THE PURIFICATION AND AMINO ACID COMPOSITION OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:341–349. doi: 10.1042/bj0890341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Bruschi M., Le Gall J. The amino acid sequence of cytochrome c(3) from Desulfovibrio desulfuricans (strain el agheila Z, NCIB 8380). FEBS Lett. 1971 Nov 1;18(2):347–350. doi: 10.1016/0014-5793(71)80483-0. [DOI] [PubMed] [Google Scholar]
- Ambler R. P., Meadway R. J. Chemical structure of bacterial penicillinases. Nature. 1969 Apr 5;222(5188):24–26. doi: 10.1038/222024a0. [DOI] [PubMed] [Google Scholar]
- Ambler R. P. The amino acid sequence of cytochrome c-551.5 (Cytochrome c(7)) from the green photosynthetic bacterium Chloropseudomonas ethylica. FEBS Lett. 1971 Nov 1;18(2):351–353. doi: 10.1016/0014-5793(71)80484-2. [DOI] [PubMed] [Google Scholar]
- Ambler R. P., Wynn M. The amino acid sequences of cytochromes c-551 from three species of Pseudomonas. Biochem J. 1973 Mar;131(3):485–498. doi: 10.1042/bj1310485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTSCH R. G., KAMEN M. D. On the new heme protein of facultative photoheterotrophs. J Biol Chem. 1958 Jan;230(1):41–63. [PubMed] [Google Scholar]
- Cusanovich M. A., Tedro S. M., Kamen M. D. Pseudomonas denitrificans cytochrome cc'. Arch Biochem Biophys. 1970 Dec;141(2):557–570. doi: 10.1016/0003-9861(70)90175-x. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Gray B. H., Fowler C. F., Nugent N. A., Fuller R. C. A reevaluation of the presence of low midpoint potential cytochrome 551.5 in the green photosynthetic bacterium Chloropseudomonas ethylica. Biochem Biophys Res Commun. 1972 Apr 28;47(2):322–327. doi: 10.1016/0006-291x(72)90715-2. [DOI] [PubMed] [Google Scholar]
- IWASAKI H., SHIDARA S., SUZUKI H., MOR T. Studies on denitrification. VII. Further purification and properties of denitrifying enzyme. J Biochem. 1963 Apr;53:299–303. [PubMed] [Google Scholar]
- Kennel S. J., Meyer T. E., Kamen M. D., Bartsch R. G. On the monohene character of cytochromes c'. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3432–3435. doi: 10.1073/pnas.69.11.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND I. W., WILKINSON J. F. Azurin: a copper protein found in Bordetella. J Gen Microbiol. 1963 Jan;30:105–112. doi: 10.1099/00221287-30-1-105. [DOI] [PubMed] [Google Scholar]
- SUZUKI H., IWASAKI H. Studies on denitrification. VI. Preparations and properties of crystalline blue protein and cryptocytochrome c, and role of copper in denitrifying enzyme from a denitrifying bacterium. J Biochem. 1962 Sep;52:193–199. doi: 10.1093/oxfordjournals.jbchem.a127596. [DOI] [PubMed] [Google Scholar]
- Strahs G. Azurin: x-ray data for crystals from Pseudomonas denitrificans. Science. 1968 Jul 4;165(3888):60–61. [PubMed] [Google Scholar]
- VERNON L. P., KAMEN M. D. Hematin compounds in photosynthetic bacteria. J Biol Chem. 1954 Dec;211(2):643–662. [PubMed] [Google Scholar]
- van Beeumen J. J., Ambler R. P. Netherlands Society for Microbiology meeting at Delft on 25 October 1972. Homologies in the amino acid sequences of cytochrome c-555 from the green photosynthetic bacteria "Chloropseudomonas ethylica" and Chlorobium thiosulfatophilium. Antonie Van Leeuwenhoek. 1973;39(2):355–356. doi: 10.1007/BF02578868. [DOI] [PubMed] [Google Scholar]


