ABSTRACT
Background
Acute lower respiratory tract infections (ALRIs) remain the leading infectious cause of death among children < 5 years, with viruses contributing to a large proportion of cases. Little is known about the epidemiology and etiology of viral ALRI in rural Bangladesh.
Methods
We enrolled 3‐ to 23‐month‐old children with ALRIs attending a subdistrict hospital outpatient clinic in Sylhet district in Bangladesh. Trained study physicians ascertained the cases and obtained nasopharyngeal swabs to detect 19 respiratory viruses by multiplex PCR using the Luminex Integrated System NxTAG Respiratory pathogen panel.
Results
Between August 2016 and September 2017, we enrolled 1477 children. Median age was 10 months; 58.1% were male. Forty‐seven percent presented during autumn (mid‐June to mid‐October). About a third had temperature ≥ 101°F, 95.4% had cough in the previous 3 days, 72.0% had fast breathing, and 80.0% had chest indrawing. Alveolar consolidation occurred in 23.9%, and 4.4% were hypoxemic (saturation < 90% on room air). Nineteen percent required hospitalization; 79.1% of them were discharged within 48 h. A respiratory virus was identified in 81.8%, majority (75.8%) with single virus isolation. Rhinoenterovirus was most commonly identified (HRV/HEV, 37.9%), followed by respiratory syncytial virus (RSV, 20.2%) and human metapneumovirus (hMPV, 11.7%). Rhinoenterovirus was detected year‐round; RSV was detected during August–November and hMPV during December–March.
Conclusions
Respiratory viruses were identified in a majority (82%) of children under 2 years of age presenting with ALRI in rural hospitals of Bangladesh. These findings have implications for future study and potentially for surveillance, antimicrobial stewardship, vaccine program planning, and policy.
Keywords: ALRI, Bangladesh, child, infant, viral
1. Background
Acute lower respiratory infections (ALRIs) are a leading cause of morbidity and mortality in children < 5 years of age globally. [1]. ALRIs are responsible for almost 20% of all deaths of children aged less than 5 years worldwide [2] and are responsible for up to 30% of pediatric admissions in low‐ and middle‐income countries (LMICs) [3].
Despite a 65% decline in under‐5 mortality between 1990 and 2015 [4], Bangladesh is still one of five countries in the world accounting for > 50% of global ALRI cases in children < 5 years. It has one of the highest population densities in the world (~1300 people/km2) [5], a risk factor for ALRI transmission, and prevalence of childhood malnutrition, a risk factor for mortality [6]. Population‐based studies in rural Bangladesh by our group previously found an ALRI incidence of 230 per 1000 child‐years in children < 5 years [7] and documented ALRI‐related hospital admission rates of 50.2–53.6 and 101.1–145 per 1000 child‐years for those < 5 and < 1 year of age, respectively [7, 8]. Another study showed that ALRIs caused 39% of pediatric hospitalizations and 40%–60% of total pediatric outpatient department visits in Bangladesh [9].
Evidence‐based public health approaches to prevent ALRI require rigorous studies to define the epidemiology and etiology of the disease. Multiple viruses, including influenza virus, respiratory syncytial virus (RSV), human metapneumovirus (hMPV), parainfluenza virus (PIV), human rhinoenterovirus (HRV/HEV), and human coronavirus (HCoV), have been implicated as etiologies of ALRI in children. The seminal PERCH study implicated multiple viruses as major causes of ALRI globally, including urban Bangladeshi children < 5 years, and found that respiratory viruses have heterogeneous circulation patterns [10]. However, data from rural settings are lacking. In this study, we sought to delineate the viral etiology and epidemiological and clinical characteristics of viral ALRI in children < 2 years of age in the rural Sylhet region of Bangladesh.
2. Methods
2.1. Study Site
Between 2014 and 2018, the Projahnmo Study group, a partnership of Johns Hopkins University (USA) with the Ministry of Health and Family Welfare (MoHFW) of the Government of Bangladesh, and Bangladeshi nongovernmental organizations conducted an assessment of national introduction of pneumococcal conjugate vaccine in Bangladesh. The study area, population, and surveillance methods were previously described [11]. In short, the study was conducted in three subdistricts (Zakiganj, Kanaighat, and Beanibazar) of Sylhet district, which have a population of ~770,000 yielding an annual birth cohort of ~20,000. Projahnmo has extensive research infrastructure, including Global Positioning System mapping, a complete census and background characteristics of the entire population, mechanisms for community‐based sampling, case identification, referral, specimen collection, transport, state‐of‐the‐art laboratories, and a data center. It has established surveillance in three subdistrict hospitals, which are staffed by trained study physicians. We used these hospitals for case detection, specimen collection, and transport. The current study was nested within the parent study described above.
2.2. Study Population
As part of the parent study [11, 12, 13], we established community surveillance for ALRI and ALRI. The study population for this study was children aged 3–23 months. Trained community health workers (CHWs) visited all households once every 2 months to teach mothers/caregivers how to recognize signs and symptoms of ALRI and asked them to take their sick children to one of the study‐designated health facilities. They also collected data on reported signs/symptoms of ALRI and care seeking.
2.3. Surveillance, Case Definition, and Study Procedures
Because respiratory viral pathogens predominate in children < 2 years of age, we screened 3–23 months old children attending the study hospitals for enrollment. Study physicians at the subdistrict hospitals were trained to identify children with clinical signs and symptoms of ALRI.
ALRI was defined as lower chest indrawing or cough or difficulty breathing and either fast breathing (respiratory rate ≥ 50 breaths/min for infants aged 3–11 months or ≥ 40 breaths/min for 12‐ to 23‐month‐olds) or another clinical sign of severe illness (persistent nasal flaring, cyanosis, head nodding or tracheal tugging, grunting, stridor while calm, hypoxemia, decision to hospitalize/refer, or WHO danger sign) [11, 14].
Children were excluded if they were previously enrolled within 30 days to avoid misclassification of a prolonged episode as a new episode. After obtaining written informed consent, demographic and clinical data, along with a nasopharyngeal swab, were collected by study physicians after consent procedures. In addition, chest radiography was performed using an analog unit (POLYMOBIL® Plus, Siemens, Erlangen, Germany) and CR Fuji Film cassette reader to digitize images. Weight was measured using standardized Tanita scales, and height or infant length (as appropriate) were measured using locally available scales by clinical assistants at the study hospitals. Outcome data were collected for children admitted to hospital.
2.4. Respiratory Pathogen Molecular Testing
Nasopharyngeal specimens collected with neonatal flocked swabs (FLOQSwabs(R)) were placed into universal transport medium tubes (UTM(R)) (Copan Italia SpA, Brescia, Italy) and stored at −80°C within 2 h of receipt. Specimens were shipped to Duke University, Durham, NC, USA, on dry ice where real‐time reverse transcription–polymerase chain reaction (PCR) with the Luminex Integrated System NxTAG Respiratory Pathogen Panel platform was performed. The Luminex platform detects 19 respiratory viruses (RSV A and B; nonspecific influenza A; influenza A subtypes H1, H3, and 2009 H1N1; influenza B; parainfluenza 1–4; human metapneumovirus (hMPV); adenovirus; human rhinoenterovirus (HRV/HEV); coronavirus types NL63, HKU1, 229E, and OC43; and human bocavirus) and three bacteria ( Chlamydophila pneumoniae , Legionella pneumophila , and Mycoplasma pneumoniae ) [15].
2.5. Statistical Analyses
Data related to baseline demographics and clinical presentation were analyzed using descriptive statistics, expressed as frequencies and proportions. Descriptive statistics were also used to describe the distribution of viruses identified and the seasonality, and the clinical characteristics of various viral infections. For seasonality, we used the classification used by Stevens et al. [16], to define summer as the period between April 15 and June 14; monsoon, June 15 and August 14; autumn, August 15 and October 14; late autumn, October 15 and December 14; winter, December 15 and February 14; and spring, February 15 and April 14. Per the WHO, we defined overcrowding as more than three persons per habitable room [17]. Chest radiographs were obtained and interpretated by a reading panel of eight Bangladeshi radiologists and pediatricians trained and calibrated to interpret images according to WHO chest radiograph methods for vaccine studies [18]. Stunting, wasting, and underweight were defined as length‐for‐age, weight‐for‐height, and weight‐for‐age z scores below −2 standard deviations (SD) from the median of the WHO child growth standards, respectively [19].
2.6. Ethics
The National Research Ethics Committee of Bangladesh Medical Research Council and the institutional review boards of the Johns Hopkins and Duke Schools of Medicine reviewed and approved the study's protocol.
3. Results
3.1. Baseline Demographics
Between August 2016 and September 2017, 2066 hospital outpatient visits were recorded for ALRI in children aged 3–23 months from the study area. Of these, 1477 (71.5%) had a nasopharyngeal specimen tested for respiratory viruses (Figure 1). Baseline demographics and clinical features of ALRI in children tested for respiratory viruses are shown in Tables 1 and 2. The median age of children enrolled was 10 months, and 58.9% (871/1477) were male. Most of the children presented with ALRI during the autumn season (mid‐June to mid‐October). Overcrowding was noted in three‐fourths of the households, and 71.7% (1060/1477) of these children were exposed to cigarette smoking (passively). A third of the children whose swabs were tested for respiratory viruses were either stunted, wasted, or underweight. The immunization coverage was high, with 93.7% (1383/1477) of the children having received at least one dose of the DPT/Pentavalent vaccine (Table 1).
FIGURE 1.
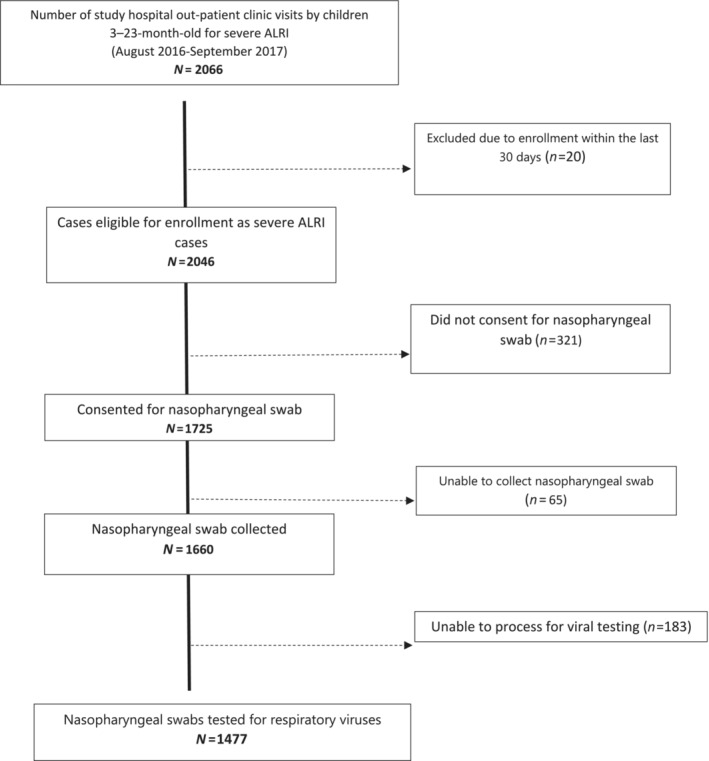
Study profile of children 3–23 months enrolled with ALRI tested for respiratory viruses, rural Bangladesh, 2016–2017.
TABLE 1.
Characteristics of children enrolled with ALRI and tested for respiratory viruses.
| Characteristics a | Total (n = 1477) |
|---|---|
| Age in months (median, IQR) | 10 (6–15) |
| Age in months (categorical) | |
| 3–5 months | 329 (22.2) |
| 6–11 months | 572 (38.7) |
| 12–17 months | 335 (22.6) |
| 18–23 months | 241 (16.3) |
| Gender, male | 871 (58.9) |
| Season of presentation b | |
| Summer | 80 (5.4) |
| Monsoon | 302 (20.4) |
| Autumn | 493 (33.3) |
| Late autumn | 238 (14.1) |
| Winter | 308 (18.3) |
| Spring | 56 (3.7) |
| Crowding status | |
| ≤ 3 people/room | 835 (56.5) |
| > 3 people/room | 642 (43.5) |
| Household wealth tertiles | |
| Upper | 468 (31.7) |
| Middle | 447 (32.3) |
| Lower | 472 (34.0) |
| Maternal education | |
| No education | 205 (13.8) |
| Primary (1–5) | 582 (39.4) |
| Secondary (6–10) | 641 (43.4) |
| Higher secondary and above (11+) | 49 (3.3) |
| Paternal education | |
| No education | 421 (28.5) |
| Primary (1–5) | 619 (41.9) |
| Secondary (6–10) | 350 (23.7) |
| Higher secondary and above (11+) | 87 (5.9) |
| Nutritional status of the child | |
| Underweight | 511 (34.6) |
| Stunted | 502 (33.9) |
| Wasted | 521 (35.2) |
| Exposure to smoking c | 1060 (71.7) |
| PCV vaccine status a | |
| No dose | 111 (7.5) |
| 1 dose | 140 (9.4) |
| 2 doses | 318 (21.5) |
| 3 doses | 908 (61.4) |
| DPT3/Pentavalent3 vaccine status a | |
| No dose | 94 (6.3) |
| 1 dose | 141 (9.5) |
| 2 doses | 177 (11.9) |
| 3 doses | 1065 (72.1) |
Percentages are all column percentages, unless indicated otherwise. Percentages are rounded off to the first decimal point.
Summer = April 15–June 14; monsoon = June 15–August 14; autumn = August 15–October 14; late autumn = October 15–December 14; winter = December 15–February 14; spring = February 15–April 14 (15).
Refers to passive cigarette smoke exposure.
TABLE 2.
Clinical features of children presenting with ALRI.
| Characteristics a | Total n = 1477 | 3–5 months n = 329 | 6–11 months n = 572 | 12–17 months n = 335 | 18–23 months n = 241 |
|---|---|---|---|---|---|
| Reported symptoms (last 3 days) | |||||
| Cough | 1410 (95.4) | 316 (96.0) | 550 (96.1) | 317 (94.6) | 227 (94.1) |
| Difficulty breathing | 970 (65.6) | 227 (68.9) | 379 (66.2) | 216 (64.4) | 148 (61.4) |
| Runny nose | 595 (40.2) | 130 (39.5) | 225 (39.3) | 141 (42.0) | 99 (41.0) |
| Reported fever | 1107 (74.9) | 225 (68.3) | 441 (77.0) | 265 (79.1) | 176 (73.0) |
| Physical examination | |||||
| Temperature | |||||
| ≥ 101°F | 566 (38.3) | 91 (27.6) | 224 (39.1) | 152 (45.3) | 99 (41.0) |
| 99.5°F–100.9°F | 216 (14.6) | 42 (1.2) | 89 (15.5) | 49 (14.6) | 36 (14.9) |
| 98.6°F–99.4°F | 294 (19.9) | 77 (23.4) | 111 (19.4) | 63 (18.8) | 43 (17.8) |
| Afebrile | 401 (27.1) | 119 (36.1) | 148 (25.8) | 71 (21.1) | 63 (26.1) |
| Observed cough | 438 (29.6) | 107 (32.5) | 160 (27.9) | 102 (30.4) | 69 (28.6) |
| Fast breathing | 1064 (72.0) | 242 (73.5) | 367 (64.1) | 257 (76.7) | 198 (82.1) |
| Chest in‐drawing | 1183 (80.0) | 279 (84.8) | 454 (79.3) | 263 (78.5) | 187 (77.5) |
| Nasal flaring | 129 (8.7) | 25 (7.5) | 52 (9.0) | 26 (7.7) | 26 (10.7) |
| Grunting | 6 (0.0) | 1 (0.0) | 2 (0.0) | 2 (0.0) | 1 (0.0) |
| Head nodding or tracheal tugging | 171 (11.5) | 40 (12.1) | 73 (12.7) | 32 (9.5) | 26 (10.7) |
| Oxygen saturation | |||||
| 94–100 | 1154 (78.1) | 260 (79.0) | 437 (76.3) | 261 (77.9) | 196 (8.1) |
| 90–93 | 110 (7.4) | 30 (9.1) | 49 (8.5) | 16 (4.7) | 15 (6.2) |
| < 90 | 66 (4.4) | 22 (6.6) | 23 (4.0) | 15 (4.4) | 6 (2.5) |
| Missing | 147 (9.9) | 17 (5.1) | 63 (11.0) | 43 (12.8) | 24 (9.9) |
| Lung auscultation | |||||
| Crepitations | 1014 (68.6) | 228 (69.3) | 405 (70.8) | 223 (66.5) | 158 (65.5) |
| Wheeze on auscultation | 426 (28.8) | 102 (31.0) | 165 (28.8) | 84 (25.0) | 75 (31.1) |
| General danger sign | |||||
| Convulsions | 42 (2.8) | 1 (0.0) | 11 (1.9) | 19 (5.6) | 11 (4.5) |
| Lethargy | 4 (0.0) | 0 (0.0) | 1 (0.0) | 3 (0.0) | 0 (0.0) |
| Unable to eat or drink | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vomits everything | 1 (0.0) | 1 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Chest radiography | |||||
| Alveolar consolidation only | 53 (3.5) | 13 (3.9) | 20 (3.4) | 11 (3.2) | 9 (3.7) |
| Other infiltrate only | 249 (16.8) | 68 (20.6) | 95 (16.6) | 57 (17.0) | 29 (12.0) |
| Alveolar consolidation and other infiltrate | 302 (20.4) | 81 (24.6) | 115 (20.1) | 68 (20.2) | 38 (15.7) |
| Admitted to hospital | 292 (19.7) | 84 (25.5) | 122 (21.3) | 60 (17.9) | 26 (10.7) |
| Duration of hospitalization b | |||||
| < 1 day | 24 (8.2) | 6 (7.1) | 10 (8.1) | 5 (8.3) | 3 (11.5) |
| 1–2 days | 207 (70.8) | 56 (66.6) | 89 (72.9) | 41 (68.3) | 21 (80.7) |
| 3–4 days | 51 (17.5) | 18 (21.4) | 18 (14.7) | 13 (21.6) | 2 (7.6) |
| ≥ 5 days | 10 (3.5) | 4 (4.7) | 5 (4.0) | 1 (1.6) | 0 (0.0) |
| Outcome of hospitalization b | |||||
| Cured and discharged | 284 (97.2) | 82 (97.6) | 118 (20.6) | 60 (96.7) | 24 (92.3) |
| Referred | 4 (1.4) | 1 (1.2) | 3 (2.4) | 0 (0.0) | 0 (0.0) |
| Left against medical advice | 3 (1.0) | 0 (0.0) | 1 (0.8) | 0 (0.0) | 2 (7.7) |
| Died | 1 (0.4) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Percentages are all column percentages, unless indicated otherwise. All percentages are rounded off to the first decimal point.
Among 292 children who required hospitalization.
3.2. Clinical Signs and Symptoms
Over one‐third (566/1477) of the children presented with temperature ≥ 101°F; older children were more often noted to have this finding. Ninety‐five percent (1410/1477) of the children's caregivers reported cough in the last 3 days, 29.6% (438/1477) had cough observed by the study clinicians, 72% (1064/1477) children had fast breathing, and 80% (1183/1477) had chest indrawing. Crepitations and wheezing were noted during lung auscultation in 68.6% (1014/1477) and 28.8% (426/1477) children, respectively. On chest radiography, alveolar consolidation with or without other infiltrates were noted in 23.9% (355/1477) of the children. Hypoxemia (SpO2 < 90%) was documented in 4.9% (66/1330) of the children with available oxygen saturation readings. Nineteen percent (292/1477) of the children required hospitalization, and the remaining cases were treated as outpatients. Of the hospitalized children, 79% (207/292) were discharged within 48 h of admission. One child died, and three children left against medical advice during hospitalization (Table 2).
3.3. Viral Etiology Identified, Seasonality, and Clinical Presentation Among Those With Specific Viruses
A respiratory virus was identified in 81.8% of children, with a majority (75.8%, 917/1209) having only one virus identified. Among those with more than one virus identified, most (85.2%, 249/292) were dual infections (Table 3).
TABLE 3.
Respiratory viruses identified among 1477 cases with ALRI.
| Virus | Total positive | Single infection a | Multiple infection a | Infection with 2 viruses b | Infection with ≥ 3 viruses b |
|---|---|---|---|---|---|
| Any c | 1209 (81.8) | 917 (75.8) | 292 (24.2) | 249 (85.2) | 43 (14.8) |
| HRV/HEV | 560 (37.9) | 362 (64.6) | 198 (35.4) | 165 (83.3) | 33 (16.7) |
| RSV A d | 111 (7.5) | 77 (69.3) | 34 (30.7) | 31 (91.1) | 3 (8.9) |
| RSV B d | 188 (12.7) | 141 (75) | 47 (25) | 41 (87.2) | 6 (12.8) |
| HMPV | 173 (11.7) | 113 (65.3) | 60 (34.7) | 44 (73.3) | 16 (26.7) |
| Influenza A e | 66 (4.4) | 51 (77.2) | 15 (22.8) | 9 (60) | 6 (40) |
| Influenza B e | 26 (1.7) | 16 (61.5) | 10 (38.5) | 8 (80) | 2 (20) |
| Adenovirus | 85 (5.7) | 28 (32.9) | 57 (67.1) | 44 (77.1) | 13 (22.9) |
| PIV 1 | 34 (2.3) | 19 (55.9) | 15 (44.1) | 10 (66.6) | 5 (33.4) |
| PIV 2 | 2 (0.1) | 1 (50) | 1 (50) | 1 (100) | 0 (0) |
| PIV 3 | 42 (2.8) | 24 (57.1) | 18 (42.9) | 15 (83.3) | 3 (16.7) |
| PIV 4 | 18 (1.2) | 14 (77.7) | 4 (22.3) | 3 (75) | 1 (25) |
| HCoV 229E | 10 (0.6) | 5 (50) | 5 (50) | 2 (40) | 3 (60) |
| HCoV NL63 | 17 (1.1) | 8 (47) | 9 (53) | 7 (77.7) | 2 (22.3) |
| HCoV OC43 | 24 (1.6) | 12 (50) | 12 (50) | 7 (58.3) | 5 (41.7) |
| HCoV HKU1 | 5 (0.3) | 1 (20) | 4 (80) | 3 (75) | 1 (25) |
| Bocavirus | 163 (11.0) | 37 (22.6) | 126 (77.4) | 99 (78.5) | 27 (21.5) |
| Chlamydophila pneumoniae | 19 (1.2) | 7 (36.8) | 12 (63.2) | 6 (50) | 6 (50) |
| Legionella pneumoniae | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Mycoplasma pneumoniae | 4 (0.2) | 1 (25) | 3 (75) | 3 (100) | 0 (0) |
Proportion in parenthesis indicates proportion of single/multiple infection cases of total positive (i.e., row percentage).
Proportion in parenthesis indicates proportion of those with infection with 2/≥ 3 viruses of those with multiple infection.
1209/1477 subjects enrolled in the study with ALRI were found to have fewer than one virus.
Taken together; 299/1477 (20.2%) subjects had RSV.
Taken together; 92/1477 (6.1%) subjects had influenza virus.
Overall, HRV/HEV was the most frequently identified virus (37.9%), followed by RSV (20.2%) and hMPV (11.7%). Among RSV, subtype B was more common than subtype A. The viruses most commonly involved in dual virus isolation were HRV/HEV (n = 165) and bocavirus (n = 99) (Table 3). Across all age group categories as shown in Figure 2, HRV/HEV was the most common virus detected, followed by RSV and hMPV.
FIGURE 2.
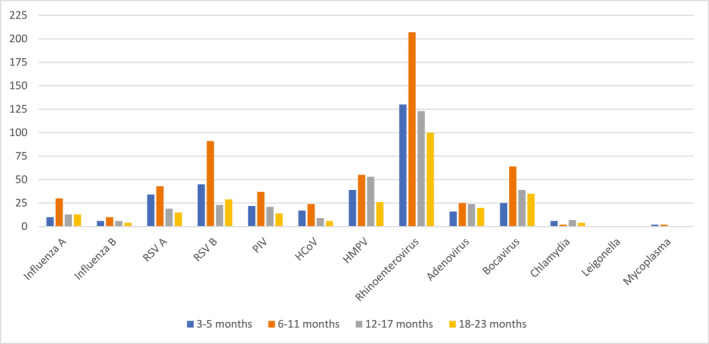
Distribution of respiratory viruses by age, rural Bangladesh 2016–2017. PIV includes PIV1, PIV2, PIV3, and PIV4; HCoV includes HCoV 229E, HCoV NL63, HCoV OC43, and HCoV HKU1.
The proportion of ALRI in which a respiratory virus was detected was highest during the autumn season (mid‐June to mid‐October). Specifically, influenza virus detection was recovered more frequently in those who presented during the months of June–August, RSV during August–November, and hMPV during December–March, following the RSV peak. For HRV/HEV, detection was year‐round, but HRV/HEV was identified more often in children enrolled during the months of November 2016 and August 2017 (Figure 3).
FIGURE 3.
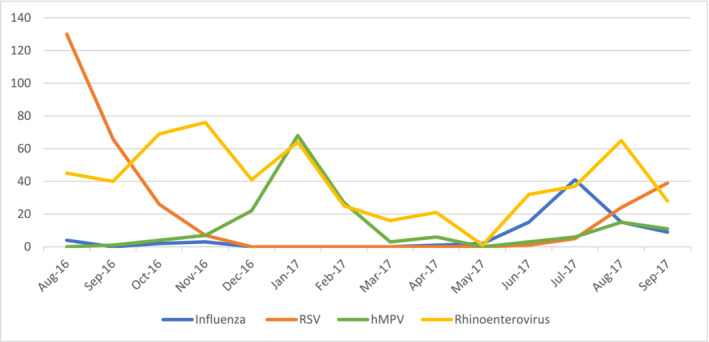
Proportion of children aged 3–23 months with viral ALRI by month, rural Bangladesh, 2016–2017.
Table 4 shows the clinical characteristics of children from whom specific viruses were recovered. History of fever was less common in those from whom HRV/HEV was recovered (Table 4); however, a large proportion of those with HRV/HEV presented with a history of cough (96.2%), age‐adjusted fast breathing (72.3%), chest indrawing (82.3%), and crepitations on lung auscultation (67.5%). Fever was most frequently noted among those infected with influenza virus (83.7%), and respiratory symptoms (such as fast breathing, chest indrawing and nasal flaring) were most common in those infected with RSV. Chest radiograph findings of alveolar consolidation with or without other infiltrates were greatest among children infected with hMPV (34.0%), followed by those infected with influenza virus. The need for in‐patient hospitalization was greatest among those infected with influenza virus (29.3%), followed by those infected with RSV (26.1%) (Table 4).
TABLE 4.
Clinical characteristics of children with specific respiratory viruses.
| Characteristics a | Influenza A + B (n = 92) b | RSV A + B (n = 299) b | PIV (n = 96) b | HMPV (n = 173) b | HRV/HEV (n = 560) b |
|---|---|---|---|---|---|
| Sex, male | 54 (58.6) | 164 (55.0) | 56 (58.3) | 91 (52.6) | 344 (61.4) |
| Median age in months (SD) | 10 (9.5) | 9 (8) | 10 (8) | 10 (9) | 10 (9) |
| Reported symptoms (last 3 days) | |||||
| History of fever | 81 (88.0) | 238 (79.8) | 76 (79.1) | 141 (81.5) | 371 (66.2) |
| History of cough | 86 (93.4) | 281 (94.2) | 93 (96.8) | 165 (95.3) | 539 (96.2) |
| History of difficulty breathing | 55 (59.7) | 197 (66.1) | 63 (65.6) | 115 (66.4) | 381 (68.0) |
| History of runny nose | 55 (59.7) | 92 (30.8) | 50 (52.0) | 87 (50.2) | 247 (44.1) |
| Degree of fever | |||||
| ≥ 101°F | 47 (16.3) | 125 (41.8) | 30 (31.3) | 74 (42.8) | 137 (24.5) |
| 99.5°F–100.9°F | 11 (11.9) | 55 (18.3) | 24 (25.0) | 24 (13.9) | 82 (14.6) |
| 98.6°F–99.4°F | 19 (20.6) | 59 (19.7) | 19 (19.8) | 30 (17.3) | 140 (25.0) |
| Afebrile | 15 (16.3) | 60 (20.0) | 23 (23.9) | 45 (26.0) | 201 (35.9) |
| Observed cough | 39 (42.3) | 89 (29.8) | 33 (34.3) | 62 (35.8) | 169 (30.1) |
| Fast breathing | 58 (63.0) | 227 (76.1) | 73 (76.0) | 128 (73.9) | 405 (72.3) |
| Chest indrawing | 64 (69.5) | 247 (82.8) | 69 (71.8) | 140 (80.9) | 461 (82.3) |
| Crepitations | 44 (47.8) | 230 (77.1) | 64 (66.6) | 136 (78.6) | 378 (67.5) |
| Wheeze on auscultation | 21 (22.8) | 85 (28.5) | 22 (22.9) | 38 (21.9) | 170 (30.3) |
| Audible wheeze | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.0) |
| Hypoxemia (< 90% sat) | 3 (3.2) | 19 (6.3) | 6 (6.2) | 8 (4.6) | 25 (4.4) |
| 94–100 | 64 (69.6) | 229 (76.8) | 72 (75.0) | 141 (81.5) | 441 (78.8) |
| 90–93 | 3 (3.2) | 35 (11.8) | 6 (6.2) | 12 (6.9) | 47 (8.4) |
| < 90 | 3 (3.2) | 19 (6.4) | 6 (6.2) | 8 (4.6) | 25 (4.4) |
| Missing | 22 (23.9) | 15 (5.0) | 12 (12.5) | 12 (6.9) | 47 (8.3) |
| Head nodding or tracheal tugging | 4 (4.3) | 49 (16.4) | 6 (6.2) | 20 (11.5) | 66 (11.7) |
| Nasal flaring | 7 (7.6) | 59 (19.7) | 5 (5.2) | 11 (6.3) | 46 (8.2) |
| Grunting | 0 (0.0) | 2 (0.0) | 0 (0.0) | 2 (1.1) | 1 (0.0) |
| General danger sign | |||||
| Convulsions | 6 (6.5) | 6 (2.0) | 0 (0.0) | 2 (1.1) | 11 (1.9) |
| Lethargy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 3 (0.0) |
| Unable to eat or drink | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vomits everything | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Chest radiography | |||||
| Alveolar consolidation only | 2 (2.1) | 7 (2.3) | 5 (5.2) | 10 (5.7) | 16 (2.8) |
| Other infiltrate only | 21 (22.8) | 49 (16.3) | 12 (12.5) | 39 (22.5) | 93 (16.6) |
| Alveolar consolidation & other infiltrate | 23 (25.0) | 56 (18.7) | 17 (17.7) | 49 (28.3) | 109 (19.4) |
| Hospitalized | 27 (29.3) | 78 (26.1) | 19 (19.7) | 34 (19.6) | 92 (16.4) |
| Duration of hospitalization | |||||
| Median (SD) in days | 2 (7.4) | 2 (0.8) | 2 (1.2) | 2 (1.2) | 2 (1.4) |
| < 1 day | 4 (14.8) | 1 (1.2) | 4 (21.0) | 1 (2.9) | 8 (8.6) |
| 1–2 days | 17 (62.9) | 64 (82.0) | 10 (52.6) | 21 (61.7) | 61 (66.3) |
| 3–4 days | 5 (18.5) | 12 (15.3) | 5 (26.3) | 10 (29.4) | 18 (19.5) |
| ≥ 5 days | 1 (3.7) | 1 (1.2) | 0 (0.0) | 2 (5.8) | 5 (5.4) |
| Outcome of hospitalization | |||||
| Discharged | 25 (92.5) | 78 (100.0) | 19 (100.0) | 33 (97.0) | 88 (95.6) |
| Referred | 1 (3.7) | 0 (0.0) | 0 (0.0) | 1 (3.0) | 1 (1.0) |
| Left against medical advice | 1 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.1) |
| Died | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) |
Percentages are all column percentages, unless indicated otherwise. Percentages are rounded off to the first decimal point.
Analyses describe cases where specific virus was detected, irrespective of single or multiple virus detection.
4. Discussion
This study provides data on viral respiratory pathogens associated with ALRI in children 3–23 months of age attending three surveillance hospitals in rural Sylhet district of Bangladesh. We detected respiratory viruses in a high proportion of children (81.8%); the top four viruses identified were HRV/HEV, RSV, hMPV, and influenza virus. Viruses were detected disproportionately in children with ALRI presenting during the autumn season, and influenza virus and RSV were most frequently associated with the need for in‐patient hospitalization.
The preponderance of virus associated‐severe ALRI in our study was noteworthy, and in keeping with findings from the Bangladesh arm of the PERCH study, where 77.7% of severe ALRI (referred to as severe or very severe pneumonia in the PERCH study defined using the modified 2005 WHO criteria) cases were attributed to respiratory viruses [10]. Similar proportions of respiratory viral etiology were found in urban Bangladesh, both in community‐based [20] and hospital‐based [21] ALRI cohorts. Other studies examining respiratory viral etiology for ALRI among children in Europe, Africa, east Asia, and southeast Asia have found similar isolation rates [22, 23, 24]. These findings are important for both epidemiologists as well as clinicians to consider, as in many settings, antibiotics are used to treat ALRIs, although the etiology may not necessarily be bacterial. Our findings call for wider availability and inclusion of testing for respiratory viruses to better understand the etiology and treatment options for pediatric ALRI.
In our study, the most commonly identified viruses were HRV/HEV, RSV, and hMPV. Although rhinoenteroviruses have been historically thought to cause mild self‐limiting upper respiratory infection, they are increasingly being identified as causes of severe bronchiolitis, and severe ALRI, including radiograph‐confirmed pneumonia [25], and are associated with long‐term sequelae including recurrent wheezing and the development of asthma in young children [26, 27]. The PERCH study, which rigorously assessed bacterial and viral etiology among children aged 1–59 months hospitalized with severe or very severe ALRI at multiple sites across the globe and included controls found that Bangladesh had the greatest prevalence of HRV/HEV across the study sites, and this is likely attributable to the greatest population density and crowding of any country [10]. The high prevalence of undernutrition could also be contributory, as was found in other settings. [28]. The PERCH study (which also included asymptomatic controls) and other Bangladeshi studies [20, 29] have also found RSV to be an important etiological agent for childhood ALRI. As observed in our study, in temperate climate regions, RSV circulation is seen typically during fall and winter [30]. RSV frequently causes severe ALRI that requires hospitalization in young children [31] and is associated with a high burden of morbidity and mortality especially among children < 2 years [32]. The recent development of an array of monoclonal antibodies as well as vaccines for RSV prevention are promising but ensuring that these interventions are financially feasible and widely available in resource‐limited regions remains a critical hurdle. Overcoming these challenges will require concerted efforts from the global health community, fostering collaborations that prioritize equitable access and affordability.
HMPV was the third most common virus detected in this study. Previous studies have reported that hMPV is prevalent during late winter and spring in temperate climates [33], and our findings were similar. The virus is highly contagious and spreads through respiratory droplets, making it a frequent cause of respiratory infections in childcare and community settings. In young children, especially those with underlying health conditions, hMPV infections can lead to severe respiratory distress and hospitalization [33]. Understanding the role of hMPV as a cause of ALRI in children less than 2 years of age is crucial for designing targeted preventive strategies, including potential vaccines and therapeutic interventions, to mitigate the impact of this respiratory virus on pediatric health. Our findings that hMPV infections peak after the RSV peak also have important implications for future vaccine programs, especially because vaccines targeting both viruses together are currently in early‐phase clinical trials [34, 35].
We found that almost one‐fourth of children with ALRI had two or more respiratory viruses detected, involving most commonly HRV/HEV, bocavirus, and hMPV. Similar rates of viral co‐infection have been reported in other studies from the region [10, 36]. In a systematic review, Goka et al. reported that the incidence of mixed viral infections ranged from 5%–62%, with a mean of 23% [37]. Although some studies have suggested that multiple infections were associated with increased morbidity and mortality, this finding is not conclusive [37]. Furthermore, the simultaneous presence of more than one virus in the same sample must be interpreted cautiously. Highly sensitive PCR assays may identify very small amounts of viral nucleic acids present during the incubation period or the convalescence phase of the illness. The identification of more than one virus can also be explained by the prolonged shedding of the virus that caused a previous infection, a coincidental upper airway infection, or the asymptomatic circulation of some viruses [38]. Bocavirus is frequently found in the presence of other respiratory viruses, making it difficult to establish a direct causal relationship with ALRI. A study of viral etiology for ALRI among hospitalized children from the Sa Kaeo Province in Thailand found that 91% of bocavirus‐positive ALRI patients < 5 years of age were co‐infected with another virus [39] and prolonged bocavirus shedding has been reported for up to 4.5 months in hospitalized children [40], making the role of bocavirus as a pathogen unclear. In contrast, RSV, influenza, hMPV, and PIV are significantly more frequent in patients with infection than in controls, and their detection by PCR is likely to be causal [41, 42]. This study was conducted prior to the emergence of the SARS‐CoV‐2 pandemic. Consequently, the data presented here may not reflect the current landscape of viral pathogens in the causation of ALRI in young children. In our study, the seasonal coronaviruses (coronavirus types NL63, HKU1, 229E, and OC43) were not frequent agents associated with ALRI, a finding similar to those reported in other studies [10, 43, 44]. Future research will help elucidate how the introduction of the novel SARS‐CoV‐2 could change the occurrence and interaction of respiratory pathogens globally and better define the role SARS‐CoV‐2 will play in the causation of pediatric ALRI the postpandemic era.
This study has several limitations. First, only a small fraction of children with ALRI from the study area sought care from study hospitals [12]. Second, we did not have data regarding bacterial etiologies, so we could not assess bacterial‐viral co‐infection. Third, we did not have control data, so we could not attribute causality to viruses that are frequently associated with colonization. In some cases, certain viruses not associated with ALRI may have been detected; however, the true etiology could have been bacterial. Fourth, a large number of children (28.5%) could not be tested for viral etiology; however, there were no major differences in the baseline demographics between those tested for respiratory viruses and those who could not be tested. Despite these limitations, this study is among the first to describe the epidemiology and clinical features of respiratory viral‐associated ALRI in rural Bangladesh and provides important insights into future studies and subsequent prevention strategies.
In conclusion, we identified respiratory viruses in a large proportion of children < 2 years presenting to care with ALRI in rural Bangladesh. The data on seasonality of respiratory viruses and associated ALRI can be valuable for determining the timing of administration of respiratory vaccines, for example, providing influenza vaccines prior to the onset of the influenza season in the monsoon and autumn seasons. Our findings suggest that further studies in which rigorous confirmation of respiratory viral agents is performed in children with ALRI are necessary, particularly as prevention strategies such as vaccine programs and antimicrobial stewardship programs are implemented.
Author Contributions
Funding acquisition: AHB and MER. Conceptualization and design: MER, AHB, EDM, LHM, SA, SS, and MS. Data curation: MER, NHC, ADR, LHM, and AHB. Data collection: ADR, JA, and NHC. Data analysis: MER, KM, NHC, and AHB. Data interpretation: MER, KM, ADR, EDM, SA, LHM, and AHB. Writing – original draft: MER and KM. Writing – review and editing: EDM, SA, NHC, ADR, MS, LHM, SS, and AHB.
Ethics Statement
The National Research Ethics Committee of Bangladesh Medical Research Council and the institutional review boards of the Johns Hopkins and Duke Schools of Medicine reviewed and approved the study's protocol.
Conflicts of Interest
The authors declare no conflicts of interest.
Peer Review
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1111/irv.70062.
Acknowledgments
We thank the children and caregivers for participating in this study. We thank Santina Castriciano and Copan Italia for the provision of nasopharyngeal swabs and media. We also thank the Projahnmo Study Group field and data management staff, the Ministry of Health and Family Welfare, the Government of Bangladesh, Thrasher Research Fund, and the Bill and Melinda Gates Foundation for their support of this study.
Funding: This study was funded by the Bill and Melinda Gates Foundation to A Baqui (OPP1084286, OPP1117483) and Thrasher Research Fund to ME Reller (14193). The funders had no role in data collection or analysis or preparation of the manuscript or decision to publish. The content is solely the responsibility of the authors and does not represent the official views of the Bill & Melinda Gates Foundation or Thrasher Research Fund.
Data Availability Statement
All relevant data are available within the paper tables and figure files.
References
- 1. GBD 2017 Causes of Death Collaborators , “Global, Regional, and National Age‐Sex‐Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017,” Lancet 392, no. 10159 (2018): 1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . The Global Health Observatory. Available at https://www.who.int/data/gho/indicator‐metadata‐registry/imr‐details/3147. Accessed July 12, 2024.
- 3. Pinzón‐Rondón Á. M., Aguilera‐Otalvaro P., Zárate‐Ardila C., and Hoyos‐Martínez A., “Acute Respiratory Infection in Children From Developing Nations: A Multi‐Level Study,” Paediatrics and International Child Health 36, no. 2 (2016): 84–90. [DOI] [PubMed] [Google Scholar]
- 4. Bangladesh Demographic and Health Survey 2014. Dhaka, Bangladesh, and Rockville, Maryland, USA: NIPORT, Mitra and Associates, and ICF International. Available at https://dhsprogram.com/pubs/pdf/FR311/FR311.pdf. Accessed July 12, 2024.
- 5. World Bank . Population density (people per sq. km of land area)—Bangladesh. Available at https://data.worldbank.org/indicator/EN.POP.DNST?locations=BD. Accessed 2 December, 2023.
- 6. Caulfield L. E., de Onis M., Blössner M., and Black R. E., “Undernutrition as an Underlying Cause of Child Deaths Associated With Diarrhea, Pneumonia, Malaria, and Measles,” American Journal of Clinical Nutrition 80, no. 1 (2004): 193–198. [DOI] [PubMed] [Google Scholar]
- 7. Zaman K., Baqui A. H., Yunus M., et al., “Acute Respiratory Infections in Children: A Community‐Based Longitudinal Study in Rural Bangladesh,” Journal of Tropical Pediatrics 43, no. 3 (1997): 133–137. [DOI] [PubMed] [Google Scholar]
- 8. Baqui A. H., Rahman M., Zaman K., et al., “A Population‐Based Study of Hospital Admission Incidence Rate and Bacterial Aetiology of Acute Lower Respiratory Infections in Children Aged Less Than Five Years in Bangladesh,” Journal of Health, Population, and Nutrition 25, no. 2 (2007): 179–188. [PMC free article] [PubMed] [Google Scholar]
- 9. Kabir L., Amin R., Mollah A. H., et al., “Respiratory Disorders in Under‐Five Children Attending Different Hospitals of Bangladesh: A Cross Sectional Survey,” Journal of Respiratory Medicine Research and Treatment 11 (2016): 183615. [Google Scholar]
- 10. Brooks W. A., Zaman K., Goswami D., et al., “The Etiology of Childhood Pneumonia in Bangladesh: Findings From the Pneumonia Etiology Research for Child Health (PERCH) Study,” Pediatric Infectious Disease Journal 40, no. 9S (2021): S79–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baqui A. H., McCollum E. D., Saha S. K., et al., “Pneumococcal Conjugate Vaccine Impact Assessment in Bangladesh,” Gates Open Research 2 (2018): 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baqui A. H., McCollum E. D., Mahmud A., et al., “Projahnmo Study Group in Bangladesh. Population‐Based Incidence and Serotype Distribution of Invasive Pneumococcal Disease Prior to Introduction of Conjugate Pneumococcal Vaccine in Bangladesh,” PLoS ONE 15, no. 2 (2020): e0228799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baqui A. H., Koffi A. K., McCollum E. D., et al., “Impact of National Introduction of Ten‐Valent Pneumococcal Conjugate Vaccine on Invasive Pneumococcal Disease in Bangladesh: Case‐Control and Time‐Trend Studies,” Vaccine 39, no. 40 (2021): 5794–5801. [DOI] [PubMed] [Google Scholar]
- 14. McCollum E. D., Ahmed S., Roy A. D., et al., “Effectiveness of the 10‐Valent Pneumococcal Conjugate Vaccine Against Radiographic Pneumonia Among Children in Rural Bangladesh: A Case‐Control Study,” Vaccine 38, no. 42 (2020): 6508–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen J. H. K., Lam H. Y., Yip C. C. Y., et al., “Clinical Evaluation of the New High‐Throughput Luminex NxTAG Respiratory Pathogen Panel Assay for Multiplex Respiratory Pathogen Detection,” Journal of Clinical Microbiology 54, no. 7 (2016): 1820–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stevens B., Watt K., Brimbecombe J., Clough A., Judd J., and Lindsay D., “The Role of Seasonality on the Diet and Household Food Security of Pregnant Women Living in Rural Bangladesh: A Cross‐Sectional Study,” Public Health Nutrition 20, no. 1 (2017): 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Principles and Recommendations for Population and Housing Censuses (Revision 2) (New York: United Nations, 2007). [Google Scholar]
- 18. Mahomed N., Fancourt N., de Campo J., et al., “Preliminary Report From the World Health Organisation Chest Radiography in Epidemiological Studies Project,” Pediatric Radiology 47 (2017): 1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization . Who Child Growth Standards: Methods and Development. Available at https://www.who.int/childgrowth/standards/technical_report/en/. Accessed July 12, 2024.
- 20. Homaira N., Luby S. P., Petri W. A., et al., “Incidence of Respiratory Virus‐Associated Pneumonia in Urban Poor Young Children of Dhaka, Bangladesh, 2009‐2011,” PLoS ONE 7, no. 2 (2012): e32056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chowdhury F., Shahid A. S. M. S. B., Ghosh P. K., et al., “Viral Etiology of Pneumonia Among Severely Malnourished Under‐Five Children in an Urban Hospital, Bangladesh,” PLoS ONE 15, no. 2 (2020. Feb 4): e0228329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tran D. N., Trinh Q. D., Pham N. T., et al., “Clinical and Epidemiological Characteristics of Acute Respiratory Virus Infections in Vietnamese Children,” Epidemiology and Infection 144, no. 3 (2016): 527–536. [DOI] [PubMed] [Google Scholar]
- 23. Guerrier G., Goyet S., Chheng E. T., et al., “Acute Viral Lower Respiratory Tract Infections in Cambodian Children: Clinical and Epidemiologic Characteristics,” Pediatric Infectious Disease Journal 32, no. 1 (2013): e8–e13. [DOI] [PubMed] [Google Scholar]
- 24. Pretorius M. A., Madhi S. A., Cohen C., et al., “Respiratory Viral Coinfections Identified by a 10‐Plex Real‐Time Reverse‐Transcription Polymerase Chain Reaction Assay in Patients Hospitalized With Severe Acute Respiratory Illness—South Africa, 2009–2010,” Journal of Infectious Diseases 206, no. Suppl 1 (2012): S159–S165. [DOI] [PubMed] [Google Scholar]
- 25. Jacobs S. E., Lamson D. M., St George K., and Walsh T. J., “Human Rhinoviruses,” Clinical Microbiology Reviews 26, no. 1 (2013): 135–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Calışkan M., Bochkov Y. A., Kreiner‐Møller E., et al., “Rhinovirus Wheezing Illness and Genetic Risk of Childhood‐Onset Asthma,” New England Journal of Medicine 368 (2013): 1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rossi G. A. and Colin A. A., “Infantile Respiratory Syncytial Virus and Human Rhinovirus Infections: Respective Role in Inception and Persistence of Wheezing,” European Respiratory Journal 45 (2015): 774–789. [DOI] [PubMed] [Google Scholar]
- 28. Taipale A., Pelkonen T., Roivainen M., et al., “Human Rhino‐ and Enteroviruses in Children With Respiratory Symptoms in Luanda, Angola,” Paediatrics and International Child Health 34, no. 2 (2014): 128–132. [DOI] [PubMed] [Google Scholar]
- 29. Chowdhury F., Shahid A. S. M. S. B., Ghosh P. K., et al., “Viral Etiology of Pneumonia Among Severely Malnourished Under‐Five Children in an Urban Hospital, Bangladesh,” PLoS ONE 15, no. 2 (2020): e0228329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGuiness C. B., Boron M. L., Saunders B., Edelman L., Kumar V. R., and Rabon‐Stith K. M., “Respiratory Syncytial Virus Surveillance in the United States, 2007‐2012: Results From a National Surveillance System,” Pediatric Infectious Disease Journal 33, no. 6 (2014): 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nair H., Nokes D. J., Gessner B. D., et al., “Global Burden of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children: A Systematic Review and Meta‐Analysis,” Lancet 375, no. 9725 (2010): 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y., Wang X., Blau D. M., et al., “Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Children Younger Than 5 Years in 2019: A Systematic Analysis,” Lancet 399, no. 10340 (2022): 2047–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kahn J. S., “Epidemiology of Human Metapneumovirus,” Clinical Microbiology Reviews 19 (2006): 546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moderna Announces mRNA‐1345, an Investigational Respiratory Syncytial Virus (RSV) Vaccine, Has Met Primary Efficacy Endpoints in Phase 3 Trial in Older Adults. Available at https://investors.modernatx.com/news/news‐details/2023/Moderna‐Announces‐mRNA‐1345‐an‐Investigational‐Respiratory‐Syncytial‐Virus‐RSV‐Vaccine‐Has‐Met‐Primary‐Efficacy‐Endpoints‐in‐Phase‐3‐Trial‐in‐Older‐Adults/default.aspx#:~:text=Moderna%20plans%20to%20initiate%20a,immunocompromised%20patients%20and%20older%20adults. Accessed 17 May, 2024.
- 35. Tan T. Q.. What's New in Pediatric Vaccines. Contemporary Pediatrics. Available at https://www.contemporarypediatrics.com/view/what‐s‐new‐in‐pediatric‐vaccines. Accessed 17 May, 2024.
- 36. Cui B., Zhang D., Pan H., et al., “Viral Aetiology of Acute Respiratory Infections Among Children and Associated Meteorological Factors in Southern China,” BMC Infectious Diseases 15, no. 1 (2015): 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goka E. A., Vallely P. J., Mutton K. J., and Klapper P. E., “Single and Multiple Respiratory Virus Infections and Severity of Respiratory Disease: A Systematic Review,” Paediatric Respiratory Reviews 15, no. 4 (2014): 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Korsun N., Angelova S., Trifonova I., et al., “Viral Pathogens Associated With Acute Lower Respiratory Tract Infections in Children Younger Than 5 Years of Age in Bulgaria,” Brazilian Journal of Microbiology 50, no. 1 (2019): 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fry A. M., Lu X., Chittaganpitch M., et al., “Human Bocavirus: A Novel Parvovirus Epidemiologically Associated With Pneumonia Requiring Hospitalization in Thailand,” Journal of Infectious Diseases 195, no. 7 (2007): 1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blessing K., Neske F., Herre U., Kreth H. W., and Weissbrich B., “Prolonged Detection of Human Bocavirus DNA in Nasopharyngeal Aspirates of Children With Respiratory Tract Disease,” Pediatric Infectious Disease Journal 28, no. 11 (2009): 1018–1019. [DOI] [PubMed] [Google Scholar]
- 41. Self W. H., Williams D. J., Zhu Y., et al., “Respiratory Viral Detection in Children and Adults: Comparing Asymptomatic Controls and Patients With Community‐Acquired Pneumonia,” Journal of Infectious Diseases 213, no. 4 (2016): 584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shi T., McLean K., Campbell H., and Nair H., “Aetiological Role of Common Respiratory Viruses in Acute Lower Respiratory Infections in Children Under Five Years: A Systematic Review and Meta‐Analysis,” Journal of Global Health 5, no. 1 (2015): 010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hasan R., Rhodes J., Thamthitiwat S., et al., “Incidence and Etiology of Acute Lower Respiratory Tract Infections in Hospitalized Children Younger Than 5 Years in Rural Thailand,” Pediatric Infectious Disease Journal 33, no. 2 (2014): e45–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krumkamp R., Kohsar M., Nolte K., et al., “Pathogens Associated With Hospitalization Due to Acute Lower Respiratory Tract Infections in Children in Rural Ghana: A Case‐Control Study,” Scientific Reports 13, no. 1 (2023): 2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are available within the paper tables and figure files.


