Abstract
Herein, we evaluated the effects of gonadotropin hormone-releasing hormone (GnRH) administration 84 h after medroxyprogesterone acetate (MAP) sponge removal on follicular growth, ovulation timing, and pregnancy per artificial insemination (AI) in cosynchronized postpartum Nili Ravi buffaloes. In this study, 58 Nili Ravi postpartum buffaloes (DIM = 103 ± 1.64) were randomly divided into two treatment groups (n = 29/treatment): GnRH-TAI-84 and TAI-84. All buffaloes were administered a MAP sponge for seven days. Upon MAP sponge removal, all the subjects received prostaglandin F2α (PGF2α) and Timed AI (TAI) was performed 84 h after sponge removal. In the GnRH-TAI-84 group, the buffaloes received GnRH alongside insemination, whereas in the TAI-84 group, the buffaloes were inseminated without GnRH administration. Follicle diameter and blood estradiol levels were measured every 6 h from 72–108 h after MAP sponge removal. The animals were checked for pregnancy using ultrasonography 40 days after AI. Animals subjected to the GnRH-TAI-84 protocol had a higher follicular growth rate and preovulatory follicle size than those in the TAI-84 group. The follicular diameter was also larger in animals that received GnRH-TAI-84 than in those that received TAI-84 90 and 96 h after MAP sponge removal. Buffaloes in the GnRH-TAI-84 group had lower estradiol concentrations at 90, 96, 102, and 108 h than those in the TAI-84 group. Ovulation in GnRH-TAI-84 buffaloes occurred 11 h earlier than that in buffaloes from the TAI-84 group. A shorter interval between AI and ovulation in GnRH-TAI-84 buffaloes (14 h vs. 25 h) led to greater pregnancies per AI (62% vs. 17%) compared to buffaloes from the TAI-84 group.
Keywords: Buffalo, Gonadotropin hormone-releasing hormone (GnRH), MAP sponge, Timed artificial insemination
In the field of reproductive management, the implementation of reproductive protocols, such as Ovsynch, G6G (Incorporation of prostaglandin F2α (PGF2α) followed by an injection of gonadotropin hormone-releasing hormone (GnRH) two days later, with the Ovsynch protocol being administered six days afterward), and Cosynch, has revolutionized the practices of large-scale cattle farming [1]. Over the course of several decades, estrus synchronization techniques adapted from the cattle industry have also been employed to enhance the reproductive efficiency of buffaloes, achieving variable success rates [2,3,4,5]. Because of the weak estrus expression in buffaloes, it is imperative to adopt an approach to enhance fertility, which involves implementing Ovsynch, or using progesterone in conjunction with GnRH and estradiol benzoate, thus eliminating the need for estrus detection, and instead employing timed artificial insemination (TAI) for both dairy cows [6,7,8] and buffaloes [9,10,11]. To successfully apply hormonal protocols and TAI in buffaloes [12,13,14], a comprehensive understanding of ovulation timing is crucial. There was variation in ovulation time following the removal of Controlled Internal Drug Release (CIDR) devices in buffaloes [15, 16]. As such, further research focusing on tightening ovulation synchrony following the removal of progesterone devices is required to optimize TAI in buffaloes. GnRH and estradiol are used in the TAI protocol to synchronize ovulation near the TAI in buffaloes [17,18,19] and bovine [20,21,22]. In this context, several studies have previously reported that increasing time from PGF2α to final GnRH administration was associated with a higher pregnancy per artificial insemination (P/AI) rate due to reduced progesterone at TAI, optimized proestrus hormonal environment, and improved endogenous estradiol concentrations, leading to synchronized ovulation in bovines. [23,24,25].
More recently, Haider et al. [26] evaluated the efficacy of the CIDR Cosynch protocol, originally developed for cows, in buffaloes, identifying a 25% P/AI. However, when a modification involving a 12-h delay in administering GnRH after CIDR removal was introduced, the P/AI significantly increased to 65%. The potential reason for this rise in P/AI in buffaloes in the 84-h CIDR-Cosynch protocol (administering GnRH and TAI concomitantly at 84 h after CIDR removal) was unclear, with the researchers being uncertain as to whether this was due to delayed AI or the administration of GnRH to larger follicles leading to higher P/AI, as described by Perry et al. [27]. It is also important to delineate the effect of the GnRH treatment based on the delay in AI timing, as both were implemented simultaneously.
Hence, the primary objective of this study was to assess the effect of the GnRH-TAI-84 (Administering GnRH and TAI concomitantly 84 h after sponge removal) and TAI-84 (timed artificial insemination performed 84 h after sponge removal) protocols on follicular size, follicle growth rate, ovulation interval, estrus intensity score, ovulation induction, and P/AI using a 7-day medroxyprogesterone acetate (MAP) sponge Cosynch in postpartum Nili Ravi buffaloes. We hypothesized that GnRH administration 84 h after sponge removal in a 7-day MAP sponge Cosynch would lead to better ovulation synchronization and, subsequently, a higher P/AI in postpartum buffaloes, as GnRH is an effective ovulation inducer and remains a reliable option for TAI.
Materials and Methods
Prior to the experiments, approval was granted by the Animal Use and Research Committee of the Buffalo Research Institute, Pattoki, Pakistan (Approval # 340118-3).
Power and sample size calculations
The 2-tailed sample size was calculated using the POWER procedure in SAS version 9.4 (SAS/STAT, SAS Institute Inc.) using data regarding the chance of pregnancy from previous studies [26, 28]. Data from these studies suggest that the P/AI ratio remains between 59% and 65% when the interval from TAI to ovulation is 8–20 h. However, the P/AI was reduced by 10–18% when the interval from TAI to ovulation was too early (4 h) or too late (28 h). In addition, Haider et al. [26] suggested that the pregnancy rate would be 65% when the interval from TAI to ovulation was 15 h after the administration of GnRH-TAI-84 h post CIDR removal. In this background, we anticipated a P/AI of ~ 60% in buffaloes administered GnRH-TAI-84 h post CIDR removal. However, the P/AI was 20% when buffaloes were bred for TAI-84 h after CIDR removal without receiving GnRH. Therefore, we calculated the sample size based on a 40%-point difference in the P/AI between treatments (α = 0.05; β = 0.20). We further conducted the power analyses for the timing of ovulation based on the previous data [26], and calculated the sample size to anticipate the mean difference of 8 h with a standard deviation of 7.5 between treatments (α = 0.05; β = 0.20). The sample size for P/AI was 23 experimental units per treatment, whereas it was 14 experimental units for the timing of ovulation. We opted for P/AI sample size calculations and enrolled additional buffaloes to accommodate potential attrition during the experiment.
Animals and management
This study was carried out at the Buffalo Research Institute, Pattoki, Punjab, Pakistan (31°1'0"N, 73°51"E), during the breeding season (September to November 2022), with average temperature ranges from 20–35ºC and a humidity level of 45–65% [29]. A total of fifty-eight adult Nili Ravi buffaloes, aged six–eight years, with a body condition score (BCS) rated on a 1–5 scale [30], weighing 540–570 kg, and with days in milk (DIM) ranging from to 90–110, were selected for this investigation, as shown in Table 1. All animals were housed in a free-stall system with unrestricted access to water and were fed a regular diet containing 30–40 Kg of green fodder and 1–2 kg of a concentrate mixture with 15% crude protein and 65% total digestible nutrients per head per day. Prior to initiating the synchronization technique, all animals were scanned to determine whether they possessed a normal reproductive tract with a small uterus and no uterine contents after parturition. Ultrasonography was performed on day –7 before the start of the protocol, and on day 0 at the initiation of the protocol to confirm the acyclic status, absence of the corpus luteum, and the absence of signs of postpartum estrus.
Table 1. Body performance and production parameters at protocol initiation, ovulation intervals, ovulation rate, estrus intensity score, and pregnancy per artificial insemination (P/AI).
| Characteristics | GnRH-TAI-84 (n = 29) |
TAI-84 (n = 29) |
SEM | P-value | |
|---|---|---|---|---|---|
| Body performance and production parameters | |||||
| Body weight (Kg) | 544.2 | 543.9 | 1.5 | 0.88 | |
| BCS (1–5) | 3.56 | 3.49 | 0.03 | 0.10 | |
| Age (years) | 6.53 | 6.68 | 0.17 | 0.83 | |
| DIM (days) | 103.5 | 103.7 | 1.64 | 0.94 | |
| Parity (n) | 2.51 | 2.62 | 0.17 | 0.68 | |
| Reproductive parameters | |||||
| Interval from PGF2α2/MAP 1 sponge removal to ovulation (h) | 98.06 | 109 | 0.63 | 0.01 | |
| Interval from TAI to ovulation (h) | 14.06 | 25 | 0.63 | 0.01 | |
| Progesterone at TAI (ng/ml) | 0.31 | 0.33 | 0.01 | 0.46 | |
| Ovulation 3; (%) n/n | 29/29 (100) | 25/29 (86) | 6.0 | 0.97 | |
| Estrus intensity score 4 | 3.82 | 3.68 | 0.14 | 0.51 | |
| P/AI 5; (%) n/n | 18/29 (62) | 5/29 (17) | 9.0 | 0.002 | |
Values shows as the least square means and associated standard error of means. 1 MAP medroxyprogesterone acetate (250 mg); 2 PGF2α, Prostaglandin F2α. 3 Total ovulation represents the number of animals ovulated divided by the total number of buffalo treated × 100. 4 Estrus intensity Score: 1 = poor sign, 2 = satisfactory sign, 3 = good sign, 4 = Very Good sign, 5 = excellent sign. 5 Pregnancy per AI; P/AI = [(number of pregnant buffaloes divided by the number of treated buffaloes) × 100].
Experimental design
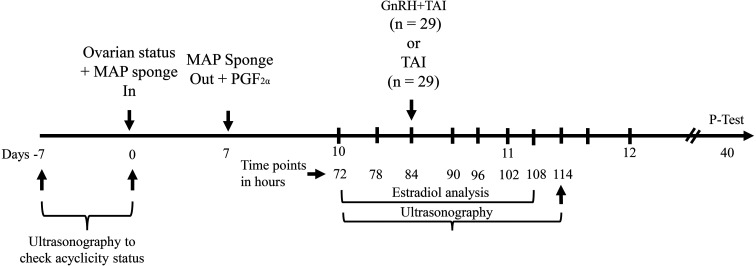
In a randomized controlled trial, randomly selected buffaloes were assigned to two treatment groups: administration of GnRH 84 h after MAP sponge removal (GnRH-TAI-84, n = 29) or untreated (TAI-84), and timed insemination 84 h after MAP sponge removal (Fig. 1). Briefly, for seven days, all animals were administered an intravaginal MAP sponge (NIAB Heat SpongeTM, containing 250 mg of medroxyprogesterone acetate/sponge, Faisalabad, Pakistan). Intramuscular administration of PGF2α (Trometamol 5 mg; DPROST, Selmore®, Lahore, Pakistan) was performed upon removal of the MAP sponge simultaneously. The buffaloes in GnRH-TAI-84 group (n = 29) were randomly assigned to receive an intramuscular GnRH injection (GnRH; 10 µg of buserelin acetate, Bosol, Selmore®, Lahore, Pakistan) concomitantly with AI at 84 h following sponge removal/PGF2α administration. Conversely, buffaloes in the TAI-84 (n = 29) group were inseminated following the TAI schedule at 84 h after sponge removal/PGF2α administration without receiving any GnRH injection.
Fig. 1.
Schematic illustration of the experimental design. MAP sponge, medroxyprogesterone acetate (250 mg) sponge; P-test, pregnancy test; TAI, timed-artificial insemination; PGF2α, prostaglandin F2α; GnRH, gonadotropin-releasing hormone.
Ultrasonography, ovulation, and pregnancy diagnosis
At the beginning of the synchronization protocol, the ovaries and uteruses of each buffalo were scanned using a B-mode ultrasound console (HS-1500V; Honda Electronics Ltd., Tokyo, Japan) with a 7.5 MHz linear probe to ascertain the presence or absence of a fetus, corpus luteum, follicle, or any structural abnormality. During the synchronization protocol, ultrasonic tracking was conducted at regular intervals of six hours, commencing at 84 and continuing until 114 h following sponge removal/PGF2α injection, including all the animals in experimental groups. Ovulation was defined as the disappearance of a preovulatory follicle observed during the last ultrasound scan [15]. A subset from each buffalo group (GnRH-TAI-84; n = 12 and TAI-84; n = 9) underwent a comprehensive study of follicle diameter, preovulatory follicle size, and follicle growth subsequent to ovulation using ultrasound at regular intervals of six hours, starting from 72 to 114 h, leading to ovulation [31]. Subsequently, ultrasonography was performed 7 days following AI to confirm ovulation by observing the corpus luteum (CL) in buffaloes. Ultrasonography was performed to confirm pregnancy on days 35–40 after AI, revealing the amniotic membrane, embryonic fluid, and heartbeat.
Measurement of estrus intensity and AI
Using the aforementioned 1–5 scale, all animals were assessed for estrus intensity. The estrus intensity score was determined based on factors such as the tone in the uterine horns, mucous secretion, edematous vulva, bellowing, restlessness, and other signs [18]. A skilled technician performed TAI following standard procedures.
Blood sampling
To determine estradiol concentration (pg/ml), six buffaloes from each group (GnRH-TAI-84 vs. TAI-84) were subjected to blood sample collection through the jugular venipuncture using disposable syringes at various time points (72 to 108 h) following sponge removal/PGF2α administration. Conversely, for the progesterone (ng/ml) assay, blood samples were collected through jugular venipuncture in the two groups at TAI (days 10.5) after MAP sponge removal/PGF2α (n = 29/treatment). The samples were maintained at room temperature for coagulation and serum harvesting. The serum was carefully collected, transferred into Eppendorf tubes after separation, and centrifuged at 2000 rpm for ten min at room temperature to remove any impurities or blood cells. The transparent supernatant serum was transferred into separate tubes and stored at –20ºC till further analysis. Radioimmunoassay (RIA) was employed to assess estradiol and progesterone levels in the serum samples. Commercially available solid-phase RIA kits (Beckman Coulter, IMMUNOTECH, Prague, Czech Republic; estradiol; Ref A21854, progesterone; Ref IM1188) were used for this assay [32]. The intra- and inter-assay coefficients of variation were 10%, 16.4%, 9.48%, and 16.8% for estradiol and progesterone levels, respectively.
Statistical analysis
Data normality was determined using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Significance was set at P < 0.05. The MIXED and GLIMMIX procedures in SAS (SAS ver. 9.4 Institute, Inc., Cary, NC, USA) was used to examine factors including body weight, BCS, age, days in milk, parity, follicular size, follicular growth rate, estradiol concentrations, progesterone concentrations, ovulatory intervals, estrus intensity score, ovulation rate, and P/AI. The model included the fixed effects of treatment, time, and their interactions. Repeated measures were used to analyze estradiol levels and follicular diameter over equal time intervals across all treatments. The choice of the autoregressive (1) covariance structure was based on the AICC values, while the experimental unit was the buffalo. Binary responses, such as ovulation and P/AI, were subjected to logistic regression analysis using the GLIMMIX procedure in SAS.
Results
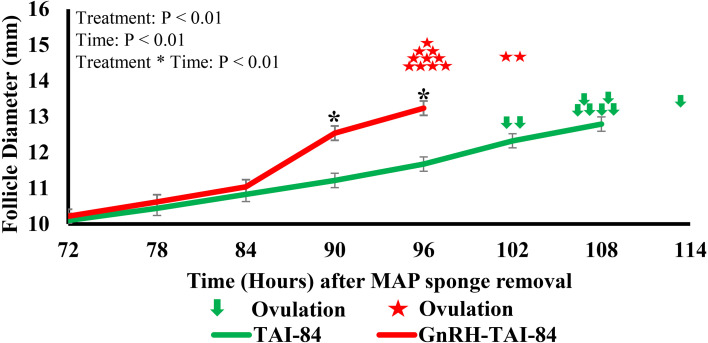
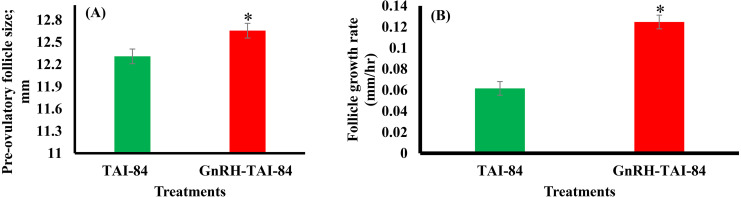
At the beginning of the protocol, the physical and production performance parameters of the treatment groups showed no significant differences (Table 1). Follicle diameter measured after sponge removal at six-hour intervals from 72 to 114 h until ovulation showed an interaction (P < 0.001) between time and treatment (Fig. 2). At 72, 78, and 84 h after sponge removal, the follicle diameter between the treatments remained the same, but significantly differed at 90 and 96 h after sponge removal. Specifically, at the 90 and 96-h time points, the follicle diameter was 1.3 and 1.5 mm larger in animals that received GnRH-TAI-84 compared with the TAI-84 group (Fig. 2). Moreover, the preovulatory follicle size was significantly larger (P < 0.002) in the GnRH-TAI-84 group than that in the TAI-84 group (Fig. 3A). The follicular growth rate, between 84 h after PGF2α/MAP sponge removal to the preovulatory follicle, was also significantly higher (P < 0.001) in GnRH-TAI-84 compared with the TAI-84 group (Fig. 3B).
Fig. 2.
Follicle diameters and ovulation times in the GnRH-TAI-84 (n = 12) and TAI-84 (n = 9) groups. * indicates a significant difference between treatments.
Fig. 3.
(A) preovulatory follicle size and (B) follicle growth rate in the GnRH-TAI-84 (n = 12) and TAI-84 (n = 9) groups. The follicular growth rate between 84 h after PGF2α /MAP sponge removal to preovulatory follicle = (Follicle size at the time of appearance of preovulatory follicle – Follicle size at 84 h after PGF2α/MAP sponge removal) / (Hours after the PGF2α /MAP sponge removal to the appearance of preovulatory follicle – (minus) 84). * indicates a significant difference between treatments.
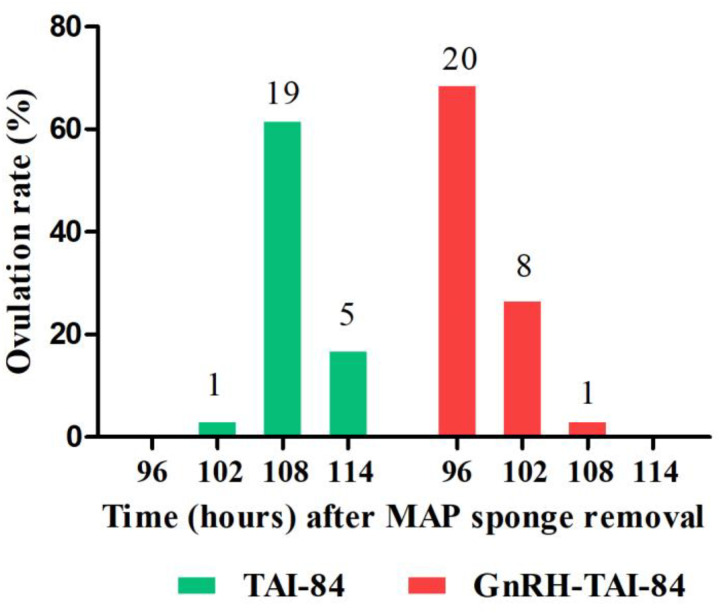
Furthermore, maximum ovulation occurred in buffaloes treated with GnRH-TAI-84 (69%) at 96 h, and in the TAI-84 group (66%) at 108 h after sponge removal (Fig. 4). Four animals in the TAI-84 group did not ovulate until the last scan, performed at 114 h after sponge removal. These animals remained anovulatory and no corpus luteum was observed on day 7 after TAI or pregnancy on the scheduled day of diagnosis.
Fig. 4.
Ovulations (%) in the GnRH-TAI-84 (n = 29) and TAI-84 (n = 25) groups. The numbers above the bars represent the number of ovulations in each treatment interval. Four animals in the TAI-84 group remained anovulatory.
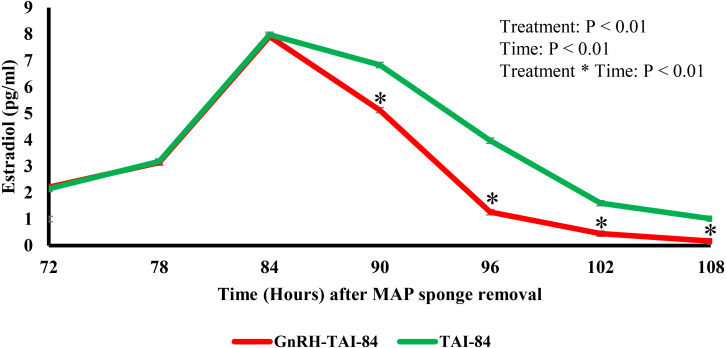
We observed a significant interaction (P < 0.001) between time and treatment for estradiol concentration in subjected animals (Fig. 5). Estradiol concentrations did not differ across treatments at 72, 78, or 84 h after sponge removal. Later, estradiol concentrations were 1.7, 2.7, 1.2, and 0.8 pg/ml lower in GnRH-TAI-84 than in the TAI-84 group at time points 90, 96, 102, and 108 h after sponge removal, respectively. Progesterone concentrations remained the same at TAI between treatments.
Fig. 5.
Serum concentrations of estradiol in the GnRH-TAI-84 (n = 6) and TAI-84 (n = 6) groups. * indicates a significant difference between treatments.
Table 1 shows the ovulatory intervals, ovulation rates, estrus intensity scores, and P/AI results in both groups. Similarly, the interval from PGF2α/MAP sponge removal to ovulation (in hours) was reduced, a 12-h duration, (P < 0.001) in the GnRH-TAI-84 group compared with TAI-84 treated animals. The ovulation rate and estrus intensity score did not differ significantly between treatments. The P/AI was significantly higher (P < 0.001) in the GnRH-TAI-84 group than that in the TAI-84 group.
Discussion
This study is one of the first reports on the success of GnRH treatment using the TAI protocol for buffalo conception. The rationale for the present experiment was based on a preliminary study conducted by our group on cyclic buffalo heifers, which revealed an increased follicular growth rate 84 h following CIDR removal and a shortened interval from TAI to ovulation [26]. Furthermore, the study by Haider et al. previously postulated whether this effect was due to the shorter ovulation time or better corpus luteum development due to larger preovulatory follicle size. The current study therefore aimed to delineate the effect of GnRH treatment in inducing ovulation 84 h after sponge removal. These findings could have an impact on the reduction of postpartum calving intervals in adult buffaloes.
In the current study, GnRH treatment improved P/AI by reducing the interval from TAI to ovulation, increasing follicular growth rate, and inducing ovulation, as indicated by a decrease in estradiol levels. Most follicles acquire LH receptors on the granulosa cells when the follicle diameter reaches approximately 8.5 mm in buffaloes [33] and 10 mm in cows [34]. Intiguingly, the follicle was medium in size and mature at the time of GnRH administration, which initiated a preovulatory LH surge and increased fertility. Moreover, follicular diameters remained similar across groups at different time points until 84 h. However, we observed an increase in follicular diameter at time point 90 in animals subjected to the GnRH-TAI-84 protocol compared to the TAI-84 protocol, indicating a sharp increase in the follicular growth rate following GnRH injection, which subsequently induced maximum ovulation at 96 h. The data on ovulation in buffaloes at 96 h in the GnRH-TAI-84 group suggests a role of GnRH in initiating a preovulatory gonadotropin surge before the dominant follicle attains physiological maturity; hence, GnRH-induced ovulation of immature follicles exerts a negative impact on P/AI [27, 35]. Thus, the success of ovulation depends on the optimal size and maturity of the dominant follicle at the time of GnRH injection [36,37,38]. The size of the preovulatory follicles in this species was consistent with previous reports [39, 40], which described that ovulation of appropriately sized follicles led to a larger CL and increased progesterone production for successful fertility.
In our study, the plasma estradiol concentration remained consistent across different time points until 84 h after sponge removal, and administering GnRH at 84 h at the peak estradiol concentration may have resulted in a higher release of LH to initiate the preovulatory LH surge during the periovulatory period. Subsequently, we observed a sharp decrease in estradiol concentration by 108 h in the GnRH-TAI-84 group compared to the TAI group. It is imperative to mention that an increase in serum estradiol concentration at TAI is positively correlated with improved P/AI owing to the increased length of proestrus, which leads to an improvement in the follicular and uterine steroid environments, as seen in bovines [41,42,43]. Consistent with our findings, previous reports have similarly reported that the administration of GnRH agonists before or during the LH surge can increase P/AI by enhancing the spontaneous preovulatory LH surge [37, 44]. Furthermore, it has been observed that administering PGF2α before GnRH can enhance the pituitary release of LH, leading to an additive effect that may further enhance the preovulatory LH surge following GnRH administration [45, 46]. Similar to the findings of Mirmahmoudi and Prakash [47], ovulation was detected in both treatments when estradiol was at basal level (1 pg/ml).
Notably, the primary concern in the Cosynch protocol utilizing progesterone (CIDR, progesterone-releasing intravaginal device (PRID), MAP sponge) is the interval to ovulation [48]. Indeed, prior research has demonstrated that application of the GnRH-TAI-84 procedure following CIDR removal shortened the ovulation interval in buffalo heifers [26]. Our study observed a similar trend of shortened interval to ovulation after sponge removal in the GnRH-TAI-84 group compared to those not receiving GnRH. Based on this information, it can be inferred that the administration of GnRH 84 h following sponge removal better synchronizes ovulation at 12 h, and TAI performed– 8–20 h before ovulation results in a higher P/AI in buffaloes, as the average time frame for sperm to reach the uterus after introduction is typically 10–14 h before ovulation [26, 49]. The estimated time of ova viability is 6–12 h after ovulation [50]. The presence of capacitated sperm at the site of fertilization within 6 to 8 h after mating in cows [51] suggests that performing AI too early or late in relation to ovulation reduces fertilization and P/AI because of the presence of premature [51] or aging oocytes at the time of fertilization [52]. Hence, ovulation time and oocyte age at fertilization are critical events for conception.
In postpartum buffaloes, exposure to subluteal progesterone stimulates the growth and maturation of the dominant follicle by increasing LH release and inducing LH receptors, resulting in increased estradiol concentration and ovulation rate [53, 54]. Moreover, progesterone pre-exposure can predict the duration and amplitude of the LH surge by GnRH injection after the progesterone device, which may, in turn, augment CL growth, endogenous progesterone production, and pregnancy maintenance [55, 56]. Initially, the MAP sponge helped to fine-tune the hypothalamus to improve gonadotropin control, folliculogenesis, and estrus expression in buffaloes [57, 58]. It further has the potential to induce ovulation of the follicle earlier due to the increased frequency of LH pulses following the removal of the MAP sponge or PGF2α administration.
The utilization of progesterone in conventional farming systems has been shown to enhance estrus intensity by increasing the sensitivity of the hypothalamus to estrogen, resulting in more pronounced estrus in buffaloes [18, 49]. The current investigation revealed that estrus intensity was uniformly high across all treatment groups, indicating the application of MAP sponges in pronounced estrus expression, as was previously observed in beef cattle [59]. In the present study, MAP sponge insertion for seven days, with GnRH administration at the time of AI (84 h after MAP sponge removal) may have enhanced the P/AI in buffaloes.
In the present study, the P/AI was significantly higher in animals in the GnRH-TAI-84 group than in those in the TAI-84 group. Indeed, similar observations of P/AI have been made in cows when the CIDR Cosynch protocol was applied [45, 60]. The GnRH-induced LH surge, which recruits more granulosa cells to become luteal cells for progesterone production, or an increase in progesterone production by existing cells [61], may also have enhanced pregnancy/AI in the current experiment. In light of our research, we confirmed that ovulation synchronized 12 h after the administration the GnRH-TAI-84 protocol. Overall, our findings provide crucial information regarding the timing of ovulation and insemination, the lifespan of sperm, and the likelihood of P/AI in this species. As such, these findings have significant implications for improving buffalo TAI protocols.
In conclusion, the administration of GnRH 84 h following sponge removal improves follicular growth rate, shortens the interval from AI to ovulation, reduces preovulatory follicle size, and sharply decreases estradiol levels, leading to ovulation, which may enhance P/AI in buffaloes. This protocol could have a major impact on the management of reproduction in postpartum buffaloes as it allows insemination at a known time of ovulation by eliminating estrus detection. In addition, it is a cost-effective and less labor-intensive approach. Future studies should be designed to test this protocol in larger buffalo herds to synchronize ovulation, which could enhance P/AI.
Conflict of interests
None of the authors have any conflicts of interest to disclose in regards to this manuscript.
Acknowledgments
We would like to express our gratitude to the staff of the Buffalo Research Institute, Pattoki for providing the animals. We also extend our gratitude to Selmore Pharmaceutical Pakistan for supplying the hormones, and thank the staff of the Nuclear Institute of Agriculture and Biology, Faisalabad, who assisted in the hormonal analysis and provided the sponges for this experiment. Major Project of Science and Technology of Nanning (20241033).
References
- 1.Stevenson JS, Britt JH. A 100-year review: practical female reproductive management. J Dairy Sci 2017; 100: 10292–10313. [DOI] [PubMed] [Google Scholar]
- 2.Neglia G, Restucci B, Russo M, Vecchio D, Gasparrini B, Prandi A, Di Palo R, D’Occhio MJ, Campanile G. Early development and function of the corpus luteum and relationship to pregnancy in the buffalo. Theriogenology 2015; 83: 959–967. [DOI] [PubMed] [Google Scholar]
- 3.Neglia G, Vecchio D, Russo M, Di Palo R, Pacelli C, Comin A, Gasparrini B, Campanile G. Efficacy of PGF(2α) on pre-ovulatory follicle and corpus luteum blood flow. Reprod Domest Anim 2012; 47: 26–31. [DOI] [PubMed] [Google Scholar]
- 4.Campanile G, Baruselli PS, Neglia G, Vecchio D, Gasparrini B, Gimenes LU, Zicarelli L, D’Occhio MJ. Ovarian function in the buffalo and implications for embryo development and assisted reproduction. Anim Reprod Sci 2010; 121: 1–11. [DOI] [PubMed] [Google Scholar]
- 5.Bhat GR, Dhaliwal GS. Estrus and ovulation synchrony of buffaloes (Bubalus bubalis): A review. Buffalo Bull 2023; 42: 239–261. [Google Scholar]
- 6.Peeler ID, Nebel RL, Pearson RE, Swecker WS, Garcia A. Pregnancy rates after timed AI of heifers following removal of intravaginal progesterone inserts. J Dairy Sci 2004; 87: 2868–2873. [DOI] [PubMed] [Google Scholar]
- 7.Pursley JR, Silcox RW, Wiltbank MC. Effect of time of artificial insemination on pregnancy rates, calving rates, pregnancy loss, and gender ratio after synchronization of ovulation in lactating dairy cows. J Dairy Sci 1998; 81: 2139–2144. [DOI] [PubMed] [Google Scholar]
- 8.Ayres H, Martins CM, Ferreira RM, Mello JE, Dominguez JH, Souza AH, Valentin R, Santos IC, Baruselli PS. Effect of timing of estradiol benzoate administration upon synchronization of ovulation in suckling Nelore cows (Bos indicus) treated with a progesterone-releasing intravaginal device. Anim Reprod Sci 2008; 109: 77–87. [DOI] [PubMed] [Google Scholar]
- 9.Vecchio D, Neglia G, Gasparrini B, Russo M, Pacelli C, Prandi A, D’Occhio MJ, Campanile G. Corpus luteum development and function and relationship to pregnancy during the breeding season in the Mediterranean buffalo. Theriogenology 2012; 77: 1811–1815. [DOI] [PubMed] [Google Scholar]
- 10.Neglia G, Gasparrini B, Di Palo R, De Rosa C, Zicarelli L, Campanile G. Comparison of pregnancy rates with two estrus synchronization protocols in Italian Mediterranean Buffalo cows. Theriogenology 2003; 60: 125–133. [DOI] [PubMed] [Google Scholar]
- 11.Chaikhun T, Tharasanit T, Rattanatep J, De Rensis F, Techakumphu M. Fertility of swamp buffalo following the synchronization of ovulation by the sequential administration of GnRH and PGF2alpha combined with fixed-timed artificial insemination. Theriogenology 2010; 74: 1371–1376. [DOI] [PubMed] [Google Scholar]
- 12.Rossi P, Vecchio D, Neglia G, Di Palo R, Gasparrini B, D’Occhio MJ, Campanile G. Seasonal fluctuations in the response of Italian Mediterranean buffaloes to synchronization of ovulation and timed artificial insemination. Theriogenology 2014; 82: 132–137. [DOI] [PubMed] [Google Scholar]
- 13.Di Francesco S, Neglia G, Vecchio D, Rossi P, Russo M, Zicarelli L, D’Occhio MJ, Campanile G. Influence of season on corpus luteum structure and function and AI outcome in the Italian Mediterranean buffalo (Bubalus bubalis). Theriogenology 2012; 78: 1839–1845. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho NA, Soares JG, Porto Filho RM, Gimenes LU, Souza DC, Nichi M, Sales JS, Baruselli PS. Equine chorionic gonadotropin improves the efficacy of a timed artificial insemination protocol in buffalo during the nonbreeding season. Theriogenology 2013; 79: 423–428. [DOI] [PubMed] [Google Scholar]
- 15.Naseer Z, Ahmad E, Singh J, Ahmad N. Fertility following CIDR based synchronization regimens in anoestrous Nili-Ravi buffaloes. Reprod Domest Anim 2011; 46: 814–817. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho NA, Soares JG, Souza DC, Vannucci FS, Amaral R, Maio JR, Sales JN, Sá Filho MF, Baruselli PS. Different circulating progesterone concentrations during synchronization of ovulation protocol did not affect ovarian follicular and pregnancy responses in seasonal anestrous buffalo cows. Theriogenology 2014; 81: 490–495. [DOI] [PubMed] [Google Scholar]
- 17.Paul V, Prakash BS. Efficacy of the Ovsynch protocol for synchronization of ovulation and fixed-time artificial insemination in Murrah buffaloes (Bubalus bubalis). Theriogenology 2005; 64: 1049–1060. [DOI] [PubMed] [Google Scholar]
- 18.Yousuf MR, Martins JP, Husnain A, Riaz U, Riaz H, Sattar A, Javed K, Ahmad N. Effect of oestradiol benzoate on oestrus intensity and pregnancy rate in CIDR treated anoestrus nulliparous and multiparous buffalo. Anim Reprod Sci 2015; 159: 104–108. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho NAT, Soares JG, Souza DC, Maio JRG, Sales JNS, Martins Júnior B, Macari RC, D’Occhio MJ, Baruselli PS. Ovulation synchronization with estradiol benzoate or GnRH in a timed artificial insemination protocol in buffalo cows and heifers during the nonbreeding season. Theriogenology 2017; 87: 333–338. [DOI] [PubMed] [Google Scholar]
- 20.Vasconcelos JLM, Jardina DT, Sá Filho OG, Aragon FL, Veras MB. Comparison of progesterone-based protocols with gonadotropin-releasing hormone or estradiol benzoate for timed artificial insemination or embryo transfer in lactating dairy cows. Theriogenology 2011; 75: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 21.Wiltbank MC, Sartori R, Herlihy MM, Vasconcelos JLM, Nascimento AB, Souza AH, Ayres H, Cunha AP, Keskin A, Guenther JN, Gumen A. Managing the dominant follicle in lactating dairy cows. Theriogenology 2011; 76: 1568–1582. [DOI] [PubMed] [Google Scholar]
- 22.Pursley JR, Kosorok MR, Wiltbank MC. Reproductive management of lactating dairy cows using synchronization of ovulation. J Dairy Sci 1997; 80: 301–306. [DOI] [PubMed] [Google Scholar]
- 23.Bridges GA, Mussard ML, Burke CR, Day ML. Influence of the length of proestrus on fertility and endocrine function in female cattle. Anim Reprod Sci 2010; 117: 208–215. [DOI] [PubMed] [Google Scholar]
- 24.Peters MW, Pursley JR. Timing of final GnRH of the Ovsynch protocol affects ovulatory follicle size, subsequent luteal function, and fertility in dairy cows. Theriogenology 2003; 60: 1197–1204. [DOI] [PubMed] [Google Scholar]
- 25.Peres RF, Claro I, Jr, Sá Filho OG, Nogueira GP, Vasconcelos JL. Strategies to improve fertility in Bos indicus postpubertal heifers and nonlactating cows submitted to fixed-time artificial insemination. Theriogenology 2009; 72: 681–689. [DOI] [PubMed] [Google Scholar]
- 26.Haider S, Chishti GA, Mehmood MU, Jamal MA, Mehmood K, Shahzad M, Tahir MZ. The effect of GnRH administration/insemination time on follicular growth rate, ovulation intervals, and conception rate of Nili Ravi buffalo heifers in 7 -day-CIDR Co-synch. Trop Anim Health Prod 2021; 53: 558. [DOI] [PubMed] [Google Scholar]
- 27.Perry GA, Smith MF, Lucy MC, Green JA, Parks TE, MacNeil MD, Roberts AJ, Geary TW. Relationship between follicle size at insemination and pregnancy success. Proc Natl Acad Sci USA 2005; 102: 5268–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haider MS, Hassan M, Khan AS, Husnain A, Bilal M, Pursley JR, Ahmad N. Effect of timing of insemination after CIDR removal with or without GnRH on pregnancy rates in Nili-Ravi buffalo. Anim Reprod Sci 2015; 163: 24–29. [DOI] [PubMed] [Google Scholar]
- 29.Nasir Hussain Shah S, Van De Wiel D, Willemse A, Engel B. Opposite breeding seasons in dairy zebu cows and dairy river buffaloes as assessed by first insemination records. Anim Reprod Sci 1989; 21: 25–35. [Google Scholar]
- 30.Ferguson JD, Galligan DT, Thomsen N. Principal descriptors of body condition score in Holstein cows. J Dairy Sci 1994; 77: 2695–2703. [DOI] [PubMed] [Google Scholar]
- 31.Baerwald AR, Walker RA, Pierson RA. Growth rates of ovarian follicles during natural menstrual cycles, oral contraception cycles, and ovarian stimulation cycles. Fertil Steril 2009; 91: 440–449. [DOI] [PubMed] [Google Scholar]
- 32.Waqas M, Mehmood MU, Shahzad Q, Kausar R, Sattar A, Naseer Z. Comparative efficacy of G6G and Ovsynch protocols on synchronization and pregnancy rate in Nili-Ravi buffalo. Anim Reprod Sci 2016; 166: 9–14. [DOI] [PubMed] [Google Scholar]
- 33.Gimenes LU, Carvalho NA, Sá Filho MF, Vannucci FS, Torres-Júnior JR, Ayres H, Ferreira RM, Trinca LA, Sartorelli ES, Barros CM, Beltran MP, Nogueira GP, Mapletoft RJ, Baruselli PS. Ultrasonographic and endocrine aspects of follicle deviation, and acquisition of ovulatory capacity in buffalo (Bubalus bubalis) heifers. Anim Reprod Sci 2011; 123: 175–179. [DOI] [PubMed] [Google Scholar]
- 34.Sartori R, Fricke PM, Ferreira JC, Ginther OJ, Wiltbank MC. Follicular deviation and acquisition of ovulatory capacity in bovine follicles. Biol Reprod 2001; 65: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 35.Vasconcelos JL, Sartori R, Oliveira HN, Guenther JG, Wiltbank MC. Reduction in size of the ovulatory follicle reduces subsequent luteal size and pregnancy rate. Theriogenology 2001; 56: 307–314. [DOI] [PubMed] [Google Scholar]
- 36.Busch DC, Atkins JA, Bader JF, Schafer DJ, Patterson DJ, Geary TW, Smith MF. Effect of ovulatory follicle size and expression of estrus on progesterone secretion in beef cows. J Anim Sci 2008; 86: 553–563. [DOI] [PubMed] [Google Scholar]
- 37.Dickinson S, Geary T, Monnig J, Pohler K, Green J, Smith M. Effect of preovulatory follicle maturity on pregnancy establishment in cattle: the role of oocyte competence and the maternal environment. Anim Reprod 2016; 13: 209–216. [Google Scholar]
- 38.Souza AH, Viechnieski S, Lima FA, Silva FF, Araújo R, Bó GA, Wiltbank MC, Baruselli PS. Effects of equine chorionic gonadotropin and type of ovulatory stimulus in a timed-AI protocol on reproductive responses in dairy cows. Theriogenology 2009; 72: 10–21. [DOI] [PubMed] [Google Scholar]
- 39.Pandey AK, Ghuman SPS, Dhaliwal GS, Honparkhe M, Phogat JB, Kumar S. Effects of preovulatory follicle size on estradiol concentrations, corpus luteum diameter, progesterone concentrations and subsequent pregnancy rate in buffalo cows (Bubalus bubalis). Theriogenology 2018; 107: 57–62. [DOI] [PubMed] [Google Scholar]
- 40.Pandey AK, Dhaliwal GS, Ghuman SP, Agarwal SK. Impact of pre-ovulatory follicle diameter on plasma estradiol, subsequent luteal profiles and conception rate in buffalo (Bubalus bubalis). Anim Reprod Sci 2011; 123: 169–174. [DOI] [PubMed] [Google Scholar]
- 41.Jinks EM, Smith MF, Atkins JA, Pohler KG, Perry GA, Macneil MD, Roberts AJ, Waterman RC, Alexander LJ, Geary TW. Preovulatory estradiol and the establishment and maintenance of pregnancy in suckled beef cows. J Anim Sci 2013; 91: 1176–1185. [DOI] [PubMed] [Google Scholar]
- 42.Bridges GA, Helser LA, Grum DE, Mussard ML, Gasser CL, Day ML. Decreasing the interval between GnRH and PGF2α from 7 to 5 days and lengthening proestrus increases timed-AI pregnancy rates in beef cows. Theriogenology 2008; 69: 843–851. [DOI] [PubMed] [Google Scholar]
- 43.Singh H, Pandey AK, Kumar S, Saini G, Duggal R, Bangar YC, Kumar S, Saini R, Kumar H. 5d CIDR-Heatsynch improves the circulatory estradiol levels, estrus expression and conception rate in anestrus buffalo (Bubalus bubalis). Anim Biotechnol 2023; 34: 4488–4499. [DOI] [PubMed] [Google Scholar]
- 44.Morgan WF, Lean IJ. Gonadotrophin-releasing hormone treatment in cattle: a meta-analysis of the effects on conception at the time of insemination. Aust Vet J 1993; 70: 205–209. [DOI] [PubMed] [Google Scholar]
- 45.Bisinotto RS, Ribeiro ES, Santos JE. Synchronisation of ovulation for management of reproduction in dairy cows. Animal 2014; 8(Suppl 1): 151–159. [DOI] [PubMed] [Google Scholar]
- 46.Pereira MH, Sanches CP, Guida TG, Rodrigues AD, Aragon FL, Veras MB, Borges PT, Wiltbank MC, Vasconcelos JL. Timing of prostaglandin F2α treatment in an estrogen-based protocol for timed artificial insemination or timed embryo transfer in lactating dairy cows. J Dairy Sci 2013; 96: 2837–2846. [DOI] [PubMed] [Google Scholar]
- 47.Mirmahmoudi R, Prakash BS. The endocrine changes, the timing of ovulation and the efficacy of the Doublesynch protocol in the Murrah buffalo (Bubalus bubalis). Gen Comp Endocrinol 2012; 177: 153–159. [DOI] [PubMed] [Google Scholar]
- 48.Colazo MG, Mapletoft RJ. A review of current timed-AI (TAI) programs for beef and dairy cattle. Can Vet J 2014; 55: 772–780. [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmad N, Arshad U. Synchronization and resynchronization strategies to improve fertility in dairy buffaloes. Theriogenology 2020; 150: 173–179. [DOI] [PubMed] [Google Scholar]
- 50.Brackett BG, Oh YK, Evans JF, Donawick WJ. Fertilization and early development of cow ova. Biol Reprod 1980; 23: 189–205. [DOI] [PubMed] [Google Scholar]
- 51.Hunter R, Wilmut I. The rate of functional sperm transport into the oviducts of mated cows. Anim Reprod Sci 1983; 5: 167–173. [Google Scholar]
- 52.Hunter R, Greve T. Could artificial insemination of cattle be more fruitful? Penalties associated with ageing eggs. Reprod Domest Anim 1997; 32: 137–141. [Google Scholar]
- 53.de Carvalho NAT, Soares JG, Baruselli PS. Strategies to overcome seasonal anestrus in water buffalo. Theriogenology 2016; 86: 200–206. [DOI] [PubMed] [Google Scholar]
- 54.Neglia G, de Nicola D, Esposito L, Salzano A, D’Occhio MJ, Fatone G. Reproductive management in buffalo by artificial insemination. Theriogenology 2020; 150: 166–172. [DOI] [PubMed] [Google Scholar]
- 55.Jacomini JO, Macedo GG, de Carvalho NAT, de Sousa Sales JN, Baruselli PS. LH surge in response to the treatment with GnRH analog or estradiol in ovariectomized buffaloes with or without progesterone pre-exposition. Livest Sci 2014; 160: 194–198. [Google Scholar]
- 56.Baruselli PS, Soares JG, Bayeux BM, Silva JCB, Mingoti RD, Carvalho NAT. Assisted reproductive technologies (ART) in water buffaloes. Anim Reprod 2018; 15(Suppl 1): 971–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kausar R, Khanum S, Hussain M, Hussain T, Ahmad N, Ahmad L, Qureshi N. Estrus synchronization and conception rates using locally prepared methylacetoxyprogesterone sponges in cyclic and acyclic Nili-Ravi buffaloes (Bubalus bubalis). Pak Vet J 2013; 33: 433–437. [Google Scholar]
- 58.Barile V. Improving reproductive efficiency in female buffaloes. Livest Prod Sci 2005; 92: 183–194. [Google Scholar]
- 59.Viñoles C, Quintans G, Paiva N, Cavestany D. Treatment of suckling beef cattle with a progestagen sponge and oestradiol benzoate or equine chorionic gonadotrophin. Vet Rec 2004; 154: 106–109. [DOI] [PubMed] [Google Scholar]
- 60.Dobbins CA, Eborn DR, Tenhouse DE, Breiner RM, Johnson SK, Marston TT, Stevenson JS. Insemination timing affects pregnancy rates in beef cows treated with CO-Synch protocol including an intravaginal progesterone insert. Theriogenology 2009; 72: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 61.Lee CN, Critser JK, Ax RL. Changes of luteinizing hormone and progesterone for dairy cows after gonadotropin-releasing hormone at first postpartum breeding. J Dairy Sci 1985; 68: 1463–1470. [DOI] [PubMed] [Google Scholar]