Abstract
The size of the ovarian reserve, an indicator of the number of primordial follicles, varies widely among individuals, and declines with age. However, the association between the ovarian reserve and fertility remains unclear. Therefore, in the present study, we analyzed the relationship between plasma concentrations of anti-Müllerian hormone (AMH), a marker of ovarian reserve, and reproductive outcomes in Japanese Black cattle. AMH level quartiles were positively associated with pregnancy following artificial insemination (AI), and the median number of days to pregnancy in Q4 (13 days, 95% confidence interval [CI] = 7–18 days) was significantly shorter than that in Q1 (21 days, 95% CI = 15–46 days). The odds ratio for the predicted pregnancy rate by logistic regression analysis in Q4 (4.06, 95% CI = 1.54–10.67) was also significantly higher than that in Q1. Plasma AMH concentrations were significantly higher in summer (June–August) than in winter (December–February). Furthermore, a strong correlation (r = 0.856, P < 0.001) was observed between plasma AMH concentrations at 2 and 14 months of age. Calves with plasma AMH concentrations of > 700 pg/ml at 2 months old showed a transient increase and maximum AMH concentration within 5 months of birth. Overall, the results of this study indicate that the plasma AMH concentration serves as a predictive marker for the probability of conception following AI in Japanese Black cattle. The current findings contribute to the reliable assessment of AMH production and the early prediction of reproductive performance in sexually mature heifers.
Keywords: Anti-Müllerian hormone (AMH), Artificial insemination, Fertility, Japanese Black cattle, Vitamin D3
Anti-Müllerian hormone (AMH) is a glycoprotein produced by the granulosa cells of the growing preantral and antral follicles in female animals [1, 2]. Thus, circulating AMH levels act as indirect indicators of ovarian reserve, reflecting the number of primordial follicles in the ovary. In cattle, the circulating AMH concentration has been extensively investigated as a donor selection criterion for in vivo fertilized embryos through superovulation and oocyte collection via ovum pickup [3, 4]. Furthermore, several studies have reported a correlation between high antral follicle count (AFC) in the ovary, another indicator of ovarian reserve, and higher pregnancy rates after artificial insemination (AI) [5,6,7]. The correlation between blood AMH concentration and pregnancy rate after AI has not been fully elucidated; however, reduced AMH concentration in Holstein cattle has been shown to be associated with shorter reproductive herd life and increased pregnancy loss during early pregnancy [8, 9]. AMH concentrations vary widely among individuals, and are influenced by characteristics induced by breed and physiological states [8, 10, 11]. Further research is therefore required to establish the value of AMH as an indicator of fertility in cattle [12].
Blood AMH concentrations vary less throughout the estrous cycle compared to the typical hormones involved in follicle development, such as follicle-stimulating hormone and estrogen [13]. However, blood AMH concentrations can be influenced by several factors when monitored over longer periods. Dennis et al. [14] previously reported that serum AMH concentrations in women were lower in winter than in summer and correlated with serum vitamin D3 (VD3) concentrations. VD3 is a steroid that promotes estrogen synthesis and AMH production in granulosa cells [14,15,16]. In cattle, VD3 synthesis in the skin decreases during winter because of the shorter daylight hours [17]. However, its effect on AMH production in cattle remains unclear. The blood AMH concentration in postnatal female cattle fluctuates until sexual maturity. Female calves further undergo transient increases in blood AMH concentration during the first few months of life [10, 18,19,20]. Understanding the long-term variation in AMH production and its influencing factors is crucial for elucidating the validity of AMH as a predictor of fertility in mature female cattle. The prediction of low fertility in cattle as industrial animals can enhance production efficiency through the selection of appropriate reproductive management programs. Assessing the ovarian reserve in calves may further enable the early prediction of fertility post-sexual maturity, with the measurement of AMH concentration being a potential method for this evaluation.
In Experiment 1 of the present study, we analyzed the relationship between plasma AMH concentration and pregnancy rate following AI in Japanese Black cattle. In Experiment 2, the correlation between seasonal variations in blood AMH and VD3 concentrations was investigated in Japanese Black cattle raised in Hokkaido, a subarctic region of Japan. In Experiment 3, the fluctuations in blood AMH concentrations in Japanese Black heifers during rearing and the early predictive utility of blood AMH concentrations following sexual maturity were examined.
Materials and Methods
Ethics
Experiments 1 and 2 used surplus serum and plasma samples, respectively, obtained from routine health checks or screening for infectious diseases, as part of standard livestock management. For Experiment 3, blood sampling was rapidly conducted by skilled technicians with minimal restraint, following the ethical guidelines of the Animal Research Center’s Animal Care and Use Committee. This committee does not issue approval numbers for research projects that focus solely on blood collection.
Blood sampling
Blood samples were collected from the tail vein, except for those extracted from young calves, which were collected from the jugular vein. Vacuum tubes containing a blood coagulation promoter (VP-P050K; Terumo Corporation, Tokyo, Japan) were used for serum collection in Experiment 1. Vacuum tubes (VP-NA070K, Terumo Corporation) containing ethylenediaminetetraacetic acid disodium salt (EDTA-2Na) were further used for plasma collection in Experiments 2 (Farm B) and 3. On Farm A in Experiment 2, blood samples were collected in vacuum tubes containing sodium heparin (VP-H070K; Terumo Corporation). The samples were centrifuged at 1,710 × g for 15 min at 4°C immediately after collection. Plasma and serum were stored at −30°C until the measurement of AMH and VD3 concentrations.
Measurement of AMH and VD3 concentrations
Serum and plasma AMH concentrations were measured in duplicate using a Bovine AMH ELISA kit (AL-114, Ansh Labs, Webster, TX, USA), in accordance with the manufacturer’s instructions, except for Farm A in Experiment 2. The samples were diluted three times with the kit buffer solution. Serum AMH concentrations were then converted to EDTA-2Na-treated plasma equivalents for analysis following the conversion formula described in the manufacturer’s instructions. In a preliminary test, the formula was validated using the serum and plasma AMH concentrations of 30 individuals. The conversion formula is as follows:
Converted plasma AMH (pg/ml) = (serum AMH (pg/ml) + 2.15) / 0.97.
In Experiment 2, Farm A, plasma AMH concentrations were measured in duplicate using the AMH Gen II ELISA kit (Beckman Coulter, Inc., Brea, CA, USA), as described previously, with some modifications to improve assay sensitivity [21].
Plasma VD3 concentrations were measured in duplicate using a Vitamin D ELISA kit (501050; Cayman Chemical, MI, USA), in accordance with the manufacturer’s instructions. This kit is primarily used to detect the metabolically stable 25-hydroxy vitamin D3 (25[OH]D3). To accurately detect vitamin D, vitamin D-binding proteins were separated from vitamin D using acetone. The plasma was mixed with a two-fold volume of acetone and centrifuged at 10,000 × g for 10 min. The supernatant was carefully collected and placed into a clean glass tube, and then evaporated by gentle blowing of nitrogen gas at 37°C. Subsequently, the samples were dissolved in a 20-fold volume of the supernatant in ELISA buffer and used for the 25(OH)D3 assay. The 25(OH)D3 concentration was measured in duplicate following the manufacturer’s instructions.
Experiment 1: Fertility assessment using plasma AMH level
Plasma AMH concentrations were measured in 292 Japanese Black cattle (112 heifers and 180 cows) from a seasonal calving herd raised between 2021 and 2023 at the Animal Research Center (Farm A) in Hokkaido, Japan. Blood samples were collected in April of each year, regardless of the estrous cycle. The mean ages of the heifers and cows were 20.8 ± 6.8 and 69.6 ± 31.0 months, respectively. The parity of the cows was 2.6 ± 1.8.
The cattle were housed in an outdoor loose-housing barn and fed a total mixed ration (TMR) from mid-November to late May. The TMR comprised grass silage and concentrate containing vitamin D. However, the vitamin D content of the concentrate was not disclosed because of trade secrecy. The cattle were fed in a pasture in a perennial ryegrass-dominated grassland for the remainder of the year.
AI for cows in natural estrus was performed between late March and mid-May each year. The cattle were visually monitored for signs of estrus by farm staff for 1 h each morning and evening. An activity meter (Velos, Nedap, Netherlands) was further used to detect estrus. Cows showing signs of estrus were inseminated with frozen semen from 26 Japanese Black bulls by 10 skilled technicians. Cows that returned to estrus following AI were re-inseminated. Ultrasonographic pregnancy diagnosis was performed 60–90 days after AI.
Cattle were categorized into four groups based on the quartiles of plasma AMH concentration: Q1 (n = 74; median: 210 pg/ml; range: 15–335 pg/ml), Q2 (n = 72; median: 453 pg/ml; range: 342–573 pg/ml), Q3 (n = 73; median: 725 pg/ml; range: 580–942 pg/ml), and Q4 (n = 73; median = 1,397 pg/ml; range: 949–4,765 pg/ml).
Experiment 2: Seasonal variation of plasma AMH and VD3 concentration
The experiment was conducted on Japanese Black cows from Farm A (43 °N), and heifers from a commercial farm (Farm B, 43 °N) in Hokkaido, Japan. On Farm A, cows were housed in an outdoor loose-housing barn, and fed grass silage or grass hay ad libitum. None of the cows were fed concentrate. AMH concentrations were measured once a month in 1–9 randomly selected cyclic cows from October 2010 to June 2013. AMH concentrations in 136 plasma samples were divided into groups by month, as follows: December–February (n = 46, 89.0 ± 36.2 months old), March–May (n = 26, 91.6 ± 37.7 months old), June–August (n = 19, 79.3 ± 36.1 months old), and September–November (n = 45, 82.3 ± 31.5 months old).
On Farm B, the heifers were housed in an indoor loose-housing barn and fed a TMR comprising grass hay, corn silage, and concentrate supplemented with vitamin D. However, the vitamin D content of the concentrate was not disclosed due to trade secrecy. Plasma AMH concentrations were measured once a month in 25–40 randomly selected heifers between August 2020 and September 2022. AMH concentrations in 709 plasma samples were divided into the following groups based on month: December–February (n = 146, 8.5 ± 1.0 months old), March–May (n = 143, 8.4 ± 1.0 months old), June–August (n = 227, 8.3 ± 1.0 months old), and September–November (n = 193, 8.4 ± 0.8 months old).
Plasma VD3 concentrations were measured in 232 randomly selected heifers from Farm B and divided into the following groups based on month: December–February (n = 59, 8.2 ± 0.6 months old), March–May (n = 29, 8.0 ± 0.6 months old), June–August (n = 58, 8.1 ± 0.6 months old), and September–November (n = 86, 7.9 ± 0.5 months old).
Experiment 3: Plasma AMH concentration during the rearing period
Experiment 3 was conducted on 19 Japanese Black heifers born on Farm A between 2020 and 2022. The calves were kept either with their dams, or reared artificially in an indoor loose-housing barn until weaning at 2.5–3 months of age. After weaning, calves were fed with grass hay and concentrate containing vitamin D ad libitum. However, the vitamin D content of the concentrate was not disclosed because of trade secrecy. Blood samples were collected 2 days after birth and monthly until 10 months of age. Blood samples were collected at the ages of 12 and 14 months. Blood samples were not obtained from four calves at 0 months of age and from two calves at 1 month of age, resulting in some missing values. Plasma AMH concentrations before 12 months of age were analyzed for correlation with those at 14 months of age, at which point sexual maturity had been reached. In addition, the calves were divided almost equally into low (n = 6), mid (n = 7), and high (n = 6) groups based on plasma AMH concentrations at 2 months of age, to analyze the changes in plasma AMH concentrations during the rearing period.
Statistical analysis
In the preliminary test, Spearman’s correlation coefficient was applied to evaluate the relationship between actual and converted plasma AMH concentrations. The Wilcoxon signed-rank test was further used to assess the difference between the actual and converted plasma AMH concentrations.
In Experiment 1, the association between AMH classification and the duration from the start of AI to pregnancy was assessed using the Cox proportional hazards model. In this statistical model, days to pregnancy were used as the time variables. Age at the first AI, birth history (yes/no), and year of blood collection were included as explanatory variables. The follow-up period lasted from the beginning to the end of the breeding season (56 or 57 day). Data on all cows that were not diagnosed as pregnant, culled, or had ceased breeding during the study period were censored. The hazard ratio indicates the probability that cattle in Q2, Q3, and Q4 will become pregnant compared with those in Q1 during the breeding season. The median number of days to pregnancy was calculated based on the Kaplan–Meier survival curve.
Logistic regression analysis was further performed to evaluate the association between pregnancy after the first AI during the breeding season and plasma AMH concentrations. The regression model incorporated pregnancy by the first AI as the objective variable, and age, birth history (yes/no), and AMH concentration category or AMH concentration as the explanatory variables. Individual and year of blood collection were included as random effects. The reference category for AMH concentration was Q1. The association between pregnancy according to the first AI and AMH concentration categories was determined using the odds ratio (OR). Furthermore, the relationship between AMH concentration and initial AI conception was assessed using both linear and quadratic regressions.
In the preliminary Cox proportional hazards and logistic regression models, the interaction between the AMH concentration category or concentration and birth history was analyzed to assess the necessity of using models based on birth history.
In Experiment 2, the Kruskal–Wallis test was applied to assess differences in AMH concentrations between seasons. The Steel–Dwass method was used for multiple comparisons. Spearman’s correlation coefficient was used to evaluate the relationship between AMH and VD3 concentrations.
In Experiment 3, Spearman’s correlation coefficient was used to evaluate the relationship between AMH concentrations and age in months.
Statistical significance was set at P < 0.05. All analyses were conducted using the R statistical package version 4.3.2 (R Core Team, 2023). All results are presented as the mean ± standard deviation.
Results
Experiment 1: Fertility assessment using plasma AMH level
In the preliminary test, the actual and converted plasma AMH concentrations to validate the conversion formula were 470 ± 305 pg/ml and 470 ± 265 pg/ml, respectively. The correlation coefficient between the actual and converted concentrations was 0.956 (P < 0.001), and there were no significant differences between the actual and converted concentrations (P = 0.352).
Considerable variability in AMH concentrations was observed among individuals (Fig. 1A). The mean ± standard deviation, median, minimum, and maximum AMH concentrations were 743 ± 642, 577, 15, and 4,765 pg/ml, respectively. The conception rates following the first AI and the pregnancy rates during the breeding period in heifers and cows were 68.5% and 56.1% and 78.6% and 68.3%, respectively.
Fig. 1.
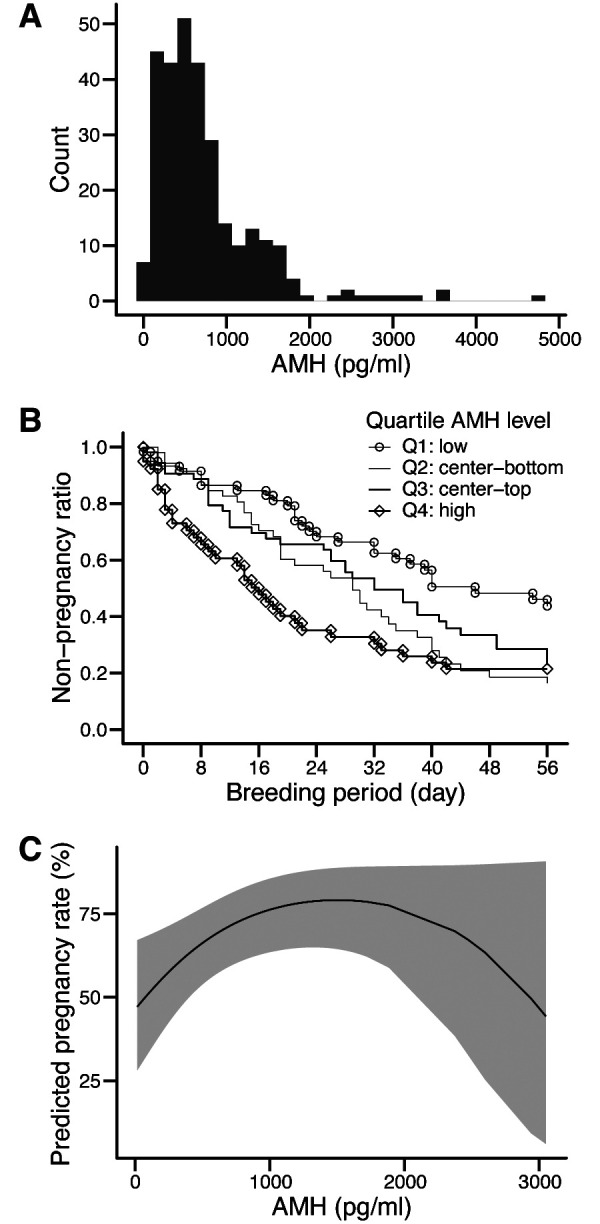
Fertility according to plasma anti-Müllerian hormone (AMH) levels in Japanese Black cattle raised on Farm A. (A) Frequency distribution of plasma AMH concentrations. (B) Survival curves of non-pregnant cows by AMH level quartile. (C) Fertility following artificial insemination, as predicted using logistic regression analysis with plasma AMH concentrations as the explanatory variable. The gray regions indicate the 95% confidence intervals.
The hazard ratio for the probability of cows becoming pregnant during the breeding season was 1.24 (95% confidence interval [CI] = 0.82–1.87) for Q2; 1.03 (95% CI = 0.66–1.60) for Q3; and 1.79 (95% CI = 1.09–2.93) for Q4. The Cox proportional hazards model revealed no significant interaction between the AMH concentration category and birth history (P > 0.05). The hazard ratio for Q4 was significantly higher than that for Q1 (P < 0.05). The median number of days to pregnancy was 21 (95% CI = 15–46 days) for Q1, 19 (95% CI = 15–33 days) for Q2, 17 (95% CI = 11–40 days) for Q3, and 13 (95% CI = 7–18 days) for Q4 (Fig. 1B).
The relationship between plasma AMH concentration and the predicted pregnancy rate at the first AI exhibited a quadratic trend, increasing with increasing AMH concentration up to 1,600 pg/ml, and decreasing thereafter (Fig. 1C). Logistic regression analysis revealed no significant interaction between AMH concentration category or concentration and birth history (P > 0.05). The odds ratio for the predicted pregnancy rate at the first AI was 1.59 (95% CI = 0.75–3.38) for Q2; 1.15 (95% CI = 0.53–2.49) for Q3; and 4.06 (95% CI = 1.54–10.67) for Q4. The odds ratio for Q4 was significantly higher than that for Q1 (P < 0.01).
Experiment 2: Seasonal variation of plasma AMH and VD3 concentration
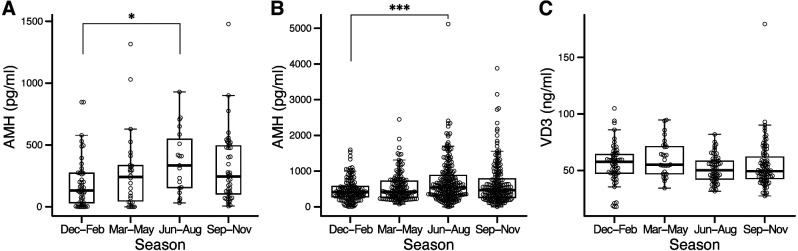
Plasma AMH concentrations at Farm A were significantly higher during June–August than from December–February (Fig. 2A). The AMH Gen II ELISA kit used in this analysis has been validated for bovine plasma samples. However, this kit was initially designed to measure human AMH concentrations [21]. Therefore, a validation experiment was conducted at Farm B using the bovine-specific ELISA kit used for Experiment 1. Plasma AMH concentrations at Farm B were also significantly higher during June–August than during December–February (Fig. 2B). No significant differences in VD3 concentrations were observed between seasons (Fig. 2C). No correlation was found between VD3 and AMH concentrations (r = −0.073, P = 0.265).
Fig. 2.
Seasonal variations in plasma anti-Müllerian hormone (AMH) and vitamin D3 (VD3) concentrations in Japanese Black cattle. Plasma AMH concentrations in cows from Farm A (A) and heifers from Farm B (B) were measured using the AMH Gen II and Bovine AMH ELISA kits, respectively. For Farm B, plasma VD3 concentrations were measured in randomly selected heifers. * P < 0.05 and *** P < 0.001.
Experiment 3: Plasma AMH concentration during the rearing period
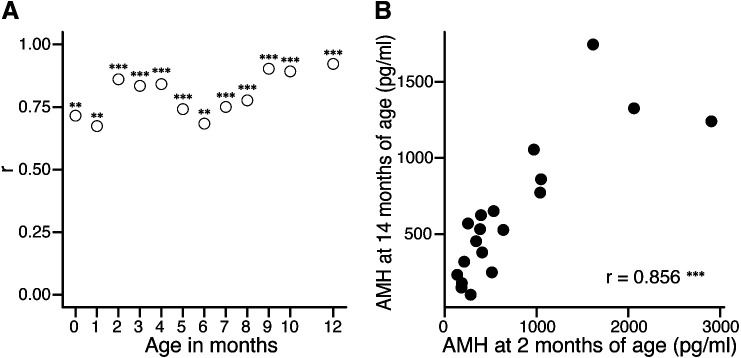
Plasma AMH concentrations during the first 12 months of life were significantly correlated with those at 14 months (Fig. 3A). The correlation coefficients (r = 0.888–0.918) between AMH concentrations at 9–12 months and 14 months of age were the highest. In young calves, high correlation coefficients (r = 0.830–0.856) were observed in those aged 2–4 months old. The correlation coefficient at 2 months of age was highest among those prior to 8 months of age (Fig. 3B).
Fig. 3.
Correlation coefficients between plasma anti-Müllerian hormone (AMH) concentrations during the rearing period (0–12 months of age) and at 14 months of age (A). Correlation between plasma AMH concentrations at 2 and 14 months (B). ** P < 0.01 and *** P < 0.001.
The low group, which was classified based on plasma AMH concentrations at 2 months of age, had low AMH levels at 14 months of age, with four of the six heifers showing plasma AMH concentrations below 300 pg/ml (Fig. 4A). The middle group, in which plasma AMH concentrations at 2 months of age ranged from 300 to 700 pg/ml, exhibited a moderate range of plasma AMH concentrations at 14 months of age (Fig. 4B). No consistency was observed in the month of age when plasma AMH concentrations were the highest in each individual in the low- and middle-age groups. The high AMH group, with plasma AMH concentrations > 700 pg/ml at 2 months of age, exhibited a transient increase, reaching the peak concentration by 5 months of age (Fig. 4C).
Fig. 4.
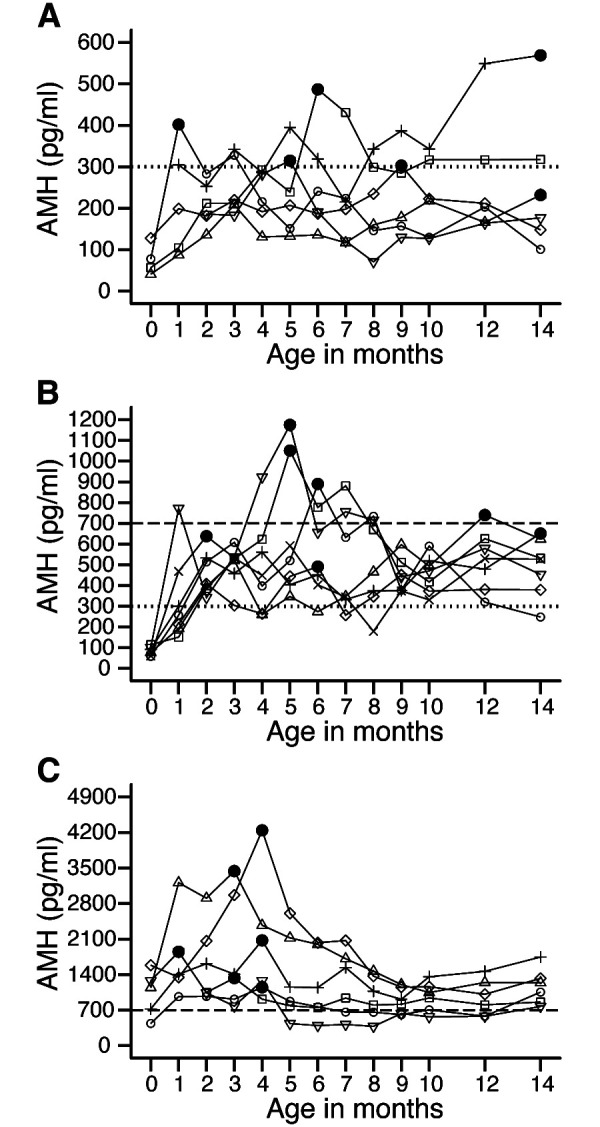
Changes in plasma anti-Müllerian hormone (AMH) concentration during the rearing period in low (A, n = 6), middle (B, n = 7), and high (C, n = 6) AMH groups. The different symbols in each panel indicate individual cows, while filled circles indicate the maximum values for individuals. The dotted and dashed horizontal lines indicate 300 and 700 pg/ml, respectively.
Discussion
The effects of the ovarian reserve on fertility in cattle is influenced by several factors, including breed and physiological conditions [8, 10]. For example, beef cows that have been separated from their calves are less susceptible to the energy imbalance and ovarian dysfunction associated with the lactation issues commonly observed in dairy cows. Indeed, the present study found that a high ovarian reserve, as assessed by blood AMH concentration, was positively associated with pregnancy rates in a seasonal breeding herd of Japanese Black cattle, a beef breed. This finding aligns with those of a previous report indicating significantly higher pregnancy rates in Aberdeen Angus cows with a high AFC (≥ 25) [6]. As such, the assessment of ovarian reserves in beef breeds may contribute to improved herd reproductive performance by enhancing the prediction of the probability of conception using AI and the selection of effective individualized reproductive management programs. As this study is the first to be conducted on a single herd of seasonally managed Japanese Black cattle, a comprehensive follow-up study of herds managed for year-round reproduction is currently underway.
The average and maximum plasma AMH concentrations in Japanese Black cattle were approximately two times higher than those in Holstein dairy cattle measured in a prior study using the same ELISA kit [22]. This inter-breed discrepancy surpassed the intra-dairy breed variation [8, 22]. A study of Holstein cows found a positive correlation between AFC and pregnancy rates [5]. However, a high AFC (≥ 25) in Holstein cows could adversely affect pregnancy rates. Several studies on dairy cattle have also reported that a high blood AMH concentration is associated with increased pregnancy rates by the end of the breeding period, along with a reduced incidence of pregnancy loss; however, it was not correlated with the pregnancy rate at the first AI [8, 22, 23]. These findings indicate that further research on dairy and beef breeds is required to investigate the effects of AMH levels on reproductive outcomes.
Long-term on-farm research has indicated that both low and excessively high ovarian reserves shorten the productive life of cows [9, 24]. In the present study, when plasma AMH concentrations exceeded 1,600 pg/ml, the pregnancy rate predicted by logistic regression analysis gradually decreased, while the 95% confidence interval was extended. Research has shown that blood AMH concentrations are increased in women with polycystic ovary syndrome, a common cause of infertility [25]. However, cystic ovarian follicular development in cattle does not correlate with increased AMH levels [26]. An increase in blood AMH concentration has been identified as a diagnostic marker for granulosa cell tumors in both women and cattle, although such occurrences are rare [27,28,29]. The number of cows with high AMH levels in a herd is relatively small. As such, additional research and careful interpretation of data are required to accurately predict fertility in cows with very high AMH levels.
Seasonal variations in plasma AMH concentrations have been observed at two farms in Hokkaido. Plasma AMH concentrations measured in cows and heifers in different years were found to be higher in summer than in winter. These findings indicate that the season of measurement should be considered when accurately assessing the AMH-producing capacity of cattle. Both farms, situated at 43 °N, experienced significant differences in sunlight hours between winter (December–February) and summer (June–August). As such, we evaluated the plasma VD3 concentration, which fluctuates with daylight hours and stimulates AMH production in follicles [16], but found no correlation with AMH production. In these regions, the average winter temperature falls below zero, with an approximate difference of 25°C between the winter and summer, leading to increased energy requirements for maintenance during winter. As such, further studies should investigate the impact of seasonal variations in energy sufficiency on AMH production.
Japanese Black heifers usually reach sexually mature and are bred at 12–14 months of age [19]. In the present study, blood samples were collected continuously during the rearing period to estimate the AMH-producing capacity of cattle after sexual maturity. Plasma AMH concentrations significantly correlated at all ages during the rearing period and at 14 months of age. The correlation coefficients at 2–4 months of age were consistently high and accurately predicted inter-individual comparisons of blood AMH concentrations at 14 months of age. This study found that the capacity of sexually mature Japanese Black heifers for high AMH production could be predicted during the rearing period, with particularly accurate predictions at 2 months of age. El-Sheikh Ali et al. [19] similarly conducted biweekly measurements of AMH concentrations in Japanese Black heifers, finding that plasma AMH concentrations were significantly higher in heifers that exhibited earlier puberty onset than in those with a later onset. However, plasma AMH concentrations during the early rearing period did not correlate with those after sexual maturity, except at 4 months of age. This discrepancy may be due to the significant variations in blood AMH concentrations among heifers. In comparison to the mean plasma AMH concentration (0.33 ± 0.10 ng/ml) at 10 weeks (2.5 months) reported by El-Sheikh Ali et al. [19], the current study found a larger individual variation in plasma AMH concentration (743 ± 737 pg/ml) at 2 months of age. The considerable variability in individual plasma AMH concentrations may have contributed to the higher correlation coefficient observed in this study.
The plasma AMH concentration in growing calves is unstable, showing a transient increase at approximately 3 months of age [10, 19, 20]. In the present study, calves were classified into three groups based on their AMH levels at 2 months of age. The highest plasma AMH concentrations during the rearing period in the low- and middle-aged groups were randomly observed up to 14 months of age. In contrast, all calves in the high-AMH group, whose plasma AMH concentrations at 2 months of age exceeded 700 pg/ml, reached the peak AMH concentrations before 5 months of age. Further, our analyses showed that transient increases in plasma AMH concentrations in young calves were characteristic of high-AMH-producing heifers. As such, calves with high plasma AMH concentrations before the age of 5 months, particularly at 2 months, may grow into heifers with high AMH levels after reaching sexual maturity.
In conclusion, this study found a positive correlation between plasma AMH concentrations and the probability of conception using AI in a seasonal breeding herd of Japanese Black cattle. Furthermore, seasonality was found to influence plasma AMH concentrations, and the AMH concentration during the rearing period was identified as a predictive indicator of AMH levels after achieving sexual maturation. These findings have significant implications for the development of reproductive management techniques based on AMH levels in cows.
Conflict of interests
The authors declare no conflict of interest.
Acknowledgments
This study was supported by a grant from MEXT KAKENHI (grant number: JP20K06436).
References
- 1.Alward KJ, Bohlen JF. Overview of Anti-Müllerian hormone (AMH) and association with fertility in female cattle. Reprod Domest Anim 2020; 55: 3–10. [DOI] [PubMed] [Google Scholar]
- 2.Iwase A, Hasegawa Y, Tsukui Y, Kobayashi M, Hiraishi H, Nakazato T, Kitahara Y. Anti-Müllerian hormone beyond an ovarian reserve marker: the relationship with the physiology and pathology in the life-long follicle development. Front Endocrinol (Lausanne) 2023; 14: 1273966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ireland JLH, Scheetz D, Jimenez-Krassel F, Themmen APN, Ward F, Lonergan P, Smith GW, Perez GI, Evans ACO, Ireland JJ. Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle. Biol Reprod 2008; 79: 1219–1225. [DOI] [PubMed] [Google Scholar]
- 4.Hirayama H, Kageyama S, Naito A, Fukuda S, Fujii T, Minamihashi A. Prediction of superovulatory response in Japanese Black cattle using ultrasound, plasma anti-Müllerian hormone concentrations and polymorphism in the ionotropic glutamate receptor AMPA1/GRIA1. J Reprod Dev 2012; 58: 380–383. [DOI] [PubMed] [Google Scholar]
- 5.Mossa F, Walsh SW, Butler ST, Berry DP, Carter F, Lonergan P, Smith GW, Ireland JJ, Evans ACO. Low numbers of ovarian follicles ≥3 mm in diameter are associated with low fertility in dairy cows. J Dairy Sci 2012; 95: 2355–2361. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira Júnior GA, Pinheiro VG, Fonseca PAS, Costa CB, Pioltine EM, Botigelli RC, Razza EM, Ereno RL, Ferraz JBS, Seneda MM, Nogueira MFG. Genomic and phenotypic analyses of antral follicle count in Aberdeen Angus cows. Livest Sci 2021; 249: 1–7. [Google Scholar]
- 7.Koyama K, Koyama T, Sugimoto M. Repeatability of antral follicle count according parity in dairy cows. J Reprod Dev 2018; 64: 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribeiro ES, Bisinotto RS, Lima FS, Greco LF, Morrison A, Kumar A, Thatcher WW, Santos JEP. Plasma anti-Müllerian hormone in adult dairy cows and associations with fertility. J Dairy Sci 2014; 97: 6888–6900. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez-Krassel F, Scheetz DM, Neuder LM, Ireland JLH, Pursley JR, Smith GW, Tempelman RJ, Ferris T, Roudebush WE, Mossa F, Lonergan P, Evans ACO, Ireland JJ. Concentration of anti-Müllerian hormone in dairy heifers is positively associated with productive herd life. J Dairy Sci 2015; 98: 3036–3045. [DOI] [PubMed] [Google Scholar]
- 10.Monniaux D, Drouilhet L, Rico C, Estienne A, Jarrier P, Touzé J-L, Sapa J, Phocas F, Dupont J, Dalbiès-Tran R, Fabre S. Regulation of anti-Müllerian hormone production in domestic animals. Reprod Fertil Dev 2012; 25: 1–16. [DOI] [PubMed] [Google Scholar]
- 11.Alward KJ, Graves WM, Palomares RA, Ely LO, Bohlen JF. Characterizing Anti-Müllerian Hormone (AMH) concentration and change over time in Holstein dairy cattle. Theriogenology 2021; 168: 83–89. [DOI] [PubMed] [Google Scholar]
- 12.Mossa F, Evans ACO. Review: The ovarian follicular reserve - implications for fertility in ruminants. Animal 2023; 17(Suppl 1): 100744. [DOI] [PubMed] [Google Scholar]
- 13.Rico C, Médigue C, Fabre S, Jarrier P, Bontoux M, Clément F, Monniaux D. Regulation of anti-Müllerian hormone production in the cow: a multiscale study at endocrine, ovarian, follicular, and granulosa cell levels. Biol Reprod 2011; 84: 560–571. [DOI] [PubMed] [Google Scholar]
- 14.Dennis NA, Houghton LA, Jones GT, van Rij AM, Morgan K, McLennan IS. The level of serum anti-Müllerian hormone correlates with vitamin D status in men and women but not in boys. J Clin Endocrinol Metab 2012; 97: 2450–2455. [DOI] [PubMed] [Google Scholar]
- 15.Dennis NA, Houghton LA, Pankhurst MW, Harper MJ, McLennan IS. Acute supplementation with high dose vitamin D3 increases serum anti-Müllerian hormone in young women. Nutrients 2017; 9: 719. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu F, Wolf S, Green O, Xu J. Vitamin D in follicular development and oocyte maturation. Reproduction 2021; 161: R129–R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson CD, Powell JL, Price DM, Hersom MJ, Yelich JV, Drewnoski ME, Bird SL, Bridges GA. Assessment of serum 25-hydroxyvitamin D concentrations of beef cows and calves across seasons and geographical locations. J Anim Sci 2016; 94: 3958–3965. [DOI] [PubMed] [Google Scholar]
- 18.Mossa F, Jimenez-Krassel F, Scheetz D, Weber-Nielsen M, Evans ACO, Ireland JJ. Anti-Müllerian Hormone (AMH) and fertility management in agricultural species. Reproduction 2017; 154: R1–R11. [DOI] [PubMed] [Google Scholar]
- 19.El-Sheikh Ali H, Kitahara G, Takahashi T, Mido S, Sadawy M, Kobayashi I, Hemmi K, Osawa T. Plasma anti-Müllerian hormone profile in heifers from birth through puberty and relationship with puberty onset. Biol Reprod 2017; 97: 153–161. [DOI] [PubMed] [Google Scholar]
- 20.Hirayama H, Naito A, Fukuda S, Fujii T, Asada M, Inaba Y, Takedomi T, Kawamata M, Moriyasu S, Kageyama S. Long-term changes in plasma anti-Müllerian hormone concentration and the relationship with superovulatory response in Japanese Black cattle. J Reprod Dev 2017; 63: 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rico C, Drouilhet L, Salvetti P, Dalbiès-Tran R, Jarrier P, Touzé J-L, Pillet E, Ponsart C, Fabre S, Monniaux D. Determination of anti-Müllerian hormone concentrations in blood as a tool to select Holstein donor cows for embryo production: from the laboratory to the farm. Reprod Fertil Dev 2012; 24: 932–944. [DOI] [PubMed] [Google Scholar]
- 22.Gobikrushanth M, Purfield DC, Canadas ER, Herlihy MM, Kenneally J, Murray M, Kearney FJ, Colazo MG, Ambrose DJ, Butler ST. Anti-Müllerian hormone in grazing dairy cows: Identification of factors affecting plasma concentration, relationship with phenotypic fertility, and genome-wide associations. J Dairy Sci 2019; 102: 11622–11635. [DOI] [PubMed] [Google Scholar]
- 23.Gobikrushanth M, Purfield DC, Colazo MG, Butler ST, Wang Z, Ambrose DJ. The relationship between serum anti-Müllerian hormone concentrations and fertility, and genome-wide associations for anti-Müllerian hormone in Holstein cows. J Dairy Sci 2018; 101: 7563–7574. [DOI] [PubMed] [Google Scholar]
- 24.Jimenez-Krassel F, Scheetz DM, Neuder LM, Pursley JR, Ireland JJ. A single ultrasound determination of ≥25 follicles ≥3 mm in diameter in dairy heifers is predictive of a reduced productive herd life. J Dairy Sci 2017; 100: 5019–5027. [DOI] [PubMed] [Google Scholar]
- 25.di Clemente N, Racine C, Pierre A, Taieb J. Anti-Müllerian hormone in female reproduction. Endocr Rev 2021; 42: 753–782. [DOI] [PubMed] [Google Scholar]
- 26.Monniaux D, Clemente N, Touzé JL, Belville C, Rico C, Bontoux M, Picard JY, Fabre S. Intrafollicular steroids and anti-mullerian hormone during normal and cystic ovarian follicular development in the cow. Biol Reprod 2008; 79: 387–396. [DOI] [PubMed] [Google Scholar]
- 27.Kanakatti Shankar R, Dowlut-McElroy T, Dauber A, Gomez-Lobo V. Clinical utility of anti-Mullerian hormone in pediatrics. J Clin Endocrinol Metab 2022; 107: 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okawa H, Tomiki M, Ishida T, Kawaguchi H, Wijayagunawardane MPB, Takagi M. Clinical diagnosis of bovine granulosa cell tumour in a holstein cow using plasma anti-müllerian hormone concentration: A case report. J Appl Anim Res 2017; 45: 529–532. [Google Scholar]
- 29.El-Sheikh Ali H, Kitahara G, Nibe K, Yamaguchi R, Horii Y, Zaabel S, Osawa T. Plasma anti-Müllerian hormone as a biomarker for bovine granulosa-theca cell tumors: comparison with immunoreactive inhibin and ovarian steroid concentrations. Theriogenology 2013; 80: 940–949. [DOI] [PubMed] [Google Scholar]