Abstract
Selenoprotein P (SeP) is synthesized in the liver and plays a vital role in maintaining selenium homeostasis via transport throughout the body. Previous studies have shown that SeP-deficient mice have severely reduced expression of selenoproteins essential for testicular function, leading to male infertility. We previously reported that the high expression of Ccdc152 in hepatocytes acts as a lncRNA, suppressing SeP expression in the liver. Ccdc152 reduces SeP translation by binding to SeP mRNA and decreasing its interaction with SECIS-binding protein 2. Although Ccdc152 is highly expressed in testes, its function remains unclear. Therefore, this study aimed to elucidate the role of Ccdc152 in the testes. Using the CRISPR/Cas9 system, we generated mice lacking all exons of Ccdc152 and found that SeP expression levels in the liver and plasma, as well as overall selenium homeostasis, remained unchanged. No significant differences were observed in the expression of glutathione peroxidase 1/4 or level of selenium in the testes. Subsequent investigation of the impact on male reproductive function revealed no abnormalities in sperm motility or Mendelian ratios of the offspring. However, a slight decrease in testicular weight and an increased rate of sperm malformations in the epididymis were observed. RNA-seq and pathway analyses identified the reduced expression of multiple genes related to kinesin and reproductive pathways. Based on these findings, Ccdc152 may not be essential for male reproductive function, but it may enhance reproductive capabilities by maintaining the expression of genes necessary for reproduction.
Keywords: Ccdc152, Glutathione peroxidase, Selenoprotein P, Spermatogenesis, Sperm morphogenesis
Spermatogenesis is a highly complex regulatory process that can be broadly classified into three stages. The first stage involves the self-renewal and differentiation of spermatogonia, which continuously undergo mitosis and maintain testicular homeostasis [1]. The second stage is meiosis, in which spermatocytes differentiate from spermatogonia in response to retinoic acid [2, 3]. The final stage is spermiogenesis, during which spermatids undergo dramatic morphological changes and become elongated. During this process, the spermatid head elongates, chromatin condenses by replacing histones with protamine [4], and acrosomes and flagella, which are responsible for fertilization, are formed [5, 6]. The continuous production of sperm throughout an individuals lifespan is supported by active self-renewal and differentiation of spermatogonia [1, 7]. High metabolic activity during these processes leads to significant mitochondrial oxygen consumption, resulting in the production of reactive oxygen species (ROS) [8]. Moreover, mitochondria are abundant in secondary spermatocytes and the middle piece of the flagella [9, 10], which actively produces ATP, resulting in high ROS production [11]. The accumulation of ROS leads to a reduction in sperm motility [12, 13] and an increase in cell death [14], thereby causing male infertility [11]. However, moderate amounts of ROS have positive effects on spermatogenesis and sperm function [15], including promoting spermatogonia self-renewal [16] and improving sperm function during capacitation [17,18,19,20,21].
The essential trace element selenium plays a pivotal role in spermatogenesis in the testes [22]. Excessive and deficient concentrations of selenium in the testicular tissue can result in defective spermatogenesis [23]. Selenoprotein P (SeP), encoded by the SELENOP gene, originates in the liver and serves as a selenium transporter. It is distributed to various organs through the systemic circulation and is specifically taken up by the testes via its receptor, Apolipoprotein E Receptor 2 (ApoER2) [24, 25]. Prior studies have demonstrated that selenium deficiency leads to reduced expression of enzymes such as glutathione peroxidase (GPx) 4 and GPx1 in the testes [26, 27]. This downregulation is associated with cytoskeletal abnormalities and diminished antioxidant capability, which compromise spermatogenesis.
In a previous study, we identified the coiled-coil domain-containing protein 152 (Ccdc152) gene, which functions as a long non-coding RNA (lncRNA) that inhibits SeP translation in the liver. We termed this novel gene product lncRNA inhibitor of SELENOP translation (L-IST). The inhibition occurs via an interaction between mRNA and SECIS-binding protein 2 (SBP2), a protein essential for SeP translation, which in turn leads to reduced ribosomal binding to SELENOP mRNA and suppression of protein expression [28]. Notably, Ccdc152/L-IST was predominantly expressed in the testes, as evidenced by Human Brain Atlas data. Furthermore, the CCDC protein family, to which Ccdc152/L-IST belongs, has been implicated in fertility, with certain mutations resulting in infertility [29, 30]. Given these findings, we hypothesized that Ccdc152/L-IST plays a regulatory role in maintaining selenium homeostasis in the testes, thereby influencing male fertility. The primary objective of this study was to elucidate the effects of Ccdc152/L-IST on selenium homeostasis and its consequent effects on male fertility in the testicular environment.
Materials and Methods
Chemicals
Sodium dodecyl sulfate (SDS) and acrylamide were purchased from Nacalai Tesque (Kyoto, Japan) and FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan), respectively. Penicillin and streptomycin were purchased from Invitrogen (Thermo Fisher Scientific, Waltham, MA, USA). ISOGEN II was purchased from Nippon Gene (Tokyo, Japan). Bouin’s solution and Myer’s hematoxylin were purchased from Sigma-Aldrich (St. Louis, MO, USA). crRNA, tracrRNA, and Cas9 nuclease were obtained from Integrated DNA Technologies (Coralville, IA, USA). All other chemicals used were of the highest commercially available quality.
Animals
C57BL/6N mice were purchased from SLC (Shizuoka, Japan) and maintained at 24 ± 1ºC and 60 ± 10% humidity, with a 12-h light/dark cycle and free access to food (MF; Oriental Yeast Co., Ltd., Tokyo, Japan) and water. All animal care and experimental procedures complied with the regulations for animal experiments and related activities of Tohoku University. The Tohoku University Institutional Animal Care and Use Committee approved this study (2019noudou-003-02).
Generation of Ccdc152 FLD mice
Ccdc152 full-locus deletion (FLD) mice were generated using i-GONAD as previously described [31]. Briefly, two crRNAs were designed with the following sequences: 5'-GAACGGCGGACCCAGAGGTGGG-3' and 3'-CCGCCTCATGCGAAGACGGTTT-5'. crRNAs and tracrRNA were annealed at 94°C for 2 min and mixed with the Cas9 nuclease. This genome-editing mixture was injected into the oviduct of pregnant mice (C57BL/6N males × females) at E0.7 and electroporated in vivo using a CUY21EditII Electroporator (BEX, Tokyo, Japan). Genotyping was performed usingPCR with primers for Ccdc152 wild type (WT) (Fw: 5'-GGCAGGCCTTTCAGAGTAAGCTAG-3' and Rv: 5'-GGTGTTAATCTGCGTACTGC-3') or FLD (Fw: 5'-AATTGGTGGGGCTCTAAGGTA-3' and Rv: 5'-GGTGTTAATCTGCGTACTGC-3') alleles. The PCR product using the Ccdc152 FLD primers was Sanger sequenced and a 22,978 bp deletion was identified. To fix the mutation in the strain, the F0 founder was mated with WT C57BL/6N mice, and the resulting F1 heterozygous male and female mice were crossed to obtain an F2 homozygous mutants.
Fertility test
A single 8-week-old male WT or Ccdc152 FLD mouse was caged with two 8-week-old female WT mice for 2 weeks. Mating was verified by the presence of vaginal plugs. Vaginal plugs and number of pups per litter were recorded.
Morphological and histological analysis
After measuring the body weights of both WT and Ccdc152 FLD mice (12–14 weeks old), the mice were euthanized. The testes and epididymides were isolated and weighed. Testes and epididymides were fixed in Bouin’s solution overnight at 4°C, gradually dehydrated by stepwise substitution with ethanol and xylene at different concentrations, and finally embedded in paraffin. Sections (4 µm) were rehydrated in xylene and ethanol at different concentrations, treated with 0.5% periodic acid for 10 min, treated with Schiff’s reagent (FUJIFILM Wako Pure Chemical Corporation) for 20 min, and counterstained with Myer’s hematoxylin for 30 sec. Sections were examined under a microscope (BX50; Olympus, Tokyo, Japan). To measure the circumference of the tubule, periodic acid-Schiff hematoxylin (PAS-H)-stained sections were examined, and images were analyzed using the ImageJ software. In total, 150 round sections of seminiferous tubules were obtained from three mice of each genotype.
Sperm motility, morphology, and count analysis
Spermatozoa from cauda epididymis were suspended in a human tubal fluid medium (101.6 mM NaCl, 4.7 mM KCl, 0.37 mM K2PO4, 0.2 mM MgSO4·7H2O, 2 mM CaCl2, 25 mM NaHCO3, 2.78 mM glucose, 0.33 mM sodium pyruvate, 21.4 mM sodium lactate, 286 mg/l penicillin G, and 228 mg/l streptomycin) and incubated at 37°C under 5% CO2 in humidified air. After incubation for 10 and 120 min, the spermatozoa were analyzed using the Sperm Motility Analysis System (SMAS; DITECT Co., Ltd., Tokyo, Japan). For morphological analysis, spermatozoa were incubated for 120 min, mounted on glass slides, air-dried, and stained with Papanicolaou stain. A total of 1200 spermatozoa were measured for normal morphology from three mice of each genotype. A hemocytometer was used for sperm counting from 1:10 dilution of spermatozoa incubated with 3% NaCl for 120 min.
Western blotting
Tissue (50 mg) was homogenized in a buffer containing 1% SDS, and proteins were extracted. After sonication, the samples were adjusted to a uniform concentration and applied to acrylamide gels at 20 µg/lane. Electrophoresis was performed at 300 V for 30 min, followed by transfer at 200 mA for 60 min. The gel was blocked with skim milk and incubated overnight with primary antibodies at the indicated concentrations. Secondary antibodies were added and shaken at room temperature for 1 h before detection. The antibodies used were as follows: rabbit anti-mSeP pAb [32], rabbit ani-GPx4 mAb (ab125066), and rabbit anti-GPx1 pAb (ab22604) from Abcam (Waltham, MA, USA) and mouse anti-GAPDH mAb (5A12) HRP-conjugated from FUJIFILM Wako Pure Chemical Corporation.
Quantitative real-time PCR (qPCR)
RNA extraction was performed using ISOGEN II following the manufacturer’s protocol. Briefly, tissues were homogenized in ISOGEN II solution, the aqueous layer was extracted, and total RNA was recovered via isopropanol precipitation. The concentration and quality of purified RNA were determined using a NanoDrop (Thermo Fisher Scientific). Reverse transcription was performed to obtain cDNA using a PrimeScript Reagent Kit (Takara Bio Inc., Shiga, Japan) with random 6mer and oligo dT primers, following the manufacturer’s protocol. qPCR was performed using Power SYBR Green PCR Master Mix (Thermo Fisher Scientific) and a CFX Connect thermal cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA). All data were normalized to GAPDH mRNA levels. The primers used were as follows: mouse SelenoP: 5'-AGCTCTGCTTGTTACAAAGCC-3' and 5'-CAGGTCTTCCAATCTGGATGC-3', mouse GPx1: 5'-AGTCCACCGTGTATGCCTTCT-3' and 5'-GAGACGCGACATTCTCAATGA-3', and mouse Gapdh: 5'-AACTTTGGCATTGTGGAAGG-3' and 5'-ACACATTGGGGGTAGGAACA-3'.
RNA sequencing (RNA-seq) analysis
Total RNA was extracted as previously described. Quality checks of the extracted RNA and RNA-seq analysis were performed using Genome-Lead (Kagawa, Japan). A total of 17 million reads were analyzed. The results were analyzed using the corresponding analysis packages in R. Differentially expressed genes were verified using padj. Differential expression was analyzed using a negative binomial distribution model.
Statistical analysis
Quantification of band intensity was performed using ImageJ software (version 1.53i). Statistical analyses for all experiments were performed using R (version 4.2.2). For all tests, statistical significance was set at P < 0.05.
Results
Establishment of Ccdc152 full-locus del. (Ccdc152 FLD) mice and its effect on selenoprotein expression
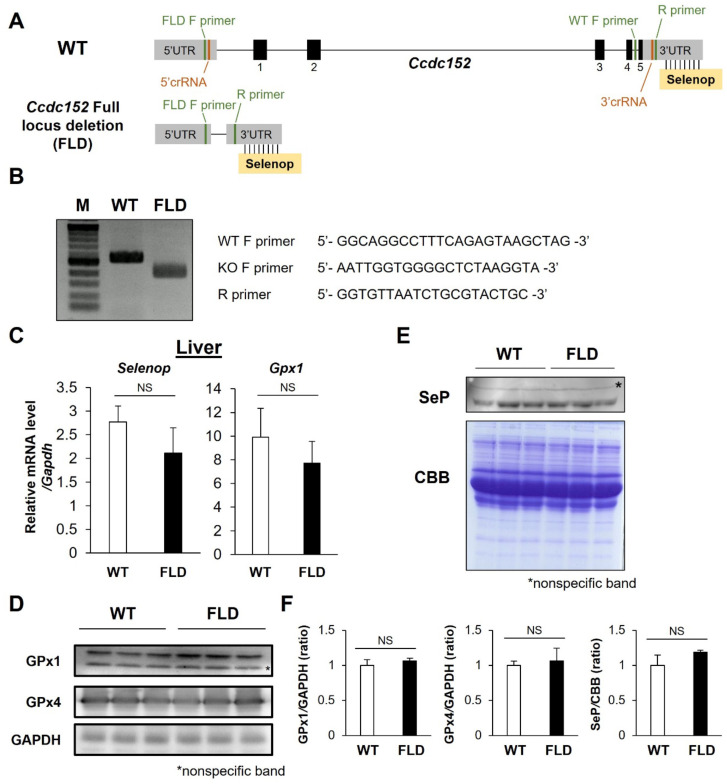
Our previous research indicated that Ccdc152 may act as a lncRNA that suppresses SeP expression in hepatocytes [28]. To investigate its physiological role in vivo, we generated Ccdc152 FLD mice using the i-GONAD method, which involves the in vivo electroporation of the Cas9 protein and sgRNA complex at the one-cell stage (Fig. 1A). Full locus deletion was verified through Sanger sequencing, utilizing primers that recognize the 5' and 3' UTR (Fig. 1B). Despite the absence of Ccdc152, we observed no significant alterations in the SeP and GPx1 mRNA levels in the liver compared to WT controls (Fig. 1C). In addition, the protein expression levels of GPx1 and GPx4 in the liver and SeP levels in the plasma were not altered by Ccdc152 FLD compared to WT controls (Fig. 1D–F). These observations suggest that Ccdc152 deficiency may not influence selenoprotein expression in the liver tissue or circulating blood.
Fig. 1.
Establishment of Ccdc152full-locus del. (FLD) mice and its effect on selenoprotein expression. (A) Schematic representation of the Ccdc152 genomic region. (B) Agarose gel electrophoresis of Ccdc152 editing in Ccdc152 FLD mice. (C–E) Expression of selenoproteins in the livers of Ccdc152 FLD mice, assessed using (C) qPCR, (D) western blotting, and (E) plasma SeP levels. (F) Quantification of the protein expression levels in (D) and (E). Data are shown as mean ± standard deviation (SD) (n = 3).
Effects of Ccdc152 on selenoprotein expression in the testis
Previous research suggests high expression of Ccdc152 in the testes [28]. Subsequent analyses were conducted on mouse testes to assess the effects of Ccdc152 deficiency on selenoprotein expression. Testicular tissue was excised from male mice and both the mRNA and protein levels of selenoproteins were quantified. No significant changes were observed in either expression metric compared to WT controls (Fig. 2A–C). These findings implied that the absence of Ccdc152 did not influence selenoprotein expression in the testes.
Fig. 2.
Effects of Ccdc152 on selenoprotein expression in the testis. (A–B) Expression of selenoproteins in the testes of Ccdc152 FLD mice, measured using (A) qPCR and (B) western blotting. (C) Quantification of the protein expression levels in (B). Data are shown as mean ± SD (n = 3).
Ccdc152 FLD mice have slight abnormalities in testicular morphology
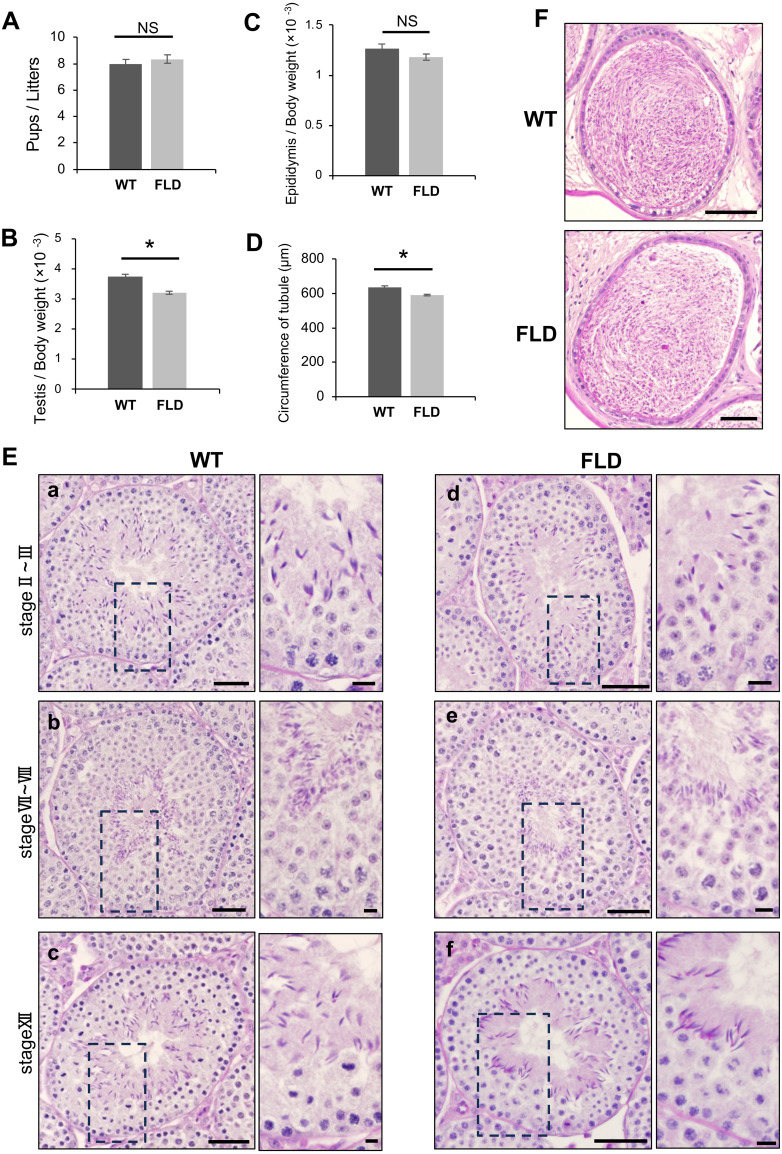
It has been reported that Ccdc152 mRNA is highly expressed in the testes [28]. We hypothesized that Ccdc152 plays an essential role in male fertility; therefore, we evaluated the fertility of Ccdc152 FLD mice. To assess fertility, we mated WT and Ccdc152 FLD male mice with WT female mice and counted the number of pups per litter over 2 weeks. As shown in Fig. 3A, the number of pups from Ccdc152 FLD male mice was comparable to that of pups from WT male mice. These results indicated that Ccdc152 FLD male mice have normal fertility. We then measured the weights of the testes and epididymis of Ccdc152 FLD mice. The testis/body weight ratio of Ccdc152 FLD mice was significantly lower than that of WT mice (Fig. 3B), whereas the epididymal/body weight ratio was not significantly different from the WT mice (Fig. 3C). Corresponding to the reduction in the testis/body weight ratio, the circumference of the seminiferous tubules in Ccdc152 FLD mice was shorter than that in WT mice (Fig. 3D). To examine spermatogenesis in Ccdc152 FLD mice, we investigated the morphology of the testes and epididymis using PAS-H staining. No significant abnormalities were detected in Ccdc152 FLD mice compared with WT mice (Fig. 3E). Furthermore, using PAS-H staining, the seminiferous tubule stages were determined based on the shape of the acrosomes of round spermatids and the nuclei of spermatocytes and elongated spermatids. As germ cells corresponding to each stage were present in the seminiferous tubules of Ccdc152 FLD mice, there was no disturbance in the seminiferous tubule stages, and spermatogenesis proceeded normally (Fig. 3Ea–f). In addition, no significant differences were observed between Ccdc152 FLD and WT mice in spermatogenesis staging of the seminiferous tubules (Fig. 3Ea–f). A large number of spermatozoa were detected in the cauda epididymis of Ccdc152 FLD, and no morphological differences were observed compared to WT mice (Fig. 3F). Testicular and epididymal histology indicated that spermatogenesis proceeded normally in Ccdc152 FLD mice. Taken together, Ccdc152 FLD mice did not show significantly affected fertility but there were slight morphological abnormalities, such as a decreased testis weight ratio and a shortened seminiferous tubule perimeter.
Fig. 3.
Ccdc152 FLD mice have slight abnormalities in testicular morphology. (A) Pups/litters in Ccdc152 FLD mice compared with WT mice from 2 weeks of mating (Student’s t-test, mean ± standard error [SE], n = 4, * P < 0.05). Litters/plugs; WT: 8/8 (n = 4), Ccdc152 FLD: 12/14 (n = 4). (B–D) Measurement of (B) testis/body weight, (C) epididymis/body weight, and (D) circumference of tubule in Ccdc152 FLD mice compared with WT mice (Student’s t-test, mean ± SE, n = 3, * P < 0.05). (E) PAS-H staining of seminiferous tubules in (a–c) WT and (d–f) Ccdc152 FLD mice. Seminiferous tubule stage comparison in (a, d) stage II–III, (b, e) stage VII–VIII, and (c, f) stage XII. Scale bars = 50 µm. In each panel, a high-magnification image of a cropped site is shown (scale bars = 10 µm). (F) PAS-H staining of cauda epididymis in WT and Ccdc152 FLD mice. Scale bars = 100 µm.
Ccdc152 FLD mice have normal sperm function
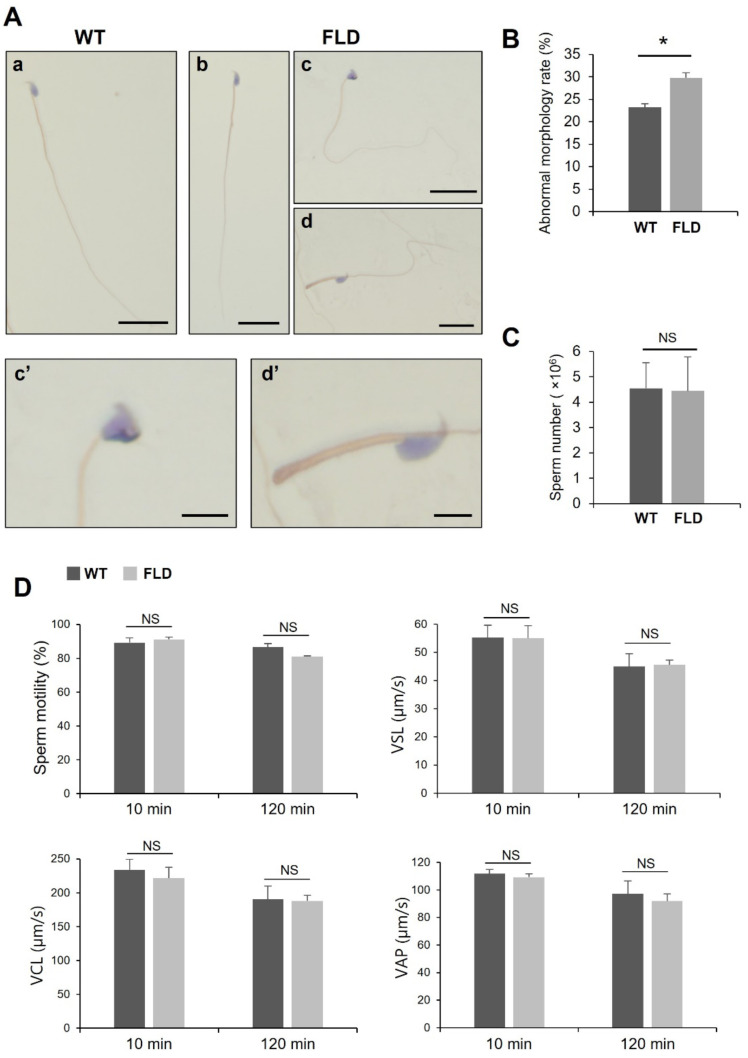
We then focused on the morphology and motility of spermatozoa. Cauda spermatozoa were collected and subjected to Papanicolaou staining for morphological analysis (Fig. 4A). The percentage of spermatozoa displaying abnormal morphology was significantly increased in Ccdc152 FLD compared to that in the WT (Figs. 4A, B). Specifically, spermatozoa head and flagella abnormalities were observed in Ccdc152 FLD mice (Figs. 4Ac’, d’). There were no changes in the number of spermatozoa in the cauda epididymis between the WT and Ccdc152 FLD mice (Fig. 4C). Sperm motility was also measured using SMAS, and no significant changes were observed in Ccdc152 FLD compared to the WT at 10 and 120 min of incubation (Fig. 4D). These results suggested that Ccdc152 is involved in spermatozoa morphogenesis, but not sperm motility.
Fig. 4.
Ccdc152full-locus del. mice have normal sperm function. (A) Papanicolaou staining of spermatozoa in (a) WT and (b–d) Ccdc152full-locus del. mice. Scale bars = 20 µm (a–d) and 5 µm (c’, d’). Panels show (b) normal and (c, d) abnormal morphologies. (c, d) High-power images of spermatozoa showing (c’) head and (d’) flagella abnormalities. (B–C) Abnormal morphology rate and sperm number in Ccdc152full-locus del. mice compared with WT mice (Student’s t-test, mean ± SE, n = 3, * P < 0.05). (D) Sperm motility analysis in Ccdc152full-locus del. mice compared with WT mice with incubation times of 10 and 120 min (Student’s t-test, mean ± SE, n = 3, * P < 0.05). VSL, Straight-line Velocity; VCL, Curvilinear Velocity; VAP, Average Path Velocity.
Ccdc152 is involved in sperm morphogenesis
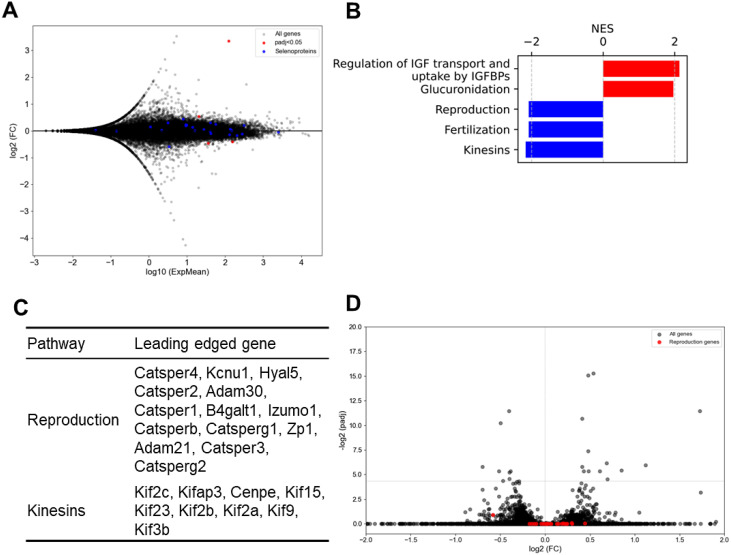
To identify the gene alterations responsible for the functional abnormalities, we conducted an RNA-seq analysis of the testes. MA plots were generated to visualize changes in gene expression, with genes showing adjusted p-values less than 0.05 highlighted in red, and selenoproteins in blue (Fig. 5A). Pathway enrichment analysis revealed an overrepresentation of pathways related to Reproduction and Kinesins, both of which are crucial for reproductive functions (Fig. 5B). Genes that exhibited particularly high levels of variability within these pathways are shown in Fig. 5C. These variable genes were further represented in volcano plots for visual inspection (Fig. 5D). Although the observed changes in gene expression were not statistically significant, consistent directional changes were observed across datasets. These findings suggest that the loss of Ccdc152 contributes to the increased prevalence of sperm with abnormal morphology, possibly through the subtle downregulation of genes crucial for sperm morphogenesis.
Fig. 5.
Ccdc152 is involved in sperm morphogenesis. RNA-seq analysis of Ccdc152full-locus del. mice compared with WT mice. (A) MA plot showing variations in gene expression. Genes showing adjusted p-values less than 0.05 are highlighted in red, and selenoproteins are in blue. (B) Results of the pathway analysis for genes with padj < 0.05. (C) Genes that significantly affected the pathways. (D) Volcano plot of the genes that showed variation in expression in (C). NES, normalized enrichment score.
Discussion
In this study, we investigated the role of Ccdc152 in testicular function. Our results indicated that the loss of Ccdc152 does not influence the expression of SeP or other selenoproteins in the liver or testes. However, RNA-seq data suggested that Ccdc152 affects testicular and sperm morphogenesis by uniformly reducing the expression of kinesin family proteins and disrupting the expression of cytoskeletal-related genes, such as Formin, Ranbp10, Actl11, and D7Ertd443e.
It has been reported that kinesin family knockout mice show abnormal sperm morphology [33]. In the present study, Ccdc152 FLD mice had downregulated expression of kinesins. Therefore, downregulation of the kinesin pathway may have caused abnormal sperm morphogenesis in Ccdc152 FLD mice. During spermiogenesis, the proteins necessary for inducing changes are transported along the microtubules and actin around the sperm head [34]. Hence, it is important for spermiogenesis that the cytoskeleton, including microtubules and actin, is formed normally. Some genes associated with cytoskeleton formation were highly differentially expressed in Ccdc152 FLD mice. Thus, another cause of the increase in sperm morphological abnormalities could be a disturbance in cytoskeleton-related gene expression. Further studies are required to determine the role of Ccdc152 in spermiogenesis.
In addition to kinesins, the reproductive pathway was downregulated in Ccdc152 FLD mice, which included many genes related to fertilization and sperm motility. In this study, Ccdc152 deficiency resulted in malformations, including abnormal sperm morphology, and affected testicular weight during spermatogenesis. Despite these changes, fertility was not affected when measured for 2 weeks, and motile sperm were still observed. However, long-term mating experiments could not be fully evaluated to count the number of total litters and pups, which is a significant subject for future study. Furthermore, these mice may exhibit increased susceptibility to stressors such as aging, environmental toxicity, and nutritional imbalances.
We have previously reported that Ccdc152 functions intracellularly as a lncRNA in hepatocytes, suppressing protein expression by binding to SeP mRNA during translation [28]. SeP is crucial for transporting selenium to the testes, and a lack of selenium in the testes is believed to lead to defects in spermatogenesis. Therefore, we anticipated fluctuations in selenoprotein expression in the testes of Ccdc152 FLD mice. However, we observed no disturbances in selenoprotein levels. Mice typically consume 3–4 g of food daily. Given that the CE-2 diet provides 0.39 µg Se/g, this equates to approximately 1.3 µg Se intake per mouse each day [35]. In comparison, the recommended daily intake of selenium for humans is between 25–30 µg. Therefore, when adjusted for body weight, mice ingested selenium at a rate more than 200 times greater than that of humans. Given this significant difference in selenium consumption, the effects of Ccdc152 FLD may not have been apparent. Moreover, scRNA-seq data showed that Ccdc152 was primarily expressed in spermatocytes, whereas SeP and its intratesticular receptor ApoER2 were expressed only in Sertoli cells and not in germ cells. Therefore, it seems unlikely that Ccdc152 and SeP interact directly in the testis because they do not coexist in the same compartment. Given that Ccdc152 is highly expressed in spermatocytes, it may also play a role in meiosis. Nevertheless, meiosis proceeded normally and numerous haploid cells were observed in Ccdc152 FLD mice, indicating that Ccdc152 is either not vital for meiosis or that a similar gene could compensate for its loss. Further analysis is required to elucidate the detailed function of Ccdc152 in the testis.
We first hypothesized that Ccdc152/L-IST plays a regulatory role in maintaining selenium homeostasis in the testes thereby, we prepared Ccdc152 FLD mice to elucidate the effects of Ccdc152/L-IST on selenium homeostasis. Based on the findings in the present study, Ccdc152 may not be essential for male reproductive function, but it may enhance reproductive capabilities by maintaining the expression of genes necessary for reproduction. Further studies are necessary to understand the physiological role of Ccdc152/L-IST and its significance on selenium metabolism and diseases.
Conflicts of interests
The authors state no conflict of interest.
Acknowledgments
This work was supported by JSPS KAKENHI [grant numbers 20H00488 and 21H05270] and the Business Incubation Program (BIP) at Tohoku University.
References
- 1.Yoshida S. Open niche regulation of mouse spermatogenic stem cells. Dev Growth Differ 2018; 60: 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science 2006; 312: 596–600. [DOI] [PubMed] [Google Scholar]
- 3.Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA 2006; 103: 2474–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rathke C, Baarends WM, Awe S, Renkawitz-Pohl R. Chromatin dynamics during spermiogenesis. Biochim Biophys Acta 2014; 1839: 155–168. [DOI] [PubMed] [Google Scholar]
- 5.Abou-Haila A, Tulsiani DR. Mammalian sperm acrosome: formation, contents, and function. Arch Biochem Biophys 2000; 379: 173–182. [DOI] [PubMed] [Google Scholar]
- 6.Lehti MS, Sironen A. Formation and function of the manchette and flagellum during spermatogenesis. Reproduction 2016; 151: R43–R54. [DOI] [PubMed] [Google Scholar]
- 7.Shinohara T, Avarbock MR, Brinster RL. β1- and α6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci USA 1999; 96: 5504–5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerriero G, Trocchia S, Abdel-Gawad FK, Ciarcia G. Roles of reactive oxygen species in the spermatogenesis regulation. Front Endocrinol (Lausanne) 2014; 5: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update 2009; 15: 553–572. [DOI] [PubMed] [Google Scholar]
- 10.da Silva AF, Mariotti FR, Máximo V, Campello S. Mitochondria dynamism: of shape, transport and cell migration. Cell Mol Life Sci 2014; 71: 2313–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chianese R, Pierantoni R. Mitochondrial Reactive Oxygen Species (ROS) Production Alters Sperm Quality. Antioxidants 2021; 10: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez JG, Storey BT. Spontaneous lipid peroxidation in rabbit epididymal spermatozoa: its effect on sperm motility. Biol Reprod 1982; 27: 1102–1108. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl 1987; 8: 338–348. [DOI] [PubMed] [Google Scholar]
- 14.Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev 2017; 84: 1039–1052. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y, Buffenstein R, Pulliam DA, Van Remmen H. Comparative studies of oxidative stress and mitochondrial function in aging. Integr Comp Biol 2010; 50: 869–879. [DOI] [PubMed] [Google Scholar]
- 16.Morimoto H, Iwata K, Ogonuki N, Inoue K, Atsuo O, Kanatsu-Shinohara M, Morimoto T, Yabe-Nishimura C, Shinohara T. ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell 2013; 12: 774–786. [DOI] [PubMed] [Google Scholar]
- 17.Aitken RJ, Paterson M, Fisher H, Buckingham DW, van Duin M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci 1995; 108: 2017–2025. [DOI] [PubMed] [Google Scholar]
- 18.Bize I, Santander G, Cabello P, Driscoll D, Sharpe C. Hydrogen peroxide is involved in hamster sperm capacitation in vitro. Biol Reprod 1991; 44: 398–403. [DOI] [PubMed] [Google Scholar]
- 19.de Lamirande E, Gagnon C. A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int J Androl 1993; 16: 21–25. [DOI] [PubMed] [Google Scholar]
- 20.O’Flaherty CM, Beorlegui NB, Beconi MT. Reactive oxygen species requirements for bovine sperm capacitation and acrosome reaction. Theriogenology 1999; 52: 289–301. [DOI] [PubMed] [Google Scholar]
- 21.Aitken RJ, Harkiss D, Knox W, Paterson M, Irvine DS. A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J Cell Sci 1998; 111: 645–656. [DOI] [PubMed] [Google Scholar]
- 22.Ahsan U, Kamran Z, Raza I, Ahmad S, Babar W, Riaz MH, Iqbal Z. Role of selenium in male reproduction - a review. Anim Reprod Sci 2014; 146: 55–62. [DOI] [PubMed] [Google Scholar]
- 23.Riaz M, Mahmood Z, Shahid M, Saeed MU, Tahir IM, Shah SA, Munir N, El-Ghorab A. Impact of reactive oxygen species on antioxidant capacity of male reproductive system. Int J Immunopathol Pharmacol 2016; 29: 421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizuno A, Toyama T, Ichikawa A, Sakai N, Yoshioka Y, Nishito Y, Toga R, Amesaka H, Kaneko T, Arisawa K, Tsutsumi R, Mita Y, Tanaka SI, Noguchi N, Saito Y. An efficient selenium transport pathway of selenoprotein P utilizing a high-affinity ApoER2 receptor variant and being independent of selenocysteine lyase. J Biol Chem 2023; 299: 105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olson GE, Winfrey VP, Nagdas SK, Hill KE, Burk RF. Apolipoprotein E receptor-2 (ApoER2) mediates selenium uptake from selenoprotein P by the mouse testis. J Biol Chem 2007; 282: 12290–12297. [DOI] [PubMed] [Google Scholar]
- 26.Imai H, Hakkaku N, Iwamoto R, Suzuki J, Suzuki T, Tajima Y, Konishi K, Minami S, Ichinose S, Ishizaka K, Shioda S, Arata S, Nishimura M, Naito S, Nakagawa Y. Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J Biol Chem 2009; 284: 32522–32532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noblanc A, Kocer A, Chabory E, Vernet P, Saez F, Cadet R, Conrad M, Drevet JR. Glutathione peroxidases at work on epididymal spermatozoa: an example of the dual effect of reactive oxygen species on mammalian male fertilizing ability. J Androl 2011; 32: 641–650. [DOI] [PubMed] [Google Scholar]
- 28.Mita Y, Uchida R, Yasuhara S, Kishi K, Hoshi T, Matsuo Y, Yokooji T, Shirakawa Y, Toyama T, Urano Y, Inada T, Noguchi N, Saito Y. Identification of a novel endogenous long non-coding RNA that inhibits selenoprotein P translation. Nucleic Acids Res 2021; 49: 6893–6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Priyanka PP, Yenugu S. Coiled-coil domain-containing (CCDC) proteins: functional roles in general and male reproductive physiology. Reprod Sci 2021; 28: 2725–2734. [DOI] [PubMed] [Google Scholar]
- 30.Wang T, Yin Q, Ma X, Tong MH, Zhou Y. Ccdc87 is critical for sperm function and male fertility. Biol Reprod 2018; 99: 817–827. [DOI] [PubMed] [Google Scholar]
- 31.Gurumurthy CB, Sato M, Nakamura A, Inui M, Kawano N, Islam MA, Ogiwara S, Takabayashi S, Matsuyama M, Nakagawa S, Miura H, Ohtsuka M. Creation of CRISPR-based germline-genome-engineered mice without ex vivo handling of zygotes by i-GONAD. Nat Protoc 2019; 14: 2452–2482. [DOI] [PubMed] [Google Scholar]
- 32.Mita Y, Nakayama K, Inari S, Nishito Y, Yoshioka Y, Sakai N, Sotani K, Nagamura T, Kuzuhara Y, Inagaki K, Iwasaki M, Misu H, Ikegawa M, Takamura T, Noguchi N, Saito Y. Selenoprotein P-neutralizing antibodies improve insulin secretion and glucose sensitivity in type 2 diabetes mouse models. Nat Commun 2017; 8: 1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao M, Qu H, Han Y, Cheng CY, Xiao X. Kinesins in mammalian spermatogenesis and germ cell transport. Front Cell Dev Biol 2022; 10: 837542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Y-L, Yang W-X. The acroframosome-acroplaxome-manchette axis may function in sperm head shaping and male fertility. Gene 2018; 660: 28–40. [DOI] [PubMed] [Google Scholar]
- 35.Haratake M, Ono M, Nakayama M. Penicillamine selenotrisulfide as a selenium- source in mice. J Health Sci 2004; 50. [Google Scholar]