Abstract
Oocyte developmental competence declines in women aged 35 and older resulting in many women resorting to IVF. The present study determined whether adding Granulocyte-macrophage colony-stimulating factor (GM-CSF) during in vitro oocyte maturation (IVM) could improve oocyte developmental competence in a mouse model of advanced maternal age. Oocytes from 12–14 month C57BL6 J × CBA mice were treated with 10 ng/ml of GM-CSF during IVM, and embryo development, mitochondrial activity, spindle formation and chromosomal alignment were examined. The addition of GM-CSF tended to increase fertilisation rates (76.19 vs. 82.03%; P = 0.07) but did not affect cumulus expansion compared with control. The addition of GM-CSF also increased blastocysts rates (51.10 vs. 61.52%; P < 0.01) and the number of good quality blastocysts (33.31 vs. 44.13%; P < 0.05) present at 96 h of culture as well as inner cell mass (12.64 vs. 15.62 ; P < 0.01) and total cell number (42.98 vs. 48.78 ; P < 0.05). GM-CSF treatment also increased mitochondrial membrane potential two to three fold in the outer (2.86 vs. 0.97; P < 0.001), intermediate (3.25 vs. 0.89; P < 0.001) and peri nuclear areas (3.62 vs. 1.08; P < 0.001). GM-CSF treatment did not influence spindle formation or chromosomal alignment. Together our results indicate that the addition of GM-CSF during IVM may improve oocyte quality in women of advanced maternal age.
Keywords: Advanced maternal age, Aneuploidy, Granulocyte macrophage colony stimulating factor (GM-CSF), In vitro oocyte maturation (IVM), Oocyte quality
Around one in four women in Australia give birth to their first child aged 35 or older [1] which is also around the time that oocyte quality declines [2, 3]. This has resulted in an increased number of women accessing assisted reproductive technology (ART) treatment such that more than half of all IVF patients in Australia are 35 years or over [1]. Similar trends have also been reported in other developed countries [2, 3]. The effect of advanced maternal age (AMA) on oocyte quality is associated with impaired energy metabolism, mitochondrial dysfunction, and redox imbalance leading to oxidative damage [2, 3]. The decline in oocyte quality also includes an increase in aneuploidy which is associated with increased rates of miscarriage [3, 4]. While pre-implantation genetic testing for aneuploidy (PGT-A) in combination with embryo freezing can increase pregnancy rates, live birth rates for women > 40 years are still less than 10% compared with around 30% for younger women [1].
In vitro oocyte maturation is increasingly used as an assisted reproductive technology and recent advances has seen success rates increase to levels similar to that for IVF [5]. In vitro oocyte maturation (IVM) also provides a potential window where treatments to improve oocyte quality can be applied and is also when the majority of aneuploidy occurs following the resumption of meiosis [4]. Numerous studies have shown that the addition of granulocyte macrophage colony stimulating factor (GM-CSF) during embryo culture can improve embryo development in a range of species including Humans and that these improvements can improve pregnancy and birth rates [6].
We have previously reported that the addition of GM-CSF during IVM can also increase embryo development as well as implantation and birth rates in mice [7]. We have also shown that GM-CSF increases mitochondrial activity [8] in mature oocytes and reduces the incidence of DNA damage in blastocysts [7] using this model. Based on these findings we hypothesise that the addition of GM-CSF during IVM will improve oocyte developmental competence and embryo development in women of advanced maternal age. The present study was undertaken to examine this hypothesis by examining embryo development, oocyte mitochondrial activity as well as spindle formation and chromosomal alignment in a previously developed mouse model of AMA [6]. This model uses 12–14 month old CBAF1 mice which approximates to women aged 35–45 years. At this age mice have an aneuploidy rate of around 50%, which is similar to that seen in women of advanced maternal age [9, 10].
Materials and Methods
All chemicals were purchased from Sigma–Aldrich (St. Louis, MO, USA) unless otherwise stated.
Mice
C57BL6 J × CBA males (6–8 weeks – sperm donors) and females (12–14 months – oocyte donors) were obtained from the University of Adelaide Laboratory Animal Services and housed under a 12 h light and 12 h dark cycle with ad libitum access to water and food. All experiments were performed in accordance with Australian Code of Practice for the care and the Use of Animals for Scientific Purpose and the study was approved by the University of Adelaide Animal Ethics Committee (M-2017-081).
In vitro oocyte maturation
Female mice were administered with 10 IU pregnant mare chorionic gonadotropin (PMSG; Folligon, Intervert, Boxmeer, The Netherlands) via intraperitoneal injection to stimulate follicle growth. 46–48 h post PMSG ovaries were collected in HEPES-buffered minimum essential medium (α-MEM; ART Lab Solutions, SA, Australia) media (supplemented with 4 mg/ml bovine serum albumin (BSA; MP Biomedicals, AlbuminNZ, Auckland, NZ) and 1 mg/ml Fetuin [11]. Cumulus oocyte complexes (COCs) were isolated from ovaries by puncturing all follicles 100–180 µm in diameter using a 30-guage needle [12]. The maturation media consisted of bicarbonate-buffered α-MEM media containing 3 mg/ml BSA and 1 mg/ml Fetuin [11]. Maturation media was filtered before adding 50 mIU/ml recombinant human FSH (Puregon-Organon, Oss, The Netherlands) [13] containing 0 and 10 ng/ml of recombinant mouse GM-CSF (R&D Systems, Minneapolis, MN, USA; Cat# 415-ML-010). GM-CSF was reconstituted as per supplier instructions in PBS plus 0.1% BSA (10 µg/ml) and frozen down in 10 μl aliquots and stored at –80oC. A dose of 10 ng/ml was chosen because we previously showed that it increases embryo development as well as implantation and birth rate in young (4 wk old) mice [7]. IVM was performed at 6% CO2, 5% O2 and 89% N2 humidified at 37oC with media pre-equilibrated for at least 4 h prior. Ten COCs were cultured in 50 μl drops of maturation media for 16 h. Post maturation cumulus expansion was assessed using a scale as previously described [14, 15]. COCs were graded as 0: no expansion, 1+: outer layer of cumulus cells expanded, 2: outer half of cumulus expanded, 3: all layers expanded apart from corona radiatae and 4+: maximum expansion of all layers of cumulus cells. The experiment was replicated five times with a minimum of 25 COCs in each group per replicate.
In vitro fertilisation and embryo culture
Male mice were sacrificed using cervical dislocation and the vas deferens along with the cauda epididymis collected in Wash Medium (Cook Medical, Bloomington, IN, USA) at 37oC, and excess tissue and fat removed using a dissecting microscope. Spermatozoa were extracted into a culture dish containing pre-equilibrated 1 ml Fertlisation Media (Cook Medical) and incubated for 45–60 min at 37oC in 6% CO2, 5% O2, and 89% N2 to allow sperm to capacitate [11]. Spermatozoa were then added to the fertilisation drop which contained the expanded COCs and co-incubated for a further 4 h at 37oC in 6% CO2, 5% O2, and 89% N2. Following fertilisation presumptive zygotes were washed in fertilisation medium and cultured in Cleave Media (20 μl drops per 10 embryos; Cook Medical) until day 5 at 37oC in 6% CO2, 5% O2, and 89% N2. Preimplantation embryo development and morphology was determined by examining cleavage rate as a measure of fertilisation at 24 h, and blastocyst development at 72 h and 96 h [16]. The experiment was replicated five times with a minimum of 25 COCs in each group per replicate.
Blastocyst differential staining
Inner cell mass (ICM) and trophectoderm (TE) cell number were determined using as previously described [17]. Blastocysts were placed in 20 μl droplets which were covered under oil and placed in prewarmed culture dish at 37oC minimum 1 h before staining. Blastocysts were incubated in 0.5% pronase for 2–3 min to remove the zona pellucida and then washed in protein free 3-(N-morpholino) propanesulfonic acid (MOPS)-buffered media (GMOPS; Vitrolife, Gothenburg, Sweden). Blastocysts where then transferred into 10 μl of 2,4,6-trinitrobenzenesulfonic acid and 90 μl of polyvinylpyrrolidone (PVP) and cultured at 4°C for 10 min. Blastocysts were then washed in MOPS medium and incubated in 20 μl of 0.1 mg/ml anti-dinitrophenyl-BSA antibody (Cat# D9656) for 10 min at 37°C followed by a third wash in MOPS medium [11]. Following third wash, blastocysts were incubated in complement 50 μl propidium iodide (10 µg/ml; PI) and 50 μl guinea pig serum diluted in MOPS media at 37°C for 5 min in the dark. Blastocysts were then transferred to 500 μl of bisbenzimide in ethanol and incubated overnight at 4°C in dark. The following day-stained blastocysts were placed in 500 μl of 100% of ethanol and mounted in a drop of glycerol and gently flattened with a coverslip for cell counting. Stained blastocysts were imaged using an Olympus BX 51 microscope fitted with a mercury lamp. Bisbenzimide was excited and emitted at 338 nm and 505 nm respectively to visualise ICM cells and PI at 537 nm and 619 nm to visualise TE cells, and the number of ICM (blue) and TE (red) cells counted manually. The experiment was replicated five times with a minimum of 10 blastocysts in each group per replicate.
Oocyte mitochondria membrane potential
Mitochondrial Membrane Potential (MMP) was measured using the fluorescent dye, JC-1 (5,5_,6,6_-tetrachloro-1,1_,3,3_-tetraethylbenzimidazolyl-carbocynanine iodide; Molecular Probes, Eugene, OR, USA) at a concentration of 1.5 mM to determine mitochondria activity in the oocyte as previously described in [18, 19]. Briefly, post IVM COCs from both groups were incubated for 15 minutes in HEPES buffered α -MEM media with JC-1 dye at 37oC in dark. After 15 min, stained oocytes were washed in HEPES buffered α -MEM and mounted on a glass slide and gently flattened with a coverslip. The inner, middle and outer distribution of mitochondria were examined as previously described [18]. Oocyte fluorescence was observed using a green filter (490–540 nm) and a red filter (570–620 nm) on The Cell Voyager CV1000 Confocal Scanner (Yokogawa, Japan) using Z-stack imaging was done. Instrument settings were kept constant for each replicate. Z-stack images of the oocyte were merged and the images analysed using Image J software for Windows (Fiji, MD, USA). The ratio of red to green fluorescence (indicating high mitochondrial membrane potential) was determined in four different regions (regions 1–4) within outer, intermediate and peri-nuclear areas of the oocyte previously described [18]. The experiment was replicated three times and a minimum of six oocytes were used for each group per replicate.
Oocyte spindle formation and chromosomal alignment
The fluorescein isothiocyanate (FITC) conjugated mouse anti-α-tubulin (1:200, Alexa Fluor 488, Thermo Fisher Scientific, Waltham, MA, USA; Cat# 322588) was used to examine spindle formation and chromosomal alignment as previously described [20]. MII oocytes were fixed in 4% paraformaldehyde (Sigma–Aldrich) and then washed for 40 min in phosphate buffered saline (PBS) with 0.3 mg/ml polyvinyl alcohol (PVA) and permeabilised in 0.25% Triton-X (Sigma–Aldrich) for 40 min at 25oC. After 40 min oocytes were blocked in PBS containing 10% BSA and then placed in 2% Tween-20 for 1 h at 25oC. After three washes oocytes were incubated with FITC conjugated mouse anti-α-tubulin for 1 h at 25oC. After three washes, chromosomes were labelled using Hoechst (10 μg/ml, Sigma–Aldrich) for 10 min. Post incubation oocytes were washed three times in PBS and groups of five oocytes were loaded onto a glass slide for imaging. Fluorescence was detected using The Cell Voyager CV1000 Confocal Scanner (Yokogawa, Japan) under 40× objective where Z-stack imaging was done. Z-stack images were merged and the analysed using Image J software for Windows. FITC excited with a band width of green-fluorescent emission of 490–540 nm while Hoechst with a band of 438–485 nm. The experiment was replicated three times and a minimum of six oocytes were used for each group per replicate.
Statistical analysis
All data is expressed as the mean ± standard error of mean (SEM) unless stated otherwise. Statistical analysis was performed using IBM SPSS Statistics for Windows, version 28 (IBM Corp., New York, NY, USA) and Graph Pad Prism version 9 with a P value of < 0.05 been statistically significant and ≤ 0.1 classified as a trend. Data were assessed for normality using the Kolmogorov-Smirnov normality test. Cumulus expansion was analysed by Poisson loglinear generalised linear models with Bonferroni post hoc test. Blastocyst development data were normalised with logarithmic transformation and assessed by using paired t-test. Blastocyst cell counts (ICM, TE, Total cell, ICM/TE ratio), spindle length and width and mitochondrial membrane potential were measured using univariate general linear models with Bonferroni post hoc test. Chromosomal alignment was analysed using binomial regression with pairwise comparison.
Results
Effect of GM-CSF during IVM on cumulus expansion and fertilisation rates
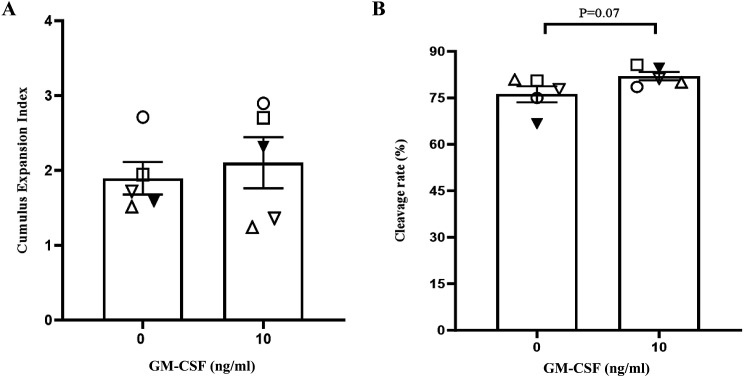
The addition of 10 ng/ml of GM-CSF during IVM had no effect on cumulus expansion (Fig. 1A; P > 0.05). However here was a trend for increase in fertilisation rates (76.19% vs. 82.03% %; Fig. 1B; P = 0.07) in the GM-CSF group compared with the control group.
Fig. 1.
The effect GM-CSF on cumulus expansion and fertilisation rates following 16 h of IVM on aged oocytes. (A) The proportion of COCs at each cumulus expansion index and (B) Proportion of 2-cells 24 h post insemination from the total number of COCs inseminated. The data is expressed as mean ± SEM of five replicates with a minimum of 25 COCs for each group per replicate. Different symbols represent the mean of each replicate across the treatment groups.
Effect of GM-CSF during IVM on blastocyst development
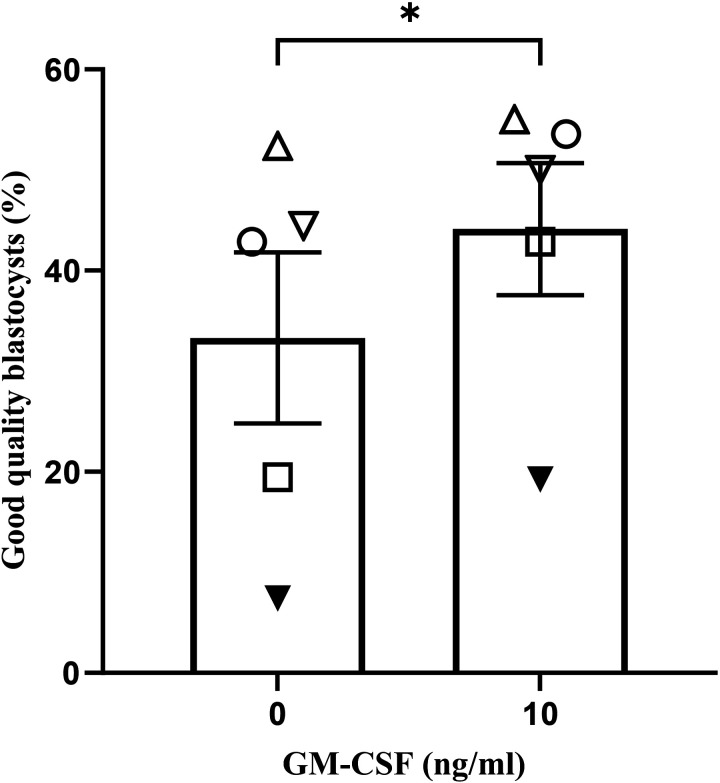
The addition of 10 ng/ml GM-CSF increased the proportion of total blastocysts at 96 h of culture (51.10% vs. 61.52%; Table1; P < 0.01). GM-CSF also increased the number of good quality blastocysts (≥ expanded; 33.31% vs. 44.13% %; Fig. 2; P < 0.05) compared with the control group.
Table 1. Effect of GM-CSF during IVM on AMA oocyte embryo development.
| GM-CSF (ng/ml) | n | 72 h post insemination early blastocyst (%) |
96 h post insemination total blastocyst (%) |
|---|---|---|---|
| 0 | 130 | 2.85 (2.85) | 51.10 (2.15) |
| 10 | 125 | 3.58 (2.21) | 61.52 (0.55) ** |
The data is expressed as a percentage of total oocytes and are the mean ± SEM of five replicates with a minimum of 25 COCs in each group per replicate (** P < 0.01).
Fig. 2.
The effect of GM-CSF during IVM on the proportion of good quality (≥ expanded) blastocyst development after 96 h of culture. The data is expressed as a percentage of good quality blastocysts to total oocytes and are the mean ± SEM of five replicates with a minimum of 25 COCs for each group per replicate. Different symbols represent the mean of each replicate across the treatment groups (* P < 0.05).
Effect of GM-CSF during IVM on blastocyst cell number
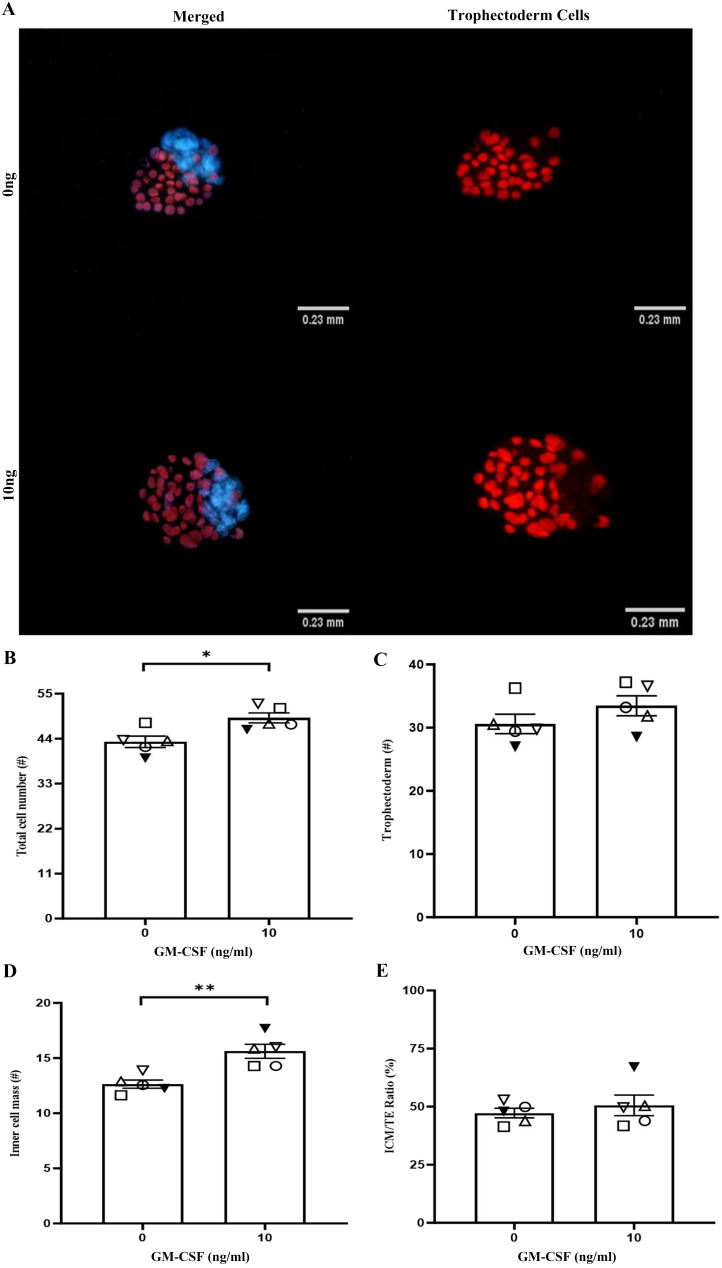
The addition of 10 ng/ml of GM-CSF increased total blastocyst cell number (42.98 vs. 48.78; Figs. 3A and 3B; P < 0.05) and blastocyst ICM cell number (12.64 vs. 15.62; Figs. 3A and 3D; P < 0.01). GM-CSF did not affect TE cell number (Figs. 3A and 3C) or the ratio of ICM/TE (Fig. 3E) compared with the control group.
Fig. 3.
The effect of GM-CSF during IVM on blastocysts cell number after 96 h of culture. (A) Representative images of blastocyst cell staining for 0 (control) and 10 ng/ml of GM-CSF. (B) The proportion of total cell number, (C) TE cell number (red), (D) ICM cell number (blue), and (E) ICM/TE ratio. The data is expressed as a percentage of total oocytes and are the mean ± SEM of five replicates with a minimum of 10 blastocysts in each group per replicate. Different symbols represent the mean of each replicate across the treatment groups (* P < 0.05, ** P < 0.01).
Effect of GM-CSF during IVM on mitochondrial activity in aged COCs
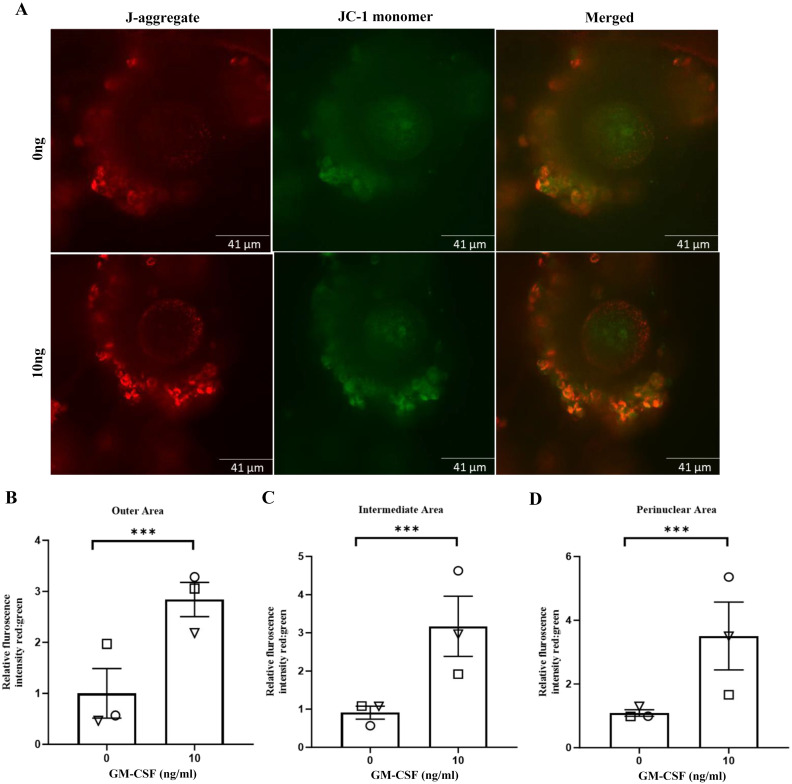
The addition of GM-CSF during IVM increased MMP in the outer (2.86 vs. 0.97; Figs. 4A and 4B; P < 0.001), intermediate (3.25 vs. 0.89; Figs. 4A and 4C; P < 0.001) and perinuclear areas (3.62 vs. 1.08; Figs. 4A and 4D; P < 0.001) compared with the control group.
Fig. 4.
The effect of GM-CSF during IVM on MMP following IVM in AMA COCs at 16 h. (A) Representative images of COCs for 0 (control) and 10 ng/ml of GM-CSF, stained with JC-1 green and red fluorescence indicate JC-1 monomers and JC-1 aggregates, respectively. (B, C and D) GM-CSF increased MMP across all three areas. The data is expressed as mean ± SEM of three replicates and was determined in a minimum of six oocytes in each group per replicate. Different symbols represent the mean of each replicate across groups (*** P < 0.001).
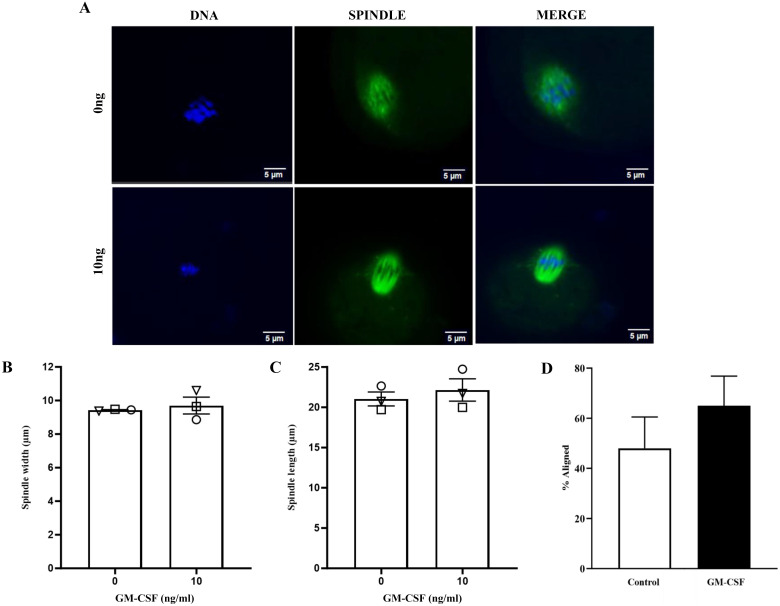
Effect of GM-CSF addition during IVM on spindle formation and chromosomal alignment
The addition of 10 ng/ml of GM-CSF to IVM had no effect on spindle formation (Figs. 5A, 5B and 5C; P > 0.05) or chromosomal alignment (Figs. 5A and 5C; P > 0.05).
Fig. 5.
The effect of GM-CSF during IVM on spindle formation following IVM in AMA oocytes. (A) Representative images of chromosomes and spindle for 0 (control) and 10 ng/ml of GM-CSF groups. (B and C) spindle width and diameter (mm) and (D) % of total oocytes with correct chromosomal alignment. Data is expressed as the mean + SEM of three replicates and was determined in a minimum of six oocytes in each group per replicate. Different symbols represent the mean of each replicate across groups (P > 0.05).
Discussion
The present study was undertaken to determine if the addition of GM-CSF during IVM could improve oocyte developmental competence in a mouse model of advanced maternal age. The addition of GM-CSF increased fertilisation rates, blastocyst development and the number of good quality blastocysts. Blastocyst ICM number and total cell number were also increased. These results are consistent with this from our previous study which showed that the addition of GM-CSF during IVM resulted in similar increases in blastocyst quantity and quality in young (4–6-week-old) mice. These improvements resulted in increased implantation and birth rates following embryo transfer. Whether GM-CSF treatment of oocytes from AMA could also improve implantation and birth rates remain to be determined.
In the present study mitochondrial function was increased two to three fold in AMA oocytes following GM-CSF treatment as measured by mitochondrial membrane potential with less COCs displaying oocytes with depolarised mitochondria (as indicated by a low red to green, fluorescent ratio). Decreased mitochondrial membrane potential is associated with opening of the mitochondrial permeability transition pore, resulting in membrane leakage and decoupling of the respiratory chain. Mitochondrial dysfunction is commonly seen in women of advanced maternal age and includes mitochondrial damage, lower mtDNA number and ATP content, increased mutation rate and elevated levels of ROS of oocytes [2, 3]. Our results show that GM-CSF may be able to be used as an in vitro therapeutic to restore mitochondrial dysfunction in women of advanced maternal age. The mechanism whereby GM-CSF improves mitochondrial function remains to be determined. However, we have shown in that GM-CSF increases glucose uptake in COCs from four week old mice which is associated with an increase in mitochondrial activity [21] presumably due to an increase in the supply of pyruvate and other energy substrates to the oocyte.
A decrease in the number and activity of mitochondria has been shown previously to result in reduced ATP production and impaired spindle activity and chromosome segregation [3]. It is also known that AMA is associated with an increase in aneuploidy [2, 4]. Oocyte aneuploidy results from either the premature separation of sister chromatid pairs or bivalent non disjunction through breakdown of the spindles [4]. As such we examined if the addition of GM-CSF during IVM could also improve spindle function and chromosomal alignment. Although, we did see numerical increases in chromosomal alignment following GM-CSF treatment this was not significant. However given the increased incidence of aneuploidy in AMA oocytes, this experiment needs to be repeated with larger number of oocytes. Future work will also determine incidence of aneuploidy in mature oocytes and embryos in our AMA using next generation sequencing.
Advanced maternal age has been shown to affect progeny growth and development [18]. Previous work using a similar AMA mouse model showed that offspring derived from blastocysts collected from AMA displayed increased body weights, increased blood pressure, altered glucose metabolism and organ allometry compared with offspring created from blastocysts of young females. These outcomes were due to the negative effects of AMA on oocyte quality as all blastocysts were transferred to surrogate young mothers, removing the effect of an aged uterus [22]. The addition of GM-CSF during in vitro embryo culture has been shown previously to improve progeny growth and development in mice and cattle [23, 24]. Whether GM-CSF during IVM can also help normalise progeny growth and development for progeny from AMA oocytes using our model warrants further investigation.
In conclusion we have shown that the addition of GM-CSF during IVM can improve embryo development in a mouse model of AMA. This is likely due to the positive benefits GM-CSF has on COC mitochondrial activity. Future work will extend our analysis of chromosomal alignment as well as examine the incidence of aneuploidy. While preliminary, our results indicate that the addition of GM-CSF during IVM may improve ART outcomes for women of advanced maternal age.
Conflict of interests
The authors declare they have no conflict of interest.
Acknowledgments
The assistance of staff from the University of Adelaide’s Laboratory Animal’s Services is gratefully acknowledged.
References
- 1.Newman JE, Paul RC, Chambers GM. Assisted reproductive technology in Australia and New Zealand 2020. National Perinatal Epidemiology and Statistics Unit. 2022. (Sydney). [Google Scholar]
- 2.Kobayashi H, Yoshimoto C, Matsubara S, Shigetomi H, Imanaka S. Altered energy metabolism, mitochondrial dysfunction, and redox imbalance influencing reproductive performance in granulosa cells and oocyte during aging. Reprod Sci 2024; 31: 906–916. [DOI] [PubMed] [Google Scholar]
- 3.Mikwar M, MacFarlane AJ, Marchetti F. Mechanisms of oocyte aneuploidy associated with advanced maternal age. Mutat Res Rev Mutat Res 2020; 785: 108320. [DOI] [PubMed] [Google Scholar]
- 4.Charalambous C, Webster A, Schuh M. Aneuploidy in mammalian oocytes and the impact of maternal ageing. Nat Rev Mol Cell Biol 2023; 24: 27–44. [DOI] [PubMed] [Google Scholar]
- 5.Gilchrist RB, Smitz J. Oocyte in vitro maturation: physiological basis and application to clinical practice. Fertil Steril 2023; 119: 524–539. [DOI] [PubMed] [Google Scholar]
- 6.Robertson SA, Chin PY, Schjenken JE, Thompson JG. Female tract cytokines and developmental programming in embryos. Adv Exp Med Biol 2015; 843: 173–213. [DOI] [PubMed] [Google Scholar]
- 7.Saini A, McPherson NO, Nottle M. Addition of GM-CSF during in vitro oocyte maturation improves embryo development and implantation and birth rate in mice. Reprod Fertil 2024; 5: e240020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saini A, McPherson NO, Nottle M. GM-CSF during in vitro oocyte maturation increases mitochondrial activity in cumulus oocyte complexes. In Program of the ESA-SRB-ANZBMS Virtual Conference; 2021. Abstract 549. [Google Scholar]
- 9.Camlin NJ, McLaughlin EA, Holt JE. The use of C57Bl/6 × CBA F1 hybrid cross as a model for human age-related oocyte aneuploidy. Mol Reprod Dev 2017; 84: 6–7. [DOI] [PubMed] [Google Scholar]
- 10.Dalton-O’Reilly J, Heazell AEP, Desforges M, Greenwood S, Dilworth M. Murine models of advanced maternal age: a systematic review and meta-analysis. Reproduction 2023; 166: M1–M12. [DOI] [PubMed] [Google Scholar]
- 11.Lim M, Brown HM, Kind KL, Breen J, Anastasi MR, Ritter LJ, Tregoweth EK, Dinh DT, Thompson JG, Dunning KR. Haemoglobin expression in in vivo murine preimplantation embryos suggests a role in oxygen-regulated gene expression. Reprod Fertil Dev 2019; 31: 724–734. [DOI] [PubMed] [Google Scholar]
- 12.Kyogoku H, Kitajima TS. The large cytoplasmic volume of oocyte. J Reprod Dev 2023; 69: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim M, Brown HM, Rose RD, Thompson JG, Dunning KR. Dysregulation of bisphosphoglycerate mutase during in vitro maturation of oocytes. J Assist Reprod Genet 2021; 38: 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanderhyden BC, Caron PJ, Buccione R, Eppig JJ. Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev Biol 1990; 140: 307–317. [DOI] [PubMed] [Google Scholar]
- 15.Wong SL, Wu LL, Robker RL, Thompson JG, McDowall MLS. Hyperglycaemia and lipid differentially impair mouse oocyte developmental competence. Reprod Fertil Dev 2015; 27: 583–592. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell M, Cashman KS, Gardner DK, Thompson JG, Lane M. Disruption of mitochondrial malate-aspartate shuttle activity in mouse blastocysts impairs viability and fetal growth. Biol Reprod 2009; 80: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handyside AH, Hunter S. A rapid procedure for visualising the inner cell mass and trophectoderm nuclei of mouse blastocysts in situ using polynucleotide-specific fluorochromes. J Exp Zool 1984; 231: 429–434. [DOI] [PubMed] [Google Scholar]
- 18.Wakefield SL, Lane M, Schulz SJ, Hebart ML, Thompson JG, Mitchell M. Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. Am J Physiol Endocrinol Metab 2008; 294: E425–E434. [DOI] [PubMed] [Google Scholar]
- 19.Zeng H-T, Richani D, Sutton-McDowall ML, Ren Z, Smitz JEJ, Stokes Y, Gilchrist RB, Thompson JG. Prematuration with cyclic adenosine monophosphate modulators alters cumulus cell and oocyte metabolism and enhances developmental competence of in vitro-matured mouse oocytes. Biol Reprod 2014; 91: 47. [DOI] [PubMed] [Google Scholar]
- 20.Al-Zubaidi U, Adhikari D, Cinar O, Zhang Q-H, Yuen WS, Murphy MP, Rombauts L, Robker RL, Carroll J. Mitochondria-targeted therapeutics, MitoQ and BGP-15, reverse aging-associated meiotic spindle defects in mouse and human oocytes. Hum Reprod 2021; 36: 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saini A, McPherson NO, Nottle M. GM-CSF during in vitro oocyte maturation increases glucose uptake in mouse cumulus oocyte complexes. In: Program of the ESA-SRB-ENSA Conference 2023; Brisbane, Queensland, Australia. Abstract 392. [Google Scholar]
- 22.Velazquez MA, Smith CGC, Smyth NR, Osmond C, Fleming TP. Advanced maternal age causes adverse programming of mouse blastocysts leading to altered growth and impaired cardiometabolic health in post-natal life. Hum Reprod 2016; 31: 1970–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sjöblom C, Roberts CT, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor alleviates adverse consequences of embryo culture on fetal growth trajectory and placental morphogenesis. Endocrinology 2005; 146: 2142–2153. [DOI] [PubMed] [Google Scholar]
- 24.Siqueira LG, Tribulo P, Chen Z, Denicol AC, Ortega MS, Negrón-Pérez VM, Kannampuzha-Francis J, Pohler KG, Rivera RM, Hansen PJ. Colony-stimulating factor 2 acts from days 5 to 7 of development to modify programming of the bovine conceptus at day 86 of gestation. Biol Reprod 2017; 96: 743–757. [DOI] [PubMed] [Google Scholar]