Abstract
Prepubertal animals are often preferred as sources of oocytes for assisted reproductive technologies (ARTs) in laboratory mice, but the normality and developmental competence of these oocytes remain controversial. This study systematically examined in vitro fertilization competence, embryo development, and fetal development after embryo transfer (ET) using oocytes from C57BL/6J mice aged 3 to 10 weeks. Oocytes were collected from superovulated females, fertilized, and cultured in vitro for 96 h or transferred at 2-cell stage to recipient females. Additionally, fetal development was compared between unfrozen and frozen-thawed in vitro-fertilized 2-cell embryos across different age groups. The number of ovulated oocytes per animal decreased with age, while the percentage of morphologically normal oocytes was highest in 3-week-old mice (99%) compared to older ages (70–86%, P < 0.05). Although fertilization percentages were consistently high (≥ 97%), blastocyst development in vitro, the nuclear counts of blastocysts and fetal development after ET were lowest for embryos from 3-week-old mice. Development of frozen-thawed embryos to fetuses was significantly reduced compared to unfrozen embryos in all age groups, except for those from 10-week-old mice. These findings suggest that oocytes from prepubertal mice, particularly from 3-week-old mice, are less developmentally competent than those from older mice. Therefore, the age of animals for oocyte source should be carefully considered based on the specific requirements of the research or ART applications.
Keywords: Animal age, Assisted reproductive technologies, C57BL/6J Mice, Embryo vitrification, In vitro fertilization and fetus development
Obtaining developmentally competent oocytes or embryos is a rate-limiting process in research of developmental biology and the application of assisted reproductive technologies (ARTs) in laboratory animals, domestic animals and human clinics. Unlike male gametes, which can be readily obtained quantitatively, female gametes are recovered in limited numbers, even after superovulation. Moreover, female animals must reach puberty before ovulation can begin, requiring substantial time, cost and labor [1, 2]. In laboratory rodents such as mice and rats, prepubertal animals aged 3–4 weeks are often preferred as oocyte donors due to their higher oocyte yield following hormonal stimulation [3]. Specifically, 3-week-old female mice are reported to ovulate more oocytes compared to animals of other ages [3,4,5,6,7], suggesting that younger females may be more efficient for oocyte retrieval than adults. However, many studies advocating the use of younger animals base their conclusions solely on the quantity of ovulated oocytes [3, 6, 7].
Conversely, oocytes from prepubertal animals differ from those of sexually mature females in several aspects, including higher rates of chromosomal abnormalities, variations in cytoplasmic morphology during oocyte growth, and differences in gene expression and methylation patterns [8,9,10,11,12]. In domestic species, embryos from prepubertal animals generally exhibit lower developmental competence and delayed development compared to those from sexually mature animals [13,14,15,16,17]. Thus, determining the optimal age for oocyte extraction in ARTs requires a comprehensive approach that considers not only the number of ovulated oocytes but also their fertilization and developmental competence.
This study systematically investigated the ideal age for obtaining oocytes for ARTs using the C57BL/6J strain; a widely utilized model in biomedical research. The numbers of ovulated oocytes, fertilization rates, in vitro developmental competence, and fetal development after embryo transfer (ET) were compared in mice aged 3 to 10 weeks. Additionally, given the routine use of embryo cryopreservation for maintaining mouse strains, especially with the explosive rise in genetically modified animals, the developmental competence of frozen-thawed embryos using in vitro fertilized (IVF) 2-cell embryos from females of various ages was examined.
Materials and Methods
Animals
C57BL/6J (B6J) and MCH(ICR)/Jcl (MCH) mice were purchased from Charles River, Japan [(currently) Jackson Laboratory Japan Inc., Kanagawa, Japan] and CLEA Japan Inc. (Tokyo, Japan), respectively. The animals were maintained as previously described [18]. All animal experiments were conducted at the National Institute of Radiological Sciences, Chiba, Japan, in accordance with the Regulations for the Handling of Laboratory Animals for Biomedical Research. The study was conducted under the authorization of the Institutional Animal Care and Use Committee for Laboratory Animal Experiments (Protocol ID: 07-1019-1) at the National Institute of Radiological Sciences.
Animals were classified by age into the following groups: 21–27 days (3 weeks old, 3W), 28–34 days (4 weeks old, 4W), 35–41 days (5 weeks old, 5W), 42–48 days (6 weeks old, 6W), 56–62 days (8 weeks old, 8W), and 70–77 days (10 weeks old, 10W). B6J females in these age groups underwent superovulation treatment and were euthanized for oocyte collection within the specified age range.
Experiment 1: examination of ovulation, in vitro fertilization and the first cleavage
Females were superovulated through intraperitoneal (i.p.) injection of 5 IU pregnant mares serum gonadotropin (PMSG, Serotropin; Aska Pharmaceutical Co., Ltd., Tokyo, Japan), followed 46–48 h later by i.p. injection of 5 IU human chorionic gonadotropin (hCG, Gonadotropin; Aska Pharmaceutical Co., Ltd.). Fifteen to sixteen hours after the hCG injection, cumulus-oocyte complexes (COCs) were collected from individual animals into a 100 µl drop of modified human tubal fluid (mHTF) [18]. The sperm, collected from the distal cauda epididymides of 12-24 weeks-old B6J males, were preincubated in modified Krebs-Ringer’s bicarbonate solution (TYH) for 1.5 h [18], then inseminated into the drops of the COCs at a concentration of 1.0–2.0 × 105 sperm/ml.
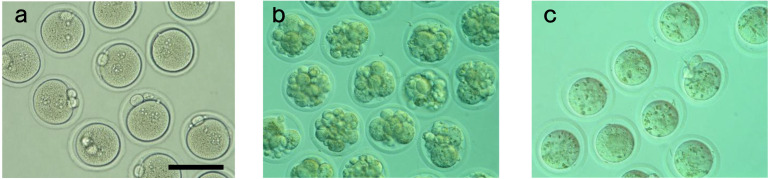
Six hours post-insemination (PI), oocytes were washed several times to remove cumulus cells and any attached sperm. They were then observed under a dissecting microscope to count and categorize the oocytes. The categories included: total number of ovulated oocytes, either fertilized or unfertilized with normal morphology (Fig. 1a), oocytes with abnormal morphology such as fragmentation (Fig.1b), and oocytes with a small diameter (< 70 µm) and larger perivitelline space (Fig. 1c). Fertilized oocytes were identified by the presence of two pronuclei and the extruded second polar body.
Fig. 1.
Representative images of (a) normal oocytes, (b) fragmented oocytes and (c) small sized oocytes are shown. Scale bar represents 100 μm.
Experiment 2: in vitro development to the blastocyst stage and fetal development after embryo transfer
Fertilized oocytes from individual animals obtained in Experiment 1 were cultured in 50 µl drops of KSOM-AA under an atmosphere of 5% CO2, 5% O2, and 90% N2 [19]. At 24 h PI, the number of 2-cell embryos was counted. Some embryos were then used for embryo transfer as described below, while the remaining embryos were further cultured until 96 h PI to assess their in vitro developmental ability. Portions of blastocysts were fixed and stained with Hoechst 33342 to count nuclear numbers, as described elsewhere [20].
Two-cell embryos were transferred to the oviducts of pseudopregnant MCH females on the day of vaginal plug formation (day 1 of pseudopregnancy). On day 19 of pregnancy, implantation and fetal development were examined by necropsy.
Experiment 3: development of frozen-thawed embryos
In vitro fertilized 2-cell embryos, obtained from each age group of animals at 24 h PI, were divided into two groups: an unfrozen control group and a frozen-thawed group. Embryos in the frozen-thawed group were vitrified in an ethylene glycol solution, following the methods described by Kasai and modified by Mochida et al. [21, 22]. The thawed embryos were immediately transferred to recipient females, and implantation and fetal development were examined as described in Experiment 2.
Statistical analysis
Percentage data were transformed using the arcsine (Tukey–Freeman) transformation to address unequal variances [23]. The data were then analyzed using analysis of variance (ANOVA). When the P-value was less than 0.05, pairwise comparisons were conducted using the Least Significant Difference (LSD) test.
Results
Experiment 1: examination of ovulation, in vitro fertilization and the first cleavage
The number of ovulated oocytes per individual animal was highest in the 3W and 4W groups, with 30 ± 6 and 31 ± 3 oocytes per animal, respectively, and decreased in the 5–10W groups, with 20–24 oocytes per animal (Table 1). Almost all oocytes from 3W mice were morphologically normal (99%), but the percentage of normal oocytes significantly decreased with age (70–86%, P < 0.05). The majority of abnormalities observed were fragmentation in oocytes from the 4W–8W groups (50–80%), whereas oocytes from the 10W group primarily exhibited smaller size (69% of abnormalities).
Table 1. Number of ovulated oocytes from various ages of C57BL/6J mice.
| Age (weeks) | No. females | No. ovulated females | Total no. oocytes (Mean ± MSE) |
No. normal oocytes at 6 h PI (% ± MSE) a |
No. abnormal oocytes |
|||
|---|---|---|---|---|---|---|---|---|
| Total | Fragmentation (% b) | Small size (% b) | Other abnormality (% b) | |||||
| 3 | 15 | 12 | 358 (30 cd ± 6) | 353 (99 c ± 1) | 5 | 1 (20) | 3 (60) | 1 (20) |
| 4 | 15 | 13 | 403 (31 c ± 3) | 324 (82 d ± 5) | 79 | 63 (80) | 16 (20) | 0 (0) |
| 5 | 15 | 13 | 285 (22 de ± 2) | 207 (76 de ± 5) | 78 | 55 (71) | 23 (29) | 0 (0) |
| 6 | 15 | 15 | 301 (20 e ± 2) | 207 (72 e ± 4) | 94 | 45 (51) | 38 (40) | 8 (9) |
| 8 | 15 | 15 | 318 (21 e ± 2) | 221 (70 e ± 4) | 97 | 77 (79) | 20 (21) | 0 (0) |
| 10 | 15 | 14 | 337 (24 cde ± 3) | 292 (86 d ± 2) | 45 | 14 (31) | 31 (69) | 0 (0) |
a Percentage and mean standard error (MSE) of total oocytes ovulated. b Percentages of total abnormal oocytes. c–e Numbers or percentages with different superscripts in the same column were significantly different (P < 0.05).
The fertilization percentages at 6 h PI and the percentage of 2-cell embryos at 24 h PI were high across all age groups (97–99% and 95–100%, respectively) (Supplementary Table 1). Although there were significant differences in the percentages of 2-cell embryos among the age groups, these differences were relatively marginal.
Experiment 2: in vitro development and fetus development after embryo transfer
After in vitro culture for 96 h PI, the development of IVF embryos to the blastocyst and expanded blastocyst stages was significantly lower in embryos from 3W animals (69 ± 3%) compared to those from other age groups (93–97%, P < 0.05) (Table 2). Nearly all blastocysts across the age groups developed into expanded blastocysts (92–100%, data not shown). The nuclear count of blastocysts from 3W animals (58.2 ± 3.1) was the lowest among the age groups and significantly lower than those from 8W (66.7 ± 1.7) and 10W animals (65.1 ± 1.5, P < 0.05) (Table 2).
Table 2. In vitro development of IVF embryos for 96 h PI from various ages of C57BL/6J mice.
| Age (weeks) | No. 2-cell cultured | No. of embryos at 96 h PI (% ± MSE) a
|
Nuclear number of blastocysts |
||
|---|---|---|---|---|---|
| Blastocysts | Expanded blastocysts | No. counted blastocysts | Nuclear number | ||
| 3 | 94 | 65 (69 b ± 3) | 60 (64 b ± 4) | 27 | 58.2 b ± 3.1 |
| 4 | 96 | 89 (93 c ± 1) | 88 (92 c ± 1) | 15 | 62.1 bc ± 3.7 |
| 5 | 36 | 35 (97 c ± 3) | 34 (95 c ± 5) | 11 | 63.5 bc ± 5.0 |
| 6 | 36 | 35 (97 c ± 3) | 35 (97 c ± 3) | 13 | 64.2 bc ± 4.0 |
| 8 | 107 | 103 (96 c ± 1) | 103 (96 c ± 1) | 40 | 66.7 c ± 1.7 |
| 10 | 125 | 121 (97 c ± 1) | 121 (97 c ± 1) | 60 | 65.1 c ± 1.5 |
a Percentage and mean standard error (MSE) of 2-cell embryos cultured. bc Percentages or numbers with different superscripts in the same column were significantly different (P < 0.05).
Percenatges of implantation and fetal development after ET of 2-cell embryos from 3W animals were the lowest among all age groups examined, with percentages of 74 ± 7% and 47 ± 9%, respectively, compared to 89–92% and 68–77% for other age groups (P < 0.05) (Table 3).
Table 3. Development after ET of IVF embryos from various ages of C57BL/6J mice.
| Age (weeks) | No. recipient | No. pregnant | No. embryos transferred | No. (% ± MSE) a |
|
|---|---|---|---|---|---|
| Implantation site | Fetus | ||||
| 3 | 6 | 6 | 96 | 71 (74 b ± 7) | 45 (47 b ± 9) |
| 4 | 6 | 6 | 87 | 80 (90 c ± 5) | 61 (68 c ± 8) |
| 5 | 6 | 5 | 62 | 56 (90 c ± 5) | 42 (68 c ± 4) |
| 6 | 6 | 5 | 64 | 61 (96 c ± 3) | 46 (75 c ± 7) |
| 8 | 6 | 6 | 84 | 74 (89 c ± 5) | 64 (77 c ± 6) |
| 10 | 6 | 6 | 88 | 81 (92 c ± 2) | 65 (74 c ± 6) |
a Percentages and mean standard error (MSE) of 2-cell embryos transferred. bc Percentages with different superscripts in the same column were significantly different (P < 0.05).
Experiment 3: development of frozen-thawed embryos
The survival of frozen-thawed embryos was not significantly affected by the age of the animals (88–97%, P > 0.05) (Supplementary Table 2). Consistent with Experiment 2, implantation sites and fetal development were lowest for embryos from 3W animals, both for unfrozen and frozen-thawed embryos. Notably, fetal development for frozen-thawed embryos from 3W animals was only 16 ± 2% (Table 4). Comparison between unfrozen and frozen-thawed embryos from the same age group revealed that fetal development of frozen-thawed embryos was significantly lower than that of unfrozen embryos, except for embryos from the 10W group.
Table 4. Development after ET of frozen-thawed IVF embryos from various ages of C57BL/6J mice.
| Age (weeks) | Treatment | No. pregnant females | No. embryos transferred | No. (% ± MSE) a |
|
|---|---|---|---|---|---|
| Implantation site | Fetus | ||||
| 3 | Unfrozen | 5 | 78 | 55 (71 ± 9) | 27 (35 ± 10) |
| Frozen | 16 | 288 | 197 (69 ± 4) | 46 (16 * ± 2) | |
| 4 | Unfrozen | 5 | 70 | 67 (94 ± 5) | 54 (74 ± 7) |
| Frozen | 13 | 249 | 218 (87 ± 3) | 122 (49 * ± 4) | |
| 5 | Unfrozen | 3 | 32 | 29 (93 ± 7) | 23 (76 ± 14) |
| Frozen | 9 | 126 | 111 (88 ± 4) | 63 (50 * ± 7) | |
| 6 | Unfrozen | 3 | 36 | 34 (94 ± 3) | 30 (83 ± 5) |
| Frozen | 4 | 63 | 55 (87 ± 4) | 35 (54 * ± 8) | |
| 8 | Unfrozen | 5 | 60 | 56 (93 ± 4) | 50 (83 ± 6) |
| Frozen | 6 | 98 | 77 (75 * ± 9) | 46 (47 * ± 4) | |
| 10 | Unfrozen | 5 | 72 | 64 (89 ± 3) | 51 (71 ± 4) |
| Frozen | 9 | 160 | 133 (84 ± 5) | 89 (55 ± 4) | |
a Percentages and mean standard error (MSE) of 2-cell embryos transferred. * Significantly different from unfrozen control within the same age group (P < 0.05).
Discussion
The use of oocytes from prepubertal animals is an important issue in various mammalian species. For domestic animals, employing prepubertal animals can shorten generation intervals, offering economic benefits. Extensive studies have been conducted using small domestic animals to enhance the productivity of in vitro-produced embryos derived from prepubertal ovaries [1, 2]. However, oocytes from prepubertal animals differ from those of adult animals in several aspects, including chromosomal normality, protein synthesis capability, cortical granule distribution, cytoskeletal kinetics, calcium oscillation patterns during sperm penetration, energy metabolism, and transcriptional activity [8,9,10,11,12, 24]. These differences may account for the compromised developmental competence observed in oocytes from prepubertal animals.
In laboratory mice, 3-week-old prepubertal animals are often recommended for oocyte donors for assisted reproductive technologies (ARTs), as they typically ovulate a higher number of oocytes compared to adults [3, 4, 6]. However, the fertilization and developmental competence of oocytes from prepubertal animals have not been systematically examined. This study aims to evaluate the fertilization and developmental competence of oocytes from C57BL/6J mice, a commonly used strain, at ages ranging from 3 to 10 weeks. Additionally, the developmental competence of frozen-thawed embryos from various age groups was assessed.
The number of ovulated oocytes was highest in 3W and 4W animals (Table 1), consistent with other studies [3, 4]. Almost all oocytes from 3W animals were morphologically normal, while the percentage of abnormal oocytes increased with age. Fragmentation was the predominant abnormality in oocytes from 4W-8W animals, whereas small size was the major abnormality in 10W animals. Endogenous gonadotropins may influence the appearance and variation of these abnormalities, as gonadotropin profiles change dynamically from birth to puberty [25].
Fertilization competence and development to the 2-cell stage were high across all age groups (Table 2). However, development to the blastocyst and expanded blastocyst stages was significantly lower in 3W animals compared to other age groups. Additionally, the nuclear count of blastocysts was significantly lower in embryos from 3W animals compared to those from 8W and 10W animals. These results suggest that while 3W oocytes have fertilization competence, they have not acquired full developmental competence to reach the blastocyst stage. Further evidence of compromised developmental competence is provided by embryo transfer (ET) results, with significantly lower fetal development of embryos from 3W animals compared with those from other ages of animas.
In vitro maturation studies on follicle growth and maturation indicated that oocytes acquire competence for various developmental stages with age, including germinal vesicle breakdown, meiotic divisions, the first cleavage division and later development [26, 27]. Developmental competence to blastocysts has been shown to achieve in oocytes from 24-days or older animals [27]. Because their study did not examine the age when oocytes acquires full developmental competence to fetus, the present study possibly compensates their study; developmental competence to fetus may be fully acquired in oocytes from 28-days or older age of animals. Precise timing of acquisition is unclear due to the difference between inbred strain in this study and F1 hybrid strain used in their study which matures faster than inbred strains. [28].
Cryopreservation of embryos or gametes is a crucial component of ARTs across various animal species. In laboratory settings, frozen embryos are essential for maintaining strains, restoring stocks in emergencies, producing genetically modified animals and facilitating transport between research institutes. The importance of cryopreservation has increased with the growing number of genetically modified animals [29]. This study provides direct comparisons of developmental competence between fresh and frozen-thawed embryos from various mouse ages.
Development of frozen-thawed embryos was reduced compared to unfrozen controls. Embryos from 3W animals were the least tolerant to the freeze-thaw process (Table 4). Previous studies have shown that vitrification increases reactive oxygen species in various animal species, which can negatively affect developmental ability. The addition of reducing agents in cryoprotectant solutions may improve the developmental competence of frozen-thawed embryos [30,31,32]. Embryos from 3W animals showed a dramatic reduction in competence. This suggests they were unable to recover adequately from oxidative damage during cryopreservation. Other factors may include increased susceptibility of the plasma membrane or cytoplasm to cryoprotectant toxicity or reduced recovering ability from damage compared to older animals [33, 34].
For practical applications, estimated efficiencies of mouse production by ARTs are calculated and shown in Table 5. Estimates for unfrozen embryos are based on results from Experiments 1 and 2, while those for frozen-thawed embryos are based on Experiment 3. Among the age groups, embryos from 10W adult females resulted in the highest number of fetuses, both for unfrozen (62.9 fetuses) and frozen-thawed embryos (45.6 fetuses). This suggests that 10W mice provide the highest developmental competence. However, when comparing fetal numbers produced from individual animals across different ages, 4W animals (16.6 fetuses unfrozen, 11.6 fetuses frozen-thawed) showed better performance than other ages, although estimates were similar to those from 10W mice (15.1 fetuses unfrozen, 11.0 fetuses frozen-thawed). From the perspective of the 3R principles of animal research, using 4W animals may reduce the number of animals sacrificed most effectively. Therefore, 4W or 10W mice are optimal choices for embryo production in this strain of mice, with the selection depending on the specific research or ART goals. Although 3W mice ovulate the highest number of oocytes, their developmental competence, particularly for frozen-thawed embryos, is not fully acquired. Thus, the age of animals used for oocyte sources should be carefully considered based on the intended research or ART applications.
Table 5. Efficiency of fetus production of oocytes from various ages of C57BL/6J mice.
| Age (week) | Treatment | Estimate no. fetus obtained from 100 ovulated oocytes | Estimate no. fetus obtained from individual animals |
|---|---|---|---|
| 3 | Fresh | 43.1 | 12.9 |
| Frozen-thawed | 13.6 | 4.0 | |
| 4 | Fresh | 53.6 | 16.6 |
| Frozen-thawed | 37.4 | 11.6 | |
| 5 | Fresh | 47.5 | 10.4 |
| Frozen-thawed | 33.5 | 7.3 | |
| 6 | Fresh | 53.0 | 10.6 |
| Frozen-thawed | 33.8 | 6.8 | |
| 8 | Fresh | 51.6 | 10.9 |
| Frozen-thawed | 30.0 | 6.3 | |
| 10 | Fresh | 62.9 | 15.1 |
| Frozen-thawed | 45.6 | 11.0 | |
Estimates were calculated from the results of Experiments 1 and 2 for fresh embryos, Experiment 3 for frozen-thawed embryos.
Conflict of Interests
The authors declare no conflict of interest in the publication of this research article.
Supplementary
Acknowledgments
The author gratefully acknowledges Ms. Mami Hayashi and Yuki Ohta-Takada for their care of the animals and their technical support with the animal experiments conducted in this research. The author also thanks to Dr. Joshua Sakon at University of Arkansas for proofreading and critically reviewing the manuscript.
References
- 1.Mastrorocco A, Cacopardo L, Lamanna D, Temerario L, Brunetti G, Carluccio A, Robbe D, Dell’Aquila ME. Bioengineering approaches to improve in vitro performance of prepubertal lamb oocytes. Cells 2021; 10: 1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Souza-Fabjan JMG, Leal GR, Monteiro CAS, Batista RITP, Barbosa NO, Freitas VJF. In vitro embryo production in small ruminants: what is still missing? Anim Reprod 2023; 20: e20230055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarrow MX, Wilson ED. The influence of age on superovulation in the immature rat and mouse. Endocrinology 1961; 69: 851–855. [DOI] [PubMed] [Google Scholar]
- 4.Sugiyama F, Kajiwara N, Hayashi S, Sugiyama Y, Yagami K. Development of mouse oocytes superovulated at different ages. Lab Anim Sci 1992; 42: 297–298. [PubMed] [Google Scholar]
- 5.Byers SL, Payson SJ, Taft RA. Performance of ten inbred mouse strains following assisted reproductive technologies (ARTs). Theriogenology 2006; 65: 1716–1726. [DOI] [PubMed] [Google Scholar]
- 6.Kolbe T, Landsberger A, Manz S, Na E, Urban I, Michel G. Productivity of superovulated C57BL/6J oocyte donors at different ages. Lab Anim (NY) 2015; 44: 346–349. [DOI] [PubMed] [Google Scholar]
- 7.Lamas S, Carvalheira J, Gartner F, Amorim I. C57BL/6J mouse superovulation: schedule and age optimization to increase oocyte yield and reduce animal use. Zygote 2021; 29: 199–203. [DOI] [PubMed] [Google Scholar]
- 8.Mitsui A, Yoshizawa M. Evaluation of Pre-maturity of mouse oocytes ovulated from prepubertal females using an in vito fertilization technique. J Mamm Ova Res 2006; 23: 114–121. [Google Scholar]
- 9.Golbus MS. The influence of strain, maternal age, and method of maturation on mouse oocyte aneuploidy. Cytogenet Cell Genet 1981; 31: 84–90. [DOI] [PubMed] [Google Scholar]
- 10.Catala V, Estop AM, Santalo J, Egozcue J. Sexual immaturity and maternal age: incidence of aneuploidy and polyploidy in first-cleavage mouse embryos. Cytogenet Cell Genet 1988; 48: 233–237. [DOI] [PubMed] [Google Scholar]
- 11.Wassarman PM, Josefowicz WJ. Oocyte development in the mouse: an ultrastructural comparison of oocytes isolated at various stages of growth and meiotic competence. J Morphol 1978; 156: 209–235. [DOI] [PubMed] [Google Scholar]
- 12.Bao S, Obata Y, Carroll J, Domeki I, Kono T. Epigenetic modifications necessary for normal development are established during oocyte growth in mice. Biol Reprod 2000; 62: 616–621. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong DT. Effects of maternal age on oocyte developmental competence. Theriogenology 2001; 55: 1303–1322. [DOI] [PubMed] [Google Scholar]
- 14.Pawlak P, Renska N, Pers-Kamczyc E, Warzych E, Lechniak D. The quality of porcine oocytes is affected by sexual maturity of the donor gilt. Reprod Biol 2011; 11: 1–18. [DOI] [PubMed] [Google Scholar]
- 15.Palmerini MG, Nottola SA, Leoni GG, Succu S, Borshi X, Berlinguer F, Naitana S, Bekmukhambetov Y, Macchiarelli G. In vitro maturation is slowed in prepubertal lamb oocytes: ultrastructural evidences. Reprod Biol Endocrinol 2014; 12: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leoni GG, Succu S, Berlinguer F, Rosati I, Bebbere D, Bogliolo L, Ledda S, Naitana S. Delay on the in vitro kinetic development of prepubertal ovine embryos. Anim Reprod Sci 2006; 92: 373–383. [DOI] [PubMed] [Google Scholar]
- 17.Salamone DF, Damiani P, Fissore RA, Robl JM, Duby RT. Biochemical and developmental evidence that ooplasmic maturation of prepubertal bovine oocytes is compromised. Biol Reprod 2001; 64: 1761–1768. [DOI] [PubMed] [Google Scholar]
- 18.Kito S, Hayao T, Noguchi-Kawasaki Y, Ohta Y, Hideki U, Tateno S. Improved in vitro fertilization and development by use of modified human tubal fluid and applicability of pronucleate embryos for cryopreservation by rapid freezing in inbred mice. Comp Med 2004; 54: 564–570. [PubMed] [Google Scholar]
- 19.Lawitts JA, Biggers JD. Culture of preimplantation embryos. Methods Enzymol 1993; 225: 153–164. [DOI] [PubMed] [Google Scholar]
- 20.Ebert KM, Hammer RE, Papaioannou VE. A simple method for counting nuclei in the preimplantation mouse embryo. Experientia 1985; 41: 1207–1209. [DOI] [PubMed] [Google Scholar]
- 21.Kasai M. Cryopreservation of mammalian embryos. Vitrification. Methods Mol Biol 1995; 38: 211–219. [DOI] [PubMed] [Google Scholar]
- 22.Mochida K, Hasegawa A, Taguma K, Yoshiki A, Ogura A. Cryopreservation of mouse embryos by ethylene glycol-based vitrification. J Vis Exp 2011; 18: 3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zar JH. 1996. Biostatistical Analysis, 3rd edition. Englewood Cliffs, NJ: Prentice-Hall. 1996: 277–305. [Google Scholar]
- 24.Catalá MG, Izquierdo D, Uzbekova S, Morató R, Roura M, Romaguera R, Papillier P, Paramio MT. Brilliant Cresyl Blue stain selects largest oocytes with highest mitochondrial activity, maturation-promoting factor activity and embryo developmental competence in prepubertal sheep. Reproduction 2011; 142: 517–527. [DOI] [PubMed] [Google Scholar]
- 25.Bronson FH, Dagg CP, Snell GD. 11 Reproduction. In: Green, EL (eds.), Biology of the laboratory mouse, 2nd edition. New York: Dover Publication Inc; 1966: 187-204. [Google Scholar]
- 26.Sorensen RA, Wassarman PM. Relationship between growth and meiotic maturation of the mouse oocyte. Dev Biol 1976; 50: 531–536. [DOI] [PubMed] [Google Scholar]
- 27.Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod 1989; 41: 268–276. [DOI] [PubMed] [Google Scholar]
- 28.Nelson JF, Karelus K, Felicio LS, Johnson TE. Genetic influences on the timing of puberty in mice. Biol Reprod 1990; 42: 649–655. [DOI] [PubMed] [Google Scholar]
- 29.Mobraaten LE. Mouse embryo cryobanking. J In Vitro Fert Embryo Transf 1986; 3: 28–32. [DOI] [PubMed] [Google Scholar]
- 30.Tatone C, Di Emidio G, Vento M, Ciriminna R, Artini PG. Cryopreservation and oxidative stress in reproductive cells. Gynecol Endocrinol 2010; 26: 563–567. [DOI] [PubMed] [Google Scholar]
- 31.Abdul Rahman NS, Mohamed Noor Khan NA, Eshak Z, Sarbandi MS, Mohammad Kamal AA, Abd Malek M, Abdullah F, Abdullah MA, Othman F. Exogenous L-glutathione improves vitrification outcomes in murine preimplantation embryos. Antioxidants 2022; 11: 2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menéndez-Blanco I, Soto-Heras S, Catalá MG, Piras AR, Izquierdo D, Paramio MT. Effect of vitrification of in vitro matured prepubertal goat oocytes on embryo development after parthenogenic activation and intracytoplasmic sperm injection. Cryobiology 2020; 93: 56–61. [DOI] [PubMed] [Google Scholar]
- 33.Lawson A, Ahmad H, Sambanis A. Cytotoxicity effects of cryoprotectants as single-component and cocktail vitrification solutions. Cryobiology 2011; 62: 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elliott GD, Wang S, Fuller BJ. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017; 76: 74–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.