Abstract
Background and Purpose
Three large, randomized trials demonstrated the benefit of short‐term dual antiplatelet therapy (DAPT) versus monotherapy after non‐cardioembolic minor stroke or high‐risk transient ischemic attack (TIA). The aim of this study was to evaluate effects of DAPT versus monotherapy on functional outcomes and safety in a real‐life setting.
Methods
Patients with minor stroke (NIHSS <4) or high‐risk TIA (ABCD2 score ≥4) of non‐cardioembolic origin without major vessel occlusion or revascularization therapy (thrombolysis or thrombectomy) treated between 2018 and 2023 were analyzed based on a prospective nationwide stroke unit registry. Data on risk factors, stroke etiology, admission stroke severity (NIHSS), functional status at 3 months (mRS), and mortality were extracted. Excellent functional outcome (mRS 0–1) at 3 months, early neurological deterioration (END), symptomatic intracranial hemorrhage (SICH) and major extracranial bleeds were defined as study endpoints and adjusted for covariates using inverse probability of treatment weighted regression models.
Results
Two Thousand Two Hundred Fifty‐four of 8546 patients with non‐cardioembolic minor stroke or high‐risk TIA received DAPT. Patients treated with DAPT had significantly more risk factors and comorbidities compared to those treated with monotherapy. After robust statistical adjustment, DAPT was significantly associated with lower occurrence of END (OR 0.50 95% CI 0.35–0.72), increased odds of excellent outcome at 3 months (aOR 1.59; 95% CI 1.20–2.09) and equivalent frequencies of SICH (aOR 1.19, 95% CI 0.30–4.73) or major extracranial bleeding (aOR 0.84; 95% CI 0.16–4.56).
Conclusions
DAPT in non‐cardioembolic minor stroke or high‐risk TIA in a real‐life setting appears to be safe and associated with improved functional outcome.
Keywords: dual antiplatelet therapy, minor stroke, outcome
INTRODUCTION
Stroke is a leading cause of morbidity and mortality worldwide representing a significant burden on healthcare systems [1]. In recent years, dual antiplatelet therapy (DAPT) has emerged as a potential strategy to improve outcomes in patients with non‐cardioembolic strokes or high‐risk TIAs. DAPT typically includes the combination of aspirin with a P2Y12 receptor inhibitor (clopidogrel or ticagrelor). In Austria, a standard DAPT regimen includes 100 mg of acetylsalicylic acid (ASA) and 75 mg of clopidogrel (loading dose: 250–300 mg ASA and 300 mg clopidogrel) for 21 days. Following the initial randomized controlled trials (RCTs) demonstrating a significant reduction in the recurrence of vascular events [2, 3, 4] short‐term DAPT has been implemented as a secondary prevention strategy for non‐cardioembolic strokes or high‐risk TIAs for a duration of 3–4 weeks in clinical routine. Only a substudy of the CHANCE trial [5] reported significant benefits of dual antiplatelet therapy (DAPT) on functional outcomes. However, data on the effects of DAPT on functional outcome after ischemic stroke in a real‐life setting are scarce.
The aim of the current study was therefore to investigate the effects of DAPT on functional outcome in patients with non‐cardioembolic strokes or high‐risk TIAs in a real‐life setting.
METHODS
The Austrian Stroke Unit Registry (ASUR) is a nationwide prospective database comprising 38 stroke units within the Austrian stroke unit network, established by the Federal Ministry of Health in 2003. Datasets were extracted from patients who were treated at one of the 38 Austrian stroke units between 2018 and 2023 for either a minor ischemic stroke (NIHSS <4) or high‐risk TIA (ABCD2 Score ≥4) with non‐cardioembolic etiology, and who did not receive thrombolysis and/or thrombectomy. Subjects with symptomatic carotid stenosis and acute carotid surgery or stent implantation, patients who presented with DAPT on admission, those prescribed oral anticoagulation as well as patients who remained on DAPT until follow‐up, were excluded. Methodological aspects of the Austrian Stroke Unit Registry have been published previously [6]. Briefly, anonymized data of all patients admitted with acute ischemic stroke are recorded on admission, discharge, and at 3‐month follow‐up. The 3‐month follow‐up is conducted either in person or via telephone. The web‐based database captures baseline characteristics, risk factors, acute treatment, and functional outcome. Supervised by an academic review board, the registry is integral to assessing the quality of stroke care. Individual informed consent was not obtained.
Variables extracted from the registry included age, sex, National Institute of Health Stroke Scale (NIHSS) scores at admission and discharge from the stroke unit, modified Rankin Scale (mRS) scores pre‐stroke, at discharge, and at the 3‐month follow‐up, as well as risk factors such as hypertension, diabetes, hypercholesterolemia, smoking, prior stroke, atrial fibrillation, coronary heart disease, and peripheral artery disease. Stroke etiology was assessed according to the TOAST (Trial of ORG 10172 in Acute Stroke Treatment) classification. Other data collected included wake‐up strokes, onset‐to‐door time (ODT), symptomatic intracerebral hemorrhage according to ECASS 3 criteria [7] (SICH) and major extracranial bleedings. For the purpose of the study patients were grouped according to DAPT or monotherapy and according to etiology. The following study endpoints were defined:
Primary endpoint: Excellent functional outcome (mRS 0–1) at 3 months after the index event
Secondary endpoint: Early neurological deterioration (END): neurological deterioration with an increase of >4 points in the NIHSS or recurrent stroke in another localization between admission and discharge from the stroke unit
Safety endpoints: SICH according to ECASS 3 criteria [7] and all‐cause mortality at 3 months follow‐up. Major extracranial bleeding at stroke unit discharge
Statistics
Results are presented as median and interquartile range (IQR) for continuous variables, while categorical variables are summarized by absolute frequencies (n) and relative frequencies (%). Differences between the follow‐up group and the lost‐to‐follow‐up group were calculated using standardized mean differences (Cohen's d). A value greater or equal 0.2 was considered statistically significant. Patients were further stratified into groups of DAPT or monotherapy. The Mann–Whitney U‐test was applied for the comparison of continuous and ordinal variables without a normal distribution, while Pearson's Chi‐Square test was used to compare the frequencies and distributions of categorical variables. To adjust for imbalances doubly robust inverse probability weighted regression adjustment (IPWRA) models were applied. IPWRA is designed to enhance the accuracy of causal inference in observational studies and involves calculating the inverse probability of treatment weights (IPW). Weights are assigned to patients based on the inverse of their probability of receiving treatment as estimated by the propensity score derived from confounders. This results in a pseudo‐population in which patients with a high probability of receiving treatment have a smaller weight and patients with a low probability of receiving treatment have a larger weight and thus the distribution of measured patient characteristics used to calculate the propensity score becomes independent of treatment assignment. IPW was then used to weight the regression models while adjusting for the following covariates: age, sex, pre‐stroke disability, common risk factors (hypertension, diabetes, myocardial infarction, hypercholerstinemia and smoking), stroke severity (NIHSS), and center. All statistical analyses were conducted using SPSS 29, IBM and Practical meta‐analysis effect size calculator, Version 2023.11.27, Campbell Collaboration.
Ethics
Ethics approval for retrospective analysis of prospectively collected data was obtained from the local ethics committee. In accordance with Austrian legislation and as part of routine observational quality registry procedures, patient consent for registration in the ASUR is not required. This study was conducted and reported in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines [8] to ensure transparency and quality in the presentation of the research findings.
RESULTS
Safety and efficacy of DAPT versus monotherapy
Between 2018 and 2023, data of 8546 patients with minor stroke (NIHSS<4) or high‐risk TIA (ABCD2 Score ≥4), non‐cardioembolic etiology were extracted (Figure 1). 2254/8546 (26.4%) were treated using DAPT. Patients treated with DAPT were less frequently female (35.5% [n = 800/2254], p < 0.01), older (median [IQR]: 74 [64, 80] vs. 72 [61, 81], p < 0.01) and had more risk factors (Table 1). There were no clinically relevant differences in pre‐stroke disability and stroke severity between the groups. For 2759 of 8546 (32.2%) of patients follow‐up data were available after 3 months (DAPT: 744/2254 [33%]; Mono: 2015/6292 [32%]). No significant differences between the groups were observed (Table S1).
FIGURE 1.
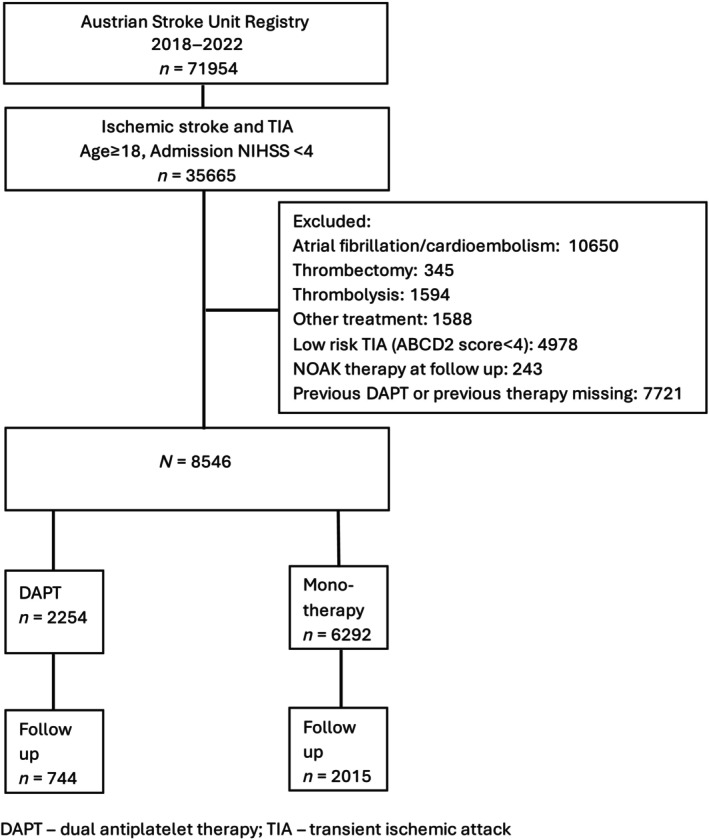
Flowchart of patient selection.
TABLE 1.
Characteristics of the study population, stratified by DAPT versus monotherapy.
| Monotherapy, n = 6292 | DAPT, n = 2254 | p‐Value | |
|---|---|---|---|
| High‐risk High‐risk TIA, n (%) | 2620 (41.6) | 921 (40.9) | 0.52 |
| Sex (female), n (%) | 2770 (44.0) | 800 (35.5) | <0.01 |
| Age, median, IQR | 72 (61, 81) | 74 (64, 80) | <0.01 |
| Hypertension, n (%) | 4953 (72.1) | 1916 (85.0) | <0.01 |
| Diabetes, n (%) | 1418 (22.5) | 648 (28.7) | <0.01 |
| Myocardial infarction, n (%) | 380 (6.1) | 223 (10.6) | <0.01 |
| Previous stroke, n (%) | 1175 (19.2) | 502 (23.6) | 0.01 |
| Hypercholersterinemia, n (%) | 4261 (70.7) | 1727 (78.9) | <0.01 |
| Smoking, n (%) | 1223 (21.8) | 546 (27.6) | <0.01 |
| Location (anterior), n (%) | 4324 (75.9) | 1991 (88.5) | <0.01 |
| Etiology | |||
| LAA, n (%) | 890 (14.1) | 483 (21.4) | <0.01 |
| SVD, n (%) | 2614 (41.5) | 931 (41.3) | |
| SUC, n (%) | 2473 (39.3) | 762 (33.8) | |
| Pre‐stroke mRS, median (IQR) | 0 (0, 0) | 0 (0, 0) | <0.01 |
| Admission NIHSS, median (IQR) | 1 (0, 2) | 1 (0, 2) | <0.01 |
Abbreviations: DAPT, dual antiplatelet therapy; IQR, interquartile range; LAA, large artery arteriosclerosis; mRS, modified Rankin Score; NIHSS, National Institute of Health Stroke Scale; SICH, symptomatic intracerebral hemorrhage; SUC, stroke of undetermined causes; SVD, small vessel disease; TIA, transient ischemic attack.
In the univariate analysis, DAPT was associated with higher rates of excellent functional outcome compared to monotherapy (612/744 [82.7%] vs. 1529/2015 [75.1%], p < 0.01) (Figure 2). END was less frequent in the DAPT group as compared to monotherapy (38/2050 [1.9%] vs. 149/4875 [3.1%], p < 0.01). Additionally, the DAPT group showed lower mortality rates (10/746 [1.3%] vs. 77/2022 [3.8%], p < 0.01) at 3 months. After multivariable adjustment, DAPT was significantly associated with excellent functional outcome at 3 months (aOR 1.59, 95% CI 1.20–2.09), lower rates of END (aOR 0.5, 95% CI 0.35–0.72) and all‐cause mortality (aOR 0.22, 95% CI 0.11–0.44). For safety endpoints (SICH and major extracranial bleeding), no significant differences were observed (SICH: 2/715 [0.3%] vs. 8/1927 [0.4%], p = 0.85; extracranial bleeding 2/2254 [0.1%] vs. 5/6292 [0.1%], p = 0.83). After adjustment, DAPT was not associated with an increased risk of SICH (aOR 1.19; 95% CI 0.30–4.73) or extracranial bleeding (aOR 0.84; 95% CI 0.16–4.56) (Table 2).
FIGURE 2.
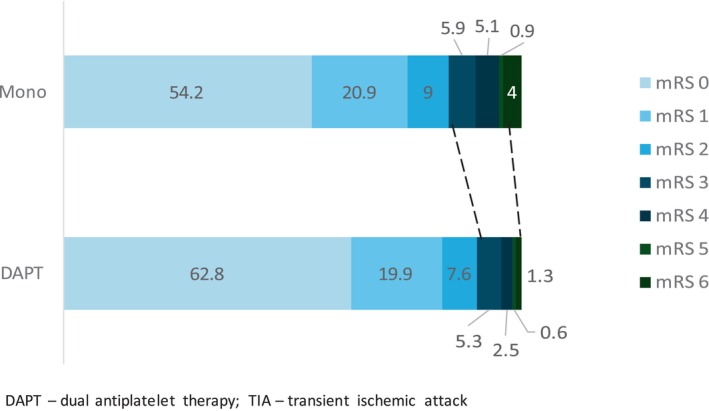
Functional outcome in non‐cardioembolic minor stroke or high‐risk TIA by DAPT versus monotherapy.
TABLE 2.
Efficacy and safety of DAPT versus monotherapy in non‐cardioembolic minor stroke or high‐risk TIA.
| Monotherapy, n = 6292 | DAPT, n = 2254 | p‐Value | aOR | 95% CI | |
|---|---|---|---|---|---|
| Early neurological deterioration, n (%) | 149 (3.4) | 38 (1.9) | <0.01 | 0.5 | 0.35–0.72 |
| mRS 0–1, n (%) |
1529 (75.9) (n = 2015) |
612 (82.3) (n = 744) |
<0.01 | 1.59 | 1.20–2.09 |
| SICH, n (%) | 8 (0.4) | 2 (0.3) | 0.84 | 1.19 | 0.30–4.73 |
| Extracranial major bleedings, n (%) | 5 (0.1) | 2 (0.1) | 0.83 | 0.84 | 0.16–4.56 |
| Mortality at 3 months, n (%) |
77 (3.8) (n = 2015) |
10 (1.3) (n = 744) |
<0.01 | 0.22 | 0.11–0.44 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; DAPT, dual antiplatelet therapy; FU, follow‐up; mRS, modified Rankin Score; SICH, symptomatic intracerebral hemorrhage.
Safety and efficacy of DAPT in patients with large artery disease
Overall, 1373/8546 (16.1%) of patients with strokes due to large vessel disease were included. The rate of patients receiving dual antiplatelet therapy (DAPT) was higher compared to other etiology groups (LAA: 35.2% vs. SVD: 27.2% vs. SUC: 23.9%). Patients with large vessel disease had more risk factors compared to other groups (Table S2). Moreover, myocardial infarction and hyperlipidemia were significantly more frequent in the DAPT group. There were no clinically relevant differences in pre‐stroke disability and stroke severity between the groups (Table S2). With DAPT significant higher rates of excellent functional outcome were observed (132/162 [81.3%] vs. 218/310 [70.3%]; p = 0.01). These results remained stable after adjustment (aOR 0.54, 95% CI 1.06–1.69) (Table 3). Additionally, no significant difference was found in the safety endpoints (SICH: 1/162 [0.6%] vs. 2/310 [0.6%], p = 0.86; aOR 0.86, 95% CI 0.06–12.98) and extracranial bleedings: (1/483 [0.2%] vs. 1/890 [0.1%], p = 0.68; aOR 0.51 95% CI 0.02–13.04). In the univariate analysis, DAPT was associated with lower mortality after 90 days (4/162 [2.5%] vs. 19/310 [6.1%], p < 0.01), respectively. However, these results did not remain stable after adjusting for imbalances (aOR 0.24, 95% CI 0.05–1.13).
TABLE 3.
Efficacy and safety outcomes of DAPT versus monotherapy by etiology.
| Monotherapy | DAPT | p‐Value | aOR | 95% CI | |
|---|---|---|---|---|---|
| Large artery atherosclerosis | |||||
| END, n (%) | 30 (4.5) | 15 (3.2) | 0.30 | 0.54 | 0.24–1.20 |
| mRS 0–1, n (%) | 218 (70.3) | 132 (81.3) | 0.01 | 0.54 | 0.31–0.94 |
| SICH, n (%) | 2 (0.7) | 1 (0.6) | 0.86 | 0.86 | 0.06–12.98 |
| Extracranial major bleeding, n (%) | 1 (0.1) | 1 (0.2) | 0.68 | 0.51 | 0.02–13.04 |
| Mortality at 3 months, n (%) | 19 (6.4) | 4 (2.3) | <0.01 | 0.20 | 0.04–0.90 |
| Small vessel disease | |||||
| END, n (%) | 63 (3) | 11 (1.3) | 0.08 | 0.48 | 0.25–0.94 |
| mRS 0–1, n (%) | 600 (74.6) | 248 (81.8) | 0.01 | 1.56 | 1.01–2.42 |
| SICH, n (%) | 3 (0.4) | 1 (0.3) | 0.80 | 0.50 | 0.03–7.57 |
| Extracranial major bleeding, n (%) | 2 (0.1) | 0 (0%) | 0.39 | — | — |
| Mortality at 3 months, n (%) | 23 (2.9) | 2 (0.7) | 0.01 | 0.09 | 0.01–0.70 |
| Stroke of undetermined cause | |||||
| END, n (%) | 11 (1.6) | 42 (2.3) | 0.29 | 0.73 | 0.37–1.45 |
| mRS 0–1, n (%) | 659 (79.3) | 220 (83.3) | 0.12 | 1.39 | 0.86–2.23 |
| SICH, n (%) | 1 (0.1) | 1 (0.4) | 0.41 | 1.76 | 0.08–40.83 |
| Extracranial major bleeding, n (%) | 2 (0.1) | 1 (0.1) | 0.70 | 0.54 | 0.04–7.04 |
| Mortality at 3 months, n (%) | 28 (3.4) | 4 (1.5) | 0.10 | 0.43 | 0.14–1.34 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; DAPT, dual antiplatelet therapy; END, early neurological deterioration; LAA, large artery atherosclerosis; mRS, modified Rankin Score; SICH, symptomatic intracerebral hemorrhage; SUC, stroke of undetermined cause; SVD, small vessel disease.
Safety and efficacy of DAPT in patients with small vessel disease
3545 (41.5%) patients with small vessel diseases were included in the analysis. Age was similarly distributed in both groups, but other risk factors were significantly more common in the DAPT group (Table S3). Rates of excellent functional outcome at 3 months were significantly higher in the DAPT group versus monotherapy (mRS 0–1: 248/303 [81.8%] vs. 600/804 [74.6%], p = 0.009; aOR 1.56, 95% CI 1.01–2.42) (Table 3). Additionally, significantly fewer ENDs were observed in the DAPT group compared to monotherapy (11/867 [1.3%] vs. 63/2114 [3%], p = 0.080; aOR 0.48, 95% CI 0.25–0.94). The occurrence of bleedings (SICH: 1/297 [0.3%] vs. 3/777 [0.4%], p = 0.8; aOR 0.50 95% CI 0.03–7.57, extracranial bleeding: 0/931 vs. 2/2612 [0.1%], p = 0.39) did not differ between the two groups. 3‐month‐mortality was significantly lower in the DAPT group (2/303 [0.7%] vs. 23/806 [2.9%], p = 0.01; OR 0.09, 95% CI 0.01–0.70).
Safety and efficacy of DAPT in stroke with undetermined source
Within the study population 3235/8546 (37.9%) patients had stroke with undetermined source (SUC). Compared to the patients with large and small vessel disease, patients in this group were significantly younger with fewer risk factors. Nonetheless, the DAPT group again showed a higher rate of vascular risk factors, similar to the other two etiologies (Table S4). Pre‐stroke disability and stroke severity were equally balanced between DAPT and monotherapy. Excellent functional outcome did not differ significantly between the groups (mRS 0–1: DAPT: 220/762 [83.3%] vs. monotherapy: 659/2473 [79.3%], p = 0.381; aOR 1.39, 95% CI 0.86–2.23) (Table 3). Although there was a trend favoring DAPT for reducing END (11/675 [1.6%] vs. 42/1843 [2.3%], p = 0.3) and mortality (4/264 [1.5%] vs. 28/830 [3.4%], p = 0.1), the difference was not statistically significant and remained non‐significant after multivariable adjustment (END: aOR 0.73, 95% CI 0.37–1.45; mortality: aOR 0.43; 95% CI 0.14–1.34). The bleeding endpoints were equivalent in both groups (SICH: 1/249 [0.4%] vs. 1/794 [0.1%], p = 0.410; OR 1.76, 95% CI 0.08–40.83) and extracranial bleeding: 1/762 (0.1%) vs. 2/2473 (0.1%); p = 0.70, aOR 0.54; 95% CI 0.04–7.04.
DISCUSSION
The main finding of this study is the significant improvement in excellent functional outcomes in the DAPT group as compared to the monotherapy group. Similarly, a sub‐analysis of the CHANCE trial [5] observed a rate of 90.1% with excellent functional outcomes in the DAPT group as compared to 88.4% in the monotherapy group after 3 months [5]. In our analysis, the rate of excellent functional outcome was generally lower than in the CHANCE study (82.7% vs. 75.1%). However, differences became even more evident after adjustment for vascular risk factors, which were more prevalent in the DAPT group. Additionally, we observed a higher mortality rate in both groups (monotherapy vs. DAPT: 4.0% vs. 1.3%) compared to the RCTs (CHANCE: 0.4% vs. 0.4%; POINT: 0.7 vs. 0.5; THALES: 0.7% vs. 0.5% [2–4]). Still, a positive effect on mortality could be demonstrated after adjusting, contrary to the RCTs [2, 3, 4]. These results indicate that DAPT is associated with better functional outcomes and lower mortality in real‐life setting. In contrast to our cohort, the RCT populations differed by younger age, lower prevalence of vascular risk factors and a different sex distribution [2, 3, 4].
The positive effect of DAPT on functional outcomes and mortality in our study population could be, at least partially, explained by the reduction of disabling recurrent strokes [5]. Another factor might be the observed reduction in END. This effect was also present in the ATAMIS RCT [9], where a combination of ASA and clopidogrel led to a 1.9% reduction of END in the first 7 days. The difference in END between the groups was comparable to our analysis (1.5%). However, the END incidence in general was lower in our study (3.4% vs. 1.9%; ATAMIS: 6.7% vs. 4.8%) [9]. This might be due to the fact that END was measured earlier (median 2 days after admission) and patients with higher stroke severity (median NIHSS 5) were included in ATAMIS trial [9]. Previous observational study showed that one point increase in admission NIHSS was associated with a 7% higher END risk [10].
Additionally, our findings suggest that the positive effects of DAPT on functional outcome were possibly driven by subjects with small vessel disease and LAA. A previous MRI based study suggested that early DAPT was associated with better functional outcome after END in lacunar strokes, but there were no data on functional outcome in LAA patients available [11]. However, large studies like INSPIRES [12] have demonstrated a significant benefit of DAPT in reducing recurrent strokes without an increase in bleeding rates among LAA patients.
Compared to patients with LAA or small vessel disease, patients with SUC likely have a different underlying pathophysiology, which is probably the explanation why the effect of DAPT on the reduction of END was neutral. This may also be due to the overall low event rate in this subgroup thus requiring a larger sample size to detect a potential effect.
For safety endpoints, we observed no significant differences between the groups. These results are comparable to those from the POINT [3] and READAPT [13] studies. Only the THALES [4] study observed a higher occurrence of SICH after the administration of DAPT with ticagrelor. In our analysis, similar to the READAPT [13] study, ASA and clopidogrel were mostly used as the standard combination. Ticagrelor had been used only in very few individual cases [13]. Unfortunately, detailed data on the DAPT regimens in ASUR are not available. An unpublished survey among Austrian stroke physicians of the participating centers indicated that typically, a loading dose of 300 mg ASA and 300 mg Clopidogrel are administered and DAPT is usually maintained for 21 days, according to the recommendation from national and European guidelines [14].
A strength of our analysis is that only the short‐term DAPT regimen has been examined. To avoid heterogeneity within the study population, patients with an indication for long‐term DAPT, carotid stent implantation, carotid endarterectomy, or large vessel occlusion (LVO) were not included. Several further limitations have to be acknowledged. Since this study is based on registry data, a selection bias has to be taken into consideration. However, doubly robust statistical adjustment has been conducted in addition to the descriptive analysis to account for possible confounders and the adjusted analysis confirmed the descriptive results in the major endpoints. It must also be noted that the decision whether to use DAPT or monotherapy was made by the respective stroke physicians on an individual basis. Therefore, differences between hospitals and potential differences during the observation period appear likely. To avoid a potential bias here, a comparably short study period was chosen, and the analyses were adjusted for center. Further, the rate of lost‐to‐follow‐up for two endpoints (mortality and mRS) has to be taken into account. However, it was found that there were no significant differences in age, sex, risk factors, disability, or stroke severity between the follow‐up group and the lost‐to‐follow‐up group. Finally, the retrospective non‐randomized nature of the study should be considered as a potential bias.
The major strength of this study is the high number of observations rigorously collected in a prospective multicentric cohort in a real‐world setting.
CONCLUSIONS
DAPT in non‐cardioembolic minor stroke or high‐risk transient ischemic attack (TIA) in a real‐life setting appears to be safe and associated with higher rates of favorable functional outcome as compared to monotherapy.
AUTHOR CONTRIBUTIONS
Stefan Krebs: Conceptualization; writing – original draft; methodology; investigation; visualization. Dominika Miksova: Formal analysis; methodology; validation. Michael Knoflach: Writing – review and editing. Thomas Gattringer: Writing – review and editing. Simon Fandler‐Höfler: Writing – review and editing. Fahrner Marlen: Data curation; investigation. Martha Marko: Writing – review and editing. Stefan Greisenegger: Writing – review and editing. Wilfried Lang: Writing – review and editing; supervision. Julia Ferrari: Supervision; writing – review and editing. Marek Sykora: Conceptualization; methodology; writing – review and editing; supervision; project administration.
FUNDING INFORMATION
Österreichische Schlaganfall‐Gesellschaft.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest associated with the publication of this article.
Supporting information
Data S1.
ACKNOWLEDGEMENTS
Non‐author contributions: Austrian Stroke Unit Registry Collaborators (Appendix).Conflict of Interest Statement The authors declare that there are no conflicts of interest associated with the publication of this article.
Krebs S, Miksova D, Knoflach M, et al. Dual antiplatelet therapy after minor strokes or high‐risk TIA: Evidence from the Austrian stroke registry. Eur J Neurol. 2025;32:e70012. doi: 10.1111/ene.70012
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. GBD 2019 Stroke Collaborators . Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20(10):795‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11‐19. [DOI] [PubMed] [Google Scholar]
- 3. Johnston SC, Easton JD, Farrant M, et al. Clinical research collaboration, neurological emergencies treatment trials network, and the POINT Investigators. Clopidogrel and aspirin in acute ischemic stroke and high‐risk TIA. N Engl J Med. 2018;379:215‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnston SC, Amarenco P, Denison H, et al. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med. 2020;383:207‐217. [DOI] [PubMed] [Google Scholar]
- 5. Wang X, Zhao X, Johnston SC, et al. Effect of clopidogrel with aspirin on functional outcome in TIA or minor stroke: CHANCE substudy. Neurology. 2015;85(7):573‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brainin M, Steiner M. Austrian stroke registry for acute stroke units. Acute stroke units in Austria are being set up on a national level following evidence‐based recommendations and structural quality criteria. Cerebrovasc Dis. 2003;15(Suppl 1):29‐32. [DOI] [PubMed] [Google Scholar]
- 7. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317‐1329. [DOI] [PubMed] [Google Scholar]
- 8. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453‐1457. [DOI] [PubMed] [Google Scholar]
- 9. Chen HS, Cui Y, Wang XH, et al. Nguyen TN; ATAMIS investigators. Clopidogrel plus aspirin vs aspirin alone in patients with acute mild to moderate stroke: the ATAMIS randomized clinical trial. JAMA Neurol. 2024;81(5):450‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siegler JE, Boehme AK, Kumar AD, et al. Identification of modifiable and nonmodifiable risk factors for neurologic deterioration after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22(7):e207‐e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berberich A, Schneider C, Herweh C, et al. Risk factors associated with progressive lacunar strokes and benefit from dual antiplatelet therapy. Eur J Neurol. 2020;27(5):817‐824. [DOI] [PubMed] [Google Scholar]
- 12. Gao Y, Chen W, Pan Y, et al. INSPIRES Investigators. Dual antiplatelet treatment up to 72 hours after ischemic stroke. N Engl J Med. 2023;389(26):2413‐2424. [DOI] [PubMed] [Google Scholar]
- 13. De Matteis E, De Santis F, Ornello R, et al. Divergence between clinical trial evidence and actual practice in use of dual antiplatelet therapy after transient ischemic attack and minor stroke. Stroke. 2023;54(5):1172‐1181. [DOI] [PubMed] [Google Scholar]
- 14. Dawson J, Merwick Á, Webb A, et al. European stroke organisation expedited recommendation for the use of short‐term dual antiplatelet therapy early after minor stroke and high‐risk TIA. Eur Stroke J. 2021;6(2):CLXXXVII‐XCI. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


