Abstract
1. The glutathione S-transferase that catalyses the reaction of 1-menaphthyl (naphth-1-ylmethyl) sulphate with GSH was purified 76-fold from rat liver. 2. The properties of the purified enzyme were studied by gel filtration and isoelectric focusing. 3. The initial-velocity pattern in the absence of products and the product-inhibition pattern have been determined. These are consistent with an Ordered Bi Bi mechanism in which the GSH adds to the enzyme before 1-menaphthyl sulphate and the products are released in the order SO42− followed by S-(1-menaphthyl)glutathione. 4. Dead-end-inhibition studies with p-aminobenzoic acid, which has been shown to be competitive with GSH and non-competitive with 1-menaphthyl sulphate, support the suggestion that an Ordered Bi Bi mechanism is operative. 5. Values were determined for some of the dissociation and Michaelis constants for the reaction of the substrates and products with the enzyme. 6. It appears that S-(1-menaphthyl)glutathione activates the enzyme when the concentration of GSH is saturating and that of 1-menaphthyl sulphate is low (of the order of its Michaelis constant).
Full text
PDF

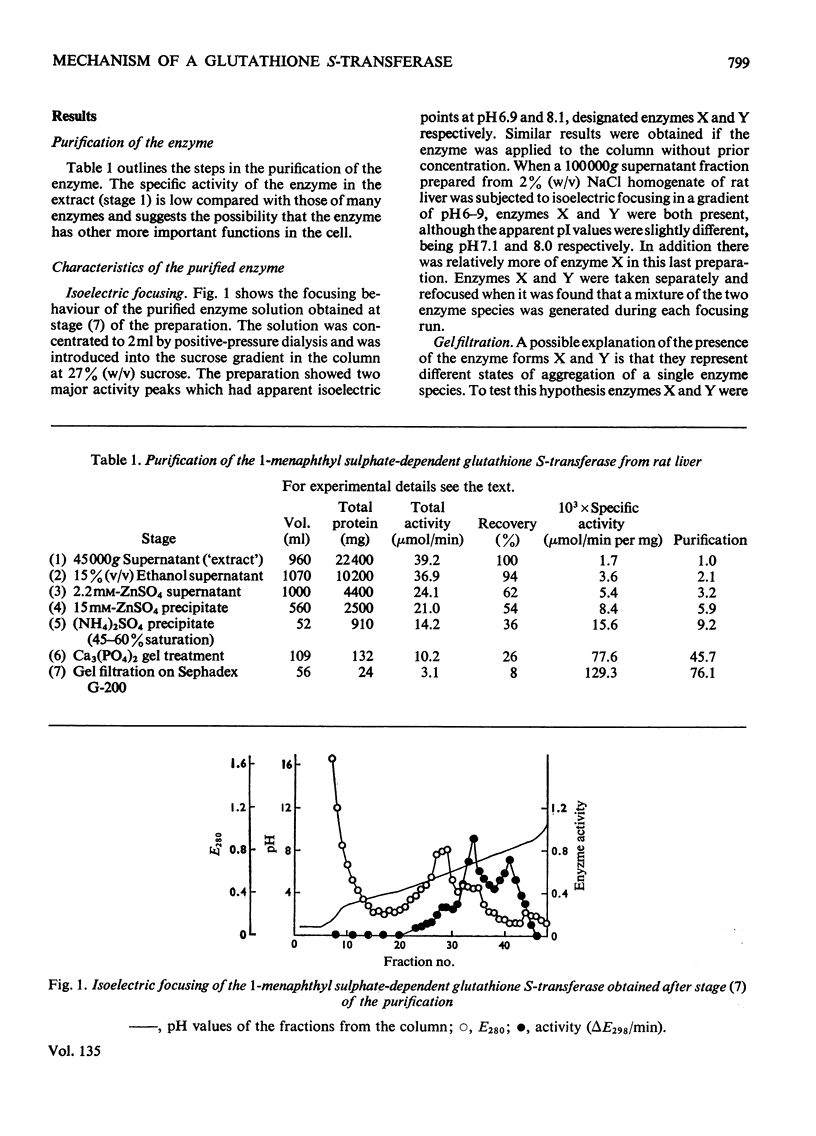
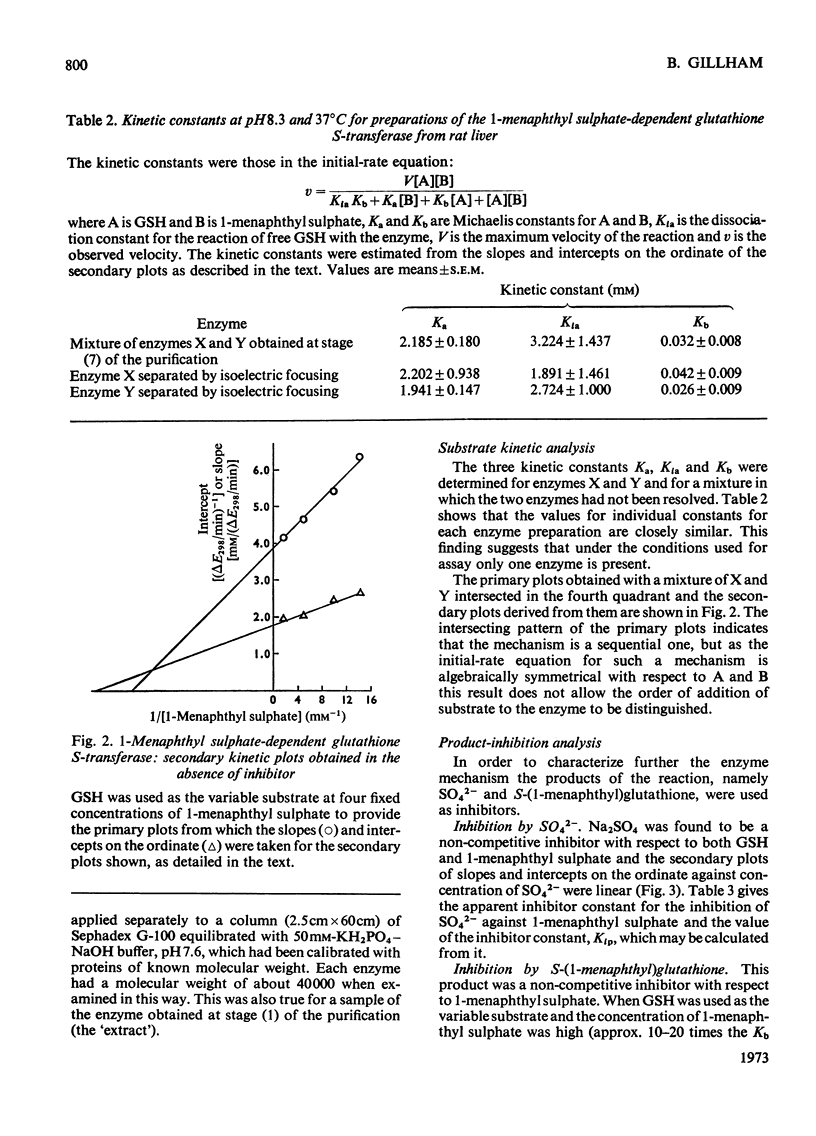
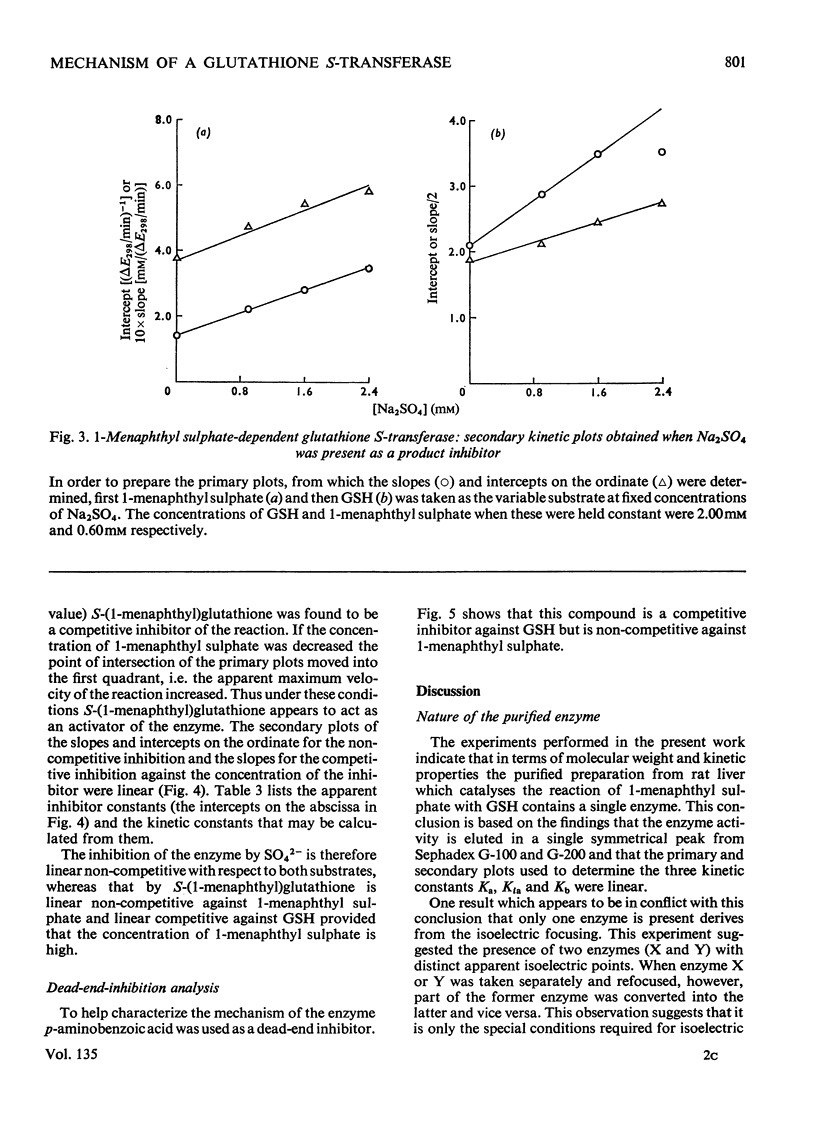

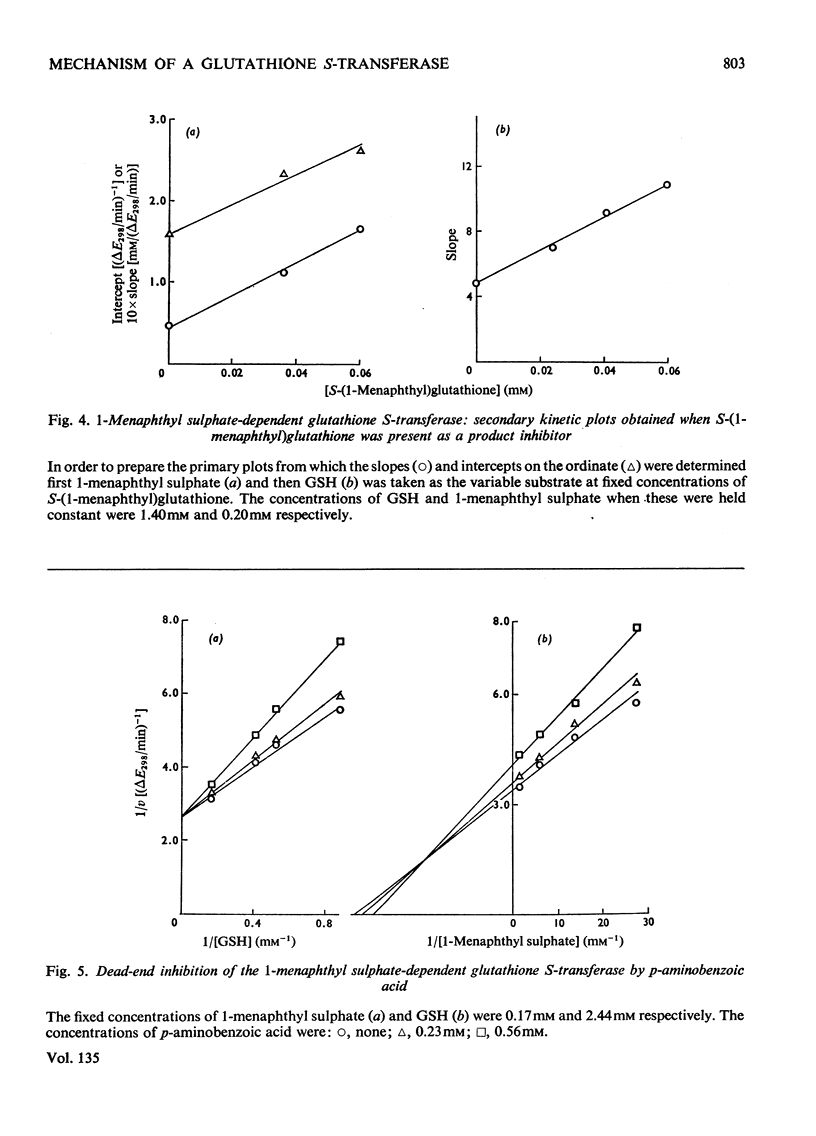

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyland E., Chasseaud L. F. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:173–219. doi: 10.1002/9780470122778.ch5. [DOI] [PubMed] [Google Scholar]
- CLELAND K. W., SLATER E. C. Respiratory granules of heart muscle. Biochem J. 1953 Mar;53(4):547–556. doi: 10.1042/bj0530547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Clapp J. J., Young L. Formation of mercapturic acids in rats after the administration of aralkyl esters. Biochem J. 1970 Aug;118(5):765–771. doi: 10.1042/bj1180765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjellstedt T. A., Allen R. H., Duncan B. K., Jakoby W. B. Enzymatic conjugation of epoxides with glutathione. J Biol Chem. 1973 May 25;248(10):3702–3707. [PubMed] [Google Scholar]
- Gillham B. The reaction of aralkyl sulphate esters with glutathione catalysed by rat liver preparations. Biochem J. 1971 Feb;121(4):667–672. doi: 10.1042/bj1210667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde C. W., Young L. Biochemical studies of toxic agents. The metabolic formation of 1- and 2-menaphthylmercapturic acid. Biochem J. 1968 Apr;107(4):519–522. doi: 10.1042/bj1070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


