Abstract
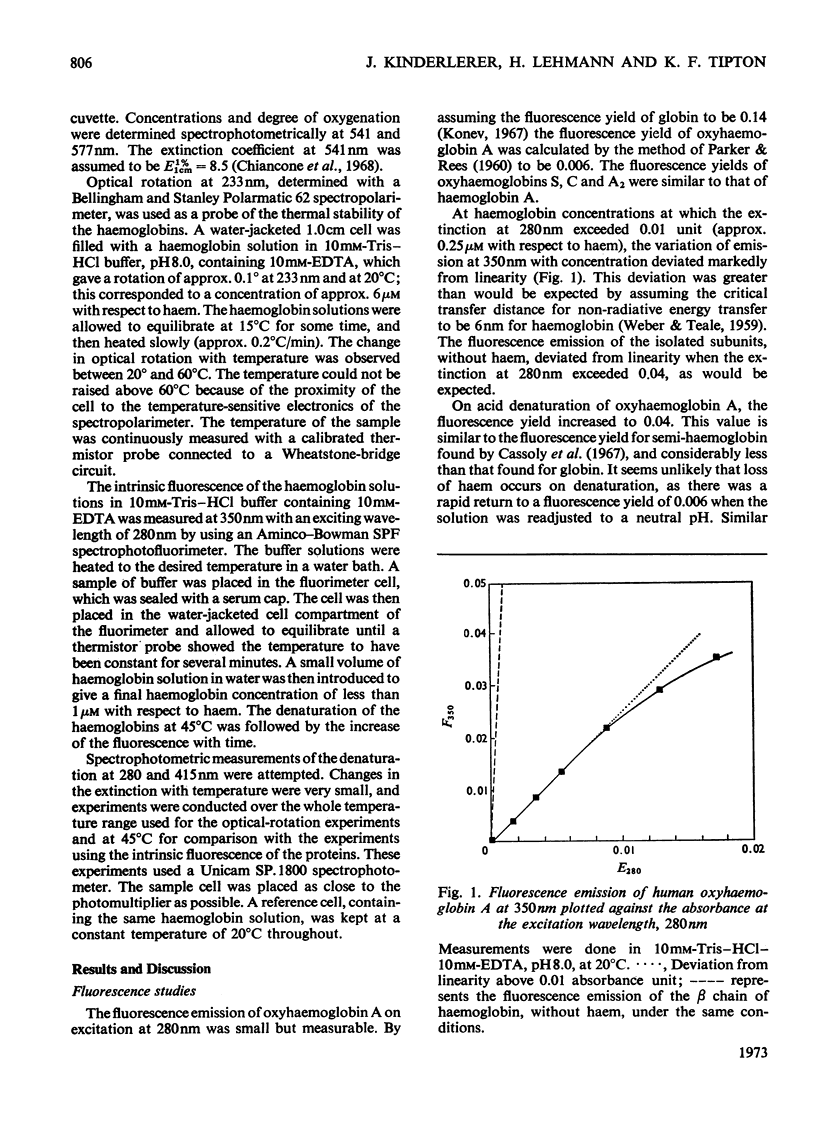
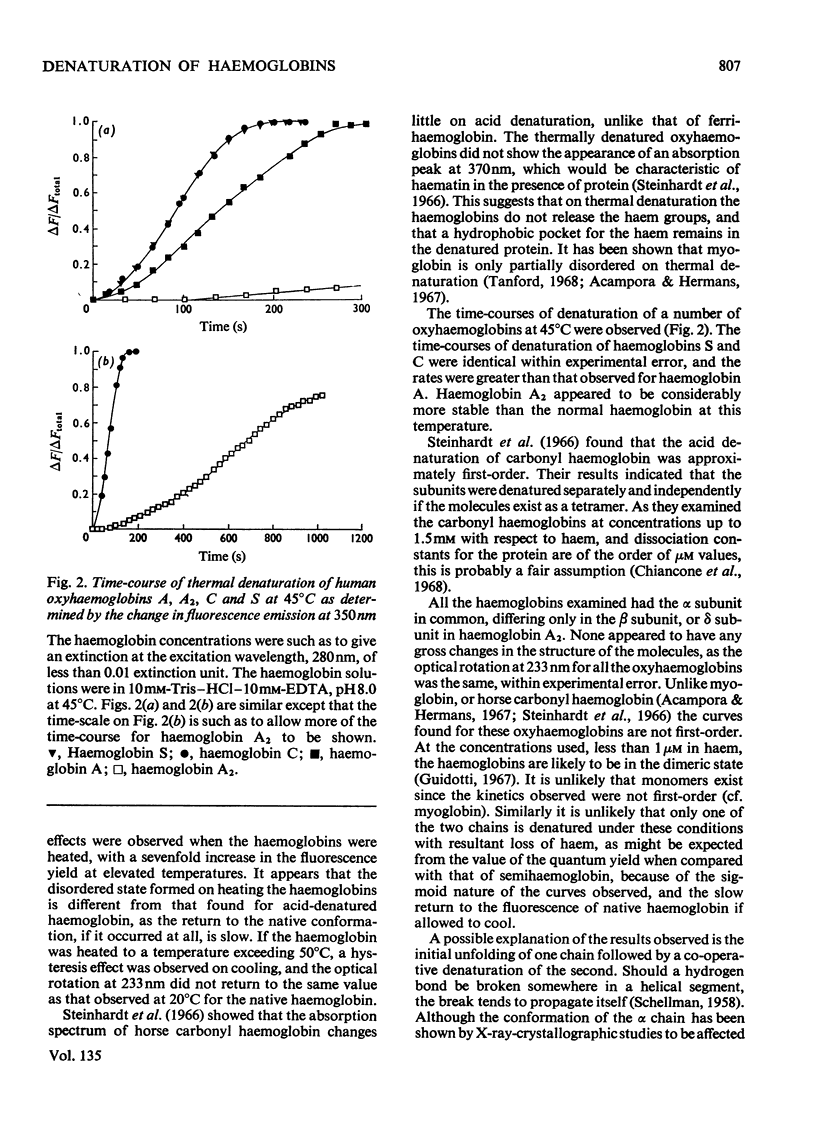
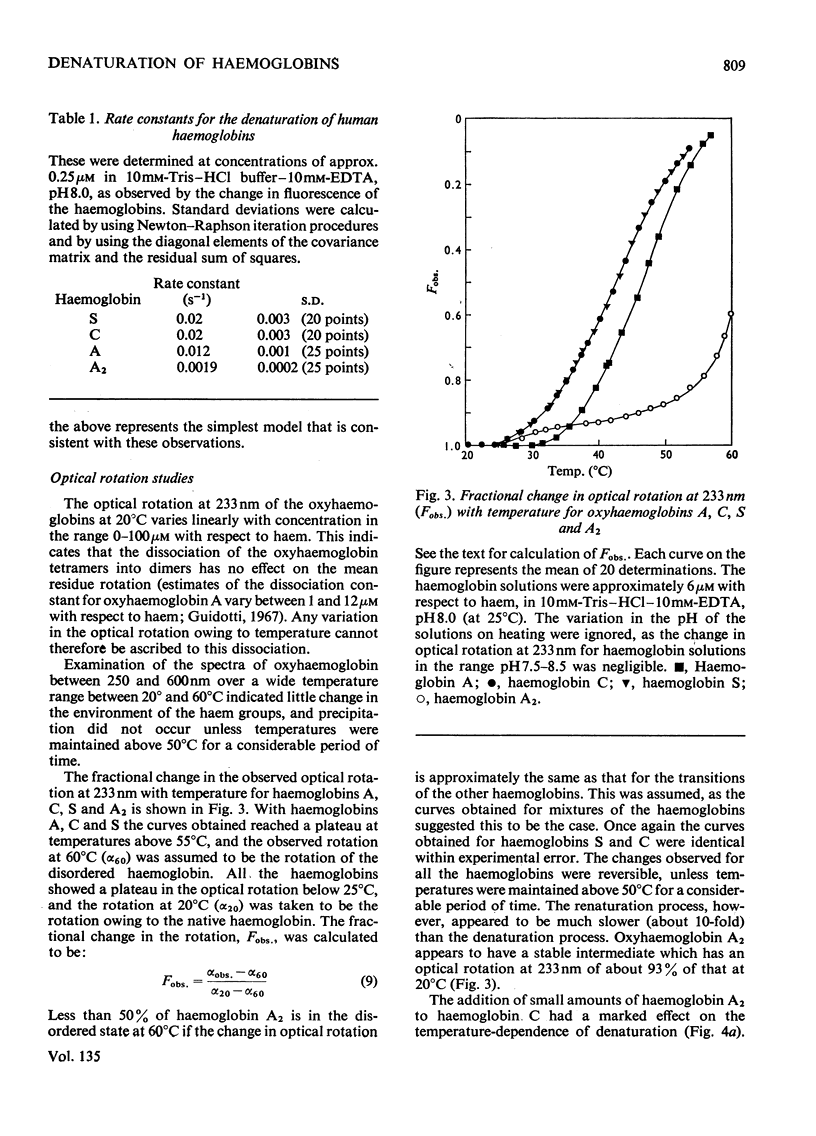
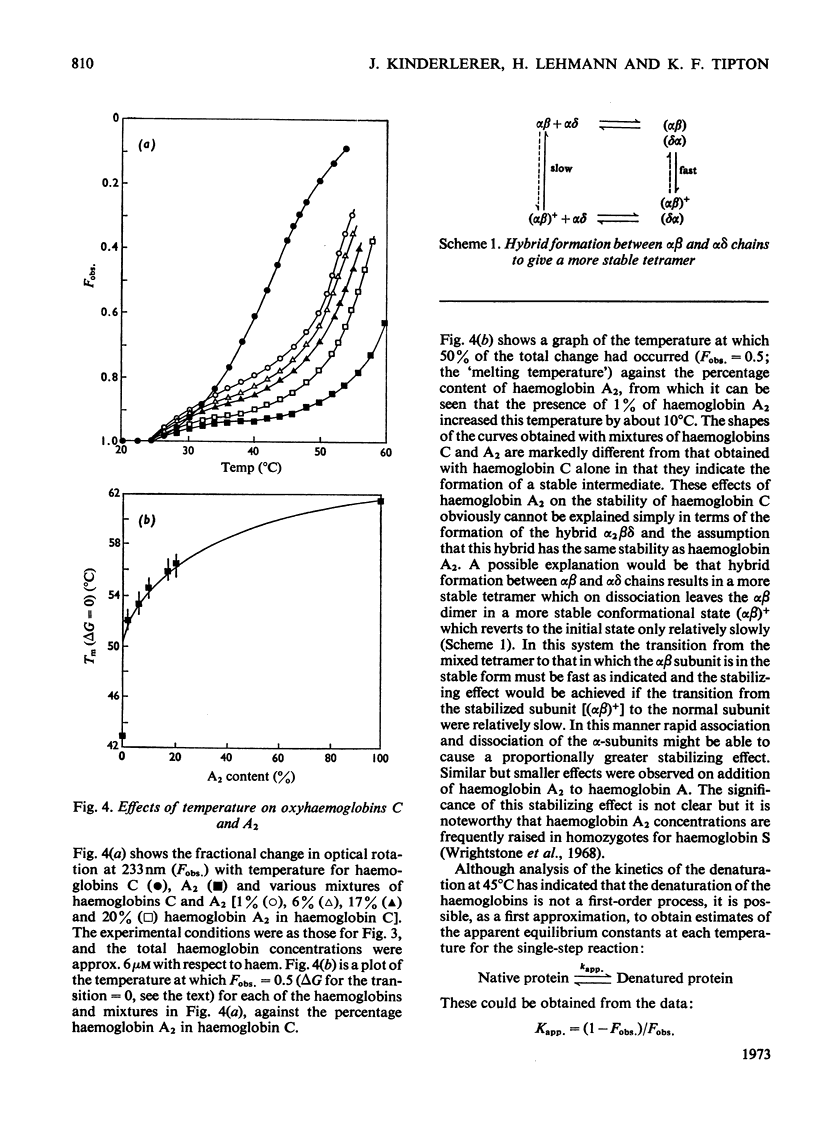
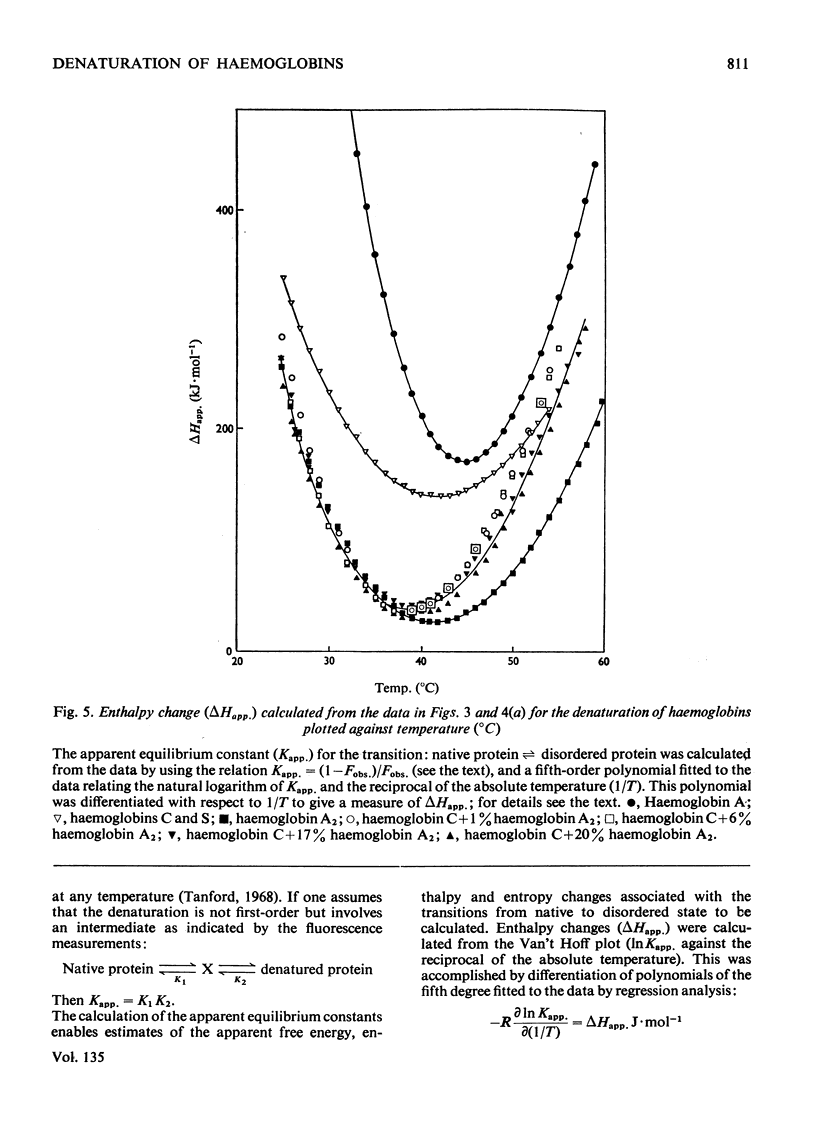
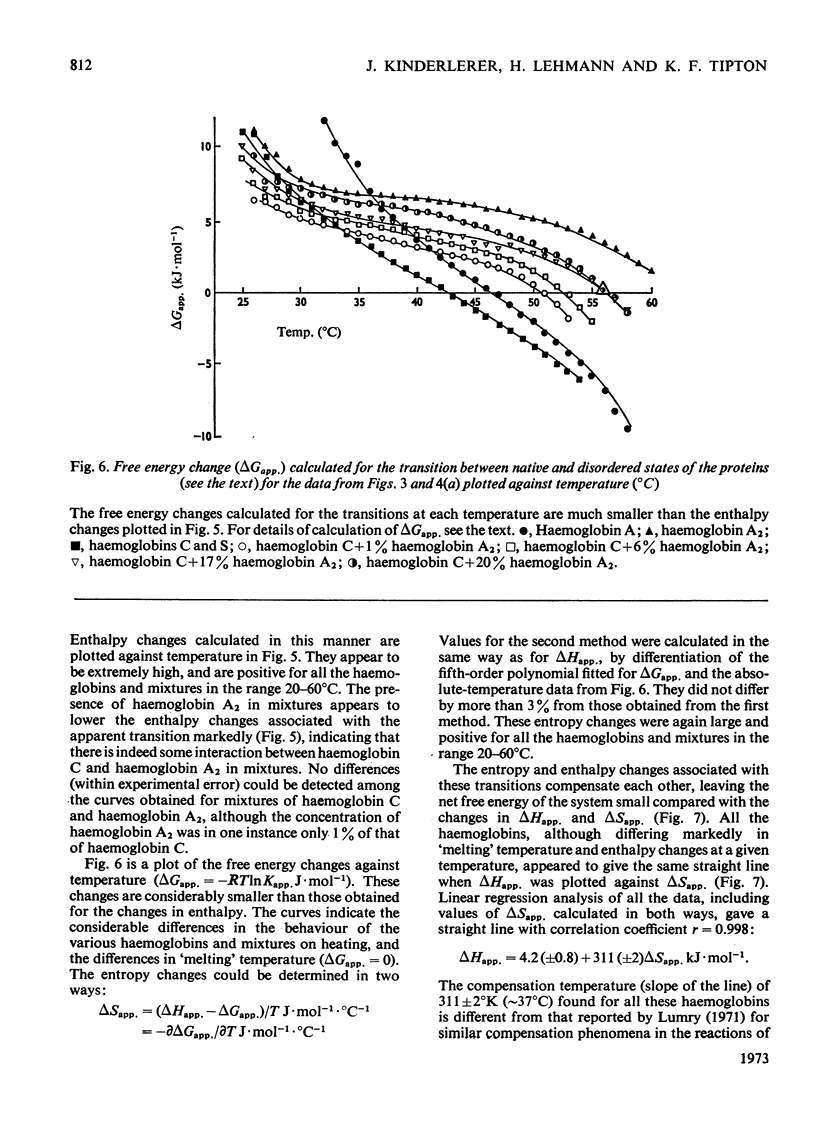
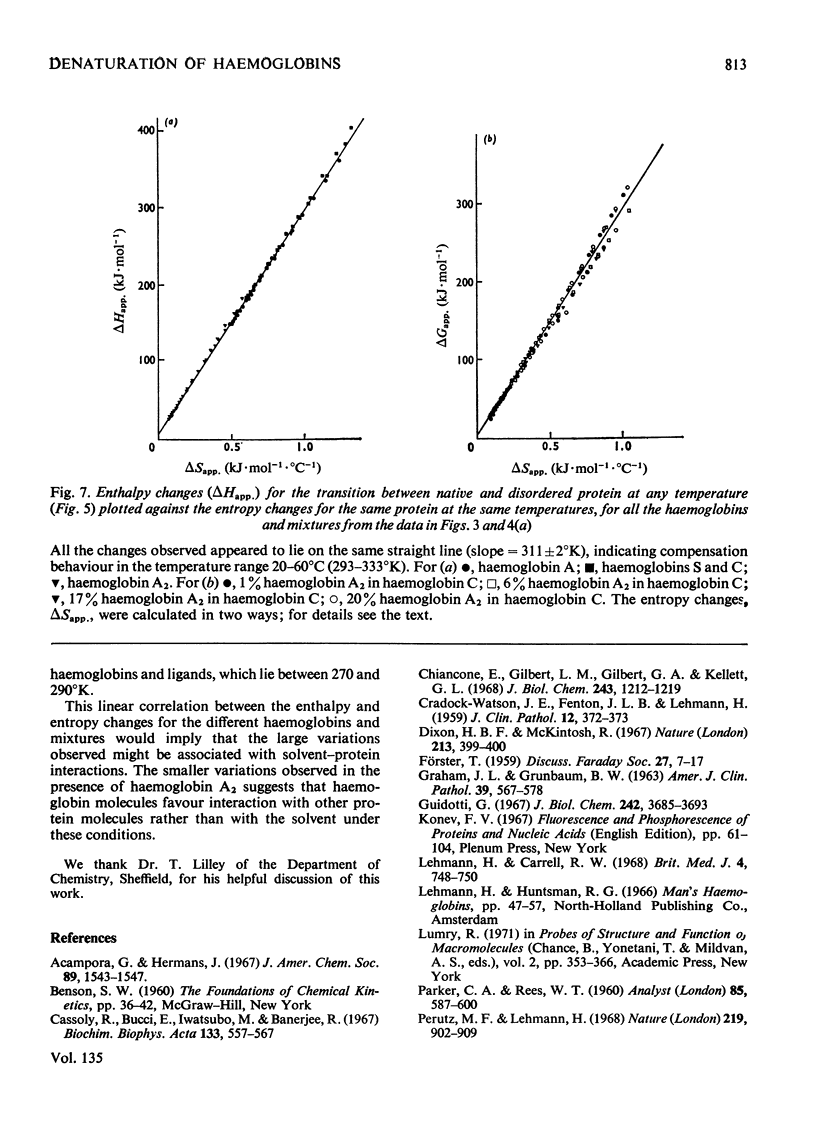
1. The time-courses of thermal denaturation of human oxyhaemoglobins A, A2, C and S at 45°C were studied by following the increase in protein fluorescence. Haemoglobins S and C were less stable than haemoglobin A, whereas haemoglobin A2 was considerably more stable. 2. The time-courses of denaturation did not follow first-order kinetics and could be fitted most simply to a co-operative scheme in which the partial denaturation of the α chain preceded that of the β chain. 3. The denaturation of these haemoglobins was studied as a function of temperature by using optical rotatory dispersion. Haemoglobin A2 was again more stable than the others. The addition of small quantities of haemoglobin A2 had a disproportionate effect on the stability of haemoglobin C. 4. The thermodynamic parameters of the denaturation process were calculated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acampora G., Hermans J., Jr Reversible denaturation of sperm whale myoglobin. I. Dependence on temperature, pH, and composition. J Am Chem Soc. 1967 Mar 29;89(7):1543–1547. doi: 10.1021/ja00983a001. [DOI] [PubMed] [Google Scholar]
- CRADOCK-WATSON J. E., FENTON J. C., LEHMANN H. Tris buffer for the demonstration of haemoglobin A2 by paper electrophoresis. J Clin Pathol. 1959 Jul;12:372–373. doi: 10.1136/jcp.12.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassoly R., Bucci E., Iwatsubo M., Banerjee R. Functional studies on human semi-hemoglobin. Biochim Biophys Acta. 1967 Apr 11;133(3):557–567. doi: 10.1016/0005-2795(67)90560-0. [DOI] [PubMed] [Google Scholar]
- Chiancone E. Dissociation of hemoglobin into subunits. II. Human oxyhemoglobin: gel filtration studies. J Biol Chem. 1968 Mar 25;243(6):1212–1219. [PubMed] [Google Scholar]
- Dixon H. B., McIntosh R. Reduction of methaemoglobin in haemoglobin samples using gel filtration for continuous removal of reaction products. Nature. 1967 Jan 28;213(5074):399–400. doi: 10.1038/213399a0. [DOI] [PubMed] [Google Scholar]
- GRAHAM J. L., GRUNBAUM B. W. A rapid method for microelectrophoresis and quantitation of hemoglobins on cellulose acetate. Am J Clin Pathol. 1963 Jun;39:567–578. doi: 10.1093/ajcp/39.6.567. [DOI] [PubMed] [Google Scholar]
- Guidotti G. Studies on the chemistry of hemoglobin. II. The effect of salts on the dissociation of hemoglobin into subunits. J Biol Chem. 1967 Aug 25;242(16):3685–3693. [PubMed] [Google Scholar]
- Lehmann H., Carrell R. W. Differences between alpha- and beta-chain mutants of human haemoglobin and between alpha- and beta-thalassaemia. Possible duplication of the alpha-chain gene. Br Med J. 1968 Dec 21;4(5633):748–750. doi: 10.1136/bmj.4.5633.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERUTZ M. F., MITCHISON J. M. State of haemoglobin in sickle-cell anaemia. Nature. 1950 Oct 21;166(4225):677–679. doi: 10.1038/166677a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968 Aug 31;219(5157):902–909. doi: 10.1038/219902a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Steinhardt J., Polet H., Moezie F. Acid denaturation of horse carbonylhemoglobin in the absence of oxygen. J Biol Chem. 1966 Sep 10;241(17):3988–3996. [PubMed] [Google Scholar]
- TEALE F. W. The ultraviolet fluorescence of proteins in neutral solution. Biochem J. 1960 Aug;76:381–388. doi: 10.1042/bj0760381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Wrightstone R. N., Huisman T. H., van der Sar A. Qualitative and quantitative studies of sickle cell hemoglobin in homozygotes and heterozygotes. Clin Chim Acta. 1968 Dec;22(4):593–601. doi: 10.1016/0009-8981(68)90108-3. [DOI] [PubMed] [Google Scholar]


