Abstract
Background
Growing evidence indicates that metal implants influence the body’s oxidative stress status, which in turn affects the degradation and stability of metal implants. The oxidative balance score (OBS) is a composite indicator, reflecting the overall oxidative balance of pro-and antioxidants of the human body. However, the associations between OBS and the level of metal ions on the population with metal implants remain to be elucidated.
Methods
We conducted a cross-sectional study using data from 2015 to 2020 National Health and Nutrition Examination Survey (NHANES). Dietary and lifestyle factors closely associated with oxidative stress were quantified to calculate the OBS. Weighted multivariate logistic regression and smooth curve fittings were performed to examine the relationship between OBS and serum cobalt levels. Subgroup analyses were stratified by age and gender. In cases where non-linearity was detected, threshold effects were assessed using a two-piecewise linear regression model.
Results
A total of 549 participants were included in this analysis. The dietary OBS was negatively associated with serum cobalt level in fully adjusted model (β = −0.179, 95%CI: −0.358 to −0.001, P: 0.04918). Stratified by age and gender, negative correlation of OBS and dietary OBS with serum cobalt level was observed only in men and age over 70 years participants. Threshold effect analysis showed linear relationships between OBS, dietary OBS and cobalt level in males. There were non-linear relationships between OBS, dietary OBS and cobalt level in age over 70 years participants, with inflection points identified at 16.3 and 8.7 for OBS and dietary OBS, respectively.
Conclusion
Our study confirms the inverse relationships between oxidative stress and serum cobalt level in individuals with metal implants, highlighting the significance of optimizing OBS to mitigate the risk of metal ion toxicity. These findings emphasize the importance of maintaining an antioxidant diet and lifestyle, particularly as they offer greater protective effect for males and the elderly population.
Keywords: metal implant, oxidative balance score, cobalt, NHANES, dietary
1. Introduction
Currently, the trend of global aging is becoming increasingly prominent due to persistently low birth rates and extended life expectancy. By 2050, it is projected that the population aged 65 and older will account for 20% of the global population (1), which suggests a significant rise in the incidence of degenerative diseases and related complications, such as osteoporosis, fractures, osteoarthritis (2). In patients with orthopedic diseases, the use of metal implants for fixation or replacement to relieve pain, correct deformities, and restore function has increased annually (2–6). It has been reported that over one million total hip and knee replacement are performed annually in the United States, with the cost exceeding $25 billion (7). However, the long-term survival rate of metal implants is not optimistic. Among younger patients undergoing total hip replacement, only 72% of the implants are able to last for 10 years (8). One of the challenges in the application of metal implants is the generation of metal debris and the release of metal ions. As is well known, these debris and ions can trigger localized adverse reactions, leading to the loosening and failure of the implants, and they may even enter the circulatory system, resulting in systemic damage (9). Numerous studies suggest that the accumulation of metal debris and ions can induce the formation of local pseudotumors (10, 11). Research conducted by Grammatopoulos et al. (12) found that out of 53 cases of metal-on-metal hip replacements, 16 required revision surgery due to the presence of pseudotumors. A prospective study carried out by British researchers revealed that, compared to preoperative values, patients who underwent metal-on-metal hip replacement experienced a significant increase in the incidence of chromosomal aneuploidy and translocations in peripheral blood, with rates rising 2-fold and 1.5-fold, respectively, within 2 years post-surgery (13). As our understanding of the adverse reactions caused by metal implants deepens, the prevention of these adverse effects has gradually become a focal point of research.
An increasing number of studies indicate that the integration process of metal implants with surrounding tissues may trigger a series of physiological and pathological changes (14). In the initial stage of implantation, the interaction between immune cells and the metal materials leads to the activation and secretion of various mediators, such as superoxide anions and hydroxyl radicals (15, 16). Additionally, metal particles generated due to fatigue, fretting, or corrosion can similarly stimulate local cells to produce excessive reactive oxygen species (ROS) (17–21). These intracellular and extracellular ROS may induce local inflammation and alter the chemical environment of the implants, thereby accelerating the degradation of metal implants and the release of metal ions. Metal micro-particles and ions not only cause localized harm but can also penetrate the bloodstream and lymphatic system, spreading throughout various tissues and organs, triggering systemic inflammatory responses and activating the immune system, resulting in tissue damage and functional impairment (22). Although existing research suggests that ROS are an important factor in the reduced stability of metal implants (5), there is currently a lack of reliable indicators to reflect the oxidative state after metal implantation and to elucidate the relationship between oxidative stress and the dissociation of metal ions.
The OBS is a comprehensive metric designed to assess the balance between oxidative stress and antioxidant capacity within the body (23–25). This indicator has been used to identify individuals at high risk for various chronic diseases, such as cardiovascular diseases, diabetes, and cancer, and to implement corresponding intervention measures. Additionally, it is considered an important monitoring indicator for evaluating treatment efficacy (24, 26–29). However, it remains unclear whether the oxidative stress status of patients with metal implants can be adequately assessed using the OBS, and whether this metric can effectively illustrate the relationship between oxidative stress and the dissociation of metal ions. Herein, we conducted a cross-sectional study to investigate the association between OBS and metal ion of the patients with metal implants using a large-scale, community population-based data from NHANES 2015–2020.
2. Materials and methods
2.1. Data source
The NHANES is a program in the United States designed to assess the health and nutritional status of adults and children. It involves a complex and comprehensive set of data methods, including physical examinations, laboratory tests, and questionnaires. The survey aims to monitor trends in various health indicators, such as diseases, nutritional deficiencies, and exposure to environmental contaminants. The National Center for Health Statistics Ethics Review Board has approved NHANES protocols, with all participants provided consenting to their data’s use in research (30).
2.2. Study population
In this study, we analyzed NHANES data from three consecutive 2-year cycles spanning 2015–2020. We included participants with metal objects inside body, and had complete data of OBS components, serum cobalt, and serum chromium. We excluded participants without metal objects (n = 32,360), with missing data for cobalt (n = 1,194), chromium (n = 2), calcium (n = 46), cotinine (n = 5), Body mass index (BMI) (n = 13), with indication of infection (WBC > 10 × 109/L; n = 86) and inflammation (CRP > 5 mg/L; n = 173). The participant screening processes is presented in Figure 1.
Figure 1.
Flowchart diagram depicting the selection strategy of study participants.
For each participant enrolled, data on OBS components, serum cobalt, and serum chromium, and covariates were extracted and analyzed.
2.3. Study variables
2.3.1. Independent variables
Based on prior research about the relationship of nutrients and lifestyle factors with oxidative stress, 16 nutrients and three lifestyle factors (alcohol consumption, smoking, and BMI) were collected to calculate OBS, including five pro-oxidants and 14 antioxidants (31, 32). Among the variables assessed, 16 nutrients and alcohol were derived from the mean of the various ingredients from the dietary interview on first day to determine the quantiles thresholds for scoring purposes. Smoking was estimated by serum cotinine, which was measured by an isotope dilution-high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry (ID HPLC-APCI MS/MS). The BMI (kg/m2) was collected from body measures and calculated as weight in kilograms divided by height in meters square. We stratified all components into three distinct groups, corresponding to the first, second, and third tertiles, respectively. Antioxidants were allocated fractional values ranging from 0 to 2, whereas the scoring for pro-oxidants were inversely distributed. Finally, the OBS scores for each participant were aggregated to yield the individual final OBS values. Supplementary Table S1 shows the distribution scheme of OBS components.
2.3.2. Dependent variables
Whole blood specimens were processed, stored, and shipped to the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, GA for analysis. The concentrations of cobalt (nmol/L) and chromium (nmol/L) in whole blood specimens were directly measured using inductively coupled plasma mass spectrometry (ICP-MS) (33).
2.3.3. Covariates
All covariates were identified based on findings from previous studies (22, 34). The demographic data, including age (year), gender (male/female), and ethnicity (Hispanic, non-Hispanic White, non-Hispanic Black, and Other Races), were collected. BMI was obtained from body measures. The criteria for identifying hypertension and diabetes were based on participants’ self-reported medical diagnoses validated by a physician. Smoking status was ascertained through Questionnaire Data. Participants who had smoked fewer than 100 lifetime cigarettes were categorized as never smokers. Those who had smoked at least 100 cigarettes, but were not smoking at the time of the survey were classified as former smokers. Conversely, participants who had exceeded the 100 cigarette threshold and were actively smoking at the time of the survey were identified as current smokers (35). The neutrophil count (1,000 cells/μL), neutrophil percentage (%), lymphocyte count (1,000 cells/μL), monocyte number (1,000 cells/μL), and platelet count (1,000 cells/μL), were quantified utilizing the Complete Blood Count with a Five-Part Differential methodology. Blood urea nitrogen (mg/dL), iron (ug/dL), phosphorus (mg/dL), total protein (g/dL), albumin (g/dL), and uric acid (mg/dL) were assessed using Beckman UniCel® DxC800 Synchron. HDL-cholesterol (mg/dL) and total cholesterol (mg/dL) were quantified using Roche/Hitachi Modular P Chemistry Analyzer. The albumin in urine (mg/dL) was measured using a solid-phase fluorescent immunoassay by sequoia-Turner Digital Fluorometer, Model 450, urine creatinine (mg/dL) was determined by Enzymatic using Roche Cobas 6,000 Analyzer. Urinary albumin-creatinine ratio (UACR) was calculated by dividing urinary albumin by creatinine (36). Monocyte/HDL cholesterol ratio (MHR) was calculated as monocyte number divided by HDL cholesterol (37). Systemic immune-inflammation indicator (SII) was calculated by multiplying the platelet count by the neutrophil count and dividing by the lymphocyte count (38). Neutrophil percentage to albumin ratio (NPAR) was calculated as neutrophil percentage divided by albumin (39). The NHANES website provides full information on the laboratory procedures, data processing, quality control, and analytic notes.
2.4. Statistical analysis
The statistical software packages R 3.4.31 and EmpowerStats 2.02 were used to conduct data analysis, with p < 0.05 was considered statistically significant. All estimates were calculated with consideration of the NHANES sample weights. Continuous variables were compared using a weighted linear regression model, while categorical variables were assessed with a weighted chi-square test. Weighted multivariable linear regression analyses were performed to investigate the relationship of OBS with serum cobalt and chromium. Further investigation was conducted through subgroup analyses, stratified by age and gender. The presence of non-linear relationships was examined using generalized additive models and smooth curve fittings. When non-linearity was detected, a two-piecewise linear regression model was employed to analyze the threshold effect.
3. Results
3.1. Baseline characteristics
In accordance with the inclusion and exclusion criteria, a total of 549 participants were deemed eligible for inclusion in the definitive analysis. These participants were divided into four groups based on OBS levels. As the levels of OBS escalate, there is a progressive decline in the levels of serum cobalt, BMI, urine creatinine, and uric acid, as well as the incidence rates of diabetes and hypertension (Table 1).
Table 1.
Baseline characteristics of study participants.
| OBS | |||||
|---|---|---|---|---|---|
| Characteristics | <10 | ≥10, <20 | ≥20, <30 | ≥30 | p-value |
| n | 66 | 203 | 225 | 55 | |
| Age (years) | 62.292 ± 12.112 | 64.521 ± 10.159 | 61.054 ± 12.267 | 61.236 ± 12.192 | 0.01531 |
| BMI (kg/m2) | 31.124 ± 5.723 | 29.693 ± 5.075 | 29.545 ± 6.778 | 25.773 ± 3.402 | <0.00001 |
| Chromium (nmol/L) | 9.512 ± 16.489 | 10.324 ± 9.739 | 8.210 ± 7.720 | 8.613 ± 7.542 | 0.13219 |
| Cobalt (nmol/L) | 10.269 ± 40.897 | 3.460 ± 7.514 | 4.370 ± 8.265 | 2.787 ± 1.290 | 0.01864 |
| Urine creatinine (mg/dL) | 122.523 ± 91.860 | 103.849 ± 62.542 | 98.079 ± 64.584 | 75.665 ± 48.977 | 0.00152 |
| Blood urea nitrogen (mg/dL) | 15.699 ± 7.220 | 16.286 ± 5.444 | 16.433 ± 4.857 | 16.791 ± 4.212 | 0.73797 |
| Iron (μg/dL) | 89.411 ± 26.580 | 85.607 ± 28.057 | 86.114 ± 33.064 | 86.230 ± 37.065 | 0.91010 |
| Phosphorus (mg/dL) | 3.673 ± 0.514 | 3.666 ± 0.494 | 3.709 ± 0.564 | 3.728 ± 0.514 | 0.77810 |
| Total Protein (g/dL) | 7.049 ± 0.413 | 6.971 ± 0.407 | 6.910 ± 0.361 | 7.055 ± 0.328 | 0.01160 |
| Uric acid (mg/dL) | 5.375 ± 1.360 | 5.591 ± 1.361 | 5.336 ± 1.362 | 4.750 ± 1.038 | 0.00016 |
| Monocyte number (1,000 cells/μL) | 0.626 ± 0.187 | 0.571 ± 0.163 | 0.574 ± 0.178 | 0.596 ± 0.298 | 0.31775 |
| HDL-cholesterol (mg/dL) | 55.236 ± 16.203 | 57.743 ± 22.547 | 56.273 ± 19.857 | 60.625 ± 16.961 | 0.39335 |
| Total cholesterol (mg/dL) | 186.738 ± 32.688 | 192.013 ± 43.755 | 193.473 ± 47.404 | 198.341 ± 32.718 | 0.55408 |
| MHR | 0.012 ± 0.005 | 0.012 ± 0.007 | 0.012 ± 0.006 | 0.011 ± 0.007 | 0.66601 |
| UACR | 0.520 ± 2.228 | 0.208 ± 1.025 | 0.559 ± 2.937 | 0.240 ± 0.622 | 0.34465 |
| NPAR | 0.949 ± 0.298 | 0.904 ± 0.262 | 0.951 ± 0.285 | 0.835 ± 0.283 | 0.01519 |
| SII | 464.666 ± 210.373 | 483.256 ± 250.736 | 520.514 ± 255.919 | 501.649 ± 215.223 | 0.33337 |
| Dietary OBS | 4.371 ± 1.365 | 11.350 ± 2.756 | 20.929 ± 3.131 | 26.585 ± 1.436 | <0.00001 |
| Lifestyle OBS | 3.077 ± 1.120 | 3.397 ± 1.302 | 3.552 ± 1.349 | 4.979 ± 1.083 | <0.00001 |
| Gender | 0.50096 | ||||
| Male | 47.250 | 43.238 | 47.368 | 37.636 | |
| Female | 52.750 | 56.762 | 52.632 | 62.364 | |
| Race | 0.11863 | ||||
| Hispanic | 9.240 | 9.561 | 7.862 | 4.006 | |
| Non-Hispanic White | 70.385 | 74.423 | 79.801 | 85.374 | |
| Non-Hispanic Black | 16.173 | 6.800 | 4.519 | 3.096 | |
| Other | 4.202 | 9.215 | 7.817 | 7.524 | |
| Diabetes | 0.02921 | ||||
| Yes | 24.417 | 19.821 | 12.504 | 5.818 | |
| No | 74.145 | 77.318 | 85.133 | 93.695 | |
| Borderline | 1.438 | 2.861 | 2.364 | 0.487 | |
| Hypertension | 0.00271 | ||||
| Yes | 68.423 | 53.094 | 45.113 | 36.083 | |
| No | 31.577 | 46.906 | 54.887 | 63.917 | |
| Smoking | 0.28010 | ||||
| Never | 37.659 | 39.608 | 47.766 | 48.476 | |
| Former | 37.057 | 41.651 | 36.792 | 41.180 | |
| Still | 25.283 | 18.742 | 15.442 | 10.343 | |
MHR, monocyte/high density lipoprotein cholesterol ratio; UACR, urinary albumin/creatinine ratio; NPAR, neutrophil percentage to albumin ratio; SII, systemic immune-inflammation indicator. Mean ± SD for continuous variables: P-value was calculated by weighted linear regression model. Percent for categorical variables: P-value was calculated by weighted chi-square test.
3.2. Association between OBS, dietary OBS, lifestyle OBS, and serum cobalt
Multiple linear regression analyses were conducted to evaluate the correlations between OBS, dietary OBS, lifestyle OBS, and serum cobalt level. The unadjusted and adjusted outcomes of these analyses are presented in Table 2. In all models examined, no significant correlation was observed between OBS and lifestyle OBS with respect to serum cobalt levels. Dietary OBS was found negatively associated with serum cobalt levels in fully adjusted model (model 3: β = −0.179, 95%CI: −0.358 to −0.001, P: 0.04918), but not in unadjusted and partially adjusted model.
Table 2.
Relationships of OBS, dietary OBS, lifestyle OBS, and the cobalt level.
| Outcome | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95%CI) | p-value | β (95%CI) | P-value | β (95%CI) | P-value | |
| OBS | −0.100 (−0.256, 0.056) | 0.20771 | −0.086 (−0.246, 0.075) | 0.29628 | −0.155 (−0.328, 0.018) | 0.07924 |
| Dietary OBS | −0.129 (−0.292, 0.035) | 0.12269 | −0.109 (−0.278, 0.059) | 0.20412 | −0.179 (−0.358, −0.001) | 0.04918 |
| Lifestyle OBS | 0.485 (−0.350, 1.320) | 0.25504 | 0.390 (−0.473, 1.253) | 0.37604 | 0.556 (−0.564, 1.676) | 0.33119 |
Model 1: no covariates were adjusted.
Model 2: age, M-HDL, and UACR were adjusted.
Model 3: age, BMI (kg/m2), diabetes, hypertension, smoking, urine creatinine (mg/dL), blood urea nitrogen (mg/dL), iron (μg/dL), phosphorus (mg/dL), total Protein (g/dL), uric acid (mg/dL), monocyte number (1,000 cells/μL), HDL-Cholesterol (mg/dL), total Cholesterol (mg/dL), M-HDL, UACR, NPAR, and SII were adjusted.
3.3. Subgroup analysis for the relationship of OBS, dietary OBS, lifestyle OBS, and serum cobalt
When stratified by gender, negative relationships of OBS (model 3: β = −0.259, 95%CI: −0.487 to −0.032, P: 0.02626) and dietary OBS (model 3: β = −0.288, 95%CI: −0.524 to −0.052, P: 0.01740) with serum cobalt were found to be statistically significant in males while not in females (Table 3).
Table 3.
Relationships of OBS, dietary OBS, lifestyle OBS, and the cobalt level stratified by gender.
| Outcome | Male | Female | ||
|---|---|---|---|---|
| β (95%CI) | P-value | β (95%CI) | P-value | |
| Model 1 | ||||
| OBS | −0.153 (−0.356, 0.051) | 0.14243 | −0.054 (−0.288, 0.180) | 0.65016 |
| Dietary OBS | −0.186 (−0.398, 0.027) | 0.08746 | −0.076 (−0.322, 0.169) | 0.54266 |
| Lifestyle OBS | 0.479 (−0.600, 1.558) | 0.38494 | 0.442 (−0.822, 1.707) | 0.49358 |
| Model 2 | ||||
| OBS | −0.124 (−0.328, 0.080) | 0.23453 | −0.046 (−0.289, 0.197) | 0.71257 |
| Dietary OBS | −0.154 (−0.368, 0.060) | 0.16057 | −0.063 (−0.318, 0.192) | 0.62932 |
| Lifestyle OBS | 0.428 (−0.662, 1.517) | 0.44209 | 0.332 (−0.981, 1.645) | 0.62048 |
| Model 3 | ||||
| OBS | −0.259 (−0.487, −0.032) | 0.02626 | −0.102 (−0.376, 0.171) | 0.46308 |
| Dietary OBS | −0.288 (−0.524, −0.052) | 0.01740 | −0.122 (−0.403, 0.158) | 0.39272 |
| Lifestyle OBS | 0.332 (−1.175, 1.839) | 0.66641 | 0.528 (−1.167, 2.222) | 0.54214 |
Model 1: no covariates were adjusted.
Model 2: age, M-HDL, and UACR were adjusted.
Model 3: age, BMI (kg/m2), diabetes, hypertension, smoking, creatinine, urine (mg/dL), blood urea nitrogen (mg/dL), iron (μg/dL), phosphorus (mg/dL), total Protein (g/dL), uric acid (mg/dL), monocyte number (1,000 cells/μL), HDL-Cholesterol (mg/dL), total cholesterol (mg/dL), M-HDL, UACR, NPAR, and SII were adjusted.
In subgroup analysis stratified by age, we observed negative relationships of OBS (model 3: β = −0.545, 95%CI: −0.982 to −0.108, P: 0.01545) and dietary OBS (model 3: β = −0.657, 95%CI: −1.110 to −0.205, P: 0.00496) with serum cobalt only in ≥70 years old participants, but not in other age groups (Table 4).
Table 4.
Relationship of OBS, dietary OBS, lifestyle OBS, and the cobalt level stratified by age.
| Outcome | <50 | ≥50, <60 | ≥60, <70 | ≥70 | ||||
|---|---|---|---|---|---|---|---|---|
| β (95%CI) | P-value | β (95%CI) | P-value | β (95%CI) | P-value | β (95%CI) | P-value | |
| Model 1 | ||||||||
| OBS | −0.032 (−0.028, 0.091) | 0.30203 | −0.019 (−0.147, 0.109) | 0.77068 | 0.103 (−0.169, 0.375) | 0.46034 | −0.332 (−0.738, 0.075) | 0.11123 |
| Dietary OBS | 0.019 (−0.043, 0.082) | 0.54829 | −0.031 (−0.168, 0.106) | 0.65973 | 0.136 (−0.136, 0.409) | 0.32842 | −0.432 (−0.868, 0.004) | 0.05367 |
| Lifestyle OBS | 0.384 (0.081, 0.688) | 0.01522 | 0.231 (−0.460, 0.923) | 0.51312 | −1.001 (−2.502, 0.500) | 0.19303 | 1.205 (−0.995, 3.404) | 0.28423 |
| Model 2 | ||||||||
| OBS | 0.026 (−0.032, 0.085) | 0.37504 | −0.025 (−0.158, 0.108) | 0.71546 | 0.109 (−0.184, 0.403) | 0.46614 | −0.413 (−0.820, −0.005) | 0.04846 |
| Dietary OBS | 0.016 (−0.045, 0.076) | 0.61676 | −0.036 (−0.178, 0.106) | 0.61878 | 0.145 (−0.145, 0.436) | 0.32802 | −0.509 (−0.942, −0.077) | 0.02212 |
| Lifestyle OBS | 0.337 (0.038, 0.637) | 0.03006 | 0.201 (−0.516, 0.918) | 0.58347 | −1.184 (−2.798, 0.431) | 0.15292 | 1.065 (−1.190, 3.321) | 0.35565 |
| Model 3 | ||||||||
| OBS | 0.022 (−0.039, 0.084) | 0.48221 | −0.098 (−0.266, 0.070) | 0.25728 | 0.216 (−0.125, 0.558) | 0.21683 | −0.545 (−0.982, −0.108) | 0.01545 |
| Dietary OBS | 0.019 (−0.043, 0.082) | 0.54491 | −0.114 (−0.291, 0.063) | 0.20996 | 0.252 (−0.083, 0.586) | 0.14332 | −0.657 (−1.110, −0.205) | 0.00496 |
| Lifestyle OBS | 0.144 (−0.257, 0.545) | 0.48342 | 0.172 (−0.843, 1.187) | 0.74097 | −1.649 (−3.722, 0.424) | 0.12152 | 2.836 (−0.191, 5.862) | 0.06798 |
Model 1: no covariates were adjusted.
Model 2: M-HDL, and UACR were adjusted.
Model 3: BMI (kg/m2), diabetes, hypertension, smoking, urine creatinine (mg/dL), blood urea nitrogen (mg/dL), iron (μg/dL), phosphorus (mg/dL), total Protein (g/dL), uric acid (mg/dL), monocyte number (1,000 cells/μL), HDL-Cholesterol (mg/dL), total Cholesterol (mg/dL), M-HDL, UACR, NPAR, and SII were adjusted.
3.4. Threshold effect analysis
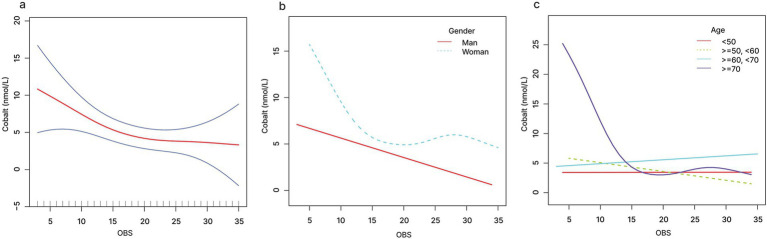
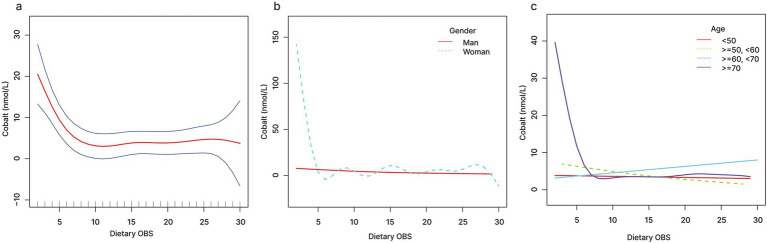
The relationship between OBS and serum cobalt exhibited a non-linear pattern, as shown by smooth curve fitting (Figure 2A). When OBS < 19.7, a one-unit increase in OBS level was associated with 0.5 units decrease in serum cobalt level. When OBS was >19.7, no significant association was observed with serum cobalt (Figure 2A and Table 5). Similarly, dietary OBS and serum cobalt exhibited a non-linear relationship as shown by smooth curve fitting (Figure 3A). When dietary OBS < 9.6, a one-unit increase in dietary OBS was associated with 2.225 units decrease in serum cobalt level. When OBS was >9.6, no significant association was observed with serum cobalt (Figure 3A and Table 5).
Figure 2.
The relationship between OBS and serum cobalt (A), stratified by gender (B), stratified by age (C).
Table 5.
Threshold effect analysis of OBS and dietary OBS on the cobalt level using the two-piecewise linear regression model.
| Adjusted β (95%CI) | P-value | |
|---|---|---|
| OBS infection point | 19.7 | |
| OBS < 19.7 | −0.500 (−0.876, −0.125) | 0.0093 |
| OBS > 19.7 | 0.154 (−0.191, 0.499) | 0.3818 |
| Log likelihood ratio | 0.039 | |
| Dietary OBS infection point | 9.6 | |
| Dietary OBS < 9.6 | −2.225 (−3.120, −1.329) | <0.0001 |
| Dietary OBS > 9.6 | 0.161 (−0.067, 0.389) | 0.1662 |
| Log likelihood ratio | <0.001 |
The age, BMI (kg/m2), diabetes, hypertension, smoking, urine creatinine (mg/dL), blood urea nitrogen (mg/dL), iron (μg/dL), phosphorus (mg/dL), total Protein (g/dL), uric acid (mg/dL), monocyte number (1,000 cells/μL), HDL-Cholesterol (mg/dL), total Cholesterol (mg/dL), M-HDL, UACR, NPAR, and SII were adjusted.
Figure 3.
The relationship between dietary OBS and serum cobalt (A), stratified by gender (B), stratified by age (C).
When stratified by gender, linear relationships of OBS and dietary OBS with serum cobalt were detected in males (Figures 2B, 3B). When stratified by age, non-linear relationships of OBS and dietary OBS with serum cobalt were observed in ≥70 years old participants (Figures 2C, 3C). When OBS < 16.3, a one-unit increase in OBS level was associated with 1.904 units decrease in serum cobalt level. When OBS was >16.3, no significant association was observed with serum cobalt. Similarly, when dietary OBS < 8.7, a one-unit increase in dietary OBS level was associated with 5.184 units decrease in serum cobalt level. When dietary OBS was >8.7, no significant association was observed with serum cobalt (Table 6).
Table 6.
Threshold effect analysis of OBS and dietary OBS on the cobalt level in ≥70 participants using the two-piecewise linear regression model.
| Adjusted β (95%CI) | P-value | |
|---|---|---|
| OBS infection point | 16.3 | |
| OBS < 16.3 | −1.904 (−3.261, −0.548) | 0.0066 |
| OBS > 16.3 | 0.060 (−0.660, 0.780) | 0.8711 |
| Log likelihood ratio | 0.029 | |
| Dietary OBS infection point | 8.7 | |
| Dietary OBS < 8.7 | −5.184 (−7.497, −2.871) | <0.0001 |
| Dietary OBS > 8.7 | 0.053 (−0.510, 0.615) | 0.8548 |
| Log likelihood ratio | <0.001 |
The age, BMI (kg/m2), diabetes, hypertension, smoking, creatinine, urine (mg/dL), blood urea nitrogen (mg/dL), iron (μg/dL), phosphorus (mg/dL), total Protein (g/dL), uric acid (mg/dL), monocyte number (1,000 cells/μL), HDL-Cholesterol (mg/dL), total Cholesterol (mg/dL), M-HDL, UACR, NPAR, and SII were adjusted.
4. Discussion
To investigate the association between oxidative stress and metal ion levels in patients with metal implants, we conducted a large-scale cross-sectional study involving 549 representative participants based on data from NHANES 2015–2020. Our results indicated that as the OBS increased, serum cobalt levels gradually decreased. These findings highlight the importance of managing OBS in individuals with metal implants. Elevated OBS and dietary OBS levels are indicative of lower cobalt levels, ultimately helping to minimize the impact of metal ions on health.
Aging is an irreversible trend that has led to a significant increase in orthopedic degenerative diseases and their complications among the elderly population, thereby resulting in a continuous rise in the demand for metal implants. Epidemiological studies indicate that the prevalence of chronic diseases, such as hypertension, coronary heart disease, and diabetes, is relatively high in this demographic. Therefore, it is crucial to explore the factors influencing the progression of chronic disease in patients who have undergone metal implantation. Oxidative stress refers to the imbalance between antioxidant defense mechanisms and the production of ROS, and it has been confirmed to be closely associated with various chronic diseases. Oxidative stress is considered a significant contributing factor to cardiovascular diseases like atherosclerosis, heart disease, and hypertension, and it also plays a vital role in the onset and progression of diabetes (40). Hyperglycemic states can trigger the production of ROS, leading to insulin resistance and β-cell dysfunction. Furthermore, oxidative stress is closely related to complications of diabetes, including cardiovascular diseases, kidney disease, and neuropathy (41). Our research has revealed that as OBS levels increase, the incidence of diabetes and hypertension gradually decreases, and renal function also shows improvement. This finding suggests that maintaining a healthy lifestyle and a diet rich in antioxidants can help mitigate the progression of chronic diseases in patients receiving metal implant therapy.
Mechanical wear and fluid corrosion have long been recognized as the primary factors leading to the generation of metallic particles and the release of metal ions (42, 43). In recent years, the role of oxidative stress in this process has garnered increasing attention. Research indicates that oxidative stress exacerbates the generation of metallic particles and the release of metal ions through various mechanisms, such as enhancing the corrosion rate of metal implants, promoting inflammatory responses, intensifying cellular damage and apoptosis, and altering the surface characteristics of materials. The increase in ROS accelerates the degradation of implants while diminishing the tissue’s ability to clear metal ions, thereby exacerbating the accumulation of metallic particles and ions in the surrounding environment (44–47). Xu et al. (44) observed extensive infiltration and accumulation of macrophages in the synovial tissue of patients with failed metal hip prostheses. Their study revealed that cobalt released from the metal implants stimulate surrounding immune cells to produce ROS, which subsequently downregulate the RhoA signaling pathway in macrophages. This alteration results in increased formation of intracellular podosome-type adhesion structures and enhanced adhesion to the extracellular matrix, ultimately leading to decreased motility of the macrophages (44). Furthermore, Kim et al. (47) found that ROS induced by metal implants could trigger apoptosis in osteoblasts and gingival fibroblasts through the activation of the Nrf2/ARE pathway and the upregulation of heme oxygenase-1. What is even worse, the significant entry of metal ions into the bloodstream can provoke systemic toxic reactions, impacting the functionality of vital organs. Metals such as cobalt, chromium, nickel, and titanium, which are major components of metal implants, have gained increasing attention due to the health issues they may cause. In a prospective study involving 100 patients with metal implants, researcher Brodner evaluated the serum cobalt concentrations in patients following metal-on-metal total hip arthroplasty, finding that the serum cobalt levels exceeded the detection limit (48). Another study described a 70-year-old patient with cobalt toxicity, whose primary symptoms included progressive hearing and vision deterioration, cataracts, and axonal sensorimotor neuropathy (49). Signorello et al. (50) found that cobalt released from metal implants enters the bloodstream and ultimately accumulates in large amounts in the bladder, resulting in a significant increase in the incidence rate of bladder cancer among patients undergoing hip replacement surgeries. Building on this, Speer et al. (46) investigated the effects of cobalt on human urothelial cells and revealed that soluble cobalt induces cell cycle arrest, leading to cytotoxicity and genotoxicity. Moreover, elevated levels of serum cobalt have also been found to closely associated with increased risks of cardiovascular diseases, hormonal imbalances, immune system suppression, and reduced capacity for infection resistance (21, 51, 52). In this study, we observed a negative correlation between OBS, dietary OBS, and the serum cobalt levels in patients with metal implant. When OBS is less than 19.7, for every unit increase in OBS, the cobalt level decreased by 0.5 units. Similarly, when dietary OBS is less than 9.6, the cobalt levels reduced by 2.225 units as the unit dietary OBS raised. This suggests that maintaining a healthy lifestyle and dietary habits may be an effective strategy to reduce ion dissociation from metal implants, lower related complications, and improve the long-term survival rate of implants.
Previous studies have indicated that there are significant differences in the response to oxidative stress based on gender and age. Before puberty, girls appear to be more susceptible to metabolic dysfunction induced by oxidative stress, whereas elevated redox markers in boys seem to offer protection against arterial stiffness and maintain lipid homeostasis (53). This phenomenon suggests that sex may play a crucial role in regulating oxidative stress-related genes, such as NCF2 and NOX3. Furthermore, research has demonstrated that sex hormones can influence the expression and activity of NADPH oxidase genes and myeloperoxidase, resulting in differences in the response to oxidative stress between males and females (54). Antioxidant lifestyles have been shown to play a significant protective role in the prevention and treatment of depression in women (23). Additionally, Cao’s research has found stronger protective effects of dietary antioxidants in women, suggesting that dietary changes can effectively prevent chronic kidney disease (55). This phenomenon may be attributed to the regulation of most proteins involved in redox status and mitochondrial function by sex hormones (56). The expression of mitochondrial related genes has been shown to be closely related to gender, suggesting a key role of sex hormone signaling in mitochondrial dynamics and cellular redox biology (57). When activated, estrogen-related receptors improve fatty acid oxidation, mitochondrial dynamics, and respiratory chain activity (58, 59). The results of our study indicate that the negative correlation between OBS and serum cobalt levels is pronounced in men but not as evident in women. This discrepancy may be related to differences in hormone levels, as estrogen is known to combat oxidative stress. Itagaki et al. (60) have found that estradiol inhibits the production of ROS and MAPK signaling, thereby preventing the activation of transcription factors and inactivating the downstream transcription processes involved in the expression and activation of TGF-β. Additionally, Sun’s research indicates that β-estradiol can enhance ROS generation and RUBICON expression, further promoting LC3B-associated phagocytosis in macrophages, which suggests a novel perspective for understanding the mechanism of trained immunity in gender differences during sepsis response (61). Furthermore, women tend to adopt healthier lifestyle choices compared to men, which may contribute to a lack of significant impact on their serum cobalt levels. Consequently, dietary modifications may be more beneficial for lowering serum cobalt levels in men.
Moreover, subgroup analyses reveal significant differences in the association between OBS and serum cobalt levels across various age groups. A notable negative correlation is observed in participants over the age of 70, while this correlation is less pronounced in other age groups. This finding is consistent with previous research by Qu, which highlighted a stronger negative correlation between OBS and periodontitis in the elderly (24). Xiao et al. (62) comprehensively identified redox-modified disease networks that are remodeled in aged mice, establishing a systemic molecular foundation for the complex links between redox dysregulation and tissue aging. They found that the bladders of aged mice exhibit baseline reactive oxygen species (ROS) accumulation and heightened oxidative stress. Mysorekar’ team discovered that d-mannose treatment reversed autophagy flux, rescued the senescence-associated secretory phenotype, and alleviated ROS and the shedding of NLRP3/Gasdermin/IL-1β-driven pyroptotic epithelial cell in elderly animals (63). These phenomena may be associated with the decline in cellular repair capacity and the efficiency of antioxidant defense systems that often accompany aging. Therefore, it is crucial for older adults to improve their OBS in order to reduce serum cobalt levels and mitigate the toxic effects of cobalt.
This study exhibits prominent strengths. Firstly, the data from NHANES were obtained by a sophisticated, multi-stage probability sampling design, strictly adhered to comprehensive quality control to ensure the effectiveness and integrity of the dataset. Therefore, based on the database, our results are highly credible when extended to non-institutionalized populations, especially, the association was validated to be robust after the adjustment for various confounders. Second, an increasing number of studies have demonstrated a significant correlation between CRP levels and biomarkers associated with oxidative stress, indicating that inflammation and infection may be potential factors influencing oxidative stress. To mitigate this impact, we implemented stringent exclusion criteria to omit individuals with CRP levels exceeding 5 mg/L, thereby enhancing the reliability of our findings (64, 65). Third, the present study concentrated on the OBS, encompassing a composite indicator of antioxidant lifestyle and diet, rather than monitoring single component in isolation. This approach enables a more thoroughgoing understanding of the intricate interplay among diverse diet and lifestyle factors in the population with metal implants, as well as their association with the serum cobalt level. Fourth, our study found the association between OBS and serum cobalt level for the first time in population with metal implants and uncovered the gender-specific and age-specific effects of OBS on the serum cobalt level. Fifth, the use of an appropriate covariate adjustment increased the representativeness and reliability of our study. Therefore, the findings carry vital public health implications in the mitigation of the metal ions toxicity on human with metal implants.
Nevertheless, there were also a few limitations in this study. Firstly, the grading criteria for physical activity lack uniformity and certain essential data are inaccessible. Therefore, physical activity was excluded from the calculation of the OBS score. Second, our design of a cross-sectional survey restricts causal inferences between OBS and serum cobalt level. So further prospectively designed studies are needed to verify the causality between OBS and serum cobalt level in human with metal objects. Meanwhile, the biases of recall and reporting using self-reported questionnaires may compromise the accuracy of OBS calculations. Third, the level of metal ions in the human body is influenced by environment and occupation. However, due to privacy concerns, the NHANES database fail to get the geographical location and living status of participants, which makes it impossible to estimate the impact of environmental and occupational exposure on metal ions of human body. Moreover, the uncertainty of implantation type, quantity, reason, or duration, determined the highly heterogeneity of the included population. Finally, the level of metal ions may alter with prolonged postoperative time, so a longer follow-up is extremely needed to further clarify the relationship between the OBS and the serum cobalt level in the population with metal implants.
5. Conclusion
In conclusion, data from a nationally representative sample uncovers the significant negative association between OBS and the level of serum cobalt in the population with metal implants. This negative correlation has been corroborated across different gender and age subgroups. Notably, the protective effects of an antioxidant diet and healthy lifestyle are particularly pronounced among males and individuals aged over 70. For the population with metal implants, maintaining good dietary and lifestyle habits may help reduce the generation of metal particles and the dissociation of metal ions, thereby improving the survival rate of metal implants and decreasing the risk of related complications.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by Postdoctoral Science Grant of School and Hospital of Stomatology Fujian Medical University (No. 202301), Startup Fund for Scientific Research, Fujian Medical University (No. 2023QH1146), and Fujian Medical University Union Hospital Talent Launch Fund Project (No. 2024XH017).
Footnotes
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics ethics review board. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from NHANES. The National Center for Health Statistics ethics review board approved all NHANES protocols, and informed consent was obtained from every participant. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
WY: Conceptualization, Writing – original draft. JC: Investigation, Writing – original draft. JS: Methodology, Writing – original draft. CS: Methodology, Writing – original draft. ZC: Conceptualization, Validation, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1485428/full#supplementary-material
References
- 1.Ni J, Ling H, Zhang S, Wang Z, Peng Z, Benyshek C, et al. Three-dimensional printing of metals for biomedical applications. Mater Today Bio. (2019) 3:100024. doi: 10.1016/j.mtbio.2019.100024, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meng M, Wang J, Huang H, Liu X, Zhang J, Li Z. 3D printing metal implants in orthopedic surgery: methods, applications and future prospects. J Orthop Translat. (2023) 42:94–112. doi: 10.1016/j.jot.2023.08.004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin S, Lin S, Xu J, Yang G, Chen H, Jiang X. Dominoes with interlocking consequences triggered by zinc: involvement of microelement-stimulated MSC-derived exosomes in senile osteogenesis and osteoclast dialogue. J Nanobiotechnol. (2023) 21:346. doi: 10.1186/s12951-023-02085-w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang P, Xu J, Xie L, Gao G, Chen S, Gong Z, et al. Improving hard metal implant and soft tissue integration by modulating the “inflammatory-fibrous complex” response. Bioact Mater. (2023) 20:42–52. doi: 10.1016/j.bioactmat.2022.05.013, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong J, Zhang L, Ruan B, Lv Z, Wang H, Wang Y, et al. NRF2 is a critical regulator and therapeutic target of metal implant particle-incurred bone damage. Biomaterials. (2022) 288:121742. doi: 10.1016/j.biomaterials.2022.121742 [DOI] [PubMed] [Google Scholar]
- 6.Huang XY, Chang LJ, Zhao H, Cai Z. Study on craniocerebral dynamics response and helmet protective performance under the blast waves. Mater Des. (2022) 224:111408. doi: 10.1016/j.matdes.2022.111408 [DOI] [Google Scholar]
- 7.Lee K, Goodman SB. Current state and future of joint replacements in the hip and knee. Expert Rev Med Devices. (2008) 5:383–93. doi: 10.1586/17434440.5.3.383, PMID: [DOI] [PubMed] [Google Scholar]
- 8.Puolakka TJ, Pajamäki KJ, Halonen PJ, Pulkkinen PO, Paavolainen P, Nevalainen JK. The Finnish arthroplasty register: report of the hip register. Acta Orthop Scand. (2001) 72:433–41. doi: 10.1080/000164701753532745, PMID: [DOI] [PubMed] [Google Scholar]
- 9.de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL acetabular system and the ASR hip resurfacing system: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. (2011) 93:2287–93. doi: 10.2106/JBJS.J.01727 [DOI] [PubMed] [Google Scholar]
- 10.Freeman MA, Swanson SA, Heath JC. Study of the wear particles produced from cobalt-chromium-molybdenum-manganese total joint replacement prostheses. Ann Rheum Dis. (1969) 28:Suppl:29 PMID: [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen D. How safe are metal-on-metal hip implants? BMJ. (2012) 344:e1410. doi: 10.1136/bmj.e1410 [DOI] [PubMed] [Google Scholar]
- 12.Grammatopoulos G, Pandit H, Kwon YM, Gundle R, McLardy-Smith P, Beard DJ, et al. Hip resurfacings revised for inflammatory pseudotumour have a poor outcome. J Bone Joint Surg Br. (2009) 91-B:1019–24. doi: 10.1302/0301-620X.91B8.22562, PMID: [DOI] [PubMed] [Google Scholar]
- 13.Ladon D, Doherty A, Newson R, Turner J, Bhamra M, Case CP. Changes in metal levels and chromosome aberrations in the peripheral blood of patients after metal-on-metal hip arthroplasty. J Arthroplast. (2004) 19:78–83. doi: 10.1016/j.arth.2004.09.010 [DOI] [PubMed] [Google Scholar]
- 14.Kalbacova M, Roessler S, Hempel U, Tsaryk R, Peters K, Scharnweber D, et al. The effect of electrochemically simulated titanium cathodic corrosion products on ROS production and metabolic activity of osteoblasts and monocytes/macrophages. Biomaterials. (2007) 28:3263–72. doi: 10.1016/j.biomaterials.2007.02.026, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Anderson JM, Miller KM. Biomaterial biocompatibility and the macrophage. Biomaterials. (1984) 5:5–10. doi: 10.1016/0142-9612(84)90060-7 [DOI] [PubMed] [Google Scholar]
- 16.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. (2005) 26:1271–86. doi: 10.1016/j.biomaterials.2004.04.035 [DOI] [PubMed] [Google Scholar]
- 17.Toledano-Serrabona J, Camps-Font O, de Moraes DP, Corte-Rodríguez M, Montes-Bayón M, Valmaseda-Castellón E, et al. Ion release and local effects of titanium metal particles from dental implants: an experimental study in rats. J Periodontol. (2023) 94:119–29. doi: 10.1002/JPER.22-0091, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandra RS, Elnahal WA, Mayne A, Brash L, McBryde CW, Treacy RBC. Birmingham hip resurfacing at 25 years. Bone Joint J. (2024) 106-B:540–7. doi: 10.1302/0301-620X.106B6.BJJ-2023-1064.R1 [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Xiang Q, Tan X, Zhang YD, Zhu HQ, Pu J, et al. Functionalized cortical bone-inspired composites adapt to the mechanical and biological properties of the edentulous area to resist fretting Wear. Adv Sci. (2023) 10:e2207255. doi: 10.1002/advs.202207255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu D, Bhalekar RM, Marsh JS, Langton DJ, Stewart AJ. Periarticular metal hypersensitivity complications of hip bearings containing cobalt-chromium. EFORT Open Rev. (2022) 7:758–71. doi: 10.1530/EOR-22-0036, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber M, Reinisch G, Trettenhahn G, Zweymuller K, Lintner F. Presence of corrosion products and hypersensitivity-associated reactions in periprosthetic tissue after aseptic loosening of total hip replacements with metal bearing surfaces. Acta Biomater. (2009) 5:172–80. doi: 10.1016/j.actbio.2008.07.032, PMID: [DOI] [PubMed] [Google Scholar]
- 22.He J, Li J, Wu S, Wang J, Tang Q. Accumulation of blood chromium and cobalt in the participants with metal objects: findings from the 2015 to 2018 National Health and nutrition examination survey (NHANES). BMC Geriatr. (2023) 23:72. doi: 10.1186/s12877-022-03710-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Liu X, Wang Y, Zeng B, Zhu B, Dai F. Association between depression and oxidative balance score: National Health and nutrition examination survey (NHANES) 2005-2018. J Affect Disord. (2023) 337:57–65. doi: 10.1016/j.jad.2023.05.071, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Qu H. The association between oxidative balance score and periodontitis in adults: a population-based study. Front Nutr. (2023) 10:1138488. doi: 10.3389/fnut.2023.1138488, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Hu J, Liu L, Zhang Y, Dang K, Cheng L, et al. Association of Dietary Inflammatory Index and Dietary Oxidative Balance Score with all-cause and disease-specific mortality: findings of 2003-2014 National Health and nutrition examination survey. Nutrients. (2023) 15:3148. doi: 10.3390/nu15143148, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahriarpour Z, Nasrabadi B, Hejri-Zarifi S, Shariati-Bafghi SE, Yousefian-Sanny M, Karamati M, et al. Oxidative balance score and risk of osteoporosis among postmenopausal Iranian women. Arch Osteoporos. (2021) 16:43. doi: 10.1007/s11657-021-00886-w, PMID: [DOI] [PubMed] [Google Scholar]
- 27.Sohouli MH, Baniasadi M, Hernández-Ruiz Á, Melekoglu E, Zendehdel M, José Soto-Méndez M, et al. Adherence to oxidative balance scores is associated with a reduced risk of breast cancer; a case-control study. Nutr Cancer. (2023) 75:164–73. doi: 10.1080/01635581.2022.2102658, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Demirer B, Yardımcı H, Erem BS. Inflammation level in type 2 diabetes is associated with dietary advanced glycation end products, Mediterranean diet adherence and oxidative balance score: a pathway analysis. J Diabetes Complicat. (2023) 37:108354. doi: 10.1016/j.jdiacomp.2022.108354, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Lee J-H, Joo YB, Han M, Kwon SR, Park W, Park KS, et al. Relationship between oxidative balance score and quality of life in patients with osteoarthritis: data from the Korea National Health and nutrition examination survey (2014-2015). Medicine. (2019) 98:e16355. doi: 10.1097/MD.0000000000016355, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Z, Chen J, Song C, Sun J, Liu W. Association between serum Iron status and muscle mass in adults: results from NHANES 2015-2018. Front Nutr. (2022) 9:941093. doi: 10.3389/fnut.2022.941093, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lei X, Xu Z, Chen W. Association of oxidative balance score with sleep quality: NHANES 2007-2014. J Affect Disord. (2023) 339:435–42. doi: 10.1016/j.jad.2023.07.040, PMID: [DOI] [PubMed] [Google Scholar]
- 32.Chen K, Yin Q, Guan J, Yang J, Ma Y, Hu Y, et al. Association between the oxidative balance score and low muscle mass in middle-aged US adults. Front Nutr. (2024) 11:1358231. doi: 10.3389/fnut.2024.1358231, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sampson B, Hart A. Clinical usefulness of blood metal measurements to assess the failure of metal-on-metal hip implants. Ann Clin Biochem. (2012) 49:118–31. doi: 10.1258/acb.2011.011141, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X, Deng Y, Fang L, Ni M, Wang X, Zhang T, et al. Association of Exposure to heavy metal mixtures with systemic immune-inflammation index among US adults in NHANES 2011-2016. Biol Trace Elem Res. (2024) 202:3005–17. doi: 10.1007/s12011-023-03901-y, PMID: [DOI] [PubMed] [Google Scholar]
- 35.Liu B, Sun Y, Xu G, Snetselaar LG, Ludewig G, Wallace RB, et al. Association between body Iron status and leukocyte telomere length, a biomarker of biological aging, in a nationally representative sample of US adults. J Acad Nutr Diet. (2019) 119:617–25. doi: 10.1016/j.jand.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nugroho P, Susanto TH, Bonar M, Rizka A, Lydia A, Koesno S, et al. The correlation of MicroRNA-21 with the Nephrin, Podocin, and urinary albumin-creatinine ratio in patients with type 2 diabetes and albuminuria: a cross-sectional study. Can J Kidney Health Dis. (2024) 11:20543581241260948. doi: 10.1177/20543581241260948, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amouzegar A, Mirzaasgari Z, Mehrabi A, Malek M, Alaei-Shahmiri F, Najafi L, et al. Association of monocyte/high-density lipoprotein cholesterol ratio and the carotid intima-media thickness in diabetic patients. BMC Endocr Disord. (2022) 22:323. doi: 10.1186/s12902-022-01246-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Meng Y, Chen M, Baral K, Fu Y, Yang Y, et al. Correlation between the systemic immune-inflammation indicator (SII) and serum ferritin in US adults: a cross-sectional study based on NHANES 2015-2018. Ann Med. (2023) 55:2275148. doi: 10.1080/07853890.2023.2275148, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Y, Zhong Z, Yang W, Yu J, Li J, Guo X, et al. Neutrophil percentage-to-albumin ratio and risk of mortality in patients on peritoneal Dialysis. J Inflamm Res. (2023) 16:6271–81. doi: 10.2147/JIR.S437256, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao R, Chen R, Zheng Q, Yao M, Li K, Cao Y, et al. Oxidative stress disrupts vascular microenvironmental homeostasis affecting the development of atherosclerosis. Cell Biol Int. (2024) 48:1781–801. doi: 10.1002/cbin.12239, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Bej E, Cesare P, Volpe AR, d’Angelo M, Castelli V. Oxidative stress and neurodegeneration: insights and therapeutic strategies for Parkinson’s disease. Neurol Int. (2024) 16:502–17. doi: 10.3390/neurolint16030037, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu JW, Zhang Y, Huang YJ, Chang L, Chen T, Ren T, et al. Dynamic response of chain mail fabrics with variable stiffness. Int J Mech Sci. (2024) 264:108840. doi: 10.1016/j.ijmecsci.2023.108840 [DOI] [Google Scholar]
- 43.Xu JW, Chang LJ, Chen TW, Ren T, Zhang Y, Cai Z. Study of the bending properties of variable stiffness chain mail fabrics. Compos Struct. (2023) 322:117369. doi: 10.1016/j.compstruct.2023.117369 [DOI] [Google Scholar]
- 44.Xu J, Yang J, Nyga A, Ehteramyan M, Moraga A, Wu Y, et al. Cobalt (II) ions and nanoparticles induce macrophage retention by ROS-mediated down-regulation of RhoA expression. Acta Biomater. (2018) 72:434–46. doi: 10.1016/j.actbio.2018.03.054, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabbioni E, Fortaner S, Farina M, del Torchio R, Olivato I, Petrarca C, et al. Cytotoxicity and morphological transforming potential of cobalt nanoparticles, microparticles and ions in Balb/3T3 mouse fibroblasts: an in vitro model. Nanotoxicology. (2014) 8:455–64. doi: 10.3109/17435390.2013.796538, PMID: [DOI] [PubMed] [Google Scholar]
- 46.Speer RM, The T, Xie H, Liou L, Adam RM, Wise JP, Sr. The cytotoxicity and genotoxicity of particulate and soluble cobalt in human urothelial cells. Biol Trace Elem Res. (2017) 180:48–55. doi: 10.1007/s12011-017-0989-z, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Kim E-C, Kim M-K, Leesungbok R, Lee SW, Ahn SJ. Co-Cr dental alloys induces cytotoxicity and inflammatory responses via activation of Nrf2/antioxidant signaling pathways in human gingival fibroblasts and osteoblasts. Dent Mater. (2016) 32:1394–405. doi: 10.1016/j.dental.2016.09.017, PMID: [DOI] [PubMed] [Google Scholar]
- 48.Brodner W, Bitzan P, Meisinger V, Kaider A, Gottsauner-Wolf F, Kotz R. Serum cobalt levels after metal-on-metal total hip arthroplasty. J Bone Joint Surg Am. (2003) 85:2168–73. doi: 10.2106/00004623-200311000-00017 [DOI] [PubMed] [Google Scholar]
- 49.Heuer C, Streit A-C, Sprengel K, Hasler RM, Ziegenhain F, Zahorecz M, et al. Cobalt intoxication: mitochondrial features and condition. J Neurol. (2022) 269:6655–7. doi: 10.1007/s00415-022-11243-3, PMID: [DOI] [PubMed] [Google Scholar]
- 50.Signorello LB, Ye W, Fryzek JP, Lipworth L, Fraumeni JF, Blot WJ, et al. Nationwide study of cancer risk among hip replacement patients in Sweden. J Natl Cancer Inst. (2001) 93:1405–10. doi: 10.1093/jnci/93.18.1405 [DOI] [PubMed] [Google Scholar]
- 51.Fleury C, Petit A, Mwale F, Antoniou J, Zukor DJ, Tabrizian M, et al. Effect of cobalt and chromium ions on human MG-63 osteoblasts in vitro: morphology, cytotoxicity, and oxidative stress. Biomaterials. (2006) 27:3351–60. doi: 10.1016/j.biomaterials.2006.01.035, PMID: [DOI] [PubMed] [Google Scholar]
- 52.Skjöldebrand C, Tipper JL, Hatto P, Bryant M, Hall RM, Persson C. Current status and future potential of wear-resistant coatings and articulating surfaces for hip and knee implants. Mater Today Bio. (2022) 15:100270. doi: 10.1016/j.mtbio.2022.100270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basnet TB, Khatri B. Oxidative stress-related genetic variation and antioxidant vitamin intake in intact and ruptured abdominal aortic aneurysm: does sex matter? Eur J Prev Cardiol. (2024) 31:59–60. doi: 10.1093/eurjpc/zwad342 [DOI] [PubMed] [Google Scholar]
- 54.Fernández-Espejo E, Rodríguez de Fonseca F, Gavito AL, Córdoba-Fernández A, Chacón J, Martín de Pablos Á. Myeloperoxidase and advanced oxidation protein products in the cerebrospinal fluid in women and men with Parkinson’s disease. Antioxidants. (2022) 11:1088. doi: 10.3390/antiox11061088, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao Y, Zhou Y, Zhong Y, Liao X, Chen X, Pi Y. Association between oxidative balance score in adults with and without chronic kidney disease: 2011-2028 NHANES. Front Nutr. (2024) 11:1374719. doi: 10.3389/fnut.2024.1374719, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang JQ, Li ZJ, Gao H, Sheng J, Liang CM, Hu YB, et al. Gender associations between phthalate exposure and biomarkers of oxidative stress: a prospective cohort study. Toxicol Ind Health. (2024) 40:312–22. doi: 10.1177/07482337241245453 [DOI] [PubMed] [Google Scholar]
- 57.di Florio DN, Sin J, Coronado MJ, Atwal PS, Fairweather DL. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. (2020) 31:101482. doi: 10.1016/j.redox.2020.101482, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Y, Liu Y, Dorn GW. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. (2011) 109:1327–31. doi: 10.1161/CIRCRESAHA.111.258723, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang T, McDonald C, Petrenko NB, Leblanc M, Wang T, Giguere V, et al. Estrogen-related receptor α (ERRα) and ERRγ are essential coordinators of cardiac metabolism and function. Mol Cell Biol. (2015) 35:1281–98. doi: 10.1128/MCB.01156-14, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Itagaki T, Shimizu I, Cheng X, Yuan Y, Oshio A, Tamaki K, et al. Opposing effects of oestradiol and progesterone on intracellular pathways and activation processes in the oxidative stress induced activation of cultured rat hepatic stellate cells. Gut. (2005) 54:1782–9. doi: 10.1136/gut.2004.053728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun Z, Qu J, Xia X, Pan Y, Liu X, Liang H, et al. 17β-estradiol promotes LC3B-associated phagocytosis in trained immunity of female mice against sepsis. Int J Biol Sci. (2021) 17:460–74. doi: 10.7150/ijbs.53050, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao H, Jedrychowski MP, Schweppe DK, Huttlin EL, Yu Q, Heppner DE, et al. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell. (2020) 180:968–983.e24. doi: 10.1016/j.cell.2020.02.012, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joshi CS, Salazar AM, Wang C, Ligon MM, Chappidi RR, Fashemi BE, et al. D-mannose reduces cellular senescence and NLRP3/GasderminD/IL-1β-driven pyroptotic uroepithelial cell shedding in the murine bladder. Dev Cell. (2024) 59:33–47.e5. doi: 10.1016/j.devcel.2023.11.017, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nemeckova I, Eissazadeh S, Rathouska JU, Silhavy J, Malinska H, Pravenec M, et al. Transgenic human C-reactive protein affects oxidative stress but not inflammation biomarkers in the aorta of spontaneously hypertensive rats. BMC Cardiovasc Disord. (2024) 24:211. doi: 10.1186/s12872-024-03870-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang X, Zhang J, Liu J, Sun L, Zhao H, Lu Y, et al. C-reactive protein promotes adhesion of monocytes to endothelial cells via NADPH oxidase-mediated oxidative stress. J Cell Biochem. (2012) 113:857–67. doi: 10.1002/jcb.23415, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.