Abstract
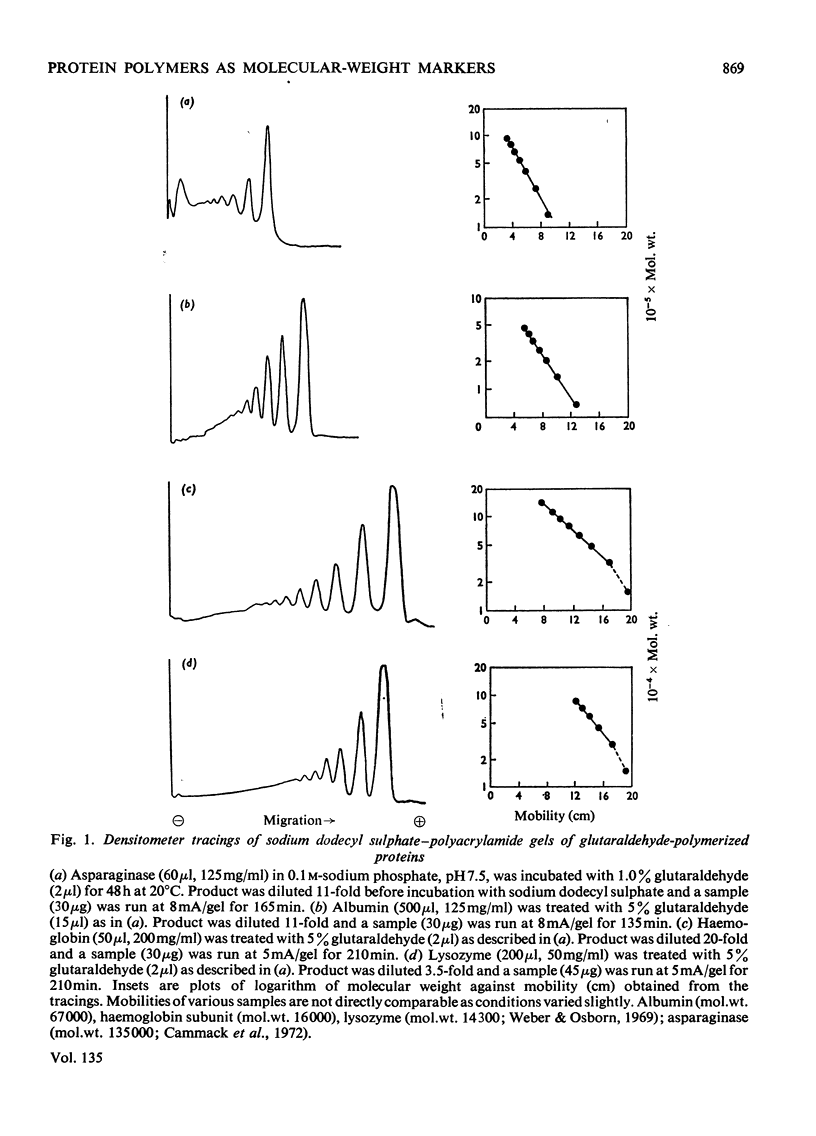
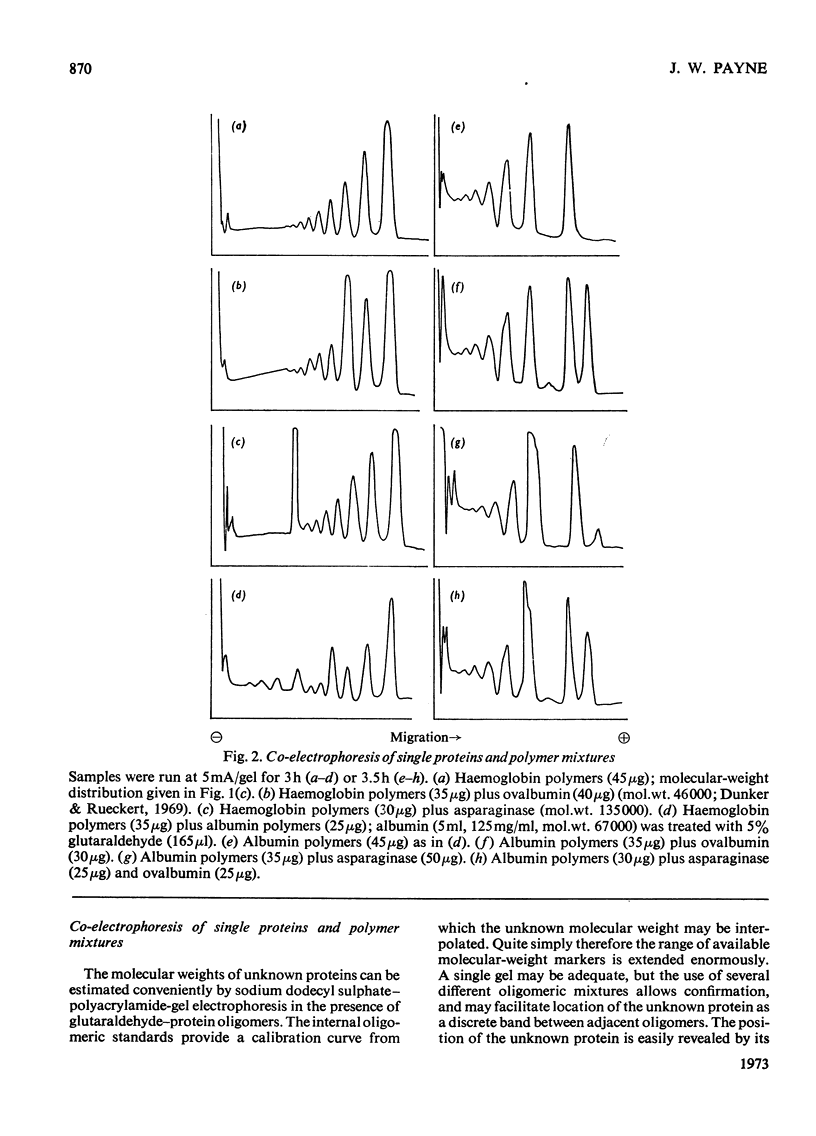
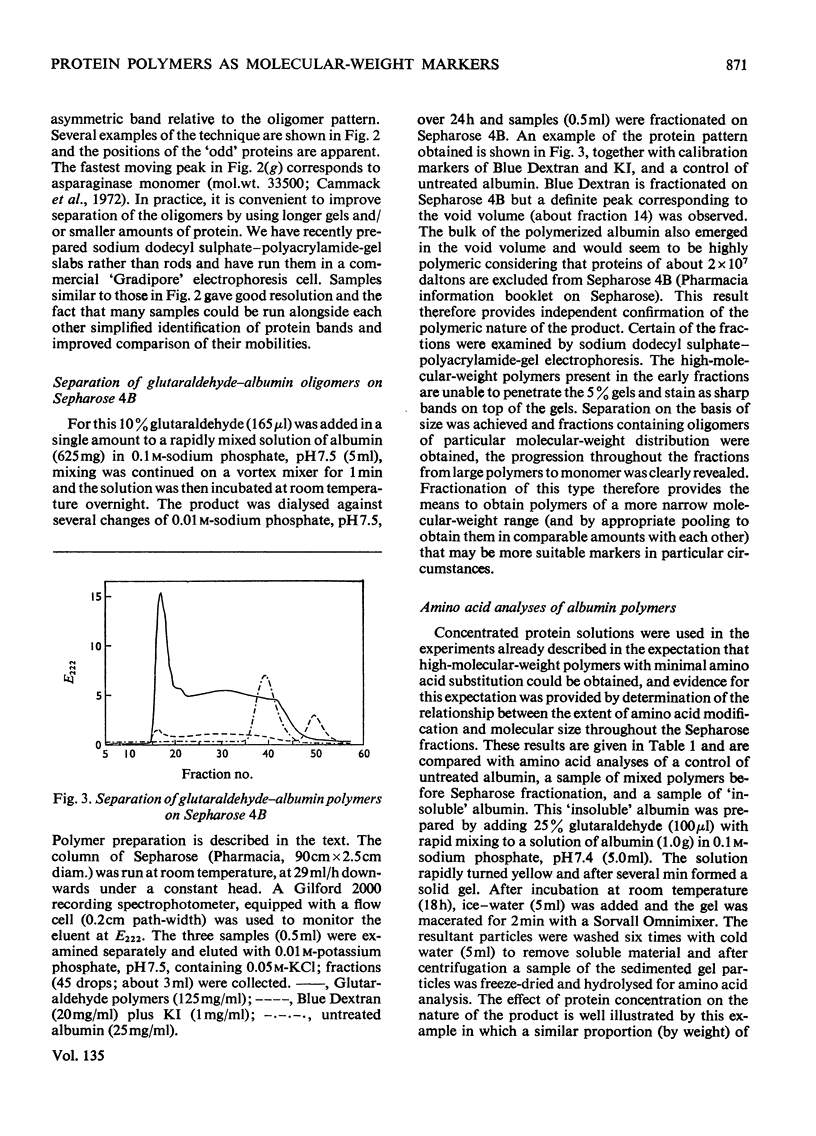
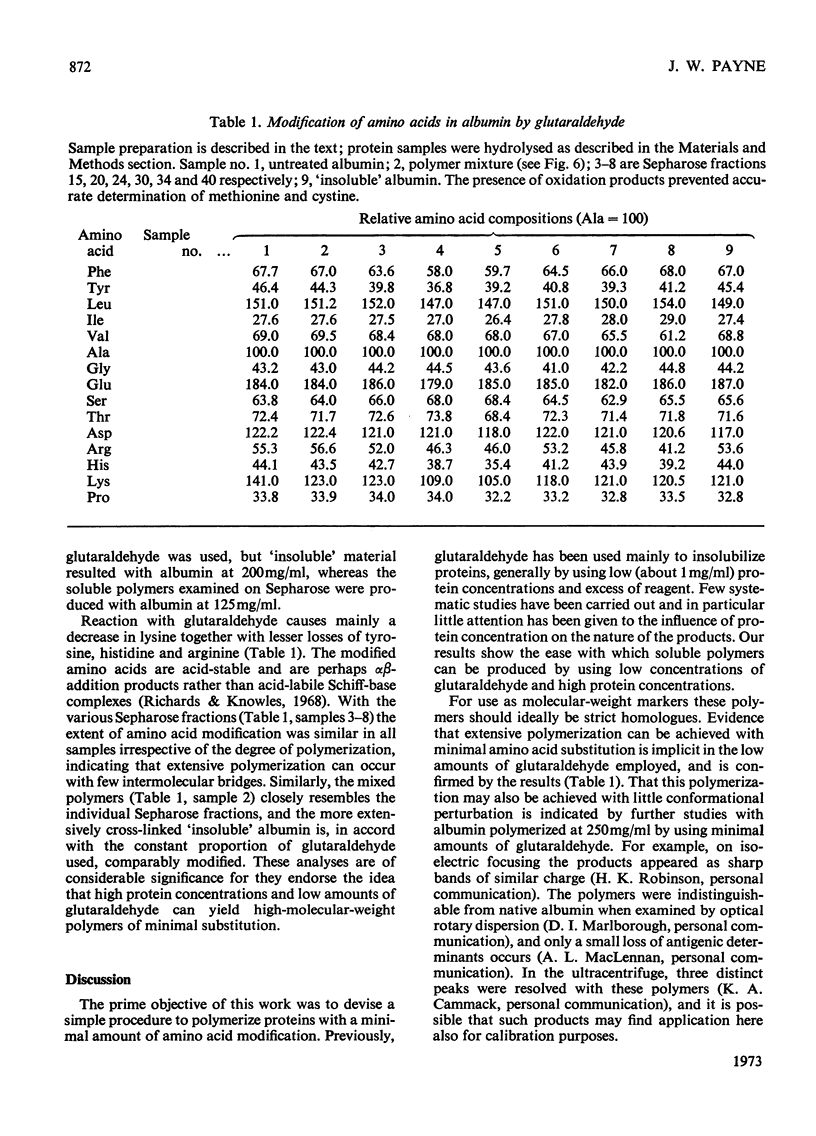
Glutaraldehyde is well known for its ability to react with proteins and to produce insoluble cross-linked aggregates. In contrast with this situation, conditions are described here which yield covalently linked soluble protein oligomers. The procedure is applicable to a wide range of proteins, and by slight variation in the reaction conditions, soluble polymers in the molecular weight range 3×104−2×107 were produced. The products are valuable aṡ molecular-weight markers, e.g. in sodium dodecyl sulphate–polyacrylamide-gel electrophoresis. The inherent similarities of these oligomers make them superior to commercial molecular-weight protein markers, which may have marked differences in composition and charge.
Full text
PDF

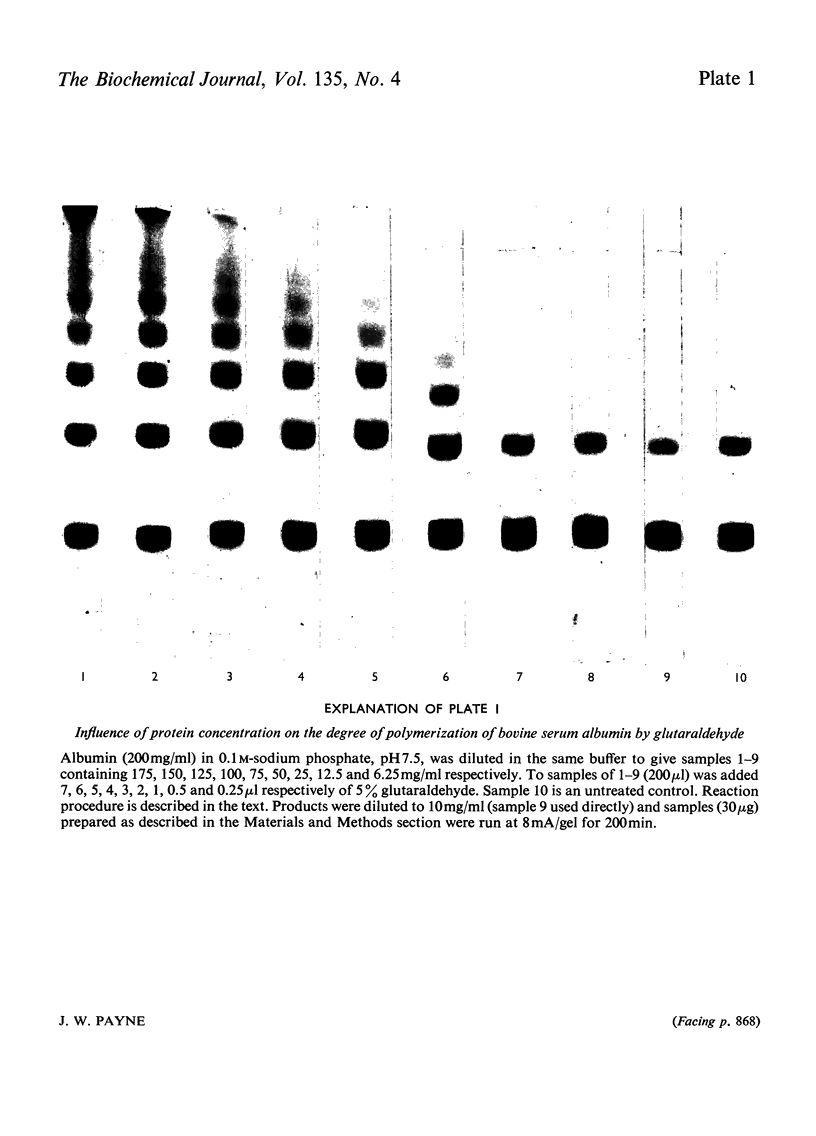
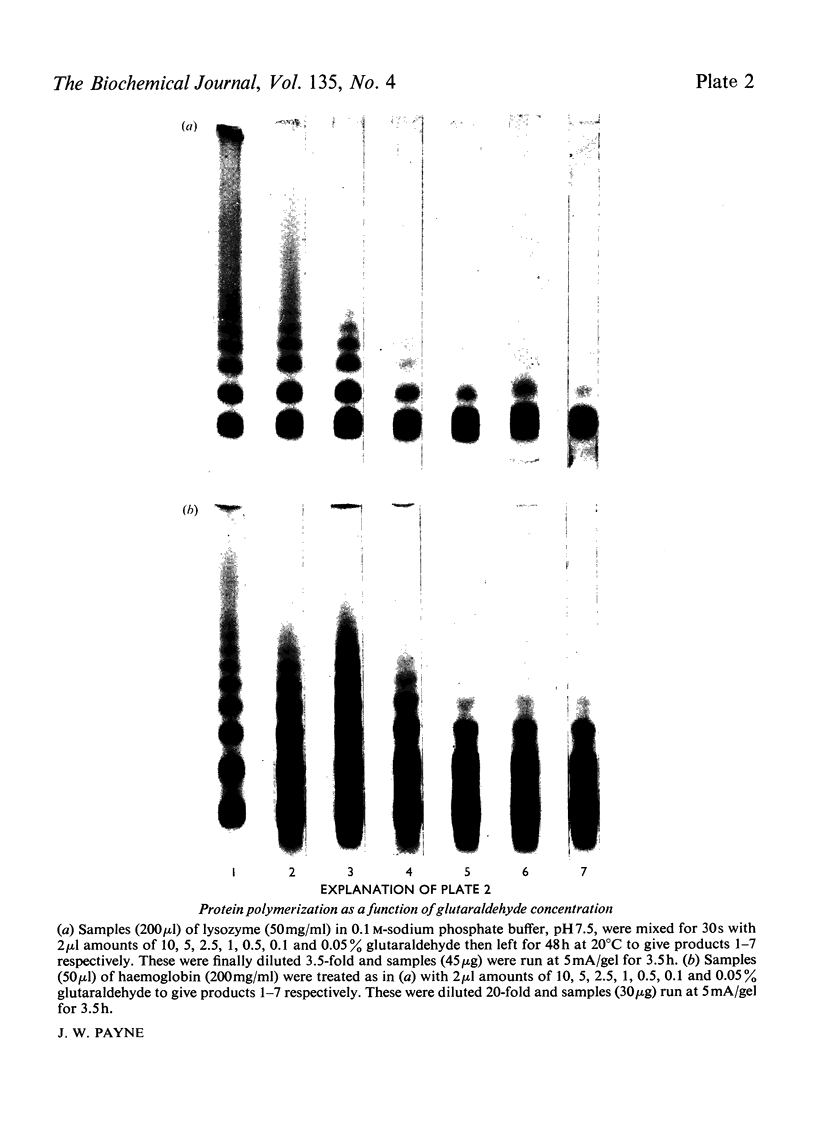

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. -O., Borg H., Mikaelsson M. Molecular weight estimations of proteins by electrophoresis in polyacrylamide gels of graded porosity. FEBS Lett. 1972 Feb 1;20(2):199–202. doi: 10.1016/0014-5793(72)80793-2. [DOI] [PubMed] [Google Scholar]
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Taudou B., Chuilon S. Glutaraldehyde, cyanuric chloride and tetrazotized O-dianisidine as coupling reagents in the passive hemagglutination test. Immunochemistry. 1969 Jan;6(1):67–76. doi: 10.1016/0019-2791(69)90179-7. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Carpenter F. H., Harrington K. T. Intermolecular cross-linking of monomeric proteins and cross-linking of oligomeric proteins as a probe of quaternary structure. Application to leucine aminopeptidase (bovine lens). J Biol Chem. 1972 Sep 10;247(17):5580–5586. [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Fasold H., Klappenberger J., Meyer C., Remold H. Bifunctional reagents for the crosslinking of proteins. Angew Chem Int Ed Engl. 1971 Nov;10(11):795–801. doi: 10.1002/anie.197107951. [DOI] [PubMed] [Google Scholar]
- Griffith I. P. The effect of cross-links on the mobility of proteins in dodecyl sulphate-polyacrylamide gels. Biochem J. 1972 Feb;126(3):553–560. doi: 10.1042/bj1260553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb A. F. Preparation of enzymically active, water-insoluble derivatives of trypsin. Arch Biochem Biophys. 1967 Mar;119(1):264–268. doi: 10.1016/0003-9861(67)90453-5. [DOI] [PubMed] [Google Scholar]
- Habeeb A. J., Hiramoto R. Reaction of proteins with glutaraldehyde. Arch Biochem Biophys. 1968 Jul;126(1):16–26. doi: 10.1016/0003-9861(68)90554-7. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A comparison of the crosslinking abilities of glutaraldehyde, formaldehyde and alpha-hydroxyadipaldehyde with bovine serum albumin and casein. Histochemie. 1969;17(2):151–161. doi: 10.1007/BF00277781. [DOI] [PubMed] [Google Scholar]
- Hopwood D., Callen C. R., McCabe M. The reactions between glutaraldehyde and various proteins. An investigation of their kinetics. Histochem J. 1970 Mar;2(2):137–150. doi: 10.1007/BF01003541. [DOI] [PubMed] [Google Scholar]
- Hopwood D. Fixatives and fixation: a review. Histochem J. 1969 May;1(4):323–360. doi: 10.1007/BF01003278. [DOI] [PubMed] [Google Scholar]
- Hopwood D. Some aspects of fixation with glutaraldehyde. A biochemical and histochemical comparison of the effects of formaldehyde and glutaraldehyde fixation on various enzymes and glycogen, with a note on penetration of glutaraldehyde into liver. J Anat. 1967 Jan;101(Pt 1):83–92. [PMC free article] [PubMed] [Google Scholar]
- Jansen E. F., Olson A. C. Properties and enzymatic activities of papain insolubilized with glutaraldehyde. Arch Biochem Biophys. 1969 Jan;129(1):221–227. doi: 10.1016/0003-9861(69)90169-6. [DOI] [PubMed] [Google Scholar]
- Ogata K., Ottesen M., Svendsen I. Preparation of water-insoluble, enzymatically active derivatives of subtilisin type Novo by cross-linking with glutaraldehyde. Biochim Biophys Acta. 1968 Jun 4;159(2):403–405. doi: 10.1016/0005-2744(68)90090-9. [DOI] [PubMed] [Google Scholar]
- Ottesen M., Svensson B. Modification of papain by treatment with glutaraldehyde under reducing and non-reducing conditions. C R Trav Lab Carlsberg. 1971;38(11):171–185. [PubMed] [Google Scholar]
- QUIOCHO F. A., RICHARDS F. M. INTERMOLECULAR CROSS LINKING OF A PROTEIN IN THE CRYSTALLINE STATE: CARBOXYPEPTIDASE-A. Proc Natl Acad Sci U S A. 1964 Sep;52:833–839. doi: 10.1073/pnas.52.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards F. M., Knowles J. R. Glutaraldehyde as a protein cross-linkage reagent. J Mol Biol. 1968 Oct 14;37(1):231–233. doi: 10.1016/0022-2836(68)90086-7. [DOI] [PubMed] [Google Scholar]
- Schejter A., Bar-Eli A. Preparation and properties of crosslinked water-insoluble catalase. Arch Biochem Biophys. 1970 Feb;136(2):325–330. doi: 10.1016/0003-9861(70)90202-x. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Wade H. E., Phillips B. P. Automated determination of bacterial asparaginase and glutaminase. Anal Biochem. 1971 Nov;44(1):189–199. doi: 10.1016/0003-2697(71)90360-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weston P. D., Avrameas S. Proteins coupled to polyacrylamide beads using glutaraldehyde. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1574–1580. doi: 10.1016/0006-291x(71)90200-2. [DOI] [PubMed] [Google Scholar]