Abstract
Amaryllidaceae alkaloid (AA) biosynthesis has garnered significant attention in recent years, particularly with the commercialization of galanthamine as a treatment for the symptoms of Alzheimer’s disease. A significant amount of research work over the last eight decades has focused on the understanding of AA biosynthesis, starting from early radiolabelling studies to recent multi-omics analysis with modern biotechnological advancements. Those studies enabled the identification of hundreds of metabolites, the characterization of biochemical pathways, and an understanding of the environmental stimuli and of the molecular regulation of these pharmaceutically and agriculturally important metabolites. Despite numerous studies, there remain significant gaps in understanding the biosynthesis of AAs in Amaryllidaceae plants. As such, further research is needed to fully elucidate the metabolic pathways and facilitate their production. This review aims to provide a comprehensive summary of the current state of knowledge on AA biosynthesis, from elicitation of expression of transcription factors in the cell nucleus to alkaloid transport in the apoplast, and to highlight the challenges that need to be overcome for further advancement.
Keywords: Amaryllidoideae, Amaryllidaceae alkaloids, biosynthesis, galanthamine, in vitro culture, isoquinoline alkaloids, multi-omic database, norbelladine, omics, specialized metabolites
This review explores the landscape of Amaryllidaceae alkaloid biosynthesis, tracing its evolution from radiolabelling studies to multi-omics analyses, emphasizing gaps and challenges, and urging further research to unlock their full potential.
Introduction
Tens of thousands of years of human civilization have depended on nature to grant the cure for illnesses and diseases. Amaryllidaceae J. St.-Hil. (sensu stricto) is a plant family that has provided beneficial medicinal value worldwide (Jin and Yao, 2019). Various ethnic groups have traditionally used this plant family to treat a range of illnesses, such as mental health issues, cancer, and respiratory and liver problems (Nair and van Staden, 2013). For instance, Crinum zeylanicum has been used in Sri Lankan folk medicine to treat rheumatism, snake-bites, and ear-aches (Jayaweera, 1981; Tsuda et al., 1984), while Zephyranthes fosteri was used in traditional Aztec medicine to treat ‘fatigue’ and ‘stress’ (Centeno-Betanzos et al., 2021). Chinese folk medicine has long utilized Lycoris radiata bulbs to treat skin and laryngeal conditions, while Amaryllis belladonna and Boophone disticha have been used in the African continent to treat cancer, inflammation, wounds, and infections (Wang et al., 2009; Nair and van Staden, 2013). The Amaryllidaceae J. St.-Hil., also known as subfamily Amaryllidoideae according to APG III, is a cosmopolitan family of bulbous monocots consisting of ~900 species shared by ~75 genera, that thrive mainly in Africa and South America (Meerow et al., 1999; APG, 2009). Their slow-blooming, exquisite flowers render them popular in horticulture. Their ability to produce a unique group of alkaloids called the Amaryllidaceae alkaloids (AAs) may explain the use of these geophytes in traditional medicine systems around the world.
AAs are basic nitrogen-containing specialized metabolites with a benzopyridine heterocyclic group. More than 650 different AAs have been elucidated so far. They derive from the metabolism of phenylalanine and tyrosine (Desgagné-Penix, 2021; Jayawardena et al., 2024). In the plant, they display defence properties to protect against abiotic or biotic stress, such as predators, targeting their nervous system (Berkov et al., 2020; Nair and van Staden, 2020), or to attract pollinators to promote seed dispersion (Berkov et al., 2020). Therapeutically, AAs exhibit various biological activities, including anti-acetylcholinesterase, antiviral, antibacterial, antifungal, anticancer, and cytotoxic activities (Ding et al., 2017; Hotchandani and Desgagne-Penix, 2017; Berkov et al., 2020; Ka et al., 2020). This wide range of potential pharmaceutical applications position AAs as attractive candidates for the development of new drugs. One of the main breakthroughs has been the approval of galanthamine as a treatment of mild symptoms of cognitive impairment and Alzheimer’s disease in at least 29 countries (Olin and Schneider, 2002; Loy and Schneider, 2006). Galanthamine selectively, reversibly, and competitively inhibits acetylcholinesterase, which leads to improved cognitive function (Olin and Schneider, 2002). Lycorine and its derivatives also attracts a lot of attention due to their strong anticancer properties (Roy et al., 2018). Many other AAs, such as cherylline, crinamine, and pancratistatin, are effective against multiple viruses, including herpes simplex virus, Rauscher leukaemia virus, coronaviruses, flaviviruses, human immunodeficiency virus, and hepatitis C virus, as reviewed in Jayawardena et al. (2024). Narciclasine, lycorine, and diverse AAs also display antifungal activities through a plethora of mechanisms (Nair and van Staden, 2020). Cripowellin, lycorine, ungeremine, and multiple AAs exhibit antibacterial activity, and are studied pharmacologically to overcome antibiotic resistance (Bendaif et al., 2018; Chen et al., 2018; Kianfe et al., 2020). AAs such as crinamine, 8α-ethoxyprecriwelline, epivittatine, lycorine, and derivatives are evaluated for their anti-inflammatory activity specific to cyclooxygenese-1 and -2 (Elgorashi et al., 2003; He et al., 2015). Their multifaceted activities highlight the relevance of these plants’ metabolites in drug discovery for improvement of human health.
The yield of an AA of interest is limited and variable in plants grown in the wild, in part due to the diversity of plant metabolic routes and to environmental stresses. In the wild, Amaryllidaceae grow in specific regions, sometimes under singular conditions, and some are classified as endangered species, such as Eucrosia stricklandii, a rare Amaryllidaceae from Ecuador, while several Narcissus species have already become extinct (Colque et al., 2002; Santos-Gally et al., 2015). In a nutshell, wild plants are not a suitable sustainable source of medicinal compounds. Organic synthesis is a challenging, less profitable, and not a sustainable alternative because of the complexity of the structure of AAs (Kohelová et al., 2021). Usually, they are extracted directly from Amaryllidaceae harvested from the field or greenhouse, or micropropagated, but the AA yield is low. Much effort is concentrated on developing in vitro systems with profitable production of AAs, and in uncovering biosynthetic pathways to acquire the knowledge to carry out metabolic engineering (Koirala et al., 2022), but much remains to be discovered with regards to their biosynthesis, and transport in their natural host.
A growing number of research papers have been exploring different aspects of AA biosynthesis, such as enzyme discovery, substrate selectivity, and pathway hypothesis. In this review, we will summarize the established biosynthetic steps, examine both in vivo and in vitro plant studies that helped unravel enzymatic reactions or their regulation, and outline the available multi-omics data. Additionally, we will discuss the latest insights into the pathway characteristics in planta and explore modern techniques and tools that can expedite pathway assembly.
Structural diversity of Amaryllidaceae alkaloids
Ever since lycorine was isolated from Narcissus pseudonarcissus, 150 years ago, scientists have identified and determined the structures of hundreds of AAs (Gerrard, 1877; Ding et al., 2017). Each year, several new alkaloids from Amaryllidaceae species are added to the list, making the puzzle of their biosynthetic route increasingly complex. For instance, in 2023 and 2024, Chaichompoo and colleagues reported 18 new AAs from the bulbs of Crinum latifolium and Crinum×amabile (Chaichompoo et al., 2023, 2024). Experts in the field of AAs have suggested various classification systems for their structure. One of the earliest classifications was introduced by Wildman, based on the presence of different types of nucleus, such as pyrrolo [de] phenanthridine, dibenzofuran, or [2] benzopyrano [3,4g] indole (Wildman, 1960). Later, Ghosal et al. presented a 12-ring system, which is still considered as the standard for numbering ring carbons (Ghosal et al., 1985). Several other ring-type classification systems were proposed based on chemical structure analysis or AA biosynthetic origin, including 42 by Berkov et al. (2020), nine by Bastida et al. (2006), 18 by Jin (2009), and nine by Desgagné-Penix (2021). These classification systems evolve through years with the periodical discovery of novel alkaloids. Hence, there is currently no universal classification system for AAs.
Amaryllidaceae alkaloid biosynthesis
Despite divergences in the classification systems of AA complex structures, the general early steps of their biosynthesis, and the precursors involved, namely tyramine and 3,4-dihydroxybenzaldehyde (3,4-DHBA) coming from the phenylpropanoid pathway, are largely accepted (Kilgore and Kutchan, 2016; Desgagné-Penix, 2021). A tyrosine decarboxylase (TYDC) catalyses decarboxylation of tyrosine into tyramine, as studied in Narcissus aff. pseudonarcissus, Lycoris radiata, and L. aurea (Kilgore, 2015; Wang et al., 2019; Hu et al., 2021); while a phenylalanine ammonia-lyase (PAL) explored in L. radiata, and a cinnamic acid 4-hydroxylase (C4H) uncovered in L. radiata and L. aurea catalyse important steps of the phenylpropanoid pathway (W. Li et al., 2018; Y. Li et al., 2018). There remain gaps in knowledge of the precursor pathway such as the synthesis of 3,4-DHBA which still awaits being uncovered. Nevertheless, these steps are beyond the scope of this review which focuses on the biosynthesis of AAs, starting from the condensation step.
Early and current evidence for biosynthesis of Amaryllidaceae alkaloids
Analytical techniques such as HPLC, GC, MS, and NMR, and in situ metabolite imaging techniques such as matrix-assisted laser desorption/ionization (MALDI) and desorption electrospray ionization- (DESI) coupled MS have allowed the detection and the elucidation of AA structures and their localization in planta (Kilgore et al., 2014; (Mehta et al., 2024). In early studies dating back to the 1950s, radioisotope studies contributed to the identification of precursors and intermediates, and to the assembly of AA metabolic pathways (Barton and Cohen, 1957). Radioactive or stable isotope labelling, random mutagenesis with ethyl methanesulfonate (EMS) or γ-radiation, gene silencing, and multi-omics techniques all contributed to identify specific gene and enzyme candidates. Their integration is decisive to metabolite pathway elucidation. For instance, this approach has enabled the assembly of the canonical pathway of vincristine and vinblastine from Catharanthus roseus, a well-studied medicinal plant, but it took >30 years (Qu et al., 2019).
In this review, the AA pathway will be divided into three sections: ‘Formation of the initial stable intermediates’; ‘Oxidative phenol coupling for diversification of the metabolites’; and ‘Downstream pathways’.
Formation of the initial stable intermediates
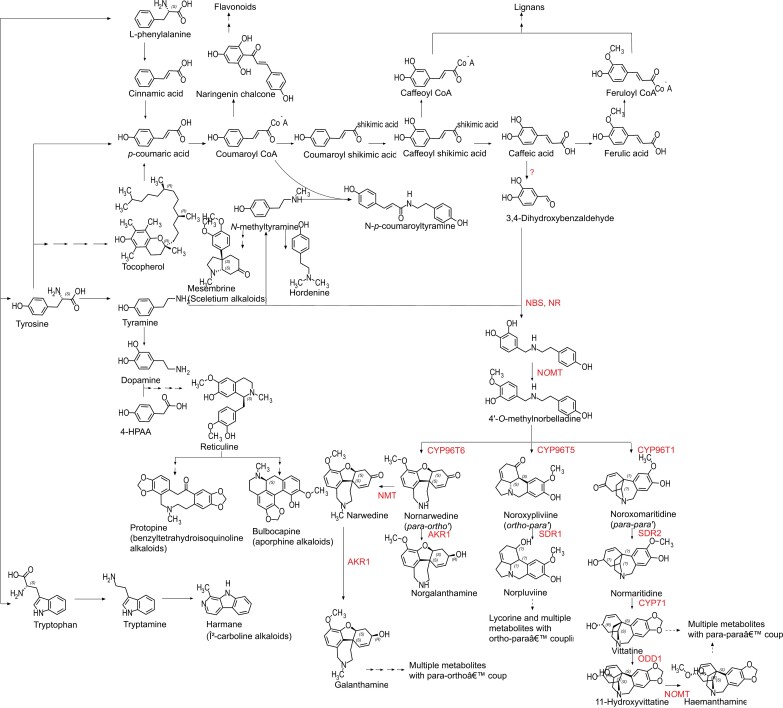
The formation of norbelladine. In the 1960s, the early radioactive isotope labelling studies gave the first insight that tyramine, as the amine group, and 3,4-DHBA, as the aldehyde partner, were incorporated into multiple AAs such as lycorine, haemanthamine, and galanthamine (Suhadolnik et al., 1962, 1963; Wildman et al., 1962; Feinstein, 1967). Another radiolabel study showed that the two precursors combined to yield norbelladine, as a scaffold reaction for the biosynthesis of all AAs (Battersby et al., 1961). Hence, to understand AA biosynthesis, the formation of norbelladine is the first key reaction to explore. Biochemically, norbelladine is synthesized through a reduction reaction that follows condensation of tyramine and 3,4-DHBA yielding norcraugsodine, a Schiff base (Fig. 1) (Majhi et al., 2023).
Fig. 1.
Amaryllidaceae metabolic pathways. Enzymatically characterized steps of the Amaryllidaceae alkaloid (AA) pathway along with other defence-related specialized metabolites and non-AA representatives recorded in the family starting from the three aromatic amino acids. A single arrow represents a single biochemical step, while multiple arrows represent multiple steps. Identified enzymes in the AA pathway are represented in red. Abbreviations are in the text.
The first enzymatic evidence for the formation of norbelladine was reported by Kilgore et al. (2016b). From the transcriptome of Narcissus aff. pseudonarcissus, they identified and cloned a short chain dehydrogenase (SDR) named noroxomaritidine/norcraugsodine reductase (NR), which is phylogenetically close to a tropinone reductase II from Datura stramonium producing tropane alkaloids. Although the major reaction catalysed by that enzyme is related to a downstream pathway step, NR can catalyse the reduction of norcraugsodine to norbelladine, using tyramine and 3,4-DHBA as substrates. Later, Singh et al. (2018) identified another candidate for this reaction from the Narcissus pseudonarcissus transcriptome. Norbelladine synthase (NBS) is an orthologue to norcoclaurine synthase (NCS), a well-characterized enzyme that catalyses the first condensation reaction in the benzylisoquinoline alkaloid (BIA) pathway via a Pictet–Spengler reaction. NBS from N. pseudonarcissus rather commits to a Mannich reaction for the condensation of tyramine and 3,4-DHBA (Singh et al., 2018). Orthologous NBS enzymes from Leucojum aestivum and Narcissus papyraceus were cloned and shown to catalyse the same reaction (Tousignant et al., 2022; Majhi et al., 2023). Interestingly, norbelladine synthesis yield is increased through the interaction between NBS and NR, that catalyse the condensation and the imine reduction, respectively, possibly through preventing the degradation of the unstable norcraugsodine (Fig. 1) (Majhi et al., 2023). None of the studies related to NBS or NR provides any information related to enzyme kinetics. This will be necessary for a better biochemical understanding of the reactions. Nonetheless, NBS and NR may be the gateway to the formation of AA, catalysing the first steps in their biosynthesis. Similar to the formation of norcoclaurine in the BIA pathway or of the 1-phenethylisoquinoline scaffold in phenethylisoquinoline (PIA) alkaloids, the synthesis of norbelladine is the step that establishes the structural features observed in AAs (Beaudoin and Facchini, 2014; Nett et al., 2020).
The formation of 4'-O-methylnorbelladine. Norbelladine 4'-O-methylation is another key step of the biosynthesis of AAs, because it is required for the subsequent oxidative phenol coupling (Kilgore et al., 2014). 4'-O-Methylnorbelladine was established as a central intermediate by radioisotope labelling experiments that aimed to uncover the origin of the methylenedioxy group in haemanthamine (Barton et al., 1962a). Mann et al. (1963) also provided the first evidence of a norbelladine-O-methyltransferase (NOMT) that catalysed this reaction, by identifying an analogous enzyme to a catechol O-methyltransferase (COMT) partially purified from Nerine bowdenii. This study established 4'-O-methylnorbelladine as the intermediate required for the downstream pathway. Thirty-five years later, a study that focused on galanthamine biosynthesis confirmed the catalysis of norbelladine O-methylation using crude enzyme extracts from six Amaryllidaceae species, with the recurrent observation that 4'-O-methylation was favoured over 3'-O-methylation (Mann et al., 1963; Eichhorn et al., 1998). The proteins extracted from the leaves of Clivia miniata and Leucojum vernum demonstrated the best activity (Eichhorn et al., 1998). Sixteen years further on, and the first NOMT, classified as a class I O-methyltransferase, was cloned and a detailed characterization of the step was performed during the assembly of the N. aff. pseudonarcissus transcriptome (Fig. 1) (Kilgore et al., 2014). Several orthologues with various substrate specificities and regioselectivities were characterized from Lycoris radiata, L. aurea, and L. longituba (Sun et al., 2018; Li et al., 2019; Li et al., 2020). The W50M/A53N/Y186K triple variant of Galanthus elwesii NOMT showed that the inversion of regioselectivity from 1:99 to 94:6 (para/meta) can be achieved through specific mutations and coordinating Ni2+ instead of Mg2+ as the metal ion partner (Su et al., 2022).
Norbelladine or 4'-O-methylnorbelladine as key intermediates
Although early isotope labelling studies established norbelladine as the first intermediate for the downstream pathways, the literature related to NOMT promiscuity raises a doubt about the order of the reactions leading to the formation of norbelladine and its methylated form. Indeed, the first NOMT candidate studied in 1963 was shown to methylate dopamine, which is a hydroxylated form of tyramine (Mann et al., 1963). NOMTs from L. radiata and L. aurea catalyse the O-methylation of 3,4-DHBA and caffeic acid in addition to norbelladine. Hence, other precursors, such as vanillin and isovanillin (the methylated products of 3,4-DHBA), could condense with tyramine to yield 3'-O-methylnorbelladine and 4'-O-methylnorbelladine, respectively, suggesting a more complex metabolic route (Sun et al., 2018; Li et al., 2019). Furthermore, O-methylation of other norbelladine derivates such as N-methylnorbelladine was observed in some of the studies mentioned above (Eichhorn et al., 1998; Kilgore et al., 2014). NR was shown to catalyse the condensation and reduction of isovanillin and tyramine to form 4'-O-methylnorbelladine, but a lower yield was observed compared with norbelladine synthesis (Kilgore et al., 2016b). The ability of NBS to catalyse the condensation of methylated precursors, such as vanillin and isovanillin, with tyramine remains to be tested.
Oxidative phenol coupling to branch into multiple directions
In plants, phenol coupling is observed in the synthesis of various specialized metabolites including lignans, flavonoids, and alkaloids. It is a key step in the synthesis of AAs. Following this reaction, the basic alkaloid structures undergo further changes to produce a range of distinct alkaloid compounds. Oxidative phenol coupling involves the formation of C–C and C–O bonds primarily catalysed by cytochrome P450 (CYP), with laccases and peroxidases playing a role in some cases. These enzymatic reactions are highly regio- and stereoselective, contributing significantly to the production of specialized metabolites (Hüttel and Müller, 2021). Barton and Cohen (1957) were the first to show evidence of phenol oxidation in the formation of AAs. They proved through radiolabelled studies that 4'-O-methylnorbelladine, not O,N-dimethylnorbelladine or N-methylnorbelladine, underwent the phenol coupling reaction to synthesize AAs, such as galanthamine and haemanthamine, in N. pseudonarcissus (Barton and Kirby, 1962; Barton et al., 1963). Depending on the C–C bond formation, three types of phenol couplings were suggested, namely para–orthoʹ, ortho–paraʹ, and para–paraʹ (Barton et al., 1962b, 1963; Fuganti and Mazza, 1971; Eichhorn et al., 1998). The formation of these bonds and the details of the biochemical reactions are well described in various literature reviews (Bastida et al., 2006; Jayawardena et al., 2024).
Transcriptome assembly from N. aff. pseudonarcissus and correlation analysis with NOMT led to the first characterization of gene candidates categorized under a novel CYP subfamily, named CYP96T (Kilgore et al., 2016a). Enzymatic characterization of the candidate CYP96T1 showed that this enzyme catalyses para–paraʹ coupling [(10bS,4aR)- and (10bR,4aS)-noroxomaritidine] of 4'-O-methylnorbelladine as the major reaction (Fig. 1), leading to AAs such as crinine, montanine, and pretazzetine, and para–orthoʹ phenol coupling (nornarwedine) as a minor reaction, leading to galanthamine (Kilgore et al., 2016a). In silico modelling, dynamics, and simulations provided an atomic understanding of the C–N coupling, and C–C bond formation that follows a diradical mechanism in the active site of CYP96T1 (W. Peng et al., 2023).
Recently, Mehta and colleagues conducted an interesting study that expanded our understanding of CYP96T enzymes in AA biosynthesis (Mehta et al., 2024). They used a combined approach of stable isotope labelling and transcriptome analysis, followed by co-expression analysis, to identify potential genes for the phenol coupling of 4'-O-methylnorbelladine. Their research suggests that three different CYP96T types are involved in para–orthoʹ, ortho–paraʹ, and para–paraʹ phenol couplings. They confirm that CYP96T1 catalyses para–paraʹ coupling, and propose that CYP96T6 leads to para–orthoʹ phenol coupling, while CYP96T5 catalyses ortho–paraʹ coupling (noroxopluviine) leading to lycorine-type AAs (Fig. 1). They also present evidence that these enzymes could be modified to alter their regioselectivity; that is, substituting Leu308 with alanine on the para–paraʹ coupling enzyme CYP96T1 yielded the same catalytic capacity as the para–orthoʹ oxidative coupling enzyme CYP96T6. If confirmed, these results will shed light on the divergent regioselectivity of CYP96T, and on the means to achieve AA molecular diversity.
Paths to galanthamine, haemanthamine, and lycorine. Intermediate compounds formed by the oxidative phenol couplings undergo further chemical changes, such as hydroxylation, methylation, reduction, oxidation, condensation, and oxygen bridge formation (Kilgore and Kutchan, 2016). Early isotope labelling and organic synthesis have helped build up multiple hypotheses for the synthesis of the intermediate and downstream metabolites, providing the basis to interpret the biosynthetic path of newly discovered compounds (Barton et al., 1962a; Eichhorn et al., 1998; Berkov et al., 2020). An alternative way has been to compare structures and reactions of the AA pathway with specialized metabolic pathways from other plant families, as this provides strong hints on the candidate enzymes. For example, enzyme families such as aldo–keto reductases (AKRs), SDRs, CYP450 monooxygenases such as CYP71, O- and N-methyltransferases (OMT, NMT), and 2-oxoglutarate-dependent dioxygenases (ODDs) are known plant enzyme superfamilies which catalyse multiple biochemical reactions diversifying alkaloid structures (Kilgore and Kutchan, 2016).
The first molecular evidence of enzymes involved in the AA downstream pathway came from studies of NR catalysing noroxomaritidine to oxomaritine, and of vittatine 11-hydroxylase catalysing vittatine to 11-hydroxyvittatine (Kilgore, 2015; Kilgore et al., 2016b). Vittatine 11-hydroxylase is an ODD homologous to an enzyme characterized in Pisum sativum that produces gibberellin (Kilgore, 2015). Isotope feeding of multiple tissue sections of Narcissus cv. ‘Tête-à-Tête’ and correlation analysis of the transcriptome suggested the involvement of multiple enzymes, such as SDR, AKR, OMT, NMT, CYP71, and ODD, in the synthesis of haemanthamine and galanthamine from 4'-O-methylnorbelladine (Fig. 1) (Mehta et al., 2024). The OMT proposed to catalyse the O-methylation of 11-hydroxyvittatine to yield haemanthamine is an orthologue to N4OMT, and the NMT catalysing nornarwedine to narwedine is a γ-tocopherol methyltransferase, homologous to an enzyme involved in colchicine synthesis (Nett et al., 2020; (Mehta et al., 2024). As the information regarding the transcripts and amino acid sequences is not yet available, it is difficult to discuss these enzymes, their mechanism, or their phylogenetic relationships further.
Future directions for enzyme discovery
On the path to discover novel enzymes, several future directions should be considered, including deepening our knowledge of already characterized steps. Further research should focus on testing and validating experimentally various hypotheses, such as resolving the involvement of multiple precursors in the formation of the first intermediates (Li et al., 2019). Furthermore, it will be important to study substrate specificity and promiscuity of O- and N-methyltransferases, hydroxylases, and dehydrogenases discovered in the early pathway, in the context of downstream steps.
Involvement in defence and in other pathways
The precursor pathway, which consists primarily of the phenylpropanoid pathway and tyramine, is not only responsible for the synthesis of alkaloids in plants, but also contributes to the production of other defence chemicals, such as lignans, flavonoids, and coumarins (Fig. 1) (de Vries et al., 2021). The phenylpropanoid pathway has multiple functions, highlighting the versatility of plant metabolic routes and their importance in protecting plants against herbivores and pathogens, helping them adapt to various environmental conditions (Dong and Lin, 2021). Understanding the synchronization of the production of different classes of specialized metabolites may help in discovering promiscuous enzymes which co-evolved in these multiple branches. For example, C. roseus 16-hydroxytabersonine-O-methyltransferase catalyses the O-methylation of both flavonoid and alkaloid synthesis, giving some insights into the evolutionary relationships of multiple pathways related to plant chemical defences (Lemos Cruz et al., 2023).
There could be relationships not only with non-alkaloid pathways, but also between some major AA groups or with other alkaloids. Cherylline and norbelladine, which do not undergo phenol coupling, could have evolved independently from other AA groups (Desgagné-Penix, 2021; Jayawardena et al., 2024). Moreover, there are other alkaloid groups reported in the Amaryllidaceae family, such as sceletium, phthalideisoquinoline, benzyltetrahydroisoquinoline, β-carboline, and aporphine alkaloids, also produced by other plant groups, such as Sceletium, Papaveraceae, and Fumariaceae (Fig. 1). The elucidated pathways of these multiple non-AA groups may help identify more candidate enzymes associated with the production of AAs.
Studying evolution of plant pathways would contribute to reinforce our knowledge on AA biosynthesis in planta. There are studies on the evolutionary origin of a few alkaloid groups such as BIA in the plant kingdom, yet no studies are available for AAs, with the exception of some studies on alkaloid diversity within the family, or within a genus such as Narcissus (Liscombe et al., 2005; Rønsted et al., 2012; Berkov et al., 2014). By investigating across different plant species, we can also gain new insights, improve our understanding of AAs, and provide a broader perspective on alkaloid biosynthesis in plants in general. For instance, as the biosynthesis of norbelladine follows a similar pathway to that of BIAs and PIA, this suggests a possible shared evolutionary history or convergent evolution.
Recently, transient expression approaches such as agroinfiltration and viral-induced gene silencing (VIGS) contributed to the discovery of specialized metabolite biosynthesis pathways, such as colchicine biosynthesis, and to the discovery of a serpentine synthase gene in C. roseus (Nett et al., 2020; Yamamoto et al., 2021). In AA biosynthesis, agroinfiltration was only used in one study, producing galanthamine and haemanthamine in Nicotiana benthamiana (Mehta et al., 2024). Application and establishment of VIGS in Amaryllidaceae plants were conducted in Narcissus tazetta for silencing MYB3 in relation to flavonoid biosynthesis, and in Lycoris chinensis for silencing Cloroplastos Alterados 1 (CLA1) and Phytoene Desaturase (PDS) genes. However, the use of VIGS for characterizing AA biosynthesis has not yet been reported (Zhou et al., 2021; Cheng et al., 2023).
Directions for further characterizations of enzymes
Enzyme structure is elucidated through techniques such as X-ray crystallography, Raman spectroscopy, or cryo-EM. There is a scarcity of enzyme crystal structures in AA biosynthesis which makes it difficult to understand their molecular mechanisms. Currently, there is only one crystallized structure of an enzyme related to an AA pathway in the Protein Data Bank (PDB), namely NR from N. aff. pseudonarcissus in complex with NADP+ and tyramine or other substrates (PDB: 5FEU, 5FF9, 5FFF) (Kilgore et al., 2016a). A conference abstract mentions the elucidation of the crystal structure of N4OMT from L. longituba, but there is no further information as of yet (Hnin et al., 2023). Advanced protein structure prediction tools such as DeepMind Alphafold2 will contribute to overcome the gap of the availability of crystallized structures (Jumper et al., 2021; C.-X. Peng et al., 2023). Although those tools are not a replacement for experimental evidence, examples such as the molecular dynamics of CYP96T1 and prediction of the effects of mutations on the inversion of regioselectivity of NOMT were achieved based on the protein structures predicted by Alphafold (Su et al., 2022; W. Peng et al., 2023).
In addition, detailed kinetic studies are required to improve our understanding of the catalytic efficiencies, substrate specificities, and regulatory mechanisms. Only then can enzyme activity be optimized with directed evolution and rational design. Surprisingly, not much effort has been put into improving the activity of enzymes that are responsible for producing alkaloids in Amaryllidaceae, with the exception of NOMT engineered by Su et al. (2022). Such research may lead to the development of biotechnological approaches for increasing the production of specific alkaloids or even the creation of new compounds (Boccia et al., 2022).
Cellular and tissue organization of the pathway
This section focuses on the molecular regulation and organization of the pathway. Understanding the mechanisms that regulate metabolite biosynthesis at the plant and cell level is crucial for its advancement. Unlike some other well-studied medicinal plants, there is limited literature available for the Amaryllidaceae family.
Organ-specific expression and subcellular localization of NBS, NR, N4OMT, and CYP96T
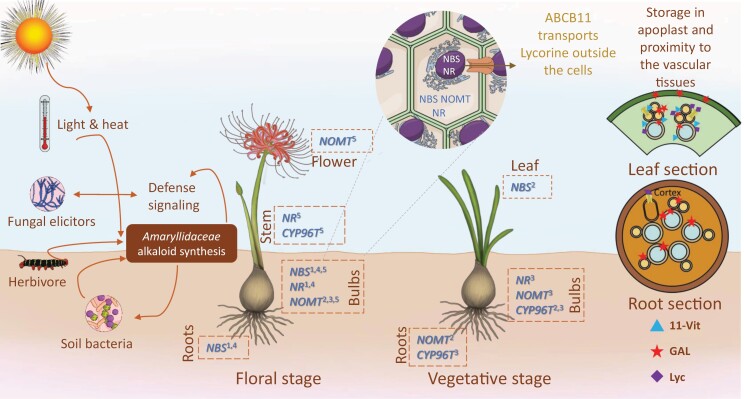
Knowledge of the subcellular localization and organ-specific expression of genes and proteins involved in specialized metabolite biosynthesis provides information on its spatial organization and regulation (Watkins and Facchini, 2022). The overall compartmentalization and regulation of the alkaloid pathways have been well described in some medicinal plants such as P. somniferum or C. roseus (Watkins and Facchini, 2022), showing that there is no common compartmentalization and regulation to plants. This highlights the need for studies on compartmentalization in Amaryllidaceae. Enzyme subcellular localization and the gene expression pattern over different tissues and developmental stages have been described in N. pseudonarcissus, L. radiata, L. longituba, and L. aestivum (Fig. 2). NBS is expressed mainly in bulbs of N. pseudonarcissus sampled at the floral stage and in bulbs and roots of L. aestivum and N. papyraceus at the floral stage, while it is enriched in leaves of L. longituba sampled at the vegetative stage (Singh et al., 2018; Li et al., 2020; Tousignant et al., 2022; Majhi et al., 2023). The expression of NR, also involved in norbelladine synthesis, is higher in bulbs during the floral stage of L. aestivum and N. papyraceus, and during the vegetative stage of L. radiata (Park et al., 2019; Majhi et al., 2023), but is increased rather in the stem of N. pseudornarcissus during the floral stage (Singh and Desgagné-Penix, 2017). N4OMT was reported as expressed mainly in bulbs of N. pseudonarcissus and L. radiata in the vegetative stage, in bulbs and flowers of N. aff. pseudonarcissus at the floral stage, and in bulbs and roots of L. longituba in the vegetative stage (Kilgore et al., 2014; Singh and Desgagné-Penix, 2017; Park et al., 2019; Li et al., 2020). In the case of CYP96T1, the highest expression was observed in the floral stems of N. pseudonarcissus in the floral stage, in roots and bulbs of L. radiata of the floral stage, and in bulbs of L. longituba in the vegetative stage (Singh and Desgagné-Penix, 2017; Park et al., 2019; Li et al., 2020). Overall, these studies suggest that these four key enzymes are often detected in bulbs, although there are differences between species and developmental stages. (Mehta et al., 2024) performed a detailed tissue analysis and argued that biosynthesis of AA starts in the leaf bases (newly forming tissues in the bulb), where they detected expression of most of the genes responsible for AA biosynthesis, starting from 4'-O-methylnorbelladine (NBS and N4OMT were not included in that study). They propose that AA biosynthesis, starting from the phenol coupling reaction, primarily occurs in leaf bases. Even though more evidence is needed to prove that hypothesis, this conclusion is consistent with observations from alkaloid biosynthesis pathway in other plants, such as Veratrum nigrum and Phlegmariurus tetrastichus (Nett et al., 2021; (Mehta et al., 2024).
Fig. 2.
A summary of Amaryllidaceae alkaloid (AA) metabolism, representing environmental stimuli initiating the biosynthesis, gene expression, and protein expression of genes associated with the AA pathway, and accumulation of a few AAs at the tissue level. 1Leucojum aestivum, 2Lycoris longituba, 3Lycoris radiata, 4Narcissus papyraceus, 5Narcissus pseudonarcissus. Abbreviations are given in the text.
At the cellular level, L. aestivum and N. papyraceus NBS and NR are localized in the cytoplasm and nucleus (Majhi et al., 2023), while L. longituba N4OMT is present only in the cytoplasm (Li et al., 2020). CYP96Ts are membrane-bound proteins that are probably accumulating in the endoplasmic reticulum membrane, even though this has not been verified yet. Overall, these findings suggest that biosynthesis of AAs may start in the cytoplasm of cells of leaf bases. In the BIA pathway of P. somniferum, NCS and multiple other enzymes such as OMT were detected mainly in the phloem sieve elements, and NMTs in laticifers from leaves or stems; but gene expression principally happens in the phloem companion cells (Beaudoin and Facchini, 2014). Further studies need to be conducted to explore these aspects regarding AAs.
Accumulation (storage) of alkaloids
Over 20 000 plants exude latex or mucilage upon physical damage or other interactions with the environment (Kekwick, 2002; Cui, 2019). The role of latex in storage and transport of alkaloids has been brought to light in medicinal plants such as C. roseus and P. somniferum (Beaudoin and Facchini, 2014; Watkins and Facchini, 2022). Plants of Amaryllidaceae secrete mucilage, which can cause skin irritations, when damaged by physical forces (Santucci and Picardo, 1992). In one study, narciclasine was isolated as a functional compound from the mucilage of N. tazetta which inhibited the seed germination and growth of rice and Chinese cabbage (Bi et al., 1998). In addition to mucilage, vascular tissues, such as xylem and phloem, are involved in the transport and storage of precursors and alkaloids. Some major reactions of alkaloid biosynthesis have been detected inside these vascular elements (Watkins and Facchini, 2022). ‘Phloem sap’, or most probably mucilage, analysis of Hippeastrum papilio revealed that it was rich in galanthamine [30.2% of the total ion chromatogram (TIC)], haemanthamine (15.5% of TIC), and 11β-hydroxygalanthamine (3.6% of TIC) (Haist et al., 2024).
Wang et al. (2007) studied the tissue distribution of galanthamine in L. aurea at the vegetative stage using fluorescent signals emitted by galanthamine They suggested that AAs may be stored in the apoplast of the tissues, mainly in the cell walls. According to the study, the primary organ of accumulation is leaf scales, and galanthamine is present in the cell walls of vascular bundles, mesophyll cells between vascular bundles, and epidermal cells of mature leaves. Multiple MS imaging (MSI) of the leaf cross-sections of N. papyraceus indicated that lycorine and 11-hydroxyvittatine are primarily found in the vicinity of vascular tissues, supporting the previous research on galanthamine accumulation (Mehta et al., 2024). Furthermore, tissue staining with Dragendorff’s reagent of H. papilio indicated that alkaloids are more concentrated in vascular bundles, vacuoles, and intracellular spaces (Haist et al., 2024). These studies indicate that AAs may be mainly produced in specialized cell types of vascular tissues, or in their proximity, and stored in the extracellular matrix such as the apoplast, highlighting the importance of the cellular transport of AAs.
Transport (trafficking) of alkaloids
Takos and Rook (2013) suggested that AA glycosides, such as lycorine-1-O-β-d-glucoside, may be the form of transportation of AAs, increasing the solubility and minimizing the toxicity. Extracellular transport may be required to protect the plant from the toxicity of the produced specialized metabolites. Different transporters involved in alkaloid trafficking have been characterized in other medicinal plants such as C. roseus, and Coptis japonica. They fall under transporter families such as ATP-binding cassette (ABC), multidrug and toxic compound extrusion (MATE), and purine uptake permease (PUP) (Shitan et al., 2014). There is only one published transporter related to Amaryllidaceae at present (R. Wang et al., 2021). The ABC transporter ABCB11 is associated with the plasma membrane and transports lycorine outside of the cell in L. aurea (Fig. 2). An in situ hybridization technique revealed that this transporter is predominantly expressed in the phloem of leaves and bulbs, as well as in the cortical cells of roots of L. aurea, supporting the hypothesis that AAs are produced in cells of leaf bases and stored in the apoplast (R. Wang et al., 2021). A comparative transcriptomic study related to methyl jasmonate (MJ) treatments, known to induce specialized metabolite production, showed changes in the expression level of 138 transporter genes. These transporters include ABC transporters (20; 14.49%), amino acid/peptide/protein transporters (23; 16.67%), and drug transporters (11; 7.97%). These changes could provide indications of AA transporters (Li et al., 2021). In conclusion, further studies that combine the characterization of enzymes, transporters, and AAs in planta will provide a mechanistic understanding that will contribute to enhancing metabolic engineering possibilities.
Insight into regulation of Amaryllidaceae alkaloid biosynthesis from in vitro culture studies
Field or greenhouse culture represents a simple approach for mass cultivation if not in competition with nutritional crops. It enables the control of environmental factors, such as nitrogen uptake, modulation of storage temperature, light wavelength, potting media, and application of fungicides, which may influence accumulation of specialized metabolites (El-Naggar and El-Nasharty, 2009; Lau et al., 2014; Zaragoza-Puchol et al., 2021). However, harvesting from cultivated Amaryllidaceae often leads to a lower yield compared with wild plants (Jin, 2013; Reis et al., 2019) because our knowledge on regulation of the biosynthetic pathways is not complete. As an alternative to field- and greenhouse-grown plants, in vitro culture enables the exploration of the effect of many more factors simultaneously.
Current methods of in vitro culture
In vitro culture was already used 70 years ago as a means of cell-free purified enzymes, from Nerine bowdenii flowering bulbs (Mann et al., 1963). The following years were unsparing in different approaches and innovations. In vitro cultivation as a means for production of AAs is rather a long and contamination-prone process whose success depends on the species, the tissue and sample quality, the growth media, the time of acclimization, and many other unknown factors. The selection of the primary plant material (tissue and clone origin) appears to have a crucial effect on the AA yields (Bogdanova et al., 2009; Georgiev et al., 2020). This emphasizes the need for more efforts in the selection and study of high alkaloid-producing cultivars. It also suggests that AAs are produced by specialized differentiated cells of specific tissues, in response to environmental factors, and that modulation of their production is possible only within this frame.
Biotic and stresses have been the subject of numerous in vitro culture studies (Fig. 2). The application of fungal elicitors on L. radiata plant cultures induced the production of AA precursors (Zhou et al., 2020). Bacterial synthetic communities applied to in vitro cultures of L. radiata suggested an interplay between AA production, bacterial endophytes, and fungal pathogens, and illustrated that AA biosynthesis could be better understood in the context of biotic interactions (Erb and Kliebenstein, 2020; Zhou et al., 2023). Interestingly, a study reported that an L. aestivum endophytic bacterium Paenibacillus lautus, that was able to produce a wide range of plant hormones simultaneously, induced higher production of AAs by the plant but also endogenously produced its own, such as galanthamine, lycorine, ismine, lycoramine, galanthine, haemanthamine, homolycorine, 1,2-dihydrochlidanthine, and hippeastrine (Ptak et al., 2022). Others have studied the effect of hormones such as jasmonic acid (JA) and 1-naphthaleneacetic acid (NAA), or specific light waves on different plant tissue (Fennell et al., 2003; Kilgore and Kutchan, 2016; Berkov et al., 2020; Ptak et al., 2020; Meena et al., 2022). Different auxins, picloram, meta-topolin, and thidiazuron were shown to regulate the regeneration rate and alkaloid profile in L. aestivum, R. bifida, and other species (Ptak et al., 2017; Reis et al., 2019). Specific combinations of hormones [6-benzylaminopurine (BAP), kinetin (KIN), and NAA] led to a specific AA increase in micropropagated Caliphruria tenera plants (Trujillo Chacón et al., 2023). Treatment of an in vitro culture of C. ×powellii ‘Album’ with different conditions (light, dark, or auxins) led to variable tissue differentiation and growth, and a rather wide range of AAs such as lycorine, crinine, and cherylline types (Koirala et al., 2023). In calli culture, light and auxin both modulated the production of many alkaloids, and AA biosynthetic genes in vitro, highlighting the delicate balance between stress and growth that must be achieved for calli to produce AAs.
All these studies emphasize the importance of discovering the biotic and abiotic elements that are involved in partial or complete activation of AA biosynthesis. Understanding the quality, quantity, and timing of the elicitors required to boost AA production is key to advance the yield range. In contrast to production for commercial purposes, the elucidation of the biosynthetic pathway does not require that AAs are produced in large amounts. It requires subtle differences in production between samples used in comparative omics studies. In this regard, harvesting samples from in vitro culture offers several advantages, such as homogeneous growth and controlled variables. The concomitant analysis of AA yield, biosynthetic genes, and culture conditions is the foundational knowledge that should be acquired to obtain a high yield of AAs in the future.
Elucidation of biosynthetic pathways in in vitro culture
There are various environmental factors that can stimulate the production of alkaloids in plants. The previous section provided a non-exhaustive list of environmental stimuli used in in vitro culture that affect alkaloid biosynthesis in the Amaryllidaceae family. Transcription factors (TFs) are proteins that bind to specific DNA sequences, such as enhancer or promoter regions, initiating the transcription process that converts DNA to RNA. They coordinate the biosynthesis of specialized metabolites in response to environmental and developmental stimuli in plants (Ziegler and Facchini, 2008; Li et al., 2024). There are many families of TFs studied in plants such as APETALA2/Ethylene-Responsive Factor (AP2/ERF), WRKY, and basic helix–loop–helix (bHLH) that contribute to alkaloid biosynthesis (Yamada and Sato, 2021). Also in upstream defence signalling, mechanisms such as JA signalling modulate the expression of TFs in response to environmental stresses (Wang et al., 2023). Transcriptome analysis of various tissues of L. longituba revealed a high percentage of TFs such as bHLH, AP2/ERF, NAC, and TCP in this galanthamine-producing plant (Li et al., 2020). A comparative transcriptomic study showed that MJ treatment was associated with an up-regulation of AA-related genes and of many TFs, such as WRKY (26 out of 32), AP2/EF (21 out of 25), and myeloblastosis (MYB) (all 14). As phenylpropanoid- and flavonoid-related genes were also up-regulated in this experiment, the identification of TFs specific to AA synthesis was not possible (Li et al., 2021). Transcriptomic analysis related to floral development and anthocyanins in L. chinensis, L. longituba, L. radiata, and L. sprengeri identified multiple TFs, such as MYB, bHLH, AP2/ERF, Cys2–His2 zinc finger (C2H2), NAM, ATAF1/2, and CUC2 (NAC); however, this study did not focus on AA synthesis (Yue et al., 2019; N. Wang et al., 2021; F. Yang et al., 2021; F. Zhang et al., 2022). Transcriptomic analysis N. pseudonarcissus calli and field-grown plants also mentions the identification of different TFs (Ferdausi et al., 2021). None of the identified sequences mentioned in all the literature detailed above are publicly available. Although most of these studies are related to anthocyanin or flavonoid synthesis in Amaryllidaceae plants, a deeper analysis could provide new insight into the AA pathway, as multiple specialized metabolite pathways are interconnected (Fig. 1). Recently, expression of heat shock factor (HSF) TFs was characterized in various tissues and flower developmental stages of L. radiata, and studied in response to hormones and abiotic stresses (Wang et al., 2024). The expression of several HSF genes, especially LrHSF5, was associated with plant development and response to abiotic and hormone stresses. The correlation with AAs remains to be characterized further. Interestingly, a recent study on TFs related to MJ treatment in L. aurea helped identify a MYC TF (LaMYC2) that up-regulated the biosynthesis of lycorine. The study demonstrated that LaMYC2 binds to the E-box motifs of the promoter region of the TYDC gene of L. aurea involved in formation of the precursors of AA biosynthesis (Zhou et al., 2024).
JA triggers the activation of TFs in response to environmental stresses (Goossens et al., 2017). Jasmonate ZIM domain (JAZ) proteins are key components in the positive regulation of the interaction of JA signalling. In the Amaryllidaceae family, identification and characterization of JAZ genes have been performed in one study in L. aurea (Wang et al., 2020). These authors cloned and characterized seven JAZ genes, and showed that the expression of the JAZ genes varied among tissues. Most of them were highly expressed in flowers, and JAZ 2, 5, and 6 were highly expressed in leaves. External MJ treatment up-regulated the expression of almost all of the JAZ genes and, at the protein level, JAZ 11 was expressed in both the nucleus and cytoplasm while JAZ 22 and 5 were expressed in the cytoplasm and JAZ 3, 4, 6, and 7 were expressed in the nucleus (Wang et al., 2020). These authors have not studied the relationship of these JAZ genes with AA synthesis, but all the data (transcript, protein sequences) are available in public databases for further studies.
Until now, AA biosynthetic genes have been mostly elucidated one gene at a time at the molecular level, based on assumption of candidate genes identified by homology in transcriptomic data from a plant or its tissues grown in strictly specific conditions (Nguyen and Dang, 2021; Majhi et al., 2023). This approach, although very useful, limits the discovery of the full potential of enzymes and of their physiological relevance. This is because the enzymes could be involved in multiple metabolic pathways and play important roles in their interaction.
In vitro culture offers a controlled platform that could help connect alkaloid, terpenoid, and phenolic compound pathways and reveal new ways to optimize AA production, but also understand the implication of AAs in cellular functions and defence-related mechanisms (Muro-Villanueva and Nett, 2023). Understanding the elements that modulate AA production would help identify specific conditions permissive or restrictive to their accumulation. These conditions and their transcriptomic and metabolomic consequences could be classified into biotic and abiotic elements, stored, and tracked in a database that would help researchers link triggers of AA production or of precursors, and thus understand new elements in the biosynthesis pathway.
Available multi-omics information on Amaryllidaceae species
The genes involved in biosynthetic pathway may be organized in gene clusters, as is the case for several well-studied plant species, such as Zea mays (2,4-dihydroxy-l,4-benzoxazin-3-one and 2,4-dihydroxy-7-methoxy-l,4-benzoxazin-3-one biosynthesis; Frey et al., 1997), Oryza sativa (momilactones and phytocassanes; Otomo et al., 2004; Shimura et al., 2007), Papaver somniferum (noscapine; Winzer et al., 2012), Arabidopsis thaliana (thalianol and marneral; Field et al., 2011), and Solanum spp. (terpenes; Matsuba et al., 2013). Unfortunately, the resources that allowed the discovery and characterization of these gene clusters, such as linkage maps and genome assemblies, are lacking for Amaryllidoideae species.
Genomic data
The cost of sequencing the nuclear genome of these species is prohibitive due to the large and complex genomes of members of this subfamily. For instance, their 1C genome size (which indicates the amount of DNA in a haploid nucleus) ranges from 6.03 Gbp in Chlidanthus fragans to 80.5 Gbp in Galanthus lagodechianus (Zonneveld et al., 2003, 2005; Leitch et al., 2019). In comparison, the Z.mays 1C genome is 2.65 Gbp and that of A. thaliana is 157 Mbp (Vu et al., 2017; Leitch et al., 2019). Also, their ploidy levels are so variable that the same species of the genus Narcissus has diploid, triploid, and tetraploid cultivars (Sochacki et al., 2022), while the ploidy of the genus Crinum varies up to octoploid (Jones and Smith, 1967; Wahkstrøm and Laane, 2009). At present, the only genomes assembled and published in the Amaryllidaceae family are those of garlic (Allium sativum; Sun et al., 2020) and onion (Allium cepa; Finkers et al., 2021), both diploid species with genome sizes of 16.24 Gbp and 13.6 Gbp, respectively. However, the Allioideae subfamily does not produce AAs, limiting the interest in use of these recent genomic resources for the study of AA biosynthesis.
As regards Amaryllidoideae, a nuclear genome assembly for Narcissus pseudonarcissus was recently submitted to the NCBI Genome database (accession JAVXUK01). However, it may not be the final version, as it consists of 3 138 040 scaffolds, has no complete or partial chromosome, and no publication is associated with it. Also, four Amaryllidaceae genome sequencing datasets are available from the Ruili Botanical Garden (Liu et al., 2019); however, the species from which the datasets originated was not provided. As these samples are part of the ‘10 000 Plant Genomes Project’ (Cheng et al., 2018; https://db.cngb.org/10kp/), they should be clearly identified and the assemblies available in the near future. Finally, there are several chloroplast genome assemblies available for this subfamily, and they have been used for phylogenetic studies (Hori et al., 2006; Jin et al., 2018; Dennehy et al., 2021; Konyves et al., 2021; Zhang et al., 2021; Cheng et al., 2022).
Transcriptomic and proteomic data
In the absence of genome assemblies, researchers have attempted to reconstruct the AA biosynthetic pathway using transcriptome sequencing combined with metabolomics or proteomics. Currently, there are transcriptomic data, in the form of raw reads or assembled transcriptomes, for 13 genera of this subfamily (Table 1), the first one being that of Lycoris aurea (Wang et al., 2013). Unfortunately, these data are not always made publicly available (Chang et al., 2011; Wang et al., 2017; Song et al., 2019; Xiang et al., 2022). In other cases, accession numbers or links to their datasets/assemblies are not provided (Pulman, 2014; Ferdausi et al., 2021) or contain mistakes (J. Yang et al., 2021, 2023; Ren et al., 2022), complicating the analysis. Park et al. (2019) published their transcriptome assembly for L. radiata in NCBI SRA, without the raw data, while J. Yang et al. (2021) reported the use of long- and short-read technology for the generation of a high-quality transcriptome assembly of Narcissus tazetta, but neither the final assembly nor the raw long reads were provided. As the use of long-read sequencing is new for Amaryllidoideae species, these datasets and assemblies should be published since they could help transcriptome and, in the future, genome annotations.
Table 1.
Transcriptomic studies from Amaryllidoideae species
| Species | Reference | Raw data | Assembly | Tissue | Developmental stage | Metabolomics |
|---|---|---|---|---|---|---|
| Amaryllis belladonna | One Thousand Plant Transcriptomes Initiative (2019) | ERR2040723 | LDMEa | Stem or leaf | NA | NA |
| Clivia miniata | Q.M. Wang et al. (2018) | PRJNA480383 | NA | Leaf | Mature striped plants, young leaves | NA |
| Y. Li et al. (2022) | PRJNA813401 | NA | Fruit peel, flower, leaf | NA | Anthocyanins, flavonoids, terpenes | |
| Crinum×powellii | Koirala et al. (2023) | PRJNA962562 | Koirala et al. (2023) | Leaf, bulb, basal plate, root, callus | Undifferentiated callus and 4-week-old plants | AA precursors and AAs |
| Galanthus elwesii | Kilgore et al. (2016a) | PRJNA306697 | b | Leaf, bulb, inflorescence | Adult, blooming | Galanthamine |
| G. sp. MBK-2015 | Kilgore et al. (2016a) | PRJNA306273 | b | Leaf, bulb, inflorescence | Adult, blooming | Galanthamine |
| Hippeastrum hybrid cultivar | X. Li et al. (2022) | PRJNA608969 | NA | Stamen | 3-year-old plants, blooming | NA |
| Y. Wang et al. (2018) | PRJNA322243 | NA | Flower | NA | NA | |
| H. vittatum | – | PRJNA862291 | NA | Bud | – | |
| Leucojum aestivum | Tousignant et al. (2022) | PRJNA720900 | NA | Bulb | Dormant | AAs |
| Lycoris aurea | Wang et al. (2013) | PRJNA188333 | NA | Stem, flower, leaf | Bud, blooming, wilting | NA |
| Ren et al. (2022) | PRJNA574869c PRJNA579847c |
NA | Bulb | Cross-cut bulb to bulblet formation | Untargeted metabolomics, sugar content, JA, ABA, and ethylene | |
| L. chinensis | F. Zhang et al. (2022) | PRJNA847051 | NA | Shoot apical meristem | 1- to 4-year-old bulbs | NA |
| L. incarnata | – | PRJNA639315 | NA | Bulb | Vegetative stage | NA |
| L. longituba | F. Zhang et al. (2022) | PRJNA490415 | NA | Tepal | Small, medium, and opening bud | Floral volatile organic compounds, anthocyanins |
| Li et al. (2020) | PRJNA590043 | NA | Bud, leaf, root | 3 years old | Galanthamine | |
| Li et al. (2021) | PRJNA720237c | NA | Seedling | 7 d old | AA precursors and AAs | |
| L. radiata | N. Wang et al. (2021) | PRJCA006232d | NA | Petal | Flowering | Protoanthocyanidins and anthocyanins |
| Park et al. (2019) | NA | PRJNA529664 | Bud, leaf, root | NA | Primary metabolites and galanthamine | |
| Xu et al. (2020) | PRJNA574731 | NA | Bulb | Dormancy, competence, bud initiation, bud enlargement, bulblet emergence and bulblet development | Hormones, starch, and soluble sugar | |
| L. sprengeri | F. Yang et al. (2021) | PRJNA714286 | NA | Petal | Adult, blooming | Anthocyanins, flavonoid-biosynthesis related metabolites, and brassinolide |
| Ren et al. (2022) | PRJNA574869c PRJNA579847c |
NA | Bulb | Cross-cut bulb to bulblet formation | Untargeted metabolomics, sugar content, JA, ABA, and ethylene | |
| Narcissus papyraceus | Hotchandani et al. (2019) | PRJNA407433 | NA | Bulb | Dormant | Heterocyclic compounds, lycorine |
| N. pseudonarcissus | Ferdausi et al. (2021); Pulman (2014) | PRJNA264603 | NA | Basal plate and callus | 4 months after planting | NA |
| Singh and Desgagné-Penix (2017) | PRJNA392294 | NA | Bulb | Adult, blooming | AAs, galanthamine | |
| X. Li et al. (2018) | PRJNA497707 | NA | Perianth and corona | S4 flowering stage | Carotenoids | |
| N. aff. pseudonarcissus | Kilgore et al. (2014) | PRJNA301357 | b | Leaf, bulb, inflorescence | Adult, blooming | Galanthamine |
| N. tazetta | Yang et al. (2023) | PRJNA891931c | NA | NA | NA | Flavonoids, proanthocyanins, and anthocyanin |
| Y. Zhang et al. (2022) | PRJNA855612 | NA | Corolla and tepal | From bud to decay | Carotenoid and flavonoid content | |
| – | PRJNA296436 | NA | Yellow petal and yellow corona | Early flowering | ||
| Ren et al. (2017) | PRJNA340092 PRJNA340090 |
NA | Tepal | 5, 12, and 20 d after planting | Chlorophyll, carotenoids, and flavonoids | |
| G. Wang et al. (2018) | PRJNA387061 | NA | Basal plate | 3-year-old bulbs | Flavonols | |
| Yan-Hong et al. (2019) | PRJNA487120 | NA | Bulb | 3-year-old bulbs | NA | |
| He et al. (2020a, b) | PRJNA523125 | GSE126727 | Corolla and petal | Early flowering and full bloom | NA | |
| J. Yang et al. (2021) | SUB10083597c PRJNA750844 |
NA | Corona and tepal | Adult, blooming | Flavonoids, carotenoids, chlorophyll, and volatile organic components | |
| N. viridiflorus | One Thousand Plant Transcriptomes Initiative (2019) | ERR2040725 ERR2040724 |
IQYY, XEUV, TRRQa | Young vegetative tissue and flower | NA | NA |
| Phycella aff. cyrtanthoides | One Thousand Plant Transcriptomes Initiative (2019) | ERR3487375 | DMINa | Young vegetative tissue | NA | NA |
| Rhodophiala pratensis | One Thousand Plant Transcriptomes Initiative (2019) | ERR2040726 | JDTYa | Young vegetative tissue | NA | NA |
| Traubia modesta | One Thousand Plant Transcriptomes Initiative (2019) | ERR2040727 | ZKPFa | Young vegetative tissue | NA | NA |
| Zephyranthes candida | Y. Zhang et al. (2022) | PRJNA796382 | NA | Flower and flower stalk | Adult, blooming | NA |
| Z. treatiae | One Thousand Plant Transcriptomes Initiative (2019) | ERR2040728 | DPFWa | Young vegetative tissue | NA | NA |
The accession number of the raw data, the assembly accession, the tissue and developmental stage sampled, and the metabolites quantified are included.
AA, Amaryllidaceae alkaloid; NA, not available
a Assembly available at GigaScience Database (http://gigadb.org/dataset/100627).
b Assembly available in the MedPlant RNASeq Database (https://medplantrnaseq.org).
c The wrong acces sion number was provided.
d Data available on https://ngdc.cncb.ac.cn/.
All transcriptome studies presented here were done using bulk RNA-seq. In some cases, a single tissue was sampled (Singh and Desgagné-Penix, 2017; Y. Wang et al., 2018; Hotchandani et al., 2019; One Thousand Plant Transcriptomes Initiative, 2019; Tousignant et al., 2022; Xiang et al., 2022). In others, tissue samples were pooled into a single library (Y. Li et al., 2022; Wang et al., 2013; C.H. Zhang et al., 2022). These tactics allowed the identification of several genes in the AA biosynthetic pathway, as well as genes in anthocyanin and phenylpropanoid pathways, and were sufficient for phylogenetic studies (Y. Wang et al., 2018; One Thousand Plant Transcriptomes Initiative, 2019). However, genes weakly expressed in a single tissue or cell type may have been missed. Coupling the study of multiple tissues and conditions with metabolomics enables co-expression analyses, using known genes of the pathway as bait to pull out new candidates from the transcriptome (Kilgore et al., 2014, 2016a; Koirala et al., 2023). This is potentiated by single-cell multi-omics, which was recently used in C. roseus and led to the identification of a new enzyme in the monoterpene indole alkaloid pathway (Li et al., 2023).
Comparative proteomic studies can help identify candidate enzymes in the biosynthetic pathway by analysing species that differ in their alkaloid composition. Of the three proteomic studies available for Amaryllidoideae, all of them have analysed Lycoris species (Ru et al., 2013; Jiang et al., 2021; Tang et al., 2023). The study by Tang et al. (2023) was the only one that focused on alkaloid biosynthesis. By comparing L. longituba, L. sprengeri, and L. incarnata, the authors were able to identify candidates for N4OMT and for the N-methyltransferase that converts norgalanthamine into galanthamine, but the sequences of these enzymes have not been published.
Multi-omics
In upcoming omics research, integrating transcriptomics and proteomics to compare tissues or populations with varying alkaloid contents (as reported for G. elwesii; Berkov et al., 2004) will be essential. It will determine whether the differences in alkaloid content are predominantly influenced by variations in the genes expressed, their expression levels within specific tissues or populations, or if these metabolic distinctions can be attributed to translational or post-translational mechanisms. Once genome sequencing becomes a more affordable avenue in the study of Amaryllidoideae species, transcriptomic and proteomic studies will help improve genome annotations. Omics toolsets also offer a powerful approach to study genetic polymorphism, evolution, and single nucleotide polymorphism in homologous genes between Amaryllidaceae species, and their link to present/absent enzymatic reactions and related metabolites (Stander et al., 2022; Méteignier et al., 2023). Then, comparative studies between species with different alkaloid compositions, or accumulating specific alkaloids in different amounts, will facilitate the search for the missing enzymes of the AA biosynthesis pathway. Furthermore, comparative genomics and phylogenetic analysis will help elucidate the evolutionary relationships between alkaloid biosynthetic pathways in different plant families, aiding in predicting undiscovered enzymes and pathways.
Importance of prediction tools and databases
Prediction tools of biosynthetic pathways and metabolic routes
Prediction tools, such as Plant Metabolic Network 15, RefMetaPlant, and MetaCyc, that forecast metabolic routes are improving the discovery of new pathways in many aspects (Caspi et al., 2020; Hawkins et al., 2021; Shi et al., 2024). The PathPred on Genome Japan tools helps predict pathways by machine learning (Moriya et al., 2010). Prediction deep learning tools could also help to discover uncharacterized plant metabolites. For example, searching for potent therapeutical compounds with similarities to AAs could help to suggest undiscovered AAs and identify their value (Sreeraman et al., 2023). A recent article describes a Self-driving Autonomous Machines for Protein Landscape Exploration (SAMPLE) platform designed to combine prediction and experimental automation to engineer proteins with zero human intervention for synthetic biology and pathway elucidation (Rapp et al., 2024). These new platforms provide insight for future scientific discoveries.
Public databases for Amaryllidaceae alkaloids
Many facets of AA biosynthesis are being covered in public database, including a TF database PlantTFDB 4.0 (Jin et al., 2016), Gene Ontology annotation through Planteome (Cooper et al., 2024), metabolomes through PMhub 1.0 or RefMetaPlant (Shi et al., 2024; Tian et al., 2024), transport through ChannelsDB (Špačková et al., 2024), and many more. However useful, these databases are not sufficient on their own to decipher AA biosynthetic pathways. A collaborative effort involving multiple research studies has been integrated into a single database that includes genomes of a few reference species, transcriptomic data in different conditions, and—most importantly—AA profiling in all available experiments. To understand the physiological fate of AAs and improve metabolic engineering strategies, data from proteomic analysis, in vitro assays, and propagation yield results according to various conditions should be further included. Such a united effort would allow gathering and visualizing valuable datasets in one platform, similarly to TAIR for Arabidopsis, The Bio-Analytic Resource for Plant Biology BAR, and Genevestigator for multiple species (Hruz et al., 2008; Lamesch et al., 2012; Waese and Provart, 2017). Building a network of AA researchers would not only allow cost sharing but also build stronger datasets and exchange of expertise and resources. Furthermore, multi-omic metadata gathered in a single platform along with datasets on biotic and abiotic experiments, phenotyping, and chemical profiling would allow faster discovery of AA enzymes and improve our definition of Amaryllidaceae plant interactions with their environment, a very useful piece of the puzzle to add to biosynthetic pathway discovery, and to in vitro production for higher AA production.
Conclusion
Knowledge of the complex genetic regulation, transport, and accumulation of AAs would solve complex questions concerning the chronological order in their synthesis and allow further technological advances such as metabolic engineering of in vitro tissues or heterologous systems.
In conclusion, the production of Amaryllidaceae alkaloids is a fascinating and intricate process that offers numerous opportunities for exploration and discovery. Studying these pathways not only sheds light on plant biochemistry, but also has implications for pharmacology and potential medicinal uses of these alkaloids. There is still much to uncover regarding the specialized metabolite production in Amaryllidaceae plants, such as identifying new enzymes, improving their activity, and understanding the interconnected pathways. The ongoing research in this area holds the possibility to unleash the full potential of these bioactive compounds for medicinal, agricultural, and industrial purposes.
Glossary
Abbreviations:
- AA
Amaryllidaceae alkaloid
- BIA
benzyl isoquinoline alkaloid
- COMT
catechol-O-methyltransferase
- 3,4-DHBA
3,4-dihydroxybenzaldehyde
- NBS
norbelladine synthase
- NCS
norcloclaurine synthase
- NOMT
norbelladine-O-methyltransferase
- N4OMT
norbelladine 4'-O-methyltransferase
- NR
noroxomaritidine reductase
- SDR
short-chain dehydrogenase
Contributor Information
Nuwan Sameera Liyanage, Department of Chemistry, Biochemistry and Physics, Université du Québec à Trois-Rivières, Trois-Rivières, QC, Canada.
Fatima Awwad, Department of Chemistry, Biochemistry and Physics, Université du Québec à Trois-Rivières, Trois-Rivières, QC, Canada.
Karen Cristine Gonçalves dos Santos, Department of Chemistry, Biochemistry and Physics, Université du Québec à Trois-Rivières, Trois-Rivières, QC, Canada.
Thilina U Jayawardena, Department of Chemistry, Biochemistry and Physics, Université du Québec à Trois-Rivières, Trois-Rivières, QC, Canada.
Natacha Mérindol, Department of Chemistry, Biochemistry and Physics, Université du Québec à Trois-Rivières, Trois-Rivières, QC, Canada.
Isabel Desgagné-Penix, Department of Chemistry, Biochemistry and Physics, Université du Québec à Trois-Rivières, Trois-Rivières, QC, Canada; Plant Biology Research Group, Trois-Rivières, Québec, Canada.
Ricarda Jost, La Trobe University, Australia.
Conflict of interest
No conflict of interest declared.
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) award number RGPIN-2021-03218 and the Canada Research Chair on plant specialized metabolism award number CRC-2018-00137. Warm thanks are extended to the Canadian taxpayers and to the Canadian government for supporting the Canada Research Chairs Program.
References
- APG. 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161, 105–121. [Google Scholar]
- Barton D, Cohen T.. 1957. Some biogenetic aspects of phenol oxidation. In: Festschrift Prof. Dr. Arthur Stoll zum siebzigsten Geburtstag. Basel: Birkhäuser, 117–143. [Google Scholar]
- Barton D, Kirby G.. 1962. Phenol oxidation and biosynthesis. Part V. The synthesis of galanthamine. Journal of the Chemical Society (Resumed) 806–817. [Google Scholar]
- Barton D, Kirby G, Taylor J.. 1962a. Origin of methylenedioxy-groups in nature. Proceedings of the Chemical Society 340–341. [Google Scholar]
- Barton D, Kirby G, Taylor J, Thomas G.. 1962b. Multiple labelling experiments in biosynthesis of Amaryllidaceae alkaloids. Proceedings of the Chemical Society 179. [Google Scholar]
- Barton D, Kirby G, Taylor J, Thomas G.. 1963. 866. Phenol oxidation and biosynthesis. Part VI. The biogenesis of amaryllidaceae alkaloids. Journal of the Chemical Society (Resumed) 4545–4558. [Google Scholar]
- Bastida J, Lavilla R, Viladomat F.. 2006. Chemical and biological aspects of Narcissus. The Alkaloids. Chemistry and Biology 63, 87–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battersby A, Fales H, Wildman W.. 1961. Biosynthesis in the Amaryllidaceae. Tyrosine and norbelladine as precursors of haemanthamine. Journal of the American Chemical Society 83, 4098–4099. [Google Scholar]
- Beaudoin GA, Facchini PJ.. 2014. Benzylisoquinoline alkaloid biosynthesis in opium poppy. Planta 240, 19–32. [DOI] [PubMed] [Google Scholar]
- Bendaif H, Melhaoui A, Ramdani M, Elmsellem H, Douez C, El Ouadi Y.. 2018. Antibacterial activity and virtual screening by molecular docking of lycorine from Pancratium foetidum Pom (Moroccan endemic Amaryllidaceae). Microbial Pathogenesis 115, 138–145. [DOI] [PubMed] [Google Scholar]
- Berkov S, Martínez-Francés V, Bastida J, Codina C, Ríos S.. 2014. Evolution of alkaloid biosynthesis in the genus Narcissus. Phytochemistry 99, 95–106. [DOI] [PubMed] [Google Scholar]
- Berkov S, Osorio E, Viladomat F, Bastida J.. 2020. Chemodiversity, chemotaxonomy and chemoecology of Amaryllidaceae alkaloids. The Alkaloids. Chemistry and Biology 83, 113–185. [DOI] [PubMed] [Google Scholar]
- Berkov S, Sidjimova B, Evstatieva L, Popov S.. 2004. Intraspecific variability in the alkaloid metabolism of Galanthus elwesii. Phytochemistry 65, 579–586. [DOI] [PubMed] [Google Scholar]
- Bi Y-R, Yung K-H, Wong Y-S.. 1998. Physiological effects of narciclasine from the mucilage of Narcissus tazetta L. bulbs. Plant Science 135, 103–108. [Google Scholar]
- Boccia M, Grzech D, Lopes AA, O’Connor SE, Caputi L.. 2022. Directed biosynthesis of new to nature alkaloids in a heterologous Nicotiana benthamiana expression host. Frontiers in Plant Science 13, 919443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova Y, Pandova B, Yanev S, Stanilova M.. 2009. Biosynthesis of lycorine by in vitro cultures of Pancratium maritimum L. (Amaryllidaceae). Biotechnology & Biotechnological Equipment 23, 919–922. [Google Scholar]
- Caspi R, Billington R, Keseler IM, Kothari A, Krummenacker M, Midford PE, Ong WK, Paley S, Subhraveti P, Karp PD.. 2020. The MetaCyc database of metabolic pathways and enzymes—a 2019 update. Nucleic Acids Research 48, D445–D453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno-Betanzos LY, Reyes-Chilpa R, Pigni NB, Jankowski CK, Torras-Claveria L, Bastida J.. 2021. Plants of the ‘Libellus de Medicinalibus Indorum Herbis’ from Mexico, 1552. Zephyranthes fosteri (Amaryllidaceae) alkaloids. Chemistry & Biodiversity 18, e2000834. [DOI] [PubMed] [Google Scholar]
- Chaichompoo W, Rojsitthisak P, Pabuprapap W, Siriwattanasathien Y, Yotmanee P, Suksamrarn A.. 2023. Alkaloids with cholinesterase inhibitory activities from the bulbs of Crinum× amabile Donn ex Ker Gawl. Phytochemistry 205, 113473. [DOI] [PubMed] [Google Scholar]
- Chaichompoo W, Rojsitthisak P, Pabuprapap W, Siriwattanasathien Y, Yotmanee P, Suksamrarn A.. 2024. Amaryllidaceae alkaloids from the bulbs of Crinum latifolium L. and their cholinesterase inhibitory activities. Phytochemistry 217, 113929. [DOI] [PubMed] [Google Scholar]
- Chang L, Chen J, Xiao Y, Xia Y.. 2011. De novo characterization of Lycoris sprengeri transcriptome using Illumina GA II. African Journal of Biotechnology 10, 12147–12155. [Google Scholar]
- Chen M-X, Huo J-M, Hu J, Xu Z-P, Zhang X.. 2018. Amaryllidaceae alkaloids from Crinum latifolium with cytotoxic, antimicrobial, antioxidant, and anti-inflammatory activities. Fitoterapia 130, 48–53. [DOI] [PubMed] [Google Scholar]
- Cheng G, Shu X, Wang Z, Wang N, Zhang F.. 2023. Establishing a virus-induced gene silencing system in Lycoris chinensis. Plants 12, 2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RY, Xie DF, Zhang XY, Fu X, He XJ, Zhou SD.. 2022. Comparative plastome analysis of three amaryllidaceae subfamilies: insights into variation of genome characteristics, phylogeny, and adaptive evolution. BioMed Research International 2022, 3909596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Melkonian M, Smith SA, et al. 2018. 10KP: a phylodiverse genome sequencing plan. GigaScience 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colque R, Viladomat F, Bastida J, Codina C.. 2002. Micropropagation of the rare Eucrosia stricklandii (Amaryllidaceae) by twin-scaling and shake liquid culture. The Journal of Horticultural Science and Biotechnology 77, 739–743. [Google Scholar]
- Cooper L, Elser J, Laporte M-A, Arnaud E, Jaiswal P.. 2024. Planteome 2024 update: reference ontologies and knowledgebase for plant biology. Nucleic Acids Research 52, D1548–D1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X. 2019. Mucilage secretion from plants: friends or foes? Molecular Plant 12, 16–17. [Google Scholar]
- Dennehy Z, Bilsborrow J, Culham A, David J, Konyves K.. 2021. The complete plastome of the South African species, Amaryllis belladonna L. (Amaryllidaceae). Mitochondrial DNA Part B: Resources 6, 3393–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgagné-Penix I. 2021. Biosynthesis of alkaloids in Amaryllidaceae plants: a review. Phytochemistry Reviews 20, 409–431. [Google Scholar]
- de Vries S, Fürst-Jansen JM, Irisarri I, Dhabalia Ashok A, Ischebeck T, Feussner K, Abreu IN, Petersen M, Feussner I, de Vries J.. 2021. The evolution of the phenylpropanoid pathway entailed pronounced radiations and divergences of enzyme families. The Plant Journal 107, 975–1002. [DOI] [PubMed] [Google Scholar]
- Ding Y, Qu D, Zhang K-M, Cang X-X, Kou Z-N, Xiao W, Zhu J-B.. 2017. Phytochemical and biological investigations of Amaryllidaceae alkaloids: a review. Journal of Asian Natural Products Research 19, 53–100. [DOI] [PubMed] [Google Scholar]
- Dong NQ, Lin HX.. 2021. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. Journal of Integrative Plant Biology 63, 180–209. [DOI] [PubMed] [Google Scholar]
- Eichhorn J, Takada T, Kita Y, Zenk MH.. 1998. Biosynthesis of the Amaryllidaceae alkaloid galanthamine. Phytochemistry 49, 1037–1047. [Google Scholar]
- Elgorashi E, Zschocke S, Van Staden J, Eloff J.. 2003. The anti-inflammatory and antibacterial activities of Amaryllidaceae alkaloids. South African Journal of Botany 69, 448–449. [Google Scholar]
- El-Naggar AH, El-Nasharty AB.. 2009. Effect of growing media and mineral fertilization on growth, flowering, bulbs productivity and chemical constituents of Hippeastrum vittatum, Herb. American-Eurasian Journal of Agricultural & Environmental Sciences 6, 360–371. [Google Scholar]
- Erb M, Kliebenstein DJ.. 2020. Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiology 184, 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein AI. 1967. The incorporation of carbon-14-labeled beta-phenylethylamine derivatives and tritium-labeled vittatine into Amaryllidaceae alkaloids. PhD thesis, Iowa State University.
- Fennell CW, Elgorashi EE, van Staden J.. 2003. Alkaloid production in Crinum moorei cultures. Journal of Natural Products 66, 1524–1526. [DOI] [PubMed] [Google Scholar]
- Ferdausi A, Chang X, Jones M.. 2021. Transcriptomic analysis for differential expression of genes involved in secondary metabolite production in Narcissus pseudonarcissus field derived bulb and in vitro callus. Industrial Crop and Products 168, 113615. [Google Scholar]
- Field B, Fiston-Lavier A-S, Kemen A, Geisler K, Quesneville H, Osbourn AE.. 2011. Formation of plant metabolic gene clusters within dynamic chromosomal regions. Proceedings of the National Academy of Sciences, USA 108, 16116–16121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkers R, van Kaauwen M, Ament K, et al. 2021. Insights from the first genome assembly of onion (Allium cepa). G3 11, jkab243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey M, Chomet P, Glawischnig E, et al. 1997. Analysis of a chemical plant defense mechanism in grasses. Science 277, 699. [DOI] [PubMed] [Google Scholar]
- Fuganti C, Mazza M.. 1971. Relative stereochemistry of protonation and hydroxylation in the biosynthesis of lycorenine and haemanthidine from protocatechualdehyde. Journal of the Chemical Society D: Chemical Communications 1196–1197. [Google Scholar]
- Georgiev V, Ivanov I, Pavlov A.. 2020. Recent progress in Amaryllidaceae biotechnology. Molecules 25, 4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard A. 1877. The proximate principles of the Narcissus pseudonarcissus. Pharmaceutical Journal and Transactions 8, 214. [Google Scholar]
- Ghosal S, Saini KS, Razdan S.. 1985. Crinum alkaloids: their chemistry and biology. Phytochemistry 24, 2141–2156. [Google Scholar]
- Goossens J, Mertens J, Goossens A.. 2017. Role and functioning of bHLH transcription factors in jasmonate signalling. Journal of Experimental Botany 68, 1333–1347. [DOI] [PubMed] [Google Scholar]
- Haist G, Sidjimova B, Yankova-Tsvetkova E, Nikolova M, Denev R, Semerdjieva I, Bastida J, Berkov S.. 2024. Metabolite profiling and histochemical localization of alkaloids in Hippeastrum papilio (Ravena) van Scheepen. Journal of Plant Physiology 296, 154223. [DOI] [PubMed] [Google Scholar]
- Hawkins C, Ginzburg D, Zhao K, et al. 2021. Plant Metabolic Network 15: a resource of genome-wide metabolism databases for 126 plants and algae. Journal of Integrative Plant Biology 63, 1888–1905. [DOI] [PubMed] [Google Scholar]
- He M, Qu C, Gao O, Hu X, Hong X.. 2015. Biological and pharmacological activities of amaryllidaceae alkaloids. RSC Advances 5, 16562–16574. [Google Scholar]
- He Y, Xu M, Chen X.. 2020a. De novo transcriptomics analysis of the floral scent of Chinese narcissus. Tropical Plant Biology 13, 172–188. [Google Scholar]
- He Y, Xu M, Li R.. 2020b. Transcriptomic investigation of the basis of corona and petal colour in Chinese narcissus. The Journal of Horticultural Science and Biotechnology 95, 565–577. [Google Scholar]
- Hnin S, Nakashima Y, Kodama T, Morita H.. 2023. Crystal structure analysis of norbelladine 4'-O-methyltransferase from Lycoris longituba. In: 4th International Conference on Natural Product Discovery and Development in the Genomic Era: SIMB.
- Hori TA, Hayashi A, Sasanuma T, Kurita S.. 2006. Genetic variations in the chloroplast genome and phylogenetic clustering of Lycoris species. Genes & Genetic Systems 81, 243–253. [DOI] [PubMed] [Google Scholar]
- Hotchandani T, Desgagne-Penix I.. 2017. Heterocyclic Amaryllidaceae alkaloids: biosynthesis and pharmacological applications. Current Topics in Medicinal Chemistry 17, 418–427. [DOI] [PubMed] [Google Scholar]
- Hotchandani T, de Villers J, Desgagne-Penix I.. 2019. Developmental regulation of the expression of amaryllidaceae alkaloid biosynthetic genes in Narcissus papyraceus. Genes 10, 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P.. 2008. Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Advances in Bioinformatics 2008, 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Li W, Liu Z, Zhang G, Luo Y.. 2021. Molecular cloning and functional characterization of tyrosine decarboxylases from galanthamine-producing Lycoris radiata. Acta Physiologiae Plantarum 43, 84. [Google Scholar]
- Hüttel W, Müller M.. 2021. Regio- and stereoselective intermolecular phenol coupling enzymes in secondary metabolite biosynthesis. Natural Product Reports 38, 1011–1043. [DOI] [PubMed] [Google Scholar]
- Jayawardena TU, Merindol N, Liyanage NS, Desgagné-Penix I.. 2024. Unveiling Amaryllidaceae alkaloids: from biosynthesis to antiviral potential—a review. Natural Product Reports doi: https://doi.org/ 10.1039/d3np00044c. [DOI] [PubMed] [Google Scholar]
- Jayaweera D. 1981. Medicinal plants (indigenous and exotic) used in Ceylon: part I. Colombo: National Science Council of Sri Lanka. [Google Scholar]
- Jiang X, Chen H, Wei X, Cai J.. 2021. Proteomic analysis provides an insight into the molecular mechanism of development and flowering in Lycoris radiata. Acta Physiologiae Plantarum 43, 4. [Google Scholar]
- Jin J, Tian F, Yang D-C, Meng Y-Q, Kong L, Luo J, Gao G.. 2016. PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Research 45, D1040–D1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SW, Park JY, Kang SJ, Park HS, Shim H, Lee TJ, Kang JH, Sung SH, Yang TJ.. 2018. The complete chloroplast genome sequence of Magic Lily (Lycoris squamigera). Mitochondrial DNA Part B: Resources 3, 1210–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z. 2009. Amaryllidaceae and Sceletium alkaloids. Natural Product Reports 26, 363–381. [DOI] [PubMed] [Google Scholar]
- Jin Z. 2013. Amaryllidaceae and Sceletium alkaloids. Natural Product Reports 30, 849–868. [DOI] [PubMed] [Google Scholar]
- Jin Z, Yao G.. 2019. Amaryllidaceae and Sceletium alkaloids. Natural Product Reports 36, 1462–1488. [DOI] [PubMed] [Google Scholar]
- Jones K, Smith JB.. 1967. Chromosome evolution in the genus Crinum. Caryologia 20, 163–179. [Google Scholar]
- Jumper J, Evans R, Pritzel A, et al. 2021. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ka S, Merindol N, Koirala M, Desgagne-Penix I.. 2020. Biosynthesis and biological activities of newly discovered Amaryllidaceae alkaloids. Molecules 25, 4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekwick R. 2002. Latex and laticifers. In: eLS. Chichester, UK: John Wiley & Sons, doi: https://doi.org/ 10.1038/npg.els. [DOI] [Google Scholar]
- Kianfe B, Kühlborn J, Tchuenguem R, Tchegnitegni B, Ponou B, Groß J, Teponno R, Dzoyem J, Opatz T, Tapondjou L.. 2020. Antimicrobial secondary metabolites from the medicinal plant Crinum glaucum A. Chev. (Amaryllidaceae). South African Journal of Botany 133, 161–166. [Google Scholar]
- Kilgore MB. 2015. The identification of alkaloid pathway genes from non-model plant species in the Amaryllidaceae. PhD thesis, Washington University, St. Loui:.
- Kilgore MB, Augustin MM, May GD, Crow JA, Kutchan TM.. 2016a. CYP96T1 of Narcissus sp. aff. pseudonarcissus catalyzes formation of the Para–Para' C–C phenol couple in the Amaryllidaceae alkaloids. Frontiers in Plant Science 7, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore MB, Augustin MM, Starks CM, O’Neil-Johnson M, May GD, Crow JA, Kutchan TM.. 2014. Cloning and characterization of a norbelladine 4'-O-methyltransferase involved in the biosynthesis of the Alzheimer’s drug galanthamine in Narcissus sp. aff. pseudonarcissus. PLoS One 9, e103223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore MB, Holland CK, Jez JM, Kutchan TM.. 2016b. Identification of a noroxomaritidine reductase with Amaryllidaceae alkaloid biosynthesis related activities. Journal of Biological Chemistry 291, 16740–16752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore MB, Kutchan TM.. 2016. The Amaryllidaceae alkaloids: biosynthesis and methods for enzyme discovery. Phytochemistry Reviews 15, 317–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohelová E, Maříková J, Korábečný J, et al. 2021. Alkaloids of Zephyranthes citrina (Amaryllidaceae) and their implication to Alzheimer’s disease: isolation, structural elucidation and biological activity. Bioorganic Chemistry 107, 104567. [DOI] [PubMed] [Google Scholar]
- Koirala M, Goncalves dos Santos CK, Gélinas S-E, Ricard S, Karimzadegan V, Lamichhane B, Sameera Liyanage N, Merindol N, Desgagné-Penix I.. 2023. Auxin and light-mediated regulation of growth, morphogenesis, and alkaloid biosynthesis in Crinum × powellii ‘Album’ callus. Phytochemistry 216, 113883. [DOI] [PubMed] [Google Scholar]
- Koirala M, Karimzadegan V, Liyanage NS, Mérindol N, Desgagné-Penix I.. 2022. Biotechnological approaches to optimize the production of Amaryllidaceae alkaloids. Biomolecules 12, 893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konyves K, Bilsborrow J, Christodoulou MD, Culham A, David J.. 2021. Comparative plastomics of Amaryllidaceae: inverted repeat expansion and the degradation of the ndh genes in Strumaria truncata Jacq. PeerJ 9, e12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamesch P, Berardini TZ, Li D, et al. 2012. The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Research 40, D1202–D1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau W, Fischbach MA, Osbourn A, Sattely ES.. 2014. Key applications of plant metabolic engineering. PLoS Biology 12, e1001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch IJ, Johnston E, Pellicer J, Hidalgo O, Bennett MD.. 2019. Angiosperm DNA C-values database. https://cvalues.science.kew.org/. [DOI] [PubMed] [Google Scholar]
- Lemos Cruz P, Carqueijeiro I, Koudounas K, et al. 2023. Identification of a second 16-hydroxytabersonine-O-methyltransferase suggests an evolutionary relationship between alkaloid and flavonoid metabolisms in Catharanthus roseus. Protoplasma 260, 607–624. [DOI] [PubMed] [Google Scholar]
- Li C, Wood JC, Vu AH, et al. 2023. Single-cell multi-omics in the medicinal plant Catharanthus roseus. Nature Chemical Biology 19, 1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Duncan S, Li Y, Huang S, Luo M.. 2024. Decoding plant specialized metabolism: new mechanistic insights. Trends in Plant Science 29, 535–545. [DOI] [PubMed] [Google Scholar]
- Li Q, Xu J, Yang L, Zhou X, Cai Y, Zhang Y.. 2020. Transcriptome analysis of different tissues reveals key genes associated with galanthamine biosynthesis in Lycoris longituba. Frontiers in Plant Science 11, 519752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Xu J, Zheng Y, Zhang Y, Cai Y.. 2021. Transcriptomic and metabolomic analyses reveals that exogenous methyl jasmonate regulates galanthamine biosynthesis in Lycoris longituba seedlings. Frontiers in Plant Science 12, 713795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Qiao C, Pang J, Zhang G, Luo Y.. 2019. The versatile O-methyltransferase LrOMT catalyzes multiple O-methylation reactions in amaryllidaceae alkaloids biosynthesis. International Journal of Biological Macromolecules 141, 680–692. [DOI] [PubMed] [Google Scholar]
- Li W, Yang Y, Qiao C, Zhang G, Luo Y.. 2018. Functional characterization of phenylalanine ammonia-lyase-and cinnamate 4-hydroxylase-encoding genes from Lycoris radiata, a galanthamine-producing plant. International Journal of Biological Macromolecules 117, 1264–1279. [DOI] [PubMed] [Google Scholar]
- Li X, Tang D, Du H, Shi Y.. 2018. Transcriptome sequencing and biochemical analysis of perianths and coronas reveal flower color formation in Narcissus pseudonarcissus. International Journal of Molecular Science 19, 4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang Z, Yang L, Xu J, Zhang Y.. 2022. Expression level of B- and C-class MADS-box genes is associated with the petaloidy of stamens in cultivated amaryllis (Hippeastrum hybridum). The Journal of Horticultural Science and Biotechnology 97, 211–223. [Google Scholar]
- Li Y, Gao R, Zhang J, et al. 2022. The biochemical and molecular investigation of flower color and scent sheds lights on further genetic modification of ornamental traits in Clivia miniata. Horticulture Research 9, uhac114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li J, Qian B, Cheng L, Xu S, Wang R.. 2018. De novo biosynthesis of p-coumaric acid in E. coli with a trans-cinnamic acid 4-hydroxylase from the Amaryllidaceae plant Lycoris aurea. Molecules 23, 3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscombe DK, MacLeod BP, Loukanina N, Nandi OI, Facchini PJ.. 2005. Evidence for the monophyletic evolution of benzylisoquinoline alkaloid biosynthesis in angiosperms. Phytochemistry 66, 1374–1393. [DOI] [PubMed] [Google Scholar]
- Liu H, Wei J, Yang T, et al. 2019. Molecular digitization of a botanical garden: high-depth whole-genome sequencing of 689 vascular plant species from the Ruili Botanical Garden. GigaScience 8, giz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy C, Schneider L.. 2006. Galantamine for Alzheimer’s disease and mild cognitive impairment. Cochrane Database of Systematic Reviews 2006, CD001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majhi BB, Gélinas S-E, Mérindol N, Ricard S, Desgagné-Penix I.. 2023. Characterization of norbelladine synthase and noroxomaritidine/norcraugsodine reductase reveals a novel catalytic route for the biosynthesis of Amaryllidaceae alkaloids including the Alzheimer’s drug galanthamine. Frontiers in Plant Science 14, 1231809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JD, Fales HM, Mudd SH.. 1963. Alkaloids and plant metabolism: VI. O-methylation in vitro of norbelladine, a precursor of amaryllidaceae alkaloids. Journal of Biological Chemistry 238, 3820–3823. [PubMed] [Google Scholar]
- Matsuba Y, Nguyen TTH, Wiegert K, et al. 2013. Evolution of a complex locus for terpene biosynthesis in Solanum. The Plant Cell 25, 2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena S, Kanthaliya B, Joshi A, Khan F, Choudhary S, Arora J.. 2022. In vitro production of alkaloids. In: Belwal T, Georgiev MI, Al-Khayri JM, eds. Nutraceuticals production from plant cell factory. Singapore: Springer Nature, 143–168. [Google Scholar]
- Meerow AW, Fay MF, Guy CL, Li QB, Zaman FQ, Chase MW.. 1999. Systematics of Amaryllidaceae based on cladistic analysis of plastid sequence data. American Journal of Botany 86, 1325–1345. [PubMed] [Google Scholar]
- Mehta N, Meng Y, Zare R, Kamenetsky-Goldstein R, Sattely E.. 2024. A developmental gradient reveals biosynthetic pathways to eukaryotic toxins in monocot geophytes. Cell 187, 5620–5637. [DOI] [PubMed] [Google Scholar]
- Méteignier L-V, Nützmann H-W, Papon N, Osbourn A, Courdavault V.. 2023. Emerging mechanistic insights into the regulation of specialized metabolism in plants. Nature Plants 9, 22–30. [DOI] [PubMed] [Google Scholar]
- Moriya Y, Shigemizu D, Hattori M, Tokimatsu T, Kotera M, Goto S, Kanehisa M.. 2010. PathPred: an enzyme-catalyzed metabolic pathway prediction server. Nucleic Acids Research 38, W138–W143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro-Villanueva F, Nett RS.. 2023. Emerging functions within the enzyme families of plant alkaloid biosynthesis. Phytochemistry Reviews. doi: https://doi.org/ 10.1007/s11101-023-09901-z [DOI] [Google Scholar]
- Nair JJ, van Staden J.. 2013. Pharmacological and toxicological insights to the South African Amaryllidaceae. Food and Chemical Toxicology 62, 262–275. [DOI] [PubMed] [Google Scholar]
- Nair JJ, van Staden J.. 2020. Insight to the antifungal properties of Amaryllidaceae constituents. Phytomedicine 73, 152753. [DOI] [PubMed] [Google Scholar]
- Nett RS, Dho Y, Low Y-Y, Sattely ES.. 2021. A metabolic regulon reveals early and late acting enzymes in neuroactive Lycopodium alkaloid biosynthesis. Proceedings of the National Academy of Sciences, USA 118, e2102949118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett RS, Lau W, Sattely ES.. 2020. Discovery and engineering of colchicine alkaloid biosynthesis. Nature 584, 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T-D, Dang T-TT.. 2021. Cytochrome P450 enzymes as key drivers of alkaloid chemical diversification in plants. Frontiers in Plant Science 12, 682181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olin JT, Schneider L.. 2002. Galantamine for Alzheimer’s disease. Cochrane Database of Systematic Reviews. 2002, CD001747. [DOI] [PubMed] [Google Scholar]
- One Thousand Plant Transcriptomes Initiative. 2019. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 574, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otomo K, Kanno Y, Motegi A, et al. 2004. Diterpene cyclases responsible for the biosynthesis of phytoalexins, momilactones A, B, and oryzalexins A–F in rice. Bioscience, Biotechnology, and Biochemistry 68, 2001–2006. [DOI] [PubMed] [Google Scholar]
- Park CH, Yeo HJ, Park YE, Baek S-A, Kim JK, Park SU.. 2019. Transcriptome analysis and metabolic profiling of Lycoris radiata. Biology 8, 63–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C-X, Liang F, Xia Y-H, Zhao K-L, Hou M-H, Zhang G-J.. 2023. Recent advances and challenges in protein structure prediction. Journal of Chemical Information and Modeling 64, 76–95. [DOI] [PubMed] [Google Scholar]
- Peng W, Li Z, Wang S, Wang B.. 2023. Unravelling the CC and CN coupling mechanism for the CYP96T1-catalyzed biosynthesis of Amaryllidaceae alkaloids. Molecular Catalysis 550, 113609. [Google Scholar]
- Ptak A, Morańska E, Saliba S, Zieliński A, Simlat M, Laurain-Mattar D.. 2017. Elicitation of galanthamine and lycorine biosynthesis by Leucojum aestivum L. and L. aestivum ‘Gravety Giant’ plants cultured in bioreactor RITA®. Plant Cell, Tissue and Organ Culture 128, 335–345. [Google Scholar]
- Ptak A, Morańska E, Skrzypek E, Warchoł M, Spina R, Laurain-Mattar D, Simlat M.. 2020. Carbohydrates stimulated Amaryllidaceae alkaloids biosynthesis in Leucojum aestivum L. plants cultured in RITA® bioreactor. PeerJ 8, e8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak A, Morańska E, Warchoł M, Gurgul A, Skrzypek E, Dziurka M, Laurain-Mattar D, Spina R, Jaglarz A, Simlat M.. 2022. Endophytic bacteria from in vitro culture of Leucojum aestivum L. a new source of galanthamine and elicitor of alkaloid biosynthesis. Scientific Reports 12, 13700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulman J. 2014. A transcriptomics approach to understanding polymorphic and transcript level differences linked to isoquinoline alkaloid production in triploid varieties of Narcissus pseudonarcissus. PhD thesis, University of Liverpool.
- Qu Y, Safonova O, De Luca V.. 2019. Completion of the canonical pathway for assembly of anticancer drugs vincristine/vinblastine in Catharanthus roseus. The Plant Journal 97, 257–266. [DOI] [PubMed] [Google Scholar]
- Rapp JT, Bremer BJ, Romero PA.. 2024. Self-driving laboratories to autonomously navigate the protein fitness landscape. Nature Chemical Engineering 1, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis A, Magne K, Massot S, Tallini LR, Scopel M, Bastida J, Ratet P, Zuanazzi JAS.. 2019. Amaryllidaceae alkaloids: identification and partial characterization of montanine production in Rhodophiala bifida plant. Scientific Reports 9, 8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Yang J, Lu B, Jiang Y, Chen H, Hong Y, Wu B, Miao Y.. 2017. Structure of pigment metabolic pathways and their contributions to white tepal color formation of Chinese Narcissus tazetta var. chinensis cv Jinzhanyintai. International Journal of Molecular Sciences 18, 1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren ZM, Zhang D, Jiao C, Li DQ, Wu Y, Wang XY, Gao C, Lin YF, Ruan YL, Xia YP.. 2022. Comparative transcriptome and metabolome analyses identified the mode of sucrose degradation as a metabolic marker for early vegetative propagation in bulbs of Lycoris. The Plant Journal 112, 115–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønsted N, Symonds MR, Birkholm T, Christensen SB, Meerow AW, Molander M, Mølgaard P, Petersen G, Rasmussen N, Van Staden J.. 2012. Can phylogeny predict chemical diversity and potential medicinal activity of plants? A case study of Amaryllidaceae. BMC Evolutionary Biology 12, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Liang L, Xiao X, Feng P, Ye M, Liu J.. 2018. Lycorine: a prospective natural lead for anticancer drug discovery. Biomedicine & Pharmacotherapy 107, 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru Q, Wang X, Liu T, Zheng H.. 2013. Physiological and comparative proteomic analyses in response to nitrogen application in an Amaryllidaceae plant, Lycoris aurea. Acta Physiologiae Plantarum 35, 271–282. [Google Scholar]
- Santos-Gally R, De Castro A, Pérez-Barrales R, Arroyo J.. 2015. Stylar polymorphism on the edge: unusual flower traits in Moroccan Narcissus broussonetii (Amaryllidaceae). Botanical Journal of the Linnean Society 177, 644–656. [Google Scholar]
- Santucci B, Picardo M.. 1992. Occupational contact dermatitis to plants. Clinics in Dermatology 10, 157–165. [DOI] [PubMed] [Google Scholar]
- Shi H, Wu X, Zhu Y, et al. 2024. RefMetaPlant: a reference metabolome database for plants across five major phyla. Nucleic Acids Research 52, D1614–D1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura K, Okada A, Okada K, et al. 2007. Identification of a biosynthetic gene cluster in rice for momilactones. Journal of Biological Chemistry 282, 34013–34018. [DOI] [PubMed] [Google Scholar]
- Shitan N, Kato K, Shoji T.. 2014. Alkaloid transporters in plants. Plant Biotechnology 31, 453–463. [Google Scholar]
- Singh A, Desgagné-Penix I.. 2017. Transcriptome and metabolome profiling of Narcissus pseudonarcissus ‘King Alfred’ reveal components of Amaryllidaceae alkaloid metabolism. Scientific Reports 7, 17356–17356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Massicotte M-A, Garand A, Tousignant L, Ouellette V, Bérubé G, Desgagné-Penix I.. 2018. Cloning and characterization of norbelladine synthase catalyzing the first committed reaction in Amaryllidaceae alkaloid biosynthesis. BMC Plant Biology 18, 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochacki D, Podwyszyńska M, Machlańska A, Dyki B.. 2022. Nuclear DNA content, selected morphological and anatomical traits of Narcissus cultivars and breeding clones. Agronomy 12, 648. [Google Scholar]
- Song J, Xu X, Chen Y, Chen G, Lai Z.. 2019. Analyses of leaft transcriptome under heat stress in Narcissus. Chinese Journal of Tropical Crops 40, 1991–2000. [Google Scholar]
- Špačková A, Vávra O, Raček T, Bazgier V, Sehnal D, Damborský J, Svobodová R, Bednář D, Berka K.. 2024. ChannelsDB 2.0: a comprehensive database of protein tunnels and pores in AlphaFold era. Nucleic Acids Research 52, D413–D418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreeraman S, Kannan MP, Singh Kushwah RB, Sundaram V, Veluchamy A, Thirunavukarasou A, Saravanan KM.. 2023. Drug design and disease diagnosis: the potential of deep learning models in biology. Current Bioinformatics 18, 208–220. [Google Scholar]
- Stander EA, de Bernonville TD, Papon N, Courdavault V.. 2022. Chromosome-scale genomes throw light on plant drug biosynthesis. Trends in Pharmacological Sciences 43, 542–545. [DOI] [PubMed] [Google Scholar]
- Su Y, Li HP, Zhang M, Ding XW, Xu JH, Chen Q, Zheng GW.. 2022. Regioselectivity inversion of an O-methyltransferase via semi-rational mutagenesis combined with metal ion substitution. ChemCatChem 14, e202200844. [Google Scholar]
- Suhadolnik R, Fischer A, Zulalian J.. 1962. The biogenetic origin of the CC unit of lycorine. Journal of the American Chemical Society 84, 4348–4349. [Google Scholar]
- Suhadolnik R, Fischer A, Zulalian J.. 1963. Biogenesis of the Amaryllidaceae alkaloids. II. Studies with whole plants, floral primordia and cell free extracts. Biochemical and Biophysical Research Communications 11, 208–212. [DOI] [PubMed] [Google Scholar]
- Sun B, Wang P, Wang R, Li Y, Xu S.. 2018. Molecular cloning and characterization of a meta/para-O-methyltransferase from Lycoris aurea. International Journal of Molecular Sciences 19, 1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhu S, Li N, et al. 2020. A chromosome-level genome assembly of garlic (Allium sativum) provides insights into genome evolution and allicin biosynthesis. Molecular Plant 13, 1328–1339. [DOI] [PubMed] [Google Scholar]
- Takos AM, Rook F.. 2013. Towards a molecular understanding of the biosynthesis of Amaryllidaceae alkaloids in support of their expanding medical use. International Journal of Molecular Sciences 14, 11713–11741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Li C, Zhang C, Cai Y, Zhang Y, Yang L, Chen M, Zhu F, Li Q, Li K.. 2023. SWATH-MS-based proteomics reveals the regulatory metabolism of Amaryllidaceae alkaloids in three Lycoris species. International Journal of Molecular Sciences 24, 4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z, Hu X, Xu Y, Liu M, Liu H, Li D, Hu L, Wei G, Chen W.. 2024. PMhub 1.0: a comprehensive plant metabolome database. Nucleic Acids Research 52, D1579–D1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousignant L, Diaz-Garza AM, Majhi BB, Gélinas S-E, Singh A, Desgagne-Penix I.. 2022. Transcriptome analysis of Leucojum aestivum and identification of genes involved in norbelladine biosynthesis. Planta 255, 30–30. [DOI] [PubMed] [Google Scholar]
- Trujillo Chacón LM, Leiva H, Zapata Vahos IC, Restrepo DC, Osorio E.. 2023. Influence of plant growth regulators on in vitro biomass production and biosynthesis of cytotoxic Amaryllidaceae alkaloids in Caliphuria tenera Baker. Biocatalysis and Agricultural Biotechnology 50, 102670. [Google Scholar]
- Tsuda Y, Kashiwaba N, Kumar V.. 1984. The alkaloidal constituents of Goda-manel (Crinum zeylanicum L.), a Sri Lankan folk medicine. Chemical and Pharmaceutical Bulletin 32, 3023–3027. [DOI] [PubMed] [Google Scholar]
- Vu GT, Cao HX, Reiss B, Schubert I.. 2017. Deletion-bias in DNA double-strand break repair differentially contributes to plant genome shrinkage. New Phytologist 214, 1712–1721. [DOI] [PubMed] [Google Scholar]
- Waese J, Provart NJ.. 2017. The bio-analytic resource for plant biology. Methods in Molecular Biology 1533, 119–148. [DOI] [PubMed] [Google Scholar]
- Wahkstrøm R, Laane MM.. 2009. Chromosome analyses in African Crinum species (Amaryllidaceae). Hereditas 91, 183–206. [Google Scholar]
- Wang G, Yang B, Wu J, Luo P, Anwar M, Allan AC, Lin-Wang K, Espley RV, Zeng L.. 2018. Identification of genes involved in flavonoid biosynthesis of Chinese narcissus (Narcissus tazetta L. var. chinensis). Plant Molecular Biology Reporter 36, 812–821. [Google Scholar]
- Wang L, Zhang X-Q, Yin Z-Q, Wang Y, Ye W-C.. 2009. Two new amaryllidaceae alkaloids from the bulbs of Lycoris radiata. Chemical and Pharmaceutical Bulletin 57, 610–611. [DOI] [PubMed] [Google Scholar]
- Wang N, Shu X, Zhang F, Song G, Wang Z.. 2024. Characterization of the heat shock transcription factor family in Lycoris radiata and its potential roles in response to abiotic stresses. Plants 13, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Shu X, Zhang F, Zhuang W, Wang T, Wang Z.. 2021. Comparative transcriptome analysis identifies key regulatory genes involved in anthocyanin metabolism during flower development in Lycoris radiata. Frontiers in Plant Science 12, 761862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Yu S, Han X, Xu J, He Q, Xu S, Wang R.. 2020. Identification, molecular characterization and expression of JAZ genes in Lycoris aurea. PLoS One 15, e0230177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QM, Cui J, Dai H, Zhou Y, Li N, Zhang Z.. 2018. Comparative transcriptome profiling of genes and pathways involved in leaf-patterning of Clivia miniata var. variegata. Gene 677, 280–288. [DOI] [PubMed] [Google Scholar]
- Wang R, Han X, Xu S, Xia B, Jiang Y, Xue Y, Wang R.. 2019. Cloning and characterization of a tyrosine decarboxylase involved in the biosynthesis of galanthamine in Lycoris aurea. PeerJ 7, e6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Liu Y, Xu S, Li J, Zhou J, Wang R.. 2021. An ATP-binding cassette transporter, LaABCB11, contributes to alkaloid transport in Lycoris aurea. International Journal of Molecular Sciences 22, 11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Xu S, Jiang Y, Jiang J, Li X, Liang L, He J, Peng F, Xia B.. 2013. De novo sequence assembly and characterization of Lycoris aurea transcriptome using GS FLX titanium platform of 454 pyrosequencing. PLoS One 8, e60449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Xu S, Wang N, Xia B, Jiang Y, Wang R.. 2017. Transcriptome analysis of secondary metabolism pathway, transcription factors, and transporters in response to methyl jasmonate in Lycoris aurea. Frontiers in Plant Science 7, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Wang PK, Huang MR, Zhou J, Han ZM.. 2007. The localization of galanthamine in vegetative organ of Lycoris aurea Herb. Journal of Molecular Cell Biology 40, 339–345. [PubMed] [Google Scholar]
- Wang Y, Chen D, He X, Shen J, Xiong M, Wang X, Zhou D, Wei Z.. 2018. Revealing the complex genetic structure of cultivated amaryllis (Hippeastrum hybridum) using transcriptome-derived microsatellite markers. Scientific Reports 8, 10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Song G, Zhang F, Shu X, Wang N.. 2023. Functional characterization of AP2/ERF transcription factors during flower development and anthocyanin biosynthesis related candidate genes in Lycoris. International Journal of Molecular Sciences 24, 14464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins JL, Facchini PJ.. 2022. Compartmentalization at the interface of primary and alkaloid metabolism. Current Opinion in Plant Biology 66, 102186. [DOI] [PubMed] [Google Scholar]
- Wildman W. 1960. Alkaloids of the Amaryllidaceae. The Alkaloids: Chemistry and Physiology 6, 289–413. [Google Scholar]
- Wildman W, Battersby A, Breuer S.. 1962. Biosynthesis in the Amaryllidaceae. Incorporation of 3-C14-tyrosine and phenylalanine in Nerine bowdenii W. Wats. Journal of the American Chemical Society 84, 4599–4600. [Google Scholar]
- Winzer T, Gazda V, He Z, et al. 2012. A Papaver somniferum 10-gene cluster for synthesis of the anticancer alkaloid noscapine. Science 336, 1704–1708. [DOI] [PubMed] [Google Scholar]
- Xiang X, Jiang X, Hu Z, Tang J, Quan M.. 2022. Comparative transcriptome analysis of the key genes associated with the accumulation of lycorine and galanthamine in Lycoris aurea. Bangladesh Journal of Botany 51, 767–777. [Google Scholar]
- Xu J, Li Q, Yang L, Li X, Wang Z, Zhang Y.. 2020. Changes in carbohydrate metabolism and endogenous hormone regulation during bulblet initiation and development in Lycoris radiata. BMC Plant Biology 20, 180–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Sato F.. 2021. Transcription factors in alkaloid engineering. Biomolecules 11, 1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Grzech D, Koudounas K, Stander EA, Caputi L, Mimura T, Courdavault V, O’Connor SE.. 2021. Improved virus-induced gene silencing allows discovery of a serpentine synthase gene in Catharanthus roseus. Plant Physiology 187, 846–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Li CH, Das D, Zheng YH, Song T, Wang LX, Chen MX, Li QZ, Zhang J.. 2021. Comprehensive transcriptome and metabolic profiling of petal color development in Lycoris sprengeri. Frontiers in Plant Science 12, 747131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ren Y, Zhang D, Chen X, Huang J, Xu Y, Aucapina CB, Zhang Y, Miao Y.. 2021. Transcriptome-based WGCNA analysis reveals regulated metabolite fluxes between floral color and scent in Narcissus tazetta flower. International Journal Molecular Science 22, 8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Wu X, Aucapiña CB, Zhang D, Huang J, Hao Z, Zhang Y, Ren Y, Miao Y.. 2023. NtMYB12 requires for competition between flavonol and (pro)anthocyanin biosynthesis in Narcissus tazetta tepals. Molecular Horticulture 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan-Hong S, Tao J, Wan-Wan Z, Wei-Hua R, Han Z, Bin Z, Hong-Mei C, Xiao-Jing C.. 2019. Study on technology and mechanism of ethylene treatment promotes the formation of more flowers of Narcissus tazetta var. chinensis. Journal of Agricultural Biotechnology 27, 1003–1015. [Google Scholar]
- Yue Y, Liu J, Shi T, Chen M, Li Y, Du J, Jiang H, Yang X, Hu H, Wang L.. 2019. Integrating transcriptomic and GC-MS metabolomic analysis to characterize color and aroma formation during tepal development in Lycoris longituba. Plants 8, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza-Puchol D, Ortiz JE, Orden AA, Sanchez M, Palermo J, Tapia A, Bastida J, Feresin GE.. 2021. Alkaloids analysis of Habranthus cardenasianus (Amaryllidaceae), anti-cholinesterase activity and biomass production by propagation strategies. Molecules 26, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CH, Shao XX, Wang XB, Shou LL, Liu YL, Xu ZG, Guo ZY.. 2022. Development of a general bioluminescent activity assay for peptide ligases. FEBS Journal 289, 5241–5258. [DOI] [PubMed] [Google Scholar]
- Zhang F, Cheng G, Shu X, Wang N, Wang Z.. 2022. Transcriptome analysis of Lycoris chinensis bulbs reveals flowering in the age-mediated pathway. Biomolecules 12, 899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang N, Cheng G, Shu X, Wang T, Zhuang W, Lu R, Wang Z.. 2021. Comparative chloroplast genomes of four Lycoris species (Amaryllidaceae) provides new insight into interspecific relationship and phylogeny. Biology 10, 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhou Y, Wu Y, et al. 2022. Balance of carotenoid synthesis and degradation contributes to the color difference between Chinese narcissus and its yellow-tepal mutant. Horticulturae 8, 727. [Google Scholar]
- Zhou J, Liu Z, Wang S, Li J, Li Y, Chen W-K, Wang R.. 2020. Fungal endophytes promote the accumulation of Amaryllidaceae alkaloids in Lycoris radiata. Environmental Microbiology 22, 1421–1434. [DOI] [PubMed] [Google Scholar]
- Zhou J, Stringlis IA, Wen J, Liu Y, Xu S, Wang R.. 2023. Interplay between Amaryllidaceae alkaloids, the bacteriome and phytopathogens in Lycoris radiata. New Phytologist 241, 2258–2274. [DOI] [PubMed] [Google Scholar]
- Zhou P, Peng J, Zeng M, Wu L, Fan Y, Zeng L.. 2021. Virus-induced gene silencing (VIGS) in Chinese narcissus and its use in functional analysis of NtMYB3. Horticultural Plant Journal 7, 565–572. [Google Scholar]
- Zhou Z, Wu M, Sun B, Li J, Li J, Liu Z, Gao M, Xue L, Xu S, Wang R.. 2024. Identification of transcription factor genes responsive to MeJA and characterization of a LaMYC2 transcription factor positively regulates lycorine biosynthesis in Lycoris aurea. Journal of Plant Physiology 296, 154218. [DOI] [PubMed] [Google Scholar]
- Ziegler J, Facchini PJ.. 2008. Alkaloid biosynthesis: metabolism and trafficking. Annual Review of Plant Biology 59, 735–769. [DOI] [PubMed] [Google Scholar]
- Zonneveld BJM, Grimshaw JM, Davis AP.. 2003. The systematic value of nuclear DNA content in Galanthus. Plant Systematics and Evolution 241, 89–102. [Google Scholar]
- Zonneveld BJM, Leitch IJ, Bennett MD.. 2005. First nuclear DNA amounts in more than 300 angiosperms. Annals of Botany 96, 229–244. [DOI] [PMC free article] [PubMed] [Google Scholar]